Abstract
BACKGROUND:
Centella asiatica (L.) is a plant with neuroprotective and neuroregenerative properties; however, its effects on the neurodifferentiation of mesenchymal stem cells (MSCs) and on peripheral nerve injury are poorly explored. This study aimed to investigate the effects of C. asiatica (L.)-neurodifferentiated MSCs on the regeneration of peripheral nerve in a critical-size defect animal model.
METHODS:
Nerve conduit was developed using decellularised artery seeded with C. asiatica-neurodifferentiated MSCs (ndMSCs). A 1.5 cm sciatic nerve injury in Sprague–Dawley rat was bridged with reversed autograft (RA) (n = 3, the gold standard treatment), MSC-seeded conduit (MC) (n = 4) or ndMSC-seeded conduit (NC) (n = 4). Pinch test and nerve conduction study were performed every 2 weeks for a total of 12 weeks. At the 12th week, the conduits were examined by histology and transmission electron microscopy.
RESULTS:
NC implantation improved the rats’ sensory sensitivity in a similar manner to RA. At the 12th week, nerve conduction velocity was the highest in NC compared with that of RA and MC. Axonal regeneration was enhanced in NC and RA as shown by the expression of myelin basic protein (MBP). The average number of myelinated axons was significantly higher in NC than in MC but significantly lower than in RA. The myelin sheath thickness was higher in NC than in MC but lower than in RA.
CONCLUSION:
NC showed promising effects on nerve regeneration and functional restoration similar to those of RA. These findings revealed the neuroregenerative properties of C. asiatica and its potential as an alternative strategy for the treatment of critical size nerve defect.
Keywords: Differentiation, Centella, Nerve regeneration, Mesenchymal stem cells
Background
Peripheral nerve injury (PNI) of critical size (greater than 3 cm) has always been a clinical challenge [1] as PNI often results in poor functional recovery and leads to socioeconomic burden [2]. The current gold standard treatment to bridge critical size PNI is nerve autograft with sural nerve [3]. However, this surgical technique in clinical practice has several drawbacks, including limited availability of donor graft, size mismatch and donor-site morbidity [4].
Tissue engineering of nerve conduit aims to create a scaffold that guides the axonal regrowth to the targeted muscle within the stipulated time to achieve functional recovery [5]. The scaffold should contain certain neurotrophic factors (NFs) [6] or viable Schwann cells [7] to achieve optimal nerve regeneration. The use of NFs could enhance nerve regeneration but also has several shortcomings, such as its high cost [8], easy degradability [9] and ability to change normal cells into malignant cells with prolonged use [10].
Collecting Schwan cells is also not preferred due to inadequate source, donor site morbidity and difficulties in culturing Schwann cells [11]. The favourable properties of mesenchymal stem cells (MSCs) such as multipotent, highly proliferative, immunosuppressive, and ease of isolation and culture, makes MSCs a suitable choice for generating Schwann cells [11]. MSCs can be differentiated into neuroectodermal lineage in the presence of neurotrophic factors, such as forskolin, fibroblast growth factor (bFGF), platelet-derived growth factor (PDGF) and heregulin [12]. Natural products that can replace synthetic NFs and overcome NFs’ disadvantages have been explored. Centella asiatica (L.) is a traditional herbal medicine with neuroprotective and neuroregenerative properties and has been long used as a nerve tonic especially in India and China [13, 14].
Previously in our laboratory, we compared the effects of (1) NFs, (2) C. asiatica extract and (3) a combination of NFs and C. asiatica extract on inducing the differentiation of MSCs into neurodifferentiated MSCs (ndMSCs) [15]. The results showed that a minimal dose of C. asiatica extract alone (400 µg/mL) is not cytotoxic and can induce the differentiation of MSCs into Schwann cell-like cells. The expression of the neural protein markers of ndMSCs was higher than that of MSCs differentiated by NFs or combined NFs and C. asiatica extract. Although the potential of C. asiatica as an alternative to NFs has been scrutinised, no study has reported the effects of such ndMSCs on PNI.
In our previous study also, we successfully developed a scaffold from human umbilical cord (HUC) artery and seeded with ndMSCs for use as a nerve conduit. The integrity of the HUC arteries remained the same after various processing stages to remove its native cell. During 12 weeks of incubation and observation, the HUC arteries did not degrade or swell. The ndMSCs were then seeded into the HUC artery [16]. Thus, the present study aimed to investigate the effect of ndMSCs seeded into decellularized HUC on the regeneration of peripheral nerve in critical-size defect animal model.
Methods
Preparation of the raw extract of C. asiatica (L.)
The raw extract of C. asiatica (L.) was prepared from powdered leaves by Prof. Dr. Mohd Ilham Adenan from Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi MARA (UiTM), Malaysia (voucher specimen no. CA-K017). The leaves were washed, cleaned and dried in an oven at 40 °C before grinding. Then, 50 kg of powdered C. asiatica leaves were extracted in five batches. In each batch, 10 kg of C. asiatica leaves were extracted in 57% denatured ethanol (60 L of 95% ethanol + 40 L deionised water) for 8 h at 60 °C. After extraction, 14.8 L of concentrated liquid extract was produced and then freeze-dried to obtain 7.96 kg of dried-powdered extract (15.92% yield). The powdered raw extract was kept at room temperature until further use [15].
Preparation of C. asiatica
Stock solution of the raw extract at a concentration of 4000 µg/mL was prepared. Then, 40 mg of powdered C. asiatica extract was diluted into 10 mL of alpha minimum essential medium-low glucose complete medium (αMEM-LG) (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum (Biowest, Riverside, MO, USA), 1% glutamax (Gibco, Grand Island, NY, USA) and 1% antibiotic-antimycotic (Gibco, Grand Island, NY, USA). This stock solution was then sterile filtered using a 0.45 µm syringe filter. Next, 4 mL of the stock solution (4000 µg/mL) was added into 36 mL of complete medium αMEM-LG (40 mL final solution) to obtain 400 µg/mL C. asiatica working solution.
Development of nerve conduit by using decellularised HUC artery seeded with C. asiatica-induced ndMSCs
The nerve conduit was developed as described previously [16]. For the isolation of human MSCs, the written informed consents were obtained from healthy women who delivered full-term infants (38–40 weeks) by elective caesarean delivery prior to the collection of umbilical cord samples. The usage of human umbilical cord samples from consenting patients in this study was approved by the Universiti Kebangsaan Malaysia Research Ethics Committee (FF-2015-175). MSCs were differentiated into ndMSCs by using 400 µg/mL C. asiatica, followed by immunocytochemistry analysis. Then, 1.5 × 106 of MSCs or ndMSCs were seeded into decellularized HUC artery conduit. Briefly, the cell pellet containing 1.5 × 106 of MSCs or ndMSCs were resuspended in 20 μL of FBS and seeded directly into the lumen of the conduit using p200 micropipette. The cell-seeded conduits were incubated at 37 °C for 2 days in a humidified 5% CO2 incubator. Haematoxylin and eosin (H&E) staining was conducted to evaluate the attachment of the cells into the lumen and wall of the conduit.
Animal experimental design
The animal study was approved by Universiti Kebangsaan Malaysia (UKM) Animal Ethics Committee (approval no: TEC/PP/2016/YOGESWARAN/18-MAY/747-MAY-2016-FEB.-2018). Eleven Sprague–Dawley (SD) rats (male, age 8–9 weeks, weight range 250–350 g) obtained from UKM Animal House, Bangi, Malaysia were housed in individually ventilated cages located inside Biobubble Clean Room, Tissue Engineering Centre, UKM, with temperature-controlled conditions (21 °C), 12 h light/dark cycle and ad libitum access to standard rat pellet (irradiated NIH 31 H Diet, Altromin, Lage, Germany) and autoclaved water. The rats were acclimatised for 2 weeks before the experiment and then divided into three groups: group 1 received implantation of reversed autograft (RA) (n = 3) and served as a positive control, group 2 received implantation of MSC-seeded conduit (MC) (n = 4) and group 3 received implantation of ndMSC-seeded conduit (NC) (n = 4). Before implantation, a 15 mm sciatic nerve injury was created at the rats’ right hind, and the procedure will be described in the following section. All rats were given immunosuppressive drug to reduce cell rejection risk in reference to the study by Leow et al., 2015 with minor modifications. Cyclosporine-A (210 mg/L) was added into the rats’ drinking water 1 day prior to implantation, and dexamethasone injections were administered intramuscularly (1.6 mg/kg, from the day of surgery) on alternate days for 2 weeks [17].
