Abstract
The loss of dopaminergic neurons of the nigrostriatal system underlies the onset of the typical motor symptoms of Parkinson’s disease (PD). Lewy bodies (LB) and Lewy neurites (LN), proteinaceous inclusions mainly composed of insoluble α-synuclein (α-syn) fibrils are key neuropathological hallmarks of the brain of affected patients. Compelling evidence supports that in the early prodromal phases of PD, synaptic terminal and axonal alterations initiate and drive a retrograde degeneration process culminating with the loss of nigral dopaminergic neurons. This notwithstanding, the molecular triggers remain to be fully elucidated. Although it has been shown that α-syn fibrillary aggregation can induce early synaptic and axonal impairment and cause nigrostriatal degeneration, we still ignore how and why α-syn fibrillation begins. Nuclear factor-κB (NF-κB) transcription factors, key regulators of inflammation and apoptosis, are involved in the brain programming of systemic aging as well as in the pathogenesis of several neurodegenerative diseases. The NF-κB family of factors consists of five different subunits (c-Rel, p65/RelA, p50, RelB, and p52), which combine to form transcriptionally active dimers. Different findings point out a role of RelA in PD. Interestingly, the nuclear content of RelA is abnormally increased in nigral dopamine (DA) neurons and glial cells of PD patients. Inhibition of RelA exert neuroprotection against (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) MPTP and 1-methyl-4-phenylpyridinium (MPP+) toxicity, suggesting that this factor decreases neuronal resilience. Conversely, the c-Rel subunit can exert neuroprotective actions. We recently described that mice deficient for c-Rel develop a PD-like motor and non-motor phenotype characterized by progressive brain α-syn accumulation and early synaptic changes preceding the frank loss of nigrostriatal neurons. This evidence supports that dysregulations in this transcription factors may be involved in the onset of PD. This review highlights observations supporting a possible interplay between NF-κB dysregulation and α-syn pathology in PD, with the aim to disclose novel potential mechanisms involved in the pathogenesis of this disorder.
Keywords: Parkinson’s disease, α-synuclein, nuclear factor-κB (NF-κB), Rel A, c-Rel ko mice
Introduction
The therapeutic management of Parkinson’s disease (PD) patients is one of the major challenges in the field of neurodegenerative diseases nowadays (Orayj and Lane, 2019). This is mostly related to two determinants. (1) Although since many years the administration of levodopa (L-Dopa) has allowed motor symptom recovery, its efficacy has a limited time window and associates with severe adverse effects, thus gathering the unmet need of novel interventions (Olanow and Stocchi, 2018). (2) In spite of more than 200 years of research in the field of PD, we still have poor discernment on the main pathogenic mechanisms. The only certainties we own relate to the neuropathological features of post-mortem PD brains. These present a prominent loss of dopaminergic nigrostriatal neurons and contain profuse insoluble proteinaceous aggregates named Lewy bodies (LB) and Lewy neurites (LN), likely originating from fibrillary α-synuclein (α-syn) protein accumulation in cell bodies and neurites, respectively (Spillantini et al., 1997; Spillantini and Goedert, 2000). This notwithstanding, we ignore the key molecular determinants initiating the degeneration of nigrostriatal neurons and/or α-syn aggregation and deposition. The molecular features of the toxic α-syn species driving neurodegeneration are also an unsolved conundrum (Longhena et al., 2019).
Investigations on experimental models reproducing human α-syn aggregate accumulation, or transgenic expression of mutant forms of the protein associated with familial PD as well as more extensive neuropathological examinations and brain imaging studies in patients, have disclosed that the most probable initiation sites for neuronal degeneration in PD are synapses and axonal projections (for review, please see Bellucci et al., 2017; Uchihara, 2017; Wong et al., 2019). Moreover, the selective neuronal vulnerability of nigral dopaminergic neurons in PD has been proposed to derive from their massive neuronal arborization and peculiar metabolic and functional profile (Surmeier et al., 2017; Wong et al., 2019). In this scenario, α-syn pathological aggregation at synapses appears to play a major role in triggering dopaminergic neuron dysfunction, flowing in a retrograde degeneration pattern involving axonal projections first and culminating with neuronal cell death (Bellucci et al., 2016, 2017; Wong et al., 2019). In particular, the pivotal role exerted by α-syn within dopaminergic neurons terminals as a regulator of neurotransmitter synthesis, reuptake and vesicle storage or motility (Bellucci et al., 2012b; Longhena et al., 2019), hints that this neuronal population may be more vulnerable to minimal perturbations of functional homeostasis induced by α-syn accumulation and aggregation. Moreover, the massive energy consumption rate of dopaminergic neurons has to be combined with an enhanced mitochondrial bioenergetics support. This is lost upon synapse degeneration in the brain of PD patients, although it appears to be upregulated with a compensatory fashion in the remaining terminals (Reeve et al., 2018). On this line, findings indicating that α-syn physiologically regulates mitochondrial homeostasis (Guardia-Laguarta et al., 2014; Faustini et al., 2019), while its aggregation prompts mitochondrial dysfunction (Nakamura et al., 2011; Tapias et al., 2017; Wang et al., 2019), support that early pathological shifts in the conformation of this protein, leading to its loss of function and accumulation, may hinder the bioenergetics profile of dopaminergic neurons, compromising their resilience along aging. This hypothesis offers a bridge between two key biological processes that are thought to participate in PD pathogenesis: mitochondrial alterations and α-syn deposition, though it is still arduous to determine who is on first, as mitochondrial impairment can also trigger α-syn pathological aggregation (Betarbet et al., 2002; Zaltieri et al., 2015; Faustini et al., 2018; Grunewald et al., 2019).
Beside α-syn pathology and mitochondrial impairment, numerous other mechanisms have been proposed to contribute to sporadic PD pathogenesis, including neuroinflammation, impaired autophagy, and oxidative stress (Pang et al., 2019). Interestingly, some of these molecular pathways appear to be cross-linked and can be regulated by nuclear factor κB (NF-κB) transcription factors (Kratsovnik et al., 2005; Djavaheri-Mergny et al., 2007; Sarnico et al., 2009a; Morgan and Liu, 2011; Lanzillotta et al., 2015; Kaminska et al., 2016; Nivon et al., 2016; Lingappan, 2018; Nandy et al., 2018). In particular, oxidative stress can activate NF-κB-mediated protective signaling (Kratsovnik et al., 2005; Lingappan, 2018), which can repress autophagy as well as autophagy-dependent apoptosis (Djavaheri-Mergny et al., 2007; Nandy et al., 2018). On the other hand, NF-κB-regulated genes play a major role in controlling the amount of ROS in the cell (Morgan and Liu, 2011; Lingappan, 2018), and by modulating autophagic activity, this factor appears as a key regulator of protein aggregate clearance (Nivon et al., 2016). Upon exposure of microglia to lipopolysaccharide (LPS), NF-κB is among the predominantly activated signaling pathways and initiates the transcription of proinflammatory gene coding for cytokines and proteolytic enzymes (Kaminska et al., 2016). However, the different composition of NF-κB dimers imprints either the protective or the noxious action of this factor. While p50/RelA dimers induce pro-apoptotic Bim and Noxa genes, c-Rel-containing dimers exert neuroprotective actions (Inta et al., 2006; Sarnico et al., 2009b).
NF-κB factors play a crucial role in the regulation of inflammation and apoptosis, are involved in the brain programming of systemic aging, as well as in the pathogenesis of several neurodegenerative diseases (Sarnico et al., 2009a; Mincheva-Tasheva and Soler, 2013; Lanzillotta et al., 2015). The NF-κB family of transcription factors consists of five different subunits (c-Rel, p65/RelA, p50, RelB, and p52), which interact to form transcriptionally active homo and heterodimers (Perkins, 1997; Chen and Greene, 2004; Ghosh et al., 2012). Different findings point to a role of NF-κB/RelA in PD. Interestingly, the nuclear content of RelA is abnormally increased in nigral dopaminergic neurons and glial cells of PD patients. Inhibition of RelA prevents dopaminergic neuron loss in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-mouse model of PD, and downregulation of RelA protects neurons from 1-methyl-4-phenylpyridinium (MPP+) toxicity, suggesting that RelA upregulation, may play a role in dopaminergic neuron degeneration (Ghosh et al., 2007). Moreover, we recently described that mice deficient for c-Rel subunit, which can exert pro-survival effects (Pizzi et al., 2002; Sarnico et al., 2009b), develop a PD-like motor and non-motor phenotype characterized by progressive brain α-syn accumulation and early synaptic changes preceding the frank loss of nigrostriatal neurons (Baiguera et al., 2012; Parrella et al., 2019). This evidence hints that a reduction in the protective function of c-Rel may render dopaminergic neurons more vulnerable to aging, the primary risk factor for PD (Collier et al., 2011; Reeve et al., 2014), thus predisposing toward the development of this disorder.
This review provides an updated critical overview of findings supporting a possible interplay between NF-κB dysregulation and α-syn pathology in PD pathogenesis, with the aim to uncover and discuss novel potential molecular mechanisms involved in this process.
α-Synuclein: Physiological Function and Role in PD
α-synuclein is a neuronal protein mainly localized at synaptic sites. Its physiological functions seem to be mostly related with regulation of neurotransmitter release and recycle, as it modulates the size, assembly, and release of synaptic vesicle pools (Murphy et al., 2000; Burre, 2015; Fusco et al., 2016), neurotransmitter reuptake (Burre, 2015; Longhena et al., 2019), exocytotic fusion pore dilation (Logan et al., 2017), and neurotransmitter vesicular uptake (Guo et al., 2008; Pifl et al., 2014; Phan et al., 2017). Remarkably, the multiplicity of α-syn interactions at synaptic sites, coupled with its intrinsic structural plasticity, can account for the cardinal role of the protein at terminals (Bendor et al., 2013; Longhena et al., 2019; Sulzer and Edwards, 2019). Studies in PD brains and experimental models have shown that α-syn overexpression and aggregation induce significant alterations of synaptic proteins, neuronal dysfunction and degeneration as well as motor deficits (Fleming et al., 2004; Garcia-Reitbock et al., 2010; Bellucci et al., 2011; Lam et al., 2011; Lundblad et al., 2012; Wang et al., 2014; Visanji et al., 2016).
Nonetheless, α-syn has been found to localize in and affect other cellular compartments aside synapses. In the nucleus, the protein physiologically interacts with and regulates histones (Goers et al., 2003; Kontopoulos et al., 2006; Schaser et al., 2019). On the other hand, its overexpression and phosphorylation modulates gene expression (Pinho et al., 2019), impairs the neuroprotective NF-κB signaling pathway (Yuan et al., 2008), and regulates the promoter of proliferator-activated receptor gamma coactivator 1 α (PGC1α), a transcription factor governing mitochondria biogenesis, to inhibit its transcription (Siddiqui et al., 2012).
