Abstract
BACKGROUND:
To regenerate tissue-engineered cartilage as a source of material for the restoration of cartilage defects, we used a human fetal cartilage progenitor cell pellet to improve chondrogenesis and modulation of the immune response in an in vivo bioreactor (IVB) system.
METHODS:
IVB was buried subcutaneously in the host and then implanted into a cartilage defect. The IVB was composed of a silicone tube and a cellulose nano pore-sized membrane. First, fetal cartilage progenitor cell pellets were cultured in vitro for 3 days, then cultured in vitro, subcutaneously, and in an IVB for 3 weeks. First, the components and liquidity of IVB fluid were evaluated, then the chondrogenesis and immunogenicity of the pellets were evaluated using gross observation, cell viability assays, histology, biochemical analysis, RT-PCR, and Western blots. Finally, cartilage repair and synovial inflammation were evaluated histologically.
RESULTS:
The fluid color and transparency of the IVB were similar to synovial fluid (SF) and the components were closer to SF than serum. The IVB system not only promoted the synthesis of cartilage matrix and maintained the cartilage phenotype, it also delayed calcification compared to the subcutaneously implanted pellets.
CONCLUSION:
The IVB adopted to study cell differentiation was effective in preventing host immune rejection.
Electronic supplementary material
The online version of this article (10.1007/s13770-019-00236-5) contains supplementary material, which is available to authorized users.
Keywords: Cartilage tissue engineering, In vivo bioreactor, Cellulose membrane, Fetal cartilage progenitor cells, Pellet culture
Introduction
Articular cartilage has a limited ability to heal after being damaged. Since it has very little blood supply, cartilage has difficulty self-repairing after damage [1, 2]. Cell-based autologous or allogeneic transplantation has been employed to treat cartilage defects [3, 4]. The method of using autologous cells requires an invasive procedure for harvesting and the number of cells harvested is limited. Moreover, the use of allogeneic mesenchymal stem cells (MSCs) has some limitations, one of which is the initiation of an immune response, leading to failure of the cell therapy [5]. Furthermore, chondrocytes dedifferentiate when expanding in vitro, resulting in the loss of phenotypic and extracellular matrix (ECM) components [6].
Three factors play an important role in the engineering of cartilage tissue, the type of cells and biomaterial and how/where the material is grown. The cells can be somatic or stem cells [7] and the biomaterials can be synthetic or natural materials. These elements have been combined optimally and cultured in a bioreactor to produce cartilage similar to natural cartilage [8]. Tissue-engineered cartilage may be an applicable resource for healing cartilage defects, which would be more promising if the cartilage could be formed in an immunocompetent host [8]. However, inflammatory reactions, which might destroy chondrocytes and subsequently deform the tissue, have prevented the reconstruction of tissue in immunocompetent animals. Moreover, host cell ingrowth impairs the quality of reconstructed tissue. Therefore, a new technique used to avoid the above problems is an in vivo (IV) bioreactor, which can effectively prevent vascular invasion, host cell invasion, and immune rejection [7]. Using the body as a bioreactor, the traditional three elements (scaffold, cells, and growth factor) were cultured and the regenerative environment of the body assisted its growth. This process can be applied to allografts because the immune system cannot recognize allografts once host cell invasion is inhibited.
Cellulose is a kind of bioinert material with good biocompatibility and low immune response that is often used in medical implants [9]. The cellulose membrane of the IV bioreactor used in this study was composed of fairly long cellulose fibrils connected to each other and had a highly porous three-dimensional network [10]. The selection of favorable stem cells in cartilage tissue-engineering is also of great importance. In previous stem cell treatments, stem cells not only had the ability to regenerate in chronic tissue damage but also had a regulatory effect on the immune environment. Implanted stem cells can regulate the immune environment during tissue repair for tissue regeneration [11]. Previously, we reported that human fetal cartilage progenitor cells (hFCPCs) were available in high-yield, could proliferate, exhibited multipotent differentiation, and maintained their chondrogenic phenotype during cartilage tissue formation [11]. Pellet culture has been widely used in chondrocyte cultures [12]. However, the chondrocyte pellet culture method can easily cause cartilage mineralization in vitro [13]. The subcutaneous environment has been used in animal models of regenerative, ectopic, tissue-engineered cartilage, but due to cell ingrowth and vascular invasion of the host, the ECM was destroyed and calcified [8].
In this study, the components and liquidity of the cellulose membrane IV bioreactor fluid were analyzed and the chondrogenesis, immunogenicity, and the ability to heal cartilage defects of FCPC pellets cultured in the bioreactor were evaluated and compared with in vitro and subcutaneous models.
Materials and methods
Cell isolation and culture
hFCPC were isolated from the human fetal cartilage tissue, as previously described [11]. All experiments were performed in accordance with relevant guidelines and regulations. Human fetal cartilage tissues (n = 2, F12w-c, M11w) were obtained from patients following elective termination at 12 weeks after gestation, and cells were isolated from the femoral head of the cartilage tissue. Cartilage pieces were digested in 0.1% collagenase type II (Worthington Biochemical Corp, Freehold, NJ, USA) in high-glucose Dulbecco’s modified Eagle Medium (DMEM; HyClone, Logan, UT, USA) containing 1% fetal bovine serum (FBS; HyClone) at 37 °C under 5% CO2. After 16 h, isolated cells were cultured in DMEM supplemented with 1% antibiotic–antimycotic and 10% FBS at a density of 8 × 103 cells/cm2. After 3 days, non-adherent cells were removed and the medium was changed. Cells were passaged at 80% confluence by 0.05% Trypsin–EDTA (Gibco, Gaithersburg, MD, USA) treatment.
Pellet culture
Aliquots of 3 × 105 cells/0.5 mL were centrifuged at 500 g for 10 min in 15 mL polypropylene tubes. After 1 day, cell pellets were cultured in chondrogenic defined medium (DMEM supplemented with ITS mixture, 50 μg/mL ascorbate-2 phosphate, 100 nM dexamethasone, 40 μg/mL proline, and 1.25 mg/mL BSA) without TGF-β, a typical chondrogenic inducer. Pellets were cultured for 3 days.
Preparation of cellulose membrane chamber
For this study, our own cellulose membrane chamber was manufactured. Cellulose membrane chamber was composed of a silicone tube (21 mm × 15 mm × 10 mm, outer diameter × inner diameter × height) (Korea Ace Scientific, Seoul, Korea) and cellulose membrane. The pore size of the cellulose membrane is about 50–400 nm (supporting information Fig. 1), provided by the bioenergy research center, college of life sciences, Kyung Hee University [10]. Both ends of the silicone tube were sealed with a cellulose membrane and fixed using 7–0 black silk. Followed by sterilization in an autoclave at 121 °C for around 20 min.
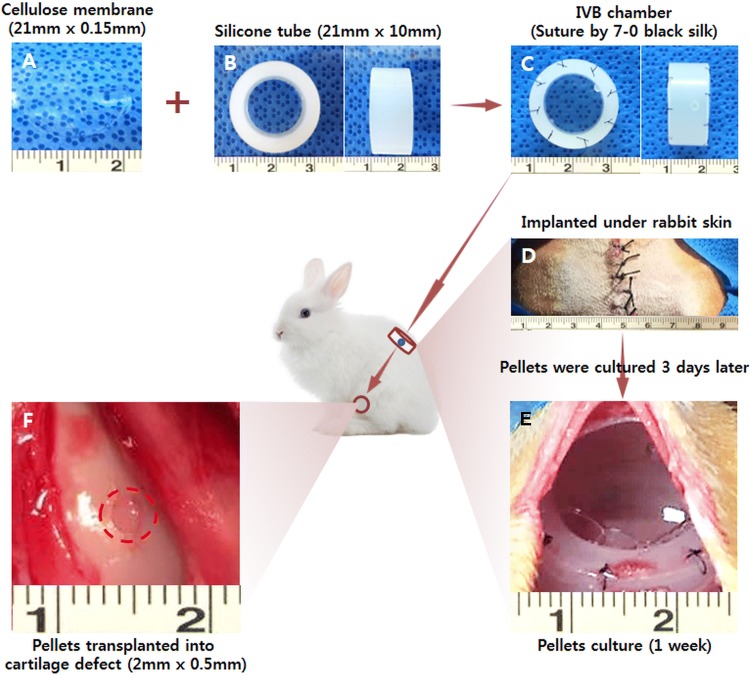
Fig. 1.