Surgical procedure
The rats were placed prone under sterile condition. The fur at the surgery area was shaved, and skin from the lateral right tight was scrubbed with povidone solution. Surgery was performed under a microsurgery microscope (Carl Zeiss, Oberkochen, Germany). A 15 mm segment of the nerve was excised with straight microscissors at mid-thigh level while the rat was under deep anaesthesia (ketamine 20 mg/mL [Troy Laboratories Pty Limited, Glendenning, NSW, Australia], xylazine 20 mg/mL [Indian Immunologicals Limited, Hyderabad, Telangana, India], 125 mg of zoletyl [Virbac, Carros, Alpes-Côte d’Azur, France]). The nerve stumps were bridged with a 19 mm conduit. The proximal and distal ends of the cut nerve were telescoped as far as 2 mm into the conduit and secured with 10/0 Dafilon micro sutures (B. Braun, Melsungen, Hesse, Germany) to the wall of the conduit to keep a 15 mm gap between the two nerve stumps. For RA implantation, the 15 mm cut nerve was reversed 180° (proximal to distal/ distal to proximal) then implanted back at the nerve gap, thereby mimicking the autograft practice. The wound was closed in two layers with 6–0 Monosyn sutures (B. Braun, Melsungen, Hesse, Germany). The rats were given antibiotics and analgesics for the first 5 days.
Functional analysis assessment on implanted rats
-
(i)
Pinch test
Pinch test was performed every 2 weeks up to 12 weeks post-implantation. Toothed forceps were used to pinch the plantar aspect (centre or lateral) of the rat’s hind paw. A forceful and reflexive withdrawal of both legs represented pain sensory recovery. Reflexes were considered positive when the paw was withdrawn.
-
(ii)
Nerve conduction study (NCS)
NCS was performed every 2 weeks up to 12 weeks post-implantation by using an electromyography machine. NCS measures the function recovery of nerve and muscle and was performed under deep anaesthesia. The sciatic and tibial nerves were stimulated at the sciatic notch and ankle, respectively. Any action of muscle twitching suggests nerve conductivity. The latencies of the responses of the musculature of the foot were measured. For quantitative analysis, (a) the peak amplitude of compound muscle action potential (CMAP), (b) the latency of CMAP onset and (c) nerve conduction velocity (NCV) values were calculated [18]. Data were normalised with 0 week (pre-implantation).
Nerve harvesting and processing
After 12 weeks of conduit implantation, the rats were euthanised with intramuscular injection of 0.1 mL/g of concoction made of ketamine (20 mg/mL), xylazil (20 mg/mL) dan zoletil (125 mg/mL) and followed by intracardiac injection of 0.1 mg/g pentabarbitol. The regenerated sciatic nerves, including the proximal and distal stump, were harvested. Macroscopic observation was performed to determine the shape, morphology and degradation of the nerve (if any). Nerve samples were then fixed in 10% formalin (Leica Biosystems, Danvers, MA, USA) 24 h for H&E staining and immunohistochemical analysis (IHC). For transmission electron microscopy (TEM), the nerve samples were fixed for 24 h in 4% glutaraldehyde (Sigma-Aldrich, St. Louis, MO, USA).
H&E staining
The nerves were processed using a series of chemicals, such as 50% and 70% alcohol (MERCK, Darmstadt, Hesse, Germany) (1 h each), 95% alcohol (1.5 h), absolute alcohol (three times for 1, 1.5 and 1.5 h), equal parts of alcohol: toluene (1.5 h), toluene (three times for 1, 1.5 and 1.5 h) and paraffin wax (2 ×, 2 h each). Tissues were then embedded into paraffin wax for histological analysis. Tissue sections of 2–5 µm thin were cut using a microtome (Leica, Bensheim, Germany) and collected onto microscopic slides. The tissue sections were then dewaxed and dehydrated with a series of chemicals: xylene (J.T.Baker, Phillisburg, NJ, USA) (three times for 5 min each) and alcohol (100%, 95% and 70% for 3 min each). Haematoxylin staining was performed for 10 min, followed by rinsing with tap water (3 min), 1% acid alcohol (three times), tap water (3 min), 1% ammonium (two times) and tap water (3 min). Eosin staining was performed for 4 min. Finally, the tissue sections were rehydrated with alcohol (95% and 100% for 3 min each) and xylene (three times for 5 min each), mounted using Depex mounting solution (Sigma Aldrich, St. Louis, MO, USA) and observed under light microscope (Olympus, CH30, Tokyo, Japan).
IHC analysis
IHC analysis was performed to evaluate the expression of neural protein markers on the regenerated nerves. The nerve tissues were processed and embedded into paraffin wax as previously described. The 5 µm-thick cut-out tissue sections were placed onto polysine adhesive microscope slides (Thermo Fisher Scientific, Waltham, MA, USA). The slides were then heated in antigen retrieval solution (pH 6) (Sigma Aldrich, St. Louis, MO, USA) for 20 min at 98 °C. The demarcated area was blocked with 10% goat serum for 1 h at 37 °C. The tissues were then incubated overnight at 4 °C with the following primary antibodies: mouse monoclonal anti-S100β (1:1000, Abcam, Cambridge, UK), rabbit polyclonal anti-NGFR (1:1000; Abcam, Cambridge, UK), mouse monoclonal anti-MBP (1:500; Thermo Fisher Scientific), mouse monoclonal anti-GFAP (2.5 µg/mL; STEMCELL Technologies, Vancouver, BC, Canada) and rabbit polyclonal anti-neurofilament (1:1000, Abcam, Cambridge, UK). The following day, the tissues were washed with 0.1% Tween 20 washing solution (Sigma Aldrich, St. Louis, MO, USA) (three times, 5 min each) and incubated with a secondary antibody, such as Texas Red anti-mouse IgG (1:300; Abcam, Cambridge, UK) or fluorescein isothiocyanate (FITC) anti-rabbit IgG (1:300, Abcam, Cambridge, UK) 2 h in the dark at 37 °C. Nuclei were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI) (1:15000, Invitrogen, Carlsbad, CA, USA) (room temp, 40 min in the dark). Finally, the tissues were rinsed with Dulbecco’s phosphate-buffered saline (DPBS) (three times) before being mounted using Depex mounting solution and examined under a fluorescence microscope (Eclipse Ti, Nikon, Tokyo, Japan).
Identification of engrafted human cells in rats
The survival of engrafted human cells was determined by IHC staining with mouse monoclonal antibody specific for human cytoplasmic marker (STEM121) (1:500, StemCells, Newark, CA, USA). IHC staining was performed as abovementioned.
Transmission electron microscopy (TEM) analysis
TEM analysis was performed as described previously with minor modifications [18]. The glutaraldehyde-fixed samples were post-fixed in 1% (w/v) PBS-buffered osmium tetroxide at 4 °C for 2 h. The samples were washed with sodium cacodylate and dehydrated with a series of 35%–100% acetone, followed by sample infiltration using a mixture of acetone and resin (ratio of 1:1 for 1 h, 1:2 for 2 h and 100% resin overnight). Polymerisation was conducted in an oven at 60 °C 24–48 h before sectioning into 1 µm-thick semi-thin sections and 50 nm-thick ultrathin sections via an ultramicrotome (Leica, Bensheim, Germany). The semi-thin sections were stained with 1% (w/v) toluidine blue/5% (w/v) sodium borate prepared in distilled water and examined via light microscopy. The ultrathin sections were cut and collected on copper slot grids with Polyform/carbon-support films and observed via TEM (Tecnai G2 Spirit BioTwin, FEI, Hillsboro, OR, USA). The average number of myelinated axons, axon and fibre diameter, myelin sheath thickness and axon-to-fibre diameter (G-ratio) were quantitatively analysed.
