Abstract
Increasing attention of plant derived therapeutic agents against cancer, investigating the anti-proliferative efficiency of plant derived chemicals have achieved increasing momentum for the design of anticancer drug. Punicalagin, dietary phytochemical altered the various cell signal transduction pathways associated with cell apoptosis and proliferation. This investigation was intended to examine the efficiency of punicalagin lying on cell viability so as to examine the molecular based punicalagin mechanism stimulated apoptosis via exploring the expression of Bcl-2 family proteins, and caspases also the cell cycle regulatory proteins p53 and NF-κB signaling in human cervical cancer cells. We also analyzed the morphological characteristic changes through mitochondrial membrane depolarization, reactive oxygen species (ROS) generation, TUNEL assay, AO/EtBr analysis in cervical cancer cells. Our findings demonstrated that punicalagin repressed the viability of cervical cancer cells in a dosereliant mode via stimulating mitochondrial mediated apoptosis. Moreover, our this study demonstrated that punicalagin blocked cervical cancer cell proliferation and stimulated cell apoptosis by suppressing NF-kappa B activity. Hence our study suggested that punicalagin exhibits opposing actions on NF-kappa B signaling networks to block cancer cell progression acts as a classical candidate for anticancer drug designing.
Keywords: Punicalagin, Cervical cancer, Apoptosis, ME-180 cells, NF-ƙB pathway
1. Introduction
Cervical cancers an importantly contribute to be global health issues, especially for women (Ginsburg et al., 2017). It is foremost common diagnosed in the 3rd cancer and the 4th cause of world-wide female cancer death (World Health Organization, 2016). It has been comprehensively investigated the function of human papillomavirus (HPV) infection believed as the cervical cancer pathogenesis as well as its precursor damage. Importantly, growing evidence designated that other non viral related molecular signaling pathways are implicated in initiation as well as progression of cancer cells (Ferlay et al., 2018, Mahdavi and Monk, 2005). Consequently, the investigation ofsignaling pathways related to cell multiplication and apoptosis is helpful to recognize the principal mechanism of cancer in order to locate prospective treatment targets (Baig et al., 2016, Sever and Brugge, 2015).
Nuclear factor-κB (NF-κB), a modulator responsible for transcription, which reins enormous gene regulating different cellular functions. The family of NF-κB contains five subunits (p50, p52, p65, RelB & c-Rel), among them, subunit p65 is an important step in a process of NF-κB stimulation (Wan and Lenardo, 2009). The NF-κB signaling pathway acts as a significant role in the inflammatory pathway and apoptosis (Xia et al., 2018). The NF-κB signaling pathway is activated in numerous cancer types e.g. cervical cancer as an important inducible tumorigenesis modulator, which making tumor cells to evade the apoptosis from cell cycle checkpoint (Stahlhut and Slack, 2013). Previously it has been documented that the NF-κB inactivation reduces the inflammatory factor levels in chemical hypoxia-treated skin dermal cells (Eltzschig and Carmeliet, 2011). Besides, it was stated that the NF-κB has a vital function in malignancies associated with the inflammatory pathway, as like activated NF-κB encourage cell proliferation, survival and angiogenesis (Park and Hong, 2016). Oncoproteins triggers the stimulation of NF-κB cascades connected with progression of cervical cancer. Constitutive activation NF-κB pathway endorses the progression of human cervical cancer as well as poor prognosis (Tilborghs et al., 2017).
Even though advances in the cervical cancer therapy, protocols for unrelenting cancers and other therapeutic decision along with littleside effects are still inneed. Since an improved knowing of the actions of the therapeutic agents has illuminated the therapy of cervical cancer, new agents, which target precise intracellular mechanisms associated with the typical features of cancer cells going on to be recognized. Cancer treatment has distorted considerably while the earlier periods for the reason that numerous novel therapies including monoclonal antibodies as well as intended anticancer drugs were being introduced (Kydd et al., 2017, Adler and Dimitrov, 2012). Conversely, attained chemo-resistivity of cancer cells resultant to the reduced curative potential is the foremost obstruction to the conservative cancer chemotherapy (Lu and Chao, 2012). Utilization of naturally derived agents as hopeful chemotherapeutic agent towards cancer was expansively examined in the preceding periods (Demain and Vaishnav, 2011). Earlier reports were stated that usage of phytochemicals against anti-cancer with various mode of functions might be highly efficient in treating the ailments with very less side-effects (Iqbal et al., 2017, Wang et al., 2012).
