Abstract
Prodigiosin is a secondary metabolite, with red pigmentation, produced by Serratia marcescens. Red pigment is a natural alkaloid whose chemical structure has three pyrrole rings. Prodigiosin has been described for several biological activities, including antitumor, inducing apotosis in T and B lymphocytes. This work aimed to evaluate the cytotoxic activity of prodigiosin in NCHI-292, HEp-2, MCF-7 and HL-60 tumor cell lines. The red pigment was isolated from Serratia marcescens UFPEDA 398 biomass whose fractions were previously separated by column chromatography, purified, identified and further characterized by GC–MS and compared with the computerized library of m/z values. The pigment corresponded to prodigiosin with maximum absorption at 534 nm, molecular weight 323 and structural formula C20H25N3O. During the prodigiosin purification process a purple absorbance fraction at 272.65 nm was also observed. Significant cytotoxic effects of prodigiosin were evidenced for NCHI-292, Hep-2, MCF-7 and HL-60 tumor cell lines. The isolated purple fraction had no cytotoxic effect (IC50 11.3 µg/mL) when compared to prodigiosin (IC50 3.4 µg/mL) for the tumor cell lines studied. The MCF-7 strain was slightly more pigment resistant (IC50 5.1 µg/mL). Therefore, further studies will be needed to elucidate the antitumor mechanisms of prodigiosin action against tumor strains from flow cytometry tests. However, although these data are preliminary, it was evidenced that prodigiosin showed cytotoxic activity in tumor cell lines suggesting promising antitumor properties. In this sense, future studies on the cytotoxic and genotoxic effects of prodigiosin produced by S. marcecsens UFPEDA 398 are suggested.
Keywords: Prodigiosin, Serratia marcescens, Cytotoxicity, Genotoxicity
Introduction
Prodigins represent a class of antibiotics oligopyrrol pigmented with high medicinal potential as immunosuppressants and tumor agents [1]. These pigments (Fig. 1) are commonly synthesized and evidenced shortly after the exponential growth phase, Log phase [2]. Actually, three classes are described for these pigments. The first brings together the group of linear tripyrols, including prodigiosin whose synthesis can be observed in a few microorganisms (Serratia marcescens, Hahella chejuensis, Pseudomonas magnesiorubra and Vibrio psychroerythreus) and undercylprodigiosin (isolated from Streptomyces longisporus ruber, Streptoverticillium rubrireticuli, Actinomadura madurae, Streptomices coelicolor and Saccharopolyspora sp.). The second class brings together the group of macrocyclic compounds, characterized by their formation between pyrroles A and C, such as cycloprodigiosin isolated from Actinomadura pelletieri and Actinomadura madurae [3]. And finally, the third class represented by the group of cyclic compounds that have only one pyrrol (pyrrol C) and includes cycloprodigiosin C (isolated from Vibrio gazogenes, Alteromonas rubra and Pseudoalteromonas denitrificans) and butylcyclohepitylprodigiosin isolated from Saccharopolyspora sp. and Streptomyces coelicolor [4].
Fig. 1.
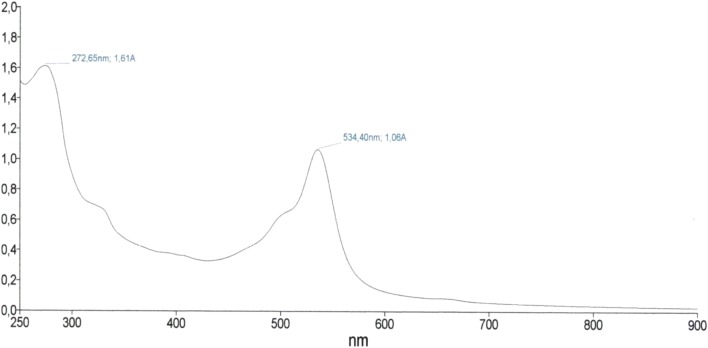
UV–Vis spectrophotometry of the red fraction with maximum absorbance at 524.40 nm and purple fraction (272.65 nm), respectively
The physiological reasons why these microorganisms synthesize the pigment are not yet well elucidated. However, it is known that highly resistant clinical isolates of the same species generally do not produce pigment while environmental strains are excellent producers. Thus, suggesting a defense mechanism for these microorganisms [5]. These natural alkaloids have been described in the literature for their antibacterial, antifungal, antiprotozoal, antimalarial, algicidal and insecticidal effects [6–10]. However, prodigiosin has aroused a growing interest in various areas such as industrial, biotechnological, medical and mainly pharmaceutical due to its antitumor activity and immunosuppressive effects on T and B lymphocytes, induction of apoptosis, antitumor effects on myelomas, lymphocytic leukemia (T-cell Leukemia), Burkitt’s lymphomas and also, in breast, colon, liver, lung, prostate and other cancers, with little adverse effects on healthy cells [8, 11–13].
The growing interest in the formulation of prodigiosin-like drugs as antitumor and immunosuppressive agents has been intensified in recent years due to numerous factors. Alkyprodigiosin branched chain prodigiosins isolated from Streptomyces coelicolor have improved potential in their immunosuppressive and antitumor activity [14]. Undercylprodigiosin, branched chain, is the best elucidated in the literature where studies show that it is the resultant of several acetate units as well as proline, glycine and serine units [15]. In this sense, as described above for the cytotoxic and antitumor activity of prodigiosin, the cytotoxic effects of the isolated Serratia marcescens UFPEDA 398 red pigment on tumor cell lines were evaluated.
Material and Method
Cells and Culture Conditions
Considering the high prevalence of breast, lung, oral cavity and leukemia cancer, according to data available from the José Gomes da Silva National Cancer Institute in 2018 [16], the following tumor cell lines were selected for this study: NCIH-292 (Lung Mucoepidermoid Carcinoma), Hep-2 (Laryngeal Epidermoid Carcinoma), MCF-7 (Breast Adenocarcinoma), and HL-60 (Human Promyelocytic Leukemia). All strains were obtained from the Laboratory of Cell Cultures and Pharmacological tests, from Department of Antibiotics, Federal University of Pernambuco—UFPE. The strains were cultured (2 × 105cells/mL) in tissue culture flasks (25 cm2/60 mL) containing cell culture medium, Minimum Essencial Medium Eagle modified Dulbecco’s (DMEN, Sigma), supplemented with 10% fetal bovine serum (Gibco), 1% antibiotic solution (penicillin 1000 IU/mL + streptomycin 250 mg/mL) and 1% of l-glutamine 200 mM and incubated at 37 °C in a humidified atmosphere containing 5% of CO2.
Prodigiosin Production
S. marcescens was inoculated into flasks (250 mL) containing 100 mL of Difco nutrient broth (10 g peptone; 5 g NaCl and 3 g/L yeast extract), then incubated at 28 °C at 150 rpm for 16 h (overnight), until an optical density of two is obtained (OD600 = 2, corresponding to 106 cells/mL). The fermentative processes for prodigiosin production were performed according to Vendruscolor et al. [17], and the solid state fermentation process was performed aiming at the maximum yield of the synthesized pigment. The previously prepared bacterial inoculum was transferred to plates containing Difco’s medium, Mannitol Agar (5 g peptone, 2 g yeast extract, 20 g mannitol and 17 g bacteriological agar in 1000 mL). The final pH of the medium was adjusted to seven (pH 7) as described by Giri et al. [5] and Wasserman et al. [15].
