Abstract
In the present study, variety of fruit vinegars were investigated in terms of their physicochemical, microbiological and bioactive properties. Total phenolic and flavonoid contents were in the range of 933–1162 mg GAE/L and 66.64–470.86 mg/L in terms of catechin equivalents, respectively. During the evaluation of antioxidant activity via DPPH and ABTS assay, samples showed the activity as in the range of 0.047–0.302 and 0.413–0.885 µg TE/mL, respectively. The counts of AAB, LAB and yeast-mold were found in the range of < 2–6.32, < 1–5.39 and < 1–3.97 log CFU/mL, respectively. Antimicrobial activity of vinegars was tested against nine bacteria by broth microdilution assay. Most of the samples were found inhibitive against test cultures at concentrations between 3.12 and 6.25% (Minimum Inhibitory Concentration, v/v), while apple vinegar was inhibitive at higher concentrations. These results indicated the high potential of fruit vinegars as antioxidant and antimicrobial agents that could be used as functional food ingredients.
Keywords: Vinegar, Phenolic, Flavonoid, Antioxidant, Antimicrobial
Introduction
Vinegar is a special kind of condiment produced from a variety of raw materials containing fermentable carbohydrates through the activity of yeasts and acetic acid bacteria. During vinegar fermentation, acetic acid bacteria mainly produce acetic acid as the basic sensorial characteristic of vinegar (Karabiyikli and Sengun, 2017). It has long been used not only as preserving and flavoring agent, but also used in traditional and natural folk medicine for treating a variety of diseases.
Bioactive compounds found in foods show antioxidative effects, which lead to minimize the occurrences of degenerative illnesses (Pandey and Rizvi, 2009). Vinegars, produced from numerous fruits rich in amino acids, organic acids, phenolics, vitamins and mineral substances, have bioactive potential and indicate antioxidant and antimicrobial properties in various levels depending on raw material and production methods used (Bakir et al., 2017; Karabiyikli and Sengun, 2017).
Acetic acid, as the predominant organic acid of vinegar cause bacterial cell death by diffusing through the cell membranes of microorganisms (Booth and Kroll, 1989). Several researchers demonstrated that the vinegars effectively inhibit the growth of microorganisms including Aeromonas hydrophila, B. cereus, E. coli O157:H7, Salmonella Enteritidis, S. Typhimurium, Staphylococcus albus, S. aureus, Vibrio parahaemolyticus, Micrococcus catarrhalis and Diplococcus pneumonia, therefore it could be used for disinfection of a variety of equipment, foods and food preparation surfaces. Furthermore, pathogens were also successfully eliminated from vegetables by vinegar rinsing or soaking (Chang and Fang, 2007; Hindi, 2013; Ramos et al., 2014; Sengun and Karapinar, 2004).
Therefore, the objectives of this study were (1) to investigate the physicochemical and bioactive properties of traditionally produced fruit vinegars, (2) to investigate the antimicrobial properties of vinegars against foodborne microorganisms by determining minimum inhibition and bactericidal concentrations.
Materials and methods
Analyze samples
Fruit vinegars, which are produced without pasteurization and additives, and sold in Turkey under the name as “organic vinegar” were purchased from local markets. Bottles of apple, plum, mandarin grape, blackberry, apricot, persimmon, pomegranate, fig and rosehip vinegars were stored in the laboratory at 20 °C prior to analysis.
Physicochemical properties of vinegar samples
The pH value of each vinegar sample was measured using a pH meter (NEL Mod 821). Titrimetric method was used for detecting total acidity of the vinegars (AOAC, 2007). Brix values of vinegars were measured by using refractometer (Hanna HI 96801) calibrated with distilled water (AOAC, 2007).
Bioactive properties of vinegar samples
Total phenolic contents of the samples were determined using the Folin-Ciocalteu colorimetric method (Cemeroglu, 2013). 75 mL of distilled water was mixed with 1 mL filtered vinegar sample (0.2 μm, Sartorius Stedim) and 5 mL of Folin-Ciocalteu’s phenol reagent (10%). Then 10 mL saturated Na2CO3 (75 g/L) was added into the mixture. The final mixture was completed to 100 mL with distilled water and incubated in the dark at room temperature for 90 min. After the incubation period, the mixture absorbance was measured at 720 nm using a spectrophotometer (Agilent Technologies, Carry60 UV–Visible). Total phenolic contents of samples were expressed as mg gallic acid equivalents (GAE)/L.
Total flavonoid contents of vinegar samples were determined according to the method described by Zhishen et al. (1999). One mL of filtered vinegar (0.2 μm, Sartorius Stedim) was mixed with 4 mL of distilled water, 0.3 mL of sodium nitrite (5%, Merck), 0.3 mL of aluminum chloride (10%, Merck) and 2 mL of sodium hydroxide (1 M, Merck). The final mixture was completed to 10 mL with distilled water and the mixture absorbance was measured at 510 nm using a spectrophotometer (Agilent Technologies, Carry60 UV–Visible). Total flavonoid contents of samples were expressed as catechin equivalents (mg catechin/L).