Pomegranate, a traditional fruit well-known to exert therapeutic benefits as a medicine, it is now being documented as a efficient chemopreventive as well as anticancer agent (Bhandari, 2012, Sharma et al., 2017). Growing proofs were evidenced the cancer therapeutic potential of pomegranate in vitro and in vivo model in different type’s cancers (Bassiri-Jahromi,, Syed et al., 2013). Punicalagin (2,3-hexahydroxydiphenoylgallagyl-D-glucose) is the abundant bioactive tannin compound isolated from the Punica granatum L. It is the largest, water soluble ellagitannin molecule that has a high bioavailability (Lin et al., 2013). It was showed to exert antioxidant, antiproliferation, antiviral, anti-inflammatory and anticancer benefits (Tang et al., 2017, Lee et al., 2010, Bialonska et al., 2010, Adams et al., 2006). It was known to stimulate apoptosis in promyelocytic leukemia cells, colon cancer lines and glioma cells (Tang et al., 2017). Earlier studies have explored that punicalagin has therapeutic action on a variety of tumor cells, which includes increasing expression of Bcl-2, Bax, Bcl-2 linked death promoterand cell cycle proteins, as well to regulating the cell propagation and apoptosis (Malik and Mukhtar, 2006, Khan et al., 2007, Wang et al., 2013). In this exploration, we aimed to investigate the anticancer potential of punicalagin through the enhancement of mitochondrial mediated apoptotic pathway and inhibition of NF-kB signaling pathway in the human cervical cancer (ME-180) cells.
2. Materials and methods
2.1. Chemicals and reagents
Punicalagin, MTT (3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide), required chemicals for cell culture comprise EDTA, penicillin–streptomycin, dulbecco’s modified eagle medium (DMEM), glutamine, FBS (fetal bovine serum), PBS (phosphate buffered saline) were procured from Sigma Aldrich. Caspase-3 & 9 was acquired from CellSignaling Technology. The antibodies (BAX & BCL-2) were purchased from SantaCruz Biotech, USA.
2.2. Maintenance of cell culture
The cells (ME-180) were cultured in a occurrence of DMEM with 10% of FBS and 1% of penicillin/streptomycin and sustained at 37 °C in a moistened atmosphere with consisting of 5% CO2 and 95% air incubation.
2.3. Determination of cytotoxicity by MTT assay
The cytotoxic activity of punicalagin was examined via MTT dependant colorimetric assay (Tolosa et al., 2015). Cells were loaded (5000–6000 cells per well) in well plates and placed incubation around 24 h at 37 °C. In order to recognize the cytotoxic activity of punicalagin, it was treated to the cells at diverse concentrations in DMSO, later placed incubation around 24 h at 37 °C. After that, MTT solution was mixed into all the wells. Then, the plates were placed incubation around 4 h at 37 °C. As a result, the medium was detached, DMSO (100 μL) was mixed with the well in an attempt in order to mix the formazan crystals. Lastly, the absorbance was determined at the range of 570 nm by the use of microplate reader.
2.4. Measurement of intracellular ROS production
ROS accretion was examined via the DCFH-DA which can substantially slice into intra-cellular matrix there it is oxidized into fluorescent dichlorofluorescein (DCF) via ROS (Annamalai et al., 2016). Consequently, the fluorescence emission power is straightly relative to the range of ROS production. ME-180 cells were placed in well plates and treated with punicalagin, then placed for incubation in the CO2 incubator around 24 h. Later, the cells were stained with DCFH-DA around 10 min. Finally, the fluorescent intensity was calculated by excitation as well as emission filters (485 ± 10 and 530 ± 12.5 nm). The results were expressed in terms of percentage enhancement of fluorescence power.
2.5. Determination of mitochondrial membrane potential (MMP)
The MMP was evaluated by means of rhodamine-123 and lipophilic cationic dye. The cells were cultured and supplemented with punicalagin (24 h). Subsequently, the cells were kept incubation with the dye (Rh-123) around 30 min. The DCM was measured qualitatively via floid cell imaging station. Later, the cells were trypsinized and the strength of fluorescence was assessed by using spectrofluorometer (at 485/530 nm).
2.6. Fluorescence microscopic examination of cell death
The staining assessment of AO/EB (acridine orange/ethidium bromide) was studied for the apoptotic finding via morphological examination (Baskić et al., 2006). The cells were kept for punicalagin treatment around 24 h. Later, the dye mixture was mixed into punicalagin treated cells and then observed instantaneously by fluorescent microscope (Inverted). However, untreated cells were treated as control; finally the findings were articulated as mean ± SD in a triplicate manner.
2.7. TUNEL assay
The cells were seeded in 6-wells plate (1 × 106 cells/well) and supplemented with punicalagin. After 24 h treatment, the cells were collected and suspended in 0.5 mL of PBS. Then, the cells were mixed with 5 mL of 1% (w/v) paraformaldehyde in PBS for 15 min. Then, the cells were centrifuged and cleaned with PBS. Then cells were suspended and mixed with 70% ethanol and incubated at −20 °C deep freezer. Ethanol was removed from cell suspension, and then the cells were sustained with the 50 μL DNA-labeling solution (10 µL reaction buffer, 0.75 µL TdT enzyme, 8.0 µL BrdUTP, and 31.25 µL dH2O) for 60 min at 37 °C. Later than the incubation, 1 mL of wash buffer was mixed and spun at 300 × g for 5 min. Then cells were maintained with 100 µL of antibody staining solution for 30 min at 37 °C. Fluorescent signals were examined using a laser scanning confocal microscopy (Nikon Microscopy).
