Abstract
Methicillin-Resistant Staphylococcus aureus (MRSA) represents one of the major causes of nosocomial infections, leading to high mortality. Surfaces in clinics, as well as the attending uniform and the hands of the dental doctor can be MRSA reservoirs. Having this in mind, the purpose of this study was to evaluate the presence of Methicillin-Sensitive Staphylococcus aureus (MSSA) and MRSA on dental medicine equipment surfaces. 354 Samples were collected from six equipment surfaces in six attendance areas before and after patient consultation and cultured in a selective medium. Polymerase Chain Reaction (PCR) was used to confirm the identity of bacterial strains as MRSA or MSSA. Data analysis was performed with chi-square tests with Bonferroni correction. It was observed 55.6% of uncontaminated samples. Contamination was: 17.5% MRSA (5.9% of samples collected before patient attendance and 11.6% after); 39.3% MSSA (14.1% collected before and 25.2% after). The prevalence of MRSA and MSSA was significantly higher after patient care. Integrated Clinic represented the most contaminated attendance area (MRSA − 41.7%, MSSA − 51.2%), the chair arm rest was the most contaminated surface for MRSA (29.7%) and the dental spittoon the most contaminated surface for MSSA (23.5%). Although a low level of contamination was observed, dental clinics, through patients possibly carrying bacteria, may be reservoirs for MRSA and MSSA transmission, and might contribute to potential nosocomial infections.
Keywords: Nosocomial infections, mecA gene, Methicillin-Sensitive Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus, Dental clinics
1. Introduction
Methicillin-Resistant Staphylococcus aureus (MRSA) was described for the first time in England in 1961 (Jevons, 1961), soon after the introduction of methicillin in clinical practice (Harkins et al., 2017). In the beginning, this antibiotic was widely used, however, due to its toxicity, it is not commercialised nowadays for human use and was replaced by similar and more stable penicillins such as oxacillin. However, the term Methicillin-Resistant S. aureus is still used (Lee et al., 2018).
S. aureus is frequently associated with skin infections, pneumonia, surgery wounds, bacteraemia, osteomyelitis and endocarditis, being considered one of the most important pathogens of the human being, both at the community level and at nosocomial infections (Shorr, 2007, Bien et al., 2011).
This bacteria colonizes the commensal flora in several regions of the human body (nasal cavities, throat, intestines and skin), but the nasal epithelium is the major colonization spot, whose prevalence reaches, in average, 20–40% of adult population (Smith et al., 2001, Lee et al., 2018).
Due to the widely empirical prescription of antibiotics, bacteria acquire several antibiotic resistances through diverse genetic mechanisms. S. aureus, specially its MRSA strain, acquires resistance to antibiotics, not only to β-lactams such as penicillin and its derivatives, methicillin/oxacillin, carbapenems and cephalosporins, but also to other classes like macrolides and tetracyclines, that makes it a multi-resistant strain. Nowadays, MRSA strains are endemic in several health units all over the world and, consequently, have become an important focus of global efforts of infection control. Due to the limited treatment options, these strains have become the biggest cause of nosocomial infections worldwide with high morbidity and mortality rates (Shorr, 2007, Pantosti and Venditti, 2009, Bhat and Tenguria, 2014).
Some clones of S. aureus evolved to MRSA by acquiring, through horizontal genetic transfer, the staphylococcal chromosomic cassette mec (SCCmec), a mobile genetic element that includes the mecA or mecC genes that confers resistance to most β-lactam antibiotics, including methicillin (García-Garrote et al., 2013, Skov et al., 2013, McCarthy et al., 2015, Milheiriço et al., 2017, Cikman et al., 2019). In fact, MRSA has a remarkable capacity to acquire resistance to any antibiotic, not only to the β-lactams, that leads to important implications to the present and future options of treatment of infections by this pathogen (Chambers and DeLeo, 2009, Lee et al., 2018).
The pathogenicity of S. aureus is related with a big number of virulence factors, that allow adhesion to surfaces, invasion or avoidance of the immune system and cause toxic effects in the host (Shorr, 2007, Bien et al., 2011). Among the most important virulence factors are adhesins and exoproteins, such as exotoxins and enzymes (nucleases, proteases, lipases, hyaluronidases and collagenases) (Bien et al., 2011, Merghni et al., 2014, Blomqvist et al., 2015).
According with the European Centre for Disease Prevention and Control (ECDC) and its annual report of the Priority Program of Infections and Resistances to Antimicrobials, although the percentage of MRSA is decreasing in Portugal, with 47.4, 46.8, 43.6 e 39.2% in the years 2014, 2015, 2016 and 2017 respectively, Portugal is considered one of the countries with the highest MRSA percentage in Europe (ECDC, 2017).
