Abstract
Alpha lipoic acid (LA) and conjugated linoleic acid (CLA) have been well-documented on a variety of functional effects in health foods. The main purpose of this study was focused on the additive anti-inflammatory activity of the combination of LA and CLA in vitro. Raw 264.7 cells induced by lipopolysaccharide were treated with LA and CLA individually or in combination at a variety of concentration ranges. Co-treating 25 μM of LA and 25 μM of CLA significantly inhibited pro-inflammatory cytokines compared to the same concentration of single LA- or CLA-treated group. The molecular mechanism of anti-inflammation by a combination of these compounds was attributed to extracellular signal-regulated kinase-1 (ERK1) and peroxisome proliferator-activated receptor gamma (PPARγ). Also, the molecular interaction between both compounds was confirmed by NMR. Our findings suggested that the combination of CLA and LA showed potential additive effect on anti-inflammation through the molecular interaction of both compounds.
Electronic supplementary material
The online version of this article (10.1007/s10068-019-00677-7) contains supplementary material, which is available to authorized users.
Keywords: Anti-inflammation, Alpha lipoic acid (LA), Conjugated linoleic acid (CLA), Extracellular signal-regulated kinase-1 (ERK1), Peroxisome proliferator-activated receptor gamma (PPARγ)
Introduction
In modern society, inflammation as a complex physiological response to pathogens or non-microbial endogenous molecules underlies the pathogenesis of many diseases such as diabetes, cardiovascular disease, bowel disease, neurodegeneration and cancer (Colotta et al., 2009; Deeks, 2011; Parimisetty et al., 2016) although it has a very complex mechanism. Currently, drug therapies have been used to suppress inflammation mainly focused on several methods such as interference with non-steroidal anti-inflammatory drugs, blocking of pro-inflammatory cytokine signaling pathway using tumor necrosis factor α (TNF- α) and interleukin-1 (IL-1) (Vane and Botting, 1987) and usage of agonists of the glucocorticoid receptor (Baschant and Tuckermann, 2010). However, some of these approaches result in therapeutic efficiency with significant side effects, although only limited numbers of key molecules are involved in each particular disease. Hence, recently nutriceutical treatments have been attracted to overcome the limitation of the drugs as an alternative.
Conjugated linoleic acid (CLA) mixed of positional and geometric octadecadienoic acid isomers derived from linoleic acid have two double bonds in the cis configuration (O’Quinn et al., 2000) although most beneficial properties of CLA were reported by its two main isomers of c9,t11-CLA and t10,c12-CLA (Pascual et al., 2005). Among the biological activities of the CLA, beneficial effects of CLA on inflammatory responses have been reported in many animal models as well as human clinical trials (Ochoa et al., 2004). It was already reported that the CLA had many immune responses such as decrease of chronic inflammation, reduction of antigen-induced cytokine production, the decrease of an adverse effect of immune challenges and modulation of inflammatory modulators (Stachowska et al., 2009).
Alpha lipoic acid (LA) as a naturally occurring dithiol compound obtained enzymatically in the mitochondrion by octanoic acid can be found in some entrails and in various vegetables (Shay et al., 2009). It was already reported that the LA modulated the plasma concentration of pro-inflammatory cytokines (Zhang and Frei, 2001) such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and suppressed genes involved in inflammatory activity such as nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) (Moura et al., 2015; Suzuki et al., 1992).
Because evidence to date suggests that the effects of CLA and LA on the anti-inflammation activity are mediated through the same cellular mechanism of the NF-κB (Cheng et al., 2004; Demarco et al., 2004), it is expected that when a combination of CLA and LA is used, there will be an additive effect in increasing anti-inflammation in immune cells. Also, it is hypothesized that the molecular interaction between both compounds can occur because the LA as the solid form can be soluble in CLA as the liquid one. The research on the molecular interaction through hydrogen bonds or hydrophobic interactions between drugs is very important to find out new drug candidates or health foods because the interaction of the drugs can affect the preparation process, improvement of solubility, physical properties, drug stability and oral bioavailability (Onakpoya et al., 2012; Teichert et al. 2003).
This research was aimed to study the additive anti-inflammation activity of the combination of CLA and LA in vitro through the molecular interaction between both compounds. We analyzed the expression of the representative inflammatory mediators and evaluated the resolving effect of fatty acids. We also elucidated the mechanism of additive effect in the view of both biological and chemical aspects. To the best of our knowledge, this is the first report that shows the improvement of the additive anti-inflammatory effect by the molecular interaction of CLA and LA.
Materials and methods
Materials
Cell culture medium DMEM and penicillin/streptomycin were provided from Hyclone (Logan, UT, USA). Fetal bovine serum was provided from GenDEPOT (Barker, TX, USA). CLA was purchased from HK Biotech (Jinju, Gyeongnam, Korea). Antibodies were purchased from Abcam (Cambridge, England). LA and other materials were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cell line and cell culture
The murine macrophage cell line Raw 264.7 (American Type Culture Collection ATCC) was cultured in DMEM high glucose containing 4.0 mM l-glutamine with 10% fetal bovine serum and 1% penicillin–streptomycin. Raw 264.7 cells were grown at 37 °C and 5% CO2 in fully humidified condition. Cell passage numbers used for this experiment were between passage 15 and 25.