2.8. Transcriptional analysis of apoptotic protein expressions
Total RNA was brought together from cells (ME-180) by using the RNeasy Mini kit based on the protocol given by manufacturer. Then, we inspected the mRNA expression pattern of Bcl-2, Bax, caspase (3 & 9) via utilizing RT-PCR. The statuses of gene expression were standardized into 18S mRNA. Lastly, the average Ct measurements (triplicate manner) were employed in order to analyse the comparative gene expression via 2−ΔΔCt formula.
2.9. Western blotting analysis
Cells were treated with lysis buffer around 30 min and then centrifuged (2,000g) around 30 min at 4 °C. Later, the supernatant was removed for the quantitative assessment of protein concentration via nanodrop spectrophotometer. Each was mixed with sample sodium dodecyl sulphate (SDS) buffer, and denatured at the range of 95 °C around 5 min. Then, the proteins were collected by using SDS-PAGE electrophoresis, followed by it was transferred into the nitrocellulose membrane, which was blocked via 5% BSA and placed overnight incubation with specific monoclonal primary antibodies (NF-κB, Caspase-3 & 9) (Cell Signaling Technology, USA) at 4 °C. Lastly, the membrane was kept incubation with 2° antibodies for 1 h at RT, washed thrice by means of PBST. Finally, it was identified with a chemiluminescent detecting system (R&D Systems, USA).
2.10. Statistical analysis
The statistical analysis was employed by means of one way ANOVA followed by DMRT analysis. Values are expressed as mean ± SD of three experiments (p < 0.05) was significantly different from the control.
3. Results
3.1. Effect of punicalagin on ME-180 cell viability
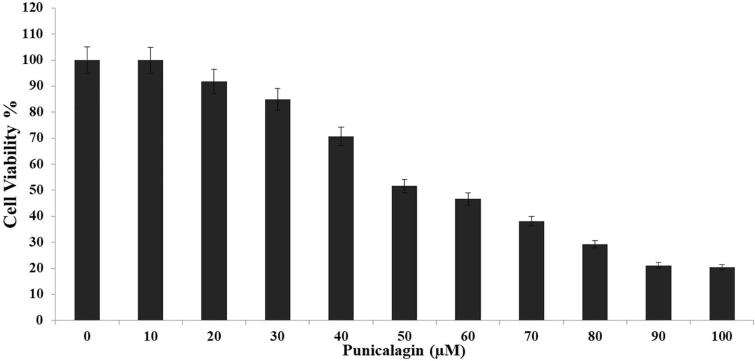
The cytotoxic level of punicalagin against the ME-180 cells were examined by using MTT assay (Fig. 1). Punicalagin was possessed a notable cytotoxicity to ME-180 cells in a concentration dependant mode (10–100 μM), the punicalagin treated cells exerted around 80% of reduction in cell viability as compared with control cells. Consequently, these findings manifestly confirmed that punicalagin treatment potentially encouraged the cell death in ME-180 cells.
Fig. 1.
The cytotoxic efficiency of punicalagin on ME-180 cells examined by MTT assay. The cells were treated with punicalagin at diverse concentrations (10–100 μM). The statistical analysis was employedby means of one way ANOVA. Values are expressed as mean ± SD of three experiments (P < 0.05) was significantly different from the control.
3.2. Effect of punicalagin on △ψM damage in ME-180 cells through rhodamine 123 staining
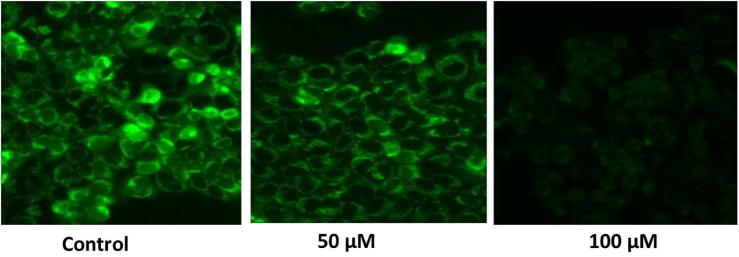
Mitochondrial membrane potential (ΔΨm) is a sign of the cellular physiological condition. Also, it act as a key marker of mitochondrial membrane reliability and reduction which is an earlier events resultant into apoptosis. In order to assess the ΔΨm reduction in punicalagin treated ME-180 cells, cells were employed for stained process with rhodamine 123 dye (Fig. 2). Control cells release augmented emission of green coloured fluorescence signifying that polarized mitochondrial membrane. Alternatively, punicalagin (at the concentration of 50&100 μM) treated ME-180 cells exhibited considerably altered mitochondrial membrane potential which is persistently to decreased the emission of green coloured fluorescence.
Fig. 2.