Although the risk of infection in dental clinics is lower than in hospitals (Petti and Polimeni, 2011, Petti et al., 2015), S. aureus strains are still responsible for a lot of pathologies at oral and perioral level such as: angular cheilitis; oral ulcers; failure of treatment with implants; prosthetic stomatitis; facial abscesses; xerostomia; osteomyelitis of the jaws among others, in addition to generalized infections discussed before, making it a public health problem (Smith et al., 2003, Klevens et al., 2008, McCormack et al., 2015, Koukos et al., 2015, Lakhundi and Zhanga, 2018). The risk factors associated with MRSA infections described in the literature include host advanced age, prolonged hospitalization, previous antibiotic therapy, severe illness, surgical procedures, among others (Al-Anazi, 2009, Irfan et al., 2018).
Aerosols contribute as a route of direct or surface contamination, leading to the increase of these strains during patient attendance in dentistry and consequently to a higher probability of cross infection (Bernardo et al., 2005, Hallier et al., 2010, Kobza et al., 2018). Surfaces in clinics, as well as the attending uniform and the hands of the dental doctor can be MRSA reservoirs. Aiming to control and reduce infection standard measures are suggested including: disinfection of hands before and after patient attendance; use of gloves, mask, cap, glasses and work uniform (Williams et al., 2003, Faden, 2019); single use protectors for the light, head board, instrument table, exhauster and also surface disinfection with 77–81% alcohol (Petti and Polimeni, 2011). Moreover, literature points to measures such as: mouthwash with 0.12% CHX or 0.05% cetylpyridinium chloride before dental procedures (Kobza et al., 2018); implementation of surgical exhausters that collect a big volume of aerosols (Kobza et al., 2018) and air cleaning systems (Hallier et al., 2010).
The goal of this study was to analyse the presence of Methicillin-Resistant (MRSA) and Methicillin-Sensitive (MSSA) Staphylococcus aureus strains in several surfaces of dental medicine equipment and in different attendance areas.
2. Materials and methods
2.1. Sampling
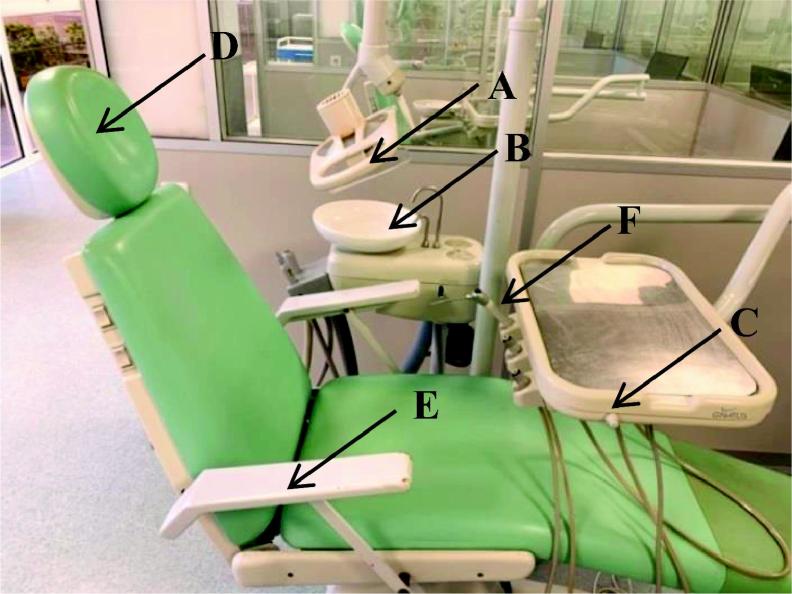
For 5 weeks 354 samples were collected from six dental equipment surfaces of the Dental Pedagogical Clinics of Health Sciences Faculty in Fernando Pessoa University, with swipes Copon Liquid Amies Elution eSwab Collection and Preservation System®. Sample collection was performed by an indirect method based on the use of commercial swabs immersed on transport culture medium. The collection of samples was performed by 4 operators that were previously instructed concerning the methodology used and covered area of each surface. After collection, samples were immediately transported to the laboratory for further processing. Per week, 12 samples were collected, in different schedules and attendance areas per day, from 6 pre-determined surfaces of each dental medicine equipment. The equipment surfaces were chosen due to their frequent interaction with the patient or the dental doctor, being the most likely surfaces to accumulate bacteria. Sampling was performed before and after attendance of one patient. Selected attendance clinical areas were: Integrated Clinic with 144 collected samples (41% of total sampling); Prosthodontic Clinic with 66 collected samples (19% of total sampling); Periodontics Clinic with 54 collected samples (15% of total sampling); Surgery Clinic with 54 samples (15% of total sampling); Odontopediatric Clinic *with 24 samples (7% of total sampling) and Special Patients Clinic with 12 samples (3% of total sampling). Differences in the number of samples collected per clinical areas result from the number of patients available in each analysed area during the period of sampling. Analysed surfaces are depicted in Fig. 1.
Fig. 1.