The overexpression of wild-type (wt) α-syn, and even more of its A53T mutated form, affects endoplasmic reticulum (ER)/Golgi transport by direct binding of the soluble proteins to ER/Golgi soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), resulting in their inhibition (Thayanidhi et al., 2010). Interestingly, this effect appears to be rescued by Rab1 overexpression and is responsible for lysosomal dysfunction onset (Cooper et al., 2006; Martinez-Vicente et al., 2008; Mazzulli et al., 2016; Hoffmann et al., 2019). Alterations in Golgi morphology and increased susceptibility to ER stress have been also found to occur in dopaminergic cells overexpressing A30P α-syn (Paiva et al., 2018).
In addition, we showed that overexpression of wt α-syn, leading to formation of insoluble aggregates within the ER, activates the protein kinase RNA-like ER kinase (PERK)-related pathway of the unfolded protein response (UPR) (Bellucci et al., 2011). Upon misfolded protein accumulation in the ER, the induced UPR inhibits protein synthesis and generation of molecular chaperones implicated in protein folding, whose activation may ultimately lead to apoptotic cell death. These findings have been further corroborated by other studies supporting that ER stress is relevant for the manifestations of synucleinopathy in vivo (Colla et al., 2012a, b; Mercado et al., 2013). For these main reasons, therapeutic strategies targeting the UPR have been proposed as PD treatments (Bellucci et al., 2012a; Mercado et al., 2018; Martinez et al., 2019).
α-synuclein can also bind mitochondria-associated ER membranes (MAM) (Guardia-Laguarta et al., 2014), and evidence indicating that its absence impairs mitochondria lipid composition, function, fusion, and trafficking (Ellis et al., 2005; Faustini et al., 2019) supports that the protein can play a physiological role in the regulation of mitochondrial homeostasis. This hypothesis is in line with findings showing that pathological α-syn aggregates or oligomers produce mitochondria fragmentation, impair mitochondrial trafficking, and lead to respiration failure (Devi et al., 2008; Nakamura et al., 2011; Tapias et al., 2017; Prots et al., 2018; Wang et al., 2019).
Therefore, although synapses, which result the sites where α-syn is most abundant, are likely the first sites to be affected by and suffer from its pathological changes, it is reasonable to prospect that α-syn aggregation and toxicity may proceed progressively through the induction of dysfunctional alterations to other cellular compartments. This view implies that a finer characterization of the pathological changes occurring after the initiation of α-syn aggregation may bring new insight into PD pathogenesis. On the other hand, it reinforces that studies exploring the factors leading or predisposing to α-syn accumulation and aggregation along aging are even more important, as they may help us to identify new targets for the development of therapies halting PD progression.
Another peculiarity of α-syn relates to its transmission from cell-to-cell and to the spreading of its pathological aggregates from the peripheral nervous system (PNS) to the central nervous system (CNS), or vice versa, in experimental models (Luk et al., 2012; Ulusoy et al., 2013, 2017; Recasens and Dehay, 2014; Dehay et al., 2016; Emmanouilidou and Vekrellis, 2016; Helwig et al., 2016; Cavaliere et al., 2017; Rutherford et al., 2017; Grozdanov and Danzer, 2018). The capability of pathological forms of α-syn to spread seems to be corroborated by neuropathological examination of post-mortem brains from patients who received fetal neuron grafts over one decade prior to death and showing the development of LB within grafted neuronal cells (Kordower et al., 2008a, b; Chu and Kordower, 2010; Li et al., 2010; Kurowska et al., 2011). These findings, together with the Braak hypothesis, suggesting that the progression of PD symptoms relates with the caudo-rostral diffusion of LB pathology in the brain (Braak et al., 2003, 2018), fed the shoot up of the prion-like hypothesis of PD, emphasizing the multiple similarities between α-syn and prion protein (Olanow and Brundin, 2013; Brundin et al., 2016; Brundin and Melki, 2017). However, studies showing that PD patients exhibit a systemic α-syn neuropathology within both PNS and CNS (Gelpi et al., 2014; Surguchov, 2016) and a critical analysis of neuronal vulnerability to α-syn accumulation, were supportive for the development of alternative hypotheses as presented by Engelender and Isacson (2017) and Surmeier et al. (2017). Very recent findings showing that systemic delivery of α-syn synthetic pre-formed fibrils in rats trigger pathological transformation of endogenous α-syn, leading to neurodegeneration in discrete CNS and PNS neuronal populations (Kuan et al., 2019), bring novel insights into this subject, opening the way to a more exhaustive comprehension of the role and relevance of α-syn spreading in PD.
NF-κB Factors: Transcriptional Regulators Governing Neuroinflammation, Apoptosis, Neuronal Function and Resilience
NF-κB is expressed in both the CNS and PNS and localizes in neurons, glial cells, and Schwann cells mostly as p50/p50 homodimers and p50/RelA heterodimers (Meffert and Baltimore, 2005). By regulating synaptic signaling and behavior, or pivotally controlling cell survival and glial cell activation, the NF-κB family of transcription factors is crucially involved in the regulation of CNS and PNS response to physiological and pathological stimuli (Meffert et al., 2003; Meffert and Baltimore, 2005; Mattson and Meffert, 2006; Mincheva-Tasheva and Soler, 2013).
NF-κB factors are ubiquitously expressed in mammalian cells, although they were first identified in lymphocytes (Meffert and Baltimore, 2005). All the NF-κB subunits, highly conserved across species, show a Rel homology domain containing the key functional regions for DNA binding, dimerization, nuclear translocation, and interaction with their inhibitory elements named IκB. Only RelA, c-Rel, and RelB show the C-terminal transactivation domain (TAD) that allows the dimer to initiate transcription (Hayden and Ghosh, 2012). Although p52 and p50 lack TADs, their heterodimerization with TAD-containing NF-κB subunits, or interaction with non-Rel proteins that have trans-activating capability, allows them to positively regulate transcription. The homodimers composed by p50 and p52 can also negatively regulate transcription by competing with TAD-containing dimers for binding to κB sites or by constitutively occupying some κB sites to increase the activation threshold for certain NF-κB target genes (Hayden and Ghosh, 2012).
Inactive NF-κB dimers localize in the cytoplasm bound to the IκB inhibitory proteins. NF-κB-inducing stimuli activate the IκB kinase complex, IKKα and IKKβ, with the regulatory IKKγ/NEMO, resulting in sequential phosphorylation, ubiquitination, and degradation of IκB. Upon the detachment of IκB, the exposure of DNA-binding domain and nuclear localization sequence allows the NF-κB dimer to translocate to the nucleus to bind the target gene promoter regions (Karin et al., 2004; Meffert and Baltimore, 2005).
A body of evidence has shown that NF-κB plays a relevant role in regulating the function of immune system by driving both the diverse inflammatory phases and the host defense (Li and Verma, 2002).
In neurons, the transcription of both p50/RelA and p50/p50 NF-κB dimers can be activated by glutamatergic synaptic inputs through Ca2+/calmodulin-dependent protein kinase II (CaMKII) and local submembranous Ca2+ increase (Meffert et al., 2003). While the p50/p50 dimers localize in the cytosol, the p50/RelA dimers are found within synaptic boutons from where, upon glutamate or N-methyl-D-aspartate (NMDA) stimulation, they translocate to reach the nucleus and translate synaptic signals into altered gene expression (Meffert et al., 2003). Consistently, RelA knockout (ko) mice exhibit spatial learning deficits, thus supporting that NF-κB nuclear translocation and gene activation govern long-term changes to adult neuronal function caused by synaptic stimulation.
NF-κB controls adult neurogenesis in CNS (Rolls et al., 2007; Koo et al., 2010), and evidence supporting that these transcriptional regulators are important for ensuring Schwann cell differentiation and myelination of peripheral axons suggests that NF-κB factors are essential differentiation signals with a prominent role also in PNS development and plasticity (Nickols et al., 2003; Tang et al., 2013).
It has been found that most of the stimuli that activate NF-κB in the immune system, such as tumor necrosis factor-α (TNF-α) or interleukin 1 (IL-1), viral infections, and oxidative stress, exert the same effect in the CNS. Though surprisingly, TNF-α-mediated NF-κB activation plays a unique role in mediating neuronal plasticity in the hippocampus without inducing neuroinflammatory changes (Albensi and Mattson, 2000; Beattie et al., 2002). The NF-κB target genes in the CNS are only partially characterized, but it is predictable that these may display significant differences in their promoter organization when compared to the canonical genes affected in the immune system. On this line, we previously showed the presence of two NF-κB sites within the regulatory region of the DA D2 receptor (Bontempi et al., 2007) as a proof of the NF-κB involvement in the regulation of neuronal responses to DA-mediated transmission.
In glial cells, basal NF-κB activity is very low. For this reason, most studies have focused glial NF-κB in models of inflammation, injury, or disease (Dresselhaus and Meffert, 2019) where it is activated in its predominant form, the p50/RelA dimer (Kiebala et al., 2010; Simmons et al., 2016; Gupta et al., 2019). In inflammation, microglia activation results in the transcription of NF-κB-target genes, nitric oxide, IL-1β, and TNFα that in turn induce NF-κB signaling with consequent enhancement of inflammatory mediators that exacerbate neuronal cell death. NF-κB pathway in microglia seems also to actively participate in plasticity mechanisms and neuronal homeostasis in response to injury (Dresselhaus and Meffert, 2019). Likewise, beside inducing pro-inflammatory gene expression, astrocytic NF-κB appears to play a role in the central control of metabolism (Zhang et al., 2017) and, by promoting the clearing of glutamate from the synapses, in the termination of excitatory signals (Ghosh et al., 2011).
NF-κB has been reported to be essential for systemic and brain aging (Adler et al., 2008; Zhang et al., 2013), with RelA subunit mediating the most significant contribution to degenerative changes associated with senescence (Tilstra et al., 2012). On this line, NF-κB dysregulation has been found to participate in brain neurodegenerative mechanisms occurring in PD (Hunot et al., 1997), Alzheimer’s disease (AD) (Kaltschmidt et al., 1997; Chen et al., 2012; Jones and Kounatidis, 2017), as well as in post-traumatic or post-ischemic brain injury (Bethea et al., 1998; Schneider et al., 1999). With regard to the regulation of neuronal cell death, diverse NF-κB dimers in response to specific stimuli can mediate distinct responses (Pizzi et al., 2005; Lanzillotta et al., 2015). We showed that the selective inhibition of RelA or c-Rel expression produces opposite effects on neuron survival. Whereas the over-activation of p50/RelA dimers promotes apoptosis, activation of c-Rel-containing dimers improves the resilience of neuronal cells after injury (Pizzi et al., 2002, 2005; Sarnico et al., 2009b). Neurotoxic stimuli, such as ischemia, high glutamate concentrations, β-amyloid, or MPP+, induce the activation of p50/RelA dimers improving the transcription of proapoptotic genes (Pizzi et al., 2002, 2005; Inta et al., 2006; Valerio et al., 2006; Sarnico et al., 2008, 2009b; Yang et al., 2010). Conversely, c-Rel-containing dimers favor the expression of anti-apoptotic genes by signals promoting neuroprotection in diverse cell-based neurotoxic settings, such as IL-1β in NMDA-mediated excitotoxicity, mGlu5 receptor agonists in β-amyloid- or MPP+-mediated toxicity and adipocyte-derived hormone leptin in oxygen and glucose deprivation (OGD)-mediated apoptosis (Pizzi et al., 2002, 2005; Kogel et al., 2004; Valerio et al., 2006; Sarnico et al., 2008). Furthermore, the over-expression of c-Rel in cultured neurons promotes anti-apoptotic effects by inducing manganese superoxide dismutase (MnSOD) and Bcl-xL (Bernard et al., 2001; Pizzi et al., 2005). Overabundance of c-Rel also limits the generation of reactive oxygen species (ROS) by inducing transcription of the mitochondrial uncoupling proteins 4 (UCP4) (Ho et al., 2012), a brain-specific mitochondrial ion channel producing mild reduction in mitochondrial membrane potential and neuroprotection (Echtay, 2007).