Prodigiosin Isolation
The pigment isolation was performed according to Nakashima et al. [18], using the chloroform: methanol solvent system in the proportions (1:1; 2:1; and 1:2 v/v), and finally extracted with methanol (100%). Then, the extracted fractions were gathered and concentrated by rota-evaporation at 45 °C with the prodigiosin extract obtained [19].
Prodigiosin Purification
After extraction, the pigment was purified by the column chromatography method (Columm chromatography, TLC) as described by Nakashima et al. [18] using a column of specific size (22 × 1 cm column) and employing Silica Gel. (Merck) as adsorbent. The purification process was performed by solubilizing the pigment in 3 mL of methanol and then eluting using the chloroform:methanol:acetic acid (5:30:65 v/v) solvent system modified to chloroform:methanol:acetone (4:2:3 v/v), where fractions were collected (10 mL) corresponding to the purified pigment. The fractions were monitored by thin layer chromatography (CCD) for subsequent identification and determination of reference values (Rf) of the samples using silica gel aluminum plates and submitted to the same solvent system (Chloroform:Methanol:Acetone 4:2:3 v/v), aiming a possible separation of the products. Then the reference value (Rf) of the fractions was determined. With the confirmation of a single substance the red fractions were gathered, corresponding to the purified pigment and the observed purple fractions were separated. Then the purified fractions were taken to be characterized [18–20].
Prodigiosin Characterization
The characterization of the isolated red fraction was performed by spectrophotometry (UV, SPEDCORD® PLUS spectrophotometer, with absorbance determined in the range of 200–700 nm). For the determination of the molecular weight of the sample, gas chromatography coupled to mass spectrometry GC–MS (GCMS-QP2010 Ultra) was employed. GC–MS mass spectrometry should be used when its known the mass spectrum of the compound such as prodigiosin and its derivatives, according to the literature. Then, the data obtained were compared with the mass values recorded in the computerized m/z value library. [3, 4, 18–20].
Cell Viability Test and Cytotoxic Activity
Cell viability and cytotoxic activity of prodigiosin in tumor cell lines were performed by the MTT [3-(4,5-dimethylthiazol-2-2,5-dipheniltetrazolium bromide)] colorimetric test after 72 h of incubation. This is a quantitative in vitro assay developed by Mosmann et al. [20] and was later adapted by Alley et al. [21] to estimate cell proliferation and survival and is effective in highlighting cytotoxicity. The test is based on the analysis of cellular enzymes that reduce the tetrazolic dye (MTT) to its insoluble form. The formazan is evidenced by the purple color and subsequently measured by the absorbance in nm. Initially, cells were cultured at a concentration of 105 cells/mL.
The positive control used was the standard doxorubicin (Dox) chemotherapy. The substances (test and control) were previously dissolved in DMSO and then serially diluted (25–0.3 μg/mL) in RPMI 1640 medium and transferred to 96-well plates (100 μL/well). The plates were incubated at 37 °C for 72 h in a humidified 5% CO2 atmosphere. Then 25 μL of MTT solution (tetrazolium salt) was added and incubated for a further 3 h. After dissolution of the precipitate with DMSO (0.2 μg/mL), the plates were read in a 595 nm plate spectrophotometer and then measured the CL50 (concentration capable of inhibiting 50% of cell growth) and their respective confidence intervals (95% CI) calculated from nonlinear regression using Prisma version 5.0 software (GraphPad, Intiuitive Software for Science, San Diego, CA).
Annexin Cytometry Assay V-FITC and Propidium Iodide (PI)
The investigation of cell death by apoptosis and necrosis was performed using annexin V-FITC and propidium iodide (PI) (Bender MedSystems, Boerhinger Maheim) according to the method described by Koopman et al. [22] and Belossillo et al. [23]. Annexin V binds to cells expressing on its membrane surfaces phosphatidylserine, while propride iodide stains the DNA of these cells with compromised nuclear membrane in the latter case, expressing the integrity of the cell membrane. Thus, it is possible to quantitatively discriminate the number of viable cells (labeled with any type of fluorochrome) in early apoptosis (marked with annexin V only) and late apoptosis and necrosis (both labeled with annexin V and PI). 105 cells per mL of NCIH-292 and Hep-2 strains were cultured in 24-well microplates containing 1 mL of medium and incubated at 48 and 72 h in DEMEN medium, 5% CO2 at 37 °C at an IC50-based prodigiosin concentration of 3.4 μg/mL. The HL-60 strain, also analyzed, was incubated at times (48 and 72 h) at an IC50-based prodigiosin concentration of 1.7 μg/mL in RPMI 1640 medium, 5% CO2, at 37 °C. As positive and negative controls, respectively, Doxorubicin (IC50 0.2 μg/mL) and only culture medium (negative control) were used. After this time, cells were transferred to microtubes and washed with saline solution (PBS, pH 7.2) at 1480 × g for 20 min at 28 °C. Then, the supernatant was discarded and the cells were resuspended in 200 μL apoptosis buffer (Anexin V Biinding Buffer-BDPharmingen). The contents of each tube were transferred to cytometry tubes where 5 μL of Annexin V-FITC and 10 μL PI were then added. Flow cytometer reading (Becton Dickinson FACS Calibur Flow Cytometer, Mountain View, CA, U.S.A.) was performed after 15 min, acquiring 20,000 cells. Data acquisition was performed using Cell Quest Pro software and analysis was performed using Flow Jo 7.6 software. Statistical analysis was performed using Graph Pad Prism 5.0 software. The statistical test used was ANOVA, followed by Dunnett’s test.
Results
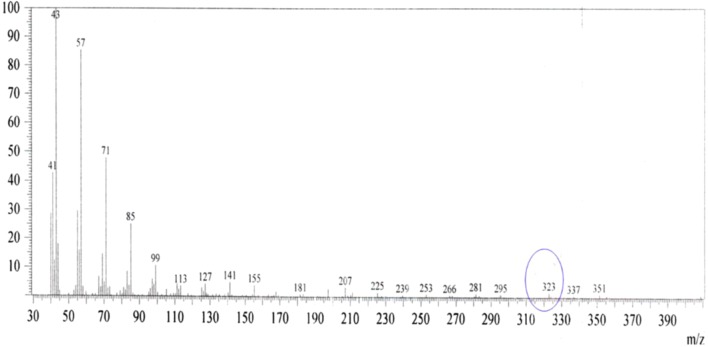
The purified red pigment showed maximum absorbance at 534.40 nm (Fig. 1) and molecular weight of 323 m/z (Fig. 2) corresponding to prodigiosin already described in the literature. Figure 3 shows the extraction process of prodigiosin. During the chromatographic column purification process (Fig. 4a, b) a second purple pigmentation fraction was obtained which had a maximum absorbance of 270 nm and a molecular weight of 149 m/z as already evidenced in the previous work described by Lapenda et al. [19]. The red pigment had its reference value (Rf) determined by CCD thin layer chromatography, and for the red fraction an Rf of 0.59 was obtained (Fig. 4c).
