Abstract
The objective of the study was to conduct a quantitative microbial risk assessment of Bacillus cereus for packaged tofu in Korea. Packaged tofu, including soft and firm tofu from six retail markets in Korea, were monitored to determine the initial contamination level of B. cereus. Predictive growth and survival models were developed to predict the change in B. cereus populations as a function of time and temperature (4–45 °C) from market to home. B. cereus was detected in 11 (12.9%) samples among 85 samples purchased at retail markets, and the average contamination level was 1.84 log CFU/g. Growth of B. cereus in tofu was observed at a temperature above 11 °C. The probability risk of B. cereus diarrheal illness due to packaged tofu consumption at the retail market is 1.0 × 10−4 per person per day. Key risk factors at the retail market and home are storage temperature and time of packaged tofu.
Keywords: Packaged tofu, Bacillus cereus, Quantitative microbial risk assessment, Predictive model, Retail market
Introduction
Tofu, a soy-based food, is known to be healthy because it has low calories and large amounts of proteins (41.3%) with all nine essential amino acids. Besides, it is an excellent source of iron, calcium, and other micro-nutrients (Préstamo and Fontecha, 2007). Tofu is one of the most consumed foods in Korea according to national food and nutrition statistics (KHIDI, 2018). According to the result of a survey on tofu consumption (MFDS, 2012), 46.8% of respondents have reported that they eat tofu once a month, 24.4% once a week, and 3.9% eat more than once a day. A total of 43.7% of these respondents were aware of the risk of foodborne illness from consumption of tofu, which was relatively high compared to that from the consumption of other foods. Besides, the consumption of tofu is expected to increase further due to the social change caused by one-person households and an aging society.
Bacillus spp. is known as the most common foodborne pathogen in tofu. B. cereus, B. subtilis, and Clostridium perfringens have been isolated in sufu (Chinese fermented tofu) (Han et al., 2001). A variety of Bacillus species have also been found in tofu, soybean, soy milk, sum-mul plate, and cutter in the manufacturing environment (Lee and Park, 2012; Lee et al., 2017; Oh et al., 2016). Many pathogenic bacteria, including B. cereus and E. coli, were also detected in tofu sold in Thailand (Ananchaipattana et al., 2012). Also, B. cereus biofilm formation has been concerned in the tofu manufacturing industry (personal communication).
Quantitative microbial risk assessment (QMRA) is a systematic process and scientific quantification of potential adverse effects resulting from human exposure to foodborne pathogens and toxins (CAC, 1999). Several quantitative risk assessment studies of B. cereus have been carried out for pasteurized milk (Acai et al., 2014; Kumari and Sarkar, 2017; Notermans et al., 1997), banked human milk (Lewin et al., 2019), Chinese-style rice (McElroy et al., 1999), and rice cake (Wang, 2014). The QMRA for Chinese style rice revealed that the risk of illness due to the emetic B. cereus was highly correlated with temperature abuse of cooked rice. In the case of rice cake, storage time was the most critical factor affecting the risk of B. cereus in rice cake. Despite the popularity and high intake of tofu in Korea, microbial risk assessment for B. cereus in tofu at the retail market has not been reported yet. Therefore, the objective of this study was to evaluate the risk of B. cereus foodborne illness from packaged tofu at the retail market in Korea.
Materials and methods
To conduct QMRA for B. cereus in tofu, each step in the risk assessment process, including hazard identification, exposure assessment, hazard characterization, and risk characterization, was performed, as shown in Fig. 1.
Fig. 1.
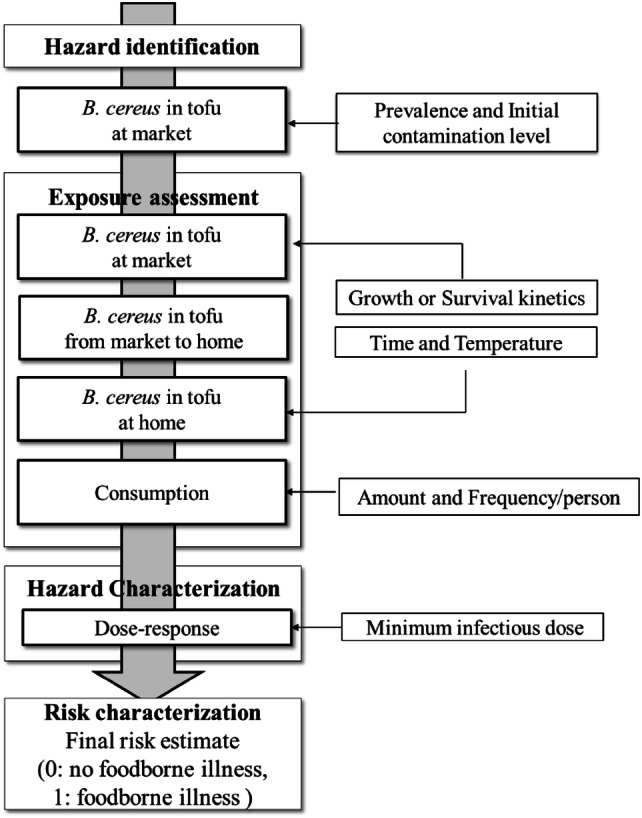
Conceptual model for conducting QMRA on B. cereus in packaged tofu
Hazard identification
To understand direct and indirect effects of B. cereus on human, general characteristics of B. cereus, minimum infective dose, dose–response model, and clinical symptoms were investigated from the literature (Acai et al., 2014; Fratamico et al., 2005; Granum and Lund, 1997; Lewin et al., 2019; Valero et al., 2007).