The dual effects of NF-κB activation on neuron survival were corroborated by studies in severe brain ischemia models. Indeed, a rapid activation of p50/RelA in both neurons and glial cells has been implicated in the pathogenesis of post-ischemic injury (Herrmann et al., 2005; Crack et al., 2006). In ischemic brain tissue of mice subjected to permanent middle cerebral artery occlusion (MCAO) and in primary cortical neurons exposed to OGD, NF-κB followed a similar pattern of activation (Pizzi et al., 2009; Lanzillotta et al., 2010) characterized by increased nuclear translocation of p50/RelA dimers (Inta et al., 2006; Pizzi et al., 2009) and decreased translocation of c-Rel-containing dimers (Sarnico et al., 2009b). In these conditions, NF-κB activity was associated with an unbalanced expression of pro-apoptotic RelA target genes, with increased expression of the pro-apoptotic members of Bcl-2 family genes, Bim and Noxa, and parallel reduction of the anti-apoptotic member Bcl-xL (Cao et al., 2002; Inta et al., 2006; Sarnico et al., 2009b). During brain ischemia, RelA induced the expression of the 1B isoform of divalent metal transporter-1 (1B/DMT1), the membrane carrier responsible for iron accumulation and brain damage after injury (Ingrassia et al., 2012). This acted as an upstream mechanism responsible for iron accumulation and contributing to neuronal cell death. Knocking-down c-Rel expression exacerbated neuronal susceptibility to OGD-mediated damage and c-Rel ko mice exposed to cerebral ischemia resulted insensitive to the neuroprotective activity of leptin, a c-Rel inducer capable of limiting cortical damage in wt mice (Valerio et al., 2006, 2009). These data strongly suggested that inhibition of c-Rel-containing dimers and activation of p50/RelA are key events in the pathogenesis of post-ischemic brain injury.
Despite these premises, p50/RelA activation per se appeared to be insufficient to drive pro-apoptotic transcription during brain ischemia. Site-specific acetylation of RelA at the Lys 310 residue was necessary to switch anti-apoptotic p50/RelA, activated after a brief preconditioning ischemia and leading to brain tolerance toward the pro-apoptotic p50/RelA activated after a prolonged harmful brain ischemia (Blondeau et al., 2001; Lanzillotta et al., 2010). In this regard, studies showing that post-stroke induction of α-syn mediates ischemic brain damage (Ishimaru et al., 1998; Hu et al., 2006; Yoon et al., 2006; Unal-Cevik et al., 2011; Surgucheva et al., 2014; Kim T. et al., 2016) and that the levels of oligomeric form of α-syn of red blood cells in ischemic stroke and PD patients are both significantly higher than in controls (Zhao et al., 2016), seem to shed light on a possible link between NF-κB dysregulation and α-syn accumulation that deserves to be addressed by ad hoc studies.
NF-κB Alterations in PD and Their Link to α-syn Pathology
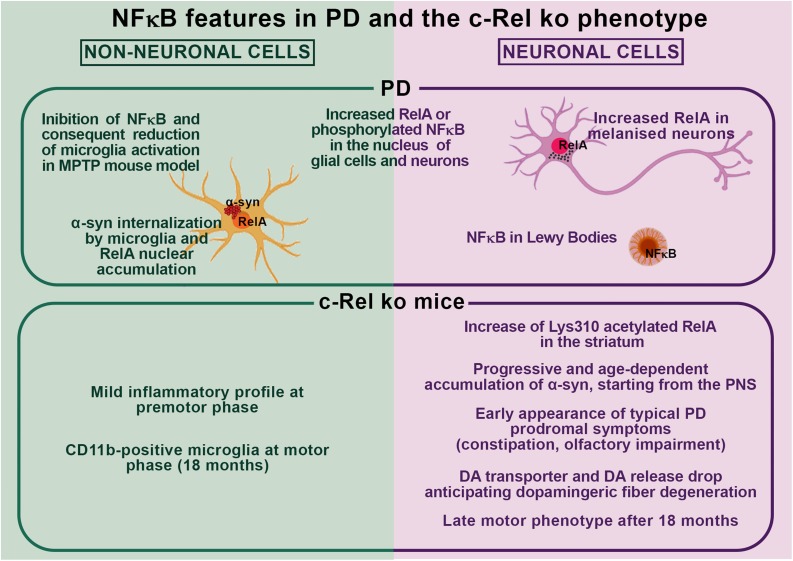
A body of evidence has highlighted the occurrence of changes and dysregulation of NF-κB in PD. Post-mortem studies showed that in the brain of patients, there is an increased RelA nuclear translocation in melanized neurons of the substantia nigra that is supportive of NF-κB activation in PD (Hunot et al., 1997). Later studies, addressing glial involvement in the degeneration process of LB-bearing neurons on the post-mortem brain of patients affected by dementia with LB (DLB), found that some LB present NF-κB immunopositivity (Togo et al., 2001), thus hinting that NF-κB may participate in the α-syn-deposition-dependent neuronal loss. These findings were corroborated by other studies showing that either RelA or active phosphorylated NF-κB can be detected in the nucleus of subpopulations of neurons and glial cells of the substantia nigra of PD patients (Ghosh et al., 2007; Garcia-Esparcia et al., 2014). On this line, it has been described that α-syn can be internalized by microglia and induces nuclear accumulation of RelA (Cao et al., 2010, 2012). Moreover, Ghosh and co-authors found NF-κB activation in the substantia nigra of MPTP-treated mice. Mice treatment with a peptide corresponding to the NEMO-binding domain (NBD) of IKKα or IKKβ, which acts as a selective NF-κB inhibitor, reduced microglia activation in the substantia nigra, prevented both nigrostriatal axis degeneration, DA loss and improved motor functions (Ghosh et al., 2007). These findings confirmed that, by modulating microglia activation, NF-κB deregulation may play a role in MPTP mouse model. Consistently, Wang et al. (2020) have recently corroborated that c-Rel is rapidly upregulated in the substantia nigra and striatum of mice acutely exposed to MPTP treatment. They also found that c-Rel can maintain neuronal survival by promoting antiapoptotic gene expression in MPP+-exposed SH-SY5Y cells and inhibits LPS-induced BV2 cell activation by suppressing inflammatory gene expression, while the c-Rel inhibitor IT901 aggravated neuronal damage and microglia activation in the acute MPTP mouse model (Wang et al., 2020). Interestingly, they also reported a significant reduction in c-Rel expression in whole blood samples from PD patients (Wang et al., 2020), thus supporting that the loss of the protective role of c-Rel could play a role in PD-related neurodegeneration. It has also been shown that the expression of RING finger protein 11 (RNF11), a negative regulator of NF-κB signaling pathway localizing in LB (Anderson et al., 2007), is reduced in PD brains, and this feature correlates with increased phosphorylated form of activated RelA. Other studies support that NF-κB dysregulation may play a role in the control of α-syn expression (Liu et al., 2014). In particular, blockade of NF-κB signaling in a rat MPTP model was found to significantly decrease histone H3 acetylation in the α-syn SNCA promoter region, thus blunting α-syn in the substantia nigra and allowing recovery of the motor dysfunction (Liu et al., 2014). We recently described that mice deficient for c-Rel factor model most of the features of human PD (Baiguera et al., 2012; Parrella et al., 2019). In particular, at 18 months of age, they develop an L-Dopa-responsive parkinsonism, which associates with significant loss of nigral neurons and striatal fibers, reduction of DA levels, increased immunoreactivity for fibrillary α-syn, and cluster of differentiation 11b (CD11b)-positive microglia as well as iron accumulation with augmented DMT1 expression in the substantia nigra (Baiguera et al., 2012; Parrella et al., 2019). At 18 months of age, c-Rel ko mice also display a striatal increase in the proapoptotic form of RelA, carrying the site-specific acetylation at Lys 310 residue (Lanzillotta et al., 2015) that, in light of our previous findings (Sarnico et al., 2009b), supports the induction of neuronal damage. From 2 months of age, c-Rel-deficient mice suffer olfactory impairment and intestinal constipation associated with α-syn accumulation in the distal colon. At 5 months, they also start to exhibit progressive age-dependent deposition of fibrillary α-syn in the olfactory bulbs, dorsal motor nucleus of vagus, and locus coeruleus. The substantia nigra is affected by α-syn accumulation only from 12 months of age, when the striatal DA transporter drops, anticipating by 6 months the dopaminergic fiber degeneration. Finally, from 12 months onward, c-Rel ko mice exhibit oxidative/nitrosative stress in the striatum that parallels the altered expression of mitochondrial homeostasis regulators in the substantia nigra (Parrella et al., 2019). To assess whether inflammation and microglia activation accompany the onset and the progression of such PD-like pathology, we investigated the expression of cytokines and microglia/macrophage activation markers, together with microglial ionized calcium-binding adapter molecule 1 (Iba1) and astrocyte glial fibrillary acidic protein (GFAP) immunoreactivity, in the substantia nigra of c-Rel-deficient mice at both premotor and motor phase (Porrini et al., 2017). We observed increased expression of markers for alternative microglia/macrophage activation [mannose receptor C-type 1 (MRC 1) and arginase 1 (Arg1)] in 4-month-old c-Rel ko mice that, however, dropped in 13-month-old mice. At this age, they rather exhibited an increased expression of pro-inflammatory IL-1β, but not IL-6 or the microglia/macrophage phagocytic marker receptor for the Fc region of complexed immunoglobulins gamma (Fcgr3)/CD16, when compared to wt. At 18 months, c-Rel-deficient mice did not show significant variations in the transcription of inflammatory and microglia/macrophage activation genes when compared to age-matched wt littermates. Immunofluorescence analysis of Iba1-positive and GFAP-positive cells in the substantia nigra revealed no morphological changes in microglia and astrocytes in c-Rel ko mice along aging, while MRC1-Iba1-positive cells were identified as non-parenchymal macrophages only in 4-month-old animals. These observations indicate that c-Rel-deficient mice exhibit a mild brain inflammatory profile at a premotor phase (12 months) without evident signs of gliosis. Unexpectedly, this finding supports that a PD-like pathology can develop also in the absence of concomitant severe brain inflammatory process.
