Abstract
Background
Studies on kidney complications in human immunodeficiency virus (HIV)-infected children are lacking. CD4 T lymphocytes are an important immune functions regulator and used as a basis for initiating antiretroviral therapy (ART) and monitoring disease progression. This study aims to determine the correlation between CD4 and estimated glomerular filtration rate (eGFR) or urine protein:creatinine ratio (uPCR) as markers of kidney complications.
Methods
This cross sectional study was conducted on HIV-infected children aged 5 to 18 years who visited the Teratai HIV Clinic at Hasan Sadikin Hospital for monthly monitoring in June 2019. CD4 count, eGFR based on the Schwartz formula, and uPCR were obtained. Correlation analysis was performed with the Pearson test.
Results
Subjects were 42 HIV-infected children, consisting of 23 males (54.8%) and 19 females (45.2%). Most children (65.0%) were in an advanced clinical stage and had been diagnosed with HIV for an average of 8 ± 3 years. All subjects had received ART, and six received tenofovir. Compliance to medications were good, and most subjects (79.0%) had normal nutritional status and CD4 count. All subjects had eGFR > 90 mL/min/1.73 m2, of which 21 (50.0%) were above normal value. Proteinuria was found in 12 patients (28.6%), and it was not significantly associated with clinical stages of HIV infection. CD4 count was correlated positively with eGFR (r = 0.473, P = 0.001) and negatively with uPCR (r = -0.284, P = 0.034).
Conclusion
The degree of immunodeficiency appears to correlate with severity of renal injury. Screening at diagnosis and periodic monitoring of kidney functions are crucial in all childhood HIV patients.
Keywords: CD4-positive T-lymphocytes, Glomerular filtration rate, Human immunodeficiency virus, Indonesia, Proteinuria
Introduction
Kidney issues are an important complication of human immunodeficiency virus (HIV) infection, with prevalence varying between 5% to 48.5% worldwide [1-3]. Deaths due to kidney diseases account for 12.2% of all HIV patient deaths [4]. In Sub-Saharan Africa, a 67% increase in mortality due to nephritis and nephrosis has been reported from 1999 to 2006 [5]. Not only are patients with HIV infection at greater risk of kidney disease, but they are also more likely to experience faster kidney dysfunction progression compared to individuals without HIV infection [6,7]. Kooij et al [7] in 2017 reported higher mortality in end-stage renal disease (ESRD) patients with compared to those without HIV infection (94% and 63%) [8]. If not treated promptly, HIV patients with renal disorder will experience rapid progression to ESRD [9].
Kidney disorders are asymptomatic at early stages; hence, early detection is important for appropriate treatment to inhibit progression of kidney disease before it becomes irreversible [5]. Guidelines from the HIV Medicine Association of the Infectious Diseases Society of America in 2014 recommend screening for early detection of kidney abnormalities in asymptomatic HIV patients at diagnosis and at least twice a year thereafter [10]. However, these are no routinely implemented screens in many countries with a high prevalence of HIV including Indonesia. Such examinations are performed only if there are indications based on clinical symptoms and selected antiretroviral therapy (ART) [11].
CD4 T lymphocytes play an important role in regulating immune functions and are the main target of HIV. Continuous destruction of CD4 by HIV results in loss of a specific immune response to HIV and, ultimately, loss of non-specific immune responses to opportunistic pathogens that are characteristic of acquired immunodeficiency syndrome (AIDS). CD4 count is used as a basis for ART and as a tool to monitor disease progression and ART effectiveness. Change in CD4 cell count is an important indicator of patient response to ART. CD4 cell count varies according to age in children younger than 5 years. Therefore, tests that can be used in this age group involve CD4%. Meanwhile, in children aged ≥ 5 years, both CD4% and absolute CD4 tests can be used, although absolute CD4 tests are preferred [12].
Studies on kidney complications in HIV-infected children are lacking, particularly in Asia. Most studies that have been performed are from Africa, as the region with the highest global HIV prevalence whose citizens are known to have a specific genetic predisposition [13]. This study aims to determine the correlation between CD4 and estimated glomerular filtration rate (eGFR) or urine protein:creatinine ratio (uPCR) as a marker of kidney disorder. Patients older than 5 years were chosen to allow uniform use of the CD4 examination.