Prevalence and initial contamination level in tofu at the retail market
To determine the prevalence and initial contamination level of B. cereus in tofu. A total of 85 commercial packaged tofu consisting of 35 soft tofu and 50 firm tofu were collected from six retail markets in the city of Seoul and Gyeonggi Province in South Korea where about 50% of the population lives. Samples were collected monthly from May to August 2017 with a constant sample size as possible. All samples were maintained in their original packaging, transported to the laboratory with ice packs within 4 h, and then subjected to microbiological analysis. Microbiological analysis was performed according to the Korea Food Code (MFDS, 2017) with the modifications. Briefly, 25 g of each sample was cut into small pieces and aseptically placed into the stomacher bags (Circulator 400 standard bags, Seward, Worthing, UK) containing 225 ml of sterile 0.85% saline or tryptic soy broth (TSB, Difco, Becton-Dickinson, Sparks, MD, USA) for quantitative or qualitative analysis, respectively. Beta distribution was used to describe the prevalence (PR) data as a monitoring result (Jeong et al., 2017; Vose, 1998). Initial B. cereus contamination level in tofu was estimated using the formula, as shown in Table 1. Probabilistic distribution of initial contamination level in tofu at the retail market was calculated using @Risk.
Table 1.
Simulation model and formulas in the Excel® spreadsheet used to calculate the risk of B. cereus in packaged tofu with @RISK
| Symbol | Unit | Definition | Formula | References |
|---|---|---|---|---|
| Product at market | ||||
| Pathogens contamination level | ||||
| PR | Prevalence of B. cereus in Tofu | RiskBeta (12, 75) | This research, Vose (1998) | |
| CL | CFU/g | Contamination level of B. cereus | − LN(1 − PR)/25 | Sanaa et al. (2004) |
| IC | Log CFU/g | Initial contamination level | Log(CL) | |
| Market | ||||
| Market storage | ||||
| Mark-Time | h | Storage time in the market | RiskPert (0.17, 48, 168) | This research |
| Mark-Temp | °C | Food temperature during storage in the market | RiskUniform (4, 11.1) | Lee et al. (2008) |
| Growth | ||||
| T1 | IF(MarkTemp ≥ 10, 1, 0) | |||
| SGR1 | Log CFU/h | Specific growth rate | IF(T1 = 1, − 0.722 + (0.0720 * MarkTemp) + (− 0.0008 * MarkTemp2), 0) | This research, McMeekin et al. (1993) |
| LT1 | h | Lag time | IF(T1 = 1, 18.07 + (− 1015/MarkTemp) + (16,071/MarkTemp2), 0) | This research, Davey (1989) |
| C | Fixed | Difference between initial and final cell numbers | 5.026 | This research |
| G1 | Log CFU/g | Growth model | IC + C * EXP(− EXP((2.718 * SGR1/C) * (LT1-MarkTime) + 1)) | This research |
| Survival | ||||
| Delta1 | h | Average of 4 °C and 9 °C, fixed | 281.08 | This research |
| p | Fixed | Average of 4 °C and 9 °C, fixed | 0.55 | This research |
| S1 | Log CFU/g | Survival model | IC − (MarkTime/Delta1)p | This research, Geeraerd et al. (2005) |
| C1 | Log CFU/g | IF(T1 = 1, G1, S1) | ||
| Transportation | ||||
| Trans-Time | h | Time (market to home) | RiskPert(0.325, 0.984, 1.643) | Jung (2011) |
| Trans-Temp | °C | Storage temperature during transportation | RiskPert(10, 18, 25) | Jung (2011) |
| Growth | ||||
| SGR2 | Log CFU/h | Specific growth rate | − 0.722 + (0.072 * TransTemp) + (− 0.0008 * TransTemp2) | This research, McMeekin et al. (1993) |
| LT2 | h | Lag time | 18.07 + (−1015/TransTemp) + (16,071/TransTemp2) | This research, Davey (1991) |
| C | Fixed | Difference between initial and final cell number | 5.026 | This research |
| G2 (C2) | Log CFU/g | Growth model | C1 + C * EXP(− EXP((2.718 * SGR2/C) * (LT2-TransTime) + 1)) | This research |
| Home | ||||
| Home storage | ||||
| Home-Time | h | Storage time until consumption | RiskPert (1,24,168) | MFDS (2012) |
| Home-Temp | °C | Storage temperature until consumption | RiskLoglogistic (− 10.407, 13.616, 8.611) | Bahk (2010) |
| Growth | ||||
| T3 | IF(HomeTemp ≥ 10, 1, 0) | |||
| SGR3 | Log CFU/h | Specific growth rate | IF(T3 = 1, − 0.722 + (0.072 * HomeTemp) + (− 0.0008 * HomeTemp2), 0) | This research, McMeekin et al. (1993) |
| LT3 | h | Lag time | IF(T3 = 1, 18.07 + (− 1015/HomeTemp) + (16,071/HomeTemp2), 0) | This research, Davey (1991) |
| C | Fixed | Difference between initial and final cell numbers | 5.026 | This research |
| G3 | Log CFU/g | Growth model | C2 + C * EXP(− EXP((2.718 * SGR3/C) * (LT3-HomeTime) + 1)) | This research |
| Survival | ||||
| Delta3 | h | Average of 4 °C and 9 °C, fixed | 281.08 | This research |
| p | Fixed | Average of 4 °C and 9 °C, fixed | 0.55 | This research |
| S3 | Log CFU/g | Survival model | C2 – (Home time/delta3)p | This research, Geeraerd et al. (2005) |
| C3 | Log CFU/g | IF(T3 = 1, G3, S3) | ||
| Consumption | ||||
| Consum | g | Daily consumption average amount | RiskInvgauss (101.66, 261.75, RiskShift(− 12.247)), 4.707, 23.985, 175.84) | MFDS (2012) |
| ConFre | %, fixed | Daily consumption frequency | 13.62 | MFDS (2012) |
| CF(0) | Daily non-consumption frequency (rate) | 1 − ConFre/100 | MFDS (2012) | |
| CF(1) | Daily consumption frequency (rate) | ConFre/100 | MFDS (2012) | |
| CF | Distribution for consumption frequency | RiskDiscrete({0, 1}, {CF(0), CF(1)}) | MFDS (2012) | |
| Amount | Daily consumption average amount considered frequency | IF(CF = 0, 0, Consum) | ||
| Hazard characterization (minimum infective dose) | ||||
| Final dose (D) | B. cereus amount | 10C3 × Amount | MFDS (2016) | |
| Risk characterization | ||||
| Risk | Probability of illness/person/day | IF(D ≥ 100,000, 1, 0) | MFDS (2016) | |
Exposure assessment
Strain preparation for the development of a predictive model
Bacillus cereus diarrheal type strains (KCTC 1014, KCTC 1092, KCTC 3624, and KCCM 11204) were purchased from the Korean Federation of Culture Collection (KFCC) and stored frozen at − 80 °C. Then 10 µL of B. cereus vegetative cells was inoculated into 10 mL of sterilized nutrient broth (NB, Difco, Sparks, MD, USA) and incubated at 36 °C for 24 h with shaking at 140 rpm using a rotary shaker (VS-8480, Vision, Daejeon, Korea) to reach a concentration of 8 log CFU/mL or more. Then 2 mL of each pre-cultured strain was mixed in a 50 mL-conical tube (SPL Life Sciences, Pocheon, Korea) to make cocktail strains for inoculation. The suspensions were centrifuged at 4000 rpm for 10 min at 4 °C. The supernatant was removed, and the pellet was washed twice with 0.1% peptone water. The inoculum was prepared by resuspending the pellets in 0.1% peptone water.