Fig. 2.
Mass spectrometry of prodigiosin (red fraction) isolated from Serratia marcercens UFPEDA 398
Fig. 3.

Extraction of prodigiosin produced by Serratia marcercens UFPEDA 398 employing chloroform: methanol solvent systems (2:1; 1:1; 1:2 and 100% methanol)
Fig. 4.
Purification of prodigiosin by Serratia marcercens UFPEDA 398 in column chromatography (A and B-TLC) and thin layer chromatography (C-CCD). At this point the separation of two fractions is observed: red corresponding to prodigiosin and one purple
Prodigiosin showed cytotoxic effect and was evidenced in the tumor cell lines studied from the cytotoxic dose measurement (IC50) (Fig. 5 and Table 1). The IC50 measured for prodigiosin showed a worrying cytotoxic effect of the pigment in all strains studied.
Fig. 5.
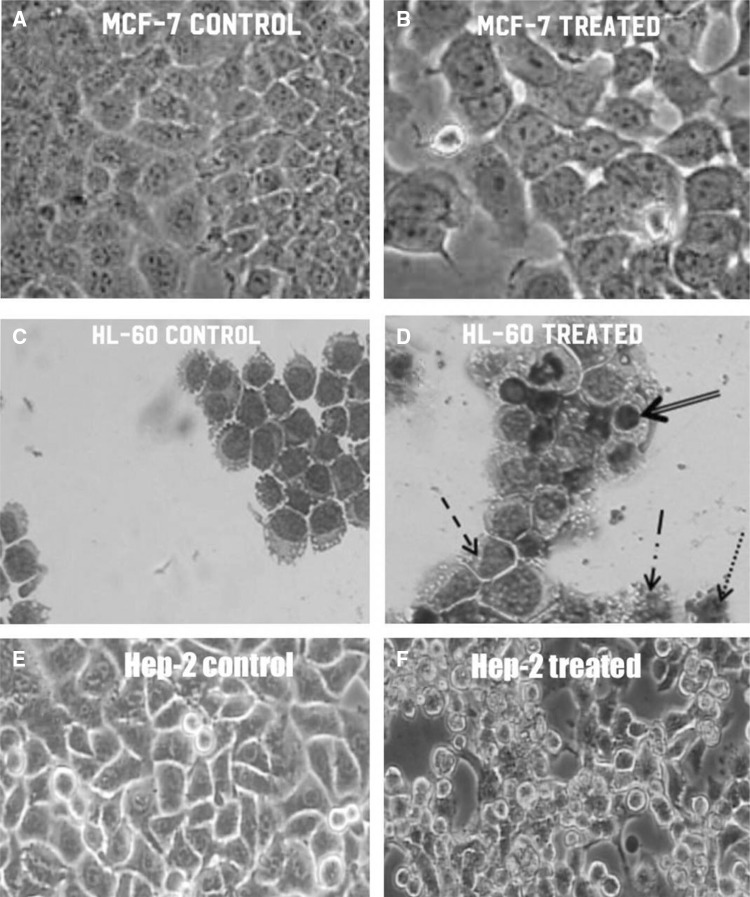
Cytotoxic effect of prodigiosin on tumoral (a and b) MCF-7, (c and d) HL-60 and (e and f) Hep-2 tumor lines, observed under × 10 and × 40 magnification. Presence of morphological changes: cellular rounding, detachment and apoptosis
Table 1.
IC50 of prodigiosin, its violet fraction and doxorubicin for different tumor cell lines
| Cell line | IC50 (μg/mL) | ||
|---|---|---|---|
| Prodigiosin | Violet fraction | Doxorubicin | |
| NCHI-292 | 3.6 | 11.6 | 0.2 |
| Hep-2 | 3.4 | 15.2 | 0.7 |
| MCF-7 | 5.1 | 15.6 | 0.2 |
| HL 60 | 1.7 | 11.7 | 0.2 |
According to Alley et al. [21], pure substances with high cytotoxic effect have an IC50 of 4 µg/mL or less.
For the MCF-7 strain an IC50 contrary to that described by Alley et al. [21] for pure substances was observed. However, this finding suggests a resistance behavior of this strain to prodigiosin (IC50 of 5.1 µg/mL), as well as to consider that the cellular behaviors are distinct, since the cell lines are equally distinct. The HL-60 strain, on the other hand, proved to be very sensitive to prodigiosin, since the IC50 found was much lower (IC50 1.7 µg/mL) than the pure substance standard described.
These data demonstrate the potential cytotoxic action of prodigiosin against these cells and are quite promising, mainly destroying the HL-60 strains responsible for Human Promyelocytic Leukemia (Table 1). Prodigiosin also presented cytotoxicity for NCIH-292 (IC50 3.6 µg/mL) and Hep-2 (IC50 3.4 µg/mL) strains when compared to the standard and synthetic cell cytotoxicity model, doxorubicin (Table 1). The cytotoxic effects of the purple fraction were also tested, however the expected results for a pure substance were not satisfactory, being obtained IC50 higher than 4.0 µg/mL, which suggests low or no cytotoxic effect.
