Abstract
The blister blight disease caused by the fungus, Exobasidium vexans has serious implications on the quality of tea production. The disease however, has been poorly studied and hence there is very limited information on the pathogen and as such the pathogenesis of blister blight infection. One of the major roadblocks in understanding E. vexans is the obligate and biotrophic nature of the fungus which limits the establishment and maintenance of in vitro cultures. To address this issue, a Central Composite Design based Response Surface Methodology (RSM) was adopted to study the modification of three fungal culture media viz. czapek dox, potato dextrose, and v8 juice, and the effect of altered media composition on growth conditions and media compositions were assessed. The response parameter for the RSM experiments was the mycelial biomass produced under different culture conditions. The uni and bi-parametric interactions among the experimental variables provided the basis for the statistically optimized conditions for maximal fungal growth. The study thus presents the recommended modifications of existing media that can lead to the successful establishment and maintenance of E. vexans in vitro cultures.
Electronic supplementary material
The online version of this article (10.1007/s12088-019-00846-6) contains supplementary material, which is available to authorized users.
Keywords: Blister blight, Exobasidium vexans, Growth media, Optimization, RSM
Introduction
Blister blight disease is regarded as a serious threat to commercial tea production, pervasive in most of the major tea-producing countries viz. India, China, Sri Lanka, Japan, and Indonesia [1]. The causal organism, Exobasidium vexans is an obligate, biotrophic basidiomycete fungus that mainly infects harvestable tender leaves thereby directly affecting the quality and production of the tea crop [2]. The pathogen is not reported to have any host other than tea and complete its life cycle within 21 days of infection [1, 3]. However, the detailed mechanism of the pathogenesis of E. vexans remains to be elucidated. The E. vexans being an obligate biotroph makes the establishment of its in vitro cultures rather challenging. An existing report on the in vitro culture of E. vexans indicates the probabilistic role of natural supplements that may help to maintain the in vitro cultures for a period of up to 48 h [4]. However, long term viable cultures through a reproducible and statistically validated optimization have not been reported so far.
In this context, three fungal culture media and their modifications were extensively studied using a response surface methodology (RSM) approach and were statistically optimized for establishing viable and long-term in vitro cultures of E. vexans. The RSM approach was chosen over the conventional single-variable method which is unable to reflect the effects of variation of one process parameter on the others and the interactions among the parameters at any given point in time [5, 6].
In the current study, E. vexans was isolated and its pure culture was developed using the single spore culture. Central Composite Design (CCD) of RSM has been applied to obtain the optimized culture conditions for the parameters using three modified fungal culture media, viz. czapek dox, potato dextrose, and v8 Juice medium. The growth parameters considered in the study includes carbon source, tea-leaf extract, calcium carbonate (CaCO3), initial pH of broth and incubation temperature.
Material and Methods
Chemicals and Reagents
Czapek dox broth (Cat. no. M076), potato dextrose broth (Cat. no. M403) and v8 juice broth (Cat. no. M895) were purchased from Himedia Laboraties and sucrose (C12H22O11), dextrose (C6H12O6), glucose (C6H12O6) and calcium carbonate (CaCO3) from Sigma-Aldrich, India.
Isolation and Study of Spore Morphology
Blister blight afflicted tea leaves were collected from Ananda Tea Estate, Lakhimpur District, Assam, India and infected parts of the tea leaves were excised using a cork borer and followed by incubation in potato dextrose agar (PDA) plates for germination of the spores. Post germination, the hyphal mass was sub-cultured in fresh PDA petri dishes for another 5–7 days at 28 °C. A spore suspension with a spore count ~ 104 spores ml− 1 was prepared from the 7 day old sub cultures in sterile distilled water and plated on fresh PDA plates. The plates were examined for single germinating events, 12–24 h post-incubation and the germinating spores were singly placed on fresh PDA plates for growth at 28 °C. Basidiospores formed from the germinating spores were examined for their morphology by covering a defined area of the slide with agar and placing a drop of the spore suspension on it. These slides were then incubated for different time intervals ranging from 0–8 h at 28 °C. Following this, the spore germination stages for the above intervals were observed under a light microscope at 40× magnification. The dimensions of the spore were measured using the Image J software.