Sample preparation and inoculation for model development
After tofu was purchased from local supermarkets in Seoul, Korea, it was aseptically cut into 10 g each (2.5 × 2 × 2 cm), and 100 µL of cocktail stains were inoculated onto the surface of tofu. An initial concentration of B. cereus strains for inoculum was 2.5–3.5 log CFU/g for the growth model and 5.5–6.5 log CFU/g for the survival model. The inoculated tofu was placed in sterile polypropylene (pp) container (30-mL, DAIHAN Scientific, Seoul, Korea) and 10 mL of sterile distilled water was added to simulate actual distribution environment of water-packaged tofu at the retail market. Each inoculated tofu was stored at 4, 9, 10, 11, 17, 30, 40, and 45 °C. At a specific time, 10 g of tofu was homogenized with sterilized 0.1% peptone water for 120 s using a stomacher (Interscience, Paris, France). Then 1 mL of the aliquot of the homogenate was serially diluted tenfold with 0.1% peptone water and spread onto mannitol egg yolk polymyxin agar (MYP, MB cell, Seoul, Korea) and incubated at 30 °C for 24 h. Results are expressed as log values of colony forming units per gram (log CFU/g) of tofu.
Development of predictive survival and growth models
Survival curve representing the viable counts of B. cereus as a function of time was fitted to the Weibull equation as shown in Eq. 1 (Geeraerd et al., 2005) using a Gina FiT V 1.5 program (Microsoft@ Excel 2010, Microsoft Corp., USA).
| 1 |
where N0 is the initial log number of cells (log CFU/g); δ (Delta) is time for the first decimal reduction of bacteria population (h); t is time (h); p is the shape of the curve. The Weibull model corresponds to a concave upward survival curve if p < 1 and concave downward if p > 1. If p = 1, the decrease is log-linear.
The primary growth model at each temperature used the modified Gompertz model, as shown in Eq. 2 (Gibson et al., 1987). The lag time (LT) and the maximum specific growth rate (SGR) were calculated using Graph Pad Prism V 4.0 (Graph-Pad Software, San Diego, CA, USA).
| 2 |
where N0 is the initial log number of cells (log CFU/g), C is the difference between initial and final cell numbers, Lag is lag time before growth (h), SGR is a specific growth rate (log CFU/h), X is sampling time (h), and Y is log cell number (log CFU/g).
Secondary models for LT and SGR were developed to evaluate the effect of storage temperature on LT and SGR with a Davey equation, as shown in Eq. 3 (Davey, 1989) and a polynomial second order equation, as shown in Eq. 4 (McMeekin et al., 1993), respectively.
| 3 |
where Y is lag time (h), a, b, and c are constant, and T is temperature (°C).
| 4 |
where Y is maximum population density (log CFU/g), a, b, and c are constant, and T is temperature (°C).
Validation of model performance
Observed values were obtained at 13 and 25 °C, which were not used for model development to verify the model performance. These observed values were compared to predicted values of B. cereus cell counts (log CFU/g) by the developed predictive model in the present study. Bias factors (Bf; Eqs. 5, 7) and accuracy factors (Af; Eqs. 6, 8) were used to assess the fit of the model.
Bias factors (Bf) and accuracy factors (Af) equations (Ross, 1996) are shown below:
| 5 |
| 6 |
| 7 |
| 8 |
where n is the total number of experimental values (values obtained from independent variables) or predicted values (values obtained from the developed growth model).
Development of scenario from market to home
Changes in contamination level of B. cereus in tofu at market
The information about storage time of tofu at the retail market was directly obtained from the interview with a retail manager. The minimum, mode, and the maximum of storage time at the retail market were 0.17 h, 48 h, and 168 h, respectively. The storage temperature at the market was set to a minimum of 4 °C and a maximum of 11.1 °C according to the study of Lee et al. (2008). Pert and uniform distributions were applied to storage time and temperature, respectively, in this work.
Changes in contamination level of B. cereus in tofu from market to home
The storage time and temperature during transportation from market to home were referred to the study of Jung (2011). The storage time of minimum, mode, and maximum for tofu were 0.325 h, 0.984 h, and 1.643 h, respectively. The storage temperature of minimum, mode, and maximum for tofu were 10, 18, and 25 °C, respectively. Pert distribution was also applied to time and temperature during transportation.
Changes in contamination level of B. cereus in tofu during storage at home before consumption
At the home storage stage before consumption, storage time was set to the minimum of 1 h, mode of 24 h, and the maximum of 168 h according to the report of the Ministry of Food and Drug Safety (2012). Pert distribution was applied to the storage time at home. The storage temperature was set to the minimum of − 5.1 °C, mode of 3.53 °C, and the maximum of 14.4 °C and storage temperature at home was applied to Loglogistic distribution (Bahk, 2010).
Consumption data of tofu in Korea
Data for the frequency and amount of tofu consumption were obtained from the report of the Ministry of Food and Drug Safety (2012). The tofu consumption data was fitted to a suitable probabilistic distribution using the fitting function of @RISK version 7.5 (Palisade, Ithaca, NY, USA).