Schematic illustration of the overall design of this study. FCPC pellets were used to construct ectopic cartilage in the IV Bioreactor system for cartilage repair. A A gross image of the cellulose membrane (size 21 mm × 0.15 mm). B A gross image of the silicone tube (size 21 mm × 10 mm). C A gross image of the IV bioreactor chamber suture by 7–0 black silk. D A gross image of the two IV bioreactor chamber implanted under the skin of a rabbit. E 3 days after IV bioreactor chamber implantation, pellets were placed into the IV bioreactor for culture, and the gross image of IV bioreactor was obtained after 1 week of pellets culture. F A gross image of the 1 week culture of pellets transplanted into the cartilage defect (defect size 2 mm × 0.5 mm)
Ectopic chondrogenesis in the subcutaneous and IV bioreactor environment
3.5 kg female adult New Zealand white rabbits (n = 24 for subcutaneous and IV bioreactor groups; OrientBio, Seongnam, Korea) were anesthetized with a mixture of Zoletil and Rumpun. manufactured cellulose membrane chambers (two chambers/rabbit) were implanted under back skins of rabbits. In this study total of 360 pellets (pellet size 1.14 ± 0.04 mm) were divided into three environments: in vitro, rabbit subcutaneous and IV bioreactor. After 3 days, a total of 240 pellets were put into the subcutaneous and cellulose membrane chamber (5 pellets/chambers). Rabbits were sacrificed at each time point of 1 day, 1, and 3 weeks post-implantation. Retrieved pellets were used for analyses, gross observation, pellets size measurement, cell viability assay, histological analysis, immunohistochemical analysis, biochemical, reverse transcription polymerase chain reaction (RT-PCR) analysis and western blot analysis, respectively.
Cartilage defect repair
The same 45 adult New Zealand White rabbits were used for cartilage defect repair (OrientBio). Under general anesthetic, the knee was exposed after lateral skin incision and muscle detachment. A cylindrical cartilage defect (diameter: 2.0 mm, depth: 0.5 mm) was created in the trochlear groove of the femur. Then, 1 week cultured engineered cartilage tissues from in vitro, autologous IV bioreactor and subcutaneous, were implanted into the defects and the wound was stitched layer to layer. The control group was implanted with nothing at the defect. After surgery, all of the rabbits were kept in individual cages at constant temperature and humidity, with unrestricted access to a standard diet and water. Rabbits could walk freely with full weight bearing. Both analgesics and antibiotics were administered for 3 days after surgery. The animals were sacrificed using an intravenous injection of a euthanasia solution at 4, 8 and 12 weeks after surgery, then the repaired cartilage and synovial membrane was harvested for gross observation, H&E staining, Safranin-O staining and Immunohistochemistry (IHC) staining of collagen type II (COL II), collagen type I (COL I) and collagen type X (COL X).
Measurement of transmittance
Initially, the transparency of each fluid was visually estimated. Subsequently, the transmission spectra of each fluid were observed using a UV–Vis spectrophotometer (Jasco V-650, Japan). The spectral distribution was measured in the visible wavelength range (400–800 nm). The results were normalized with water.
Permeability assay
In order to test diffusion across the cellulose membrane, we confirmed the diffusion patterns of glucose through the cellulose membrane using a two-chamber system developed previously in our laboratory [14]. Briefly, the cellulose membrane was fixed between two specific chambers. We loaded 4500 mg/L glucose DMEM on one side of the chamber and loaded distilled water (DW) on the other side of the chamber. After 2, 4, 8, 12, and 24 h measured the glucose concentration in the DW in the Ajou University Hospital.
In vivo bioreactor fluid component analysis
Total protein and glucose in IV BIOREATOR (Cellulose membrane chamber, see 2.3) fluid were measured at the Ajou University Hospital. Hyaluronan (HA) content in IV BIOREATOR fluid was measured using ELISA kit according to the manufacture’s instructions (R&D Systems, Minneapolis, MN, USA). Lactate content in IV Bioreactor fluids was measured using EnzyChromTM l-Lactate Assay Kit (ECLE-100) according to the manufacturer’s instructions (BioAssay Systems, Hayward, CA, USA). Experiments were conducted in triplicate, and optical densities were used to normalize the lactate production results.
Gross observation and size measurement of the pellets
Retrieved pellets (n = 5/time point/group) were observed in terms of their shape, color, and size. The size of the pellets was determined using the Image J program.
Cell viability assay
The pellets (n = 5/time point/group) were incubation at 48-well plate in serum-free DMEM medium 200 μL alamarBlue (Invitrogen, Carlsbad, CA, USA) 20 μL, 37 °C in a 5% CO2 incubator. After 5 h of incubation, the absorbance of duplicate samples (100 μL) of each well was measured in a 96 well plate, at wavelengths of 570 and 600 nm, using a microplate reader (Infinite M200, Tecan, Männedorf, Switzerland).
Histology and immunohistochemistry
Samples (n = 5/time point/group) were fixed with 4% formaldehyde for 24 h, dehydrated, and then embedded in paraffin wax. Sections, each 4 μm in thickness, were prepared and stained with hematoxylin/eosin for visualizing cell distribution and morphology (for assessment of synovitis using Krenn’s synovitis score system [15], supporting information Table 1), Safranin-O/fast green for accumulation of sulfated proteoglycans (for the evaluation of Safranin O-Fast green stained cartilaginous pellet using the Bern score [16], supporting information Table 2 and cartilage repair score using the O’Driscoll score system [17], supporting information Table 3), and Alizarin Red for calcified pellets (for quantitative analysis of the calcification area using the Image J program). For immunohistochemical analyses, sections of samples prepared as described above were treated with 3% Hydrogen peroxide (H2O2) for 10 min and reacted with a pepsin solution (Golden Bridge International, Inc., Mukilteo, WA, USA) for 10 min. After blocking the sections with 1% BSA in phosphate-buffered saline (PBS) for 1 h, sections were incubated with primary antibodies (1:200) for 1.5 h at room temperature. The primary antibodies used were type II, type I and type X collagen (all from Abcam, Cambridge, UK; supporting information Table 4). With 2 times washing in PBS, sections were incubated with a biotinylated secondary antibody (1:200) for 30 min and peroxidase-conjugated streptavidin solution for 30 min at room temperature (both from Golden Bridge International). Finally, sections were reacted with a 3,3′-diaminobenzidine (DAB) solution (Golden Bridge International) and counterstained with Mayer’s hematoxylin (Sigma, St. Louis, MO, USA) and then mounted for microscopic observation (Nikon E600, Tokyo, Japan).
Biochemical assay
For DNA and sulfated glycosaminoglycan (sGAG) contents assays, pellets (n = 5/time point/group) were fully digested for 24 h at 60 °C in papain digestion solution containing 125 μg/mL of papain with 5 mM l-cysteine-HCl and 5 mM EDTA in 100 mM Na2HPO4 (all from Sigma, USA). The DNA content was measured using the Picogreen assay. Total sGAG content was measured using the Blyscan GAG assay kit (Biocolor, Carrickfergus, UK). The papain-digested pellets were reacted with Blyscan dye reagent for 30 min and centrifuged for 10 min (12,000 rpm). The deposits were dissolved with dissociation reagent and absorbance was read at 656 nm. For collagen content assay, pellets (n = 5/time point/group) were fully digested for 2 days at 4 °C in 0.1 M of HCl digestion solution containing 1 mg/mL pepsin (Sigma). Collagen content was measured using the Sircol collagen assay kit (Biocolor). The HCL-digested pellets were reacted with Sircol dye reagent for 30 min and centrifuged for 10 min (12,000 rpm). The deposits were washed with ice–cold acid–salt wash reagent and centrifuged for 10 min (12,000 rpm). The deposits were dissolved with Alkali reagent and absorbance was read at 555 nm.
Reverse transcription polymerase chain reaction (RT-PCR)
The pellets (n = 5/time point/group) were rinsed in PBS and lysed in 500 μL TRIzol (Invitrogen) for 10 min. Total RNA was extracted according to the manufacturer’s instructions. Then, cDNA was synthesized using total RNA and iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA). Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed for human leukocyte antigens-ABC (HLA-ABC), CD80, CD86 and glyceraldehyde-6-phosphate dehydrogenase (GAPDH) using AccuPower PCR PreMix (Bioneer, Seongnam, Korea) according to the manufacturer’s instructions. The sequences of the primers and the reaction conditions are described with detailed information in Table 1.
Table 1.