Optimization of the Ideal Concentration of Media Components and Culture Conditions
RSM using CCD was applied in order to determine the ideal concentration/composition of media components effective establishment of in vitro establishment of E. vexans culture. For RSM the Design-Expert v7.0.0 software (https://www.statease.com/, Stat-Ease, Inc Minneapolis, MN USA) was used to design the experiments and to analyze the experimental results. The CCD was based on five-level-five factors (55) leading to 32 experimental runs and the values of the response were obtained as a mean of the triplicate runs. The five process parameters used in the optimizations were the concentrations of supplemented carbon sources, tea-leaf extract, CaCO3 (used for the modification of the three basal media), pH and incubation temperature. The tea-leaf extract was prepared by crushing the requisite amount of tea leaves (0–30 g), as per the CCD matrix, for each media in 100 ml of distilled H2O to obtain the desired % (w/v) of the extract. The boundary conditions for each parameter were designed based on the preliminary studies and are depicted in Table S1. The calculation of the optimal growth environment was based on a second order polynomial function fitted using the following equation, to correlate the relationship between the mycelial weight, medium components and growth conditions:
where Y is the predicted response variable, b0 is the model constant, bi is the linear co-efficient, bii is the interaction coefficients, bij is the quadratic co-efficient and Xi and Xj are the coded values. The significance of the model equation and model terms were evaluated by F test, while the analysis of variance (ANOVA) was used to evaluate the statistical significance of the model [7]. The quality of the quadratic model equation was expressed by determining the goodness of fit and the optimal values were obtained by solving the regression equation and analyzing the response surface plot.
Further, pareto analysis was computed to analyze the sensitivity of the individual parameter on the response variable within a design model. The following equation was used for pareto computation to evaluate the relative significance of individual factor
where Pi is the percentage influence of individual factor on the response [8].
Results
Isolation and Study of Spore Morphology
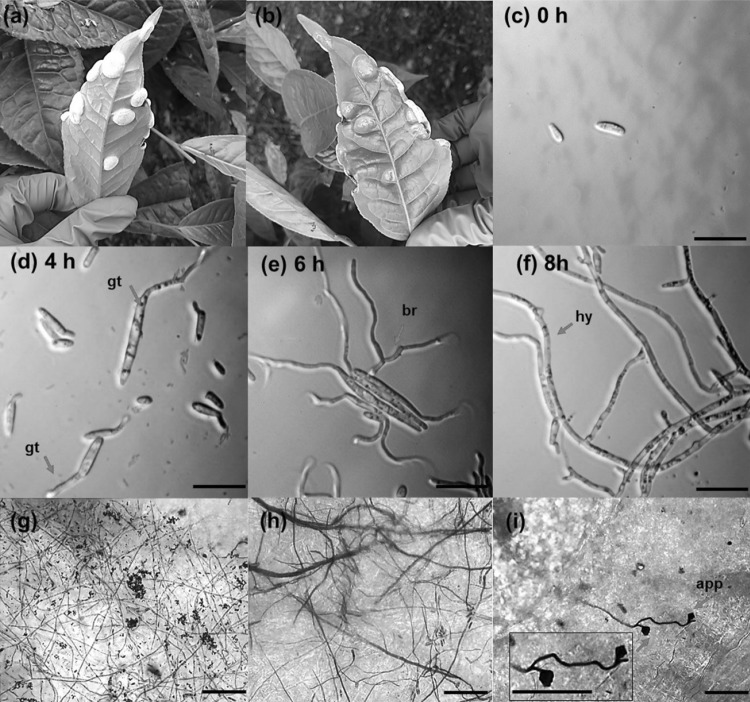
Isolation of E. vexans was carried out from samples collected from the Blister blight afflicted tea leaves in different locations of Ananda Tea Estate, Lakhimpur District, Assam, India (Fig. 1a, b). The light micrographs of fully developed E. vexans basidiospores are shown in Fig. 1c which were ellipsoid to ovoid in shape and somewhat curved, with slightly tapered ends. The average size of the basidiospores was 10.07 ± 3.44 × 3.034 ± 1.00 µm. The different stages of basidiospore germination were examined for an incubation period of 0–8 h. The basidiospores germinating on the agar surface, 4 h post-incubation were seen to have the germ tubes emerging from either one or both ends of the basidiospore (Fig. 1d, e). The basidiospores were aseptate during the initial stages of germination, which was followed by the appearance of 1–4 transverse septa during the later stages. This process of septation and the emergence of germ tube from the basidiospores precisely matches the detailed description of the E. vexans basidiospore germination, reported by Gadd and Loos [9]. Further, the hyphae observed after an incubation period of 8 h appeared to differentiate further into branches (Fig. 1f). The fungal colonization on the infected tea leaves collected from blister blight effected tea plantations was also studied using modified trypan blue staining method [10]. Extensive colonization of the fungus was observed on the infected leaf part with the presence of appressorium formation (Fig. 1g–i). The molecular identification of the fungus was also carried out by developing ITS barcode which confirmed the isolate to be E. vexans (Accession no. MN307382, data not shown).