The effect of punicalagin on △ψM damage was estimated with ME-180 cells through Rhodamine 123. 50 μM and 100 μM of punicalagin treated ME-180 cells was shown decreased fluorescence intensity as indicated distorted mitochondrial matrix. The images were viewed from fluid cell imaging station.
3.3. The effect of punicalagin on intracellular ROS production was assessed with ME-180 cells through DCFH-DA staining
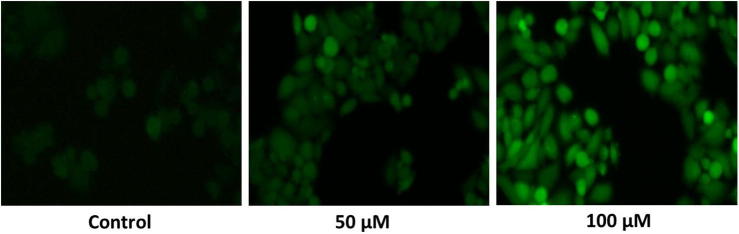
It is eminent that apoptosis is motivated through the increased generation of ROS. As like, we found that punicalagin efficiently improved the ROS generation in ME-180 cells by means of DCFH-DA dye (Fig. 3). In punicalagin treated ME-180 cells, ROS production was corroborated by the measurement of prominent fluorescence emission as compared to control cells. Therefore, DCFH-DA staining assay clearly revealed that increased ROS production in ME-180 cells as a result of punicalagin treatment.
Fig. 3.
The effect of punicalagin on intracellular ROS production was assessed with ME-180 cells through DCFH-DA staining. A photo micrographic image exhibited increased ROS production in 50 and 100 μM of punicalagin treated ME-180 cells was indicate deep DCF fluorescence intensity. The images were acquired by floid cell imaging station.
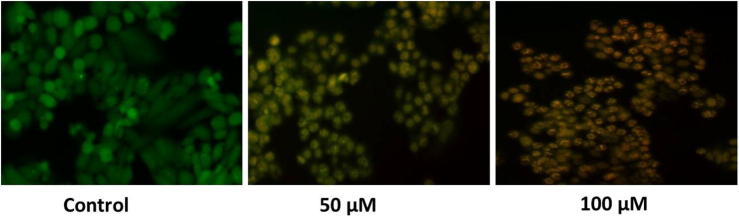
3.4. Effect of punicalagin on apoptotic occurrence by staining assay (AO/EB) staining in ME-180 cells.
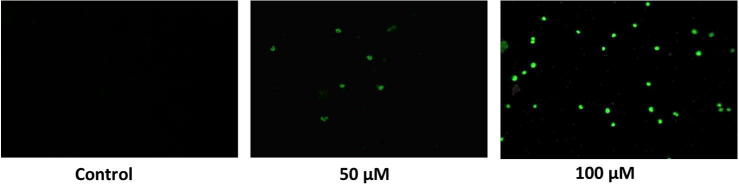
Predominantly, the nuclei were found to be splitted into lesser fragments, thereby which signifying that the formation of apoptosis bodies in punicalagin treated ME-180 cells. Punicalagin treatment (50&100 μM) resultant into an increased mass of late apoptotic cells, which has recognized by means of staining cells (orange red coloured) due to damaged membrane (Fig. 4). These alterations were not examined in vehicle control cells, which exhibited stained nuclei with intact cell structure. Therefore, our findings suggested that punicalagin have a powerful impact on the nuclear morphology, which is intimately related with apoptosis. In addition, the TUNEL assay exhibited that punicalagin induced apoptosis was regulated positively following punicalagin treatment (24 h) in ME-180 cancer cells (Fig. 5).
Fig. 4.
Effect of punicalagin on apoptotic occurrence by staining assay (AO/EB) staining in ME-180 cells. Illustrated fluorescence microscopy images shows punicalagin (50 & 100 μM) treated ME-180 cells exhibits enhanced % of apoptotic cells in a various concentration dependent manner.
Fig. 5.
DNA and nuclear damage by punicalagin was examined by fluorescence microscopy by means of TUNEL staining in ME-180 cells. ME-180 cells were stained with FITC using an apoptosis Assay kit. FITC binds to phosphatidylserine, which appears on the outer leaflet of the cell membrane as a delayed sign of apoptosis.
