Abstract
The phenomenal increase in the demand of herbal drugs, leads to over exploitation of medicinal plants which ultimately resulted in the scarcity and endangerment of many valuable plant species. On observing the difficulties in procuring genuine herbal drugs arose the concept of substitution which was documented in many classical Ayurvedic texts. The present study made a comparative evaluation of the gastroprotective potential of hydroalcoholic extracts of an original drug Aconitum heterophyllum (HAAH) and its substitute Cyperus rotundus (HACR) in the treatment of gastric ulcer under in vivo experimental conditions. The anti-ulcer property of the plant extracts was investigated against pylorus ligation induced ulcer in Wistar albino rats. The results confirmed that both A. heterophyllum and C. rotundus deliver comparable significant protection against gastric ulcer, indicated by a decrease in the free and total acidity, volume of gastric content, total proteins and increase in pH of gastric content, total carbohydrates and total carbohydrates to total proteins ratio. The observed anti-ulcer potential of both the drugs is attributed mainly to prevention of the generation of damaging free radical cascades and oxidant radical release.
Keywords: Acute toxicity, Cytotoxicity, Drug substitution, Histopathology, Lipid peroxidation
1. Introduction
Gastric ulcer is considered as a chronic disease disturbing the normal life activities of millions of people throughout the world (Klein-Junior et al., 2010), which is a deep lesion penetrating through the entire thickness of the gastrointestinal mucosa and muscularis mucosa (Tarnawski et al., 2001). Reports indicated that ulcer is caused when there is variability in balance between the defensive agents of gastric mucosa like intestinal barrier, mucus discharge etc and aggressors such as HCl/pepsin secretion associated with digestive mechanism or excessive liquor intake, anxiety and other gastric infections (Tulassay and Hersnzenyi, 2010, Oliveira et al., 2011). Over the past few decades, there has been an upsurge in pharmaceutical research towards the development of promising as well as safer synthetic/natural drugs against ulcer. Herbal drugs are currently regarded as promising choice of drug materials in the quest of new medications for ulcer treatment (Bighetti et al., 2005, Santin et al., 2010). The incredible rise in the demand of drugs of plant origin leads to over exploitation of medicinal plants which ultimately resulted in the scarcity and endangerment of many valuable plant species. This paved way to short supply, increased prices, forced import as well as adulteration of herbal drugs. On observing the difficulties in procuring genuine herbal drugs arouse the concept of substitution which was documented in classical Ayurvedic texts. The concept of herbal drug substitution has already been mentioned in ancient works such as Bhaishajya ratnavali, Bhavaprakasha, Yogaratnakara, Ayurveda Sarasamgraha etc which were written during 14-17th centuries. The particulars of more than 60 herbal drugs and their substitutes were cited in Bhavaprakasha, more than 70 in Yogaratnakara, about 75 in Bhaishajya Ratnavali, and above 110 were described in Sarasamgraha (Vaghela et al., 2013). Though the list of drugs mentioned as substitutes have been in use for years there has been not much scientific validation of the same.
The present study aims to evaluate and compare the ability of the root tubers of Aconitum heterophyllum and its recommended substitute Cyperus rotundus, according to Bhavaprakasha, against gastric ulcer. A comparative study on the anti-diarrheal efficacy of the methanolic extracts of roots of A. heterophyllum and C. rotundus showed that both these drugs exhibited good anti-diarrheal effect, A. heterophyllum inhibiting 53% and C. rotundus inhibiting 46% diarrheal activity in Swiss albino mice (Venkatasubramanian et al., 2010). Both these medicinal plants are reported with related biological activities against fever, liver and spleen disorders, skin infections, urinary tract diseases and diabetes (Seethapathy et al., 2014).
2. Materials and methods
2.1. Plant samples and extraction
The root tubers of C. rotundus and A. heterophyllum were collected from the Raw drug division of Aryavaidyasala, Kottakkal and authenticated by Prof. Benil PB, Department of Agadatantra, VPSV Ayurveda college, Kottakkal. The drug samples were washed with tap water to remove dust particles and finally using distilled water. After cleaning they were shade dried, coarse powdered and sieved. A known quantity of the powdered samples was soaked for a day in 1: 1 solution of ethanol and water in a percolator. The extracts were then filtered, dried under vacuum and stored.