Sequences of primers used for RT-PCR analysis. The expected size of products and annealing temperatures of the PCR reactions are also presented
| Gene name | Sequence | Product size (bp) | Annealing temp. (°C) |
|---|---|---|---|
| GAPDH |
Forward 5′-GGTCATGAGTCCTTCCACGAT-3′ Reverse 5′-GGTGAAGGTCGGAGTCAACGG-3′ |
520 | 59 |
| HLA-ABC (MHC class I) |
Forward 5′-GATTCTCCCCAGACGCCGAG-3′ Reverse 5′-CCTGGGCACTGTCACTGCTT-3′ |
1084 | 62 |
| CD80 |
Forward 5′-CATCACGGAGGGTCTTCTAC-3′ Reverse 5′-AGGATCTTGGGAAACTGTTGT-3′ |
710 | 57 |
| CD86 |
Forward 5′-TGCAAACTCTCAAAACCAAAG-3′ Reverse 5′-AAA4CACGCTGGGCTTCATCA-3′ |
816 | 55 |
Western blot analysis
Western blotting for HLA-ABC, CD80, CD86, and β-actin. Total proteins were extracted from pellets and using radioimmunoprecipitation assay (RIPA) lysis buffer (Rockland, Gilbertsville, PA, USA) and the protein concentrations were quantified using the Bradford assay (Bio-Rad). The protein extracts were subjected to electrophoresis through a 4–20% precast polyacrylamide gel (Bio-Rad). Subsequently, the proteins were transferred onto polyvinylidene fluoride (PVDF) membranes (Bio-Rad) and the membranes were blocked in 5% nonfat dry milk in Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST). Membranes were incubated for 2 h at 37 °C with primary antibodies against β-actin (GeneTex, Irvine, CA, USA; dilution of 1:2000), HLA-ABC, CD80, and CD86 (all from Abcam; dilution of 1:2000; supporting information Table 4). The membranes were then incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or anti-mouse secondary antibodies (both from GeneTex; dilution of 1:2000) at 37 °C for 1 h.
Statistical analysis
Data were analyzed for statistical significance by one-way and two-way analysis of variance (ANOVA) Tukey’s multiple comparisons test using GraphPad Prism 7.00 software. The experiments were repeated at least three times (n = 5). Data are expressed as mean ± standard deviation (SD). Statistical significance was assigned as *p < 0.05.
Results
Set-up of the in vivo bioreactor
To evaluate the capacity to self-regenerate new cartilage, we created an in vivo bioreactor using a cellulose membrane and a silicone tube in rabbit subcutaneous skin (Fig. 1A–D). The FCPC pellet culture within the chamber at 3 days after implantation, a thin fibrous tissue encapsulated with a vascular network surrounded all the chambers and only minimal fibrotic reaction was seen in the subcutaneous pockets surgically created. The IV bioreactor chamber was full of body fluid (Fig. 1E). After 1 week of culture, the pellet was transplanted into an autologous cartilage defect (Fig. 1F). The pellet was opaque and whitish, like native cartilage, and no vascular invasion was observed. The shape of the newly formed tissue changed little compared to its appearance before implantation.
IV bioreactor fluid characteristics and liquidity
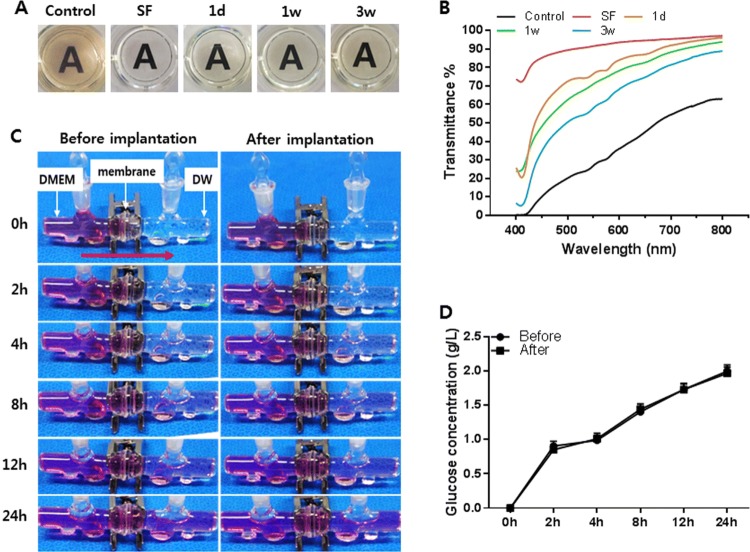
The appearance and transparency of the IV bioreactor fluid and native synovial fluid (SF) are shown in Fig. 2A and B. The IV bioreactor fluid recovered after the pellets were cultured for 1 day and 1 and 3 weeks. The control was a failed cellulose membrane chamber that was yellow–brown, turbid, and contained small particles, whereas the IV bioreactor fluid was clear, slightly yellow, and free of particulates, at all time points, similar to SF. Synovial fluid had the highest light transmittance, followed by the IV bioreactor fluid at 1 day, 1 and 3 weeks, and the control. The transmittance was 97%, 96%, 94%, 89%, and 63% respectively, at 800 nm. The total protein, glucose, and hyaluronic acid content in the IV bioreactor fluid were measured before pellet culture and compared to rabbit SF and serum. The results are compared to the contents of human SF and serum as references in Table 2. The results showed that these components in the IV bioreactor group were closer to synovial fluid than serum. Before and 3 weeks after implantation, the permeability of the cellulose membrane used in the IV bioreactor was compared for 24 h (Fig. 2C). The color of the right chamber (DW) thickened with time and the color of both chambers changed similarly by 24 h in both groups. The glucose concentration in the right chamber was similarly increased with time in both groups (Fig. 2D). It increased quickly in the first 2 h, then increased slowly. The total protein, glucose, and lactic acid content in the IV bioreactor fluid with and without pellets were compared at 3, 6, 9, and 12 days (Table 3). The contents of these components were similar between both groups at the same time point and each group maintained similar levels at all time points. These results indicated that the IV bioreactor fluid content was closer to SF than serum. And after 3 weeks of implantation, the permeability of the cellulose membrane was as good as before and the IV bioreactor system maintained homeostasis.
Fig. 2.
IV bioreactor fluid characteristics and confirm the fluid liquidity. The IV bioreactor fluid appearance A and transparency (transmittance%/wavelength) B. C The permeability of the cellulose membrane. D The concentration of glucose that was transferred from the left chamber to the right through the cellulose membrane (glucose concentration (g/L)/h)
Table 2.
IVB fluid component analysis
| Group | Rabbit | Humana | |||
|---|---|---|---|---|---|
| Serum | SF | IYB fluid | Serum | SF | |
| Total protein (mg/mL) | 63 ± 3 9 | 25 5 ± 3.2 | 35.2 ± 3.1 | 67.2 ± 3.1 | 19.9 ± 5 |
| Glucose (mg/dL) | 114 ± 16 | 97 ± 9 | 106 ± 12 | 108 ± 18 | 93 ± 17 |
| Hyahionan (μg/mL) | 0.2 ± | 30 ± 2.9 | 5.1 ± 0.3 | 0.3 ± | 2500 ± 790 |
aSource: [25] Balazs et al. Arthritis Rheum. 1967;10(4):357–76
Table 3.
