Abstract
Background:
Inflammation induces dysfunction of endothelial cells via inflammatory cell adhesion, and this phenomenon and reactive oxygen species accumulation are pivotal triggers for atherosclerosis-related vascular disease. Although exosomes are excellent candidate as an inhibitor in the inflammation pathway, it is necessary to develop exosome-mimetic nanovesicles (NVs) due to limitations of extremely low release rate and difficult isolation of natural exosomes. NVs are produced in much larger quantities than natural exosomes, but due to the low flexibility of the cell membranes, the high loss caused by hanging on the filter membranes during extrusion remains a challenge to overcome. Therefore, by making cell membranes more flexible, more efficient production of NVs can be expected.
Methods:
To increase the flexibility of the cell membranes, the suspension of umbilical cord-mesenchymal stem cells (UC-MSCs) was subjected to 5 freeze and thaw cycles (FT) before serial extrusion. After serial extrusion through membranes with three different pore sizes, FT/NVs were isolated using a tangential flow filtration (TFF) system. NVs or FT/NVs were pretreated to the human coronary artery endothelial cells (HCAECs), and then inflammation was induced using tumor necrosis factor-α (TNF-α).
Results:
With the freeze and thaw process, the production yield of exosome-mimetic nanovesicles (FT/NVs) was about 3 times higher than the conventional production method. The FT/NVs have similar biological properties as NVs for attenuating TNF-α induced inflammation.
Conclusion:
We proposed the efficient protocol for the production of NVs with UC-MSCs using the combination of freeze and thaw process with a TFF system. The FT/NVs successfully attenuated the TNF-α induced inflammation in HCAECs.
Keywords: Exosome-mimetic nanovesicles (NVs), Freeze and thaw (FT) procedure, Anti-inflammation, Endothelial cell, TNF-α
Introduction
Atherosclerosis is a major reason for cardiovascular disease which is the main cause of death and disability recently worldwide [1]. Over the last 30 years, the role of inflammation in atherosclerosis has burgeoned [2]. Inflammation induces dysfunction of endothelial cells (ECs) through various inflammatory cells, and this phenomenon and reactive oxygen species (ROS) accumulation are crucial triggers for atherosclerosis-related vascular disease [2–4]. Many research articles have indicated that tumor necrosis factor-α (TNF-α) is a pivotal cytokine in the inflammatory cascade, which induces leukocyte migration onto the surface of ECs, finally triggers endothelial apoptosis [5–7]. Additionally, TNF-α induces subsequent intracellular signaling, including the expression of various chemokines and adhesion molecules like vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), and E-selectin [8–10]. The inflammatory transcription factor, nuclear factor κ enhancer-binding protein (NF-κB) plays a significant role in controlling these pro-inflammatory genes [11]. NF-κB is a transcription factor that regulates the expression of several inflammatory mediators such as TNF-α, interleukin-6 (IL-6), IL-8, and IL-1β [12]. Activation of NF-κB enhances the production of TNF-α, adhesion molecules, and eventually induces endothelial dysfunction. Moreover, in cardiac injury and heart diseases, c-Jun N-terminal kinases (JNK) and p38 mitogen-activated protein kinases (MAPKs) are a critical signal to participate in ECs’ changes [13–16]. Various candidates, including natural products [17, 18], and genes [19], are being conducted to prevent damage of ECs by inflammation through controlling these pathways. Among them, exosomes have been utilized with the expectation of paracrine effects. Moreover, exosomes contain functional miRNAs, long noncoding RNAs (lncRNA), and various surface proteins and deliver many subsets of genetic materials and important messages to alter the gene expression and cellular functions of distant organs [20–24].
Exosomes can deliver their cargo to the other cells and can thereby induce the phenotype changes of the recipient cells. The various physiological roles of exosomes in intercellular communication can be explained by their properties to carry genetic and proteomic information between cells [25, 26]. Recently, umbilical cord-mesenchymal stem cells (UC-MSCs) derived exosomes have been utilized to prevent aging-induced cardiac dysfunction and unravel the mechanism related to NF-κB/TNF-α pathway [12]. They utilized UC-MSCs derived natural exosomes to control the dysfunction of ECs. However, the production yield of natural exosome from mammalian cells is too low due to the low release rate and difficulty in purification [27–29]. As an alternative to natural exosomes, exosome-mimetic nanovesicle (NV) has been developed [30]. The nano-sized vesicles, NVs, have similar characteristics with natural exosomes in terms of size, morphology, and protein contents [31–33]. While strategies for NVs production offer much higher yields compared to the isolation of natural exosomes, currently the generation method of NVs has not been fully optimized. These production techniques suffer from relatively low cost-effectiveness still. Due to the need for specialized equipment or high costs, the production of NVs may still be prohibitive to many researchers. Herein, we suggested a higher production method of NVs with modification of the process of NV production. Freeze and thaw (FT) process can help cell more flexible to penetrate extrusion membrane more easily, which enable more efficient production of NVs (FT/NVs). And tangential flow filtration (TFF) system is used in place of density gradient ultracentrifugation to isolate pure NVs from residual proteins. To demonstrate the biological properties of FT/NVs, we pretreated FT/NVs to ECs followed by TNF-α treatment for inflammation. The results indicate that FT/NVs can be a good candidate for developing agents that can attenuate endothelial inflammation.