Determination of gastrocnemius muscle atrophy
The gastrocnemius muscle of the rats (right side [experimental] and left side [normal]) were harvested at 12 weeks post-implantation and weighed. Muscle atrophy was determined on the basis of the differences between the right and left muscle weight.
Statistical analysis
Data were expressed as mean ± standard error of the mean. One-way ANOVA, two-way ANOVA and Tukey’s multiple comparison test were performed using GraphPad Prism version 7.0 (GraphPad Software, Inc., San Diego, CA, USA) to determine the significant differences between groups and time point. Significance level was defined as p < 0.05.
Results
Development of nerve conduit
Immunocytochemistry analysis showed the positive expression of S100β, P75 NGFR, MBP and GFAP in ndMSCs similar to that in Schwann cells. In MSCs, only S100β was expressed (Data not shown). The positive expression of neural markers after treatment with 400 µg/mL C. asiatica showed that MSCs were successfully differentiated into ndMSCs. H&E staining after the 2-day incubation of the seeded conduits showed that MSCs or ndMSCs were successfully seeded into the decellularized HUC artery conduit. The seeded cells, MSCs or ndMSCs, adhered, spread well in the inner wall of the decellularized artery conduit (Data not shown) and did not clump or aggregate, same as in our previous study [16]. These results showed the biocompatibility between the HUC artery conduit and the seeded cells.
Creation of nerve injury and implantation
A 15 mm sciatic nerve injury was successfully created in immunosuppressed SD rats. For the RA group, the donor sciatic nerve obtained during the creation of 15 mm sciatic nerve injury did not shrink after cutting. The diameter of the artery conduits used for MC and NC implantation was similar to that of the sciatic nerve; as such, implantation was performed smoothly.
Pinch test
The earliest response of the rats was at weeks 6 (n = 2) and 8 (n = 1) for those in RA group, at weeks 8 (n = 1) and 12 (n = 2) for those treated with MC implantation and at week 6 (n = 3) for those treated with NC implantation. The results showed that NC implantation improved the rats’ sensory sensitivity similar to that in RA. A positive response revealed that sensory function was recovered amongst the rats that experienced sciatic nerve injury. The pinch test could not be performed on the rats that exhibited self-autotomy during MC (week 8) and NC implantations (week 4) (Table 1).
Table 1.
Pinch test from 2 weeks post-implantation
| Groups/weeks | 0 (Pre-implantation) | 2 | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|---|---|
| RA1 | + | – | – | + | + | + | + |
| RA2 | + | – | – | + | + | + | + |
| RA3 | + | – | – | – | + | + | + |
| MC1 | + | – | – | – | – | – | + |
| MC2 | + | – | – | – | + | + | + |
| MC3 | + | – | – | – | – | – | + |
| MC4 | + | – | – | – | N/A | N/A | N/A |
| NC1 | + | – | – | + | + | + | + |
| NC2 | + | – | N/A | N/A | N/A | N/A | N/A |
| NC3 | + | – | – | + | + | + | + |
| NC4 | + | – | – | + | + | + | + |
(–) shows negative response, whilst (+) shows positive response
N/A (not applicable) shows that the rats experienced self-autotomy; thus, the pinch test could not be performed. Reversed autograft (RA) (n = 3), MSC-seeded conduit (MC) (n = 4), ndMSC-seeded conduit (NC) (n = 4)
Nerve conduction study
Amplitude
The RA group presented the highest amplitude compared with the MC and NC groups at all weeks (Fig. 1A). Meanwhile, the amplitude in the NC group was higher than that of the MC group at all weeks except in week 6. All groups exhibited increasing amplitude almost every week. High amplitude measurements indicate high state of nerve myelination, whereas low amplitude indicates the loss of axons. However, the differences amongst the three groups were not significant (Fig. 1A). In terms of functional recovery percentage, the CMAP of the RA group showed neurophysiological recovery of 100% of the normal baseline values at week 12 and was the highest for all the groups. Meanwhile, the CMAP of the NC group showed neurophysiological recovery of 89.12%, which was higher than that of the MC group (70.66%) (Table 2).
-
(b)
Latency
The NC group showed the lowest latency amongst all groups in all weeks except at week 4. The RA group presented the lowest latency at week 4 (1.58 ± 0.58 ms) compared with MC (1.84 ± 0.43 ms) and NC groups (1.71 ± 0.03 ms) (Fig. 1B). All groups exhibited decreasing latency almost every week. A low latency indicates that the electrical impulse travels fast through the nerve with a high state of nerve myelination. However, the difference amongst the three groups at the same time point was not significant. The only significant difference in latency was between the 2nd and 12th weeks in the RA group (p < 0.05) (Fig. 1B). At week 12, the latency of the NC group recovered up to 96.15% of the normal baseline values and was the highest compared with that of RA (95%) and MC (94.12%). The MC group showed the lowest recovery amongst the three groups (Table 2).
-
(c)
NCV
The NCV of the NC group was higher than that of the RA and MC groups at weeks 2, 6 and 12 (Fig. 1C). All groups showed increasing NCV throughout the 12 weeks. Statistical analysis showed that the NC group has significantly higher NCV at week 12 than at weeks 2, 4 and 6 (p < 0.05). Meanwhile, the RA group had significantly higher NCV at week 12 than at week 2 (p < 0.05). The NCV of the MC group was not significantly different in all weeks. The differences amongst the groups were not significant (Fig. 1C). At week 12, the NC group recovered up to 93.63% of the normal baseline values compared with RA (91.32%) and MC (73.66%). The MC group showed the lowest recovery amongst the three groups (Table 2).
Fig. 1.
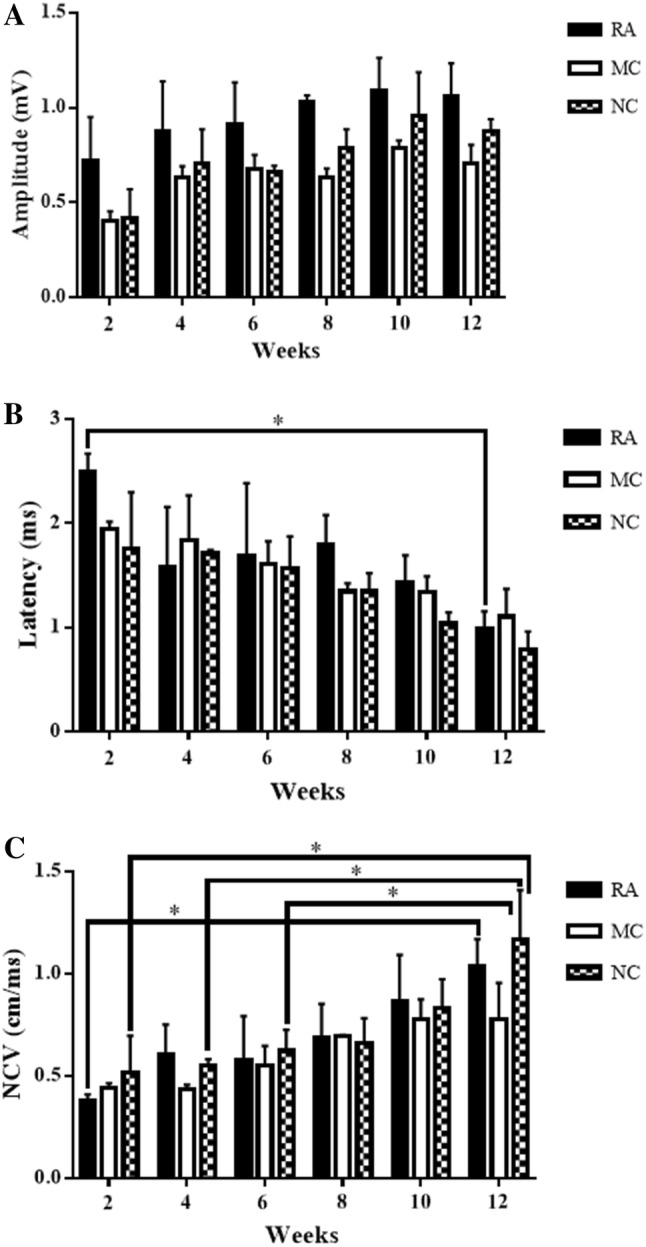
NCS. A Mean values of the peak amplitude of CMAP from 2 weeks post-implantation. P value < 0.05. n=3. B. Mean values of latency of CMAP from 2 weeks post-implantation. p value < 0.05. n=3. C. Mean values of NCV from 2 weeks post-implantation. P value < 0.05. n=3
Table 2.