Fig. 1.
Dorsal (a) and ventral (b) surfaces of blister blight infected tea leaves (Ananda Tea Estate, Lakhimpur, India). Light micrographs of E. vexans basidiospores at different phases of germination on agar. Basidiospores (c); basidiospores with germ tube growth on single end (d); basidiospores with germ tube growth from both ends (e); and hyphal growth of E. vexans showing branching (f). Extensive hyphal growth observed on infected leaf part (g–h) and appressorium formation (i) (Scale: 10 µm, gt germ tube, br branching of hyphae, hy hyphae, app appresorium)
Optimization of the Ideal Concentration of Media Components and Culture Conditions
To optimize the culture conditions for the in vitro growth of E. vexans, RSM experiments were conducted for the CCD matrix with parameters used to modify the three fungal culture media viz. czapek dox, potato dextrose, and V8 juice. After incorporating the experimental value for response of mycelial biomass, CCD matrix provided the optimal culture conditions with five independent variables for each of the media, in the form of statistically significant fitted models. The fitted models had P values in the range of ≤ 0.0001 indicating their satisfactory confidence levels. For all the three media an α value of 0.05 was used for the analysis that corresponds to a confidence level of 95%. The P values were found to be less than the α value for the fitted models which rejects the null hypothesis and corroborates the statistical significance of the fitted models for all the three media. Further, the fitted models were found with high F values whereas the values for lack of fit, were non-significant, reiterating the goodness of fit [11, 12].
Czapek Dox Medium
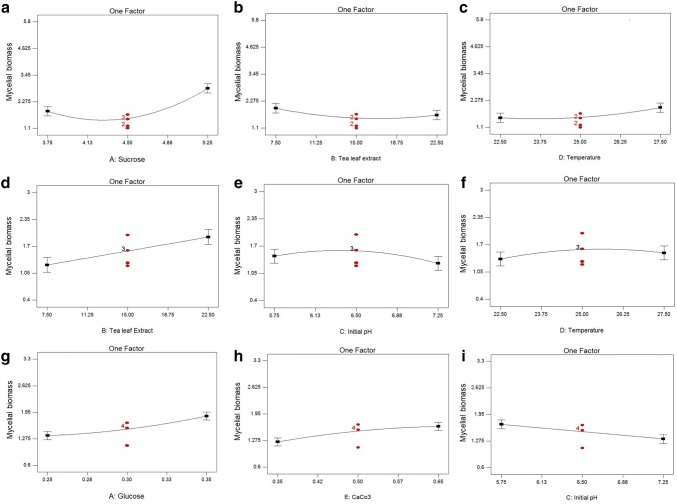
For the czapek dox broth medium, the variation of sucrose, incubation temperature and tea-leaf extract in the media, significantly influenced the response parameter, in the fitted single factor interactions (Fig. 2a–c). The optimized conditions for czapek dox broth medium showing the highest mycelium growth were obtained for 6.0 g of carbon source, 15% w/v tea-leaf extract, and 0.4 g of CaCO3 at pH 6.5 and 25 °C incubation temperature (Table S2).
Fig. 2.
Effect of univariate interactions described by the model for czapek dox broth a–c, potato dextrose broth d–f and v8 juice broth g–i
The quadratic model, which was the best fitted model for the czapex dox media was subjected to analysis of variance (ANOVA) which resulted in the model determination coefficient R2 = 0.94, indicating that 94% of the variability can be described by the quadratic model (Table S3) [6]. The R2 value was therefore deemed as significant goodness of fit. The predicted R2 = 0.75 was in reasonable agreement with the adjusted R2 = 0.90, demonstrating the accuracy of the model [13]. The adequate precision of 17, which is far greater than the standard precision value of 4, proving that the quadratic model can be used to predict the response to change in culture parameters, with high accuracy [14].