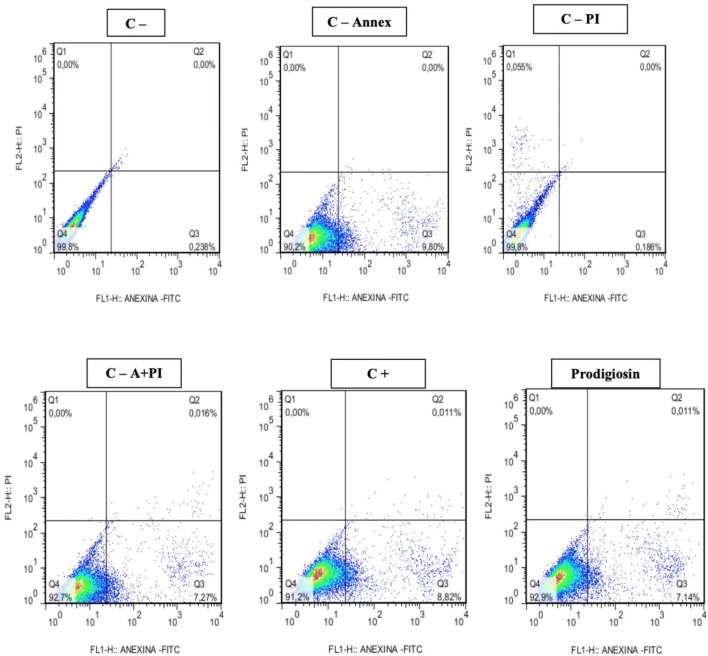
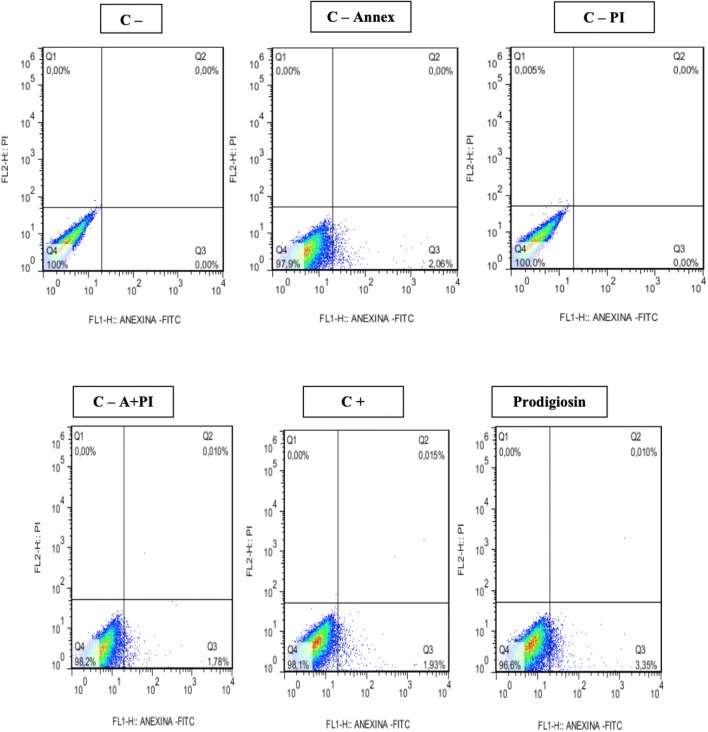
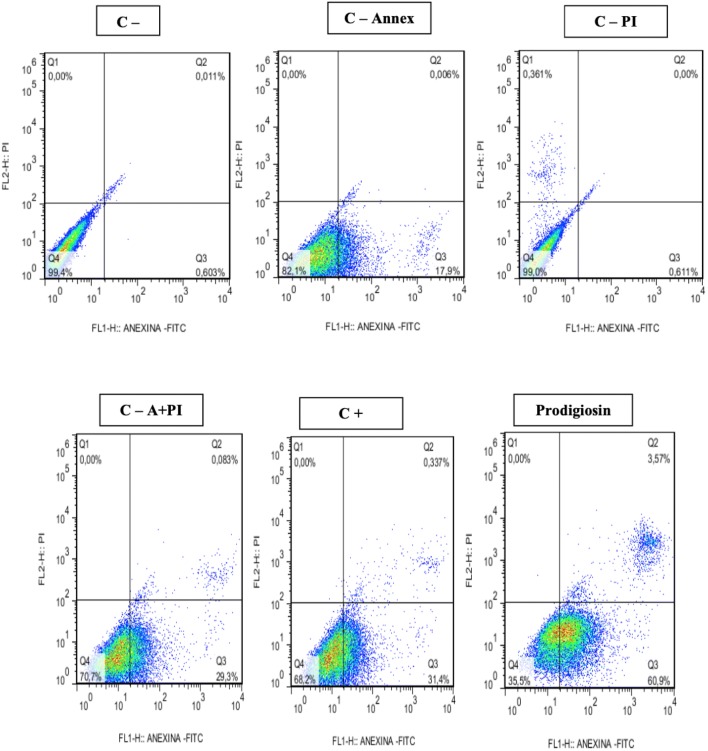
In confirmation of the cytotoxicity tests, promising results were also obtained regarding the cytotoxic effects measured by flow cytometry. Apoptosis induction was observed for prodigiosin-treated tumor cell lines at 48 and 72 h. The results obtained for the 48-h treatments (Figs. 6, 7, 8) showed induction of apoptosis only for NCIH-279 tumor lines with 60% apoptosis when compared to controls (Table 2).
Fig. 6.
Induction of apoptosis and necrosis in Hep-2 line after exposure to prodigiosin (IC50%: 3.4 μg/mL) for 48 h
Fig. 7.
Induction of apoptosis and necrosis in HL-60 line after exposure to prodigiosin (IC50%: 3.4 μg/mL) for 48 h
Fig. 8.
Induction of apoptosis and necrosis in NCIH-279 line after exposure to prodigiosin (IC50%: 3.4 μg/mL) for 48 h
Table 2.
Induction of apoptosis in tumor lines exposed to prodigiosin (IC50: 3.4 µg/mL) for 48 h
| Samples | Time (h) | Mean + SD (%) | |||
|---|---|---|---|---|---|
| ANEX | PI | A + PI | VC | ||
| Hep-2 control | 48 | 0.2135 ± 0.009 | 0.0 ± 0.0 | 0.0 ± 0.0 | 99.75 ± 0.05 |
| Hep-2. control—annex | 48 | 9.3 ± 0.5 | 0.0 ± .00 | 0.0 ± 0.0 | 90.7 ± 0.35 |
| Hep-2. control—PI | 48 | 0.186 ± 0.0 | 0.0655 ± 0.0105 | 0.0 ± 0.0 | 99.75 ± 0.05 |
| Hep-2. control—A +PI | 48 | 7.57 ± 0.93 | 0.0 ± 0.0 | 0.0245 ± 0.008 | 92.4 ± 0.9 |
| Hep-2. control + | 48 | 7.57 ± 0.93 | 0.0 ± 0.0 | 0.0245 ± 0.008 | 92.4 ± 0.9 |
| Hep-2. prodigiosin | 48 | 5.71 ± 1.01 | 0.0 ± 0.0 | 0.019 ± 0.014 | 94.25 ± 1.05 |
| HL 60 control | 48 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100.0 ± 0.0 |
| HL 60. control—annex | 48 | 3.495 ± 0.586 | 0.0 ± 0.0 | 0.0 ± 0.0 | 96.25 ± 0.212 |
| HL 60. control—PI | 48 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100.0 ± 0.0 |
| HL 60. control—A + PI | 48 | 2.7 ± 0.02 | 0.0 ± 0.0 | 0.014 ± 0.024 | 97.2 ± 0.57 |
| HL 60. control + | 48 | 4.99 ± 2.66 | 0.0 ± 0.0 | 0.0336 ± 0.0334 | 94.91 ± 2.78 |
| HL 60. prodigiosin | 48 | 3.55 ± 0.43 | 0.0008 ± 0.0020 | 0.0298 ± 0.0174 | 96.33 ± 0.40 |
| NCIH 279 control | 48 | 0.496 ± 0.230 | 0.0 ± 0.0 | 0.0061 ± 0.0063 | 98.58 ± 2.001 |
| NCIH 279. control—annex | 48 | 17.55 ± 0.49 | 0.0 ± 0.0 | 0.0 ± 0.0 | 82.45 ± 0.49 |
| NCIH 279 control—PI | 48 | 0.581 ± 0.042 | 0.364 ± 0.004 | 0.0 ± 0.0 | 99.05 ± 0.070 |
| NCIH 279. control—A + PI | 48 | 27.97 ± 0.974 | 0.0 ± 0.0 | 0.0842 ± 0.0389 | 71.95 ± 0.91 |
| NCIH 279. control + | 48 | 53.06 ± 7.02 | 0.0 ± 0.0 | 2.073 ± 1.452 | 44.87 ± 8.31 |
| NCIH 279. prodigiosin | 48 | 60.56 ± 0.81 | 0.0 ± 0.0 | 3.25 ± 1.22 | 36.03 ± 1.30 |
Annex = annexin V-FITC; PI = propidium iodine; VC = viable cells