3.5. Effect of punicalagin NF-κB protein expression in ME-180 cells
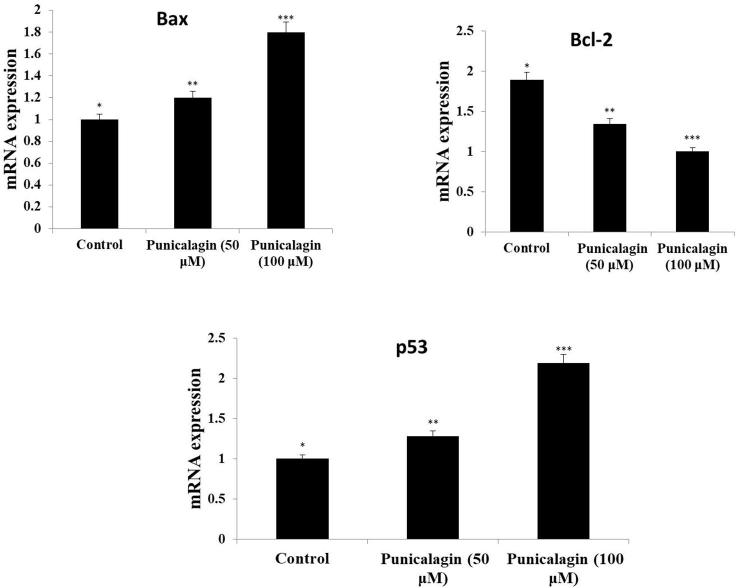
The expression level of protein (NF-κB) in ME-180 cells was presented in Fig. 6. Our findings confirmed that punicalagin considerably down-regulated the protein expression (NF-κB)at various range of dose concentration and as well punicalagin reduced the Iκ-B expression in a dose reliant mode in ME-180 cancer cells (Fig. 7). Further, we have evaluated punicalagin stimulated apoptosis in cervical cancer cells. Punicalagin drastically up-regulated the protein expression of caspase-3 & 9 and also it upregulated the expression of Bax, p53 mRNA in ME-180 cells (Fig. 8). Moreover, punicalagin significantly downregulated the mRNA expression level of Bcl-2 in ME-180 cervical cancer cells.
Fig. 6.
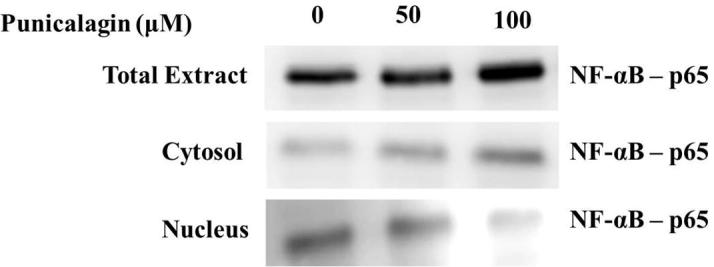
Effect of punicalagin NF-κB protein expression in ME-180 cells. Cells were treated with punicalagin (50, 100 μM) for 24 h.After different treatment conditions, cells were lysed, and NF-κB protein expression was analysed by Western blotting with NF-κB monoclonal antibody.
Fig. 7.
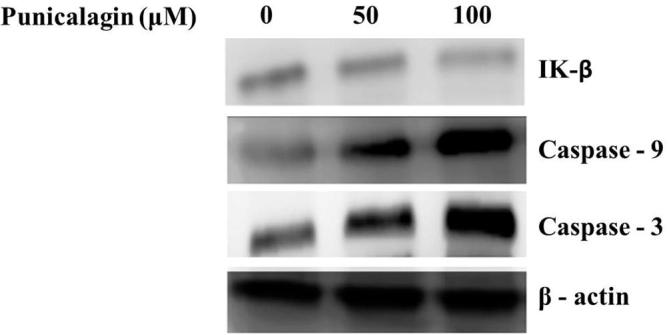
Effect of punicalagin treatment on IK-β, Caspase-3 and Caspase-9 in ME-180 cells. The cells were treated with punicalagin for 24 h and then collected. The protein levels were measured by western blot. The details are illustrated in materials and methods; the figures represent the data of three experiments, each conducted in duplicate.
Fig. 8.
Effect of punicalagin treatment on Bcl-2, Bax and p53 in ME-180 cells. The cells were treated with punicalagin (50&100 μM) around 24 h. Later thandiverse treatment conditions, total cellular mRNA was isolated as well astranscribedreversely. The mRNA levels were examined by RT-PCR analysis. Expression levels were standardized with GAPDH. Each column shows themean ± S.D. of three independent experiments performed in triplicate. Values not sharing a common markings, differ significantly at p < 05 vs. control (DMRT).
4. Discussion
Globally, cervical cancer is being one among the lethal and frequently detected cancers. In the current decades, cervical cancer incidence has improved drastically and is probable to augment in future (Denny et al., 2015). Current therapeutic strategies are inadequate and also associated with an assortment of side effects. Hence, there is an insistent necessity to seek novel therapeutic targets to restrain the rising cervical cancer incidence (Kori and YalcinArga, 2018). Over the years, secondary metabolites derived from plants have achieved substantial consideration as bioactive molecules. They have been revealed to exert anticancer properties in opposition to various cancers (Seca and Pinto, 2018, Sharma, 2019). Our findings suggested that punicalagin treatment strongly inhibited the MW-180 cell multiplication in a dose reliant way. As a major form of cell death, apoptosis provide a decisive task in sustaining the stability of the cell to mediate growth and development (Elmore, 2007). The normal apoptotic mechanisms are evaded in a various cancer cells, leading to uninhibited growth (Wong, 2011). Therefore, apoptosis have become a focus in life-science research, predominantly in tumor research.