2.2. Experimental animals
Wistar albino rats weighing 120 g ± 20 g body weight were procured from S.D.M Ayurvedic Research Centre, Udupi, Karnataka. The animals were accommodated in polypropylene cages with dimensions of 525 × 330 × 230 mm. Six rats were housed in a cage provided with temperature 250 ± 2 °C and humidity 50 ± 5% throughout the study period. Rats were nourished with normal rat food materials and water ad libitum. Prior to the in vivo studies animal ethical committee approval from the institute was made (SDMCRA/IAEC/RJ18).
2.3. Acute oral toxicity studies
To assess acute toxicity of the hydroalcoholic extracts of A. heterophyllum (HAAH) and C. rotundus (HACR), oral formulations were made using 0.5% carboxy methyl cellulose and evaluated for single dose acute toxicity following OECD guidelines.
2.4. In vitro cytotoxicity study by MTT assay
A malignant cell line – MCF-7 and a non-malignant cell line HEK-293 were selected to analyse cytotoxicity. The cells were maintained at standard experimental conditions and the study was done according to a standard protocol (Mosmann, 1983).
2.5. Anti-ulcer studies using pylorus ligation model
The selected drugs have been widely mentioned in the disease spectrum of Gastro-intestinal tract. The drugs have been indicated in vague symptoms like dyspepsia, abdominal distension etc to specific symptoms like abdominal pain from peptic ulcers. Considering all the symptoms it was decided to perform a gastroprotective effect of the drug through injury induced by pyloric ligation.
A day prior to dosing, the selected animals were randomly separated into 4 different groups with 6 animals in each group.
Group I- Ulcer control
Group II- Standard group (pyloric ligation + pantoprazole)
Group III- Test group III (pyloric ligation + HAAH 200 mg/kg)
Group IV- Test group IV (pyloric ligation + HAAH 400 mg/kg)
Group V- Test group III (pyloric ligation + HACR 200 mg/kg)
Group VI- Test group IV (pyloric ligation + HACR 400 mg/kg)
HAAH and HACR at two different doses were administered for 10 consecutive days and Pantoprazole was administered prior to pyloric ligation at 24, 48 and 72 h intervals followed by withdrawal of food for a period of 36 h by maintaining the animals in a metabolic cage. Water was provided ad libitum to all the animals. Pyloric ligation was performed using the standard protocol after one hour of drug administration (Shay et al. 1945). The animals were ether anaesthetized and the pyloric portion of the stomach was ligated after lifting it through a midline incision starting just below the xiphoid process without damage or traction to its blood supply. The stomach was replaced carefully after ligation with a cotton thread. The incision was closed in layers using interrupted sutures and the animals were fasted without food and water during the post-operative period. The animals were euthanized in ether chambers at the end of the tenth hour. The abdomen was removed from the body cavity after ligating at the level of oesophagus. The contents of the stomach were measured by draining them into a graduated centrifuge tube and biochemical studies were performed.
2.6. Biochemical parameters examined
The gastric tissue homogenate was subjected for biochemical characterization to study volume and pH, free and total acidity (Hawk, 1947), total proteins (Lowry et al., 1951) and total carbohydrates (Nair, 1976). To evaluate the anti-oxidant effect of the selected drugs, gastric tissue homogenate was also analyzed for anti-oxidant enzyme markers like catalase (Sinha, 1972), glutathione peroxidase (Rotruck et al., 1973) and lipid peroxidase (Ohkawa et al., 1997).
2.7. Histopathology of gastric tissue
Histopathological screening of gastric tissue sections were done to evaluate the protective effect of HAAH and HACR. The gastric tissues of the experimental rats were fixed in 10% formalin, processed and stained with hematoxylin and eosin according to a standard protocol (Bancroft and Stevens, 1977).
2.8. Statistical analysis
To carry out interpretation of the data one way ANOVA followed by Dunnet’s T3 multiple comparison was performed using the statistical software IBM SPSS Version 22.
3. Results
3.1. Evaluation of acute toxicity
In order to check the toxicity of the selected drugs, three doses each of HAAH and HACR at 175, 550 and 2000 mg/kg were selected. All these doses were safe without any mortality or other negative effects on the appearance and behaviour in experimental rats. Therefore, the LD50 of the studied drugs was determined to exceed 2000 mg/kg which the highest dose used.