Cytotoxicity assay
Mitochondrial reduction of MTT to formazan was determined as an indicator of cell viability. Briefly, after removing the supernatant for measuring nitrite accumulation, the cells were incubated with MTT (0.5 mg/mL) for 1 h at 37 °C and solubilized in DMSO. The extent of formazan production was determined at 560 nm.
Measurement of nitrite accumulation by Griess assay
RAW 264.7 cells were cultured in 96-well plates (Corning, NY, USA). Cells were treated with bacterial LPS (E. coli serotype 055:B5, 1 μg/mL) in the presence or absence of various concentrations of CLA and LA (0–200 μM) for 18 h (Cheng et al., 2004). CLA and LA were firstly dissolved in ethyl alcohol (EtOH) and further diluted with the medium. Final EtOH concentrations on the cells were ≤ 0.1 vol% and were shown not to interfere with the assay. After 20 h, the concentration of nitrite, a stable metabolite of nitric oxide, was measured in the culture supernatant by the Griess assay. Griess reagent (Sigma-Aldrich, St. Louis, MO, USA) was mixed with an equal volume of sample in 96 wells, then read the absorbance at 540 nm after 15 min.
Enzyme-linked immunosorbent assay (ELISA)
Supernatants were collected from the wells from which total RNA was harvested immediately. Cells were stimulated with bacterial LPS for 24 h, both with and without a 1 h pretreatment of CLA and LA. Control wells were treated with the same volume of the EtOH vehicle used to dilute these compounds. Supernatants were obtained by centrifugation (5 min, 12,902 g at 4 °C), aliquoted and immediately stored at − 70 °C until used. These samples were thawed once and used in ELISA assays to quantify the secreted cytokine. The assays were performed according to the manufacturer’s instructions (Komabiotech, Seoul, Korea) and the target protein concentration was normalized to the total protein content determined by the Pierce BCA protein assay kit (Thermo Fisher Scientific, San Jose, CA, USA). Pro-inflammatory cytokine level was measured using TNF-α/IL-1β and IL-6/IL-12 combo ELISA kit (Komabiotech, Seoul, Korea). All groups were measured with TECAN infinite pro-2000 at 450 nm absorbance.
Quantification of mRNA expression
A total of 3 × 105 cells were grown for 24 h in each well of six-well plates, de-induced by incubation for 6 h in culture medium without fetal bovine serum and stimulated with LPS (1 μg/mL) for 24 h, both with and without a 1 h pretreatment of CLA and LA. Control wells were treated with the same volume of the EtOH used to dilute these compounds. Total RNA was isolated from the cells with Trizol reagent (Invitrogen Corp., Carlsbad, CA, USA) according to the manufacturer’s instructions. The RNA was quantified by spectrophotometry, and 1 μg RNA was reverse-transcribed into cDNA. The relative abundance of the transcripts of the candidate inflammatory genes described in supplement 1, were determined by quantitative real-time reverse transcription-PCR (qRT-PCR) with SYBR green (Thermo Fisher Scientific, San Jose, CA, USA).
Western blot analysis
Cell culture for Western blot analysis was also progressed with the same pattern as previously described in the quantification of mRNA expression. Lysates were harvested by scraping the cells in sodium dodecyl sulfate sample buffer on ice, followed by heat-denaturation at 95 °C for 5 min. For the preparation of nuclear and cytoplasmic extracts, the cells were washed and scraped into PBS. Cells were lysed with RIPA buffer following the presented protocol, and total protein was quantified by Pierce BCA assay. For Western blotting, 20 μg of total protein were separated on polyacrylamide gels at 110 V for 50 min. Subsequently, the sample was transferred to nitrocellulose membranes for another 60 min. The membranes were blocked with 5% skim milk for 1 h at room temperature. The blocked membrane was probed with the primary antibodies overnight at 4 °C. The primary antibodies were detected using horseradish peroxidase-conjugated secondary antibodies. Western blot imaging was performed with GelDoc XT Bio-Rad and quantified by the image analysis software.
Solubility test
The solubility in this study was defined as the amount of LA that is soluble in CLA solvent to achieve a saturated solution at 25 °C and 1 atm. The oversaturated amount of LA was added to CLA, and then vortexed for 15 min to dissolve LA at the saturated level. The solution was filtered by 25 mm hydrophilic nylon net filter with a 30 μm pore size and weighed the remaining LA to substrate from the initial weight of LA. The final concentration of LA in CLA was expressed in g/L.
T1 analysis of 1H NMR
For 1H NMR analysis, 5 mg CLA and LA were dissolved in 0.7 ml of CDCl3 in a 5 mm NMR tube. All spectra were recorded at 25 ± 0.1 °C under temperature control, using an Avance III HD 850 MHz nuclear magnetic resonance spectrometer (Bruker, Rheinstetten, Germany). Chemical shift values were reported in ppm by referencing them to TMS used as the internal standard. Longitudinal relaxation, T1, was measured using a conventional inversion recovery pulse program. T1 values were estimated with Bruker Dynamics center 2.4.9 in Topspin 3.5 software.