Hazard characterization
Because dose–response models for B. cereus is limited, no reliable dose–response model for B. cereus is available in the literature (McElroy et al., 1999; Park et al., 2014), previous microbial risk assessment studies showed the limitation of a full risk assessment (Acai et al., 2014; Wang, 2014). In this work, we used the minimum infective dose of B. cereus (105 cells) for hazard characterization (Granum and Lund, 1997; Valero et al., 2007). Simulation conditions according to the assumption were calculated using @ RISK version 7.5 as follows: if the number of the final ingested bacteria is more than the minimum infective dose, then foodborne illness occurred (risk is one). If the number of the final ingested bacteria is less than the minimum infective dose, then no foodborne illness occurred (risk is zero) (MFDS, 2016).
Risk characterization
To estimate the probability risk of foodborne illness per person per day for the intake of B. cereus with consumption of tofu, a Monte Carlo simulation model was used with a set of probabilistic distributions of prevalence and initial contamination levels, predictive model as a function of temperature and time, and frequency and amount of tofu consumption. For dose–response model, the minimum infective dose for B. cereus was used from the literature and input variables in each step of the conceptual model were placed in Excel spreadsheet program (Microsoft@ Excel 2010, Microsoft Corp., USA) and modeled as Table 1. Simulations were conducted using @RISK 7.5 version. Median Latin Hypercube sampling was selected. Iteration was set to more than 10,000 and used as the final simulation result. The sensitivity analysis of the final simulation results was also conducted.
Results and discussion
Hazard identification
Bacillus cereus is a facultative aerobic Gram-positive, spore-forming rod-shaped bacteria, which is widespread in the environment. B. cereus produces endospores and can withstand many food processing conditions, including heat, high pressure, chemical, and low pH. Although its optimal growth temperature is 30–37 °C, it can survive at a minimum temperature of 5 °C and a maximum temperature of 50 °C. Its minimum infectious dose is 105 cells (Granum and Lund, 1997; Valero et al., 2007). B. cereus causes different types of illness by producing two types of toxin-emetic (vomiting) and diarrheal toxins. Diarrheal syndrome is most frequently associated with proteinaceous foods, vegetables, processed meat, and dairy products (Fratamico et al., 2005). Recently, two dominant spoilage bacterial strains, T-1 and T-2 were isolated from spoiled tofu stored at 37 °C for 24 h, which were confirmed as B. cereus by 16 S rRNA sequence and fatty acid composition (Lee et al., 2017).
Prevalence and initial contamination level of B. cereus in tofu at the retail market
As a result of monitoring study in the present study, B. cereus was detected in 11(12.9%) samples among 85 samples purchased at retail markets and diarrheal type B. cereus was in six out of eleven samples, indicating that diarrheal B cereus is important in tofu, which agrees with previous studies. Thirteen cases of the diarrheal type of food poisoning from 2001 to 2007 was reported in Korea (Kim et al., 2010). The diarrheal type of food poisoning is caused by the non-hemolytic enterotoxin (nhe) and hemolysin BL (hbl) genes (Granum and Lund, 1997). Kim et al. (2011) reported that nhe and hbl genes are the primary toxins among the tested 120 B. cereus isolates from clinical and food samples in Korea.
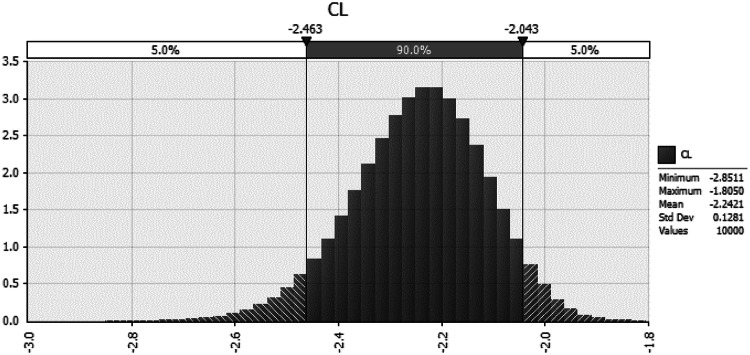
The average contamination level of packaged tofu at the retail market was 1.84 log CFU/g in this work. Beta distribution was selected to describe the prevalence (PR) data from the results of the microbial analysis in this work (Vose, 1998). The Beta distribution describes the true probability (P) of an event occurring, given n (number of trials) and s (number of positive) as follows: (P) = Beta[α1(s + 1), α2 (n − s + 1)]. The probabilistic density for initial concentration (log CFU/g) of B. cereus in tofu in Korea was calculated using the following formula (Sanaa et al., 2004), Log(− LN(1 − PR)/25) as shown in Table 1. As a result, the mean initial contamination level (CL) of B. cereus was estimated to be − 2.242 log CFU/g (Fig. 2).
Fig. 2.