Percentage of functional recovery (amplitude, latency and NCV) in RA, MC and NC groups between week 0 (pre-implantation) and weeks 12
| Parameters/groups | Weeks | 0 | 12 | % of functional recovery |
|---|---|---|---|---|
| Amplitude CMAP (mV) | RA | 9.13 ± 1.74 | 9.17 ± 0.91 | 100.44 |
| MC | 12.10 ± 0.38 | 8.55 ± 0.78 | 70.66 | |
| NC | 9.65 ± 1.45 | 8.6 ± 2.00 | 89.12 | |
| Latency (ms) | RA | 0.95 ± 0.35 | 1.00 ± 0.50 | 95.00 |
| MC | 0.8 ± 0.10 | 0.85 ± 0.15 | 94.12 | |
| NC | 1.25 ± 0.05 | 1.30 ± 0.30 | 96.15 | |
| NCV (cm/ms) | RA | 2.19 ± 0.31 | 2.00 ± 1.00 | 91.32 |
| MC | 2.05 ± 0.18 | 1.51 ± 0.22 | 73.66 | |
| NC | 1.57 ± 0.31 | 1.47 ± 0.24 | 93.63 |
Reversed autograft (RA) (n = 3), MSC-seeded conduit (MC) (n = 4), ndMSC-seeded conduit (NC) (n = 4)
Macroscopic observation of sciatic nerve at 12 weeks post-implantation
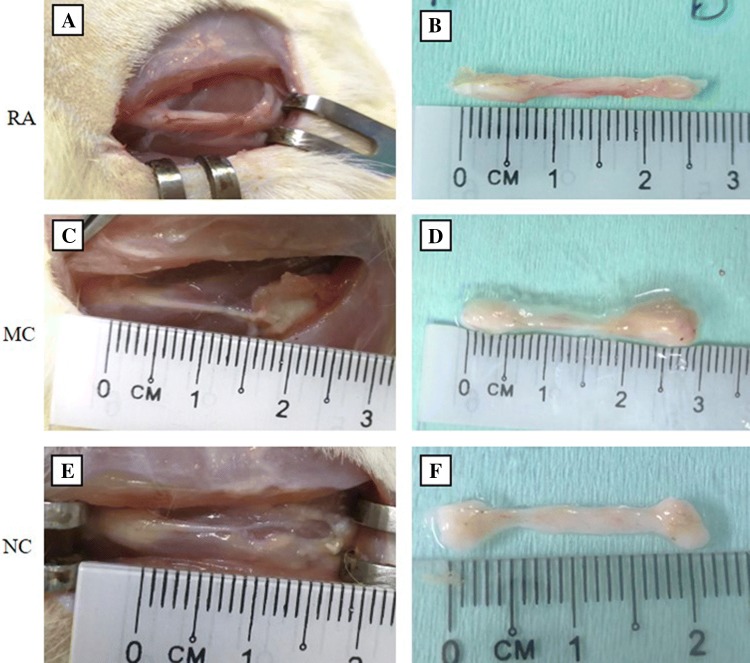
At 12 weeks post-implantation, nerve regeneration was observed in the RA, MC and NC groups (Fig. 2). The nerves in the RA group were intact, and blood vessels surrounding the nerve were evident. The implanted sciatic nerve in the RA group did not degrade, and the diameter of the nerve was preserved (Fig. 2A, B). The conduits in the MC and NC implantation groups did not degrade but unfortunately shrunk, especially in the middle part (Fig. 2C–F).
Fig. 2.
Sciatic nerve 12 weeks post-implantation. A, B Sciatic nerve in RA treatment (n = 3). C, D Sciatic nerve in MC implantation (n = 4). E, F Sciatic nerve in NC implantation (n = 4)
H&E staining
H&E staining showed cell migration and nerve regeneration in all groups at 12 weeks post-implantation as indicated by the presence of the cells with purple-stained nucleus at the proximal, middle and distal sites of the harvested nerves. The presence of red blood cells in the proximal site of the RA and NC groups confirmed that angiogenesis occurred (Fig. 3).
Fig. 3.
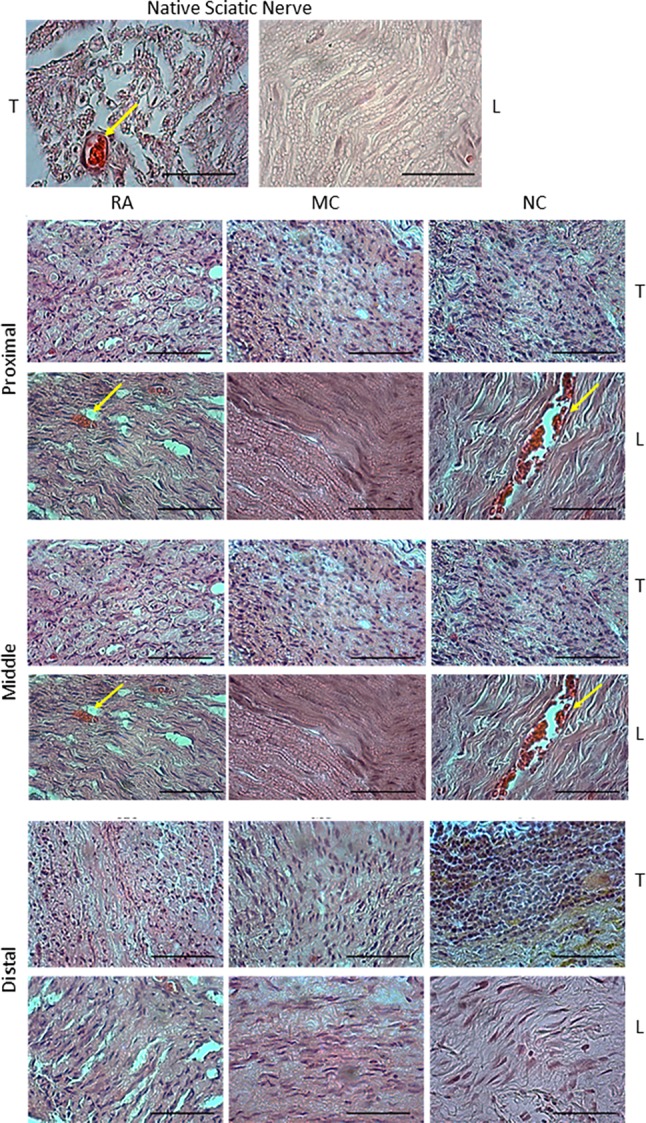
H & E staining of the harvested sciatic nerve for RA (n = 3), MC (n = 4) and NC (n = 4) groups (proximal, middle and distal). Arrows show red blood cells on the proximal part of RA and NC similar to those of the native sciatic nerve. Magnification, 20×. Scale bar, 100 μm. T = transverse section, L = longitudinal section
IHC analysis
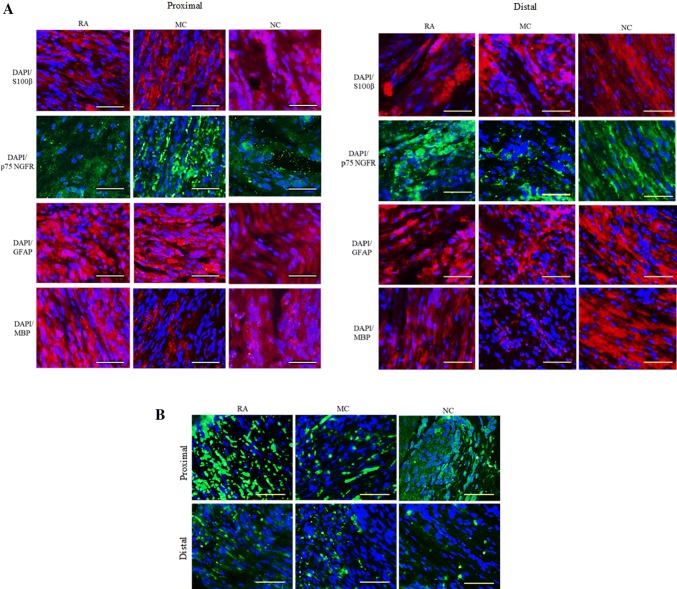
IHC analysis revealed that the neural marker proteins were not expressed in all groups, and axonal regeneration was enhanced in NC and RA as indicated by the MBP expression in the proximal and distal sites of such groups. The expression of MBP in the MC group in the proximal and distal sites was lower than that in the RA and NC groups. Meanwhile, the expression of S100β, P75 NGFR and GFAP was similar for all groups (Fig. 4A). Additionally, the expression of neurofilament in the proximal site was higher than that in the distal site in all groups. Thus, the nerve had regenerated from the proximal to the distal site (Fig. 4B).