Taken together, our studies on c-Rel ko mice offer new insights into the pathogenesis of PD. They support that NF-κB unbalance increases the susceptibility of neurons to aging by promoting pathological changes, such as accumulation of α-syn and nigral neuronal loss, which are peculiar features of frail aged subjects over and above PD patients’ brain (Buchman et al., 2013, 2014). Indeed, the LB pathology incidentally detected in brain of people dying from non-neurological causes support that PD is an exacerbated version of aging, and if subjects could live longer, they would all develop the disease (Hawkes, 2008; Reeve et al., 2014). Therefore, alterations in c-Rel function may predispose people to PD development. What we miss are the mechanistic insights linking this phenomenon to α-syn accumulation and neurodegeneration. This notwithstanding, multiple pathways could be responsible for the onset of synucleinopathy in the brain of c-Rel ko mice. The peculiar energy-demanding profile of dopaminergic neurons, which depends on their distinctive morphological/structural and physiological features, predisposes them to mitochondrial shortfalls (Surmeier, 2018). The energy production by mitochondria and ER in the dopaminergic neurons of the substantia nigra associates with the generation of large amounts of ROS (Pacelli et al., 2015). ROS can be constantly neutralized by anti-oxidant systems, including superoxide dismutases (SODs), catalases, glutathione peroxidase, and UCP4 and UCP5. Remarkably, UCP4 and MnSOD are known to mediate the protective effects of c-Rel (Chen et al., 2000; Ho et al., 2012), supporting that, by blunting the expression of these genes, the absence of c-Rel might enhance ROS accumulation during aging in substantia nigra neurons. This process can synergize with reduced c-Rel-dependent expression of anti-apoptotic Bcl-xL (Sarnico et al., 2009a) to affect neuronal resilience. It is also conceivable that mitochondria impairment associated with c-Rel deficiency may first switch the acetylation state of RelA during aging to elevate Bim, DMT1, iron, as well as α-syn intracellular levels (Halliwell, 2006; Surmeier, 2018), which result into deposition of α-syn aggregates and neuronal damage.
It is worth mentioning that NFκB appears as a central and major regulator of protein aggregate clearance by modulating autophagic activity. Misincorporation of amino acid analogs into proteins as well as inhibition of proteasomal activity or expression of mutated SOD1 can induce a non-canonical NFκB activation that, in turn, upregulates the expression of two activators of selective autophagy, Bcl-2-associated athanogene 3 (BAG3), and heat shock protein B8 (HspB8), thus allowing clearance of protein aggregates (Nivon et al., 2016). Therefore, we may advance the hypothesis that a dysregulation of NF-κB deriving from c-Rel deficiency may predispose cells to misfolded protein accumulation, an effect that in the context of aging and PD may produce enhancement of α-syn accumulation as a main fall-out.
In addition to these possible explanations to justify how c-Rel deficiency may lead to PD, relevant clues may also derive from the fact that this factor is a key regulator of immunity by affecting T-cell differentiation and regulatory T-cell (Treg) function (Hilliard et al., 2002; Visekruna et al., 2012; Grinberg-Bleyer et al., 2017; Luu et al., 2017). Either innate or adaptive immune responses are implicated in PD (Kannarkat et al., 2013; Sulzer et al., 2017; Kustrimovic et al., 2019; Li et al., 2019; Tansey and Romero-Ramos, 2019). PD patients exhibit both brain and peripheral inflammation, and it has been found that inflammatory bowel disease predisposes to PD (Brudek, 2019). Notably, neuroinflammation promotes α-syn prion-like behavior, and along with aging, both the gastrointestinal tract and olfactory epithelium, which have been proposed as the initiation sites for α-syn spreading in the prion hypothesis, are mostly vulnerable to inflammation (Lema Tome et al., 2013). A body of evidence shows that by secreting antimicrobial peptides such as mucin or defensins or sensing pathogens via Toll-like receptors, enterocytes or intestinal epithelial cells represent the first barrier against gut microbiota (Magrone and Jirillo, 2013; Nagpal et al., 2018). Intestinal microbiota and gut immune system interact with each other, maintaining a condition of homeostasis in the context of the intestinal habitat. In daily interplay between normal microbiota (Balfour Sartor, 1997) and innate and adaptive immune cells, the more harmful bacteria species induce the release of pro-inflammatory IL-17 by T helper 17 (Th17) cells, which lead to IL-8 production by intestinal epithelial cells (Wu et al., 2009). Conversely, protective bacteria stimulate anti-inflammatory IL-10 by Treg cells that, by counteracting the activity of Th17 cells, avoid the noxious reaction to the host (Magrone and Jirillo, 2013).
As c-Rel is a crucial determinant of Treg identity and function (Grinberg-Bleyer et al., 2017), it may be easily conceivable that compromised Treg-mediated activity make c-Rel-deficient mice more vulnerable to microbiota-dependent bowel inflammation. The enteric inflammatory microenvironment, together with LPS, present at the outer membrane of Gram-negative bacteria, would, in turn, promote α-syn conformational shifts, aggregation, and spreading (Kim C. et al., 2016; Fitzgerald et al., 2019). Our observations, showing that young c-Rel ko mice exhibit α-syn accumulation in the distal colon (Parrella et al., 2019) anticipating the progressive α-syn brain deposition, sound in line with this hypothesis. Studies on patients with early stage diagnosed PD, showing that α-syn staining in the enteric nervous system correlates with compromised intestinal barrier integrity (Lema Tome et al., 2013) seem to further corroborate the above postulate.
Conclusion
Collectively, these findings support the occurrence of a possible interplay between NF-κB dysregulation and α-syn pathology in PD as observed in c-Rel ko mice (Figure 1). In particular, by affecting key molecular pathways driving neuronal resilience to stressors, neuroinflammation, and protein misfolding, unbalances in the activation of NF-κB factors may significantly contribute to PD pathogenesis by favoring α-syn accumulation, aggregation and spreading, and promoting glial cell activation and neuronal cell death. These same processes may boost NF-κB activation, initiating a vicious circle perpetrating disease progression. Further studies are compelling to elucidate the causes and features of NF-κB alterations as well as whether and how NF-κB unbalance may lead to α-syn pathology and neurodegeneration in PD.
FIGURE 1.
Schematic overview of nuclear factor-κB (NF-κB) features in Parkinson’s disease (PD) and in the c-Rel ko mouse model.
Author Contributions
AB wrote the first draft of the manuscript. AB, LB, FL, GF, CM, EP, FB, VP, and MP revised the manuscript. AB, FL, FB, and EP prepared the figure and integrated it in the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. We are grateful to the Italian MIUR PRIN 2017-1065 and the MIUR PNR 2015-2020 PerMedNet. VP Researcher Fellowship is covered by Fondazione Cariplo–Giovani Ricercatori – Research Support GR 2018-0391.
References
- Adler A. S., Kawahara T. L., Segal E., Chang H. Y. (2008). Reversal of aging by NFkappaB blockade. Cell Cycle 7 556–559. 10.4161/cc.7.5.5490 [DOI] [PubMed] [Google Scholar]
- Albensi B. C., Mattson M. P. (2000). Evidence for the involvement of TNF and NF-kappaB in hippocampal synaptic plasticity. Synapse 35 151–159. [DOI] [PubMed] [Google Scholar]
- Anderson L. R., Betarbet R., Gearing M., Gulcher J., Hicks A. A., Stefansson K., et al. (2007). PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J. Neuropathol. Exp. Neurol. 66 955–964. 10.1097/nen.0b013e3181567f17 [DOI] [PubMed] [Google Scholar]
- Baiguera C., Alghisi M., Pinna A., Bellucci A., De Luca M. A., Frau L., et al. (2012). Late-onset Parkinsonism in NFkappaB/c-Rel-deficient mice. Brain 135(Pt 9), 2750–2765. 10.1093/brain/aws193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour Sartor R. (1997). Enteric microflora in IBD: pathogens or commensals? Inflamm. Bowel Dis. 3 230–235. 10.1097/00054725-199709000-00008 [DOI] [PubMed] [Google Scholar]
- Beattie E. C., Stellwagen D., Morishita W., Bresnahan J. C., Ha B. K., Von Zastrow M., et al. (2002). Control of synaptic strength by glial TNFalpha. Science 295 2282–2285. 10.1126/science.1067859 [DOI] [PubMed] [Google Scholar]
- Bellucci A., Antonini A., Pizzi M., Spano P. (2017). The end is the beginning: Parkinson’s disease in the light of brain imaging. Front. Aging Neurosci. 9:330. 10.3389/fnagi.2017.00330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellucci A., Mercuri N. B., Venneri A., Faustini G., Longhena F., Pizzi M., et al. (2016). Review: Parkinson’s disease: from synaptic loss to connectome dysfunction. Neuropathol. Appl. Neurobiol. 42 77–94. 10.1111/nan.12297 [DOI] [PubMed] [Google Scholar]
- Bellucci A., Navarria L., Zaltieri M., Falarti E., Bodei S., Sigala S., et al. (2011). Induction of the unfolded protein response by α-synuclein in experimental models of Parkinson’s disease. J. Neurochem. 116 588–605. 10.1111/j.1471-4159.2010.07143.x [DOI] [PubMed] [Google Scholar]
- Bellucci A., Navarria L., Zaltieri M., Missale C., Spano P. (2012a). alpha-synuclein synaptic pathology and its implications in the development of novel therapeutic approaches to cure Parkinson’s disease. Brain Res. 1432 95–113. 10.1016/j.brainres.2011.11.031 [DOI] [PubMed] [Google Scholar]
- Bellucci A., Zaltieri M., Navarria L., Grigoletto J., Missale C., Spano P. (2012b). From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 1476 183–202. 10.1016/j.brainres.2012.04.014 [DOI] [PubMed] [Google Scholar]