The total antioxidant capacities of vinegar samples were determined by DPPH and ABTS radical scavenging assays.
DPPH Assay DPPH radical scavenging capacity of the vinegars was determined according to the modified protocol described by Cemeroglu (2013). The different volume of filtered vinegar (0.2 μm, Sartorius Stedim) (20–100 µL) was mixed with 300 µL of 0.1 mM 2,2-Diphenyl-1-picrylhydrazyl (DPPH, Sigma-Aldrich). The final mixture was completed to 3 mL with methanol and stored in the dark at room temperature for 15 min. Then the mixture absorbance was measured at 517 nm using a spectrophotometer (Agilent Technologies, Carry60 UV–Visible). The percentage inhibition (Abs, %) was described by the Equation given below:
where Ac is absorbance of control (methanol), As is absorbance of sample. The antioxidant activity of vinegars was expressed as µg Trolox equivalents/mL (µg TE/mL).
ABTS Assay Antioxidant activity of the vinegars was also determined by ABTS assay (Re et al., 1999). Briefly, to obtain ABTS radical cation (ABTS+) solution, 10 mg of 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS, Sigma-Aldrich) dissolved in 2.57 mL of distilled water and 37.5 mg of potassium persulfate (Sigma-Aldrich) dissolved in 1 mL of distilled water was mixed and stored in the dark at room temperature for 12–16 h. Then 1 mL of ABTS+ solution was diluted with approximately 88 mL of ethanol to achieve absorbance value of 0.70 ± 0.02 (initial absorbance value) at 734 nm. Furthermore, 3 ml of ABTS+ solution and 300 μL of filtered vinegar (0.2 μm, Sartorius Stedim) with different concentrations (100–500 μg/mL) were mixed and the mixture was kept in the dark at room temperature for 6 min. Then the mixture absorbance was measured at 734 nm using a spectrophotometer (Agilent Technologies, Carry60 UV–Visible). The percent inhibition of ABTS+ was calculated according to the Equation given below:
where Ac is initial absorbance value, As is final absorbance value. The antioxidant capacity of vinegars was expressed as µg of Trolox equivalents/ml (µg TE/ml).
Color properties of vinegar samples
Color values of vinegars were measured by using a HunterLab Colorflex (Management Company, USA) and the results were expressed as L* (lightness-darkness), a* (redness-greenness), and b* (yellowness-blueness) (Rommel et al., 1990).
Microbiological properties of vinegar samples
After transferring 25 mL of vinegar into 225 mL of 0.1% Peptone Water (PW, pH 6.3 ± 0.2, Oxoid, England), the sample dilutions were prepared with PW. Appropriate growth media was used for evaluating the microbial counts.
The counts of acetic acid bacteria (AAB) were determined on Glucose Yeast Extract Calcium Carbonate Agar (GYC, 10% glucose, 2% calcium carbonate, 1% yeast extract, 1.5% agar, pH 6.8 ± 0.2) by using surface plate method and the plates were incubated at 30 °C for 5–10 days (De Vero et al., 2006). Double plated Man Rogosa and Sharp Agar (MRS, pH 6.2 ± 0.2, Oxoid) was used for counting lactic acid bacteria (LAB) and the plates were incubated at 30 °C for 3–5 days (ISO 15214, 1998). Yeast and mold counts were determined on acidified (with tartaric acid-10%, Merck, Germany) Potato Dextrose Agar (PDA, pH 5.6 ± 0.2, Oxoid) by using pour plate method and the plates were incubated at 25 °C for 3–5 days (FDA-BAM 2001).
Antimicrobial properties of vinegar samples
Antimicrobial activity of vinegar samples was determined by detecting minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC).
Bacillus cereus No 8, Bacillus subtilis ATCC 6037, Enterococcus faecalis ATCC 29212, Escherichia coli ATCC 1103, Escherichia coli O157:H7 ATCC 43895, Listeria monocytogenes Scott A, Pediococcus acidilactici ATCC 8042, Salmonella Typhimurium NRRLB4420 and Staphylococcus aureus ATCC 6538P were used as test cultures for evaluating the antimicrobial activity of vinegar samples. In the study, it is mainly focused on the pathogens associated with food-borne diseases, but representative species for food spoilage (B. subtilis), hygiene indicator (E. faecalis) and fermentative microorganism (P. acidilactici) have also been included. All microorganisms were obtained from Food Microbiology Laboratory, Food Engineering Department, Ege University, Turkey. The bacterial cultures stored at − 20 °C were regenerated for several times in Mueller-Hilton Broth (MHB, pH 7.3 ± 0.2, CM405-Oxoid) at 37 °C for 18–24 h. The optimized bacterial cultures (DEN-1 McFarland Densitometer, Grant-bio), equivalent to 0.5 McFarland turbidity standard, were used in the analyses.