Methods
A cross-sectional study was conducted on pediatric HIV patients at Teratai HIV Clinic Dr. Hasan Sadikin General Hospital Bandung in June 2019. The inclusion criteria were: 1) children aged 5 to 18 years and 2) known diagnosis of HIV infection according to the World Health Organization (WHO) criteria [14]. Exclusion criteria were: 1) history of previous kidney abnormality or 2) urinary tract infection, characterized by fever and urinary complaints.
Informed consent was obtained from parents of the subjects who met the study criteria. Identified subject characteristics consisted of age, sex, age at diagnosis, time since diagnosis, duration of ART, type of ART, route of transmission, clinical stage at diagnosis, and nadir CD4 count. Weight was measured in kilograms (kg) using a weight scale, while height was measured in centimeters (cm) using a stadiometer. Nutritional status was determined by the WHO body mass index (BMI) for age chart. Two blood samples, each 3 milliliters (mL), were collected for CD4 and serum creatinine examination. Serum creatinine was then used to calculate eGFR using the Schwartz formula [15]. Five mL of morning urine samples were collected from each subject for examination of urine protein and urine creatinine and calculation of uPCR.
Normal eGFR values by age were determined based on the guidelines from the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (NKF KDOQI). Impaired renal function was defined as eGFR < 90 mL/min, while proteinuria was defined as uPCR ≥ 0.2, and nephrotic proteinuria was uPCR ≥ 1.0 [15,16]. Correlation analysis between CD4 and eGFR was carried out with the Pearson correlation test, while correlation analysis between CD4 and uPCR was performed by the Spearman correlation test. Data analysis was performed using the IBM SPSS Statistics for Windows version 20.0 (IBM Corp., Armonk, NY, USA). This study was approved by the Research Ethics Committee of Dr. Hasan Sadikin General Hospital, Bandung (approval number: LB.02.01/X.6.5/163/2019).
Results
During the study period in June 2019, 45 subjects who met the inclusion criteria were enrolled. Three subjects younger than 5 years of age were excluded. Table 1 shows the demographic and clinical characteristics of the study population. Male and female proportions were almost the same (54.8% vs. 45.2%). The youngest age of the subjects was 5 years 7 months, while the oldest was 16 years 11 months. The median age at diagnosis was 3 years, with an average time since diagnosis of 8 years. All patients received ART, one of which consisted of lamivudine. Six subjects who used tenofovir previously experienced first line regimen treatment failure or started treatment during adolescence. Most subjects (79%) had normal nutritional status. Only 40 of the 42 subjects had available clinical stage at diagnosis. Two patients were initially diagnosed with HIV infection in a district hospital and private clinic, and we were not able to trace their initial history. Twenty-six (65.0%) of the 40 subjects were in advanced stage (clinical stage III or IV) at the time of diagnosis.
Table 1.
Subject characteristics (n = 42)
| Characteristic | Value |
|---|---|
| Sex | |
| Male | 23 (54.8) |
| Female | 19 (45.2) |
| Current age (yr) | 11 ± 3 |
| Age at diagnosis (yr) | 3 (0.5-8) |
| Time since diagnosis (yr) | 8 ± 3 |
| ART duration (yr) | 8 (3-14) |
| Type of ART | |
| Lamivudine | 42 (100.0) |
| Zidovudine | 38 (90.5) |
| Tenofovir | 6 (14.3) |
| Nevirapine | 21 (50.0) |
| Efavirenz | 19 (45.2) |
| Lopinavir/Ritonavir | 2 (4.8) |
| Nutritional status | |
| Normal | 33 (78.6) |
| Moderate malnutrition | 6 (14.3) |
| Severe malnutrition | 3 (7.1) |
| Clinical stage at diagnosis (n = 40) | |
| I | 5 (12.5) |
| II | 9 (22.5) |
| III | 13 (32.5) |
| IV | 13 (32.5) |
Data are presented as number (%), mean ± standard deviation, or median (minimum-maximum).
ART, antiretroviral therapy.