Confirm the IVB fluid liquidity
| Group | 3 days | 6 days | 9 days | 12 days |
|---|---|---|---|---|
| Total protein (mg/mL) | ||||
| IVB fluid (− P) | 27.5 ± 0.7 | 25.5 ± 2.1 | 21 ± 1.8 | 24 ± 4.2 |
| IVB fluid (+ P) | 29 ± 1.3 | 26 5 ± 2.1 | 22 ± 1.4 | 24 ± 2.2 |
| Glucose (mg/dL) | ||||
| IVB fluid (− P) | 145 ± 13 | 140 ± 15 | 139 ± 13 | 138 ± 18 |
| IVB fluid (+ P) | 119 ± 16 | 112 ± 15 | 115 ± 17 | 116 ± 16 |
| Lactate (mM) | ||||
| IVB fluid (− P) | 7.9 ± 0.4 | 7.9 ± 1.2 | 7.7 ± 1.8 | 7.9 ± 1.7 |
| IVB fluid (+ P) | 8.3 ± 0.7 | 8.5 ± 0.4 | 8.3 ± 1 | 8.4 ± 0 6 |
(− P): without outpellets, (+ P): with pellets
Gross observation, size measurement, and cell viability of the pellets
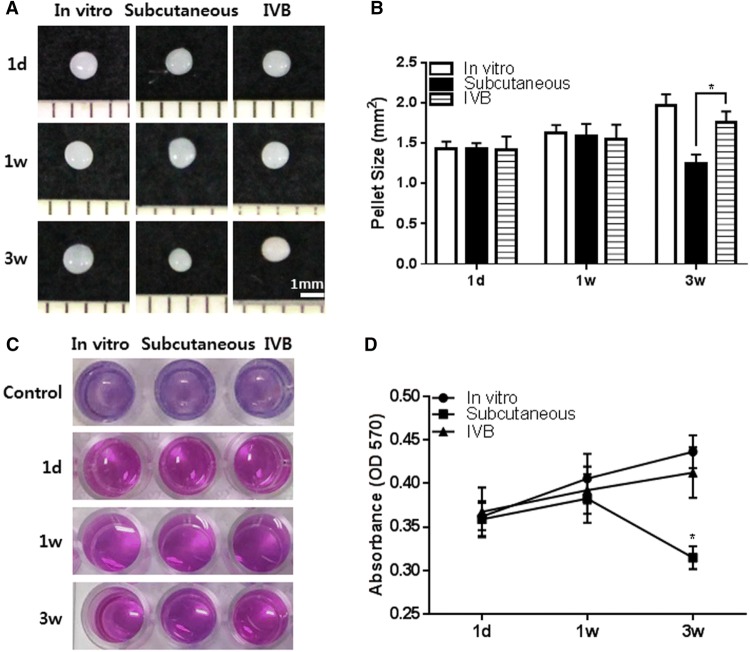
The effect of the IV bioreactor was compared to the growth of FCPC pellets cultured in vitro and subcutaneously on the growth of FCPC pellets. The pellets were first cultured in vitro for 3 days, then cultured in vitro, subcutaneously or in the IV bioreactor for 1 day, 1 and 3 weeks. All pellet groups showed whitish, hyaline cartilage-like circular morphology and no subcutaneous tissue adhesion or blood-stained red color appearred at any time point (some subcutaneous groups showed adhesions, data not shown) (Fig. 3A). In the gross images and size measurement, the size of the in vitro and IV bioreactor groups significantly increased with time, but the size of the pellets in the subcutaneous group were decreased at 3 weeks. The estimated size of the pellets in the IV bioreactor group (1.762 ± 0.132 mm2) was larger than that of the subcutaneous group (1.244 ± 0.115 mm2) (p < 0.05) and similar to the in vitro group (1.969 ± 0.135 mm2) at 3 weeks (Fig. 3B). The pellet viability was also determined (Fig. 3C). The cell viability reagent changed from purple to pink in all groups at all time points and the absorbance was measured (Fig. 3D). The absorbance increased with time in the in vitro and IV bioreactor groups, but the absorbance in the subcutaneous group was decreased at 3 weeks. The gross examination and viability results indicated the ability of the IV bioreactor to maintain the growth of FCPC pellets.
Fig. 3.
Gross observation, size measurement and cell viability of pellets retrieved from the in vitro, subcutaneous and IV bioreactor experiment. The pellets were cultured for 3 days in vitro before implantation in the subcutaneous and IV bioreactor of rabbit. The implanted pellets (n = 5/time point/group) were retrieved at 1 day, 1 and 3 weeks after implantation. A The gross images of pellets are presented. B The size of each pellet was measured in the in vitro (open bar), subcutaneous (solid bar) and IV bioreactor (horizontal striped bar) groups using Image J program. C The cell viability was examined using Alamar blue assay. D Absorbance of Alamar blue at 570 nm. The data are presented as a mean ± standard deviation (SD) from 5 independent experiments (n = 5). *p < 0.05
Histological observation of the pellets
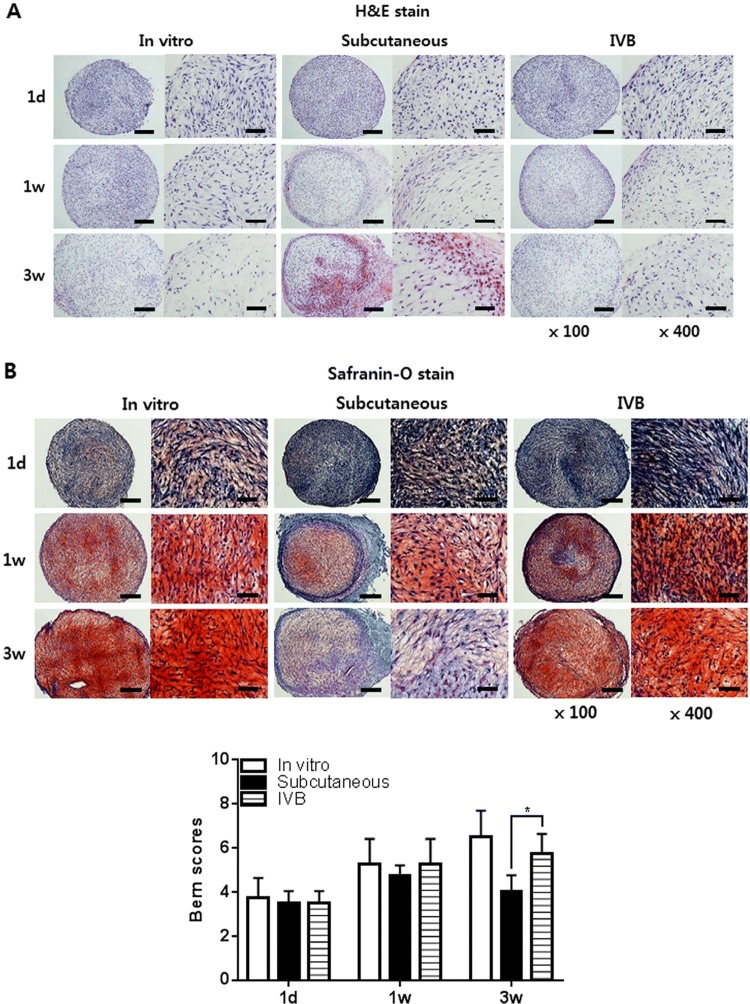
Chondrogenesis of the pellets cultured in vitro, subcutaneously, and in the IV bioreactor was further examined histologically (Fig. 4A, B). Safranin-O/fast green staining showed that the accumulation of sulfated GAGs was significantly increased with time and no significant difference was noted in the intensity and area of safranin-O staining between the in vitro and IV bioreactor groups until 3 weeks. Staining was decreased in the subcutaneous group at 3 weeks. The Bern score of the IV bioreactor group (5.8 ± 0.9) was larger than that of the subcutaneous group (4.0 ± 0.8) (p < 0.05) and similar to the in vitro group (6.5 ± 1.2) at 3 weeks (Fig. 4B). In the high magnification images (400×), the differentiated cells in the metachromatically stained area of the in vitro and IV bioreactor groups were round or long fusiform during the entire culture process and lacunar structures characteristic of native cartilage were not seen (Fig. 4A, B). This morphology was observed for differentiated cells of the subcutaneous group at 1 week of culture, but the cells structures were severely degraded by 3 weeks in culture.
Fig. 4.