The MIC value of the vinegars was determined by microdilution method using standard 96-well microtiter plates, according to the modified protocol described by Deng et al. (2014). Serial two-fold dilutions of filtered vinegar samples (0.2 μm, Sartorius Stedim) prepared with MHB (a total volume of 200 μL), were dispensed into wells of the microplate. In wells of the prepared microplate, the final concentrations of the vinegar were; 50%, 25%, 12.5%, 6.25%, 3.12%, 1.56%, 0.78%, 0.39%, 0.20% and 0.10% (v/v), respectively. After dilution of the samples, 10 µL of bacterial culture was inoculated into each well. Wells containing only MHB and the test culture were used as negative and positive controls, respectively. The dilution and inoculation procedure described was repeated for each vinegar sample and test microorganism, separately. After incubating the plates at 37 °C for 18–24 h, 20 μL of 0.5% (w/v) 2,3,5-triphenyl tetrazolium chloride (TTC, Merck, 108380, Germany) aqueous solution was added into the wells and the color change of the wells were interpreted after 30 min at 37 °C. The lowest concentration of the vinegar required to inhibit visible growth of the test culture (no color formation) was selected as the MIC value.
After detecting the MIC values, samples were taken from the first wells where no growth was observed and streaked on Mueller-Hilton Agar (MHA, pH 7.3 ± 0.2, CM337-Oxoid) to determine MBC of vinegar samples. The plates incubated at 37 °C for 24 h were checked for colony formation (Tomas-Menor et al., 2013).
Statistical analysis
Three replicates and two parallels were applied during analysis. Data were examined by one-way analysis of variance (ANOVA) and the differences among the means were compared by Duncan’s Multiple Range Test using the SPSS software for Windows Version 15 at significance level of p < 0.05. All data obtained in the study were as mean value ± standard deviation (S.D.) in Figs. 1 and 2.
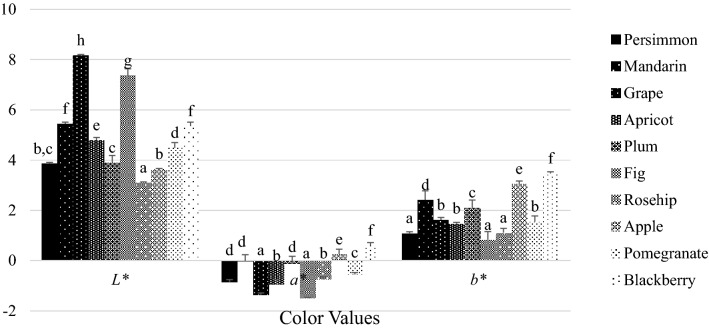
Fig. 1.
Color properties of vinegar samples (Different letters on data bars indicate a significant difference (p < 0.05). Statistical analysis was applied separately for L*, a*, b* values)
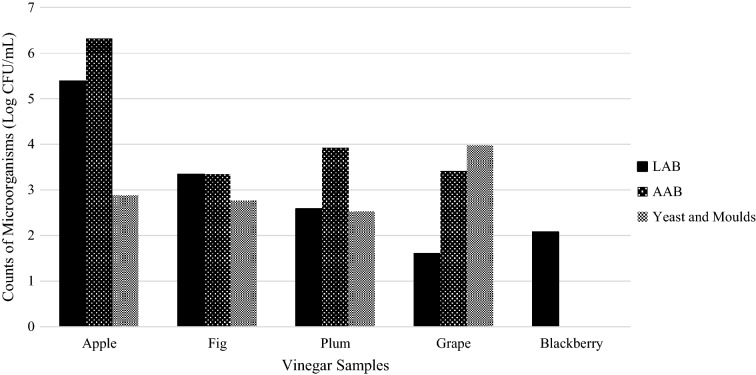
Fig. 2.
Microbiological properties of vinegar samples (the counts were found under detection limits for apricot, mandarin, persimmon, pomegranate and rosehip vinegars)
Results and discussion
The pH value and total acidity of vinegar samples ranged from 3.22 to 3.85 and 1.11 to 5.61% acetic acid (w/v), respectively (p < 0.05). According to Turkey and United States standards, the acidity of vinegars should be at least 4% acetic acid (w/v) (Anon, 2016; FDA, 1995). The results showed that acidity of vinegar samples was in conformity with the standard regulations, except apple vinegar (Table 1). In the study performed by Bakir et al. (2017), the pH and total acidity of different fruit vinegars ranged from 2.8 to 3.9 and 0.7 to 6.6% acetic acid. In another study, pH and total acidity of the traditional and industrial vinegar samples varied from 2.63 to 3.90 and 0.32 to 7.20% acetic acid, respectively, and the high majority (80%) of the traditional vinegar samples (grape, apple, artichoke, pomegranate, apple-lemon and hawthorn vinegar) was not in conformity with the standards (Ozturk et al., 2015).