Enormous research has examined that flavonoid possess suitable therapeutic properties, especially encouraging of tumour cell apoptosis, including antiviral, antioxidant, anti-inflammatory and antiproliferative functions (Abotaleb et al., 2018, Chahar et al., 2011). It is well documented that the stimulation of apoptosis via endogenous antioxidant depletion or increased reactive oxygen species (ROS) generation (Liou and Storz, 2010). Cells are accredited to staying alive in lesser ROS levels, but a relative ROS development which in turn supports the cell cycle arrest or apoptosis. ROS altering drugs are being predictable as therapeutic strategies so as to target the cancer cell destruction (Watson, 2013). In this research investigation, we found that punicalagin considerably stimulated ROS production in ME-180 cells in a concentration based mode. Numerous research confirmations have revealed anticancer drugs stimulated cytotoxic effects towards cancerous cell through the apoptotic stimulation via mitochondrial dysfunction and also reduced levels of ΔΨm (Abotaleb et al., 2018, Cheng et al., 2019). Our findings signified that punicalagin reduced the ΔΨm levels in ME-180 cells at various dose concentration manner. It has been previously investigated that anticancer drugs stimulated apoptosis via the reduced ΔΨm level named intrinsic signaling pathway (Pistritto et al., 2016). In this research investigation, we found that punicalagin stimulates the structural alteration in the ME-180 cells, which are confirmed by AO/EtBr assessment. Also, we found that the apoptotic cells with cell blebbing, shrinkage, fragmentation as well as nuclear margination. The combinational therapy of AO/EtBr staining method has been used as a reliable assessment of cellular degeneration. After the staining process, it was illuminated that viable cells were consistently stained green colour, thereby it signifies that the cells with early apoptosis symbolize the greenish-yellow colour or else green-yellow coloured fragments, whereas cells with late apoptosis symbolize orange-coloured fragments.
The regulation of apoptosis is an intricate process principally stimulated via two pathways, the intrinsic (mitochondrial) and extrinsic (cell-death receptor) cascade (Reed, 2000). Additionally, these two pathways have a range of intersections which may be regulated by a number of factors. Earlier report stated that pro-apoptotic and anti-proliferative action of punicalagin in various cancer cell lines via modification in the apoptosis-associated protein expression (Lin et al., 2013, Khan et al., 2007). Pomegranate extract has been mentioned that up-regulating protein expression range of pro-apoptotic marker (Bax), down-regulating protein expression status of anti-apoptotic markers (Bcl-XL & Bcl-2) in prostate cancer cell line (Koyama et al., 2010). Punicalagin treatment reduced the Bcl-2 expression level, and enhanced the caspase-9 (activated) and PARP expression levels in glioma cells (Khan et al., 2007). In the same way, punicalagin stimulates apoptosis in colon cancer cells via caspase-9 & −3 stimulation, and Bcl-XL down regulation. In this current research investigation, punicalagin extensively reduced the expression of anti-apoptotic protein (Bax) and improved the expression of pro-apoptotic protein (Bcl-2) in ME-180 cells.
NF-κB signaling pathway is well thought-out to be allied with multiple cell functions (Oeckinghaus and Ghosh, 2009). During inactive condition, NF-κB locates in the cytoplasm and is attached to inhibitory protein, IκB which guards the nuclear localization signal. Later, this complex molecules enters into the nucleus, there it attached into its consensus sequence in order to activate its downstream genes upon stimulation (Cooper, 2000). In this current research investigation, it was signified that punicalagin treatment decreased the activity of NF-κB in cervical cancer cells. Also, it was suggested that punicalagin treatment noticeably attenuated the inhibition of NF-κB and subsequently induced cervical cancer apoptosis. Overall, our findings demonstrated that punicalagin blocked cervical cancer cell proliferation and stimulated cell apoptosis by suppressing NF-kappa B activity. These findings offer the indication for a role of punicalagin in preventing or treating cervical cancer through modulation of NF-kappa B singling pathway.