Statistical analysis
All experiments were performed at least three times in independent experiments. Data were expressed ± SEM. Values with P ≤ 0.05 were considered statistically different compared to 100% (LPS-treated cells) by one sample or student’s t test. Statistical analysis was performed with Graph Pad Prism (version 5.00).
Results and discussion
Optimization of the treatment concentration of CLA and LA with factorial design
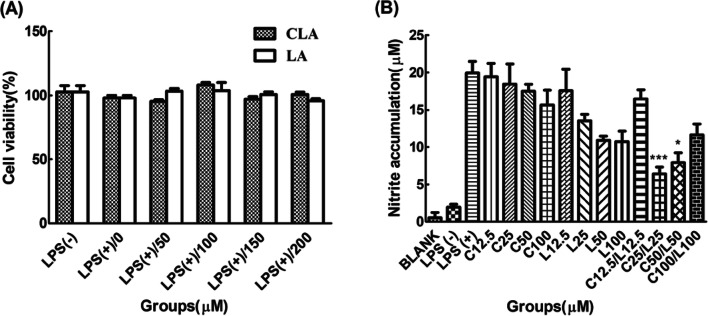
Experiments related to cell growth and toxicity are the most necessary experiments to obtain stable and uniform result values. Most fatty acid compounds have cytotoxicity due to their hydrophobic property and damages in the cellular membrane (Ibarguren et al., 2014). The treatment of CLA and LA in the presence of LPS did not affect cytotoxicity up to 200 μM of each compound as shown in Fig. 1A and 1B although each compound was found to be cytotoxic above 250 μM or more. Based on the results, we decided that the proper maximum treatment range of each compound was 250 μM, suggesting that the subsequent fatty acid treatment did not affect the results of the experiment.
Fig. 1.
A Cytotoxicity of CLA and LA with LPS. B Optimization of concentration by factorial design with nitrite accumulation. Cell viability was determined by mitochondrial reduction of MTT test as described in ‘Materials and methods’. The viability of cells treated with LPS only was referred to as 100%. N = 5. ***P < 0.001 and *P < 0.05 represent significant differences compared to the values seen in LPS-activated cells
Then, the factorial design was progressed with measuring nitrite accumulation because of an indicator of anti-inflammation and simplicity of experiment. Total groups were divided into three experimental groups as low, medium and high concentration. There are numerical ten folds difference among each concentration group. Efficient and optimum concentration was determined at medium-range of both compounds. So, both medium-range concentration, such as 25 μM and 50 μM were fixed as final concentration candidates.
Effects of combined CLA and LA on pro-inflammatory activity by ELISA assay
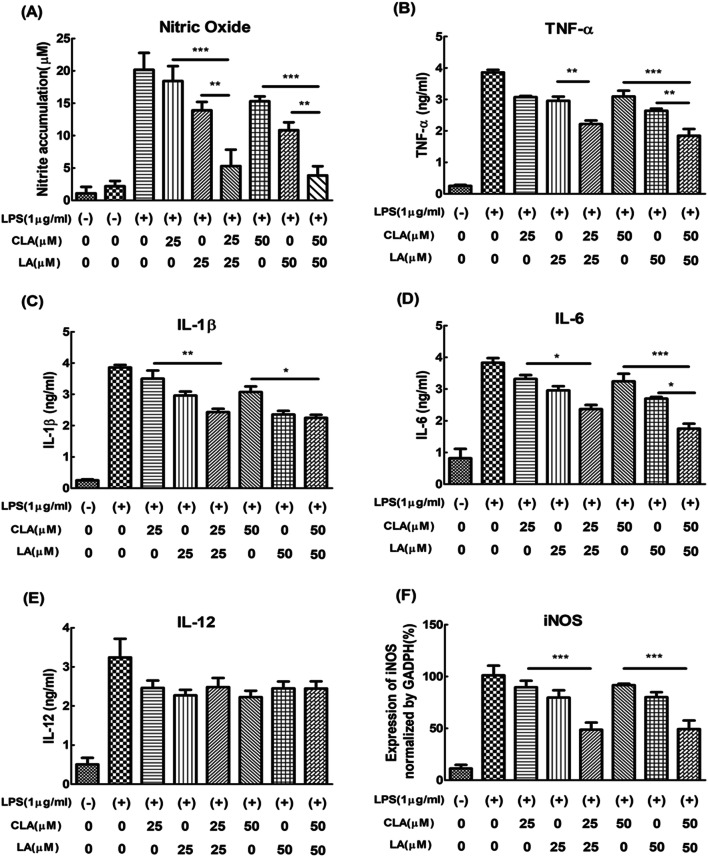
Nitric oxide (NO), as a representative index of inflammation, was confirmed by Griess assay. The optimum concentration of fatty acid treatment was also determined by the factorial design of nitrite accumulation (Chen and Kitts, 2008). When CLA 25 uM and LA 25 uM were combined, it was found to be a more effective decrease of nitrite accumulation than 25 μM of CLA and 25 μM of LA alone as well as 50 μM of CLA and 50 μM of LA alone (Fig. 2A). Optimal concentrations of CLA and LA were determined as 25 and 50 μM, respectively, and the best effect of the decreasing nitrite accumulation was obtained when the ratio of CLA to LA was from 1:1 to 2:1.