Image of the 6 analysed surfaces of dental medicine equipment: A-light; B- dental spittoon; C-table; D-head board; E-chair arm rest; F-air/water syringe.
Of the 354 samples only 300 correspond to observation prior to (150 samples) and after patient attendance (150 samples). In some cases, it was not possible to obtain the prior or the after for every attendance area. Those 54 samples are used in further analysis where pairwise analysis is not mandatory.
After collection, samples were cultured in biplates of selective chromogenic medium – chrmID®MRSA/chromID S. aureus- following manufacturer instructions (Biomérieux).
It is a fast detection method, being considered positive the growth of any green colony, indicative of MRSA or MSSA in accordance with the side of the plate where the colony grew (Zurita et al., 2010, Datta et al., 2011). The plates were incubated at 37 °C for 48 h.
The presence of MRSA strains was confirmed by PCR, through the detection of the mecA gene, considered the gold standard by literature (Datta et al., 2011, Stegger et al., 2011, Coban, 2012, Pereira et al., 2014, Chen et al., 2017). Strains that were negative for the presence of the mecA gene, were further analysed for the presence of the nuc gene, that allows the identification of MSSA (Chen et al., 2017).
Identification of the mecA or nuc genes was performed in accordance with Koukos et al. (2015). PCR products were analysed by electrophoresis in 1% agarose gel.
2.2. Statistical analysis
Statistical analysis of the results was performed using the IBM SPSS Statistics vs.25.0 software (IBM Corp. Released 2017, Armonk, NY: IBM Corp.). Counts and percentages were used to describe all qualitative variables. Pairwise comparison of before-after patient attendance positive sample’ collection was performed using the McNemar test. Comparison of positive MRSA or MSSA samples after clinical attendance per clinical attendance clinic (due to low counts, analysis per clinical attendance clinic were performed excluding the Odontopediatric Clinic and the Special Patients Clinic) or per dental medicine equipment surface were performed with chi-square tests with Bonferroni correction. All comparisons were performed using a 5% level of significance.
3. Results
3.1. Quantitative analysis of MRSA and MSSA results
From the 354 collected samples (100%), 197 were non-contaminated (negative for MRSA and MSSA) samples (55.6%) and 157 are contaminated samples. From the contaminated samples, 62 were MRSA positive corresponding to 17.5% of total sampling and 139 were MSSA positive corresponding to 39.3% of total sampling. It is important to mention that samples can be positive for both MRSA and MSSA. In this way, 44 samples are positive for both strains and are counted individually for each strain.
3.1.1. Analysis of positive results observed before and after patient attendance
From a strictly before-after patient consultation comparison it was possible to conclude that a significantly higher percentage of MRSA (McNemar test, p = 0.010) and MSSA (McNemar test, p < 0.001) was observed after patient attendance (Table 1) with 24.7% of MRSA and 53.3% MSSA positive samples.
Table 1.
Number of samples and percentage of negative and positive MRSA and MSSA in equipment surface before and after clinical patient attendance.
| Cleaning state of the equipment |
||||
|---|---|---|---|---|
| Before clinical attendance |
After clinical attendance |
p* | ||
| n (%) | n (%) | |||
| MRSA | Negative | 130ª (86.7) | 113b (75.3) | 0.010 |
| Positive | 20b (13.3) | 37a (24.7) | ||
| MSSA | Negative | 110a (73.3) | 70b (46.7) | <0.001 |
| Positive | 40b (26.7) | 80a (53.3) | ||
a, b - different letters indicate significant differences in counts/% of MRSA or MSSA, where 'a' indicates the highest % and 'b' the lowest %.
McNemar test for pairwise observations.
3.1.2. Quantitative analysis of colonies
In order to evaluate the presence of MRSA and MSSA, it is important to analyse positive results in a quantitative perspective of number of colonies present. In this analysis, samples were distributed as shown in Fig. 2 and Table 2.
Fig. 2.
Example plates with MRSA and MSSA colonies. (A) <5 colonies; (B) 5 to 15 colonies; (C) >15 colonies.
Table 2.
Analysis of number and percentage of positive MRSA and MSSA colonies obtained in samples collected after clinical patient attendance.
| MRSA | MSSA | |
|---|---|---|
| n (%) | n (%) | |
| <5 colonies | 37 (90) | 75 (84) |
| 5 to 15 colonies | 1 (2) | 7 (8) |
| >15 colonies | 3 (7) | 7 (8) |
| Total | 41 (11.6) | 89 (25.1) |
From the MRSA positive samples collected after clinical patient attendance (11.6%), it was observed that 90% corresponded to plates with < 5 colonies and from MSSA positive samples (25.1%), 84% were plates with < 5 colonies (Table 2). This data clearly shows a low level of contamination.
3.2. Analysis of results obtained in different clinical attendance areas
Concerning MRSA positive samples (Table 3), significant differences were observed among clinical attendance areas (Chi2-test, p = 0.038). The Integrated Clinic represented the attendance area with highest percentage of MRSA positive samples (41.7%), being significantly higher than the other attendance areas.