The results of CD4, eGFR, and uPCR examinations are shown in Table 2 and are classified according to clinical stage in Table 3. No statistically significant difference was observed among clinical stages. Thirty-three subjects (78.6%) were immunocompetent based on CD4 count, 6 (14.3%) experienced mild immunodeficiency, and 3 (7.1%) experienced severe immunodeficiency [14]. Distributions of eGFR and uPCR were abnormal, with extreme values in several subjects. One subject with uPCR results far exceeding other subjects (0.65 mg/mg) was severely stunted in growth (WHO height for age z score, -3.46 standard deviation, SD), with a CD4 count of 60 cell/mm3 (%CD4+, 5.15%). Another subject with eGFR far below that of the other subjects (118 mL/min/m2) was the oldest subject (aged 16 years 11 months) and was severely undernourished (WHO BMI for age z score, -4.4 SD) with proteinuria (uPCR 0.39 mg/mg).
Table 2.
Laboratory results
| Examination | Value |
|---|---|
| CD4 count (cell/mm3) | 796 ± 383 |
| Percentage CD4+ (%) | 27.8 (0.7-37.5) |
| eGFR (mL/min/1.73 m2) | 158 (118-215) |
| uPCR (mg/mg) | 0.15 (0.01-0.65) |
Data are presented as mean ± standard deviation or median (minimum-maximum).
eGFR, estimated glomerular filtration rate; uPCR, urine protein: creatinine ratio.
Table 3.
Clinical characteristics according to clinical stage
| Clinical stage | n | CD4 count | eGFR | uPCR |
|---|---|---|---|---|
| I | 5 | 724 ± 156 | 165 ± 12 | 0.138 ± 0.095 |
| II | 9 | 929 ± 417 | 165 ± 22 | 0.100 ± 0.099 |
| III | 13 | 754 ± 359 | 153 ± 14 | 0.151 ± 0.106 |
| IV | 13 | 785 ± 454 | 168 ± 29 | 0.236 ± 0.160 |
| P value | 0.716 | 0.344 | 0.087 |
Data are presented as mean ± standard deviation. Analysis was performed using one-way ANOVA.
eGFR, estimated glomerular filtration rate; uPCR, urine protein:creatinine ratio.
All subjects had eGFR > 90 mL/min/m2. Twenty-one subjects (50.0%) had eGFR above the normal value according to age. Twelve subjects (28.6%) had proteinuria, while none experienced nephrotic proteinuria. Five of the 6 subjects (83.3%) who received tenofovir experienced proteinuria (P = 0.005), while only 8 (21.1%) of the 38 subjects who received zidovudine experienced proteinuria (P = 0.004). Five of the six subjects who used tenofovir and experienced proteinuria had been diagnosed with HIV stage IV at diagnosis (2 subjects), had mild immunodeficiency at examination (2 subjects), or were severely stunted in growth (2 subjects).
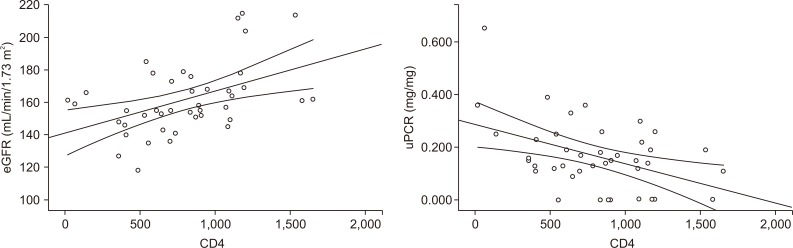
Correlations between CD4 and eGFR or uPCR are shown in Table 4 and Fig. 1. CD4 count was positively correlated with eGFR (r = 0.473, P = 0.001) and negatively correlated with uPCR (r = -0.284, P = 0.034).
Table 4.
Correlation between CD4 and eGFR or uPCR
| Variable | CD4 count | ||
|---|---|---|---|
| Correlation coefficient (r) | P value | 95% CI | |
| eGFR (mL/min/1.73 m2) | 0.473 | 0.001a | 0.198 to 0.679 |
| uPCR (mg/mg) | -0.284 | 0.034b | -0.075 to -0.469 |
Analysis was performed using the aPearson, brank Spearman correlation test, significant if P < 0.05.
CI, confidence interval; eGFR, estimated glomerular filtration rate; uPCR, urine protein:creatinine ratio.
Figure 1. Correlation between CD4 and estimated glomerular filtration rate (eGFR) or urine protein:creatinine ratio (uPCR).