Probabilistic distribution of initial contamination level of B. cereus in tofu
Exposure assessment
Development of predictive model and validation
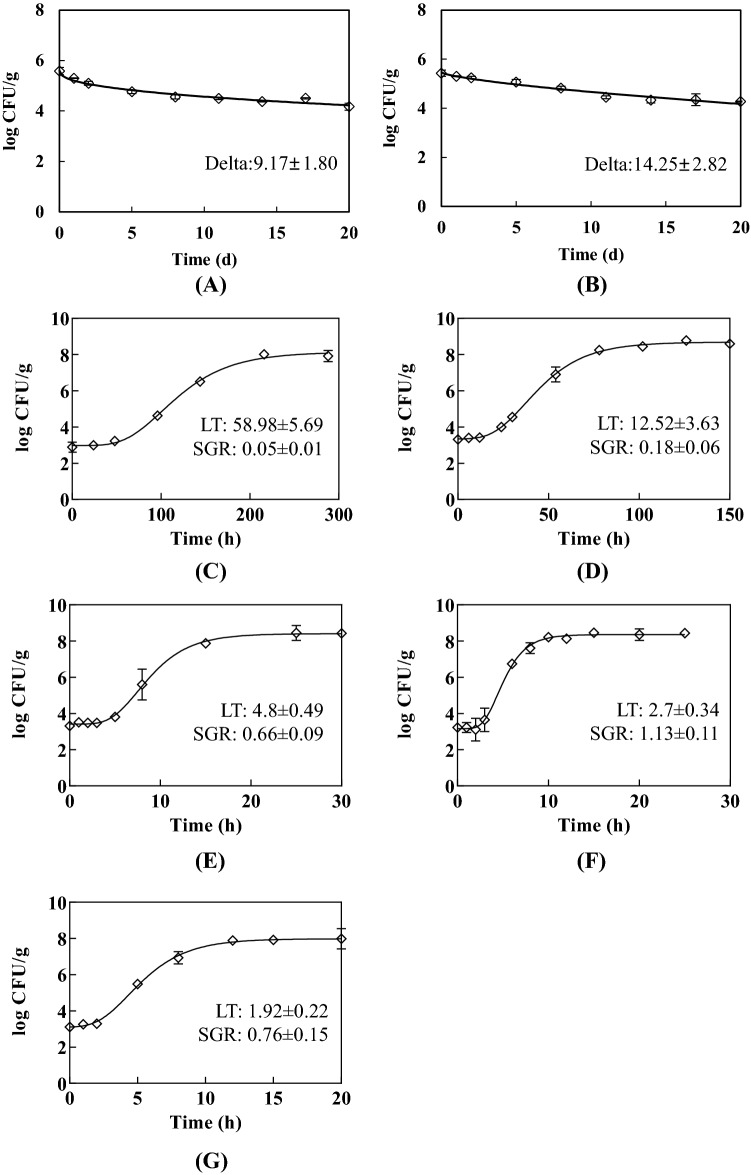
Results for the primary survival model of B. cereus in tofu using the Weibull equation are shown in Fig. 3(A) and (B). B. cereus in tofu showed a tendency to grow above 11 °C and survive below 9 °C. However, B. cereus did not grow or die in tofu stored at 10 °C (data not shown). Thus, B. cereus at 10 °C is considered to be a boundary temperature between growth and survival. Delta values (treatment time for the first decimal reduction) of tofu stored at 4 and 9 °C were 9.17 and 14.25 days, respectively. The lower the storage temperature, the smaller the delta value. However, there was no significant difference in delta value between these two temperatures. p Values (representing the shape of the graph) at 4 and 9 °C were 0.42 and 0.69, respectively. If the p value is greater than 1, it indicates a convex curve in which microorganisms slowly die at the beginning of the storage. If the p value is less than 1, it indicates a concave curve and microorganisms die rapidly at the beginning of the storage (Albert and Mafart, 2005). In this study, the p value was less than one at both temperatures, indicating that these microorganisms decreased rapidly at the beginning of the storage. There was a significant difference in the p value between these two temperatures (p < 0.05). These results indicated that the storage of tofu at the retail market below 9 °C inhibits the growth of B. cereus. To prevent the growth of B. cereus in tofu in the retail market, monitoring the distribution temperature of tofu is needed.
Fig. 3.
Primary predictive models of B. cereus in tofu. (A) 4 °C, (B) 9 °C, (C) 11 °C, (D) 17 °C, (E) 30 °C, (F) 40 °C, (G) 45 °C. Unit: Delta (d), LT(h), SGR(log cfu/h)
Results for the primary growth model of B. cereus in tofu using the modified Gompertz model are shown in Fig. 3(C)–(G). LT values of B. cereus in tofu stored at 11, 17, 30, 40, and 45 °C were 58.98, 12.52, 4.8, 2.7, and 1.92 h, respectively. LT values decreased with increasing storage temperature. SGR values of B. cereus in tofu stored at 11, 17, 30, 40, and 45 °C were 0.05, 0.18, 0.66, 1.13, and 0.76 log CFU/h, respectively. The highest SGR value was observed at 40 °C, and overall SGR values increased with increasing storage temperature. Recently, B. cereus was isolated from spoiled tofu and growth model of B. cereus on tofu were developed at 15, 20, 25, and 30 °C (Lee et al., 2017). LT values of B. cereus were reported to be 9.1, 6.9, 4.9, and 2.9 h, and SGR values were 0.113, 0.327, 0.536, and 1.134 log CFU/h, respectively, indicating that two isolated B. cereus from tofu seem to grow faster than B. cereus in tofu in the present study. However, these different results might also be explained with different methods between these two studies, including different strain and storage method of tofu. Growth models of B. cereus in the bracken at 17, 24, and 35 °C have also been developed in a previous study. LT values were 55.09, 12.35, and 5.37 h, while SGR values were 0.11, 0.33, and 0.34 log CFU/h at 17, 24, and 35 °C, respectively (Enkhjargal et al., 2013). At 13 °C, the LT was 71.65 h, and the SGR was 0.03 log CFU/h in Doraji (Jo et al., 2012). Both studies showed lower growth rates of B. cereus in root vegetables compared to tofu, which has better intrinsic growth conditions. The growth of bacteria is affected by various factors, such as nutrients, pH, water activity, and initial contamination level. Despite the distribution and storage guideline of refrigerated food, 93.5% of tofu in the retail market was sold at room temperature according to results of the survey for distribution temperatures in discount stores, convenience stores, and department stores. It has also been reported that surface and core temperatures of tofu stored in refrigerator range from 6.1 to 10.6 °C and 4 to 11.1 °C, respectively (Yoon et al., 2017), indicating that the growth of B. cereus in the tofu may occur at the retail market.
Growth model performance was validated by the bias factor (Bf) and accuracy factor (Af) (Ross, 1996). Bf is a measure of the relative deviation between the experimental value and the predicted value by the model (appropriate range: 0.70–1.15). On the other hand, Af is a measure of how close the predicted value is to the experimental value (the farther from 1, the more inaccurate) (Oscar, 2005). In the present study, Bf and Af of LT were 0.94 and 1.95, respectively, while Bf and Af of SGR were 1.06 and 1.31, respectively. Both LT and SGR Bf values were in the range of 0.70–1.15. Thus, the developed model in this work was considered to be appropriate. Values of LT and SGR Af were close to 1. Thus, the developed model is accurate to predict the growth of B. cereus in tofu and was used for microbial risk assessment.