Table 3.
Number of samples and percentage of negative and positive MRSA and MSSA per clinical attendance area after clinical patient attendance.
| Negative |
Positive |
||
|---|---|---|---|
| Attendance area | n (%) | n (%) | |
| MRSA | Integrated Clinic | 69a (57.5) | 15a (41.7) |
| Prosthodontic Clinic | 15b (12.5) | 9b (25.0) | |
| Periodontics Clinic | 15b (12.5) | 9b (25.0) | |
| Surgery Clinic | 21a (17.5) | 3c (8.3) | |
| Odontopediatric Clinic* | 11 | 1 | |
| Special Patients Clinic* | 7 | 5 | |
|
Samples under comparison Total of samples |
120 (100.0) 138 |
36 (100.0) 42 |
|
| MSSA | Integrated Clinic | 41a (56.9) | 43a (51.2) |
| Prosthodontic Clinic | 5b (6.9) | 19b (22.6) | |
| Periodontics Clinic | 12a (16.7) | 12b (14.3) | |
| Surgery Clinic | 14a (19.4) | 10b (11.9) | |
| Odontopediatric Clinic* | 11 | 1 | |
| Special Patients Clinic* | 5 | 7 | |
|
Samples under comparison Total of samples |
72 (100.0) 88 |
84 (100.0) 92 |
|
a, b, c: different letters indicate significant differences in counts/% of MRSA or MSSA per surface of dental medicine equipment, where 'a' indicates the highest %, 'b' the next % and 'c' the lowest %.
Bold values indicate maximum MRSA and MSSA positive values.
Odontopediatric Clinic and Special Patients Clinic were left out of the analysis due to very low sample size of the group.
Regarding MSSA positive samples (Table 3) detected per clinical attendance area, significant differences were observed between those areas (Chi2-test, p = 0.002). Once again, the Integrated Clinic represented the attendance area with the significantly highest percentage of MSSA positive samples (51.2%), being significantly higher than the others.
3.3. Analysis of results obtained per surface of dental medicine equipment
MRSA positive samples collected on equipment surfaces, were distributed as portrayed in Table 4. Significant differences (Chi2-test, p = 0.002) were detected between analysed surfaces. The chair arm rest (29.7%), the head board (27.0%) and the dental spittoon (24.3%) were the surfaces with higher % of MRSA positive samples, and were not significantly different from each other. The second highest surface was the air/water syringe (8.1%) and the lowest counts were detected on the light and table (5.4% each).
Table 4.
Number of samples and percentage of negative and positive MRSA and MSSA per surface of dental medicine equipment after clinical patient attendance.
| Equipment surface | MRSA+ | MSSA+ |
|---|---|---|
| Light | 2c (5.4%) | 13a (15.3%) |
| Dental spittoon | 9a(24.3%) | 20a(23.5%) |
| Table | 2c (5.4%) | 4b (4.7%) |
| Head board | 10a (27.0%) | 18a (21.2%) |
| Chair arm rest | 11a (29.7%) | 17a (20.0%) |
| Air/water syringe | 3b (8.1%) | 13a (15.3%) |
| Total of samples | 37 (100%) | 85 (100%) |
a, b, c: different letters indicate significant differences in counts/% of MRSA or MSSA per surface of dental medicine equipment, where 'a' indicates the highest %, 'b' the next % and 'c' the lowest %.
Bold values indicate highest MRSA and MSSA positive values without significant differences.
Concerning MSSA positive samples, significant differences (Chi2-test, p < 0.001) were also detected between analysed surfaces. The dental spittoon (with 23.5%) was the equipment surface with the highest percentage of MSSA positive samples, and did not differ significantly from the head board, the chair arm rest, the light or the air/water syringe. The table was the surface with the lowest percentage (4.7%) of MSSA positive samples.
4. Discussion
From the total of collected samples (354), the percentage of MRSA positive samples was 17.5% and of MSSA was 39.3%. MRSA and MSSA negative samples corresponded to 55.6% of total sampling. Similar results were obtained in the work of Faden (2019) where a MRSA contamination percentage of 11% was observed. This study did not analyse the presence of MSSA.
Before patient attendance, it was observed a MRSA contamination of 13.3% and 26.7% for MSSA. Samples collected after attendance showed an increase in contamination by MRSA (24.7%) and by MSSA (53.3%). These results confirm a significant increase of samples contaminated by MSSA or MRSA after patient attendance, that is in accordance with other published studies (Williams et al., 2003, Petti et al., 2012, Faden, 2019, Kobza et al., 2018). Study of Motta et al. (2007) analysed six dental clinic surfaces, before, during and after patient attendance and detected a rise in MSSA contamination levels during attendance. However, only 2% of collected samples were positive for MRSA (Motta et al., 2007). This difference in MRSA results could be explained by the size of the tested sample. The study of Motta et al. (2007) has a small sampling size, with a total of 10 samples collected per equipment surface.