3.2. Cell cytotoxicity studies by MTT assay
In the present study, cytotoxicity of HAAH and HACR were checked against malignant MCF-7 cells and non-malignant HEK-293 cells (Table 1). Result showed that both HAAH and HACR do not exhibit cytotoxicity on the cell lines studied.
Table 1.
Cytotoxic effect of HAAH and HACR on MCF-7 cell line.
| Drugs | Concentration (µg) |
IC50 | ||||||
| 0 | 20 | 50 | 100 | 200 | 500 | 1000 | ||
| % Viability | ||||||||
| HAAH | 90.59 ± 1.44 | 84.87 ± 1.43 | 77.63 ± 2.71 | 69.22 ± 0.89 | 61.69 ± 2.11 | 55.84 ± 3.79 | 45.99 ± 1.75 | 771.37 |
| HACR | 87.24 ± 1.63 | 69.68 ± 2.11 | 66.84 ± 1.39 | 63.28 ± 0.19 | 55.75 ± 3.53 | 50.38 ± 4.39 | 39.75 ± 0.38 | 603.16 |
3.3. Effect of HAAH and HACR on pH and volume of gastric fluid
The pH of the gastric juice was found to be 1.5 in the control group. When compared with the control group, Pantoprazole administered group has shown a significant elevation in pH. In Test group IV there was a significant rise in pH was observed while test group III has only statistically non-significant increase in pH. Even though test groups V and VI have shown an increase gastric pH, the result was insignificant (Table 2).
Table 2.
Effect of Pantoprazole, HAAH and HACR on various gastric parameters.
| Groups | pH | Gastric juice volume (ml) | Free acidity (mEq/L) | Total acidity (mEq/L) | Total carbohydrates (µg/ml) | Total proteins (µg/ml) | CP Ratio |
|---|---|---|---|---|---|---|---|
| Ulcer Control | 1.5 ± 0.5 | 9.58 ± 1.01 | 26.33 ± 2.39 | 83.83 ± 6.74 | 534.22 ± 80.37 | 726.00 ± 99.66 | 0.73 ± 0.04 |
| Pantoprazole | 6.50 ± 0.42*** | 4.46 ± 0.33** | 12.67 ± 0.88* | 41.17 ± 3.98** | 958.82 ± 153.34** | 280.96 ± 43.06** | 3.40 ± 0.06** |
| HAAH 200 | 3.08 ± 0.27 | 5.56 ± 1.02 | 15.83 ± 1.74 | 47.00 ± 3.59* | 739.25 ± 72.85 | 248.13 ± 35.57** | 2.96 ± 0.05** |
| HAAH 400 | 4.83 ± 0.48** | 3.52 ± 0.22** | 11.17 ± 1.42** | 28.50 ± 2.46** | 1283.16 ± 219.75** | 309.98 ± 10.47** | 4.14 ± 0.09** |
| HACR 200 | 3.75 ± 0.30 | 6.35 ± 0.89 | 17.50 ± 1.73 | 52.66 ± 3.86* | 704.8 ± 96.75 | 270.75 ± 20.84** | 2.62 ± 0.03** |
| HACR 400 | 3.16 ± 0.44 | 4.98 ± 1.05* | 13.06 ± 1.42* | 43.17 ± 4.05** | 983.34 ± 119.75** | 273.07 ± 18.44** | 3.61 ± 0.08** |
Data: MEAN ± SEM, ***P < 0.001; **P < 0.01; *P < 0.05 in comparison to ulcer control group.
Pantoprazole administered group has shown considerable significant decrease in gastric juice volume than the control group. Test group III shows insignificant decrease in volume in contrast to group IV which exhibited significant reduction in the gastric volume against control group. The test drug HACR decrease gastric juice volume at both low and high doses but only the effect of high dose was significant (Table 2).
3.4. Effect of HAAH and HACR on acidity
In standard drug administered group, free acidity was found to be significantly reduced against control group. The test drug HAAH group has shown dose dependent significant reduction in the concentration of free acidity. The rats administered with HACR at both the dose levels has shown reduction in the concentration of free acidity but the effect of the higher dose was only significant. Almost identical result was obtained for total acidity also (Table 2).