Fig. 2.
A Nitrite accumulation, B TNF-α, C IL-1β, D IL-6, and E IL-12. F mRNA gene epxression level of iNOS. Nitrite accumulation in the supernatant was measured 20 h after LPS stimulation by the Griess reaction. ELISA assay was measured 24 h after LPS stimulation by cytokine combo ELISA kit as described in ‘Methods’. Data were expressed as 100% of LPS-treated cells and represent the mean ± SEM of three experiments performed in triplicate. N = 3. ***P < 0.001, **P < 0.01 and *P < 0.05 represent significant differences compared to the values seen in LPS-activated cells
Additionally, we confirmed the gene expression of inducible nitric oxide synthase (iNOS), the source of NO, and it was found that the expression of the iNOS was inhibited. Furthermore, pro-inflammatory cytokines such as TNF-α (Fig. 2B), IL-1β (Fig. 2C), and IL-6 (Fig. 2D) were significantly decreased by co-treatment of 25 and 50 μM of CLA and LA compared to the same concentration of single treatment of CLA and LA, indicating additive effect of anti-inflammatory activity although IL-12 as the pro-inflammatory cytokine was not much changed by co-treatment (Fig. 2E).
When the inflammatory reaction proceeds under LPS induction, resting macrophages are activated in the form of M1 macrophages (Martinez and Gordon, 2014). The M1 macrophages produce TNF-α, IL-1β, IL-6, IL-12 and NO through NF-κB and activator protein 1 (AP-1) signaling transcription factors (Chen et al., 2007). TNF-α, IL-1β, IL-6, and IL-12 transmit signals to various type of cells to boost the immune system, while NO is produced from arginine with the help of iNOS, as a type II NO enzyme. The TNF-α primarily produced by macrophages has some actions on various organ systems. Also, the TNF-α functions together with IL-1β and IL-6. It was already reported that IL-1β and IL-6 showed the similar repression tendency with TNF-α whereas the IL-12 known as an important factor for priming Th1 cells and stimulating NK cells was not directly related to the production of TNF-α (Zwirner and Ziblat, 2017). Therefore, it can be speculated that the co-treatment of CLA and LA does not affect the activation of NK cells.
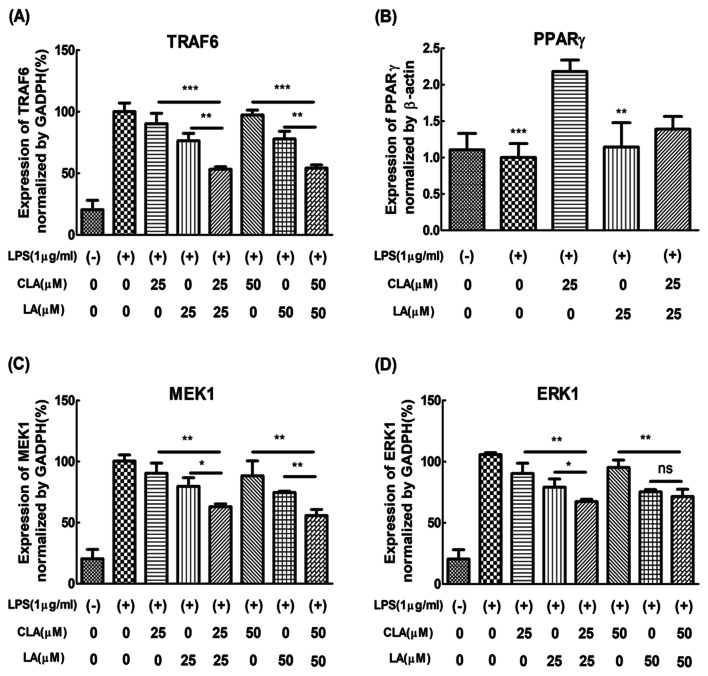
Effects of combined CLA and LA on anti-inflammatory activity by qRT-PCR
When the combined CLA and LA were treated, the inhibitory effect of the inflammation-related indicators was higher than that of each single treatment group. To clarify more the additive anti-inflammatory activity by the combination of CLA and LA, the mRNA expression level was confirmed by qRT-PCR. As shown in Fig. 3A, expression of TNF receptor associated factor 6 (TRAF6) by co-treatment of 25 μM of CLA and LA and 50 μM of CLA and LA was lower than a single treatment of 25 μM or 50 μM of CLA and LA, indicating the additive anti-inflammatory activity of CLA and LA. However, the expression of PPARγ was not affected by the co-treatment of CLA and LA (Fig. 3B), because CLA itself increased the expression of PPARγ. Also, the expression of MEK and ERK were also checked (Fig. 3C, D). The expression of IL-6 and TRAF6 by co-treatment of 25 or 50 μM of CLA and LA was lower than by a single treatment of 25 or 50 μM of CLA and LA.
Fig. 3.