Materials and methods
Preparation of NVs and FT/NVs
Umbilical cord-mesenchymal stem cells (UC-MSCs) were obtained from CHA Biotech Co., Ltd. (Seongnam, Korea). Cells were cultured in alpha-MEM containing 10% FBS and 1% antibiotic-antimycotic mixture and maintained in a humidified atmosphere with 5% CO2 at 37 °C. The eighth passage (P8) cells were detached with Accutase (Stemcell technology, Vancouver, BC, Canada). The cells were suspended at a concentration of 3.3 × 106 cells/mL in phosphate-buffered saline (PBS) solution, and then sequentially extruded through different pore sizes of 10 μm, 1 μm, and 200 nm polycarbonate membrane filter (Whatman, Maidstone, KNT, UK) using a Liposofast LF-50 (Avestin, Ottawa, ON, Canada). To concentrate and purify them, a tangential flow filtration (TFF) system was used with 300 K hollow fiber filter (Repligen, Waltham, MA, USA). To make FT/NVs, the cell suspension was subjected to 5 cycles of rapid freeze in liquid nitrogen and thaw in 37 °C water for 5 min each before serial extrusion. The rest of the process was the same as NVs.
Characterization of NVs and FT/NVs
The morphology of NVs and FT/NVs was analyzed by transmission electron microscopy (TEM; FEI Tecnai Spirit G2, Hillsboro, OR, USA). The samples were loaded onto a carbon-coated copper grid (Electron Microscopy Sciences, Fort Washington, PA, USA). Three minutes later, 2% uranyl acetate was dropped to grid and dried. The images were recorded by FEI Tecnai Spirit G2 at an acceleration voltage of 80 kV. The size and concentration of NVs and FT/NVs were measured with nanoparticle tracking analysis using the Nanosight NS300 (Malvern Instruments, Malvern, UK). NVs and FT/NVs were dispersed in PBS solution at 500 ng total proteins/mL and assessed with a sCMOS camera at level 12, a slide shutter of 1200, and a slider gain of 146. The chamber temperature was maintained at 25.4 °C. The data were analyzed using nanoparticle tracking analysis software version 3.2 with a detection threshold of 3, auto set to blur size, and max jump distance. The total protein amount was examined by BCA protein assay kit (Pierce, Rockford, IL, USA).
Cell culture
Human coronary artery endothelial cells (HCAECs) were obtained from the Lonza (Cambrex, Walkersville, MD, USA). Cells were cultured in EGM-2 media with MV bullet kit and maintained in a humidified atmosphere with 5% CO2 at 37 °C.
Leukocyte adhesion assay
HCAECs monolayers, grown as described earlier, were established in culture plates. Then NVs or FT/NVs (50 μg/mL) were added. After being incubated for 2 h, cells of each well were treated with TNF-α (10 ng/ml) for 6 h. HCAECs were then incubated with 2 × 105 THP1 cells labeled with calcein-AM for 1 h. After incubation, non-adherent cells were removed by washing with PBS solution twice. A total of three random regions were photographed, and the numbers of adhesion cells were directly counted.
Quantitative real-time PCR
Total RNA was reverse transcribed using a PrimeScript RT reagent kit (Takara, Japan). Real-time PCR was performed using SYBR green PCR mater mix (Applied Biosystems, Foster City, CA, USA). Reactions were carried out on the QuantStudio 3 (Applied Biosystems) with the following primers. ICAM-1: forward, 5′-ccttcctcaccgtgtactgg-3′ and reverse, 5′-agcgtagggtaaggttcttgc-3′; VCAM-1: forward, 5′-tgcacagtgacttgtggacata-3′ and reverse, 5′-gccaccactcatctcgattt-3′; IL-1β: forward, 5′-tacctgtcctgcgtgttgaa-3′ and reverse, 5′-tctttgggtaatttttgggatct-3′; IL-6: forward, 5′-gatgagtacaaaagtcctgatcca-3′ and reverse, 5′- ctgcagccactggttctgt-3′; IL-8: forward, 5′-agacagcagagcacacaagc-3′ and reverse, 5′-atggttccttccggtggt-3′; 18 s rRNA: forward, 5′-gcaattattccccatgaacg-3′ and reverse, 5′-gggacttaatcaacgcaagc-3′. The data were quantified using 2−ΔΔCt method with 18 s rRNA as a reference.