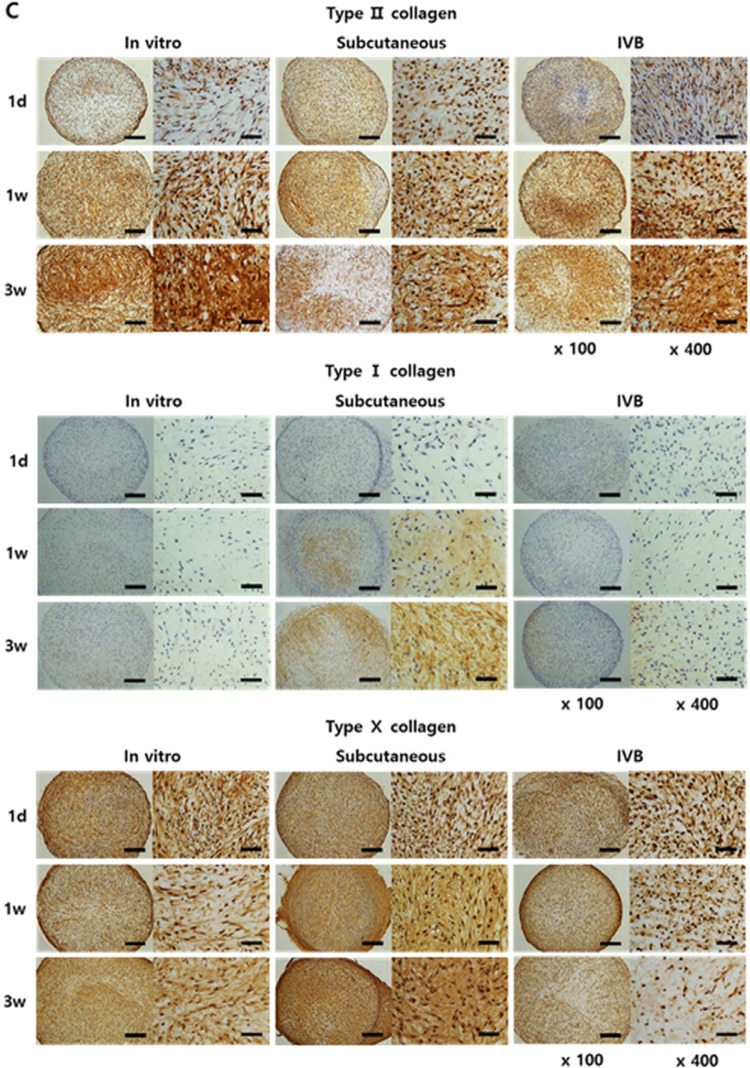
Histological and immunohistochemical observation of the pellets. The pellets were cultured for 3 days in vitro before implantation in the subcutaneous and IV BIOREATOR of rabbit. The implanted pellets (n = 5/time point/group) were retrieved at 1 day, 1 and 3 weeks after implantation. The pellets were processed to prepare thin sections, each 4 μm in thickness. A Hematoxylin/eosin to observe the distribution of cells. B Safranin-O/fast green to observe accumulation of sulfated proteoglycans, and the Bern scores were evaluated. C Immunostained with an antibody against rabbit type II, type I and type X collagen. D Alizarin Red stain to observe calcium deposits in the pellets, and the Alizarin Red stained area/total area (%) were evaluated using Image J program. The stained images are presented as a whole pellet (left columns, × 100) and at high magnification (right columns, × 400). Scale bar = 200 μm for × 100 and 50 μm for × 400 images. Data are presented as a mean ± SD from 5 independent experiments in the histograms (n = 5). *p < 0.05
Immunohistochemical analysis of the pellets
Immunostaining for type II, type I, and type X collagen was conducted to confirm the chondrogenic phenotypes of the pellets (Fig. 4C). Type II collagen increased with time in the in vitro and IV bioreactor groups but it was decreased at 3 weeks in the subcutaneous group, reaching about 50% of the intensity and stained area seen at 1 week. The IV bioreactor group showed strong expression of type II collagen in the whole pellets at 3 weeks that was stronger than the subcutaneous group, but weaker than the in vitro group. The type I and type X collagen showed weak expression at all time points in the in vitro and IV bioreactor groups but increased with time in the subcutaneous group.
Calcification of the pellets
To examine if the loss of chondrogenic phenotypes was correlated with calcification of the matrix, Alizarin Red staining was performed and the Image J program confirmed the percentage of the calcifications (Fig. 4D). In both the subcutaneous and the IV bioreactor groups, red stain, indicative of calcified mineral deposits, was first observed in the peripheral region at 1 week and spread into the central region at 3 weeks. The in vitro group showed weak staining in the peripheral region at 3 weeks. The stained area was more intense and broader in the subcutaneous group (9.17 ± 5.04%) than in the IV bioreactor (1.43 ± 0.83%) (p < 0.05) and in vitro groups (0.88 ± 0.57%) (p < 0.05) at 3 weeks. These results indicated that the IV bioreactor not only supported the chondrogenesis better but also maintained the chondrogenic phenotype of the FCPC pellets.
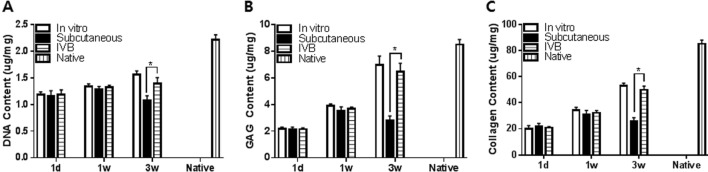
Biochemical analysis for the content of DNA, GAGs and collagen
The content of DNA, GAGs, and collagen was measured quantitatively by chemical assays of the retrieved pellets (Fig. 5). The DNA content increased with time after culture in vitro and IV bioreactor groups, but subcutaneous group was decreased at 3 weeks (Fig. 5A). The DNA content of the IV bioreactor group (1.40 ± 0.11 μg/mg) was higher than of the subcutaneous group (1.08 ± 0.08 μg/mg) (p < 0.05), but lower than that of the in vitro group (1.56 ± 0.07 μg/mg) (p < 0.05) at 3 weeks. This result suggests that the number of cells increased with time in vitro and IV Bioreactor groups, but the subcutaneous group was decreased at 3 weeks. The content of GAGs and collagen showed a similar pattern overall to that of DNA content. The GAGs content increased with time after culture in vitro and IV bioreactor groups, but the subcutaneous group was decreased at 3 weeks (Fig. 5B). The IV bioreactor group (6.15 ± 0.27 μg/mg) was higher than of the subcutaneous group (2.79 ± 0.35 μg/mg) (p < 0.05), and similar to in vitro group (6.98 ± 0.26 μg/mg) (p < 0.05) at 3 weeks. The collagen content increased with time after culture in vitro and IV bioreactor groups, but the subcutaneous group was decreased at 3 weeks (Fig. 5C). The IV bioreactor group (50 ± 3 μg/mg) was higher than of the subcutaneous group (26 ± 3 μg/mg) (p < 0.05), and similar to in vitro group 53 ± 2 μg/mg) (p < 0.05) at 3 weeks. These results support the ability of the IV bioreactor to maintain chondrogenesis of FCPC pellets observed above by biochemical analysis.
Fig. 5.
Biochemical analysis of in vitro, subcutaneous, IV bioreactor pellets and native rabbit cartilage (vertical striped bar) for total content of DNA A, GAGs B and collagen C. The pellets were cultured for 3 days in vitro before implantation in the subcutaneous and IV Bioreactor of rabbit. The implanted pellets (n = 5/time point/group) were retrieved at 1 day, 1 and 3 weeks after implantation. The amount of each component was calculated using standard curves for each assay and normalized by the wet weight of each pellet. Data are presented as a mean ± SD from 5 independent experiments in the histograms (n = 5). *p < 0.05
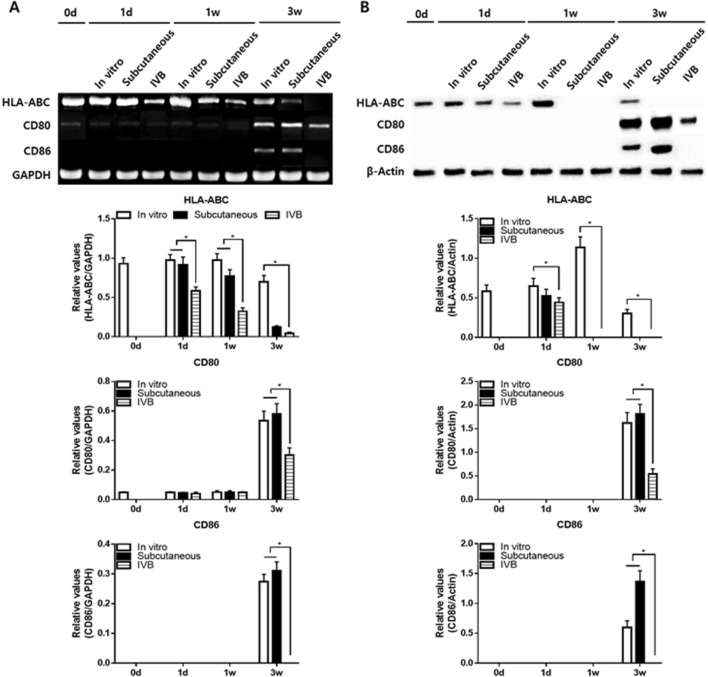
Molecular analysis of immunogenicity
The RT-PCR analysis showed that the mRNA of HLA-ABC was maintained significantly low levels in IV bioreactor group than in the in vitro group at all time points, and it decreased with time in all groups (Fig. 6A). The mRNA of CD80 was low levels only at 1 day and 1 week but increased at 3 weeks in all groups. However, its level of the IV Bioreactor was significantly lower than in vitro and subcutaneous groups. The mRNA pattern of CD86 showed a similar to that of the CD80. The mRNA of CD86 was no expression only at 1 day and 1 week but increased at 3 weeks in vitro and subcutaneous groups. However, its level of the IV bioreactor has maintained no expression at all time points. The western blotting analysis of protein showed a similar pattern overall to that of the mRNA (Fig. 6B). The protein of HLA-ABC in vitro group showed increase at 1 day and 1 week, and decrease at 3 weeks. But IV bioreactor and subcutaneous groups showed relatively low levels at 1 day, and it no expression at 1 and 3 weeks. The protein of CD80 was no expression only at 1 day and 1 week but increased at 3 weeks in all groups. However, its level of the IV bioreactor was significantly lower than in vitro and subcutaneous groups. The protein pattern of CD86 showed a similar to that of the CD80. The protein of CD86 was no expression only at 1 day and 1 week but increased at 3 weeks in vitro and subcutaneous groups. However, its level of the IV Bioreactor was maintained no expression at all time points. These results suggest that the IV Bioreactor not only better maintain the chondrogenic phenotype of FCPC pellets but also decreased the immunogenicity at a longer time when compared with the in vitro and subcutaneous.