Although not performed in our study, some researchers still analysed the presence of these strains on surfaces over time and observed a drastic decrease of contamination in the 15 min after exposure to the environment and without cleaning. These studies observed a decrease of 90% in contamination level (Petti et al., 2012), probably because environmental conditions were not adequate for bacterial survival. Petti et al. (2012) also observed higher bacterial survival rates in non-artificial surfaces such as textiles and low survival in artificial materials such as plastic containers and bottles. However, these strains could still be detected, with 1% of survival rate, for a period of 4 months in strips of upholstery of dental chairs inoculated with MRSA (Petti et al., 2012). Motta et al. (2007) detected a lower degree of contamination by these strains 1 h after attendance when compared with during the attending period.
Through the quantitative analysis of colonies present on culture medium, 90% of MRSA plates and 84% of MSSA plates had<5 colonies. This shows a low level of contamination. These results are in accordance with other studies that detected most MRSA contamination with less than 10 colonies (Petti et al., 2012) and less than five colonies per culture medium (Decraene et al., 2008). These studies do not mention results for the MSSA strain.
Integrated Clinic was the clinical attendance area with highest percentage of MRSA and MSSA positive samples with 41.7 and 51.2% respectively, which can be explained by the diversity of treatment types performed in this attendance.
The statistical analysis revealed significant differences between attendance areas. Faden (2019) considered the oral surgery department the most relevant concerning MRSA detection while Roberts et al. (2011) referred the existence of MRSA positive samples in a dental care unit in Japan and Petti and Polimeni 2012 referred a dental emergency unit in Brazil and an oral surgery unit in London (Roberts et al., 2011, Petti and Polimeni, 2012, Faden, 2019). Khairalla et al. (2017) pointed as attendance areas with higher prevalence of MRSA the Prosthodontic (2.3%), the Periodontology (1.9%), the Dentistry (1.8%) and the Oral Surgery (0.98%) departments, being the Endodontic department the one having lowest contamination (0.60%). In the study of Motta et al. (2007), the Pediatric department was the most contaminated clinical area.
Concerning the analysed equipment surface with highest contamination, chair arm rest was the most MRSA contaminated surface (29.7%). This surface did not show significant differences when compared with the dental spittoon (24.3%) and the head board (27%). Concerning MSSA, the dental spittoon corresponded to the clinical surface with highest percentage of positive samples (23.5%). It did also not show significant differences when compared with the chair arm rest (20%) and the head board (21.2%). These equipment surfaces have direct contact with the patient and direct skin, hand and finger contact is known to be the most common route for S. aureus transmission (Mulligan et al., 1993, Bradley, 1999). Observed results may indicate that patients are possible carriers of these strains, contributing to cross contamination in the dental clinic.
The table, light and air/water syringe showed the lowest percentages of contamination by the two strains. Published data varies in accordance with the analysed surfaces. In the study of Horiba et al. (1995) no MRSA positive samples were detected, however these researchers observed MSSA in the chair and in samples collected from the air and floor. Williams et al. (2003) stated as the most contaminated surfaces the floor, the head board, the light and also the working uniform. Kurita et al. (2006) observed positive samples in the chair arm rest and the air/water syringe. Moreover, Motta et al. (2007) revealed a higher MSSA contamination in the attendance chair buttons, in the light and in the air/water syringe during clinical procedures. Negrini et al. (2009) state as MRSA positive results the table and the storage room in a Pediatric clinic. On the other hand, Roberts et al. (2011) found in 4 of 7 analysed clinics MRSA positive samples in the chair, floor and air/water syringe. Petti et al. (2015) found positive samples in the air pressure system, chair, light, in the air/water syringe and in air samples. In the study of Khairalla et al. (2017), in accordance with the analysed surfaces, the dentist chair, the rotating working equipment and the dental spittoon revealed the highest contamination by MSSA with 8%, 7.1% and 6.2%, respectively. For the MRSA positive samples, the same study revealed that the attendance chair and the dental spittoon were the most contaminated surfaces, both with 8.82%. On the other hand, the equipment light (with 2.9%) represented the least contaminated surface. A recent study of Faden (2019) obtained similar results, observing highest positive samples in paper medical registrations of the patient, followed by the chair arm rest, dental spittoon and head board. The light and table correspond to the two lowest contaminated surfaces (Faden, 2019).
The main limitation found in the methodology used in this study was the number of collected samples that should be similar in the different attendance areas analysed.
5. Conclusion
It is well known that the dental practice is associated with a high risk of exposure of the patient and the healthcare workers, to several bacteria.
The present results suggest that the different clinical attendance areas, as well as the equipment surfaces, may be reservoirs for the transmission of these strains and that the use of individual and surface protection as well as the protocols of disinfection after each attendance period, are very important to minimize the risk of cross infection in the dental clinic.