Western blot analysis
Cells were lysed in RIPA buffer. After centrifugation, the protein concentration of the supernatant was assayed using a BCA protein assay kit (Pierce, Rockford, IL, USA). Then, proteins (20 μg) were subjected to 10% SDS-PAGE and transferred to NC membranes. After blocking, sequential incubation with primary antibodies and HRP-linked secondary antibodies, blots were developed using enhanced chemiluminescence solution for 5 min, after which they were scanned with Image Lab software (Bio-Rad, Hercules, CA, USA). The antibodies against VCAM-1, ICAM-1, Alix, TSG101, and GAPDH were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Phospho-JNK and phospho-p38 MAPK antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). CD63 and CD9 antibodies were purchased from Abcam (Cambridge, MA, USA).
Measurement of reactive oxygen species (ROS)
HCAECs were pretreated with exosomes for 2 h and then incubated with TNF-α (10 ng/mL) for 3 h. Oxidative stress in HCAECs was assessed by measuring the levels of ROS with 2,7-dichlorodihydrofluorescein diacetate stain (DCFH-DA; Abcam), as recommended by the manufacturer. After staining, cells were subsequently analyzed by a CytoFLEX flow cytometer (Indianapolis, IN, USA).
Immunocytochemistry
Cells grown on chamber slide (SPL Life science, Korea) were fixed in 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.3% Triton X-100 in PBS solution. Then, cells were immersed in a blocking solution containing 5% BSA in PBS solution for 1 h followed by the incubation with 1:100 dilution of monoclonal antibody against NF-κB p65 (Santa Cruz Biotechnology, Inc.) in a blocking solution for overnight at 4 °C. After washing, cells were incubated in blocking solution containing Alexa Fluor 555-conjugated anti-mouse IgG (Invitrogen, Carlsbad, CA, USA) for 1 h followed by three washes in PBS solution. The slide was then mounted with Vectashield mounting solution containing DAPI (Vector Labs, Burlingame, CA, USA), analyzed by Zeiss 880 confocal laser scanning microscope (Carl Zeiss, Oberkochen, Germany).
Results
Efficient production of exosome-mimetic nanovesicles (NVs) by freeze and thaw (FT) process
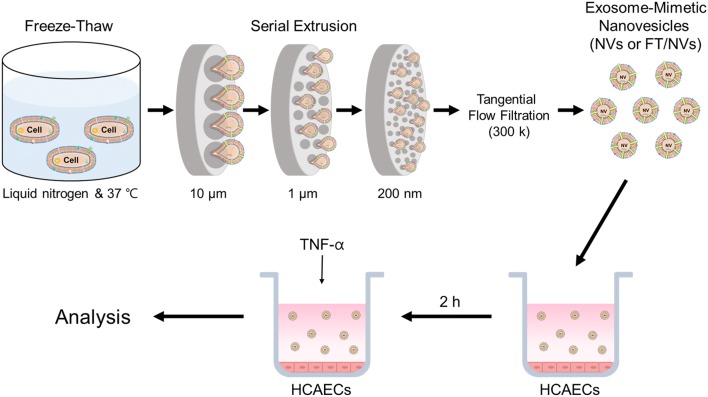
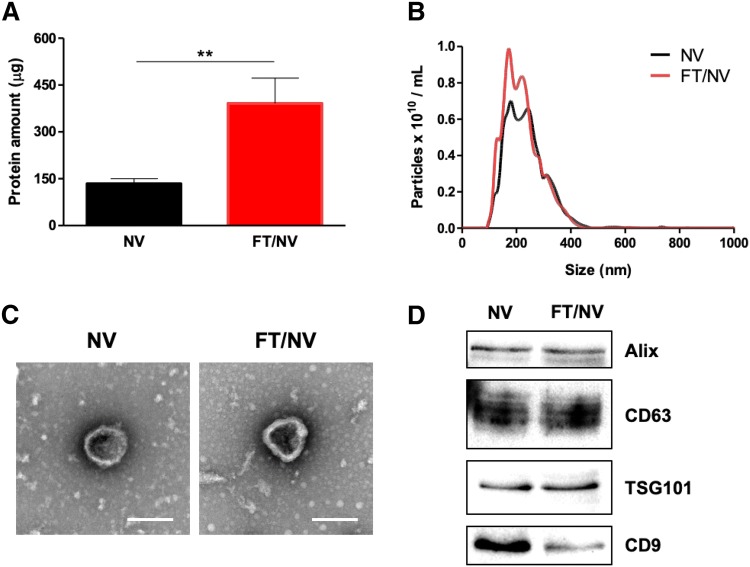
The procedure for the preparation of exosome-mimetic nanovesicles (NVs) from UC-MSC is illustrated in Fig. 1. First, the NVs were extruded serially using various sizes of the polycarbonate membranes. Then, a tangential flow filtration (TFF) system was utilized to isolate purified NVs. In general, the production yield of NVs is much more than that of natural exosomes. The amount of protein of NVs was evaluated using the BCA assay. The total amount of protein was about 150 μg from 1 × 107 cells. Although it was much more than that of natural exosomes, higher efficient methods for NVs production could be effective for various biomedical applications. The rigidity of cells could disturb complete penetration and dissociation during their passing through the membrane. Freeze and thaw (FT) procedure could help the cell to penetrate the membrane with high yield. It consists of quickly freezing at liquid nitrogen and thawing at 37 °C warm water in sequence. The FT process represents a mild homogenization procedure that enables cellular membranes more flexible. With the process, exosome-mimetic nanovesicles (FT/NVs) could produce about 3 times more proteins than NVs, as shown in Fig. 2. Then, characterization of the NVs and FT/NVs by transmission electron microscopy (TEM) and nanoparticle tracking analysis (NTA) revealed that the vesicle structure was similar to exosomes. From the NTA, about 1 × 1010 vesicles were obtained and the size of the vesicles was around 200 nm. Additionally, Western blotting was used to characterize the NVs and FT/NVs, and the results showed that both contained common exosomal markers, such as CD9, CD63, Alix, and TSG101. All these results suggest that NVs and FT/NVs have similarities with natural exosomes in size, morphology, and protein contents. Moreover, the production yield of FT/NVs was about 3-fold higher than NVs.