Fig. 4.
IHC analysis (ICC) of the sciatic nerve 12 weeks post-implantation for RA, MC and NC groups (proximal and distal). Nuclei were counter-stained with DAPI (blue). Analysis of the longitudinal section. Magnification, 20×. Scale bar, 100 μm. n = 3. A ICC of the sciatic nerve for protein S100β (red), p75NGFR (green), GFAP (red) and MBP (red). B ICC of the sciatic nerve for protein NF (green)
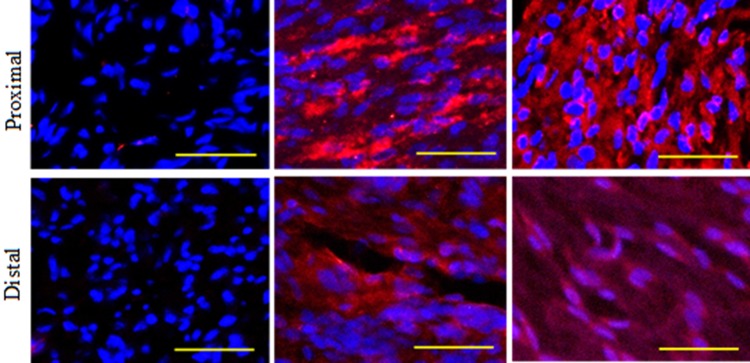
Identification of engrafted human cells in rats
The expression of STEM121 was not detected in the RA group in the proximal and distal sites. STEM121 was expressed in the MC and NC groups in the proximal and distal sites. As such, the engrafted human cells could survive in the rats and were not rejected until 12 weeks post-implantation (Fig. 5).
Fig. 5.
Identification of engrafted human cells in SD rats by the expression of protein STEM 121 (red). Nuclei were counter-stained with DAPI (blue). Analysis of the longitudinal section. Magnification, 20×. Scale bar, 100 μm. n = 3
Toluidine blue staining
The myelinated axons and myelin sheath on the semi-thin transverse section of the regenerated nerves were identified via histological analysis with toluidine blue at 12 weeks post-implantation. The myelinated axons were more abundant with an even distribution, had superior morphological appearances, and were more organised in the RA and NC groups than in the MC group. The unmyelinated axons in the MC group were more abundant than those in the RA and NC groups, and the unmyelinated and myelinated axons were unevenly distributed and compressed (Fig. 6).
Fig. 6.
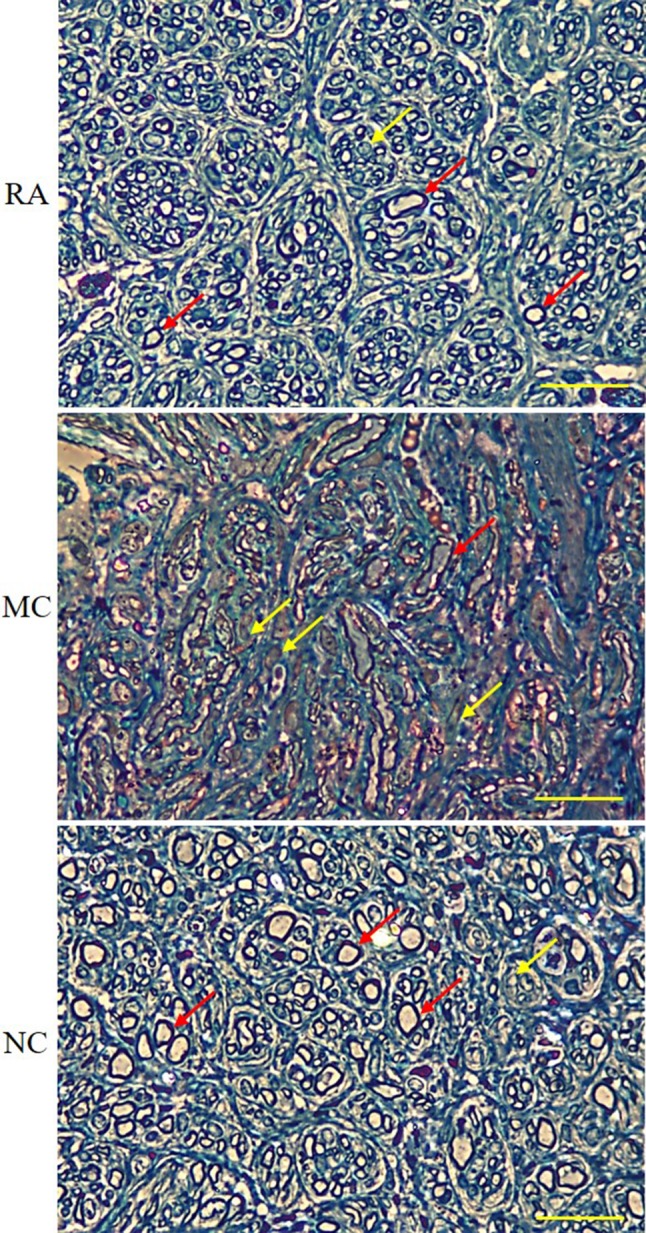
Transverse section of toluidine blue-stained sciatic nerve under a light microscope. Red arrows show myelinated axons, whereas yellow arrows show non-myelinated axons. Magnification, 40×. Scale bar, 200 μm. n = 3
Morphometric analysis of sciatic nerve
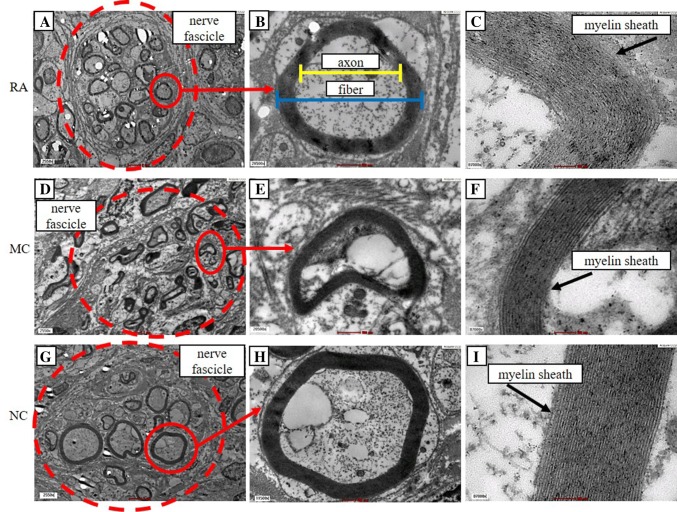
Morphometric analysis was conducted on the ultrathin section of the nerve to assess axonal regeneration. TEM showed that the myelinated axons in RA and NC groups were circular in shape, organised and evenly distributed in the nerve fascicle, whereas those in the MC group were compressed, disorganised and unevenly distributed in the nerve fascicle. The myelin sheath in the RA and NC groups were thicker than that in the MC group (Fig. 7).