A TRAF6, B PPARγ, C MEK1 and D ERK1. Expression level was measured 24 h after LPS stimulation with or without CLA & LA by the SYBR green qRT-PCR as described in ‘Methods’. For the statistical analysis, one-way ANOVA and Tukey’s HSD test were carried out. Data were expressed as a percentage of relative expression in LPS-treated groups and represent the mean ± SEM of three experiments performed in triplicate. N = 3. ***P < 0.001, **P < 0.01 and *P < 0.05 represent significant differences compared to the values seen in LPS-activated cells
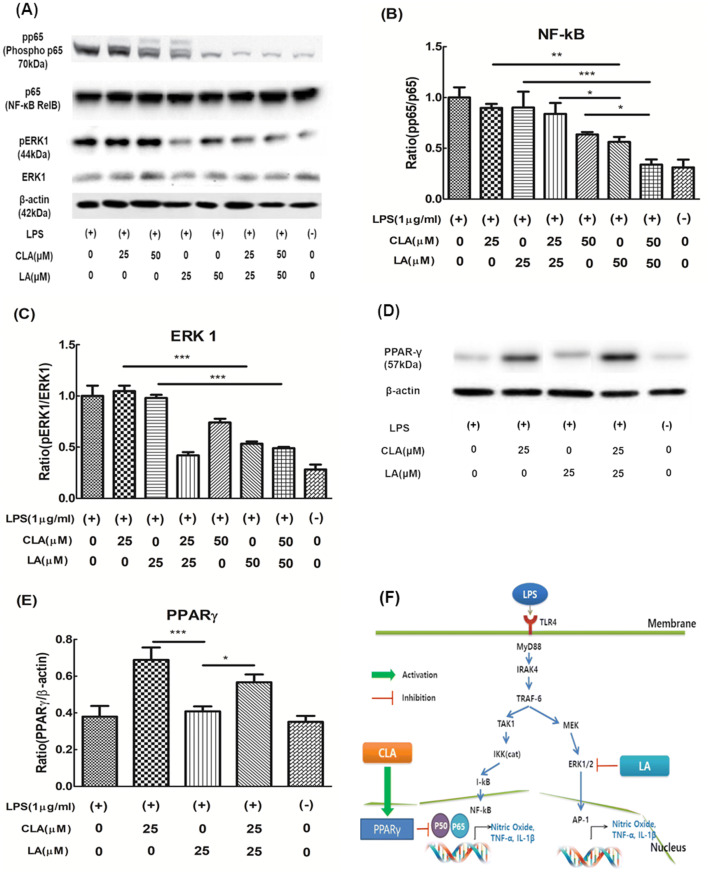
To understand the criteria for selecting these gene expressions, it must be taken into consideration the mechanism of anti-inflammation. There are two well-known signaling pathways that control the inflammation activity genes such as NF-κB (Bouwmeester et al., 2004) and AP-1(Wang et al., 2013a). The inhibition of the expression level of NF-κB by the co-treatment of CLA and LA may be attributed to two main reasons. First, The TNF receptor- associated factor 6 (TRAF-6) and mitogen-activated protein kinase kinase (MEK1) as the upper signaling pathway of NF-κB and AP-1 were downregulated by the co-treatment of CLA and LA. Second, a SUMOylation-dependent pathway mediated transrepression of inflammatory response genes by PPAR gamma (Pascual et al., 2005). The activated PPAR gamma-induced SUMOylation of NF-κB transcription factor downregulated NF-κB expression. Another master regulatory gene of inflammation of AP-1 may be regulated by MAPK signaling pathway (Karin, 1995). Especially, extracellular signal-regulated kinase 1 (ERK1) signaling may be highly related to the anti-inflammatory effect of CLA and LA (Maeng et al., 2006). Both mechanisms may induce the suppression of NO and pro-inflammatory cytokines. Schematic additive mechanism of anti-inflammation by co-treatment of CLA and LA in Raw 264.7 cells was shown in Fig. 4F.
Fig. 4.
A Western blot analysis of pp65/p65 and pERK/ERK, B and C Imaging analysis data for quantification of pp65/p65 and pERK/ERK, D Western blot analysis of PPARγ/β-actin, E Imaging analysis data for quantification of PPARγ/β-actin. Data were expressed as a percentage of only LPS-treated cells (100%) and represent the mean ± SEM of three experiments performed in triplicate. N = 3. ***P < 0.001, **P < 0.01 and *P < 0.05 represent significant differences compared to the values seen in LPS activated cells. F Schematic additive effect mechanism of anti-inflammation by co-treatment of CLA and LA in Raw 264.7 cells
The effect of CLA and LA on the inflammatory signaling pathway
To make clear the effect of CLA and LA on anti-inflammatory signaling pathways, NF-κB, ERK1 and PPARγ were analyzed at the protein level by Western blot analysis. The ratio of pp65 to p65 (the subunit of NF-κB) was smaller in co-treatment of CLA and LA than a single treatment of CLA and LA as shown in Fig. 4A, B. Also, the ratio of pERK1/ERK1 was lower in the co-treatment of CLA and LA than a single treatment of CLA and LA, indicating that co-treatment of CLA and LA additively suppressed the ERK1 signal although the LA inhibited more ERK1 signal than the CLA (Fig. 4A, C). The expression of NF-κB and pERK1 was found to be additive by the co-treatment group.