We performed multivariate analysis to determine the correlations of CD4 and tenofovir use with eGFR and uPCR, as shown in Tables 5 and 6. We observed that the correlation between CD4 and eGFR was 0.415 after being controlled by tenofovir. Every 1 cell/mm3 increase of CD4 increased eGFR by 0.023 mL/min/1.73 m2. Meanwhile, the correlation between CD4 and uPCR was -0.390 after being controlled by tenofovir. Every 1 cell/mm3 increase of CD4 decreased uPCR by 0.00013 mg/mg.
Table 5.
Multivariate analysis of correlation between tenofovir-controlled CD4 and eGFR
| Variable | Unstandardized coefficients | Standardized coefficients | P value | |
|---|---|---|---|---|
| B | SE | Beta | ||
| (Constant) | 144.779 | 7.271 | < 0.001 | |
| CD4 | 0.023 | 0.008 | 0.415 | 0.005 |
| Tenofovir | -13.28 | 8.566 | -0.218 | 0.129 |
Dependent variable: eGFR.
eGFR, estimated glomerular filtration rate; SE, standard error.
Table 6.
Multivariate analysis of correlation between tenofovir-controlled CD4 and uPCR
| Variable | Unstandardized coefficients | Standardized coefficients | P value | |
|---|---|---|---|---|
| B | SE | Beta | ||
| (Constant) | 0.25901 | 0.04319 | < 0.001 | |
| CD4 | -0.00013 | 0.00005 | -0.390 | 0.008 |
| Tenofovir | 0.10016 | 0.05088 | 0.275 | 0.056 |
Dependent variable: uPCR.
SE, standard error; uPCR, urine protein:creatinine ratio.
Discussion
This study found that 29% of subjects had proteinuria by means of uPCR examination. This result is greater than that in a study conducted by Gupta et al [17] (11.5%) in 2017. Differences in results between these studies may be due to differences in clinical stage and time from diagnosis among the subjects. In addition, the previous study involved a greater number of subjects (139 subjects) and a longer study period (1 year 4 months). The results of this study are similar to those of studies conducted by Chaparro et al [18] in the United States (33%) in 2008 and Ikpeme et al [19] in Nigeria (31.6%) in 2012. All these studies used uPCR ≥ 0.2 to determine proteinuria. This high prevalence indicates that impaired renal function is indeed an important complication in HIV patients [20].
This study found that CD4 positively correlated with eGFR and negatively correlated with uPCR. Previous studies have reported mixed results related to CD4 correlation with kidney disorders. Gupta et al [17] found no correlation between CD4 and proteinuria, while Chaparro et al [18] found a correlation between CD4 and nephrotic proteinuria but no correlation with intermediate proteinuria (uPCR ≥ 0.2, < 1.0). These findings might be due to differences in subject characteristics between studies. The majority (86%) of subjects in India were in the early clinical and immunological stages, and proteinuria was found in only 11.5% [17]. Meanwhile, Chaparro et al. involved a greater number of subjects (286 subjects) and a wider age range (the youngest being 0.2 years, the oldest being 22 years). Chaparro et al. used the same proteinuria examination method and found an incidence of proteinuria of 33%, of which 11.2% were nephrotic (uPCR > 1.0). In that study, there were no subjects with nephrotic proteinuria [18].
Ikpeme et al [19] found similar results to the present study: a correlation between CD4 and proteinuria, with a coefficient of -0.2. That study involved a greater number of subjects (98 subjects) and a longer study period (6 months). Proteinuria was determined by urine albumin:creatinine ratio (uACR) test to detect microalbuminuria that reflects damage to the glomerular vascular endothelium in early stages and decreased tubular ability to reabsorb urine albumin. Meanwhile, uPCR examination includes all non-albumin proteins of both glomerular and tubular origins. Fisher et al [21] in 2013 compared uACR and uPCR tests in chronic kidney disease populations (with no HIV infection) and found a very good correlation between the two examinations (r = 0.92). uPCR was chosen in this study because prior research by Antonello et al [16] reports a very good correlation between uPCR and 24-hour quantitative protein test as the gold standard examination for proteinuria (r = 0.957) in HIV children.