Fig. 7.
TEM analysis of the sciatic nerve. The dashed circles in A, D and G show axon in a nerve fascicle. Red arrows show an enlarged myelinated axon. The image in B shows a representative of axonal and fibre diameter. The images in C, F and I show layers of myelin that form myelin sheath. Analysis of the transverse section. A, D, G Magnification, 2550×. Scale bar, 2 μm. B, E, H Magnification, 20,500×. Scale bar, 500 nm. (C, F, I Magnification, 87,000×. Scale bar, 100 nm. n = 3
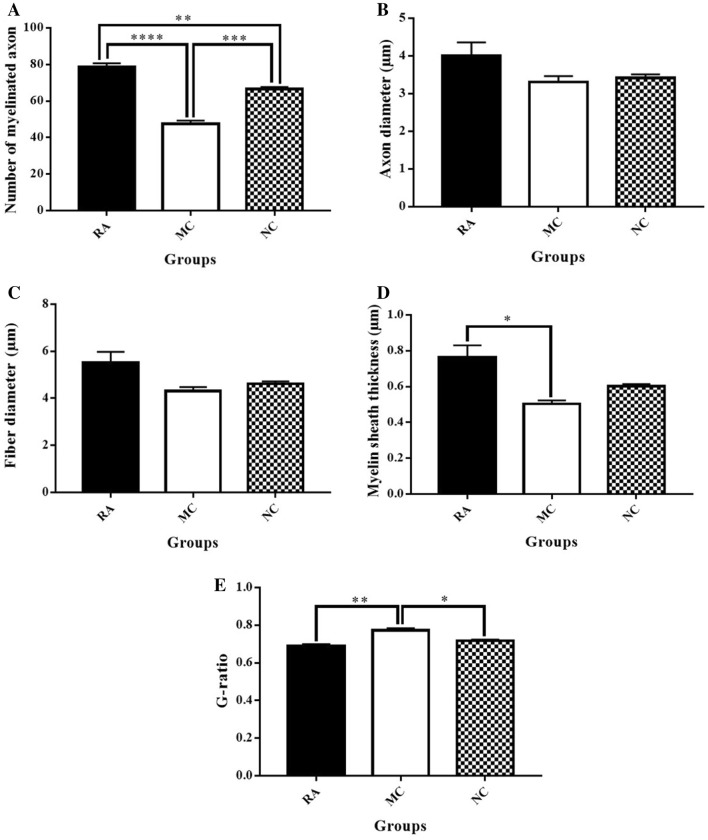
The RA group had significantly higher number of myelinated axons and axon and fibre diameters (myelinated axons: 78.5 ± 2.1, axon diameter: 3.99 ± 0.36 µm, fibre diameter: 5.52 ± 0.46 µm) than the NC (myelinated axons: 66.5 ± 1.2, axon diameter: 3.42 ± 0.09, fibre diameter: 4.62 ± 0.10 µm) and MC groups (myelinated axons: 47.5 ± 1.8, axon diameter: 3.31 ± 0.16 µm, fibre diameter: 4.31 ± 0.17 µm) (Fig. 8A–C). However, the differences were not significant. The myelin sheath thickness of the RA group was higher (0.76 ± 0.07 µm) than that of the NC (0.60 ± 0.01 µm) and MC groups (0.50 ± 0.02 µm; significantly different) (Fig. 8D). The G-ratio of the RA (0.69 ± 0.01) and NC groups (0.72 ± 0.01) was significantly lower (higher myelination) than that of MC (0.77 ± 0.01) (Fig. 8E).
Fig. 8.
Morphometric analysis of sciatic nerve. A Number of myelinated axons. p value: **p = 0.0068, ***p = 0.0006, ****p = < 0.0001. B Axon diameter. C Fiber diameter. D Myelin sheath thickness. p value: *p = 0.0115. E G-ratio. p value: *p = 0.0149, **p = 0.0018. n = 3
Gastrocnemius muscle atrophy analysis
The gastrocnemius muscle weights of the right side (experimental) were normalised with the gastrocnemius muscle weight of the left side (normal) to examine the extent of muscle atrophy. The results of the gastrocnemius muscle atrophy analysis 12 weeks post-implantation revealed that the RA group had lower muscle atrophy (higher weight of muscle), which is 0.93 ± 0.04 g compared with that of NC (0.83 ± 0.01 g) and MC (0.81 ± 0.03 g). However, the differences were not significant.
Discussion
The effect of ndMSCs in promoting the regeneration of peripheral nerve in a critical-size defect animal model was evaluated. Human Wharton’s jelly MSCs (hWJMSCs) are attractive candidates in regenerative medicine due to their unique properties, including high proliferative rate, multipotency, immunosuppressive effects, low expression of major histocompatibility complex (MHC) I molecules and non-expression of MHC II molecules [19, 20]. Furthermore, hWJMSCs could form neural cells, such as neurons, oligodendrocytes and astrocyte [21].
Following our success in constructing a nerve conduit by using HUC artery seeded with C. asiatica ndMSCs [16], this conduit was further tested by bridging PNI in rats and utilising this nerve graft. Given the 12 weeks of recovery period, the study on a 15 mm transectional sciatic nerve injury in SD rat model revealed that (1) the HUC artery conduit provided a conducive environment for ndMSC adherence and supported its proliferation and (2) C. asiatica-induced ndMSCs promoted axonal regrowth and peripheral nerve regeneration towards the distal targeted muscle through the conduit.
In this study, a 15 mm sciatic nerve injury was created because it represents the critical size of PNI in rats [22, 23]. The use of the RA group as the standard procedure for positive control group could prevent second injury when a donor nerve is obtained from another nerve segment [24]. After the nerve conduits MC or NC were implanted, no conduit rejection was detected on the rats throughout the 12 weeks of post-implantation recovery period. In the first 2 weeks, the rats experienced self-mutilation (autotomy). The degree of autotomy following the sciatic nerve injury and conduit implantation was different among the three groups, and the levels of autotomy in the RA group were lower than those in the MC and NC groups. Although this autotomy behaviour was resolved after 3 weeks, the severe extent of autotomy led to the loss of toes that rendered gait analysis impossible to perform. Autotomy is often caused by the peripheral neuropathic pain which results from nerve injury. This condition is caused by compounds, including neurotrophic molecules, cytokines and chemokines, which are produced during Wallerian degeneration and play important roles in axonal regeneration and neuropathic pain initiation [25, 26].