Fig. 1.
Schematic illustration of the experimental procedure for the preparation of exosome-mimetic nanovesicles (NVs or FT/NVs) and treatment NVs or FT/NVs to human coronary artery endothelial cells (HCAECs)
Fig. 2.
Characterization of NVs and FT/NVs. A Total amount of protein in NVs and FT/NVs from 1 × 107 cells (n = 3, **p < 0.01). B Size distribution of NV and FT/NV was evaluated by NTA. C Representative TEM images of NV and FT/NV (scale bars = 200 nm). D Western blot analysis of exosomal markers (Alix, CD63, TSG101, and CD9) on NV and FT/NV
NVs inhibit TNF-α induced leukocyte adhesion and cell adhesion molecule expression
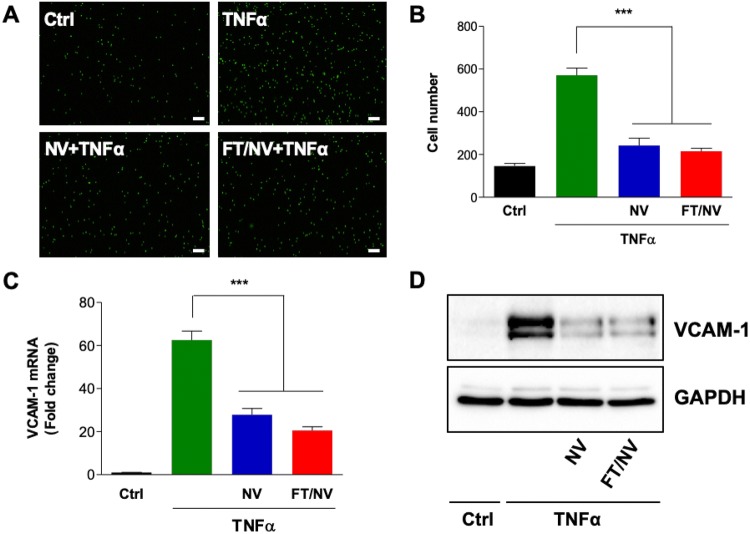
The adhesion of leukocytes to vascular endothelium is a hallmark of the inflammatory process. To investigate the anti-inflammatory effects of NVs on endothelial inflammation, the adhesion of leukocyte to endothelial cells was observed. In both NVs and FT/NVs, pretreatment of NVs (50 μg/mL) dramatically inhibited calcein-AM labeled leukocyte adhesion to TNF-α-stimulated HCAECs (Fig. 3A, B). Leukocyte recruitment to vascular endothelium relies on the interplays of ECs surface proteins, such as VCAM-1, with their ligands expressed on leukocytes. We next investigated the effect of NVs and FT/NVs on VCAM-1 expression in TNF-α-stimulated HCAECs. As shown in Fig. 3C, D, both mRNA and protein expression levels of VCAM-1 decreased, suggesting the inhibitory effect of NVs on TNF-α-induced leukocyte adhesion to HCAECs. The difference was not detected between NVs and FT/NVs.
Fig. 3.