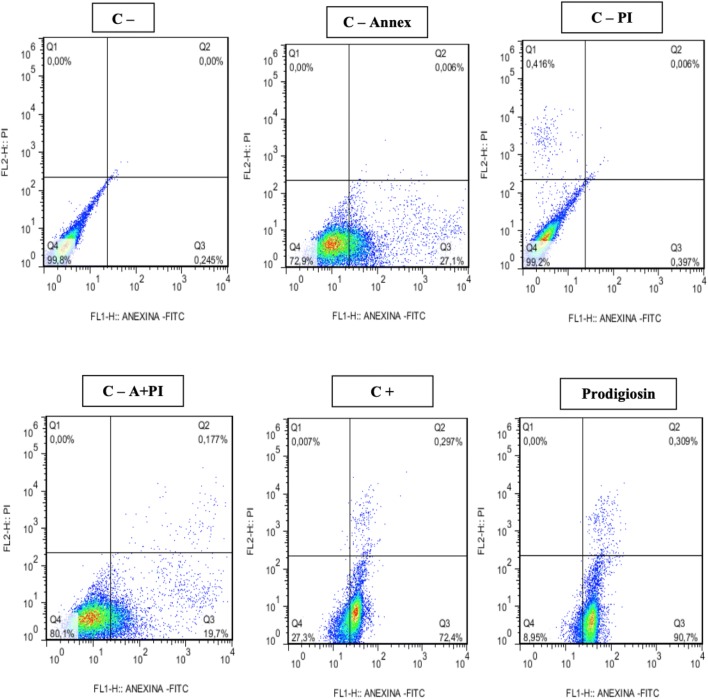
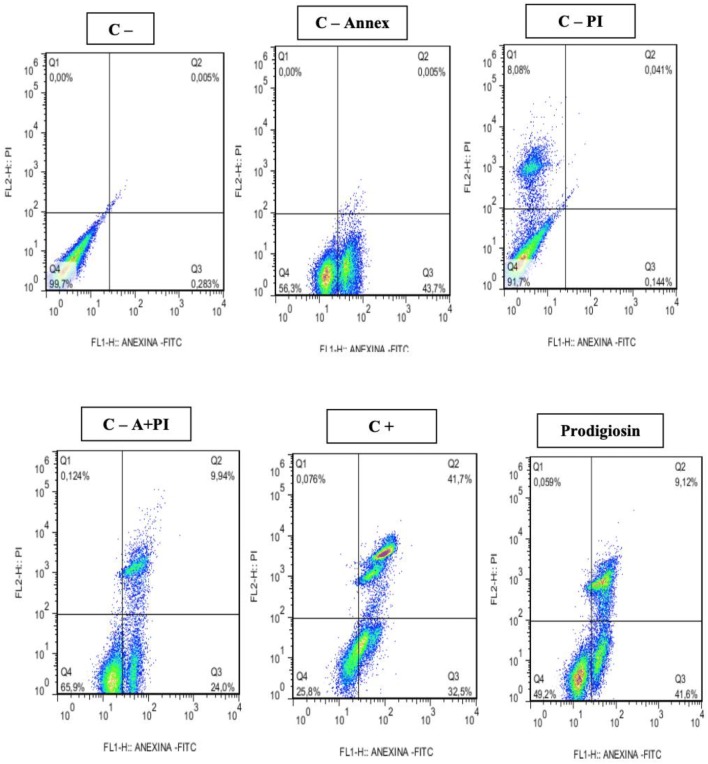
In the 72 h treatments (Table 3), highly significant results were observed for induction of apoptosis in the evaluated tumor lines. For the Hep-2 strain (Fig. 9) an apoptosis percentage of 80.5% (80.55 ± 10.15) was observed in relation to the negative controls Annexin V of 26.3% (26.3 ± 0.8), PI propidium iodide 0% (0.362 ± 0.035), 19% Annexin V + PI (19.0 ± 0.7) and for the 67% Annexin V + PI positive control (67.55 ± 4.85). For the NCIH 279 strain the apoptosis percentage (Fig. 10) corresponded to 42% (42.20 ± 6.11) being quite significant when the controls were observed (Table 3). However, for the HL-60 strain this percentage was very low 5% (5.26 ± 2.88) not being considered apoptotic effect for these cells.
Table 3.
Induction of apoptosis in tumor lines exposed prodigiosin (IC50%: 3.4 µg/mL) for 72 h
| Samples | Time (h) | Mean (%) + SD (%) | |||
|---|---|---|---|---|---|
| ANNEX | PI | A + PI | VC | ||
| Hep-2 control | 72 | 0.2445 ± 0.0005 | 0 ± 0 | 0 ± 0 | 99.8 ± 0.0 |
| Hep-2. control—annex | 72 | 26.3 ± 0.8 | 0 ± 0 | 0.003 ± 0.003 | 73.7 ± 0.8 |
| Hep-2. control—PI | 72 | 0.362 ± 0.035 | 0.4325 ± 0.0165 | 0.0125 ± 0.0065 | 99.2 ± 0.0 |
| Hep-2. control—A +PI | 72 | 19.0 ± 0.7 | 0.0 ± 0.0 | 0.162 ± 0.015 | 80.85 ± 0.75 |
| Hep-2. control + | 72 | 67.55 ± 4.85 | 0.0035 ± 0.0035 | 0.0224 ± 0.073 | 32.15 ± 4.95 |
| Hep-2. prodigiosin | 72 | 80.55 ± 10.15 | 0.0 ± 0.0 | 0.3895 ± 0.0805 | 19.02 ± 10.07 |
| HL-60 control | 72 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100.0 ± 0.0 |
| HL 60. control—annex | 72 | 3.85 ± 0.07 | 0.0 ± 0.0 | 0.0 ± 0.0 | 96.2 ± 0.14 |
| HL 60. control—PI | 72 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 100.0 ± 0.0 |
| HL 60. control—A + PI | 72 | 2.03 ± 1.35 | 0.0 ± 0.0 | 0.011 ± 0.020 | 97.27 ± 0.05 |
| HL 60. control + | 72 | 3.64.0 ± 0.45 | 0.0 ± 0.0 | 0.0315 ± 0.0162 | 96.4 ± 0.44 |
| HL 60. prodigiosin | 72 | 5.26 ± 2.88 | 0.0 ± 0.0 | 0.0562 ± 0.0472 | 94.68 ± 2.92 |
| NCIH-279 control | 72 | 0.2513 ± 0.0314 | 0.0 ± 0.0 | 0.0008 ± 0.0020 | 99.73 ± 0.051 |
| NCIH 279. control—annex | 72 | 44.15 ± 0.63 | 0.0 ± 0.0 | 0.005 ± 0.007 | 55.85 ± 0.63 |
| NCIH 279 control—PI | 72 | 0.1825 ± 0.054 | 8.045 ± 0.049 | 0.0565 ± 0.0219 | 91.7 ± 0.0 |
| NCIH 279. control—A + PI | 72 | 24.85 ± 1.20 | 0.127 ± 0.004 | 9.85.0 ± 0.1202 | 65.15 ± 1.06 |
| NCIH 279. control + | 72 | 39.14 ± 2.06 | 2.36 ± 5.16 | 8.8 ± 4.38 | 51.2 ± 1.73 |
| NCIH 279. prodigiosin | 72 h | 42.20 ± 6.11 | 0.0605 ± 0.0306 | 25.50 ± 14.27 | 35.50 ± 13.88 |
Annex = Annexin V-FITIC; PI = propidium iodine; VC = viable cells
Fig. 9.