3.5. Effect of HAAH and HACR on total carbohydrates
The standard drug administered group has shown statistically significant rise in total carbohydrates against control group. Both the test drugs HAAH and HACR at their higher doses (400 mg/kg) has shown statistically significant elevation in the total carbohydrate contents (Table 2).
3.6. Effect of HAAH and HACR on total proteins
The pantoprazole administered group rats have shown significant reduction in total protein contents. Both the test drugs also significantly reduced the total proteins and it was noted that both test drugs at their lower dose prevented protein leakage from gastric mucosa better than their higher doses (Table 2).
3.7. Effect of HAAH and HACR on total carbohydrates: Total proteins ratio
The standard drug administered group has shown an increase in the ratio of total carbohydrates to total proteins. Data also shows that there was significant increase in ratio when administered with both lower and higher therapeutic test doses of HAAH and HACR (Table 2).
3.8. Anti-oxidant effect of HAAH and HACR
To elucidate the gastroprotective efficacy of hydroalcoholic extracts of Aconitum heterophyllum and Cyperus rotundus, the redox-sensitivity of HAAH and HACR was examined. The oxidative stress markers like catalase, glutathione peroxidase and malondialdehyde were analysed in gastric tissue homogenates of control and treated animals.
The concentration of catalase in group I rats was 18.95 ± 0.89 which was significantly elevated in pantoprazole treated rats to 30.62 ± 0.79, an increase by 61.58%. The higher doses of test drugs HAAH and HACR also significantly improved the catalase concentration in rats. The effect of the high dose of HACR was more than that of the standard drug. The lower dose of drugs exhibited insignificant increase in catalase level (Table 3).
Table 3.
Effect of Pantoprazole, HAAH and HACR on antioxidant parameters.
| Groups | Catalase (µM of H2O2 consumed/min/mg protein) | Glutathione peroxidase (µM of GSH oxidized/min/mg protein) | Lipid peroxidation (µM of MDA formed/g wet tissue) |
|---|---|---|---|
| Ulcer Control | 18.95 ± 0.89 | 2.10 ± 0.22 | 9.91 ± 0.48 |
| Pantoprazole | 30.62 ± 0.79** | 4.76 ± 0.34** | 3.69 ± 0.23** |
| HAAH 200 mg/kg | 25.58 ± 2.38 | 2.92 ± 0.18 | 5.25 ± 0.19 |
| HAAH 400 mg/kg | 29.89 ± 1.13* | 4.37 ± 0.41** | 2.52 ± 0.16** |
| HACR 200 mg/kg | 21.71 ± 1.54 | 3.14 ± 0.29 | 6.00 ± 0.84 |
| HACR 400 mg/kg | 42.32 ± 1.46** | 3.79 ± 0.37* | 3.42 ± 0.69** |
Data: MEAN ± SEM, **P < 0.01; *P < 0.05 in comparison to ulcer control group.
The level of glutathione peroxidase in ulcer control rats was 2.10 ± 0.22 μmol/mg protein, which was significantly increased to 4.76 ± 0.34 μmol/mg protein following the administration of pantoprazole. Pre-treatment with HAAH higher dose significantly elevated the concentration of glutathione peroxidase (4.37 ± 0.41 μmol/mg protein) which is equivalent to the effect of standard drug. The lower dose of HAAH and both doses of HACR better the concentration of glutathione peroxidase but was not significant (Table 3).
Malondialdehyde (MDA) levels in gastric mucosa which is assumed to be an index of lipid peroxidation was found raised in group I. The pantoprazole treated group (3.69 ± 0.23 μmol/g of tissue) had significant reduction in MDA concentration against the ulcer control group. HAAH and HACR at higher dose diminished the MDA content (2.52 ± 0.16 μmol/g and 3.42 ± 0.69 μmol/g) significantly (Table 3).