Table 1.
Physicochemical properties of vinegar samples
| Vinegar samples | pH | Total acidity* | Brix |
|---|---|---|---|
| Persimmon | 3.325 ± 0.007d | 4.79 ± 0.01f | 3.00 ± 0.00e |
| Mandarin | 3.545 ± 0.007e | 5.61 ± 0.00g | 3.50 ± 0.00f |
| Grape | 3.22 ± 0.00a | 4.32 ± 0.03c | 3.00 ± 0.00e |
| Apricot | 3.33 ± 0.007d | 4.27 ± 0.01c | 2.00 ± 0.00b |
| Plum | 3.56 ± 0.007e | 4.78 ± 0.04f | 5.00 ± 0.00g |
| Fig | 3.22 ± 0.00a | 4.73 ± 0.05e,f | 3.00 ± 0.00e |
| Rosehip | 3.24 ± 0.00b | 4.68 ± 0.02e | 2.00 ± 0.00b |
| Apple | 3.85 ± 0.007f | 1.11 ± 0.02a | 1.00 ± 0.00a |
| Pomegranate | 3.30 ± 0.007c | 4.19 ± 0.02b | 2.80 ± 0.00d |
| Blackberry | 3.32 ± 0.007d | 4.56 ± 0.01d | 2.70 ± 0.00c |
*g/100 mL as acetic acid. Standard deviation of means is shown as ± SD
Values in the same column with different superscripts are significantly different (p < 0.05)
Brix values shows the percentage of soluble solid content in an aqueous sample and it is a characteristic that change with depending on the type of vinegar. In the study, brix values of the samples were ranged from 1.00 to 5.00 (Table 1). The highest brix value was found in plum vinegar while the lowest value was in apple vinegar (p < 0.05). In other studies, brix value was reported as 1.02–20.80 for fruit vinegars (Budak, 2015; Ozturk et al., 2015).
Total phenolic content, total flavonoid content and antioxidant activity of vinegar samples were determined by spectrophotometric methods and the results were shown in Table 2. Among the vinegar samples, the highest total phenolic content and total flavonoid content were found in the blackberry (1162 mg GAE/L) and plum vinegar (470.86 mg catechin/L), respectively. Mandarin vinegar had the lowest levels in terms of total phenolic (933 mg GAE/L) and flavonoid content (66.64 mg catechin/L). It was previously detected that the total phenolic and flavonoid contents in traditional vinegar samples ranged from 40.44 to 2228.79 mg GAE/L and 10.89 to 349.05 mg catechin/L, respectively (Ozturk et al., 2015). In another study, total phenolic and flavonoid contents of different fruit vinegars were determined in the range of 17–255 mg GAE/100 mL and 2.4–96 mg catechin/100 mL, respectively, while the highest values were found in balsamic vinegar and the lowest values were found in apple vinegar (Bakir et al., 2017). Various researchers reported total phenolic contents of vinegars as 0.89–1.11 mg GAE/mL for blueberry vinegar (Su and Chien, 2007), 0.80 mg GAE/mL for persimmon vinegar (Sakanaka and Ishihara, 2008), 1.61 mg GAE/mL for strawberry vinegar (Ubeda et al., 2013) and total flavonoid contents of vinegars as 0.58 mg/mL for traditional balsamic vinegar (Verzelloni et al., 2007) and 1.10 mg/mL for Zhenjiang vinegar (Qui et al., 2010). Kelebek et al. (2017) reported that the substrate selection for vinegar production is an important parameter to take into account the final phenolic content of fruit vinegars.
Table 2.