On the other hand, in the case of PPARγ, both CLA and LA were expected to show an increasing pattern because both substances as the fatty acid family shared similar receptors such as free fatty acid receptor 1 (Wang et al., 2013a; 2013b). However, only the CLA group increased the expression of PPARγ (Fig. 4D, E), which is different results from the previous report that LA increased the expression of PPARγ (Hara et al., 2013). It is believed that there may be an interference with each other by their molecular interaction between CLA and LA.
Molecular interaction between CLA and LA
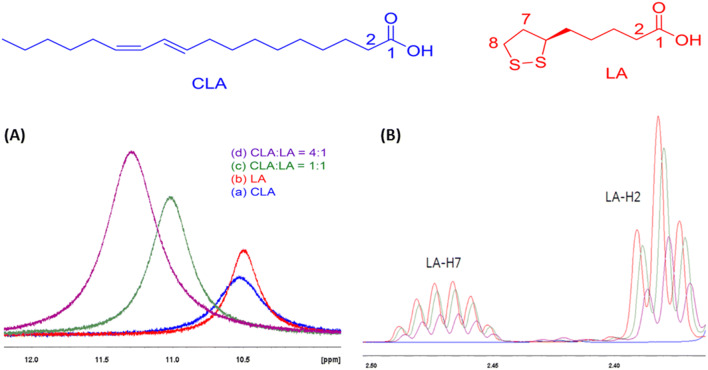
The molecular interaction of CLA and LA is one of the important clues to explain the mechanism of anti-inflammatory additive effect because co-treatment of them were effective in improving anti-inflammation. The solubility test of LA in CLA as a solvent was carried out before performing the 1H NMR experiment. By saturating LA at 53.56 g/L (0.26 mol/L) in CLA, the mole ratio of LA and CLA in the solution of LA/CLA was determined as 4 to 1. The molecular interactions of CLA and LA were confirmed through the change of chemical shift and T1 relaxation time by NMR measurement. The conditions of 1H NMR and chemical structure of LA and CLA are shown in Fig. 5. The corresponding peak shifts of 1H NMR are summarized in the Table 1. With the addition of LA to CLA, the peak shifts of LA were observed. The LA carboxylic acid peak at 10.49 ppm was shifted to 11.02 ppm (CLA:LA = 1:1) and 11.29 ppm (CLA:LA = 4:1) of higher frequency (deshielding). The T1 relaxation time of LA carboxylic acid peak was also decreased from 4.05 (LA) to 3.69 s (CLA:LA = 1:1) and 3.33 s (CLA:LA = 4:1). The changes of chemical shift and T1 relaxation time of carboxylic acid peaks indicate the interaction of LA and CLA with each other through hydrogen bonds. In addition, a shielding of chemical shift and decreasing of T1 relaxation time were found a little bit in the protons of LA 2-H and 3-H near the acid. A shielding of chemical shift and decreasing of T1 relaxation time were also slightly observed in LA 7-H and 8-H of sulfur ring, suggesting the minor interaction of hydrophobic aliphatic long chains of two materials. Also, 1H NMR experiments were performed by changing the temperature conditions (5 °C, 20 °C and 35 °C) to find the dominant interaction groups. The chemical shift change (1.16 ppm) of LA carboxylic acid peak in CLA at 35 °C is much more deshielding than the chemical shift change (0.73 ppm) of LA carboxylic acid peak at 5 °C (data not shown). On the other hands, the chemical shift change of the hydrophobic long chain and sulfur ring did not change significantly at various temperatures. Also, the predominant interaction between CLA and LA suggests hydrogen bonding of the acid as the result of chemical shift and T1 relaxation variation of 1H NMR (Nanny and Maza, 2001). The temperature-dependent T1 NMR results are very useful indicators of the dominance of the chemical reactor (Bottomley et al., 1984). Thus, these results suggested that strong hydrogen and hydrophobic interaction between the two substances influenced the anti-inflammatory activity in co-treatment group. The corresponding peak shifts of 1H NMR and T1 relaxation time are summarized in the supplementary materials from number 3 to 5.
Fig. 5.
1H NMR (CDCl3, 25 °C) of (a) 5 mM of CLA, (b) 5 mM of LA, (c) 5 mM of CLA and 5 mM of LA (CLA:LA = 1:1, mole ratio) and (d) 20 mM of CLA and 5 mM of LA (CLA:LA = 4:1, mole ratio). A1H NMR chemical shift change of acid peaks, and B1H NMR chemical shift change of LA
Table 1.