NVs and FT/NVs suppressed TNF-α-induced inflammation in HCAECs. HCAECs were pretreated with NV or FT/NV for 2 h and then stimulated with TNF-α (10 ng/mL) for 6 h. A Calcein-AM-labeled THP-1 cells were incubated with stimulated-HCAECs for 1 h. Adherent THP-1 cells on HCAECs were detected by a fluorescence microscope (× 40, scale bars = 200 μm). B Adherent THP-1 cells were counted and quantified. The C mRNA and D protein expression levels of VCAM-1 were analyzed by real-time qPCR and Western blot, respectively. Data are mean ± SD from 3 independent experiments. ***p < 0.001
NVs decrease the production of proinflammatory cytokines caused by TNF-α
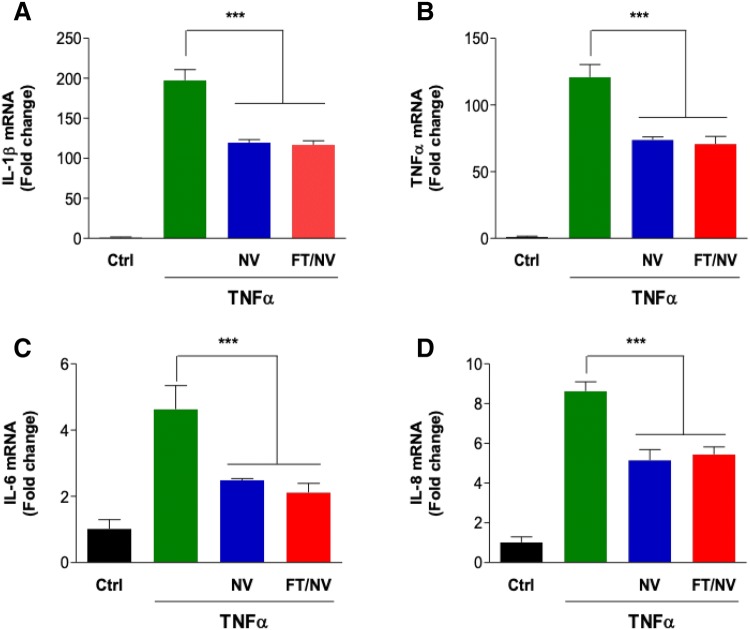
The activation of leukocytes is a complex process involving the release of various pro-inflammatory cytokines, such as TNF-α, IL-6, IL-8, and IL-1β. These cytokines are important regulators of the inflammatory reaction in the vessel wall. To evaluate the effect of NVs on the production of inflammatory cytokines, the mRNA expression levels of pro-inflammatory cytokines were determined using real-time qPCR analysis. As shown in Fig. 4, the production of pro-inflammatory cytokines in HCAECs was effectively reduced in the NVs-pretreated group compared with TNF-α group. These results demonstrated that FT/NVs, as well as NVs, reduced endothelial inflammation induced by TNF-α through modulation of inflammatory response-related factors.
Fig. 4.
Nanovesicles reduced the production of pro-inflammatory cytokines. HCAECs were pretreated with NV or FT/NV for 2 h. Then, cells were incubated with TNF-α for 6 h. The mRNA expression levels of A IL-1β, B TNF-α, C IL-6, and D IL-8 were determined by real-time qPCR. Data are mean ± SD from 3 independent experiments. ***p < 0.001
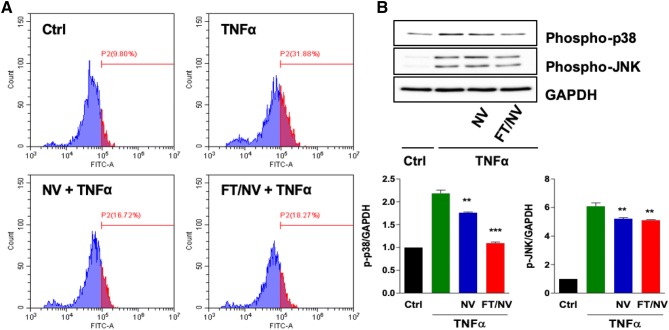
NVs inhibit oxidative stress by suppressing p38/JNK MAPK pathway induced by TNF-α
Because TNF-α increased ROS production in various cell lines, the intracellular ROS levels were analyzed in TNF-α-treated ECs by flow cytometric analysis after DCFH-DA staining. About half the level of ROS was detected in TNF-α stimulated HCAECs pretreated with FT/NVs (Fig. 5A). Moreover, the relationship among NVs, p38, and JNK activities was examined to identify the signaling pathway which facilitates the anti-inflammatory effect of NVs. As expected, the result indicated that NVs inhibited TNF-α-induced p38 and JNK phosphorylation, which means that the p38 and JNK pathway plays a pivotal role in facilitating the effects of NVs (Fig. 5B). Taken together, NVs prevented the accumulation of intracellular ROS and the downstream p38/JNK MAPK signaling activation in response to TNF-α in HCAECs.
Fig. 5.