Bioactive properties of vinegar samples
| Vinegar samples | Total phenolic contents (mg GAE/L) | Total flavonoid contents (mg catechin/L) | Antioxidant activity (DPPH, µg TE/mL) | Antioxidant activity (ABTS, µg TE/mL) |
|---|---|---|---|---|
| Persimmon | 1006.5 ± 0.707c | 153.9 ± 7.306b | 0.0907 ± 0.008b | 0.783 ± 0.006f |
| Mandarin | 933 ± 1.414a | 66.64 ± 3.334a | 0.217 ± 0.007e | 0.776 ± 0.008f |
| Grape | 1025 ± 2.828d | 221.81 ± 3.435d | 0.119 ± 0.023b,c,d | 0.441 ± 0.003b |
| Apricot | 1005 ± 11.313c | 166.62 ± 3.502b,c | 0.1302 ± 0.024c,d | 0.885 ± 0.012g |
| Plum | 1057 ± 4.242f | 470.86 ± 3.637g | 0.302 ± 0.006f | 0.538 ± 0.003d |
| Fig | 935.5 ± 6.363a | 178.45 ± 7.104c | 0.047 ± 0.003a | 0.595 ± 0.007e |
| Rosehip | 1103.5 ± 6.363g | 234.21 ± 9.80d | 0.111 ± 0.003b,c | 0.547 ± 0.005d |
| Apple | 988 ± 2.828b | 174.79 ± 3.401c | 0.147 ± 0.003d | 0.523 ± 0.01c |
| Pomegranate | 1044 ± 4.242e | 265.19 ± 3.30e | 0.143 ± 0.006d | 0.515 ± 0.003c |
| Blackberry | 1162 ± 1.414h | 321 ± 7.88f | 0.099 ± 0.014b | 0.413 ± 0.003a |
Standard deviation of means is shown as ± SD. Values in the same column with different superscripts are significantly different (p < 0.05)
There are several analytical methods that have been developed for determining antioxidant activity. DPPH and ABTS are the most commonly used methods, which are different in reaction kinetics and side reactions appearance that based on the deactivation of radicals. As shown in Table 2, antioxidant activities of samples were varied from 0.047 to 0.302 µg TE/mL by DPPH assay, and 0.413 to 0.885 µg TE/mL by ABTS assay (p < 0.05). The values were higher in ABTS assay than in DPPH assay for each vinegar sample analyzed. The highest value obtained with ABTS and DPPH assays was found in apricot vinegar and plum vinegar, respectively. The difference between ABTS and DPPH assay results could be due to the different mechanism of reaction involved. Previous studies were also reported a wide range of antioxidant activity values for different vinegars samples. For example, antioxidant activity was found in the range of 2–6 µmol TE/mL for apple cider vinegars, 16.88–33.52 µM TE/mL for traditional balsamic vinegar, 13–189 mg Trolox/100 mL for different fruit vinegars (Bakir et al., 2017; Bertelli et al., 2015; Budak et al., 2011). It was reported that the differences in antioxidant capacities among vinegars were originated from their different phenolic contents and the compounds found in the samples showing antioxidants properties (Davalos et al., 2005).
Color properties of fruit vinegars are given in Fig. 1. L*, a*, b* values of the vinegars were found in the range of 3.11–8.17, − 1.50–0.60 and 0.82–3.47, respectively (p < 0.05). L*(whiteness/darkness) values were found higher in grape and fig vinegars among all samples while the highest a* (redness/greenness) and b* (yellowness/blueness) values were determined in blackberry vinegar (p < 0.05). The results showed that no significant correlation was found between the color values and total phenolic/flavonoid contents of vinegars. However, the highest redness/greenness and yellowness/blueness values were found in blackberry vinegar, which has the highest total phenolic content. Furthermore, a wide range of color properties for various types of vinegars were reported by many researchers. Cruz et al. (2018) reported that vinegars with higher phenolic content tend to have lower whiteness/darkness, but higher values of the red component color.
Microbiological properties of fruit vinegars were represented in Fig. 2. The number of LAB was found in the range of between 1.60 and 5.39 log CFU/mL for apple, fig, plum, grape and blackberry vinegars. The numbers of AAB and yeast-mold were found in the range of 3.34–6.32 and 2.53–3.97 log CFU/mL, respectively for apple, fig, plum and grape vinegar. On the other hand, LAB, AAB and yeast-mold counts of apricot, mandarin, persimmon, pomegranate and rosehip vinegars were found under detection limits. In the previous study, the counts of AAB, LAB and yeast-mold of 20 traditional fruit vinegars (including grape, apple, artichoke, pomegranate, apple-lemon and hawthorn vinegars) were found in the range of < 10–7.2 × 106, < 10–1.1 × 109 and < 10–3.9 × 106 CFU/mL, respectively (Ozturk et al., 2015). It was reported that microbiological status of vinegar may change depending on intrinsic conditions (media composition) and extrinsic parameters, such as temperature and humidity. Ethanol and acid, produced in the initial stages of spontaneous fermentation, mainly by yeast and LAB (Saccharomyces cerevisiae), respectively, inhibit the growth of undesirable microorganisms and extend the shelf-life of vinegar. Following that in the second stage of vinegar fermentation, acetic acid is produced by AAB and inhibit acid-intolerant microorganisms. However, there is a great risk of spoilage for vinegar produced by spontaneous fermentation due to the difficulty of control (Solieri and Giudici, 2009).
In the study, antimicrobial activity of vinegar samples was investigated against nine bacterial strains by MIC and MBC assays. Vinegar samples exhibited growth-inhibitory effect for all test microorganisms (Table 3). Most of the samples were found inhibitive at concentrations between 3.12 and 6.25% (MIC, v/v), while apple vinegar showed inhibitive effect at higher concentrations. The most sensitive bacteria to all vinegar samples was P. acidilactici (Table 3).