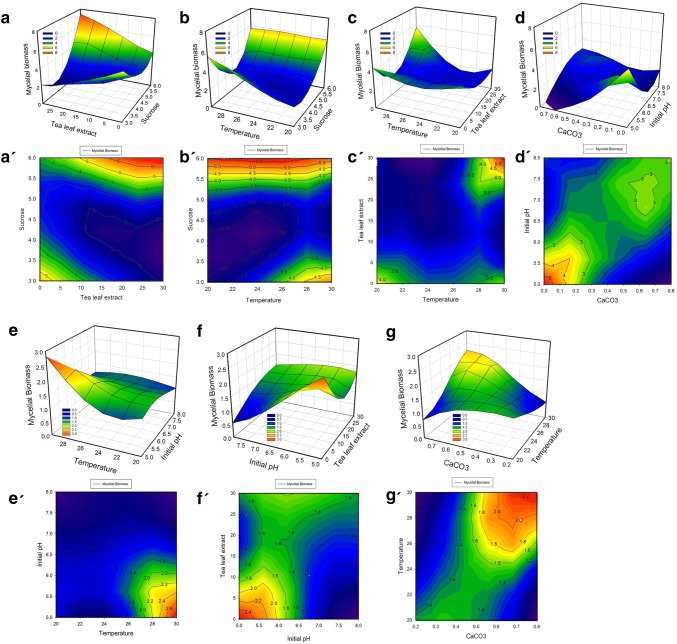
During the estimation of the response to alteration in parameters, the P value < 0.05, indicates high significant, whereas P value > 0.1, indicates that the variable is non-significant [15]. For czapex dox, the variables, sucrose and temperature, were found to be significant for the mycelial production (Table S3). The quadratic terms AB, AD, BD, CE, A2, B2 and D2 were found to have a significant effect on the growth of E. vexans (Table S3). The effects of interaction among the significant variables were represented in the form of the single factor interaction plots (Fig. 2a–c). The 3D plots and their corresponding contour plots for the significant multi-variate interactions among the parameters are shown in Fig. 3a–d and a′–d′ respectively.
Fig. 3.
Effect of multivariate interactions: Response surface 3D plots and response surface contour plot described by the model for czapek dox broth a–d and a′–d′ and v8 juice broth e–g and e′–g′ respectively
For the single factor interactions, an increase in the amount of sucrose/temperature in czapek dox results in the increase in mycelial biomass (Fig. 2a, c). The tea-leaf extract influences minor variations in mycelial yield, when added within the boundary conditions to czapek dox (Fig. 2b).
For the multivariate interactions, the 3D plots show the influence of tea-leaf extract on the mycelial growth, when in conjunction with either sucrose (Fig. 3a) or incubation temperature (Fig. 3c). Sucrose, on the other hand, was also able to influence the mycelial growth in association with the incubation temperature (Fig. 3b). Further, the CaCO3 concentration and pH of czapek dox bore significant influence on the mycelial growth, wherein the pH 5.75 and 0.2 g of CaCO3 showed the highest biomass accumulation (Fig. 3d).