Nanovesicles inhibited the TNF-α-induced oxidative stress. HCAECs were pretreated with NV or FT/NV for 2 h, and the cells were stimulated with TNF-α (10 ng/mL) for 6 h. A Intracellular ROS levels were detected by flow cytometry after DCFH-DA staining. B Phosphorylation of p38 and JNK was detected by Western blot analysis. Data are mean ± SD from 3 independent experiments. **p < 0.01; ***p < 0.001
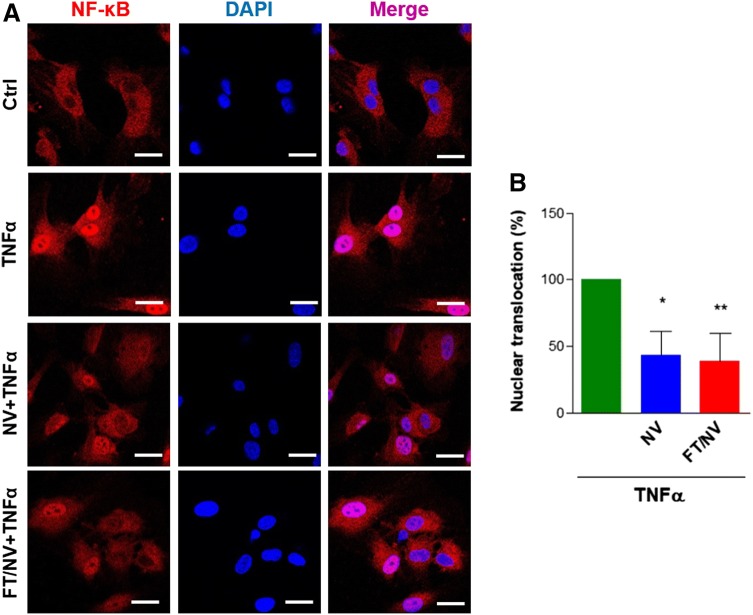
NVs inhibit nuclear translocation of NF-κB induced by TNF-α
Activation of the NF- κB transcription family, by nuclear translocation of cytoplasmic complexes, plays a central role in inflammation. To investigate the effect of FT/NVs on the activation of NF-κB, nuclear translocation of p65 subunit of NF-κB was examined via confocal microscopy. As shown in Fig. 6, TNF-α induced nuclear translocation of NF-κB compared with the control group. However, pretreatment of NVs or FT/NVs significantly reduced NF-κB translocation into the nucleus. These results implied that the anti-inflammatory effects of NVs are related to the ability to modulate NF-κB activity.
Fig. 6.
NVs and FT/NVs diminished TNF-α-induced NF-κB activation. A HCAECs were incubated with NV or FT/NV for 2 h, and then cells were stimulated with TNF-α for 1 h. The localization of p65 in cells was detected by immunofluorescence and visualized by confocal laser scanning microscope (× 400, scale bars = 20 μm) B Quantification of nuclear translocation of p65 as the percentage compared with the TNF-α-treated group. *p < 0.05; **p < 0.01
Discussion
In this study, we proposed higher efficient production methods for exosome-mimetic nanovesicles with additional freeze and thaw process (FT/NVs). Although the potential of exosomes in biological applications is widely understood, the amount of natural exosomes isolated from conventional cell culture is extremely small, and the purification process is also very complicated, limiting their use. The preparation methods for exosome-mimetic nanovesicles (NVs) have been established in recent years for these reasons [30]. Although the methods for NVs preparation have been developed with similar properties with natural exosomes, more efficient production methods would be helpful for various biomedical applications. The problems with previously reported methods for exosome-mimetic nanovesicles are the inefficiency of extrusion and ultracentrifugation. In order to overcome these limitations, we suggested a method of serial extrusion followed by FT. After serial extrusion with polycarbonate membrane, pure NVs and FT/NVs could be isolated using a TFF system. In general, the freeze and thaw process has been utilized for cell homogenization via cell membrane rupture [34]. This process is beneficial for cellular membranes more flexible and eases penetration. TFF system is the filtration system that the fluid is passed parallel to the filter membrane, rather than being pushed through a membrane perpendicularly which can clog the filter media [35]. The impurities, such as extracellular proteins are separated by the TFF system, resulting in concentrated pure exosomes. Finally, the combination of freeze and thaw process and TFF system produced about 3 times more than general NVs and about 250 times more than natural exosomes [2, 30]. The size, shape, and surface exosomal protein markers of FT/NVs are the same as NVs, and attenuating inflammation property is also similar.
The proinflammatory cytokine, TNF-α, can increase ROS level and induce infiltration of various inflammatory cells into injury sites [2, 10]. TNF-α has been widely utilized in inflammation experiments because TNF-α stimulates the release of many pro-inflammatory cytokines, such as IL-1β, TNF-α, IL-6, and IL-8 [36]. Herein, we showed TNF-α triggered leukocyte adhesion on the HCAECs through NF-κB activation and VCAM-1 upregulation. And NVs and FT/NVs could diminish TNF-α-induced adhesion between leukocyte and ECs by blocking mRNA and protein expression of VCAM-1 and NF-κB activation.
UC-MSC derived exosome has been tried for inflammation inhibition in aging-induced cardiac dysfunction [12]. However, the low production yield of exosomes makes it difficult to translate into clinical practice. Although the effect of our NVs and FT/NVs on inhibition of inflammations was shown, the exact inhibition mechanism should be unraveled.