Acknowledgments
This project was supported by Researchers Supporting Project number (RSP-2019/5) King Saud University, Riyadh, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ginsburg O., Bray F., Coleman M.P., Vanderpuye V., Eniu A., Kotha S.R., Sarker M., Huong T.T., Allemani C., Dvaladze A., Gralow J., Yeates K., Taylor C., Oomman N., Krishnan S., Sullivan R., Kombe D., Blas M.M., Parham G., Kassami N., Conteh L. The global burden of women's cancers: a grand challenge in global health. Lancet. 2017;389(10071):847–860. doi: 10.1016/S0140-6736(16)31392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . World Health Organization; 2016. World health statistics 2016: monitoring health for the SDGs sustainable development goals. [Google Scholar]
- Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. International Agency for Research on Cancer; Lyon, France: 2018. Global Cancer Observatory: Cancer Today. [Google Scholar]
- Mahdavi A., Monk B.J. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005;10(7):528–538. doi: 10.1634/theoncologist.10-7-528. [DOI] [PubMed] [Google Scholar]
- Baig S., Seevasant I., Mohamad J., Mukheem A., Huri H.Z., Kamarul T. Potential of apoptotic pathway-targeted cancer therapeutic research: Where do we stand? Cell Death Dis. 2016;7(1) doi: 10.1038/cddis.2015.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring HarbPerspect Med. 2015;5(4) doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan F., Lenardo M.J. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring HarbPerspect Biol. 2009;1(4) doi: 10.1101/cshperspect.a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia L., Tan S., Zhou Y., Lin J., Wang H., Oyang L., Tian Y., Liu L., Su M., Wang H., Cao D., Liao Q. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018;11(11):2063–2073. doi: 10.2147/OTT.S161109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut C., Slack F.J. MicroRNAs and the cancer phenotype: profiling, signatures and clinical implications. Genome Med. 2013;5(12):111. doi: 10.1186/gm516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364(7):656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.H., Hong J.T. Roles of NF-κB in cancer and inflammatory diseases and their therapeutic approaches. Cells. 2016;5(2):15. doi: 10.3390/cells5020015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilborghs S., Corthouts J., Verhoeven Y., Arias D., Rolfo C., Trinh X.B., van Dam P.A. The role of nuclear factor-kappa B signaling in human cervical cancer. Crit Rev OncolHematol. 2017;120:141–150. doi: 10.1016/j.critrevonc.2017.11.001. [DOI] [PubMed] [Google Scholar]
- Kydd J., Jadia R., Velpurisiva P., Gad A., Paliwal S., Rai P. Targeting strategies for the combination treatment of cancer using drug delivery systems. Pharmaceutics. 2017;9(4):46. doi: 10.3390/pharmaceutics9040046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler M.J., Dimitrov D.S. Therapeutic antibodies against cancer. HematolOncolClin North Am. 2012;26(3):447–481. doi: 10.1016/j.hoc.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu HP, Chao CC. Cancer cells acquire resistance to anticancer drugs: an update. Biomed J. 2012 Nov-Dec;35(6):464-72. [DOI] [PubMed]
- Demain A.L., Vaishnav P. Natural products for cancer chemotherapy. MicrobBiotechnol. 2011;4(6):687–699. doi: 10.1111/j.1751-7915.2010.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J., Abbasi B.A., Mahmood T., Kanwal S., Ali B., Shah S.A., Khalil A.T. Plant-derived anticancer agents: a green anticancer approach. Asian Pacific J. Tropical Biomed. 2017;7(12):1129–1150. [Google Scholar]
- Wang H., Khor T.O., Shu L., Su Z.Y., Fuentes F., Lee J.H., Kong A.N. Plants vs. cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12(10):1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari PR. Pomegranate (Punica granatum L). Ancient seeds for modern cure? Review of potential therapeutic applications. Int. J. Nutr., Pharmacol., Neurol. Dis. 2012 Sep 1;2(3):171.
- Sharma P., McClees S.F., Afaq F. Pomegranate for prevention and treatment of cancer: an update. Molecules. 2017;22(1):177. doi: 10.3390/molecules22010177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassiri-Jahromi S. Punica granatum (Pomegranate) activity in health promotion and cancer prevention. Oncol. Rev. 2018;12(1). [DOI] [PMC free article] [PubMed]
- Syed D.N., Chamcheu J.C., Adhami V.M., Mukhtar H. Pomegranate extracts and cancer prevention: molecular and cellular activities. Anticancer Agents Med Chem. 2013;13(8):1149–1161. doi: 10.2174/1871520611313080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L., Chen T., Lin S., Chung C., Lin T., Wang G., Anderson R., Lin C., Richardson D. Broad-spectrum antiviral activity of chebulagic acid and punicalagin against viruses that use glycosaminoglycans for entry. BMC Microbiol. 2013;13:187. doi: 10.1186/1471-2180-13-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J., Li B., Hong S., Liu C., Min J., Hu M., Li Y., Liu Y., Hong L. Punicalagin suppresses the proliferation and invasion of cervical cancer cells through inhibition of the β-catenin pathway. Mol. Med. Rep. 2017;16(2):1439–1444. doi: 10.3892/mmr.2017.6687. [DOI] [PubMed] [Google Scholar]
- Lee C.J., Chen L.G., Liang W.L., Wang C.C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010;118:315–322. [Google Scholar]