The scenario for risk assessment
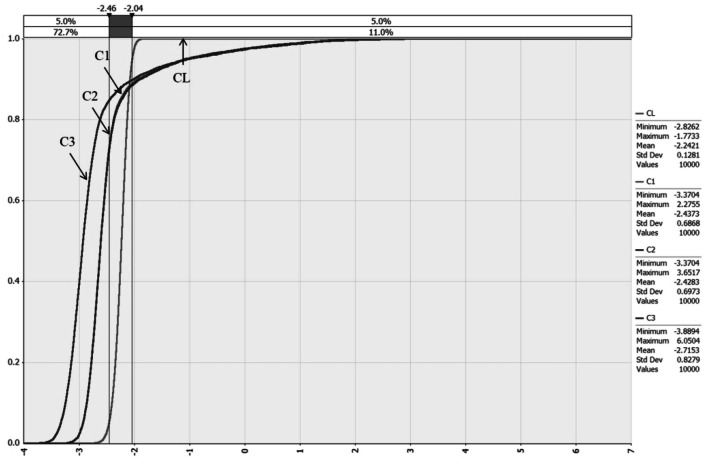
The exposure assessment assumed the worst-case scenario but reflected possible realistic exposure scenarios. According to the results of a survey (MFDS, 2012), 61.7% of respondents ate tofu immediately after purchase, and 38.3% of respondents stored tofu for 20.8 h in the refrigerator. The worst scenario was set that consumers do not eat tofu immediately after purchase. Figure 4 shows changes in the contamination level of B. cereus according to the initial contamination level and distribution scenarios from the retail market to home. The initial contamination level of B. cereus in tofu was − 2.24 log CFU/g (CL) at the retail market. During storage at the market, B. cereus contamination level was slightly decreased to − 2.44 log CFU/g (C1). During transport from the market to home (Table 1), the contamination level was changed to − 2.43 log CFU/g (C2) because of the transportation temperature (minimum 10 °C, mode 18 °C, and maximum 25 °C). Before consumption at home, the level of contamination was finally estimated to be − 2.72 log CFU/g (C3) because of refrigerator temperature at home. In conclusion, the contamination level of B. cereus in tofu decreased during storage from the retail market to home before consumption.
Fig. 4.
Cumulative distribution for comparing the contamination level of B. cereus in tofu from the initial contamination level to home IC, Initial contamination level; C1, Market storage; C2, Transportation; C3, Before consumption
Tofu consumption data in Korea
The daily average frequency and consumption amount of tofu in Korea were based on survey data from MFDS (2012). A total of 1000 adults aged over 18 years who were at six major provinces [Seoul, Incheon (Gyeonggi), Gangwon, Daejeon (Chungcheong), Gwangju (Jeolla), Daegu (Kyungbuk), Busan, Ulsan (Kyungnam)] in Korea were surveyed. RiskInvGauss distribution was considered to be the most suitable one for estimating the average consumption amount of tofu by @RISK distribution fitting function. The daily average consumption amount of tofu was 89.42 g, and the daily average frequency was 13.62%.
Hazard characterization and risk characterization
Because there are no reliable human or animal feeding studies for the B. cereus, we considered the minimum infectious dose for B. cereus instead of the exposure-dose model to evaluate the risk of B. cereus foodborne illness from packaged tofu at the retail market in this work. The final dose (D) of B. cereus consumed by the consumer was calculated based on the final contamination level of B. cereus, the daily consumption average amount (89.42 g), and the frequency (13.62%) using the formula of 10^final contamination level × Amount (Table 1). The final risk was expressed as 1 if the final dose was more than 105 (minimum infective dose) or 0 if it was less than 105. The closer the risk value is to 1, the more food poisoning may occur. The probability risk of B. cereus foodborne illness from tofu consumption per person per day was 1.0 × 10−4 in this work, which can be considered low. This study was based on the assumption that packaged tofu at the retail market was contaminated with diarrheal type B. cereus vegetative cells and consumed without heating. Bacillus enterotoxin causes diarrheal illness, which is frequently linked with protein foods such as tofu and meat product. B. cereus spore and the emetic type B. cereus toxin are heat resistant and survive cooking. Thus the risk may be changed according to the state (vegetative cell vs. spore) and type (diarrheal vs. emetic) of B. cereus in packaged tofu. Although the enterotoxin type of B. cereus is predominant in clinical and food samples (Kim et al., 2011), it is necessary to monitor for B. cereus contamination in tofu periodically.
In comparison, the mean number of annual cases of B. cereus for pasteurized milk produced in Slovakia was 0.054/100,000 populations (Acai et al., 2014). The risk of emetic type B. cereus associated with the consumption of Chinese-style rice was 6.11 × 10−3 for overall handling scenarios (McElroy et al., 1999). The probability risk of Staphylococcus aureus in processed cheese, causing foodborne illness per person per day is 2.24 × 10−9 (Lee et al., 2015). Since the assumption of microbial risk assessment varies among studies, it is not appropriate to compare the risk of foodborne illness. The limitations of the present microbial risk assessment of B. cereus in tofu are that we did not consider spore or emetic type toxin present in packaged tofu.
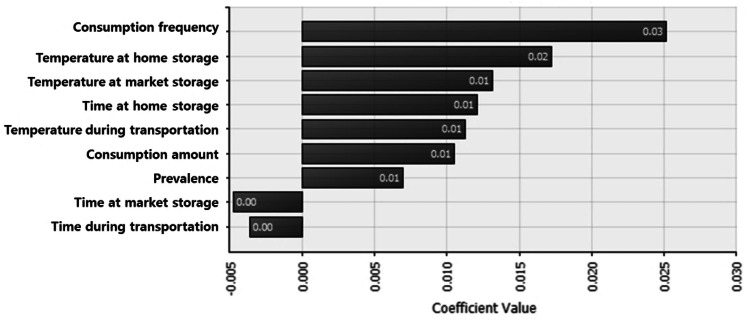
Sensitivity analysis was used to show the influence of input distribution on the change in the value of the output (Fig. 5). The most influential factor for B. cereus risk in tofu was the consumption frequency, followed by the storage temperature at home and retail market. Although the consumption frequency had the most significant impact, it cannot be controlled. Therefore, careful attention to temperature management of packaged tofu at home and the retail market may reduce the risk of B. cereus. Besides, storage time at home is also a very critical factor for the risk of B. cereus in tofu under the worst scenario case (168 h storage at 14.4 °C).