- Bendor J. T., Logan T. P., Edwards R. H. (2013). The function of alpha-synuclein. Neuron 79 1044–1066. 10.1016/j.neuron.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard D., Quatannens B., Begue A., Vandenbunder B., Abbadie C. (2001). Antiproliferative and antiapoptotic effects of crel may occur within the same cells via the up-regulation of manganese superoxide dismutase. Cancer Res. 61 2656–2664. [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., Di Monte D. A., Greenamyre J. T. (2002). Mechanistic approaches to Parkinson’s disease pathogenesis. Brain Pathol. 12 499–510. 10.1111/j.1750-3639.2002.tb00468.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea J. R., Castro M., Keane R. W., Lee T. T., Dietrich W. D., Yezierski R. P. (1998). Traumatic spinal cord injury induces nuclear factor-kappaB activation. J. Neurosci. 18 3251–3260. 10.1523/jneurosci.18-09-03251.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blondeau N., Widmann C., Lazdunski M., Heurteaux C. (2001). Activation of the nuclear factor-kappaB is a key event in brain tolerance. J. Neurosci. 21 4668–4677. 10.1523/jneurosci.21-13-04668.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontempi S., Fiorentini C., Busi C., Guerra N., Spano P., Missale C. (2007). Identification and characterization of two nuclear factor-kappaB sites in the regulatory region of the dopamine D2 receptor. Endocrinology 148 2563–2570. 10.1210/en.2006-1618 [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici K., Rub U., de Vos R. A., Jansen Steur E. N., Braak E. (2003). Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 24 197–211. 10.1016/s0197-4580(02)00065-9 [DOI] [PubMed] [Google Scholar]
- Braak H., Del Tredici-Braak K., Gasser T. (2018). Special issue “Parkinson’s disease”. Cell Tissue Res. 373 1–7. [DOI] [PubMed] [Google Scholar]
- Brudek T. (2019). Inflammatory bowel diseases and Parkinson’s disease. J. Parkinsons Dis. 9 S331–S344. 10.3233/JPD-191729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Ma J., Kordower J. H. (2016). How strong is the evidence that Parkinson’s disease is a prion disorder? Curr. Opin. Neurol. 29 459–466. 10.1097/WCO.0000000000000349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P., Melki R. (2017). Prying into the prion hypothesis for Parkinson’s disease. J. Neurosci. 37 9808–9818. 10.1523/JNEUROSCI.1788-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S., Yu L., Wilson R. S., Boyle P. A., Schneider J. A., Bennett D. A. (2014). Brain pathology contributes to simultaneous change in physical frailty and cognition in old age. J. Gerontol. A Biol. Sci. Med. Sci. 69 1536–1544. 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchman A. S., Yu L., Wilson R. S., Schneider J. A., Bennett D. A. (2013). Association of brain pathology with the progression of frailty in older adults. Neurology 80 2055–2061. 10.1212/WNL.0b013e318294b462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burre J. (2015). The synaptic function of alpha-synuclein. J. Parkinsons Dis. 5 699–713. 10.3233/JPD-150642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G., Pei W., Ge H., Liang Q., Luo Y., Sharp F. R., et al. (2002). In vivo delivery of a Bcl-xL fusion protein containing the TAT protein transduction domain protects against ischemic brain injury and neuronal apoptosis. J. Neurosci. 22 5423–5431. 10.1523/jneurosci.22-13-05423.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Standaert D. G., Harms A. S. (2012). The gamma chain subunit of Fc receptors is required for alpha-synuclein-induced pro-inflammatory signaling in microglia. J. Neuroinflammation 9:259. 10.1186/1742-2094-9-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Theodore S., Standaert D. G. (2010). Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol. Neurodegener. 5:42. 10.1186/1750-1326-5-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaliere F., Cerf L., Dehay B., Ramos-Gonzalez P., De Giorgi F., Bourdenx M., et al. (2017). In vitro alpha-synuclein neurotoxicity and spreading among neurons and astrocytes using Lewy body extracts from Parkinson disease brains. Neurobiol. Dis. 103 101–112. 10.1016/j.nbd.2017.04.011 [DOI] [PubMed] [Google Scholar]
- Chen C., Edelstein L. C., Gelinas C. (2000). The Rel/NF-kappaB family directly activates expression of the apoptosis inhibitor Bcl-x(L). Mol. Cell. Biol. 20 2687–2695. 10.1128/mcb.20.8.2687-2695.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. H., Zhou W., Liu S., Deng Y., Cai F., Tone M., et al. (2012). Increased NF-kappaB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 15 77–90. 10.1017/S1461145711000149 [DOI] [PubMed] [Google Scholar]
- Chen L. F., Greene W. C. (2004). Shaping the nuclear action of NF-kappaB. Nat. Rev. Mol. Cell Biol. 5 392–401. 10.1038/nrm1368 [DOI] [PubMed] [Google Scholar]
- Chu Y., Kordower J. H. (2010). Lewy body pathology in fetal grafts. Ann. N. Y. Acad. Sci. 1184 55–67. 10.1111/j.1749-6632.2009.05229.x [DOI] [PubMed] [Google Scholar]
- Colla E., Coune P., Liu Y., Pletnikova O., Troncoso J. C., Iwatsubo T., et al. (2012a). Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J. Neurosci. 32 3306–3320. 10.1523/JNEUROSCI.5367-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colla E., Jensen P. H., Pletnikova O., Troncoso J. C., Glabe C., Lee M. K. (2012b). Accumulation of toxic alpha-synuclein oligomer within endoplasmic reticulum occurs in alpha-synucleinopathy in vivo. J. Neurosci. 32 3301–3305. 10.1523/JNEUROSCI.5368-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier T. J., Kanaan N. M., Kordower J. H. (2011). Ageing as a primary risk factor for Parkinson’s disease: evidence from studies of non-human primates. Nat. Rev. Neurosci. 12 359–366. 10.1038/nrn3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., et al. (2006). Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313 324–328. 10.1126/science.1129462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crack P. J., Taylor J. M., Ali U., Mansell A., Hertzog P. J. (2006). Potential contribution of NF-kappaB in neuronal cell death in the glutathione peroxidase-1 knockout mouse in response to ischemia-reperfusion injury. Stroke 37 1533–1538. 10.1161/01.STR.0000221708.17159.64 [DOI] [PubMed] [Google Scholar]
- Dehay B., Vila M., Bezard E., Brundin P., Kordower J. H. (2016). Alpha-synuclein propagation: new insights from animal models. Mov. Disord. 31 161–168. 10.1002/mds.26370 [DOI] [PubMed] [Google Scholar]
- Devi L., Raghavendran V., Prabhu B. M., Avadhani N. G., Anandatheerthavarada H. K. (2008). Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J. Biol. Chem. 283 9089–9100. 10.1074/jbc.M710012200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djavaheri-Mergny M., Amelotti M., Mathieu J., Besancon F., Bauvy C., Codogno P. (2007). Regulation of autophagy by NFkappaB transcription factor and reactives oxygen species. Autophagy 3 390–392. 10.4161/auto.4248 [DOI] [PubMed] [Google Scholar]
- Dresselhaus E. C., Meffert M. K. (2019). Cellular specificity of NF-kappaB function in the nervous system. Front. Immunol. 10:1043. 10.3389/fimmu.2019.01043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay K. S. (2007). Mitochondrial uncoupling proteins–what is their physiological role? Free Radic. Biol. Med. 43 1351–1371. 10.1016/j.freeradbiomed.2007.08.011 [DOI] [PubMed] [Google Scholar]
- Ellis C. E., Murphy E. J., Mitchell D. C., Golovko M. Y., Scaglia F., Barcelo-Coblijn G. C., et al. (2005). Mitochondrial lipid abnormality and electron transport chain impairment in mice lacking alpha-synuclein. Mol. Cell. Biol. 25 10190–10201. 10.1128/MCB.25.22.10190-10201.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmanouilidou E., Vekrellis K. (2016). Exocytosis and spreading of normal and aberrant alpha-synuclein. Brain Pathol. 26 398–403. 10.1111/bpa.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelender S., Isacson O. (2017). The threshold theory for Parkinson’s disease. Trends Neurosci. 40 4–14. 10.1016/j.tins.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Faustini G., Longhena F., Varanita T., Bubacco L., Pizzi M., Missale C., et al. (2018). Synapsin III deficiency hampers alpha-synuclein aggregation, striatal synaptic damage and nigral cell loss in an AAV-based mouse model of Parkinson’s disease. Acta Neuropathol. 136 621–639. 10.1007/s00401-018-1892-1 [DOI] [PubMed] [Google Scholar]
- Faustini G., Marchesan E., Zonta L., Bono F., Bottani E., Longhena F., et al. (2019). Alpha-synuclein preserves mitochondrial fusion and function in neuronal cells. Oxid. Med. Cell. Longev. 2019:4246350. 10.1155/2019/4246350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald E., Murphy S., Martinson H. A. (2019). Alpha-synuclein pathology and the role of the microbiota in Parkinson’s disease. Front. Neurosci. 13:369. 10.3389/fnins.2019.00369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming S. M., Salcedo J., Fernagut P. O., Rockenstein E., Masliah E., Levine M. S., et al. (2004). Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J. Neurosci. 24 9434–9440. 10.1523/JNEUROSCI.3080-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fusco G., De Simone A., Arosio P., Vendruscolo M., Veglia G., Dobson C. M. (2016). Structural ensembles of membrane-bound alpha-synuclein reveal the molecular determinants of synaptic vesicle affinity. Sci. Rep. 6:27125. 10.1038/srep27125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Esparcia P., Llorens F., Carmona M., Ferrer I. (2014). Complex deregulation and expression of cytokines and mediators of the immune response in Parkinson’s disease brain is region dependent. Brain Pathol. 24 584–598. 10.1111/bpa.12137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Reitbock P., Anichtchik O., Bellucci A., Iovino M., Ballini C., Fineberg E., et al. (2010). SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain 133(Pt 7), 2032–2044. 10.1093/brain/awq132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpi E., Navarro-Otano J., Tolosa E., Gaig C., Compta Y., Rey M. J., et al. (2014). Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov. Disord. 29 1010–1018. 10.1002/mds.25776 [DOI] [PubMed] [Google Scholar]
- Ghosh A., Roy A., Liu X., Kordower J. H., Mufson E. J., Hartley D. M., et al. (2007). Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 104 18754–18759. 10.1073/pnas.0704908104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh G., Wang V. Y., Huang D. B., Fusco A. (2012). NF-kappaB regulation: lessons from structures. Immunol. Rev. 246 36–58. 10.1111/j.1600-065X.2012.01097.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh M., Yang Y., Rothstein J. D., Robinson M. B. (2011). Nuclear factor-kappaB contributes to neuron-dependent induction of glutamate transporter-1 expression in astrocytes. J. Neurosci. 31 9159–9169. 10.1523/JNEUROSCI.0302-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goers J., Manning-Bog A. B., McCormack A. L., Millett I. S., Doniach S., Di Monte D. A., et al. (2003). Nuclear localization of alpha-synuclein and its interaction with histones. Biochemistry 42 8465–8471. 10.1021/bi0341152 [DOI] [PubMed] [Google Scholar]
- Grinberg-Bleyer Y., Oh H., Desrichard A., Bhatt D. M., Caron R., Chan T. A., et al. (2017). NF-kappaB c-Rel is crucial for the regulatory T cell immune checkpoint in cancer. Cell 170 1096–1108.e13. 10.1016/j.cell.2017.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozdanov V., Danzer K. M. (2018). Release and uptake of pathologic alpha-synuclein. Cell Tissue Res. 373 175–182. 10.1007/s00441-017-2775-9 [DOI] [PubMed] [Google Scholar]