NC implantation could improve rat’s sensory sensitivity in a similar manner to RA, where the rats exhibited withdrawal response to pinch test as early as 6 weeks. The sensory recovery in MC group was confirmed only at 8 and 12 weeks post-implantation. The toe withdrawal response was observed in all groups possibly due to the reflex from saphenous nerve, originating from the femoral nerve and distributed to the foot dorsum [22]. Electrophysiological studies revealed that the mean amplitude of CMAP and the nerve conduction velocity were higher in the NC group than in the MC group. Additionally, the mean latency result in the NC group was lower than that of the MC group. At 12 weeks post-implantation, the NC group exhibited higher nerve conduction velocity and lower latency than the RA group. Nerve conduction velocity is a reliable index for assessing the conduction of action potential in peripheral nerves. Compound muscle action potential can only be measured when sufficient regenerated nerve fibres grow across the nerve gap and innervate the distal target muscle [27]. This result indicated that NC implantation increased the nerve regeneration by increasing the nerve fibre density from the proximal to the distal site and innervating the targeted muscle. This discovery is in agreement with a previous study stating that the oral administration of C. asiatica extract accelerates nerve regeneration and exhibits rapid functional recovery [14]. In summary, the neurological functional recovery of the NC group was comparable with that of the RA group.
After 12 weeks of implantation, the collected and processed conduits revealed that the regenerated nerves in all groups were intact and did not undergo degeneration. However, some nerves in the MC and NC groups slightly shrunk at the middle possibly because of the interactions with the surrounding muscle or stretching of the nerves. Additionally, the presence of angiogenesis could be due to nerve regeneration that produced molecules, such as cytokines and chemokines, during Wallerian degeneration. Histology and IHC analyses of the regenerated nerve provided further evidence on the degree of axonal regeneration and myelination. H&E staining revealed the formation of neural tissues in all groups; however, the observation was negligible. IHC staining showed that the axonal regeneration in the RA and NC groups was superior to that in MC, especially in the expression of MBP protein at the proximal and distal sites of the graft. This finding can be attributed to the ndMSCs seeded in the conduit, which could have acted as Schwann cells and aided in nerve regeneration. MBP is an important myelination protein in the nervous system [28], and its expression is directionally proportional to myelinated axonal regeneration.
The expression levels of S100β, p75NGFR and GFAP proteins were comparable in all groups. Transection of an axon could release neurotrophic factors [29]. A previous study also suggested that undifferentiated MSCs could differentiate into glial cells in vivo by releasing cytokines and neurotrophic factors at the injury site, thereby explaining the positive expression of S100β and other neural markers [30]. Moreover, S100β is a positive marker for Schwann cells [31]. As the main glial cells in peripheral nerve, Schwann cells proliferate and migrate to the injured nerve site and release bioactive substrates and neurotrophic factors, such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) [32, 33]. The positive expression of NF indicated the presence of nerve fibres [34]. NF forms neuronal cytoskeleton and provides structural support for axons. According to a previous study, 400 µg/mL C. asiatica induces the differentiation of MSCs into Schwann cells and to other neural lineages, such as oligodendrocytes and astrocytes, thereby explaining the positive expression of NF [15]. Furthermore, the positive detection of engrafted human cells in rats of MC and NC groups by STEM 121 expression revealed that the cells could survive inside the rats’ body. This phenomenon is attributed to the immunosuppressive treatment with cyclosporin that inhibited calcineurin, which is responsible for activating an immune reaction [35].
Toluidine blue staining, TEM and morphometric analysis further revealed that the number of myelinated axons in RA and NC groups was significantly higher than that in the MC group. The presence of native Schwann cells in the RA group contributed to axonal regeneration and increased number of myelinated axons compared with those of the NC and MC groups. On the other hand, the seeded C. asiatica-neurodifferentiated ndMSCs in the NC groups could have enhanced axonal regeneration and increased the number of myelinated axons compared with undifferentiated MSCs. Previous studies stated that differentiated MSCs by glial growth factors in vitro enhance the axonal regenerations in vivo [30, 36]. However, the number of myelinated axons was still significantly higher in the RA group than in the NC group due to the presence of host Schwann cells inside the nerve graft. The myelinated axons of the RA and NC groups had superior morphological appearance over those of the MC group. This finding could be due to the seeded cells in the conduit that play an important role in determining the degree of axon myelination. The native Schwann cells in RA and ndMSCs in NC could enhance the regeneration of nerve fibres [34].
The insignificant difference between the G-ratio of RA and NC showed that the degree of myelination in NC was similar to that in RA and better than that in MC. As such, C. asiatica-induced ndMSCs can enhance the myelination of axons and prompt neurologic functional recovery. Hence, C. asiatica-induced ndMSCs could replace Schwann cells, and the whole conduit could replace the autograft for PNI treatment.
In this study, the extent of muscle atrophy was evaluated by measuring the weight of the gastrocnemius muscle. The lower muscular atrophy showed that extensive movement was made when the sciatic nerve innervated the muscle, and the nerve was functional. Although the sciatic nerve had innervated the gastrocnemius muscle which was frequently used to study motor function, the weight of this muscle is not a definite parameter for determining its recovery. The gastrocnemius muscle is a skeletal muscle composed of slow type I and fast type II muscle fibres. The composition and distribution of these different types of muscle fibres determine their function in terms of speed of contraction, fatigability and the degree of force production [37].
NC showed promising effects on nerve regeneration and functional restoration similar to RA and better than MC. These observations revealed the neuroregenerative capabilities of C. asiatica and its potential as an alternative treatment for critical nerve defect. Thus, C. asiatica ndMSCs promotes the regeneration of the peripheral nerve in a critical-size defect animal model. Future studies with a large sample size and extended period of implantation may produce significant outcomes of treatment with ndMSC in promoting the regeneration of peripheral nerves in a critical-size defect.
Acknowledgements
This study was funded by the Ministry of Agriculture and Agro-based Industry Malaysia under NKEA Research Grant Scheme (NRGS) (Project Code: NH 1014 D048) and Universiti Kebangsaan Malaysia (Project Code: FF-2017-175 and GUP-2017-007). The author would like to thank Prof. Dr. Mohd Ilham Adenan from Atta-ur-Rahman Institute for Natural Product Discovery, Universiti Teknologi MARA, Malaysia, who kindly provided C. asiatica extract for use in this experiment.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Ethical statement
The animal study was approved by Universiti Kebangsaan Malaysia (UKM) Animal Ethics Committee (TEC/PP/2016/YOGESWARAN/18-MAY/747-MAY-2016-FEB.-2018). For the isolation of human mesenchymal stem cell (hMSCs), the written informed consents were obtained from healthy women who delivered full-term infants (38–40 weeks) by elective caesarian delivery prior to the collection of umbilical cord samples. The usage of human umbilical cord samples from consenting patients in this study was approved by the Universiti Kebangsaan Malaysia Research Ethics Committee (FF-2015-175).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kaizawa Y, Kakinoki R, Ikeguchi R, Ohta S, Noguchi T, Takeuchi H, et al. A nerve conduit containing a vascular bundle and implanted with bone marrow stromal cells and decellularized allogenic nerve matrix. Cell Transplant. 2017;26:215–228. doi: 10.3727/096368916X692951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffman PN, Lasek RJ. Axonal transport of the cytoskeleton in regenerating motor neurons: constancy and change. Brain Res. 1980;202:317–333. doi: 10.1016/0006-8993(80)90144-4. [DOI] [PubMed] [Google Scholar]