This study found a correlation between CD4 cell count with eGFR (r = 0.473, P = 0.001) and serum creatinine (r = -0.334, P = 0.024). To our knowledge, this is the first study investigating the correlation between CD4 and eGFR in HIV children. Previous studies were conducted on adult HIV patients. Among them are studies by Verma and Singh [22] in India (r = -0.26, P = 0.02) and Adedeji et al [23] in Nigeria (r = -0.228, P = 0.025). The difference in results may be due to differences in subject age, the greater number of subjects in the previous studies, and the various mechanisms possibly underlying kidney complications. Kidney complications in HIV can occur due to hemodynamic disorders such as severe dehydration or hypovolemic shock, nephrotoxic ART, direct HIV infection in epithelial cells and renal mesangium, or deposition of immune complexes into kidney tissue. Nephropathy due to direct infection of kidney cells (HIV-associated nephropathy) is the most common cause of kidney disease in children and adolescents infected with HIV in the world. These complications can be either mediated or not mediated by CD4 [24,25]. This is reflected in the results of our study showing that, even though the degree of immunodeficiency represented by CD4 can affect the degree of renal complications, the low correlation coefficient indicates the presence of other factors that influence kidney complications.
In this study, most of our subjects were in clinical stage III or IV at the time of diagnosis. Due to good adherence to medications, most of the subjects exhibited normal nutritional status and CD4 count. Nonetheless, clinical stage reflects prognosis and increased susceptibility to HIV-related complications, including kidney complications [26]. This may explain the considerably high numbers of subjects with proteinuria in this study despite normal CD4 count in most.
Proteinuria was found in all clinical stages, irrespective of sex, time since diagnosis, duration of therapy, and nutritional status. Tenofovir use increased the incidence of proteinuria, but proteinuria also appeared to occur with use of other drugs, though without any statistical significance. This underlines the importance of monitoring kidney function from diagnosis and periodically thereafter as a method of early detection of impaired kidney function in all HIV patients. Urine protein testing is an choice for monitoring patients with HIV infection.
Though CD4 cell count is the main parameter determining ART initiation, ART is administered to children at clinical stages III and IV regardless of CD4 count. The presence of nephropathy in HIV is one of the criteria for diagnosing stage 4 HIV, which requires renal biopsy to establish a definitive diagnosis. However, this procedure is not performed routinely in children. Definitions that are widely used in many epidemiological studies consist of persistent proteinuria (> 1+ protein on dipstick or uPCR ≥ 0.2 for more than three months) with enlarged echogenic kidneys on ultrasonography and abnormal microscopic examination of urine [6,25].
In Indonesia, examination of kidney function is not routinely performed until there are clinical symptoms [11]. If persistent proteinuria is detected and confirmed by ultrasonography or kidney biopsy, ART initiation can be performed earlier without waiting for CD4 count decline [25]. This is particularly important in children, because HIV in children has unique properties compared to HIV in adults. Although early manifestations are often mild and nonspecific, due to their immature immune systems with higher CD4 count, children are more susceptible to common childhood and opportunistic infections and may experience rapid progression of HIV infection if treatment is delayed [27]. In children, ART is said to be the most important management tool to inhibit progression to ESRD [28].
One of the limitations of this study is the cross-sectional design, which prevented us from evaluating the persistence of proteinuria. However, of the 27 adolescents involved in this study, only 2 were severely undernourished. Furthermore, urine samples were collected in the morning, and the subjects rested for at least half an hour before collecting urine specimens.
In addition, the results of serum creatinine, eGFR, and uPCR had an abnormal distribution. This may be due to the nonhomogeneous characteristics of the subjects, resulting in extreme values in several subjects. There may be confounding factors that influenced the results because of the non-strict exclusion criteria. We observed that eGFRs in this study were very high. Theoretically, this may possibly have been caused by low muscle mass of the subjects. However, we were certain that our subjects did not experience muscle hypertrophy. Most of our subjects had normal nutritional status, with only three being severely undernourished and showing eGFR values that were not very high. Therefore, we have reason to believe that serum creatinine remains a reliable measurement to reflect renal function. Nonetheless, it can still be considered a limitation that we did not measure other indicators of eGFR, which are not always available in our country.
Moreover, as mentioned above, kidney complications in HIV can occur due to various factors, and kidney biopsy is the gold standard to establish the underlying cause. However, it is not always feasible to perform biopsy in all settings. Urine protein testing can be an easy alternative for screening and to determine the need for further examination in children with HIV.
Further research is needed with a larger sample size, a longer study period, and a cohort method to evaluate whether proteinuria is persistent in children with HIV.