- Grunewald A., Kumar K. R., Sue C. M. (2019). New insights into the complex role of mitochondria in Parkinson’s disease. Prog. Neurobiol. 177 73–93. 10.1016/j.pneurobio.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Guardia-Laguarta C., Area-Gomez E., Rub C., Liu Y., Magrane J., Becker D., et al. (2014). alpha-synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 34 249–259. 10.1523/JNEUROSCI.2507-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J. T., Chen A. Q., Kong Q., Zhu H., Ma C. M., Qin C. (2008). Inhibition of vesicular monoamine transporter-2 activity in alpha-synuclein stably transfected SH-SY5Y cells. Cell. Mol. Neurobiol. 28 35–47. 10.1007/s10571-007-9227-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Waters M. R., Biswas D. D., Brown L. N., Surace M. J., Floros C., et al. (2019). RelB controls adaptive responses of astrocytes during sterile inflammation. Glia 67 1449–1461. 10.1002/glia.23619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. (2006). Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 97 1634–1658. 10.1111/j.1471-4159.2006.03907.x [DOI] [PubMed] [Google Scholar]
- Hawkes C. H. (2008). Parkinson’s disease and aging: same or different process? Mov. Disord. 23 47–53. 10.1002/mds.21766 [DOI] [PubMed] [Google Scholar]
- Hayden M. S., Ghosh S. (2012). NF-kappaB, the first quarter-century: remarkable progress and outstanding questions. Genes Dev. 26 203–234. 10.1101/gad.183434.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwig M., Klinkenberg M., Rusconi R., Musgrove R. E., Majbour N. K., El-Agnaf O. M., et al. (2016). Brain propagation of transduced alpha-synuclein involves non-fibrillar protein species and is enhanced in alpha-synuclein null mice. Brain 139(Pt 3), 856–870. 10.1093/brain/awv376 [DOI] [PubMed] [Google Scholar]
- Herrmann O., Baumann B., de Lorenzi R., Muhammad S., Zhang W., Kleesiek J., et al. (2005). IKK mediates ischemia-induced neuronal death. Nat. Med. 11 1322–1329. 10.1038/nm1323 [DOI] [PubMed] [Google Scholar]
- Hilliard B. A., Mason N., Xu L., Sun J., Lamhamedi-Cherradi S. E., Liou H. C., et al. (2002). Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J. Clin. Invest. 110 843–850. 10.1172/JCI15254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho J. W., Ho P. W., Liu H. F., So D. H., Chan K. H., Tse Z. H., et al. (2012). UCP4 is a target effector of the NF-kappaB c-Rel prosurvival pathway against oxidative stress. Free Radic. Biol. Med. 53 383–394. 10.1016/j.freeradbiomed.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Hoffmann A. C., Minakaki G., Menges S., Salvi R., Savitskiy S., Kazman A., et al. (2019). Extracellular aggregated alpha synuclein primarily triggers lysosomal dysfunction in neural cells prevented by trehalose. Sci. Rep. 9:544. 10.1038/s41598-018-35811-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Rea H. C., Wiktorowicz J. E., Perez-Polo J. R. (2006). Proteomic analysis of hypoxia/ischemia-induced alteration of cortical development and dopamine neurotransmission in neonatal rat. J. Proteome Res. 5 2396–2404. 10.1021/pr060209x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunot S., Brugg B., Ricard D., Michel P. P., Muriel M. P., Ruberg M., et al. (1997). Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 94 7531–7536. 10.1073/pnas.94.14.7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingrassia R., Lanzillotta A., Sarnico I., Benarese M., Blasi F., Borgese L., et al. (2012). 1B/(-)IRE DMT1 expression during brain ischemia contributes to cell death mediated by NF-kappaB/RelA acetylation at Lys310. PLoS One 7:e38019. 10.1371/journal.pone.0038019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta I., Paxian S., Maegele I., Zhang W., Pizzi M., Spano P., et al. (2006). Bim and Noxa are candidates to mediate the deleterious effect of the NF-kappa B subunit RelA in cerebral ischemia. J. Neurosci. 26 12896–12903. 10.1523/JNEUROSCI.3670-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru H., Ueda K., Takahashi A., Maruyama Y. (1998). Changes in presynaptic protein NACP/alpha-synuclein in an ischemic gerbil hippocampus. Brain Res. 788 311–314. 10.1016/s0006-8993(98)00033-x [DOI] [PubMed] [Google Scholar]
- Jones S. V., Kounatidis I. (2017). Nuclear factor-Kappa B and Alzheimer disease, unifying genetic and environmental risk factors from cell to humans. Front. Immunol. 8:1805. 10.3389/fimmu.2017.01805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B., Uherek M., Volk B., Baeuerle P. A., Kaltschmidt C. (1997). Transcription factor NF-kappaB is activated in primary neurons by amyloid beta peptides and in neurons surrounding early plaques from patients with Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 94 2642–2647. 10.1073/pnas.94.6.2642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B., Mota M., Pizzi M. (2016). Signal transduction and epigenetic mechanisms in the control of microglia activation during neuroinflammation. Biochim. Biophys. Acta 1862 339–351. 10.1016/j.bbadis.2015.10.026 [DOI] [PubMed] [Google Scholar]
- Kannarkat G. T., Boss J. M., Tansey M. G. (2013). The role of innate and adaptive immunity in Parkinson’s disease. J. Parkinsons Dis. 3 493–514. 10.3233/JPD-130250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Yamamoto Y., Wang Q. M. (2004). The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 3 17–26. 10.1038/nrd1279 [DOI] [PubMed] [Google Scholar]
- Kiebala M., Polesskaya O., Yao Z., Perry S. W., Maggirwar S. B. (2010). Nuclear factor-kappa B family member RelB inhibits human immunodeficiency virus-1 Tat-induced tumor necrosis factor-alpha production. PLoS One 5:e11875. 10.1371/journal.pone.0011875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Lv G., Lee J. S., Jung B. C., Masuda-Suzukake M., Hong C. S., et al. (2016). Exposure to bacterial endotoxin generates a distinct strain of alpha-synuclein fibril. Sci. Rep. 6:30891. 10.1038/srep30891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Mehta S. L., Kaimal B., Lyons K., Dempsey R. J., Vemuganti R. (2016). Poststroke induction of alpha-synuclein mediates ischemic brain damage. J. Neurosci. 36 7055–7065. 10.1523/JNEUROSCI.1241-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogel D., Peters M., Konig H. G., Hashemi S. M., Bui N. T., Arolt V., et al. (2004). S100B potently activates p65/c-Rel transcriptional complexes in hippocampal neurons: clinical implications for the role of S100B in excitotoxic brain injury. Neuroscience 127 913–920. 10.1016/j.neuroscience.2004.06.013 [DOI] [PubMed] [Google Scholar]
- Kontopoulos E., Parvin J. D., Feany M. B. (2006). Alpha-synuclein acts in the nucleus to inhibit histone acetylation and promote neurotoxicity. Hum. Mol. Genet. 15 3012–3023. 10.1093/hmg/ddl243 [DOI] [PubMed] [Google Scholar]
- Koo J. W., Russo S. J., Ferguson D., Nestler E. J., Duman R. S. (2010). Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl. Acad. Sci. U.S.A. 107 2669–2674. 10.1073/pnas.0910658107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower J. H., Chu Y., Hauser R. A., Freeman T. B., Olanow C. W. (2008a). Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 14 504–506. 10.1038/nm1747 [DOI] [PubMed] [Google Scholar]
- Kordower J. H., Chu Y., Hauser R. A., Olanow C. W., Freeman T. B. (2008b). Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov. Disord. 23 2303–2306. 10.1002/mds.22369 [DOI] [PubMed] [Google Scholar]
- Kratsovnik E., Bromberg Y., Sperling O., Zoref-Shani E. (2005). Oxidative stress activates transcription factor NF-kB-mediated protective signaling in primary rat neuronal cultures. J. Mol. Neurosci. 26 27–32. [DOI] [PubMed] [Google Scholar]
- Kuan W. L., Stott K., He X., Wood T. C., Yang S., Kwok J. C. F., et al. (2019). Systemic alpha-synuclein injection triggers selective neuronal pathology as seen in patients with Parkinson’s disease. Mol. Psychiatry 10.1038/s41380-019-0608-9 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurowska Z., Englund E., Widner H., Lindvall O., Li J. Y., Brundin P. (2011). Signs of degeneration in 12-22-year old grafts of mesencephalic dopamine neurons in patients with Parkinson’s disease. J. Parkinsons Dis. 1 83–92. 10.3233/JPD-2011-11004 [DOI] [PubMed] [Google Scholar]
- Kustrimovic N., Marino F., Cosentino M. (2019). Peripheral immunity, immunoaging and neuroinflammation in Parkinson’s disease. Curr. Med. Chem. 26 3719–3753. 10.2174/0929867325666181009161048 [DOI] [PubMed] [Google Scholar]
- Lam H. A., Wu N., Cely I., Kelly R. L., Hean S., Richter F., et al. (2011). Elevated tonic extracellular dopamine concentration and altered dopamine modulation of synaptic activity precede dopamine loss in the striatum of mice overexpressing human alpha-synuclein. J. Neurosci. Res. 89 1091–1102. 10.1002/jnr.22611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillotta A., Porrini V., Bellucci A., Benarese M., Branca C., Parrella E., et al. (2015). NF-kappaB in innate neuroprotection and age-related neurodegenerative diseases. Front. Neurol. 6:98. 10.3389/fneur.2015.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanzillotta A., Sarnico I., Ingrassia R., Boroni F., Branca C., Benarese M., et al. (2010). The acetylation of RelA in Lys310 dictates the NF-kappaB-dependent response in post-ischemic injury. Cell Death Dis. 1:e96. 10.1038/cddis.2010.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lema Tome C. M., Tyson T., Rey N. L., Grathwohl S., Britschgi M., Brundin P. (2013). Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease–is there a link? Mol. Neurobiol. 47 561–574. 10.1007/s12035-012-8267-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. Y., Englund E., Widner H., Rehncrona S., Bjorklund A., Lindvall O., et al. (2010). Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov. Disord. 25 1091–1096. 10.1002/mds.23012 [DOI] [PubMed] [Google Scholar]
- Li Q., Verma I. M. (2002). NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2 725–734. 10.1038/nri910 [DOI] [PubMed] [Google Scholar]