Induction of apoptosis and necrosis in Hep-2 line after exposure to prodigiosin (IC50%: 3.4 μg/mL) for 72 h
Fig. 10.
Induction of apoptosis and necrosis in NCIH 279 line after exposure to prodigiosin (IC50%: 3.4 μg/mL) for 72 h
Discussion
Prodigiosine in acidic medium as well as in neutral medium has a reddish color with a maximum absorption spectrum between 535 and 540 nm. In an alkaline medium, the pigment has a yellowish-orange color and an absorption spectrum with a maximum peak at 470 nm [24].
After the pigment purification step by the chromatographic column method (TLC), the separation of two fractions was observed, which attracted attention because they present different pigments. The first is pink-reddish and the second purple.
According to Williamson et al. [8], the red colored pigment is the tipirrol alkaloid, prodigiosin (4-methoxy-5-[(Z)-(5-methil-4-pentyl-2H-pyrrol-2-ilidine) methil]-1H, 1′H-2,2′-bipyrrole) whose structural formula and molecular weight are respectively C20H25N3O and 323 g/mol.
In studies, Guryanov et al. [2], after characterizing the pigment by infrared NMR spectrophotometer, describe the isolated red fraction of Serratia marcecsens ATCC9986 as being prodigiosin whose molecular weight found corresponded to 322.9 m/z. In our data, a second purple fraction was observed. This fraction, whose biological properties have not yet been described, had a molecular weight corresponding to 149 m/z. However, Guryanov et al. [2], suggest that is one of the precursors of prodigiosin the monopyrrole (Monopirrol—MAP) formed from a parallel biosynthetic route that converge with a second route of formation of a second precursor, the bipyrrole (Bipyrrole—BMC) resulting in the red pigment called prodigiosin. Corroborating Isaka et al. [25] and Jeong et al. [4], say that the purple fraction corresponds to the monopyrrole (MAP) precursor of prodigiosin.
Da Silva et al. [26] describe the red pigment obtained from medium using glycerol as a carbon source as being prodigiosin with a molecular weight equal to 323 m/z. In addition, Asano et al. [27] and Parani et al. [28] corroborate the data described by Da Silva et al. [26] regarding the characterization of prodigiosin.
In this study, the cytotoxic activity of prodigiosin produced by S. marcescens UFPEDA 398 in cell lines isolated from solid tumors (NCIH-292, Hep-2 and MCF-7) and leukemia (HL-60) was evaluated. The results of in vitro cell exposure to prodigiosin after 72 h demonstrated that the pigment induced a cytotoxic effect in the tumor cell lines studied and that these results were quite significant (Table 1), when compared with the control (standard chemotherapy, doxorubicin). The HL-60 strain was more susceptible to the cytotoxic action of prodigiosin when compared to MCF-7 and the control.
Corroborating the findings of this study for the cytotoxic effect of prodigiosin, Elahian et al. [10], claim that prodigiosin was cytotoxic for tumor cell lines A2780 (ovarian epithelial cancer) and EPG85-257 (gastric carcinoma), being found IC50 of 8.92 μg/mL and 8.45 μg/mL, respectively, when compared to doxorubicin (IC50 0.07 μg/mL). These data are similar to those obtained in our study, considering that the following values were found for the IC50 at 3.6 μg/mL in all studied tumor cell lines, except the MCF-7 line (IC50 5.5 μg/mL).
Ho et al. [29], describe cytotoxic action for prodigiosin against MCF-7 strain (IC50 3.5 μg/mL) and significant cytotoxic action for MCF-10 strain when observed IC50 higher than 10 (IC50 > 10).
In antitumor studies Williamnson et al. [8] corroborates the findings in this research describing the cytotoxic and antitumor effects of prodigiosin as well as Manderville et al. [30], Liu et al. [31] that describe the cytotoxic and antitumor of metacycloprodigiosin and Pérez-Tómaz et al. [12] describing the apoptotic effects in corroboration with Montaner et al. [32].
According to Nakashima et al. [18], among the cell lines tested, U937 cells showed the greatest susceptibility to prodigiosin toxicity. The red pigment induced typical apoptotic nuclear morphological changes and single cell gel electrophoresis revealed that prodigiosin caused DNA fragmentation in U937 cells. The author also describes that in U937 cells treated with pigment, the acidic compartment, like lysosomes, has disappeared, suggesting that the intracellular pH compartmentalization disorder induced by prodigiosin may trigger an apoptotic signal. Since the p38 MAP kinase inhibitor specifically prevented prodigiosin-mediated cell death, p38 MAP kinase may be involved in the cytotoxic mechanism.
Cytotoxic effects of prodigiosin were also evidenced by Montaner et al. [11, 32] being this effect observed from the induction of cell apoptosis through DNA fragmentation. These data corroborate those evidenced in this study from the analysis of flow cytometry, where cells in apoptosis and necrosis were detected. The prodigiosin pigment induced early and late apoptosis (necrosis) in tumor cell lines exposed to 72 h at a concentration of 3.4 μg/mL.
In the last years, the mechanism of natural and programmed cell death called apoptosis has been differentiated by its morphological characteristics of necrosis. It is known that the induction of apoptosis in the cell is regulated by a gene action that involves the expression of the regulatory protein called p53. This regulation of apoptosis involves two classes of proteins: the pro-apoptotic (Family of Caspases; Bcl-2; Bid that promote the release of cytochromes C from the mitochondria to the cytoplasm) and the anti-apoptotic (IAPs) that inhibit the caspases and consequently, non-induction of apoptosis [33, 34]. Apoptotic mechanisms have been extensively studied considering that they represent a form of suppression of different tumor stages [35].
Prodigiosin is known mainly for its apoptotic activity in tumor cell lines. This apoptotic effect of prodigiosin suggests its selective action for malignant cells regardless of the action of p53 and cellular resistance to antitumor drugs, thus suggesting that it is a potent atitumor [25, 36, 37].
In studies Lorenzo et al. [38], claim that chromosomal breaks that lead to irreversible DNA damage induce the cell to activate the apoptotic mechanism. In our studies, it was observed that cell lines exposed to prodigiosin in 48 h did not show significant apoptotic effect, except for the NCHI 279 line (60% of initial apoptosis). The absence of apoptosis in the other strains exposed in 48 h does not eliminate the hypothesis of evidence of genomic damage. However, after 72 h, significant results were observed for apoptosis, which suggests that in this 72-h period, once exposed to prodigiosin, these cells may have suffered irreversible damage to DNA, which induced the activation of the apoptotic mechanisms.