3.9. Histopathological examination
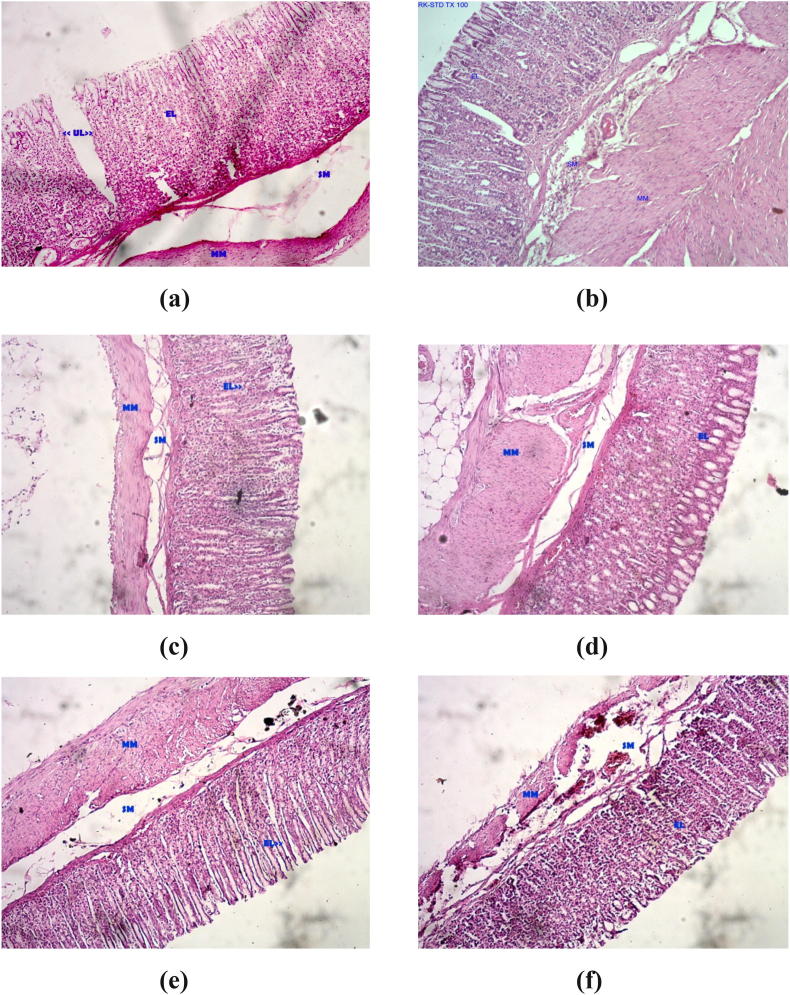
Microscopic investigation of the stomach segments of the pyloric ligated untreated rats showed deep ulceration, severely eroded epithelium and disoriented villi and crypts. Edema of the lamina propria (sub-mucosal layer-SM) was observed. In some sections the changes were observed even in muscularis mucosa layer (MM). Mucosal and sub-mucosal congestion were also noted. A dense inflammatory infiltrate in the lamina propria and hemorrhagic patches were observed in severely ulcerated sections (Fig. 1A).
Fig. 1.
Histopathological examinations of gastric mucosal tissue sections of rats (a) Ulcer control group (b) Pantoprazole treated (c) HAAH 200 mg/kg treated (d) HAAH 400 mg/kg treated (e) HACR 200 mg/kg treated (f) HACR 400 mg/kg treated.
The pantoprazole induced change seen in the gastric mucosa is the parietal cell hyperplasia with granular cytoplasm and apical snouts. In the pantoprazole induce group the mucosal lining was found to be normal with focally denuded epithelium. There was mild inflammatory infiltrate in the lamina propria and congested vessels were observed in the submucosa (Fig. 1B).
In HAAH (200 mg/kg) given group, moderate ulceration was observed in two rats and in others ulceration severity was very less and limited to epithelial erosion (Fig. 1C). In HAAH (400 mg/kg) treated groups, deep penetrating ulcers were absent, epithelial erosion, sub-mucosal edema were less in comparison to sections from pyloric ligated control group (Fig. 1D).
In HACR (200 mg/kg) given group, deep ulcers were observed in one rat and moderate ulceration was observed in another. In rest of the rats ulceration severity was limited to epithelial erosion (Fig. 1E). In HACR (400 mg/kg) given group, moderate ulceration was observed in one rat and in rest of them epithelial erosion with superficial ulceration was observed (Fig. 1E). It is observed that the severity of ulceration was negligible in contrast to the pyloric control and HAAH (200 mg/kg) groups.