- Li Y. I., Wong G., Humphrey J., Raj T. (2019). Prioritizing Parkinson’s disease genes using population-scale transcriptomic data. Nat. Commun. 10:994. 10.1038/s41467-019-08912-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappan K. (2018). NF-kappaB in oxidative stress. Curr. Opin. Toxicol. 7 81–86. 10.1016/j.cotox.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Gao J., Tu S., Xu S., Liu Y., Shang W., et al. (2014). Neuroprotective effects of jitai tablet, a traditional Chinese medicine, on the MPTP-induced acute model of Parkinson’s disease: involvement of the dopamine system. Evid. Based Complement. Alternat. Med. 2014:542383. 10.1155/2014/542383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan T., Bendor J., Toupin C., Thorn K., Edwards R. H. (2017). alpha-synuclein promotes dilation of the exocytotic fusion pore. Nat. Neurosci. 20 681–689. 10.1038/nn.4529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longhena F., Faustini G., Spillantini M. G., Bellucci A. (2019). Living in promiscuity: the multiple partners of alpha-synuclein at the synapse in physiology and pathology. Int. J. Mol. Sci. 20:141. 10.3390/ijms20010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk K. C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J. Q., et al. (2012). Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science 338 949–953. 10.1126/science.1227157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M., Decressac M., Mattsson B., Bjorklund A. (2012). Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc. Natl. Acad. Sci. U.S.A. 109 3213–3219. 10.1073/pnas.1200575109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu M., Jenike E., Vachharajani N., Visekruna A. (2017). Transcription factor c-Rel is indispensable for generation of thymic but not of peripheral Foxp3(+) regulatory T cells. Oncotarget 8 52678–52689. 10.18632/oncotarget.17079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrone T., Jirillo E. (2013). The interaction between gut microbiota and age-related changes in immune function and inflammation. Immun. Ageing 10:31. 10.1186/1742-4933-10-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A., Lopez N., Gonzalez C., Hetz C. (2019). Targeting of the unfolded protein response (UPR) as therapy for Parkinson’s disease. Biol. Cell 111 161–168. 10.1111/boc.201800068 [DOI] [PubMed] [Google Scholar]
- Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A. C., Mazzulli J., Mosharov E. V., et al. (2008). Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J. Clin. Invest. 118 777–788. 10.1172/JCI32806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M. P., Meffert M. K. (2006). Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 13 852–860. 10.1038/sj.cdd.4401837 [DOI] [PubMed] [Google Scholar]
- Mazzulli J. R., Zunke F., Isacson O., Studer L., Krainc D. (2016). alpha-synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc. Natl. Acad. Sci. U.S.A. 113 1931–1936. 10.1073/pnas.1520335113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meffert M. K., Baltimore D. (2005). Physiological functions for brain NF-kappaB. Trends Neurosci. 28 37–43. 10.1016/j.tins.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Meffert M. K., Chang J. M., Wiltgen B. J., Fanselow M. S., Baltimore D. (2003). NF-kappa B functions in synaptic signaling and behavior. Nat. Neurosci. 6 1072–1078. 10.1038/nn1110 [DOI] [PubMed] [Google Scholar]
- Mercado G., Lopez N., Martinez A., Sardi S. P., Hetz C. (2018). A new model to study cell-to-cell transfer of alpha-synuclein in vivo. Biochem. Biophys. Res. Commun. 503 1385–1393. 10.1016/j.bbrc.2018.07.053 [DOI] [PubMed] [Google Scholar]
- Mercado G., Valdes P., Hetz C. (2013). An ERcentric view of Parkinson’s disease. Trends Mol. Med. 19 165–175. 10.1016/j.molmed.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Mincheva-Tasheva S., Soler R. M. (2013). NF-kappaB signaling pathways: role in nervous system physiology and pathology. Neuroscientist 19 175–194. 10.1177/1073858412444007 [DOI] [PubMed] [Google Scholar]
- Morgan M. J., Liu Z. G. (2011). Crosstalk of reactive oxygen species and NF-kappaB signaling. Cell Res. 21 103–115. 10.1038/cr.2010.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. D., Rueter S. M., Trojanowski J. Q., Lee V. M. (2000). Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20 3214–3220. 10.1523/jneurosci.20-09-03214.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal R., Mainali R., Ahmadi S., Wang S., Singh R., Kavanagh K., et al. (2018). Gut microbiome and aging: physiological and mechanistic insights. Nutr. Healthy Aging 4 267–285. 10.3233/NHA-170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nemani V. M., Azarbal F., Skibinski G., Levy J. M., Egami K., et al. (2011). Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J. Biol. Chem. 286 20710–20726. 10.1074/jbc.M110.213538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandy A., Lin L., Velentzas P. D., Wu L. P., Baehrecke E. H., Silverman N. (2018). The NF-kappaB factor relish regulates Atg1 expression and controls autophagy. Cell Rep. 25 2110–2120.e3. 10.1016/j.celrep.2018.10.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickols J. C., Valentine W., Kanwal S., Carter B. D. (2003). Activation of the transcription factor NF-kappaB in Schwann cells is required for peripheral myelin formation. Nat. Neurosci. 6 161–167. 10.1038/nn995 [DOI] [PubMed] [Google Scholar]
- Nivon M., Fort L., Muller P., Richet E., Simon S., Guey B., et al. (2016). NFkappaB is a central regulator of protein quality control in response to protein aggregation stresses via autophagy modulation. Mol. Biol. Cell 27 1712–1727. 10.1091/mbc.E15-12-0835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow C. W., Brundin P. (2013). Parkinson’s disease and alpha synuclein: is Parkinson’s disease a prion-like disorder? Mov. Disord. 28 31–40. 10.1002/mds.25373 [DOI] [PubMed] [Google Scholar]
- Olanow C. W., Stocchi F. (2018). Levodopa: a new look at an old friend. Mov. Disord. 33 859–866. 10.1002/mds.27216 [DOI] [PubMed] [Google Scholar]
- Orayj K., Lane E. (2019). Patterns and determinants of prescribing for Parkinson’s disease: a systematic literature review. Parkinsons Dis. 2019:9237181. 10.1155/2019/9237181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacelli C., Giguere N., Bourque M. J., Levesque M., Slack R. S., Trudeau L. E. (2015). Elevated mitochondrial bioenergetics and axonal arborization size are key contributors to the vulnerability of dopamine neurons. Curr. Biol. 25 2349–2360. 10.1016/j.cub.2015.07.050 [DOI] [PubMed] [Google Scholar]
- Paiva I., Jain G., Lazaro D. F., Jercic K. G., Hentrich T., Kerimoglu C., et al. (2018). Alpha-synuclein deregulates the expression of COL4A2 and impairs ER-Golgi function. Neurobiol. Dis. 119 121–135. 10.1016/j.nbd.2018.08.001 [DOI] [PubMed] [Google Scholar]
- Pang S. Y., Ho P. W., Liu H. F., Leung C. T., Li L., Chang E. E. S., et al. (2019). The interplay of aging, genetics and environmental factors in the pathogenesis of Parkinson’s disease. Transl. Neurodegener. 8:23. 10.1186/s40035-019-0165-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parrella E., Bellucci A., Porrini V., Benarese M., Lanzillotta A., Faustini G., et al. (2019). NF-kappaB/c-Rel deficiency causes Parkinson’s disease-like prodromal symptoms and progressive pathology in mice. Transl. Neurodegener. 8:16. 10.1186/s40035-019-0154-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins N. D. (1997). Achieving transcriptional specificity with NF-kappa B. Int. J. Biochem. Cell Biol. 29 1433–1448. 10.1016/s1357-2725(97)00088-5 [DOI] [PubMed] [Google Scholar]
- Phan J. A., Stokholm K., Zareba-Paslawska J., Jakobsen S., Vang K., Gjedde A., et al. (2017). Early synaptic dysfunction induced by alpha-synuclein in a rat model of Parkinson’s disease. Sci. Rep. 7:6363. 10.1038/s41598-017-06724-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifl C., Rajput A., Reither H., Blesa J., Cavada C., Obeso J. A., et al. (2014). Is Parkinson’s disease a vesicular dopamine storage disorder? Evidence from a study in isolated synaptic vesicles of human and nonhuman primate striatum. J. Neurosci. 34 8210–8218. 10.1523/JNEUROSCI.5456-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinho R., Paiva I., Jercic K. G., Fonseca-Ornelas L., Gerhardt E., Fahlbusch C., et al. (2019). Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein. Hum. Mol. Genet. 28 31–50. 10.1093/hmg/ddy326 [DOI] [PubMed] [Google Scholar]
- Pizzi M., Goffi F., Boroni F., Benarese M., Perkins S. E., Liou H. C., et al. (2002). Opposing roles for NF-kappa B/Rel factors p65 and c-Rel in the modulation of neuron survival elicited by glutamate and interleukin-1beta. J. Biol. Chem. 277 20717–20723. 10.1074/jbc.M201014200 [DOI] [PubMed] [Google Scholar]
- Pizzi M., Sarnico I., Boroni F., Benarese M., Steimberg N., Mazzoleni G., et al. (2005). NF-kappaB factor c-Rel mediates neuroprotection elicited by mGlu5 receptor agonists against amyloid beta-peptide toxicity. Cell Death Differ. 12 761–772. 10.1038/sj.cdd.4401598 [DOI] [PubMed] [Google Scholar]
- Pizzi M., Sarnico I., Lanzillotta A., Battistin L., Spano P. (2009). Post-ischemic brain damage: NF-kappaB dimer heterogeneity as a molecular determinant of neuron vulnerability. FEBS J. 276 27–35. 10.1111/j.1742-4658.2008.06767.x [DOI] [PubMed] [Google Scholar]
- Porrini V., Mota M., Parrella E., Bellucci A., Benarese M., Faggi L., et al. (2017). Mild inflammatory profile without gliosis in the c-Rel deficient mouse modeling a late-onset Parkinsonism. Front. Aging Neurosci. 9:229. 10.3389/fnagi.2017.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prots I., Grosch J., Brazdis R. M., Simmnacher K., Veber V., Havlicek S., et al. (2018). alpha-synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc. Natl. Acad. Sci. U.S.A. 115 7813–7818. 10.1073/pnas.1713129115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recasens A., Dehay B. (2014). Alpha-synuclein spreading in Parkinson’s disease. Front. Neuroanat. 8:159. 10.3389/fnana.2014.00159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A., Simcox E., Turnbull D. (2014). Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res. Rev. 14 19–30. 10.1016/j.arr.2014.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve A. K., Grady J. P., Cosgrave E. M., Bennison E., Chen C., Hepplewhite P. D., et al. (2018). Mitochondrial dysfunction within the synapses of substantia nigra neurons in Parkinson’s disease. NPJ Parkinsons Dis. 4:9. 10.1038/s41531-018-0044-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A., Shechter R., London A., Ziv Y., Ronen A., Levy R., et al. (2007). Toll-like receptors modulate adult hippocampal neurogenesis. Nat. Cell Biol. 9 1081–1088. 10.1038/ncb1629 [DOI] [PubMed] [Google Scholar]
- Rutherford N. J., Dhillon J. S., Riffe C. J., Howard J. K., Brooks M., Giasson B. I. (2017). Comparison of the in vivo induction and transmission of alpha-synuclein pathology by mutant alpha-synuclein fibril seeds in transgenic mice. Hum. Mol. Genet. 26 4906–4915. 10.1093/hmg/ddx371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnico I., Boroni F., Benarese M., Sigala S., Lanzillotta A., Battistin L., et al. (2008). Activation of NF-kappaB p65/c-Rel dimer is associated with neuroprotection elicited by mGlu5 receptor agonists against MPP(+) toxicity in SK-N-SH cells. J. Neural Transm. 115 669–676. 10.1007/s00702-007-0007-2 [DOI] [PubMed] [Google Scholar]