According to Montaner et al. [11], in vitro prodigiosin promotes breaks in the DNA facilitating the oxidation of the double strand, which suggests a direct correlation with cytotoxic activity. These breaks in cellular DNA induce accumulation of the tumor-suppressing protein p53. However, it was observed that the cytotoxic action of prodigiosin occurs independently of p53 in B lymphocytes, HL-60 and in the Jurkat cells.
Guryanov et al. [2], claim that prodigiosin produces a strong cytotoxic effect even at the lowest concentration of 1.0 μg/mL. Thus, suggesting future studies of this pigment to measure possible levels of genomic damage in tumor and normal strains in order to better elucidate the target-specific mechanism of prodigiosin.
According to Lins et al. [39], prodigiosin induced genomic damage in the tumor lines NCIH-292, Hep-2, MCF-7 and HL-60 and, mainly, in the control (BGMK). The pigment induced the formation of micronuclei in tumor and normal cells, resulting from possible chromosomal breaks or delays in the anaphase phase during cell division. These data are worrying, since these events to the cell nucleus can promote mutational events, which are the main causes of cancer induction.
Recently, Lapenda et al. [40], measured the genotoxic effects of prodigiosin in normal human cell lines (PBMC), demonstrating that the pigment induced the formation of micronucleus and damage to cellular DNA at different levels.
The cytotoxic effect of the purple fraction was also evaluated. The purple pigment showed a low cytotoxic effect in the tumor lines studied, evidenced by the measurement of the IC50 whose lowest result was 11.6 μg/mL for NCIH-292 and the highest IC50 of 15.6 μg/mL for the MCF-7 strain. The low cytotoxic effect of the purple fraction was based on those described by Alley et al. [21] which establishes a pattern of cytotoxicity for pure substances (equal to or less than IC50 3.4 μg/mL). Thus, it was observed that the purple fraction did not show significant cytotoxic effects for the tumor cell lines studied when compared with doxorubicin and prodigiosin.
Conclusion
The isolated red pigment from Serratia marcescens UFPEDA 398 corresponds to prodigiosin similar to the data described in the literature. The purple fraction obtained did not show significant cytotoxic activity. Prodigiosin, which showed significant cytotoxic effect inducing apoptosis in tumor cell lines, suggesting promising antitumor activity. All tumor lines studied were sensitive to the cytotoxic effect of prodigiosin, except the MCF-7 strain, which was slightly more resistant than the other strains. However, despite the evident cytotoxic activity of prodigiosin, this study is preliminary, and future studies of genotoxics that better elucidate such mechanisms of cell action and their target-specific action are necessary before their application in antitumor therapies.
Acknowledgements
The authors thank the responsible technical team, the Federal University of Pernambuco for the physical and structural support, giving their laboratories to perform the tests and the fundamentally important funding agencies such as CNPq and CAPES.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cerdeno AM, Bibb MJ, Charlis GL. Analysis of the prodiginine biosynthesis gene cluster of Streptomyces coelicolor A3 (2): new mechanisms for chain initiation and termination in modular multienzymes. Chem Biol. 2001;8:817–829. doi: 10.1016/S1074-5521(01)00054-0. [DOI] [PubMed] [Google Scholar]
- 2.Guryanov DI, Karamova NS, Yusupova DV, Gnezdilov OI, Koshkarova AL. Bacterial pigment prodigiosin and its genotoxic effect. Russ J Bioorg Chem. 2013;39:106–111. doi: 10.1134/S1068162012060040. [DOI] [PubMed] [Google Scholar]
- 3.Davaraj N, Dharumaduari D, Noorudin T, Annamalai P. Production of prodigiosin from Serratia marcescens and its cytotoxicity activity. J Pharm Res. 2009;2:590–593. [Google Scholar]
- 4.Jeong H, Yim H, Lee C, Choi H, Park Y, Yoon S, Hur G, Kang H, Kim D, Lee H, Park K, Park S, Lee K, Kim J. Genomic blueprint of Hahella chejuensis, a marine microbe producing an algicidal agent. Nucleic Acids Res. 2005;33:7066–7073. doi: 10.1093/nar/gki1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giri AV, Anandkumar N, Muthukumaran G, Pennathur G. A novel medium for the enhanced cell growth and production of prodigiosin from Serratia marcescens isolated from soil. BMC Microbiol. 2004;4:11. doi: 10.1186/1471-2180-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett JW, Bentley R. Seeing red: the story of prodigiosin. Adv Appl Microbiol. 2000;47:1–32. doi: 10.1016/S0065-2164(00)47000-0. [DOI] [PubMed] [Google Scholar]
- 7.Harris AKP, Williamnson NR, Slater H, Cox A, Abbasi S, Fouds I, Simonsen H, Fj Leeper, Salmond GPC. The Serratia gene cluster encoding biosynthesis of the red antibiotic, prodigiosin, shows species- and strain-dependent genome context variation. Microbiology. 2004;150:3547–3560. doi: 10.1099/mic.0.27222-0. [DOI] [PubMed] [Google Scholar]
- 8.Williamnson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol. 2007;2:605–618. doi: 10.2217/17460913.2.6.605. [DOI] [PubMed] [Google Scholar]
- 9.Kim D, Kim JF, Yim JH, Kwon SK, Lee CH, Lee HK. Red to red-the marine bacterium Hahella chejuensis and its product prodigiosin for mitigation of harmful algal blooms. J Microbiol Biotechnol. 2008;18:1621–1629. [PubMed] [Google Scholar]
- 10.Elahian F, Moghimi B, Dinmohammadi F, Ghamghami M, Hamidi M, Mirzaei SA. The anticâncer gente Prodigiosin is not a multidrug resistance protein substrate. DNA Cell Biol. 2013;32:90–97. doi: 10.1089/dna.2012.1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montaner B, Pérez-Tomáz R. The prodigiosins: a new family of anticancer drugs. Curr Cancer Drug Tergets. 2003;3:57–65. doi: 10.2174/1568009033333772. [DOI] [PubMed] [Google Scholar]
- 12.Pérez-Tomáz Ricardo, et al. The prodigiosins, proapoptotic drugs with anticancer properties. Biochem Pharmacol. 2003;66:1447–1452. doi: 10.1016/S0006-2952(03)00496-9. [DOI] [PubMed] [Google Scholar]