Integrated Clinic was the clinical attendance area with highest contamination by MRSA or MSSA.
The three most contaminated surfaces by MRSA and MSSA (dental spittoon, the head board and the chair arm rest) represent the three surfaces with direct contact with the patient.
Further studies are needed for the analysis of more surfaces in dental clinics, as well as aerosol collection and the identification of the reservoir of MRSA and MSSA strains, by performing sample collection from the nose and skin of patients and health professionals. Furthermore, the analysis of working uniforms could also be performed to elucidate the possible role of the dental doctor as a carrier of bacterial strains. Moreover, it would be also interesting to test the presence of these bacterial strains overtime, after patient attendance.
6. Sources of financing
This work was supported by national funds through FCT – Fundação para a Ciência e a Tecnologia, I.P. in the project UID/Multi/04546/2019.
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
All authors have read and approved the final article.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Anazi A.R. Prevalence of Methicillin-Resistant Staphylococcus aureus in a teaching hospital in Riyadh, Saudi Arabia. Biomed. Res. 2009;20(1):7–14. [Google Scholar]
- Bernardo W.L., Boriollo M.F., Gonçalves R.B., Hofling J.F. Staphylococcus aureus ampicillin-resistant from the odontological clinic environment. Rev. Inst. Med. Trop. Sao Paulo. 2005;47(1):19–24. doi: 10.1590/s0036-46652005000100004. [DOI] [PubMed] [Google Scholar]
- Bhat J.A., Tenguria R. Significance of MRSA in nosocomial infections. Int. J. Appl. Sci. 2014;1(1):27–36. [Google Scholar]
- Bien J., Sokolova O., Bozko P. Characterization of virulence factors of Staphylococcus aureus: novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. J. Pathogens. 2011;601905 doi: 10.4061/2011/601905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist S., Leonhardt A., Arirachakaran P., Carlen A., Dahlén G. Phenotype, genotype, and antibiotic susceptibility of Swedish and Thai oral isolates of Staphylococcus aureus. J. Oral Microbiol. 2015;7(1):26250. doi: 10.3402/jom.v7.26250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley S. Methicillin-resistant Staphylococcus aureus: long-term care concerns. Am. J. Med. 1999;106:2S–10S. doi: 10.1016/s0002-9343(98)00349-0. [DOI] [PubMed] [Google Scholar]
- Chambers H.F., DeLeo F.R. Waves of resistance: staphylococcus aureus in the Antibiotic Era. Nat. Rev. Microbiol. 2009;7(9):629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zhao Q., Guo J., Li Y., Chen Q. Identification of methicillin-resistant Staphylococcus aureus (MRSA) using simultaneous detection of mecA, nuc, and femB by loop-mediated isothermal amplification (LAMP) Curr. Microbiol. 2017;74(8):965–971. doi: 10.1007/s00284-017-1274-2. [DOI] [PubMed] [Google Scholar]
- Cikman A., Aydin M., Gulhan B., Karakecili F., Kurtoglu M.G., Yuksekkaya S., Parlak M., Gultepe B.S., Cicek A.C., Bilman F.B., Ciftci I.H., Kara M., Atmaca S., Ozekinci T. Absence of the mecC gene in methicillin-resistant Staphylococcus aureus isolated from various clinical samples: The first multi-centered study in Turkey. J. Infect. Public Health. 2019;12:528–533. doi: 10.1016/j.jiph.2019.01.063. [DOI] [PubMed] [Google Scholar]
- Coban A.Y. Rapid determination of methicillin resistance among Staphylococcus aureus clinical isolates by colorimetric methods. J. Clin. Microbiol. 2012;50(7):2191–2193. doi: 10.1128/JCM.00471-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta P., Gulati N., Singla N., Vasdeva H.R., Bala K., Chander J., Gupta V. Evaluation of various methods for the detection of methicillin-resistant Staphylococcus aureus strains and susceptibility patterns. J. Med. Microbiol. 2011;60:1613–1616. doi: 10.1099/jmm.0.032219-0. [DOI] [PubMed] [Google Scholar]
- Decraene V., Ready D., Pratten J., Wilson M. Air-borne microbial contamination of surfaces in a UK dental clinic. J. Gen. Appl. Microbiol. 2008;54(4):195–203. doi: 10.2323/jgam.54.195. [DOI] [PubMed] [Google Scholar]
- ECDC, 2017. Surveillance of antimicrobial resistance in Europe 2017. European Centre for Disease Prevention and Control. Available in <https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2017>.