- Sarnico I., Lanzillotta A., Benarese M., Alghisi M., Baiguera C., Battistin L., et al. (2009a). NF-kappaB dimers in the regulation of neuronal survival. Int. Rev. Neurobiol. 85 351–362. 10.1016/S0074-7742(09)85024-1 [DOI] [PubMed] [Google Scholar]
- Sarnico I., Lanzillotta A., Boroni F., Benarese M., Alghisi M., Schwaninger M., et al. (2009b). NF-kappaB p50/RelA and c-Rel-containing dimers: opposite regulators of neuron vulnerability to ischaemia. J. Neurochem. 108 475–485. 10.1111/j.1471-4159.2008.05783.x [DOI] [PubMed] [Google Scholar]
- Schaser A. J., Osterberg V. R., Dent S. E., Stackhouse T. L., Wakeham C. M., Boutros S. W., et al. (2019). Alpha-synuclein is a DNA binding protein that modulates DNA repair with implications for Lewy body disorders. Sci. Rep. 9:10919. 10.1038/s41598-019-47227-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A., Martin-Villalba A., Weih F., Vogel J., Wirth T., Schwaninger M. (1999). NF-kappaB is activated and promotes cell death in focal cerebral ischemia. Nat. Med. 5 554–559. 10.1038/8432 [DOI] [PubMed] [Google Scholar]
- Siddiqui A., Chinta S. J., Mallajosyula J. K., Rajagopolan S., Hanson I., Rane A., et al. (2012). Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free Radic. Biol. Med. 53 993–1003. 10.1016/j.freeradbiomed.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons L. J., Surles-Zeigler M. C., Li Y., Ford G. D., Newman G. D., Ford B. D. (2016). Regulation of inflammatory responses by neuregulin-1 in brain ischemia and microglial cells in vitro involves the NF-kappa B pathway. J. Neuroinflammation 13:237. 10.1186/s12974-016-0703-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini M. G., Goedert M. (2000). The alpha-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann. N. Y. Acad. Sci. 920 16–27. 10.1111/j.1749-6632.2000.tb06900.x [DOI] [PubMed] [Google Scholar]
- Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997). Alpha-synuclein in Lewy bodies. Nature 388 839–840. 10.1038/42166 [DOI] [PubMed] [Google Scholar]
- Sulzer D., Alcalay R. N., Garretti F., Cote L., Kanter E., Agin-Liebes J., et al. (2017). T cells from patients with Parkinson’s disease recognize alpha-synuclein peptides. Nature 546 656–661. 10.1038/nature22815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Edwards R. H. (2019). The physiological role of alpha-synuclein and its relationship to Parkinson’s disease. J. Neurochem. 150 475–486. 10.1111/jnc.14810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surgucheva I., He S., Rich M. C., Sharma R., Ninkina N. N., Stahel P. F., et al. (2014). Role of synucleins in traumatic brain injury - an experimental in vitro and in vivo study in mice. Mol. Cell. Neurosci. 63 114–123. 10.1016/j.mcn.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Surguchov A. (2016). Parkinson’s disease: assay of phosphorylated alpha-synuclein in skin biopsy for early diagnosis and association with melanoma. Brain Sci. 6:17. 10.3390/brainsci6020017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J. (2018). Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J. 285 3657–3668. 10.1111/febs.14607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Obeso J. A., Halliday G. M. (2017). Selective neuronal vulnerability in Parkinson disease. Nat. Rev. Neurosci. 18 101–113. 10.1038/nrn.2016.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Wang Y., Zhou S., Qian T., Gu X. (2013). Signaling pathways regulating dose-dependent dual effects of TNF-alpha on primary cultured Schwann cells. Mol. Cell. Biochem. 378 237–246. 10.1007/s11010-013-1614-x [DOI] [PubMed] [Google Scholar]
- Tansey M. G., Romero-Ramos M. (2019). Immune system responses in Parkinson’s disease: early and dynamic. Eur. J. Neurosci. 49 364–383. 10.1111/ejn.14290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapias V., Hu X., Luk K. C., Sanders L. H., Lee V. M., Greenamyre J. T. (2017). Synthetic alpha-synuclein fibrils cause mitochondrial impairment and selective dopamine neurodegeneration in part via iNOS-mediated nitric oxide production. Cell. Mol. Life Sci. 74 2851–2874. 10.1007/s00018-017-2541-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayanidhi N., Helm J. R., Nycz D. C., Bentley M., Liang Y., Hay J. C. (2010). Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol. Biol. Cell 21 1850–1863. 10.1091/mbc.E09-09-0801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilstra J. S., Robinson A. R., Wang J., Gregg S. Q., Clauson C. L., Reay D. P., et al. (2012). NF-kappaB inhibition delays DNA damage-induced senescence and aging in mice. J. Clin. Invest. 122 2601–2612. 10.1172/JCI45785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togo T., Iseki E., Marui W., Akiyama H., Ueda K., Kosaka K. (2001). Glial involvement in the degeneration process of Lewy body-bearing neurons and the degradation process of Lewy bodies in brains of dementia with Lewy bodies. J. Neurol. Sci. 184 71–75. 10.1016/s0022-510x(00)00498-6 [DOI] [PubMed] [Google Scholar]
- Uchihara T. (2017). An order in Lewy body disorders: retrograde degeneration in hyperbranching axons as a fundamental structural template accounting for focal/multifocal Lewy body disease. Neuropathology 37 129–149. 10.1111/neup.12348 [DOI] [PubMed] [Google Scholar]
- Ulusoy A., Phillips R. J., Helwig M., Klinkenberg M., Powley T. L., Di Monte D. A. (2017). Brain-to-stomach transfer of alpha-synuclein via vagal preganglionic projections. Acta Neuropathol. 133 381–393. 10.1007/s00401-016-1661-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulusoy A., Rusconi R., Perez-Revuelta B. I., Musgrove R. E., Helwig M., Winzen-Reichert B., et al. (2013). Caudo-rostral brain spreading of alpha-synuclein through vagal connections. EMBO Mol. Med. 5 1119–1127. 10.1002/emmm.201302475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal-Cevik I., Gursoy-Ozdemir Y., Yemisci M., Lule S., Gurer G., Can A., et al. (2011). Alpha-synuclein aggregation induced by brief ischemia negatively impacts neuronal survival in vivo: a study in [A30P]alpha-synuclein transgenic mouse. J. Cereb. Blood Flow Metab. 31 913–923. 10.1038/jcbfm.2010.170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A., Boroni F., Benarese M., Sarnico I., Ghisi V., Bresciani L. G., et al. (2006). NF-kappaB pathway: a target for preventing beta-amyloid (Abeta)-induced neuronal damage and Abeta42 production. Eur. J. Neurosci. 23 1711–1720. 10.1111/j.1460-9568.2006.04722.x [DOI] [PubMed] [Google Scholar]
- Valerio A., Dossena M., Bertolotti P., Boroni F., Sarnico I., Faraco G., et al. (2009). Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke 40 610–617. 10.1161/STROKEAHA.108.528588 [DOI] [PubMed] [Google Scholar]
- Visanji N. P., Brotchie J. M., Kalia L. V., Koprich J. B., Tandon A., Watts J. C., et al. (2016). alpha-synuclein-based animal models of Parkinson’s disease: challenges and opportunities in a new era. Trends Neurosci. 39 750–762. 10.1016/j.tins.2016.09.003 [DOI] [PubMed] [Google Scholar]
- Visekruna A., Volkov A., Steinhoff U. (2012). A key role for NF-kappaB transcription factor c-Rel in T-lymphocyte-differentiation and effector functions. Clin. Dev. Immunol. 2012:239368. 10.1155/2012/239368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Das U., Scott D. A., Tang Y., McLean P. J., Roy S. (2014). alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling. Curr. Biol. 24 2319–2326. 10.1016/j.cub.2014.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Becker K., Levine N., Zhang M., Lieberman A. P., Moore D. J., et al. (2019). Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol. Commun. 7:41. 10.1186/s40478-019-0696-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Dong H., Wang J., Huang Y., Zhang X., Tang Y., et al. (2020). Pro-survival and anti-inflammatory roles of NF-kappaB c-Rel in the Parkinson’s disease models. Redox Biol. 30:101427. 10.1016/j.redox.2020.101427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong Y. C., Luk K., Purtell K., Burke Nanni S., Stoessl A. J., Trudeau L. E., et al. (2019). Neuronal vulnerability in Parkinson disease: should the focus be on axons and synaptic terminals? Mov. Disord. 34 1406–1422. 10.1002/mds.27823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Rhee K. J., Albesiano E., Rabizadeh S., Wu X., Yen H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15 1016–1022. 10.1038/nm.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C., Atkinson S. P., Vilella F., Lloret M., Armstrong L., Mann D. A., et al. (2010). Opposing putative roles for canonical and noncanonical NFkappaB signaling on the survival, proliferation, and differentiation potential of human embryonic stem cells. Stem Cells 28 1970–1980. 10.1002/stem.528 [DOI] [PubMed] [Google Scholar]
- Yoon D. K., Hwang I. K., Yoo K. Y., Lee Y. B., Lee J. J., Kim J. H., et al. (2006). Comparison of alpha-synuclein immunoreactivity and protein levels in ischemic hippocampal CA1 region between adult and aged gerbils and correlation with Cu, Zn-superoxide dismutase. Neurosci. Res. 55 434–441. 10.1016/j.neures.2006.04.014 [DOI] [PubMed] [Google Scholar]
- Yuan Y., Jin J., Yang B., Zhang W., Hu J., Zhang Y., et al. (2008). Overexpressed alpha-synuclein regulated the nuclear factor-kappaB signal pathway. Cell. Mol. Neurobiol. 28 21–33. 10.1007/s10571-007-9185-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaltieri M., Longhena F., Pizzi M., Missale C., Spano P., Bellucci A. (2015). Mitochondrial dysfunction and alpha-synuclein synaptic pathology in Parkinson’s disease: who’s on first? Parkinsons Dis. 2015:108029. 10.1155/2015/108029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Li J., Purkayastha S., Tang Y., Zhang H., Yin Y., et al. (2013). Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature 497 211–216. 10.1038/nature12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Reichel J. M., Han C., Zuniga-Hertz J. P., Cai D. (2017). Astrocytic process plasticity and IKKbeta/NF-kappaB in central control of blood glucose, blood pressure, and body weight. Cell Metab. 25 1091–1102.e4. 10.1016/j.cmet.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H. Q., Li F. F., Wang Z., Wang X. M., Feng T. (2016). A comparative study of the amount of alpha-synuclein in ischemic stroke and Parkinson’s disease. Neurol. Sci. 37 749–754. 10.1007/s10072-016-2485-1 [DOI] [PubMed] [Google Scholar]