The corresponding contour plots for the aforementioned 3D plots (Fig. 3a–d) are represented in Fig. 3a′–d′. An elliptical contour plot denotes that the interactions between the corresponding variables are significant whereas a circular contour plot indicates that the interactions between the corresponding variables are negligible [16]. The contour plots for the fitted models Fig. 3a′–d′ show an elliptical nature, thereby establishing the significant interactions between the variables. The interactions between these variables can be expressed by the following second-order polynomial model equation:
Potato Dextrose Medium
The CCD matrix based RSM for potato dextrose broth medium (Table S4) show pH and temperature to be the significant variables, among others, in influencing the increased yield of mycelial biomass. The highest mycelial growth was found for the condition with 3.0 g of carbon source, 30% w/v of tea-leaf extract and 0.4 g of CaCO3 at pH 6.5 and temperature 25 °C. The coefficient of determination, R2 = 0.73, indicates a good agreement between predicted and experimental values, with an adequate precision value of 10.93 (Table S5). Further, the ANOVA shows that the predicted R2 = 0.51 and adjusted R2 = 0.62, were in close proximity reflecting the model’s accuracy [13]. The corresponding P value of < 0.05 for tea-leaf extract, pH and temperature indicate that these variables are significant factors for mycelium production. The single factor interaction plots for these three factors are depicted in Fig. 2d–f. Tea-leaf extract had a prominent effect on the growth of E. vexans (Fig. 2d) where the amount of mycelial biomass is directly proportional to % w/v of tea-leaf extract. The optimal pH and temperature for the growth of E. vexans in potato dextrose broth were found to be around 6.5 and 25 °C respectively (Fig. 2e, f). However, none of the multivariate interactions between the variables were significant for the growth of E. vexans, (P value of the quadratic terms > 0.05) and therefore the 3D plots for the same have not been shown. The influence of the variables on potato dextrose broth can be expressed by the following second-order polynomial model equation:
V8 Juice Medium
The carbon source, media pH, incubation temperature and CaCO3 were observed to be the most significant parameters influencing mycelial growth response for the v8 juice (Table S7). The highest mycelial growth was obtained for 0.35 g of carbon source, 7.5% w/v tea-leaf extract and 0.6 g of CaCO3 at 5.75 pH and 27.5 °C temperature in the CCD matrix (Table S6). The ANOVA with predicted R2 = 0.62 and adjusted R2 = 0.79, were in reasonable agreement with each other, with an adequate precision value of 15.1 (Table S7). This provides an acceptable signal for using the model to navigate the design space [13, 15]. The fitted quadratic model had an R2 = 0.87 which is in good agreement for a significant correlation between the predicted and observed responses [17]. Additionally, the interaction between the terms BC, CD and DE were found to be significant with a P Value of < 0.05 (Table S7).
The single factor interaction plots for glucose and CaCO3 indicates the proportionate accumulation of mycelial biomass in response to an increase in glucose and CaCO3 concentrations in the v8 media (Fig. 2g, h). Figure 2i shows that lower broth pH favors the biomass accumulation of E. vexans, with the boundary conditions being set at pH 5.75. The 3D response surface plots and their corresponding contour plots showing interactions between the process parameters based on the fitted model are depicted in Fig. 3e–g and e′–g′ respectively. The interaction between tea-leaf extract and initial pH was found to be significant with a P value = 0.003. (Fig. 3e, Table S7). Further, the influence of temperature on the growth of E. vexans was found to be significant while interacting with the media pH and CaCO3 (Fig. 3f, g). The elliptical contour plot corresponding to the 3D plot for the fitted model (Fig. 3e′–g′) indicates significant interactions between the process parameters [18]. The interactions of the variables studied can be expressed by the following second-order polynomial model equation:
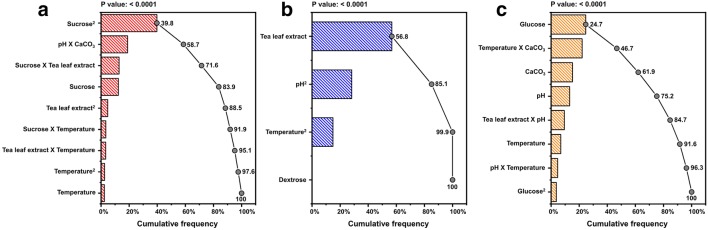
The pareto graphic analysis was performed to determine the percentage effect of the most influential parameters which were used to modify the three basal media as described by the individual model equations. The squared effect of sucrose and glucose was found to be the most significant parameter for czapek dox and v8 juice media respectively (Fig. 4a, c). For potato dextrose media tea leaf extract was found to have the highest influence on the response, mycelial biomass growth (Fig. 4b).
Fig. 4.
Pareto graphic analysis for mycelial biomass described by model for czapek dox (a), potato dextrose (b) and v8 juice (c)
A normal probability for the czapex dox, potato dextrose, and v8 juice media was plotted to analyze the adequacy of the models, and it examines the standard deviation between the predicted and experimental responses (Fig. S1 a–c). The plots show an approximately linear distribution of the data points, forming a straight line, for all the three media and are in good agreement for normally distributed residuals of the response [19].