Table 3.
The minimum inhibition concentration (MIC) and minimum bactericidal concentration (MBC) values of vinegar samples
| Vinegar samples | MIC and MBC values of vinegar samples (%, v/v) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. monocytogenes | E. faecalis | B. subtilis | S. aureus | E. coli O157:H7 | S. Typhimurium | E. coli | B. cereus | P. acidilactici | ||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| Persimmon | 3.12 | 50 | 3.12 | 50 | 6.25 | 50 | 6.25 | > 50 | 6.25 | > 50 | 6.25 | > 50 | 3.12 | 50 | 12.5 | 50 | 3.12 | 50 |
| Mandarin | 6.25 | 50 | 6.25 | 50 | 6.25 | 50 | 6.25 | > 50 | 3.12 | > 50 | 3.12 | > 50 | 3.12 | 50 | 3.12 | 50 | 3.12 | 50 |
| Grape | 3.12 | 50 | 3.12 | 50 | 6.25 | > 50 | 6.25 | 50 | 6.25 | 25 | 6.25 | 25 | 6.25 | 50 | 6.25 | 50 | 3.12 | 50 |
| Apricot | 6.25 | > 50 | 12.5 | 50 | 6.25 | > 50 | 6.25 | 50 | 6.25 | 50 | 6.25 | > 50 | 6.25 | > 50 | 6.25 | > 50 | 3.12 | 50 |
| Plum | 3.12 | 50 | 6.25 | 50 | 6.25 | 25 | 6.25 | 25 | 6.25 | 50 | 6.25 | 50 | 3.12 | 50 | 3.12 | 50 | 3.12 | 50 |
| Fig | 3.12 | 50 | 6.25 | 50 | 6.25 | > 50 | 6.25 | 50 | 6.25 | 50 | 3.12 | 50 | 3.12 | 50 | 3.12 | 25 | 3.12 | 50 |
| Rosehip | 3.12 | 25 | 3.12 | 50 | 6.25 | > 50 | 6.25 | 50 | 3.12 | 50 | 3.12 | 50 | 3.12 | 50 | 6.25 | 25 | 3.12 | 50 |
| Apple | 12.5 | > 50 | 25 | > 50 | 25 | > 50 | 12.5 | > 50 | 12.5 | > 50 | 12.5 | > 50 | 12.5 | > 50 | 12.5 | > 50 | 12.5 | > 50 |
| Pomegranate | 3.12 | 50 | 6.25 | 50 | 6.25 | 50 | 6.25 | 50 | 6.25 | > 50 | 6.25 | > 50 | 3.12 | 50 | 3.12 | 50 | 3.12 | 50 |
| Blackberry | 3.12 | 25 | 6.25 | 50 | 6.25 | > 50 | 6.25 | 25 | 6.25 | 50 | 6.25 | 50 | 3.12 | 12.5 | 3.12 | 25 | 3.12 | 50 |
Furthermore, most of the samples were found bactericidal at concentrations between 25 and 50% (MBC, v/v). However, bactericidal effect was not observed only in apple vinegar sample for all test cultures (Table 3). Antimicrobial activity of vinegars was also reported by various researchers only by disk diffusion assay. In a study performed by Karaagac et al. (2016), antimicrobial activity of mulberry vinegar produced traditionally were tested on S. aureus, Streptococcus pyogenes, Klebsiella oxytoca, E. faecalis, B. subtilis, B. cereus, Erwinia carotovora, E. coli and Candida albicans by disk diffusion method and S. aureus (28 mm), K. oxytoca (24.6 mm) and B. subtilis (23.3 mm) were determined as the most sensitive strains to mulberry vinegar. In another study, various microorganisms (B. cereus, E. coli, E. coli O157:H7, Klebsiella pneumoniae, L. monocytogenes, Pseudomonas aeruginosa, Proteus vulgaris, S. Typhimurium, S. aureus, Yersinia enterocolitica) showed sensitivity to traditionally produced vinegars (grape, apple, lemon, artichoke, pomegranate, hawthorn) at varying rates (6.18–23.56 mm) and the most sensitive strain was B. cereus (Ozturk et al., 2015). Vinegar samples (apple, grape, pomegranate, balsamic, gilaburu, blackberry, artichoke, lemon, rosehip, hawthorn, blueberry, date, mulberry, apricot and rice) showed antibacterial activity against S. aureus, S. Typhimurium and E. coli (8.58–15.81 mm) (Bakir et al., 2017). Similar results were also detected by Kelebek et al. (2017), who investigated apple and grape vinegars in terms of antimicrobial activity against S. aureus, E. coli and Pseudomonas aeruginosa (9.00–1.50 mm). These studies showed that antimicrobial activities of vinegar samples could partly be related to their pH values, acetic acid and phenolic contents.