- Faden A. Methicillin-resistant Staphylococcus aureus (MRSA) screening of hospital dental clinic surfaces. Saudi J. Biol. Sci. 2019;26(7):1795–1798. doi: 10.1016/j.sjbs.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Garrote F., Cercenado E., Marín M., Bal M., Trincado P., Corredoira J., Ballesteros C., Pita J., Alonso P., Vindel A. Methicillin-resistant Staphylococcus aureus carrying the mecC gene: emergence in Spain and report of a fatal case of bacteraemia. J. Antimicrob. Chemother. 2013;69(1):45–50. doi: 10.1093/jac/dkt327. [DOI] [PubMed] [Google Scholar]
- Hallier C., Williams D.W., Potts A.J., Lewis M.A.O. A pilot study of bioaerosol reduction using an air cleaning system during dental procedures. Br. Dental J. 2010;209(8):E14. doi: 10.1038/sj.bdj.2010.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkins C.P., Pichon B., Doumith M., Parkhill J., Westh H., Tomasz A., Lencastre H., Bentley S., Kearns A.M., Holden M.T.G. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017;18(1):130. doi: 10.1186/s13059-017-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiba N., Yoshida T., Suzuki K., Maekawa Y., Ito M., Matsumoto T., Nakamura H. Isolation of methicillin-resistant staphylococci in the dental operatory. J. Endod. 1995;21(1):21–25. doi: 10.1016/S0099-2399(06)80552-2. [DOI] [PubMed] [Google Scholar]
- Irfan S., Ahmed I., Lalani F.K., Anjum N., Mohammad N., Owais M., Zafar A. Methicillin resistant Staphylococcus aureus outbreak in a neonatal intensive care unit. East. Mediterr Health J. 2018 doi: 10.26719/emhj.18.058. [DOI] [PubMed] [Google Scholar]
- Jevons M.P. “Celbenin”-resistant staphylococci. Br. Med. J. 1961;1:124–125. [Google Scholar]
- Khairalla A.S., Wasfi R., Ashour H.M. Carriage frequency, phenotypic, and genotypic characteristics of methicillin-resistant Staphylococcus aureus isolated from dental health-care personnel, patients, and environment. Sci. Rep. 2017;7(1):7390. doi: 10.1038/s41598-017-07713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevens R.M., Gorwitz R.J., Collins A.S. Methicillin-resistant Staphylococcus aureus: a primer for dentists. J. Am. Dental Assoc. 2008;139(10):1328–1337. doi: 10.14219/jada.archive.2008.0044. [DOI] [PubMed] [Google Scholar]
- Kobza J., Pastuszka J.S., Brągoszewska E. Do exposures to aerosols pose a risk to dental professionals? Occup. Med. 2018;68:454–458. doi: 10.1093/occmed/kqy095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koukos G., Sakellari D., Arsenakis M., Tsalikis L., Slini T., Konstantinidis A. Prevalence of Staphylococcus aureus and methicillin resistant Staphylococcus aureus (MRSA) in the oral cavity. Arch. Oral Biol. 2015;60(9):1410–1415. doi: 10.1016/j.archoralbio.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Kurita H., Kurashina K., Honda T. Nosocomial transmission of methicillin resistant Staphylococcus aureus via the surfaces of the dental operatory. Br. Dental J. 2006;201(5):297–300. doi: 10.1038/sj.bdj.4813974. [DOI] [PubMed] [Google Scholar]
- Lakhundi S., Zhanga K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin. Microbiol. Rev. 2018;31(4):e00020–e118. doi: 10.1128/CMR.00020-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.S., Lencastre H., Garau J., Kluytmans J., Malhotra-Kumar S., Peschel A., Harbarth S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers. 2018;4:18030. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- McCarthy H., Rudkin J.K., Black N.S., Gallagher L., O'Neill E., O'Gara J.P. Methicillin resistance and the biofilm phenotype in Staphylococcus aureus. Front. Cell. Infect. Microbiol. 2015;5:1. doi: 10.3389/fcimb.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack M.G., Smith A.J., Akram A.N., Jackson M., Robertson D., Edwards G. Staphylococcus aureus and the oral cavity: an overlooked source of carriage and infection? Am. J. Infect. Control. 2015;43(1):35–37. doi: 10.1016/j.ajic.2014.09.015. [DOI] [PubMed] [Google Scholar]
- Merghni A., Nejma M.B., Hentati H., Mahjoub A., Mastouri M. Adhesive properties and extracellular enzymatic activity of Staphylococcus aureus strains isolated from oral cavity. Microb. Pathog. 2014;73:7–12. doi: 10.1016/j.micpath.2014.05.002. [DOI] [PubMed] [Google Scholar]