Fig. 6.
A Total RNA was isolated from the pellets and expression levels of leukocyte antigen HLA-ABC, CD80 and CD86 were examined in the in vitro, subcutaneous and IV Bioreactor groups by RT-PCR analysis. Band intensities from 5 independent pellets (n = 5) were measured by the Image J program to obtain quantitative data. The intensities of HLA-ABC, CD80 and CD86 were normalized to that of GAPDH (a house-keeping gene) in the histograms. B Protein was isolated from the pellets and expression levels of HLA-ABC, CD80 and CD86 were examined in the in vitro, subcutaneous and IV bioreactor groups by Western blotting. Band intensities from 5 independent pellets (n = 5) were measured by the Image J program to obtain quantitative data. The intensities of HLA-ABC, CD80 and CD86 were normalized to that of β-actin (a house-keeping gene) in the histograms
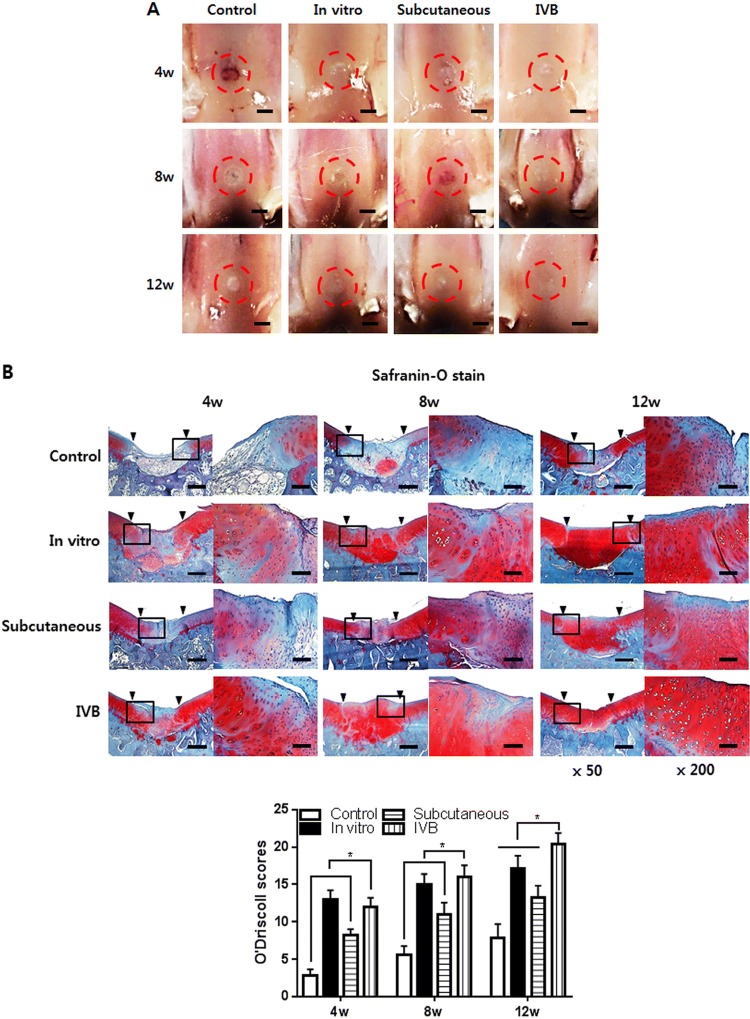
Macroscopic and histological observation of the ectopic engineered cartilage for cartilage repair in vivo
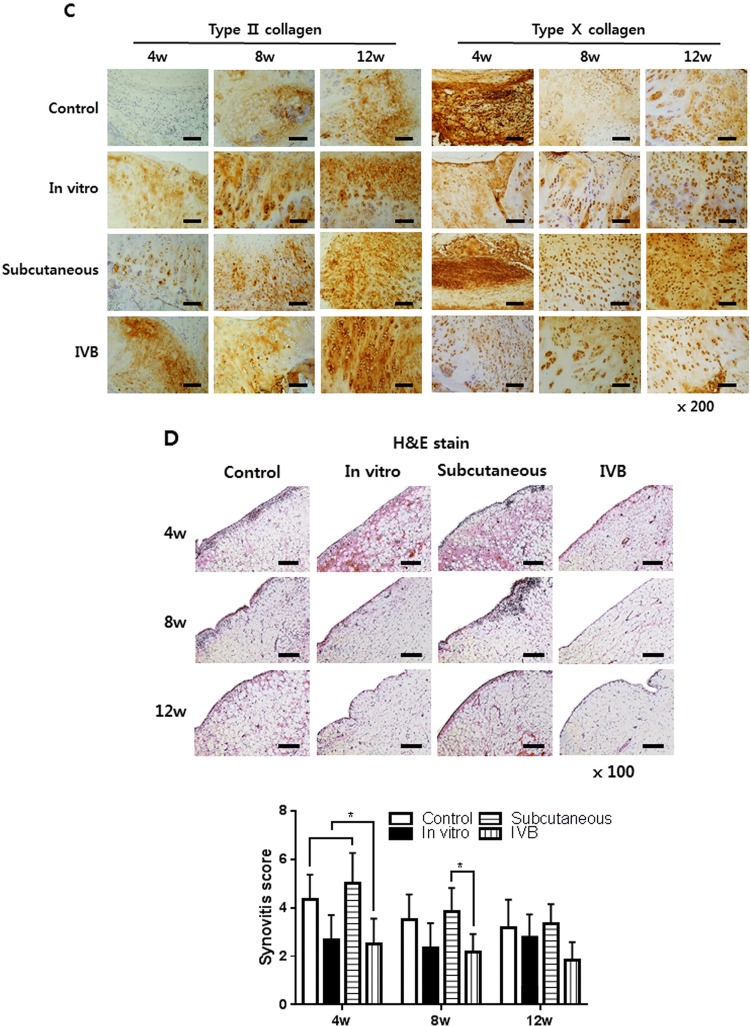
The ectopic engineered cartilage was then applied to treat cartilage defect in the rabbit model. There was no infection, wound dehiscence, disability, and death throughout the in vivo experiment. The new tissue gradually formed and filled the defect from 4 to 12 weeks (Fig. 7A). In the control group, fiber-like tissue was gradually formed from 4 to 12 weeks. But the formation of new tissue was scarce and the defect center still has obvious depression at 12 weeks. Semi-transparent tissue gradually filled with defects in vitro, subcutaneous and IV bioreactor groups at 4–12 weeks. However, at 8 and 12 weeks, the similarity between neocartilage and native cartilage in the IV bioreactor group was better than that subcutaneous and in vitro groups. Specifically, the IV bioreactor group was almost completely covered with cartilage-like tissue, similar to the surrounding native cartilage with indistinct margins, after 12 weeks. But for the subcutaneous and in vitro groups, the margins between the native cartilage and the neocartilage was still obvious. The histological examination with safranin-O staining is shown (Fig. 7B). In the control group, fiber-like tissue formed in the defect, the integration between newly formed tissue and adjacent native cartilage can be easily identified with an obvious gap at 4–12 weeks. The thickness of neocartilage is similar to adjacent native cartilage and the staining intensity gradually increases in IV bioreactor, subcutaneous and in vitro groups at 4–12 weeks. In the IV bioreactor and in vitro groups, the neocartilage gradually became smooth of the surface, the cartilage lacuna density and distribution were similar to native cartilage, and the integration with adjacent native cartilage was complete. The IV bioreactor group at 12 weeks was better than in vitro group. However, in the subcutaneous group, the neocartilage surface was not smooth, the staining intensity and cartilage lacuna density were lower, and the integration with adjacent native cartilage was not complete. Consistently with the results of safranin-O staining examination, there was a statistically significant difference in the O’Driscoll score between the IV bioreactor, subcutaneous and in vitro group at each time point.
Fig. 7.