- Bialonska D., Ramnani P., Kasimsetty S.G., Muntha K.R., Gibson G.R., Ferreira D. The influence of pomegranate by-product and punicalagins on selected groups of human intestinal microbiota. Int. J. Food Microbiol. 2010;140:175–182. doi: 10.1016/j.ijfoodmicro.2010.03.038. [DOI] [PubMed] [Google Scholar]
- Adams L.S., Seeram N.P., Aggarwal B.B., Takada Y., Sand D., Heber D. J. Agric. Food Chem. 2006;54(3):980–985. doi: 10.1021/jf052005r. [DOI] [PubMed] [Google Scholar]
- Malik A., Mukhtar H. Prostate cancer prevention through pomegranate fruit. Cell Cycle. 2006;5:371–373. doi: 10.4161/cc.5.4.2486. [DOI] [PubMed] [Google Scholar]
- Khan N., Hadi N., Afaq F., Syed D.N., Kweon M.H., Mukhtar H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis. 2007;28:163–173. doi: 10.1093/carcin/bgl145. [DOI] [PubMed] [Google Scholar]
- Wang S.G., Huang M.H., Li J.H., Lai F.I., Lee H.M., Hsu Y.N. Punicalagin induces apoptotic and autophagic cell death in human U87MG glioma cells. Acta Pharmacol. Sin. 2013;34:1411–1419. doi: 10.1038/aps.2013.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolosa L., Donato M.T., Gómez-Lechón M.J. General cytotoxicity assessment by means of the MTT assay. Methods Mol. Biol. 2015;1250:333–348. doi: 10.1007/978-1-4939-2074-7_26. [DOI] [PubMed] [Google Scholar]
- Annamalai G., Kathiresan S., Kannappan N. [6]-Shogaol, a dietary phenolic compound, induces oxidative stress mediated mitochondrial dependant apoptosis through activation of proapoptotic factors in Hep-2 cells. Biomed. Pharmacother. 2016;82:226–236. doi: 10.1016/j.biopha.2016.04.044. [DOI] [PubMed] [Google Scholar]
- Baskić D., Popović S., Ristić P., Arsenijević N.N. Analysis of cycloheximide-induced apoptosis in human leukocytes: fluorescence microscopy using annexin V/propidium iodide versus acridin orange/ethidium bromide. Cell Biol. Int. 2006;30(11):924–932. doi: 10.1016/j.cellbi.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Denny L., Herrero R., Levin C. Cervical Cancer. In: Gelband H., Jha P., Sankaranarayanan R., editors. Cancer: Disease Control Priorities. Third ed. The International Bank for Reconstruction and Development / The World Bank; Washington (DC): 2015. p. 1. [Google Scholar]
- Kori M., YalcinArga K. Potential biomarkers and therapeutic targets in cervical cancer: Insights from the meta-analysis of transcriptomics data within network biomedicine perspective. PLoS ONE. 2018;13(7) doi: 10.1371/journal.pone.0200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seca A.M.L., Pinto D.C.G.A. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018;19(1):263. doi: 10.3390/ijms19010263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, editor. Bioactive Natural Products for the Management of Cancer: from Bench to Bedside. 2019.
- Elmore S. Apoptosis: a review of programmed cell death. ToxicolPathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.S. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30(1):87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abotaleb M., Samuel S.M., Varghese E., Varghese S., Kubatka P., Liskova A., Büsselberg D. Flavonoids in cancer and apoptosis. Cancers (Basel). 2018;11(1):28. doi: 10.3390/cancers11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chahar M.K., Sharma N., Dobhal M.P., Joshi Y.C. Flavonoids: A versatile source of anticancer drugs. Pharmacogn. Rev. 2011;5(9):1–12. doi: 10.4103/0973-7847.79093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou G.Y., Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–496. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J. Oxidants, antioxidants and the current incurability of metastatic cancers. Open Biol. 2013;3(1) doi: 10.1098/rsob.120144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M.H., Huang H.L., Lin Y.Y., Tsui K.H., Chen P.C., Cheng S.Y., Chong I.W., Sung P.J., Tai M.H., Wen Z.H., Chen N.F., Kuo H.M. BA6 Induces apoptosis via stimulation of reactive oxygen species and inhibition of oxidative phosphorylation in human lung cancer cells. Oxid Med Cell Longev. 2019;7(2019):6342104. doi: 10.1155/2019/6342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistritto G., Trisciuoglio D., Ceci C., Garufi A., D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8(4):603–619. doi: 10.18632/aging.100934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.C. Mechanisms of apoptosis. Am. J. Pathol. 2000;157(5):1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S., Cobb L.J., Mehta H.H., Seeram N.P., Heber D., Pantuck A.J., Cohen P. Pomegranate extract induces apoptosis in human prostate cancer cells by modulation of the IGF-IGFBP axis. Growth Horm IGF Res. 2010;20(1):55–62. doi: 10.1016/j.ghir.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeckinghaus A., Ghosh S. The NF-kappaB family of transcription factors and its regulation. Cold Spring HarbPerspect Biol. 2009;1(4) doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM. The Cell: A Molecular Approach. 2nd edition. Sunderland (MA): Sinauer Associates. The Nuclear Envelope and Traffic between the Nucleus and Cytoplasm. 2000.
Further reading
- Kaurinovic B., Vastag D. InAntioxidants. IntechOpen; 2019. Flavonoids and phenolic acids as potential natural antioxidants. [Google Scholar]