In conclusion, fruit vinegars used in this study exhibited important differences in terms of total acidity, pH, brix, color, total phenolic content, total flavonoid content, antioxidant and antimicrobial capacities. In general, the results showed that fruit vinegars contain high amount of total phenolic and flavonoid contents, which lead them to show high antioxidant and antimicrobial activities. Vinegars, except apple vinegar, exhibited the most inhibitive effect against P. acidilactici. Large differences exist in the properties of fruit vinegars could be mainly originated from raw material and production technology used. This study proved that fruit vinegars are important source of bioactive compounds. In the further analysis of fruit vinegars, individual phenolic composition should be investigated.
Acknowledgements
No funding was received.
Compliance with ethics standards
Conflict of interest
The authors declare no conflict of interest.
Human and animal rights
This article does not contain any studies with human or animal subjects.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ilkin Yucel Sengun, Email: ilkin.sengun@ege.edu.tr.
Gulden Kilic, Email: gulden-gk@hotmail.com.
Berna Ozturk, Email: ozturkberna5@gmail.com.
References
- Anonymous. Vinegar-product made from liquids of agricultural origin-definition, requirements, marking, Turkish Standards Institüte, Ankara, (Vol. TS 1880 EN 13188/D1: 2016) (2016)
- AOAC. Official Analytical Chemists. 18th ed. Association of Official Analytical Chemists, AOAC International, Arlington, VA, USA (2007)
- Bakir S, Devecioglu D, Kayacan S, Toydemir G, Karbancioglu-Guler F, Capanoglu E. Investigating the antioxidant and antimicrobial activities of different vinegars. Euro. Food Res. Tech. 2017;243:2083–2094. doi: 10.1007/s00217-017-2908-0. [DOI] [Google Scholar]
- Bertelli D, Maietti A, Papotti G, Tedeschi P, Bonetti G, Graziosi R, Brandolini V, Plessi M. Antioxidant activity, phenolic compounds, and NMR characterization of balsamic and traditional balsamic vinegar of Modena. Food An. Met. 2015;8:371–379. doi: 10.1007/s12161-014-9902-y. [DOI] [Google Scholar]
- Booth IR, Kroll RG. The preservation of foods by low pH. New York: Elsevier Science Publishers; 1989. [Google Scholar]
- Budak NH. Total antioxidant activity and phenolic contents with advanced analytical techniques in the mulberry vinegar formation process. Fruit Sci. 2015;2:27–31. [Google Scholar]
- Budak HN, Kumbul DD, Savas CM, Seydim AC, Kok-Tas T, Ciris IM, Guzel-Seydim ZB. Effects of apple cider vinegars produced with different techniques on blood lipids in high-cholesterol-fed rats. J. Agric. Food Chem. 2011;59:6638–6644. doi: 10.1021/jf104912h. [DOI] [PubMed] [Google Scholar]
- Cemeroglu B. Food analysis. Ankara: Food Technology Association Publications; 2013. pp. 87–157. [Google Scholar]
- Chang JM, Fang TJ. Survival of Escherichia coli O157:H7 and Salmonella enterica serovars Typhimurium in iceberg lettuce and the antimicrobial effect of rice vinegar against E. coli O157:H7. Int. J. Food Microbiol. 2007;24:745–751. doi: 10.1016/j.fm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Cruz M, Correia AC, Gonçalves FJ, Jordão AM. Phenolic composition and total antioxidant capacity analysis of red wine vinegars commercialized in Portuguese market. Ciencia E. Tecnica. 2018;33:102–115. [Google Scholar]
- Davalos A, Bartolome B, Gomez-Cordoves C. Antioxidant properties of commercial grape juices and vinegars. Food Chem. 2005;93:325–330. doi: 10.1016/j.foodchem.2004.09.030. [DOI] [Google Scholar]
- De Vero L, Gala E, Gullo M, Solieri L, Landi S, Giudici P. Application of denaturing gradient gel electrophoresis (DGGE) analysis to evaluate acetic acid bacteria in traditional balsamic vinegar. Food Microbiol. 2006;23:809–813. doi: 10.1016/j.fm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Deng Y, Yang G, Yue J, Qian B, Liu Z, Wang D, Zhong Y, Zhao Y. Influences of ripening stages and extracting solvents on the polyphenolic compounds, antimicrobial and antioxidant activities of blueberry leaf extracts. Food Control. 2014;38:184–191. doi: 10.1016/j.foodcont.2013.10.023. [DOI] [Google Scholar]
- FDA (Food and Drug Administration). Vinegar, definitions—adulteration with vinegar eels, 1995. Available from: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-ice/documents/webcontent/ucm074471.pdf. Accessed 9 Jan 2018.