Physical growth behaviour of E. vexans was evaluated on czapex dox, potato dextrose, and v8 juice agar media wherein the highest radial mycelial growth was observed for czapek dox agar media with a colony diameter of 4 cm after 7 days post incubation. This was followed by v8 juice agar media with a colony diameter of 2.8 cm and 2.5 cm for potato dextrose agar media (Fig. S2).
Discussion
Owing to the biotrophic nature of the blister blight causal organism, E. vexans, this study mediated the optimization of media components and growth conditions to support the in vitro growth of the pathogen in the three modified basal media. After the first report on E. vexans by Gadd and Loss in 1948, Ezuka in 1955 reported in vitro growth of E. vexans on home-made Potato Sucrose Agar (PSA). This study supported the formation of Chlamydospore like bodies after germination, in line with the work carried out by Gadd and Loss in 1950. However, these studies report detectable colony growth only after an incubation period of 20 days. In this context, the present study, addresses the gap by optimizing the media components of three culture media using CCD matrix of RSM to optimize the in vitro growth of E. vexans. The highest mycelial biomass was obtained for czapek dox media wherein the supplemented sucrose was found to be the most influential parameter for the growth, followed by the combined effect of pH, CaCO3 and tea leaf extract. For v8 juice media the glucose concentration was found to be the most significant component for the growth of E. vexans. Under natural condition E. vexans completes its life cycle only in Tea under specific climatic conditions common to elevated regions. The optimum temperature range of 25–28 °C for the in vitro growth of E. vexans found in the study is in accordance with the microclimate of low temperature and high humidity that is known to favor blister blight infection in tea plantation. It is interesting to note that visible mycelial growth was observed 5 days post incubation and was found to be viable for up to five weeks, before they needed to be sub-cultured. Thus, the establishment of viable in vitro culture of the pathogen would undeniably facilitate the downstream molecular characterization of the pathogen to understand its pathogenic mechanism and to design various disease mitigation strategies including the development of environment friendly fungicides.
Conclusion
This study demonstrates the chemometric modelling of the effect of the growth parameters carbon source, tea-leaf extract, initial pH of broth, incubation temperature and CaCO3 on the in vitro growth of E. vexans for the three different modified basal media czapek dox, potato dextrose and v8 Juice. The influence of interaction among the media components and growth conditions to mediate in vitro growth of E. vexans was efficiently characterized and explained using the CCD matrix. The significant parameters differed for each of the modified basal growth media with carbon source the most significant parameter for czapek dox and v8 juice while tea leaf extract for potato dextrose. The optimal temperature and pH range for the in vitro growth of E. vexans was found to be 25–27 °C and 7–8 respectively. The optimization of media components and growth conditions enabled visible mycelial growth post 5 days of incubation and the viability of the culture was upto four-five weeks on the modified basal media.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors wish to acknowledge DBT, Govt. of India, for the Twinning Research Grant (Grant No. BT/427/NE/TBP/2013). Author C.C. would like to acknowledge DST, Govt. of India for her DST INSPIRE Junior Research Fellowship (IF-150964). The authors thank Ananda Tea Estate, Lakhimpur District, Assam for providing the blister blight infected tea leaf samples used in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chandra S, Chakraborty N, Panda K, Acharya K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol Biochem. 2017;115:298–307. doi: 10.1016/j.plaphy.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Sowndhararajan K, Marimuthu S, Manian S. Biocontrol potential of phylloplane bacterium Ochrobactrum anthropi BMO-111 against blister blight disease of tea. J Appl Microbiol. 2013;114:209–218. doi: 10.1111/jam.12026. [DOI] [PubMed] [Google Scholar]