In conclusion, degree of immunodeficiency correlates with degree of kidney complications. Screening at diagnosis and periodic monitoring of kidney functions are crucial in all HIV patients.
Footnotes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Nanan Sekarwana, Almira Aliyannissa, Djatnika Setiabudi, and Anggraini Alam participated in the data collection. Nanan Sekarwana, Almira Aliyannissa, Rahmat Budi Kuswiyanto, and Heda Melinda Nataprawira wrote the manuscript. Nanan Sekarwana, Almira Aliyannissa, and Rahmat Budi Kuswiyanto participated in the study design and performed the statistical analysis. Nanan Sekarwana, Almira Aliyannissa, Rahmat Budi Kuswiyanto, Djatnika Setiabudi, Heda Melinda Nataprawira, and Anggraini Alam participated in the conception, analysis, and interpretation of data. Nanan Sekarwana, Almira Aliyannissa, Djatnika Setiabudi, and Anggraini Alam provided intellectual content of critical importance to the work and technical support. All authors read and approved the final manuscript.
References
- 1.Calza L, Vanino E, Magistrelli E, et al. Prevalence of renal disease within an urban HIV-infected cohort in Northern Italy. Clin Exp Nephrol. 2014;18:104–112. doi: 10.1007/s10157-013-0817-5. [DOI] [PubMed] [Google Scholar]
- 2.Ekat MH, Courpotin C, Diafouka M, et al. [Prevalence and factors associated with renal disease among patients with newly diagnoses of HIV in Brazzaville, Republic of Congo] Med Sante Trop. 2013;23:176–180. doi: 10.1684/mst.2013.0170. French. [DOI] [PubMed] [Google Scholar]
- 3.Gupta V, Gupta S, Sinha S, et al. HIV associated renal disease: a pilot study from north India. Indian J Med Res. 2013;137:950–956. [PMC free article] [PubMed] [Google Scholar]
- 4.Adih WK, Selik RM, Hu X. Trends in diseases reported on US death certificates that mentioned HIV infection, 1996-2006. J Int Assoc Physicians AIDS Care (Chic) 2011;10:5–11. doi: 10.1177/1545109710384505. [DOI] [PubMed] [Google Scholar]
- 5.Arendse CG, Wearne N, Okpechi IG, Swanepoel CR. The acute, the chronic and the news of HIV-related renal disease in Africa. Kidney Int. 2010;78:239–245. doi: 10.1038/ki.2010.155. [DOI] [PubMed] [Google Scholar]
- 6.Bhimma R, Purswani MU, Kala U. Kidney disease in children and adolescents with perinatal HIV-1 infection. J Int AIDS Soc. 2013;16:18596. doi: 10.7448/IAS.16.1.18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kooij KW, Vogt L, Wit FWNM, et al. Higher Prevalence and faster progression of chronic kidney disease in human immunodeficiency virus-infected middle-aged individuals compared with human immunodeficiency virus-uninfected controls. J Infect Dis. 2017;216:622–631. doi: 10.1093/infdis/jix202. [DOI] [PubMed] [Google Scholar]
- 8.Trullàs JC, Cofan F, Barril G, et al. Spanish HIV Infection in Dialysis Study Group. Outcome and prognostic factors in HIV-1-infected patients on dialysis in the cART era: a GESIDA/SEN cohort study. J Acquir Immune Defic Syndr. 2011;57:276–283. doi: 10.1097/QAI.0b013e318221fbda. [DOI] [PubMed] [Google Scholar]
- 9.Anochie IC, Eke FU, Okpere AN. Human immunodeficiency virus-associated nephropathy (HIVAN) in Nigerian children. Pediatr Nephrol. 2008;23:117–122. doi: 10.1007/s00467-007-0621-0. [DOI] [PubMed] [Google Scholar]
- 10.Lucas GM, Ross MJ, Stock PG, et al. HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update by the. Clin Infect Dis. 2014;59:e96–e138. doi: 10.1093/cid/ciu617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kementerian Kesehatan Republik Indonesia. Kementerian Kesehatan Republik Indonesia; Jakarta: 2014. Pedoman penerapan terapi HIV pada anak. Indonesian. [Google Scholar]
- 12.World Health Organization. Laboratory Guidelines for enumerating CD4 T Lymphocytes in the context of HIV/AIDS [Internet] World Health Organization; New Delhi (Republic of India): 2007. [cited 2019 Apr 29]. Available from: https://www.who.int/hiv/amds/LaboratoryGuideEnumeratingCD4TLymphocytes.pdf. [Google Scholar]