1H NMR chemical shift (CS) and T1 relaxation time at 25 °C
| # of carbon | LA | CLA:LA = 1:1 (mole ratio) | CLA:LA = 4:1 (mole ratio) | |||
|---|---|---|---|---|---|---|
| CS (ppm) | T1 (s) | CS (ppm) | T1 (s) | CS (ppm) | T1 (s) | |
| Acid-OH | 10.4958 | 4.05 | 11.0156 | 3.69 | 11.2877 | 3.33 |
| 1 | – | – | – | – | – | – |
| 2 | 2.3819 | 1.58 | 2.3796 | 1.51 | 2.3775 | 1.46 |
| 3 | 1.7108 | 1.45 | 1.7096 | 1.40 | 1.7084 | 1.33 |
| 4 |
1.5185 1.4811 |
1.52 1.46 |
1.5172 1.4796 |
1.42 1.38 |
1.5161 1.4787 |
1.32 1.28 |
| 5 | 1.6777 | 1.45 | 1.6759 | 1.40 | 1.6744 | 1.33 |
| 6 | 3.5773 | 2.96 | 3.5764 | 2.95 | 3.5752 | 2.82 |
| 7 |
2.4702 1.9171 |
2.50 2.35 |
2.4692 1.9136 |
2.43 2.32 |
2.4679 1.9152 |
2.35 2.20 |
| 8 |
3.1883 3.1218 |
3.60 3.68 |
3.1874 3.1208 |
3.61 3.65 |
3.1861 3.1196 |
3.48 3.65 |
In this study, Our study group confirmed that the two fatty acids resolved the inflammation and showed the additive effect of the anti-inflammation when the two substances were treated together. We analyzed the expression of pro- and anti-inflammatory cytokines and searched the molecular mechanism. The combination of CLA and LA could reduce inflammatory state by reducing the expression level of NF-κB and the PPARγ. Also, it was explored that the molecular interaction of hydrogen bonding and hydrophobic bonding between CLA and LA influenced the additive effect of the anti-inflammation. As far as we know, this is the first report to demonstrate the additive effect of the anti-inflammation by the combination of CLA and LA through the molecular interaction. Also, the combination of anti-inflammatory additive effects of both fatty acids is expected to help to develop functional health foods and feed additives in the future.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) and Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (115084-2). Ki-June Lee was supported by Brain Korea 21 Plus program.
Abbreviations
- AP-1
Activator protein 1
- BCA
Bicinchoninic acid
- CDCl3
Deuterated chloroform
- CLA
Conjugaeted linoleic acid
- DMEM
Dulbecco’s modified Eagle’s Media
- DMSO
Dimethyl sulfoxide
- ELISA
Enzyme linked Immunosorbent Assay
- ERK
Extracellular signal-regulated kinase
- EtOH
Ethanol
- IL
Interleukin
- iNOS
inducible nitric oxide synthase
- LA
Lipoic acid
- LPS
Lipopolysaccharide
- MAPK
Mitogen-activated protein kinase
- MEK
Mitogen-activated protein kinase kinase
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NMR
Nuclear magnetic resonance
- NO
Nitric oxide
- NF-κB
Nuclear factor kappa-light chain-enhancer of activated B cells
- PBS
Phosphate buffered saline
- PPARγ
Peroxisome proliferator-activated receptor gamma
- qRT-PCR
Quantitative real-time polymerase chain reaction
- RIPA
Radioimmunoprecipitation assay
- SEM
Standard error of the mean
- SUMO
Small ubiquitin-like modifier
- TMS
Tetramethylsilane
- TNF-α
Tumor necrosis factor alpha
- TRAF
Tumor necrosis factor receptor-associated factor
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ki-June Lee, Email: zenith09@snu.ac.kr.
Yoon-Joo Ko, Email: yjko@snu.ac.kr.
Sang-Kee Kang, Email: kangsk01@snu.ac.kr.
Whee-Soo Kim, Email: gnltngnltn@snu.ac.kr.
Chong-Su Cho, Email: chocs@snu.ac.kr.
Yun-Jaie Choi, Email: cyjcow@snu.ac.kr.
References
- Baschant U, Tuckermann J. The role of the glucocorticoid receptor in inflammation and immunity. J. Steroid Biochem. Mol. Biol. 2010;120:69–75. doi: 10.1016/j.jsbmb.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Bottomley PA, Foster TH, Argersinger RE, Pfeifer LM. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1 to 100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 1984;11:425–448. doi: 10.1118/1.595535. [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G, Drewes G, Gavin AC, Jackson DB, Joberty G, Neubauer G, Rick J, Kuster B, Superti-Furga G. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat. Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- Chen CY, Peng WH, Tsai KD, Hsu SL. Luteolin suppresses inflammation-associated gene expression by blocking NF-kappaB and AP-1 activation pathway in mouse alveolar macrophages. Life Sci. 2007;81:1602–1614. doi: 10.1016/j.lfs.2007.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen XM, Kitts DD. Determining conditions for nitric oxide synthesis in Caco-2 cells using Taguchi and factorial experimental designs. Anal. Biochem. 2008;381:185–192. doi: 10.1016/j.ab.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Cheng WL, Lii CK, Chen HW, Lin HW, Liu KL. Contribution of conjugated linoleic acid to the suppression of inflammatory responses through the regulation of the NF-kappaB pathway. J. Agric. Food Chem. 2004;52:71–78. doi: 10.1021/jf0348626. [DOI] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- Deeks SG. HIV infection, inflammation, immunosenescence, and aging. Annu. Rev. Med. 2011;62:141–155. doi: 10.1146/annurev-med-042909-093756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarco VG, Scumpia PO, Bosanquet JP, Skimming JW. Alpha-lipoic acid inhibits endotoxin-stimulated expression of iNOS and nitric oxide independent of the heat shock response in RAW 264.7 cells. Free Radic. Res. 2004;38:675–682. doi: 10.1080/10715760410001702503. [DOI] [PubMed] [Google Scholar]