- Milheiriço C., Lencastre H., Tomasz A. Full-genome sequencing identifies in the genetic background several determinants that modulate the resistance phenotype in methicillin-resistant Staphylococcus aureus strains carrying the novel mecC Gene. Antimicrob. Agents Chemother. 2017;61(3):1–9. doi: 10.1128/AAC.02500-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motta R.H.L., Groppo F.C., Bergamaschi C.C., Ramacciato J.C., Baglie S., Mattos-Filho T.R. Isolation and antimicrobial resistance of Staphylococcus aureus isolates in a dental clinic environment. Infect. Control Hosp. Epidemiol. 2007;28(2):185–190. doi: 10.1086/510867. [DOI] [PubMed] [Google Scholar]
- Mulligan M.E., Murray-Leisure K.A., Ribner B.S. Methicillin resistant Staphylococcus aureus: a consensus review of the microbiology, pathogenesis, and epidemiology with implications for prevention and management. Am. J. Med. 1993;94:313–328. doi: 10.1016/0002-9343(93)90063-u. [DOI] [PubMed] [Google Scholar]
- Negrini T.C., Duque C., Oliveira A.C., Hebling J., Spolidorio L.C., Spolidorio D.M. Staphylococcus aureus contamination in a pediatric dental clinic. J. Clin. Pediatr. Dent. 2009;34(1):13–18. doi: 10.17796/jcpd.34.1.n435k10291222035. [DOI] [PubMed] [Google Scholar]
- Pantosti A., Venditti M. What is MRSA? Eur. Respir. J. 2009;34:1190–1196. doi: 10.1183/09031936.00007709. [DOI] [PubMed] [Google Scholar]
- Petti S., Polimeni A. Risk of Methicillin-resistant Staphylococcus aureus transmission in the dental healthcare setting: a narrative review. Infect. Control Hosp. Epidemiol. 2011;32(11):1109–1115. doi: 10.1086/662184. [DOI] [PubMed] [Google Scholar]
- Petti S., Polimeni A. Methicillin-resistant Staphylococcus aureus infection transmission in dental health care settings: Myths and facts. Am. J. Infect. Control. 2012;40(3):287–288. doi: 10.1016/j.ajic.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Petti S., Giusti M., Moroni C., Polimeni A. Long-term survival curve of methicillin-resistant Staphylococcus aureus on clinical contact surfaces in natural-like conditions. Am. J. Infect. Control. 2012;40(10):1010–1012. doi: 10.1016/j.ajic.2011.11.020. [DOI] [PubMed] [Google Scholar]
- Petti S., Kakisina N., Volgenant C.M.C., Messano G.A., Barbato E., Passariello C., Soet J.J. Low methicillin-resistant Staphylococcus aureus carriage rate among Italian dental students. Am. J. Infect. Control. 2015;43(2):89–91. doi: 10.1016/j.ajic.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Pereira L.A., Harnett G.B., Hodge M.M., Cattell J.A., Speers D.J. Real-time PCR assay for detection of blaZ genes in Staphylococcus aureus clinical isolates. J. Clin. Microbiol. 2014;52(4):1259–1261. doi: 10.1128/JCM.03413-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M.C., Soge O.O., Horst J.A., Ly K.A., Milgrom P. Methicillin-resistant Staphylococcus aureus from dental school clinic surfaces and students. Am. J. Infect. Control. 2011;39(8):628–632. doi: 10.1016/j.ajic.2010.11.007. [DOI] [PubMed] [Google Scholar]
- Shorr A.F. Epidemiology of Staphylococcal resistance. Clin. Infect. Dis. 2007;45(3):171–176. doi: 10.1086/519473. [DOI] [PubMed] [Google Scholar]
- Skov R., Larsen A.R., Kearns A., Holmes M., Teale C., Edwards G., Hill R. Phenotypic detection of mecC-MRSA: cefoxitin is more reliable than oxacillin. J. Antimicrob. Chemother. 2013;69(1):133–135. doi: 10.1093/jac/dkt341. [DOI] [PubMed] [Google Scholar]
- Smith A.J., Robertson D., Tang M.K., Jackson M.S., MacKenzie D., Bagg J. Staphylococcus aureus in the oral cavity: a three-year retrospective analysis of clinical laboratory data. Br. Dental J. 2003;195(12):701–703. doi: 10.1038/sj.bdj.4810832. [DOI] [PubMed] [Google Scholar]
- Smith A.J., Jackson M.S., Bagg J. The ecology of Staphylococcus species in the oral cavity. J. Med. Microbiol. 2001;50(11):940–946. doi: 10.1099/0022-1317-50-11-940. [DOI] [PubMed] [Google Scholar]
- Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G., Laurent F., Teale C., Skov R., Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecA (LGA251) Clin. Microbiol. Infect. 2011;18(4):395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- Williams H.N., Singh R., Romberg E. Surface contamination in the dental operatory: a comparison over two decades. J. Am. Dental Assoc. 2003;134(3):325–330. doi: 10.14219/jada.archive.2003.0161. [DOI] [PubMed] [Google Scholar]
- Zurita J., Mejía C., Guzmán-Blanco M. Diagnóstico e teste de sensibilidade para Staphylococcus aureus resistente à meticilina na América Latina. Braz. J. Infect. Dis. 2010;14(2):97–106. [Google Scholar]