- 13.Kopp JB, Nelson GW, Sampath K, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–2137. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children [Internet] World Health Organization; Geneva (Switzerland): 2007. [cited 2019 Apr 29]. Available from: https://www.who.int/hiv/pub/guidelines/HIVstaging150307.pdf. [Google Scholar]
- 15.National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1–S266. [PubMed] [Google Scholar]
- 16.Antonello VS, Poli-De-Figueiredo CE, Antonello IC, Tovo CV. Urinary protein-to-creatinine ratio versus 24-h proteinuria in the screening for nephropathy in HIV patients. Int J STD AIDS. 2015;26:479–482. doi: 10.1177/0956462414543939. [DOI] [PubMed] [Google Scholar]
- 17.Gupta G, Hemal A, Saha A, Kapoor K, Goyal P, Upadhyay AD. Proteinuria in HIV-infected Indian children. Trop Doct. 2017;47:230–233. doi: 10.1177/0049475516668963. [DOI] [PubMed] [Google Scholar]
- 18.Chaparro AI, Mitchell CD, Abitbol CL, et al. Proteinuria in children infected with the human immunodeficiency virus. J Pediatr. 2008;152:844–849. doi: 10.1016/j.jpeds.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Ikpeme EE, Ekrikpo UE, Akpan MU, Ekaidem SI. Determining the prevalence of human immunodeficiency virus-associated nephropathy (HIVAN) using proteinuria and ultrasound findings in a Nigerian paediatric HIV population. Pan Afr Med J. 2012;11:13. [PMC free article] [PubMed] [Google Scholar]
- 20.Antonello VS, Antonello IC, Herrmann S, Tovo CV. Proteinuria is common among HIV patients: what are we missing? Clinics (Sao Paulo) 2015;70:691–695. doi: 10.6061/clinics/2015(10)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fisher H, Hsu CY, Vittinghoff E, Lin F, Bansal N. Comparison of associations of urine protein-creatinine ratio versus albumin-creatinine ratio with complications of CKD: a cross-sectional analysis. Am J Kidney Dis. 2013;62:1102–1108. doi: 10.1053/j.ajkd.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verma B, Singh A. Clinical spectrum of renal disease in hospitalized HIV/AIDS patients: a teaching hospital experience. J Family Med Prim Care. 2019;8:886–891. doi: 10.4103/jfmpc.jfmpc_98_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adedeji TA, Adedeji NO, Adebisi SA, Idowu AA, Fawale MB, Jimoh KA. Prevalence and pattern of chronic kidney disease in antiretroviral-naïve patients with HIV/AIDS. J Int Assoc Provid Aids Care. 2015;14:434–440. doi: 10.1177/2325957415587570. [DOI] [PubMed] [Google Scholar]
- 24.Ross MJ. Advances in the pathogenesis of HIV-associated kidney diseases. Kidney Int. 2014;86:266–274. doi: 10.1038/ki.2014.167. [DOI] [PubMed] [Google Scholar]
- 25.Jindal AK, Tiewsoh K, Pilania RK. A review of renal disease in children with HIV infection. Infect Dis (Lond) 2018;50:1–12. doi: 10.1080/23744235.2017.1371852. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso CA, Pinto JA, Candiani TM, Carvalho IR, Dantas AG, Goulart EM. Assessment of the prognostic value of the World Health Organization clinical staging system for HIV/AIDS in HIV-infected children and adolescents in a cohort in Belo Horizonte, Brazil. J Trop Pediatr. 2012;58:353–359. doi: 10.1093/tropej/fmr110. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization; World Health Organization, editor. Manual on paediatric HIV care and treatment for district hospitals: addendum to the pocket book of hospital care of children. World Health Organization; Geneva: 2011. Introduction: infants, children and HIV-infection; pp. 44–45. [PubMed] [Google Scholar]
- 28.McCulloch MI, Ray PE. Kidney disease in HIV-positive children. Semin Nephrol. 2008;28:585–594. doi: 10.1016/j.semnephrol.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]