- Hara T, Kimura I, Inoue D, Ichimura A, Hirasawa A. Free fatty acid receptors and their role in regulation of energy metabolism. Rev. Physiol. Biochem. Pharmacol. 2013;164:77–116. doi: 10.1007/112_2013_13. [DOI] [PubMed] [Google Scholar]
- Ibarguren M, Lopez DJ, Escriba PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim. Biophys. Acta. 2014;1838:1518–1528. doi: 10.1016/j.bbamem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- Maeng YS, Min JK, Kim JH, Yamagishi A, Mochizuki N, Kwon JY, Park YW, Kim YM, Kwon YG. ERK is an anti-inflammatory signal that suppresses expression of NF-kappaB-dependent inflammatory genes by inhibiting IKK activity in endothelial cells. Cell. Signal. 2006;18:994–1005. doi: 10.1016/j.cellsig.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura FA, de Andrade KQ, dos Santos JC, Goulart MO. Lipoic Acid: its antioxidant and anti-inflammatory role and clinical applications. Curr. Top Med. Chem. 2015;15:458–483. doi: 10.2174/1568026615666150114161358. [DOI] [PubMed] [Google Scholar]
- Nanny MA, Maza JP. Noncovalent interactions between monoaromatic compounds and dissolved humic acids: a deuterium NMR T1 relaxation study. Environ. Sci. Technol. 2001;35:379–384. doi: 10.1021/es0012927. [DOI] [PubMed] [Google Scholar]
- O’Quinn PR, Nelssen JL, Goodband RD, Tokach MD. Conjugated linoleic acid. Anim. Health Res. Rev. 2000;1:35–46. doi: 10.1017/S1466252300000049. [DOI] [PubMed] [Google Scholar]
- Ochoa JJ, Farquharson AJ, Grant I, Moffat LE, Heys SD, Wahle KW. Conjugated linoleic acids (CLAs) decrease prostate cancer cell proliferation: different molecular mechanisms for cis-9, trans-11 and trans-10, cis-12 isomers. Carcinogenesis. 2004;25:1185–1191. doi: 10.1093/carcin/bgh116. [DOI] [PubMed] [Google Scholar]
- Onakpoya IJ, Posadzki PP, Watson LK, Davies LA, Ernst E. The efficacy of long-term conjugated linoleic acid (CLA) supplementation on body composition in overweight and obese individuals: a systematic review and meta-analysis of randomized clinical trials. Eur. J. Nutr. 2012;51:127–134. doi: 10.1007/s00394-011-0253-9. [DOI] [PubMed] [Google Scholar]
- Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, d’Hellencourt CL. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflamm. 2016;13:67. doi: 10.1186/s12974-016-0530-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-gamma. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay KP, Moreau RF, Smith EJ, Smith AR, Hagen TM. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta. 2009;1790:1149–1160. doi: 10.1016/j.bbagen.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowska E, Dolegowska B, Dziedziejko V, Rybicka M, Kaczmarczyk M, Bober J, Rac M, Machalinski B, Chlubek D. Prostaglandin E2 (PGE2) and thromboxane A2 (TXA2) synthesis is regulated by conjugated linoleic acids (CLA) in human macrophages. J. Physiol. Pharmacol. 2009;60:77–85. [PubMed] [Google Scholar]
- Suzuki YJ, Aggarwal BB, Packer L. Alpha-lipoic acid is a potent inhibitor of NF-kappa B activation in human T cells. Biochem. Biophys. Res. Commun. 1992;189:1709–1715. doi: 10.1016/0006-291X(92)90275-P. [DOI] [PubMed] [Google Scholar]
- Teichert J, Hermann R, Ruus P, Preiss R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J. Clin. Pharmacol. 2003;43:1257–1267. doi: 10.1177/0091270003258654. [DOI] [PubMed] [Google Scholar]
- Vane J, Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987;1:89–96. doi: 10.1096/fasebj.1.2.3111928. [DOI] [PubMed] [Google Scholar]
- Wang A, Al-Kuhlani M, Johnston SC, Ojcius DM, Chou J, Dean D. Transcription factor complex AP-1 mediates inflammation initiated by Chlamydia pneumoniae infection. Cell. Microbiol. 2013;15:779–794. doi: 10.1111/cmi.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KC, Tsai CP, Lee CL, Chen SY, Lin GJ, Yen MH, Sytwu HK, Chen SJ. alpha-Lipoic acid enhances endogenous peroxisome-proliferator-activated receptor-gamma to ameliorate experimental autoimmune encephalomyelitis in mice. Clin. Sci. (Lond) 2013;125:329–340. doi: 10.1042/CS20120560. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Frei B. Alpha-lipoic acid inhibits TNF-alpha-induced NF-kappaB activation and adhesion molecule expression in human aortic endothelial cells. FASEB J. 2001;15:2423–2432. doi: 10.1096/fj.01-0260com. [DOI] [PubMed] [Google Scholar]
- Zwirner NW, Ziblat A. Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol. 2017;8:25. doi: 10.3389/fimmu.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.