Fig. 5.
The correlation coefficient for sensitivity analysis affecting illness by B. cereus with consumption of tofu numbers in the bars represent coefficient value
In conclusion, quantitative microbial risk assessment was conducted to evaluate the risk of diarrheal type B. cereus in packaged tofu from the retail market to home for safe distribution management. Growth and survival of B. cereus in packaged tofu were observed at the temperature above 11 °C and below 9 °C, respectively. B. cereus did not grow or die in tofu stored at 10 °C in this work. The probability of exposure assessment data and daily consumption amount and frequency were numerically combined, and a minimum infective dose of B. cereus was applied to the @RISK program. The probability risk of diarrheal type B. cereus foodborne illness from tofu consumption is 1.0 × 10−4 per person per day. In this work, a low level of B. cereus (less than 100 per g) was found in packaged tofu at the retail market, and thus the probability risk of food poisoning was low although the high consumption rate and growth potential of B. cereus in tofu during transportation from the retail market to home were observed. Since the microbiological standard for B. cereus in tofu should be less than 1000 per gram, storage temperature should be carefully monitored to prevent the growth of B. cereus in packaged tofu at the retail market and the traditional market as well. Moreover, various control measure should be emphasized to minimize B. cereus contamination during tofu manufacturing and thus reduce the risk of B. cereus foodborne illness in Korea.
Acknowledgements
This research was supported by a Grant (17162MFDS035) from the Ministry of Food and Drug Safety in 2017.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest regarding the publication of this paper.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mi Jin Kwon, Email: kmj9303@hanmail.net.
Min Suk Rhee, Email: rheems@korea.ac.kr.
Ki Sun Yoon, Email: ksyoon@khu.ac.kr.
References
- Acai P, Valik L, Liptakova D. Quantitative risk assessment of Bacillus cereus in pasteurized milk produced in the Slovak Republic. Czech J. Food Sci. 2014;2:122–131. doi: 10.17221/106/2013-CJFS. [DOI] [Google Scholar]
- Albert I, Mafart P. A modified Weibull model for bacterial inactivation. Int. J. Food Microbiol. 2005;100:197–211. doi: 10.1016/j.ijfoodmicro.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Ananchaipattana C, Hosotani Y, Kawasaki S, Pongswat S, Latiful BM, Isobe S, Inatsu Y. Bacterial contamination of soybean curd (tofu) sold in Thailand. Food Sci. Technol. Res. 2012;18:843–848. doi: 10.3136/fstr.18.843. [DOI] [Google Scholar]
- Bahk GJ. Statistical probability analysis of storage temperatures of domestic refrigerator as a risk factor of foodborne illness outbreak. Korean J. Food Sci. Technol. 2010;42:373–376. [Google Scholar]
- CAC (Codex Alimentarius Commission). Principles and guidelines for the conduct of a microbiological risk assessment. CAC/GL-30, Rome, Italy: FAO/WHO (1999)
- Davey KR. A predictive model for combined temperature and water activity on microbial growth during the growth phase. J. Appl. Microbiol. 1989;67:483–488. doi: 10.1111/j.1365-2672.1989.tb02519.x. [DOI] [PubMed] [Google Scholar]
- Davey KR. Applicability of the Davey (linear Arrhenius) predictive model to the lag phase of microbial growth. J Appl Bacteriol. 1991;70:253–257. doi: 10.1111/j.1365-2672.1991.tb02933.x. [DOI] [Google Scholar]
- Enkhjargal L, Min KJ, Yoon KS. Development and validation of predictive model for foodborne pathogens in preprocessed namuls and wild root vegetables. J. Korean Soc. Food Sci. Nutr. 2013;42:1690–1700. doi: 10.3746/jkfn.2013.42.10.1690. [DOI] [Google Scholar]
- Fratamico PM, Bhunia AK, Smith JL. Foodborne pathogens: microbiology and molecular biology. Norwich: Caister Academic Press; 2005. [Google Scholar]
- Geeraerd AH, Valdramidis VP, Van Impe JF. GInaFiT, a freeware tool to assess non-log-linear microbial survivor curves. Int. J. Food Microbiol. 2005;102:95–105. doi: 10.1016/j.ijfoodmicro.2004.11.038. [DOI] [PubMed] [Google Scholar]
- Gibson AM, Bratchell N, Roberts TA. The effect of sodium chloride and temperature on the rate and extent of growth of Clostridium botulinum type A in pasteurized pork slurry. J. Appl. Microbiol. 1987;62:479–490. doi: 10.1111/j.1365-2672.1987.tb02680.x. [DOI] [PubMed] [Google Scholar]
- Granum PE, Lund T. Bacillus cereus and its food poisoning toxins. FEMS Microbiol. Lett. 1997;157:223–228. doi: 10.1111/j.1574-6968.1997.tb12776.x. [DOI] [PubMed] [Google Scholar]
- Han BZ, Beumer RR, Rombouts FM, Nout MJR. Microbiological safety and quality of commercial sufu–a Chinese fermented soybean food. Food Control. 2001;12:541–547. doi: 10.1016/S0956-7135(01)00064-0. [DOI] [Google Scholar]
- Jeong J, Lee J, Lee H, Lee S, Kim S, Ha J, Yoon K, Yoon Y. Quantitative microbial risk assessment for Campylobacter foodborne illness in raw beef offal consumption in South Korea. J. Food Prot. 2017;4:609–618. doi: 10.4315/0362-028X.JFP-16-159. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Hong SH, Kim YG, Shin DB, Oh MH, Hwang JH, Lkhagvasarnai E, Yoon KS. Validation of broth model for growth of Bacillus cereus in blanched vegetables. J. East Asian Soc. Diet. Life. 2012;22:558–565. [Google Scholar]