Macroscopic, histological and immunohistochemical observation of neocartilage in the cartilage defect at 4, 8 and 12 weeks post-implantation of ectopic engineered cartilage are shown. A Macroscopic observation of the newly formed tissue. The red circle indicates the location of the cartilage defect. Scale bar = 200 μm. B Safranin-O and fast green staining of neocartilage in vivo. The square indicates the enlarged position. The arrows indicate the boundary between normal and regenerated cartilage. And O’Driscoll scores were evaluated. C Immunohistochemical staining of type II and type X collagen. D H&E staining of synovium in the cartilage defect joint. And synovitis evaluated by Krenn’s synovitis scoring system. Scale bar = 400 μm for × 50, 200 μm for × 100 and 100 μm for × 200 images. Data are presented as a mean ± SD from 5 independent experiments in the histograms (n = 5). *p < 0.05
Immunohistochemical observation of the ectopic engineered cartilage for cartilage repair in vivo
IHC staining of type II and type X collagen was performed to confirm the chondrogenic phenotype of implanted ectopic engineered cartilage and to evaluate the composition of newly formed tissues (Fig. 7C). As shown in the figure, the staining of type II collagen in the IV bioreactor and in vitro group was stronger and uniform than the subcutaneous and control group, while the staining of type X was weaker at the same time point. This suggests that the neocartilage of IV bioreactor group was close to hyaline cartilage. These results indicating that IV bioreactor can help ectopic engineered cartilage maintain better chondrogenic phenotype during cartilage repair in vivo.
Histological observation of the synovium
At 4 weeks, HE staining of the synovium in the subcutaneous and control groups showed an enlargement of the synovial lining cell layer, inflammatory cell infiltration and an increase of the cellularity, but gradually decreased with time (Fig. 7D). The synovium of the IV Bioreactor and in vitro groups were basically normal at each time point. Krenn’s synovitis score showed that there was a statistical difference between the IV bioreactor group and the subcutaneous group at 4 and 8 weeks, and no difference between the groups at 12 weeks. These results indicating that IV bioreactor not only helps ectopic engineered cartilage maintain better chondrogenic phenotype during cartilage repair in vivo but also may help reduce synovitis.
Discussion
Previous studies have reported that ectopic cartilage reconstruction was not successful in immunocompetent animals, which was mainly the result of host immune responses to the allogeneic or xenogeneic implants and cells, vascular invasion that caused cartilage degradation and bone formation, and host tissue ingrowth [18]. The results demonstrated that the IV bioreactor was a useful experimental system for the reconstruction of cartilage in immunocompetent hosts [5, 19, 20]. The IV bioreactor culture technique has mainly been applied to investigate cell differentiation. Most nutrients can pass through the pores of the cellulose membrane, but cells cannot. Cellulose is a hydrophilic material, with low non-specific adsorption of proteins. Furthermore, mammalian cells are not easily adsorbed to the cellulose surface, so they do not block the pores [21–24].
Recently, several studies have demonstrated that the nutrient supply for natural articular cartilage mainly comes from synovial fluid [25]. Component analysis of the IV bioreactor fluid in this study showed that the protein, glucose, and HA contents were closer to synovial fluid than serum (Table 2). Glucose plays an important nutritional role in chondrogenesis and maintenance [26]. When HA was added to the culture medium, DNA, sulfated glycosaminoglycans, and type II collagen synthesis increased [27]. The IV bioreactor environment promoted the synthesis of cartilage matrix and maintained the cartilage phenotype for 3 weeks, whereas the subcutaneous cultured pellet degenerated and its phenotypic expression was reduced after 3 weeks (Figs. 4, 5). This may have been because the IV bioreactor fluid containd substances required for chondrogenesis. The IV bioreactor environment not only promoted the synthesis of cartilage matrix and maintained the cartilage phenotype, but it also delayed calcification compared to the subcutaneous group (Fig. 4D). In this study, calcification in the subcutaneous group may be associated with host cell ingrowth and vascular invasion [28], while the reasons for delayed calcification in the IV bioreactor group may be related to some serum protein contained in the IV bioreactor that may play an inhibitory role in the early stage of the calcification process after penetrating the matrix [29]. Moreover, although vascular invasion was avoided, osteogenic differentiation could not be completely prevented. It was inevitable that factors directing osteogenesis were transmitted through body fluid during implantation. Calcification is a multifactorial process caused by an imbalance between inhibitors and pro-mineralization factors. Loss of proteoglycans often leads to articular cartilage calcification because proteoglycans are effective mineralizing inhibitors [30]. The loss of chondrogenic phenotypes appears to be associated with increased matrix calcification (Alizarin Red staining) and hypertrophy (expression of type I and type X collagen). Chondrocyte phenotypic changes include hypertrophy differentiation, apoptosis, altered responses to growth factors, inflammatory cytokines, and mediators [31]. Type I and type X collagen genes are related to the induction of chondrocyte hypertrophy [32].
In this study, the RT-PCR and Western blot results showed that the expression levels of HLA-ABC, CD80, and CD86 in pellets cultured for 1 week were lower, especially in the IVB group than in the in vitro and subcutaneous groups (Fig. 6). HLA-ABC is a major histocompatibility class I complex (MHC I) and CD80 and CD86 are co-stimulatory molecules. They are present on the cell surface and activate T-cells when they are bound to T cell receptors (TCRs), initiating an immune response to destroy the foreign material [33]. Therefore, the presence of HLA-ABC, CD80, and CD86 on donor cells are important constituents involved in the immune rejection of implanted tissue-engineered constructs [34]. Many researchers believe that the ECM shields MHC molecules from recognition by host cells, thereby protecting the chondrocytes from host immune responses [35]. In conclusion, this study successfully reconstructed cartilage in an immunocompetent host using an IV bioreactor system. The results revealed that allogeneic stem cells might also be applicable, which would resolve the problem caused by the shortage of resources for cartilage repair.
Compared to the in vitro and subcutaneous groups, the IV bioreactor group healed the cartilage defect better, which was confirmed by macroscopic, histological, and immunohistochemical observations (Fig. 7). Repair of the cartilage defects using engineered cartilage in an IV bioreactor in vivo showed that neocartilage formed around the original cartilage and had a favorable boundary, whereas the constructs without the in vivo culture did not perform as well. This implied the superiority of in vivo engineered cartilage for repairing cartilage defects. According to previous reports, ectopic cartilage reconstruction has not been successful in immunocompetent animals, which is mainly manifested as host immune response to allogeneic or xenogeneic implants and cells [18]. To prevent an immunological rejection, the scaffold and cells were encapsulated within the diffusion chamber before implantation into the cartilage defect [36]. Thus, the IV bioreactor may act as a mechanical barrier between the cell and the host, thereby hiding the surface antigens [5, 19, 20]. The results of H&E synovial staining and synovitis scoring showed that the IV bioreactor group had lower synovitis scores than the IV bioreactor and subcutaneous groups (Fig. 7D). The synovia of the subcutaneous and control groups showed enlargement of the synovial lining cell layer, increased cellularity, and inflammatory cell infiltration. It is well known that the immune response of the joint to implanted neocartilage is mediated by the synovial membrane. Defects in articular cartilage can trigger an innate immune response, even in the absence of an implant [37]. In this study, an IV bioreactor was used to protect the pellet from host interference and create a suitable environment for cartilage regeneration.
In conclusion, this study successfully reconstructed ectopic cartilage in an immunocompetent host using a IV bioreactor system and obtaining satisfiable results applying for initial repair of cartilage defect. In addition, it was speculated that, because the reconstructed tissue to repair the defect of host was formed right in host, it might be preferred to extracted allografts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
This paper was funded by the Ministry of Health and Welfare (HI17C2191) in 2017.
Compliance with ethical standards
Conflict of interests
The authors declare that they have no conflict of interest.
Ethical statement
Human fetal cartilage progenitor cell (hFCPC) were isolated from the human fetal cartilage tissue. The study was approved by the institutional review board of the Ajou University Medical Center (AJIRB-CRO-07-139) and was carried out with the informed consent of all donors. This study was approved by the ethics committee for animal research of the Laboratory Animal Research Center of Ajou University Medical Center (Approval No. 2014-0068). Authors complied with institutional ethical use protocols (NIH Guide for Care and Use of Laboratory Animals).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue Guang Li and In-Su Park contributed equally to this work.