- 13.Francisco R, Pérez-Tomás R, Gimènez-Bonafé P, Soto-Cerrato V, Giménez-Xavier P, Ambrosio S. Mechanisms of prodigiosin cytotoxicity in human neuroblastoma cell lines. Eur J Pharmacol. 2007;572:111–119. doi: 10.1016/j.ejphar.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 14.Mo SJ, Kim BS, Reynolds KA. Production of branched-chain alkylprodiginines in streptomyces coelicolor by replacement of the 3-ketoacyl ACP synthase III initiation enzyme, RedP. Chem Biol. 2005;12:191–200. doi: 10.1016/j.chembiol.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 15.Wasserman HH, Shaw CK, Sykes RJ. The biosynthesis of metacycloprodigiosin and undecyl prodigiosin. Tetrahedron Lett. 1974;33:2787–2790. doi: 10.1016/S0040-4039(01)91743-5. [DOI] [Google Scholar]
- 16.de Oliveira Santos M. Estimativa 2018: incidência de câncer no Brasil. Rev Bras Cancerol. 2018;64:119–120. [Google Scholar]
- 17.Vendruscolo F, Tosin I, Giachini AJ, Schmidell W, Ninow JL. Antimicrobial activity of M onascus pigments produced in submerged fermentation. J Food Process Preserv. 2014;38:1860–1865. doi: 10.1111/jfpp.12157. [DOI] [Google Scholar]
- 18.Nakashima T, Myiazaki Y, Matsuyam Y, Muraoka W, Yamaguchi K, Oda T. Producing mechanism of an algicidal compound against red tide phytoplankton in a marine bacterium γ-proteobacterium. Appl Microbiol Biotechnol. 2006;73:684–690. doi: 10.1007/s00253-006-0507-2. [DOI] [PubMed] [Google Scholar]
- 19.Lapenda JC, et al. Antimicrobial activity of prodigiosin isolated from Serratia marcescens UFPEDA 398. World J Microbiol Biotechnol. 2015;31:399–406. doi: 10.1007/s11274-014-1793-y. [DOI] [PubMed] [Google Scholar]
- 20.Mosmann T. Rapid colorimetric assay for cellular growth and survival. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 21.Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212–216. doi: 10.1097/00001622-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Koopman G, et al. Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood. 1994;84:1415–1420. doi: 10.1182/blood.V84.5.1415.bloodjournal8451415. [DOI] [PubMed] [Google Scholar]
- 23.Bellosillo B, Villamor N, López-Guillermo A, Marcé S, Esteve J, Campo E, Ontserrat E. Complement-mediated cell death induced by rituximab in B-cell lymphoproliferative disorders is mediated in vitro by a caspase-independent mechanism involving the generation of reactive oxygen species. Blood. 2001;98:2771–2777. doi: 10.1182/blood.V98.9.2771. [DOI] [PubMed] [Google Scholar]
- 24.Andreyeva IN, Gorodnikova TI. Pigmentation of Serratia marcescens and spectral properties of prodigiosin. Microbiology. 2015;84:28–33. doi: 10.1134/S0026261715010026. [DOI] [PubMed] [Google Scholar]
- 25.Isaka M, Jaturapat A, Kramyu J, Tanticharoen M, Thebtaranonth Y. Potent in vitro antimalarial activity of metacycloprodigiosin isolated from Streptomyces spectabilis BCC 4785. Antimicrob Agents Chemother. 2002;46:1112–1113. doi: 10.1128/AAC.46.4.1112-1113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Da Silva GP, Mack M, Contiero J. Glycerol: a promising and abundant carbon source for industrial microbiology. Biotechnol Adv. 2009;27:30–39. doi: 10.1016/j.biotechadv.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Asano S, et al. Prodigiosin produced by Serratia marcescens enhances the insecticidal activity of Bacillus thuringiensis delta-endotoxin (Cry1C) against common cutworm ,Spodoptera litura. Nippon Noyaku Gakkaishi. 1999;24:381–385. [Google Scholar]
- 28.Parani K, et al. Optimization of prodigiosin production from a strain of Serratia marcescens SR1 and screening for antifungal activity. J Biol Control. 2008;22:73–79. [Google Scholar]
- 29.Ho TF, Peng YT, Chuang SM, Lin SC, Feng BL, Lu CH, Yu WJ, Chang JS, Chang CC. Prodigiosin down-regulates survivin to facilitate paclitaxel sensitization in humanbreast carcinoma cell lines. Toxicol Appl Pharmacol. 2009;235:253–260. doi: 10.1016/j.taap.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 30.Manderville RA. Synthesis, proton-affinity and anti-cancer properties of the prodigiosin-group natural products. Curr Med Chem Anti Cancer Agents. 2001;1:195–218. doi: 10.2174/1568011013354688. [DOI] [PubMed] [Google Scholar]
- 31.Liu R, et al. Potent in vitro anticancer activity of metacycloprodigiosin and undecylprodigiosin from a sponge-derived actinomycete Sac-charopolyspora sp. nov. Arch Pharm Res. 2005;28:1341–1344. doi: 10.1007/BF02977899. [DOI] [PubMed] [Google Scholar]
- 32.Montaner B, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131:585–593. doi: 10.1038/sj.bjp.0703614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandey R, Chander R, Sainis KB. Prodigiosins as anti cancer agents: living up to their name. Curr Pharm Des. 2009;15:732–741. doi: 10.2174/138161209787582192. [DOI] [PubMed] [Google Scholar]
- 34.Chang CC, Wang YH, Chern CM, Liou KT, Hou YC, Peng YT, Shen YC. Prodigiosin inhibits gp91 and iNOS expression to protect mice against the oxidative/nitrosative brain injury induced by hypoxia–ischemia. Toxicol Appl Pharmacol. 2011;257:137–147. doi: 10.1016/j.taap.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 35.Lu CH, Lin SC, Yang SY, Pan MY, Lin YW, Hsu CY, Wei YH, Chang JS, Chang CC. Prodigiosin-induced cytotoxicity involves RAD51 down-regulation through the JNK and p38 MAPK pathways in human breast carcinoma cell lines. Toxicol Lett. 2012;212:83–89. doi: 10.1016/j.toxlet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Silva Júnior END, de Moura MAB, Pinto AV, Pinto MDCF, de Souza MCB, Araújo AJ, Goulart MO. Cytotoxic, trypanocidal activities and physicochemical parameters of nor-2-lapachone-based 1, 2, 3-triazoles. J Braz Chem Soc. 2009;20:635–643. doi: 10.1590/S0103-50532009000400007. [DOI] [Google Scholar]
- 37.Hsieh HY, Shieh JJ, Chen CJ, Pan MY, Yang SY, Lin SC, Chang CC. Prodigiosin down-regulates SKP2 to induce p27KIP1 stabilization and antiproliferation in human lung adenocarcinoma cells. Br J Pharmacol. 2012;166:2095–2108. doi: 10.1111/j.1476-5381.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorenzo Y, Azqueta A, Luna L, Bonilla F, Domínguez G, Collins AR. The carotenoid β-cryptoxanthin stimulates the repair of DNA oxidation damage in addition to acting as an antioxidant in human cells. Carcinogenesis. 2009;30:308–314. doi: 10.1093/carcin/bgn270. [DOI] [PubMed] [Google Scholar]
- 39.Lins JCL, De Melo MEB, Do Nascimento SC, Adam ML. Differential genomic damage in different tumor lines induced by prodigiosin. Anticancer Res. 2015;35:3325–3332. [PubMed] [Google Scholar]
- 40.Lapenda JCL, et al. Evidence of genomic damage induced by prodigiosin produced by Serratia marcenscens UFPEDA 395 in human peripheral blood mononuclear cells. Biomed J Sci Tech Res. 2019;13:10039–10046. [Google Scholar]