- Jung H. Consumer survey and hazard analysis for the improvement of food hygiene and safety in purchase. MS thesis, Korea University, Seoul, South Korea (2011)
- Kim JB, Jeong HR, Park YB, Kim JM, Oh DH. Food poisoning associated with emetic-type of Bacillus cereus in Korea. Foodborne Pathog. Dis. 2010;7:555–563. doi: 10.1089/fpd.2009.0443. [DOI] [PubMed] [Google Scholar]
- Kim JB, Kim JM, Cho SH, Oh HS, Choi NJ, Oh DH. Toxin genes profiles and toxin production ability of Bacillus cereus isolated from clinical and food samples. J. Food Sci. 2011;76:25–29. doi: 10.1111/j.1750-3841.2010.01958.x. [DOI] [PubMed] [Google Scholar]
- Korea Health Industry Development Institute (KHIDI). National food & nutrition statistics: High-frequency foods. Available from: http://www.khidi.or.kr/kps/dhraStat/result15?menuId=MENU01669&year=. Accessed April 03, 2018
- Kumari S, Sarkar PK. Quantitative risk assessment of human exposure to Bacillus cereus group associated with household refrigerated storage of pasteurized milk in India. Indian J. Dairy Sci. 2017;70:186–192. [Google Scholar]
- Lee SH, Park YS. Analysis of microflora profile in the manufacturing process of commercial tofu. Food Eng Prog. 2012;16:270–277. [Google Scholar]
- Lee YS, Ha JH, Park KH, Lee SY, Choi YJ, Lee DH, Park SH, Moon ES, Ryu K, Shin HS, Ha SD. Survey on storage temperature of domestic major chilled foods in refrigerator. J. Food Hyg. Saf. 2008;23:304–308. [Google Scholar]
- Lee H, Kim K, Choi KH, Yoon Y. Quantitative microbial risk assessment for Staphylococcus aureus in natural and processed cheese in Korea. J. Dairy Sci. 2015;98:5931–5945. doi: 10.3168/jds.2015-9611. [DOI] [PubMed] [Google Scholar]
- Lee DY, Kwon KH, Chai C, Oh SW. Microbial contamination of tofu in Korea and growth characteristics of Bacillus cereus isolates in tofu. LWT-Food Sci. Technol. 2017;78:63–69. doi: 10.1016/j.lwt.2016.11.081. [DOI] [Google Scholar]
- Lewin A, Delage G, Bernier F, Germain M. Banked human milk and quantitative risk assessment of Bacillus cereus infection in premature infants: a simulation study. Canadian J. Infect. Dis. Med. Microbiol. 2019 doi: 10.1155/2019/6348281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy DM, Jaykus LA, Foegeding PM. A quantitative risk assessment for Bacillus cereus emetic disease associated with the consumption of Chinese-style rice. J. Food Saf. 1999;19:209–229. doi: 10.1111/j.1745-4565.1999.tb00246.x. [DOI] [Google Scholar]
- McMeekin TA, Olley J, Ross T. Predictive microbiology: theory and application. Taunton: Wiley; 1993. [Google Scholar]
- Ministry of Food and Drug Safety (MFDS). Food intake for microbial risk assessment (2012). Available from: http://academic.naver.com/article.naver?doc_id=59503552. Accessed Oct. 01, 2017
- Ministry of Food and Drug Safety (MFDS). Understanding of microbial risk assessment for emergency response (2016) Available from http://www.nifds.go.kr Accessed Oct 01, 2017
- Ministry of Food and Drug Safety (MFDS). Korea Food Code. Available from: https://www.foodsafetykorea.go.kr/portal/safefoodlife/food/foodRvlv/foodRvlv.do. Accessed Jan. 20
- Notermans S, Dufrenne J, Teunis P, Beumer R, Te Giffel M, Peeters Weem P. A risk assessment study of Bacillus cereus present in pasteurized milk. Food Microbiol. 1997;13:145–151. [Google Scholar]
- Oh KY, Ahn SA, Oh SM. A study of shelf-life and antimicrobial activity on putrefactive microorganisms related to soybean curd of Persicaria hydropiper L extracts. Culi. Sci. & Hos. Res. 2016;22:198–211. [Google Scholar]
- Oscar TP. Development and validation of primary, secondary, and tertiary models for the growth of Salmonella typhimurium on sterile chicken. J. Food Prot. 2005;68:2606–2613. doi: 10.4315/0362-028X-68.12.2606. [DOI] [PubMed] [Google Scholar]
- Park MS, Cho JI, Lee SH, Bahk GI. A study on dose-response models for foodborne disease pathogens. J. Fd. Hyg. Safety. 2014;4:299–304. doi: 10.13103/JFHS.2014.29.4.299. [DOI] [Google Scholar]
- Préstamo G, Fontecha J. High-pressure treatment on the tofu fatty acids and acylglycerols content. Innov. Food Sci. Emerg. 2007;8:188–191. doi: 10.1016/j.ifset.2006.10.002. [DOI] [Google Scholar]
- Ross T. Indices for performance evaluation of predictive models in food microbiology. J. Appl. Microbiol. 1996;81:501–508. doi: 10.1111/j.1365-2672.1996.tb01946.x. [DOI] [PubMed] [Google Scholar]
- Sanaa M, Coroller L, Cerf O. Risk assessment of listeriosis linked to the consumption of two soft cheeses made from raw milk: Camembert of Normandy and Brie of Meaux. Risk Analysis. 2004;24:389–399. doi: 10.1111/j.0272-4332.2004.00440.x. [DOI] [PubMed] [Google Scholar]
- Valero M, Hernandez-Herrero LA, Giner MJ. Survival, isolation, and characterization of a psychrotrophic Bacillus cereus strain from a mayonnaise-based ready-to-eat vegetable salad. Food Microbiol. 2007;24:671–677. doi: 10.1016/j.fm.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Vose DJ. The application of quantitative risk assessment to microbial food safety. J. Food Prot. 1998;61:640–648. doi: 10.4315/0362-028X-61.5.640. [DOI] [PubMed] [Google Scholar]
- Wang J. Development of quantitative microbial risk assessment for Bacillus cereus on rice cake. MS thesis, Kangwon University, Chuncheon, South Korea (2014)
- Yoon Y, Yoon KS, Kim HJ, Lee MS, Ha SD. Risk assessment of low-risk foodborne pathogens for food and livestock products: 2017 Annual report (2017)