- 3.Bhorali P, Gohain B, Gupta S, Bharalee R, Bandyopadhyay T, Das SK, Agarwal N, Singh HR, Bhagawati P, Bhattacharyya N, Ahmed P. Molecular analysis and expression profiling of blister blight defense-related genes in tea. Indian J Genet Pl Br. 2012;72:226. doi: 10.13140/2.1.2468.3527. [DOI] [Google Scholar]
- 4.Nagao H. Effect of aqueous vitamin B on the growth of blister blight pathogen, Exobasidium vexans. Songklanakarin J Sci Technol. 2012;34:601–606. [Google Scholar]
- 5.Qiu J, Song F, Qiu Y, Li X, Guan X. Optimization of the medium composition of a biphasic production system for mycelial growth and spore production of Aschersonia placenta using response surface methodology. J Invertebr Pathol. 2013;112:108–115. doi: 10.1016/j.jip.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Wu W, Ahn B. Statistical optimization of ultraviolet irradiate conditions for vitamin D2 synthesis in oyster mushrooms (Pleurotus ostreatus) using response surface methodology. PLoS ONE. 2014;9:e95359. doi: 10.1371/journal.pone.0095359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Oliveira CC, de Souza AKS, de Castro RJS. Bioconversion of chicken feather meal by Aspergillus niger: simultaneous enzymes production using a cost-effective feedstock under solid state fermentation. Indian J Microbiol. 2019;59:209–216. doi: 10.1007/s12088-019-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okoye CC, Onukwuli OD, Okey-Onyesolu CF. Predictive capability evaluation of RSM and ANN models in adsorptive treatment of crystal violet dye simulated wastewater using activated carbon prepared from Raphia hookeri seeds. J Chin Adv Mater Soc. 2018;6:478–496. doi: 10.1080/22243682.2018.1497534. [DOI] [Google Scholar]
- 9.Gadd CH, Loos CA. The basidiospores of exobasidium vexans. T Brit Mycol Soc. 1948;31:220–233. doi: 10.1016/S0007-1536(48)80004-5. [DOI] [Google Scholar]
- 10.Yang H, Zhao X, Liu C, Bai L, Zhao M, Li L. Diversity and characteristics of colonization of root-associated fungi of Vaccinium uliginosum. Sci Rep. 2018;8:15283. doi: 10.1038/s41598-018-33634-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang H, Dong H, Cai T, Zheng P, Li H, Zhang D, Sun J. In vitro optimization of enzymes involved in precorrin-2 synthesis using response surface methodology. PLoS ONE. 2016;11:e0151149. doi: 10.1371/journal.pone.0151149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang QY, Zhou WW, Zhou Y, Wanga XF, Xu JF. Response surface methodology to design a selective co-enrichment broth of Escherichia coli. PCR Microbiol Res. 2012;167:405–412. doi: 10.1016/j.micres.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Jadhav SB, Surwase SN, Phugare SS, Jadhav JP. Response surface methodology mediated optimization of Remazol Orange decolorization in plain distilled water by Pseudomonas aeruginosa BCH. Int J Environ. 2013;10:181–190. doi: 10.1007/s13762-012-0088-9. [DOI] [Google Scholar]
- 14.Liu W, Ji J, Chen H, Ye C. Optimal color design of psychological counseling room by design of experiments and response surface methodology. PLoS ONE. 2014;9:e90646. doi: 10.1371/journal.pone.0090646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel M, Abigail EA, Chidambaram R. Isotherm modelling, kinetic study and optimization of batch parameters using response surface methodology for effective removal of Cr (VI) using fungal biomass. PLoS ONE. 2015;10:e0116884. doi: 10.1371/journal.pone.0116884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shang H, Zhou H, Duan M, Li R, Wu H, Lou Y. Extraction condition optimization and effects of drying methods on physicochemical properties and antioxidant activities of polysaccharides from comfrey (Symphytum officinale L.) root. Int J Biol Macromol. 2018;112:889–899. doi: 10.1016/j.ijbiomac.2018.01.198. [DOI] [PubMed] [Google Scholar]
- 17.Chandra M, Oro I, Ferreira-Dias S, Malfeito-Ferreira M. Effect of ethanol, sulfur dioxide and glucose on the growth of wine spoilage yeasts using response surface methodology. PLoS ONE. 2015;10:e0128702. doi: 10.1371/journal.pone.0128702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Acikel U, Erşan M, Acikel YS. Optimization of critical medium components using response surface methodology for lipase production by Rhizopus delemar. Food Bioprod Process. 2010;88:31–39. doi: 10.1016/j.fbp.2009.08.003. [DOI] [Google Scholar]
- 19.Makadia AJ, Nanavati JI. Optimisation of machining parameters for turning operations based on response surface methodology. Measurement. 2013;46:1521–1529. doi: 10.1016/j.measurement.2012.11.026. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.