- 3.Ray WZ, Mackinnon SE. Management of nerve gaps: autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp Neurol. 2010;223:77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 5.Gu X, Ding F, Williams DF. Neural tissue engineering options for peripheral nerve regeneration. Biomaterials. 2014;35:6143–6156. doi: 10.1016/j.biomaterials.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 6.Dezawa M, Kanno H, Hoshino M, Cho H, Matsumoto N, Itokazu Y, et al. Specific induction of neuronal cells from bone marrow stromal cells and application for autologous transplantation. J Clin Invest. 2004;113:1701–1710. doi: 10.1172/JCI200420935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jonsson S, Wiberg R, McGrath AM, Novikov LN, Wiberg M, Novikova LN, et al. Effect of delayed peripheral nerve repair on nerve regeneration, Schwann cell function and target muscle recovery. PLoS One. 2013;8:e56484. doi: 10.1371/journal.pone.0056484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwong KW, Sivakumar T, Wong WK. Intein mediated hyper-production of authentic human basic fibroblast growth factor in Escherichia coli. Sci Rep. 2016;6:33948. doi: 10.1038/srep33948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li D, Yuan T, Zhang X, Xiao Y, Fan Y, Wang R. Icariin: a potential promoting compound for cartilage tissue engineering. Osteoarthritis Cartilage. 2012;20:1647–1656. doi: 10.1016/j.joca.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Raghavan RN, Vignesh G, Kumar BS, Selvaraj R, Dare BS. Phytochemicals: do they hold the future in stem cell differentiation. Int J Res Pharm. 2015;6:379–381. [Google Scholar]
- 11.Wakao S, Matsuse D, Dezawa M. Mesenchymal stem cells as a source of Schwann cells: their anticipated use in peripheral nerve regeneration. Cells Tissues Organs. 2014;200:31–41. doi: 10.1159/000368188. [DOI] [PubMed] [Google Scholar]
- 12.Jadalannagari S, Aljitawi OS. Ectodermal differentiation of wharton’s jelly mesenchymal stem cells for tissue engineering and regenerative medicine applications. Tissue Eng Part B Rev. 2015;21:314–322. doi: 10.1089/ten.teb.2014.0404. [DOI] [PubMed] [Google Scholar]
- 13.Lokanathan Y, Omar N, Ahmad Puzi NN, Saim A, Hj Idrus R. Recent updates in neuroprotective and neuroregenerative potential of Centella asiatica. Malays J Med Sci. 2016;23:4–14. [PMC free article] [PubMed] [Google Scholar]
- 14.Soumyanath A, Zhong YP, Gold SA, Yu X, Koop DR, Bourdette D, et al. Centella asiatica accelerates nerve regeneration upon oral administration and contains multiple active fractions increasing neurite elongation in-vitro. J Pharm Pharmacol. 2005;57:1221–1229. doi: 10.1211/jpp.57.9.0018. [DOI] [PubMed] [Google Scholar]
- 15.Norazzila O, Yogeswaran L, Mohd Razi ZR, Bt Haji Idrus R. The effects of Centella asiatica (L.) Urban on neural differentiation of human mesenchymal stem cells in vitro. BMC Complement Altern Med. 2019;19:167. doi: 10.1186/s12906-019-2581-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hussin HM, Idrus RH, Lokanathan Y. Development of nerve conduit using decellularized human umbilical cord artery seeded with Centella asiatica induced-neurodifferentiated human mesenchymal stem cell. Sains Malays. 2018;47:2789–2798. doi: 10.17576/jsm-2018-4711-22. [DOI] [Google Scholar]
- 17.Leow SN, Luu CD, Hairul Nizam MH, Mok PL, Ruhaslizan R, Wong HS, et al. Safety and efficacy of human Wharton’s Jelly-derived mesenchymal stem cells therapy for retinal degeneration. PLoS One. 2015;10:e0128973. doi: 10.1371/journal.pone.0128973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Qi F, Zhu S, Ye Z, Ma T, Hu X, et al. A synthetic oxygen carrier in fibrin matrices promotes sciatic nerve regeneration in rats. Acta Biomater. 2013;9:7248–7263. doi: 10.1016/j.actbio.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 19.Bongso A, Fong CY. The therapeutic potential, challenges and future clinical directions of stem cells from the Wharton’s jelly of the human umbilical cord. Stem Cell Rev Rep. 2013;9:226–240. doi: 10.1007/s12015-012-9418-z. [DOI] [PubMed] [Google Scholar]
- 20.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, VanderWerff I, et al. Immune properties of human umbilical cord Wharton’s jelly-derived cells. Stem Cells. 2008;26:2865–2874. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell KE, Weiss ML, Mitchell BM, Martin P, Davis D, Morales L, et al. Matrix cells from Wharton’s jelly form neurons and glia. Stem Cells. 2003;21:50–60. doi: 10.1634/stemcells.21-1-50. [DOI] [PubMed] [Google Scholar]
- 22.Yurie H, Ikeguchi R, Aoyama T, Kaizawa Y, Tajino J, Ito A, et al. The efficacy of a scaffold-free Bio 3D conduit developed from human fibroblasts on peripheral nerve regeneration in a rat sciatic nerve model. PLoS One. 2017;12:e0171448. doi: 10.1371/journal.pone.0171448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lokanathan Y, Ng MH, Hasan S, Ali A, Mahmod M, Htwe O, et al. Olfactory ensheathing cells seeded muscle-stuffed vein as nerve conduit for peripheral nerve repair: a nerve conduction study. J Biosci Bioeng. 2014;118:231–234. doi: 10.1016/j.jbiosc.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Costa MP, Teixeira NH, Longo MV, Gemperli R, Costa HJ. Combined polyglycolic acid tube and autografting versus autografting or polyglycolic acid tube alone A comparative study of peripheral nerve regeneration in rats. Acta Cir Bras. 2015;30:46–53. doi: 10.1590/S0102-86502015001000006. [DOI] [PubMed] [Google Scholar]
- 25.Carr MM, Best TJ, Mackinnon SE, Evans PJ. Strain differences in autotomy in rats undergoing sciatic nerve transection or repair. Ann Plast Surg. 1992;28:538–544. doi: 10.1097/00000637-199228060-00008. [DOI] [PubMed] [Google Scholar]
- 26.Klusáková I, Dubový P. Experimental models of peripheral neuropathic pain based on traumatic nerve injuries–an anatomical perspective. Ann Anat. 2009;191:248–259. doi: 10.1016/j.aanat.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Ao Q, Fung CK, Tsui AY, Cai S, Zuo HC, Chan YS, et al. The regeneration of transected sciatic nerves of adult rats using chitosan nerve conduits seeded with bone marrow stromal cell-derived Schwann cells. Biomaterials. 2011;32:787–796. doi: 10.1016/j.biomaterials.2010.09.046. [DOI] [PubMed] [Google Scholar]
- 28.Martini R, Mohajeri MH, Kasper S, Giese KP, Schachner M. Mice doubly deficient in the genes for P0 and myelin basic protein show that both proteins contribute to the formation of the major dense line in peripheral nerve myelin. J Neurosci. 1995;15:4488–4495. doi: 10.1523/JNEUROSCI.15-06-04488.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proc Natl Acad Sci U S A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tohill M, Mantovani C, Wiberg M, Terenghi G. Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci Lett. 2004;362:200–203. doi: 10.1016/j.neulet.2004.03.077. [DOI] [PubMed] [Google Scholar]
- 31.Michetti F, Corvino V, Geloso MC, Lattanzi W, Bernardini C, Serpero L, et al. The S100B protein in biological fluids: more than a lifelong biomarker of brain distress. J Neurochem. 2012;120:644–659. doi: 10.1111/j.1471-4159.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- 32.Haastert K, Mauritz C, Matthies C, Grothe C. Autologous adult human Schwann cells genetically modified to provide alternative cellular transplants in peripheral nerve regeneration. J Neurosurg. 2006;104:778–786. doi: 10.3171/jns.2006.104.5.778. [DOI] [PubMed] [Google Scholar]
- 33.Novikova LN, Pettersson J, Brohlin M, Wiberg M, Novikov LN. Biodegradable poly-β-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials. 2008;29:1198–1206. doi: 10.1016/j.biomaterials.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 34.Dezawa M, Takahashi I, Esaki M, Takano M, Sawada H. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14:1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- 35.Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant. 2012;2012:230386. doi: 10.1155/2012/230386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brohlin M, Mahay D, Novikov LN, Terenghi G, Wiberg M, Shawcross SG, et al. Characterisation of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64:41–49. doi: 10.1016/j.neures.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Koning M, Harmsen MC, van Luyn MJ, Werker PM. Current opportunities and challenges in skeletal muscle tissue engineering. J Tissue Eng Regen Med. 2009;3:407–415. doi: 10.1002/term.190. [DOI] [PubMed] [Google Scholar]