In conclusion, we have made the high yield of exosome-mimetic nanovesicle with freeze and thaw process before the serial extrusion process. In addition, the TFF system was introduced for the purification of NVs and FT/NVs. By combining these processes, we could obtain the FT/NVs with high yield and high purity. FT/NVs are characterized and compared with NVs using TEM, NTA, and Western blotting analysis. Moreover, NVs and FT/NVs were utilized for attenuating TNF-α-induced inflammation in endothelial cells as well as the expression of pro-inflammatory cytokines, including IL-1β, TNF-α, IL-6, and IL-8. The efficiency of FT/NVs for inflammation relief is similar to NVs, which exhibited 2-fold decreased compared with the TNF-α-treated group. Moreover, this study revealed that TNF-α-induced NF-κB activation was also inhibited by NVs and FT/NVs pretreatment at the concentration of 50 μg/mL. Based on these results, it is concluded that the FT/NVs could attenuate TNF-α induced endothelial inflammation effectively.
Acknowledgements
This work was supported by Basic Science Research Program (2017R1A6A3A04012362 and 2020R1A2B5B03002344) and Bio & Medical Technology Development Program (2018M3A9E2024579) through the National Research Foundation of Korea funded by the Ministry of Science and ICT (MSIT) and a grant of the Korea Health Technology R&D Project (HI18C0089) through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical statement
There are no animal or human experiments carried out for this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kyoung-Won Ko and Yong-In Yoo have equally contributed to this work.
Contributor Information
Won-Kyu Rhim, Email: wkrhim@cha.ac.kr.
Dong Keun Han, Email: dkhan@cha.ac.kr.
References
- 1.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Allen SD, Liu YG, Kim T, Bobbala S, Yi S, Zhang X, et al. Celastrol-loaded PEG-b-PPS nanocarriers as an anti-inflammatory treatment for atherosclerosis. Biomater Sci. 2019;7(2):657–668. doi: 10.1039/C8BM01224E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y, He R, Wang P, Shi Y, Zhao L, Liang J. Exosomes from LPS-stimulated macrophages induce neuroprotection and functional improvement after ischemic stroke by modulating microglial polarization. Biomater Sci. 2019;7(5):2037–2049. doi: 10.1039/C8BM01449C. [DOI] [PubMed] [Google Scholar]
- 5.Erl W, Weber C, Wardemann C, Weber PC. Alpha-Tocopheryl succinate inhibits monocytic cell adhesion to endothelial cells by suppressing NF-kappa B mobilization. Am J Physiol. 1997;273:H634–H640. doi: 10.1152/ajpcell.1997.273.2.C634. [DOI] [PubMed] [Google Scholar]
- 6.Collins T, Read MA, Neish AS, Whitley M, Thanos D, Maniatis T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. doi: 10.1096/fasebj.9.10.7542214. [DOI] [PubMed] [Google Scholar]
- 7.Mackay F, Loetscher H, Stueber D, Gehr G, Lesslauer W. Tumor necrosis factor alpha (TNF-alpha)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55. J Exp Med. 1993;177:1277–1286. doi: 10.1084/jem.177.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang L, Froio RM, Sciuto TE, Dvorak AM, Alon R, Luscinskas FW. ICAM-1 regulates neutrophil adhesion and transcellular migration of TNF-α-activated vascular endothelium under flow. Blood. 2005;106:584–592. doi: 10.1182/blood-2004-12-4942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharief MK, Hentges R. Association between tumor necrosis factor-α and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 10.Walczak H. TNF and ubiquitin at the crossroads of gene activation, cell death, inflammation, and cancer. Immunol Rev. 2011;244:9–28. doi: 10.1111/j.1600-065X.2011.01066.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang C. The role of inflammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu B, Zhang L, Liang C, Liu B, Pan X, Wang Y, et al. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-κB/TNF-α signaling pathway. Oxid Med Cell Longev. 2019;2019:9739258 [DOI] [PMC free article] [PubMed]
- 13.Kawano H, Sakamoto T, Ito T, Miyata K, Hashiguchi T, Maruyama I. Hyaluronan protection of corneal endothelial cells against extracellular histones after phacoemulsification. J Cataract Refract Surg. 2014;40:1885–1893. doi: 10.1016/j.jcrs.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Zhang C, Kawauchi J, Adachi MT, Hashimoto Y, Oshiro S, Aso T, et al. Activation of JNK and transcriptional repressor ATF3/LRF1 through the IRE1/TRAF2 pathway is implicated in human vascular endothelial cell death by homocysteine. Biochem Biophys Res Commun. 2001;289:718–724. doi: 10.1006/bbrc.2001.6044. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto T, Turesson I, Book M, Gerwins P, Claesson-Welsh L. p38 MAP kinase negatively regulates endothelial cell survival, proliferation, and differentiation in FGF-2–stimulated angiogenesis. J Cell Biol. 2002;156:149–160. doi: 10.1083/jcb.200103096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McMullen ME, Bryant PW, Glembotski CC, Vincent PA, Pumiglia KM. Activation of p38 has opposing effects on the proliferation and migration of endothelial cells. J Biol Chem. 2005;280:20995–21003. doi: 10.1074/jbc.M407060200. [DOI] [PubMed] [Google Scholar]