- FDA-BAM (Food and Drug Administration-Bacteriological Analytical Manual). Yeasts, molds and mycotoxins. Chapter:18, 2001. Available from: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm071435.htm Accessed 15 Jul 2017.
- Hindi NK. In vitro antibacterial activity of aquatic garlic extract, apple vinegar and apple vinegar-garlic extract combination. Am. J. Phytomed. Clin. Theraps. 2013;1:42–51. [Google Scholar]
- ISO 15214. International organization for standardization, microbiology of food and animal feeding stuffs-Horizontal method for the enumeration of mesophilic lactic acid bacteria-colony count technique at 30 °C. International Organization for Standardization, Geneva, Switzerland. (1998)
- Karaagac RA, Aydogan MN, Koseoglu MS. An investigation on antimicrobial and antioxidant activities of naturally produced mulberry vinegar. Pharm. Biol. 2016;6:34–39. [Google Scholar]
- Karabiyikli S, Sengun IY. Beneficial effects of acetic acid bacteria and their food products. In: Sengun IY, editor. Acetic acid bacteria: fundamentals and food applications. Boca Raton: CRC Press Inc; 2017. pp. 221–242. [Google Scholar]
- Kelebek H, Kadiroğlu P, Demircan NB, Selli S. Screening of bioactive components in grape and apple vinegars: antioxidant and antimicrobial potential. J. Inst. Brew. 2017;123:407–416. doi: 10.1002/jib.432. [DOI] [Google Scholar]
- Ozturk I, Calıskan O, Tornuk F, Sagdıc O. Antioxidant, antimicrobial, mineral, volatile, physicochemical and microbiological characteristics of traditional home-made Turkish vinegars. Food Sci. Technol LEB. 2015;63:144–151. doi: 10.1016/j.lwt.2015.03.003. [DOI] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxf. Med. Cell. Long. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qui J, Ren C, Fan J, Li Z. Antioxidant activities of aged oat vinegar in vitro and in mouse serum and liver. J. Sci. Food Agr. 2010;90(11):1951–1958. doi: 10.1002/jsfa.4040. [DOI] [PubMed] [Google Scholar]
- Ramos B, Brandão TRS, Teixeira P, Silva CLM. Balsamic vinegar from Modena: An easy and effective approach to reduce Listeria monocytogenes from lettuce. Food Control. 2014;42:38–42. doi: 10.1016/j.foodcont.2014.01.029. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolarization assay. Free Rad. Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Rommel A, Heatherbell DA, Wrolstad RE. Red raspberry juice and wine: Effect of processing and storage on anthocyanin pigment composition, color and appearance. J. Food Sci. 1990;55:1011–1017. doi: 10.1111/j.1365-2621.1990.tb01586.x. [DOI] [Google Scholar]
- Sakanaka S, Ishihara Y. Comparison of antioxidant properties of persimmon vinegar and some other commercial vinegars in radical scavenging assays and on lipid oxidation in tuna homogenates. Food Chem. 2008;107:739–744. doi: 10.1016/j.foodchem.2007.08.080. [DOI] [Google Scholar]
- Sengun IY, Karapinar M. Effectiveness of lemon juice, vinegar and their mixture in elimination of Salmonella Typhimurium on carrots. Int. J. Food Microbiol. 2004;96:301–305. doi: 10.1016/j.ijfoodmicro.2004.04.010. [DOI] [PubMed] [Google Scholar]
- Solieri L, Giudici P. Vinegars of World. Milano: Imprint Springer; 2009. [Google Scholar]
- Su MS, Chien PJ. Antioxidant activity, anthocyanins and phenolics of rabbiteye blueberry (Vaccinium ashei) fluid products as affected by fermentation. Food Chem. 2007;104:182–187. doi: 10.1016/j.foodchem.2006.11.021. [DOI] [Google Scholar]
- Tomas-Menor L, Morales-Soto A, Barrajón-Catalán E, Roldán-Segura C, Segura-Carretero A, Micol V. Correlation between the antibacterial activity and the composition of extracts derived from various Spanish Cistus species. Food Chem. Toxicol. 2013;55:313–322. doi: 10.1016/j.fct.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Ubeda C, Callejón RM, Hidalgo C, Torija MJ, Troncoso AM, Morales ML. Employment of different processes for the production of strawberry vinegars: Effects on antioxidant activity, total phenols and monomeric anthocyanins. LWT Food Sci. Technol. 2013;52:139–145. doi: 10.1016/j.lwt.2012.04.021. [DOI] [Google Scholar]
- Verzelloni E, Tagliazucchi D, Conte A. Relationship between the antioxidant properties and the phenolic and flavonoid content in traditional balsamic vinegar. Food Chem. 2007;105:564–571. doi: 10.1016/j.foodchem.2007.04.014. [DOI] [Google Scholar]
- Zhishen J, Mengcheng T, Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]