References
- 1.Hunziker EB. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15–28. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 2.Hunziker EB. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432–463. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 3.Bartha L, Vajda A, Duska Z, Rahmeh H, Hangody L. Autologous osteochondral mosaicplasty grafting. J Orthop Sports Phys Ther. 2006;36:739–750. doi: 10.2519/jospt.2006.2182. [DOI] [PubMed] [Google Scholar]
- 4.Rose T, Craatz S, Hepp P, Raczynski C, Weiss J, Josten C, et al. The autologous osteochondral transplantation of the knee: clinical results, radiographic findings and histological aspects. Arch Orthop Trauma Surg. 2005;125:628–637. doi: 10.1007/s00402-005-0010-8. [DOI] [PubMed] [Google Scholar]
- 5.Revell CM, Athanasiou KA. Success rates and immunologic responses of autogenic, allogenic, and xenogenic treatments to repair articular cartilage defects. Tissue Eng Part B Rev. 2009;15:1–15. doi: 10.1089/ten.teb.2008.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelttari K, Steck E, Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39:S58–S65. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 7.Huang RL, Kobayashi E, Liu K, Li Q. Bone graft prefabrication following the in vivo bioreactor principle. EBioMedicine. 2016;12:43–54. doi: 10.1016/j.ebiom.2016.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng L, Sun J, Chen X, Wang G, Jiang B, Fan H, et al. In vivo cartilage engineering with collagen hydrogel and allogenous chondrocytes after diffusion chamber implantation in immunocompetent host. Tissue Eng Part A. 2009;15:2145–2153. doi: 10.1089/ten.tea.2008.0268. [DOI] [PubMed] [Google Scholar]
- 9.Courtenay JC, Johns MA, Galembeck F, Deneke C, Lanzoni EM, Costa CA, et al. Surface modified cellulose scaffolds for tissue engineering. Cellulose. 2017;24:253–267. doi: 10.1007/s10570-016-1111-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang YJ, Shin JM, Kang TH, Kimura S, Wada M, Kim UJ. Cellulose dissolution in aqueous lithium bromide solutions. Cellulose. 2014;21:1175–1181. doi: 10.1007/s10570-014-0183-9. [DOI] [Google Scholar]
- 11.Choi WH, Kim HR, Lee SJ, Jeong N, Park SR, Choi BH, et al. Fetal cartilage-derived cells have stem cell properties and are a highly potent cell source for cartilage regeneration. Cell Transplant. 2016;25:449–461. doi: 10.3727/096368915X688641. [DOI] [PubMed] [Google Scholar]
- 12.Ito A, Nagai M, Tajino J, Yamaguchi S, Iijima H, Zhang X, et al. Culture temperature affects human chondrocyte messenger RNA expression in monolayer and pellet culture systems. PLoS One. 2015;10:e0128082. doi: 10.1371/journal.pone.0128082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farquharson C, Whitehead CC. Differentiation and mineralization in chick chondrocytes maintained in a high cell density culture: a model for endochondral ossification. In Vitro Cell Dev Biol Anim. 1995;31:288–294. doi: 10.1007/BF02634003. [DOI] [PubMed] [Google Scholar]
- 14.Li TZ, Jin CZ, Choi BH, Kim MS, Kim YJ, Park SR, et al. Using cartilage extracellular matrix (CECM) membrane to enhance the reparability of the bone marrow stimulation technique for articular cartilage defect in canine model. Adv Funct Mater. 2012;22:4292–4300. doi: 10.1002/adfm.201102695. [DOI] [Google Scholar]
- 15.Krenn V, Morawietz L, Burmester GR, Kinne RW, Mueller-Ladner U, Muller B, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–364. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 16.Grogan SP, Barbero A, Winkelmann V, Rieser F, Fitzsimmons JS, O’Driscoll S, et al. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006;12:2141–2149. doi: 10.1089/ten.2006.12.2141. [DOI] [PubMed] [Google Scholar]
- 17.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68:1017–1035. doi: 10.2106/00004623-198668070-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ding J, Chen B, Lv T, Liu X, Fu X, Wang Q, et al. Bone marrow mesenchymal stem cell-based engineered cartilage ameliorates polyglycolic acid/polylactic acid scaffold-induced inflammation through M2 polarization of macrophages in a pig model. Stem Cells Transl Med. 2016;5:1079–1089. doi: 10.5966/sctm.2015-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskalewski S, Hyc A, Osiecka-Iwan A. Immune response by host after allogeneic chondrocyte transplant to the cartilage. Microsc Res Tech. 2002;58:3–13. doi: 10.1002/jemt.10110. [DOI] [PubMed] [Google Scholar]
- 20.Weinand C, Peretti GM, Adams SB, Jr, Randolph MA, Savvidis E, Gill TJ. Healing potential of transplanted allogeneic chondrocytes of three different sources in lesions of the avascular zone of the meniscus: a pilot study. Arch Orthop Trauma Surg. 2006;126:599–605. doi: 10.1007/s00402-005-0100-7. [DOI] [PubMed] [Google Scholar]
- 21.Brash JL, Ten Hove P. Protein adsorption studies on ‘standard’ polymeric materials. J Biomater Sci Polym Ed. 1993;4:591–599. doi: 10.1163/156856293X00230. [DOI] [PubMed] [Google Scholar]
- 22.Pelton R. Bioactive paper provides a low-cost platform for diagnostics. Trends Analyt Chem. 2009;28:925–942. doi: 10.1016/j.trac.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu CY, Suen SY, Chen SC, Tzeng JH. Analysis of protein adsorption on regenerated cellulose-based immobilized copper ion affinity membranes. J Chromatogr A. 2003;996:53–70. doi: 10.1016/S0021-9673(03)00531-4. [DOI] [PubMed] [Google Scholar]
- 24.Zou H, Luo Q, Zhou D. Affinity membrane chromatography for the analysis and purification of proteins. J Biochem Biophys Methods. 2001;49:199–240. doi: 10.1016/S0165-022X(01)00200-7. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Wei L, Zeng L, He D, Wei X. Nutrition and degeneration of articular cartilage. Knee Surg Sports Traumatol Arthrosc. 2013;21:1751–1762. doi: 10.1007/s00167-012-1977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mobasheri A, Vannucci SJ, Bondy CA, Carter SD, Innes JF, Arteaga MF, et al. Glucose transport and metabolism in chondrocytes: a key to understanding chondrogenesis, skeletal development and cartilage degradation in osteoarthritis. Histol Histopathol. 2002;17:1239–1267. doi: 10.14670/HH-17.1239. [DOI] [PubMed] [Google Scholar]
- 27.Akmal M, Singh A, Anand A, Kesani A, Aslam N, Goodship A, et al. The effects of hyaluronic acid on articular chondrocytes. J Bone Joint Surg Br. 2005;87:1143–1149. doi: 10.1302/0301-620X.87B8.15083. [DOI] [PubMed] [Google Scholar]
- 28.Britt JC, Park SS. Autogenous tissue-engineered cartilage: evaluation as an implant material. Arch Otolaryngol Head Neck Surg. 1998;124:671–677. doi: 10.1001/archotol.124.6.671. [DOI] [PubMed] [Google Scholar]
- 29.Heiss A, DuChesne A, Denecke B, Grötzinger J, Yamamoto K, Renné T, et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 30.Chen CC, Boskey AL, Rosenberg LC. The inhibitory effect of cartilage proteoglycans on hydroxyapatite growth. Calcif Tissue Int. 1984;36:285–290. doi: 10.1007/BF02405332. [DOI] [PubMed] [Google Scholar]
- 31.Ea HK, Nguyen C, Bazin D, Bianchi A, Guicheux J, Reboul P, et al. Articular cartilage calcification in osteoarthritis: insights into crystal-induced stress. Arthritis Rheum. 2011;63:10–18. doi: 10.1002/art.27761. [DOI] [PubMed] [Google Scholar]
- 32.Girkontaite I, Frischholz S, Lammi P, Wagner K, Swoboda B, Aigner T, et al. Immunolocalization of type X collagen in normal fetal and adult osteoarthritic cartilage with monoclonal antibodies. Matrix Biol. 1996;15:231–238. doi: 10.1016/S0945-053X(96)90114-6. [DOI] [PubMed] [Google Scholar]
- 33.Fangmann J, Dalchau R, Fabre JW. Rejection of skin allografts by indirect allorecognition of donor class I major histocompatibility complex peptides. J Exp Med. 1992;175:1521–1529. doi: 10.1084/jem.175.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi HL. Immunobiology of rejection and adaptation. Transplant Proc. 2007;39:647–652. doi: 10.1016/j.transproceed.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 35.Lattermann C, Romine SE. Osteochondral allografts: state of the art. Clin Sports Med. 2009;28:285–301. doi: 10.1016/j.csm.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X, Liu J, Liu Q, Lu Z, Zheng L, Zhao J, et al. Therapy for cartilage defects: functional ectopic cartilage constructed by cartilage-simulating collagen, chondroitin sulfate and hyaluronic acid (CCH) hybrid hydrogel with allogeneic chondrocytes. Biomater Sci. 2018;6:1616–1626. doi: 10.1039/C8BM00354H. [DOI] [PubMed] [Google Scholar]
- 37.Arzi B, DuRaine GD, Lee CA, Huey DJ, Borjesson DL, Murphy BG, et al. Cartilage immunoprivilege depends on donor source and lesion location. Acta Biomater. 2015;23:72–81. doi: 10.1016/j.actbio.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.