- 17.Zhou P, Lu S, Luo Y, Wang S, Yang K, Zhai Y, et al. Attenuation of TNF-α-induced inflammatory injury in endothelial cells by ginsenoside Rb1 via inhibiting NF-κB, JNK and p38 signaling pathways. Front Pharmacol. 2017;8:464. doi: 10.3389/fphar.2017.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bedair TM, Bedair HM, Ko KW, Park W, Joung YK, Han DK. Persulfated flavonoids accelerated re-endothelialization and improved blood compatibility for vascular medical implants. Colloids Surf B Biointerfaces. 2019;1(181):174–184. doi: 10.1016/j.colsurfb.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 19.Muslin AJ. MAPL Signaling in cardiovascular health and disease: molecular mechanism and therapeutic targets. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 21.Chun SY, Lim JO, Lee EH, Han MH, Ha YS, Lee JN, et al. Preparation and characterization of human adipose tissue-derived extracellular matrix, growth factors, and stem cells: a concise review. Tissue Eng Regen Med. 2019;16(4):385–393. doi: 10.1007/s13770-019-00199-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathivanan S, Ji H, Simpson RJ. Exosomes: extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Teng X, Chen L, Chen W, Yang J, Yang Z, Shen Z. Mesenchymal stem cell-derived exosomes improve the microenvironment of infarcted myocardium contributing to angiogenesis and anti-inflammation. Cell Physiol Biochem. 2015;37:2415–2424. doi: 10.1159/000438594. [DOI] [PubMed] [Google Scholar]
- 24.van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, et al. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood. 2013;121:3997–4006. doi: 10.1182/blood-2013-02-478925. [DOI] [PubMed] [Google Scholar]
- 25.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 26.Lim W, Kim HS. Exosomes as therapeutic vehicles for cancer. Tissue Eng Regen Med. 2019;16(3):213–223. doi: 10.1007/s13770-019-00190-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ, et al. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 2012;161:635–644. doi: 10.1016/j.jconrel.2011.11.021. [DOI] [PubMed] [Google Scholar]
- 28.Zhang M, Jin K, Gao L, Zhang Z, Li F, Zhou F, et al. Methods and technologies for exosome isolation and characterization. Small Methods. 2018;2:1800021. doi: 10.1002/smtd.201800021. [DOI] [Google Scholar]
- 29.Kim M, Yun HW, Park DY, Choi BH, Min BH. Three-dimensional spheroid culture increases exosome secretion from mesenchymal stem cells. Tissue Eng Regen Med. 2018;15(4):427–436. doi: 10.1007/s13770-018-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jang SC, Kim OY, Yoon CM, Choi DS, Roh TY, Park J, et al. Bioinspired exosome-mimetic nanovesicles for targeted delivery of chemotherapeutics to malignant tumors. ACS Nano. 2013;7:7698–7710. doi: 10.1021/nn402232g. [DOI] [PubMed] [Google Scholar]
- 31.Hwang DW, Choi H, Jang SC, Yoo MY, Park JY, Choi NE, et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using 99 m Tc-HMPAO. Sci Rep. 2015;5:15636. doi: 10.1038/srep15636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh WJ, Zou S, Ong WY, Torta F, Alexandra AF, Schiffelers RM, et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci Rep. 2017;7:14322. doi: 10.1038/s41598-017-14725-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu JY, Ji AL, Wang ZX, Qiang GH, Qu Z, Wu JH, et al. Exosome-mimetic nanovesicles from hepatocytes promote hepatocyte proliferation in vitro and liver regeneration in vivo. Sci Rep. 2018;8:2471. doi: 10.1038/s41598-018-20505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phuengkham H, Song C, Um SH, Lim YT. Implantable synthetic immune niche for spatiotemporal modulation of tumor-derived immunosuppression and systemic antitumor immunity: postoperative immunotherapy. Adv Mater. 2018;30:e1706719. [DOI] [PubMed]
- 35.Genovesi CS. Several uses for tangential-flow filtration in the pharmaceutical industry. J Parenter Sci Technol. 1983;37:81–86. [PubMed] [Google Scholar]
- 36.Compton SJ, Cairns JA, Holgate ST, Walls AF. The role of mast cell tryptase in regulating endothelial cell proliferation, cytokine release, and adhesion molecule expression: tryptase induces expression of mRNA for IL-1β and IL-8 and stimulates the selective release of IL-8 from human umbilical vein endothelial cells. J Immunol. 1998;161:1939–1946. [PubMed] [Google Scholar]