4. Discussion
The current study corroborates the potential of hydroalcoholic extracts of the root tubers of Aconitum heterophyllum and Cyperus rotundus against gastric ulcer under in vivo experimental conditions in Wistar albino rats induced with pylorus ligation induced ulcer. It was reported that pylorus ligation leads to the accumulation of acid and pepsin which ultimately results in the self-digestion of gastric mucosa and ulceration (Bharti et al., 2010). The pathogenesis of pylorus ligation also involves the generation of reactive oxygen species (Kaur et al. 2014).
Acute toxicity study of HAAH and HACR revealed that they did not have any toxicity effects or mortality even at a dose of 2000 mg/kg in Wistar rats confirming the safety of test drugs. In the present study cytotoxicity assay was performed to find out potential toxicity of HAAH and HACR using malignant (MCF-7) as well as non-malignant (HEK-293) cultured cells. The activity of a herbal drug extract to prevent cell growth and viability is considered to be an indication of its toxicity (Ifeoma and Oluwakanyinsola, 2013). According to ANCI (American National Cancer Institute) guidelines a crude extract is considered highly cytotoxic only if its IC50 value is less than 20 µg/ml (Abdel-Hameed et al., 2012). The present study reveals that both HAAH and HACR were not at all cytotoxic as their IC50 values were about 20–25 times more than the set value against MCF-7 cells. When tested against HEK-293 cells, even at 1000 µg/ml, they were unable to destroy 50% of the treated cells.
Gastric acid is a key element in the formation of ulcer in pylorus-ligated rat models. Regarding the rise in gastric acid secretion during pylorus ligation it is reported that the excitation of pressure receptors in the mucosa of the gastric antrum paves way to the activation of vagus-vagal reflux in the over secretion model of pylorus ligature (Baggio et al., 2001). The gastric juice volume reduction by HAAH at higher dose was much more than that produced by standard drug. HAAH showed dose related response in this regard but HACR showed lesser effect when compared to HAAH and standard drug. The drugs were probably acting by enhancing the mucosal barrier by increasing the mucosal secretion.
When compared to pantoprazole, the HAAH and HACR increased the pH to a moderate level only. The significant increase in pH by the standard drug might impair the normal digestive process by inhibiting the enzymes requiring lower pH, which actually required lower pH for optimal activities. At the same time HAAH and HACR increase pH only moderately, ensuring normal enzyme activities.
One of the major reasons for ulcer induction due to pyloric ligation is supposed to be the rise in intestinal secretion of HCl which is mainly stress induced (Rao et al., 1998). In the case of gastric ulcer, generally acid secretion ranges from normal to high and acts as an aggressive factor leading to ulceration, decrease of which is one of the main approaches to treat ulcer. Standard drug being a proton pump inhibitor significantly reduces free and total acidity. The rise in pH and decline in acidity as well as volume of gastric fluid in pre-treated rats confirms that both HAAH and HACR comprises of some bioactive agents that can decrease the gastric acidity by controlling the action of H+−K+ ATPase and pepsin.
The current study revealed that there arise a sharp increase in content of total carbohydrates on treatment with pantoprazole, HAAH and HACR in pylorus ligation induced ulcerated rats. The higher doses of HAAH and HACR were more significant than lower doses. In group I rats, the concentration of total proteins was fairly high and on treatment with the standard as well as the HAAH and HACR extracts, there was significant reduction in the total proteins. This reduction in protein content is due to the drop in protein leakage from the mucosal cells specifying mucosal resistance action of the drugs. It was earlier reported that the decline in the protein contents of gastric fluid is related to the decreased leak of plasma proteins into gastric juice (Grossman, 1978). The total carbohydrate/total protein ratio is a key index of the amount of mucopolysaccharides present in the intestinal fluid. Also the elevated carbohydrate/protein ratio is considered to be a reliable and consistent factor indicating the presence of an efficient mucosal barrier (Sanyal et al., 1971) as well as a direct reflection of mucin activity (Jain et al., 1994).
Gastric ulcer still remains as a pathological condition of the gastrointestinal tract in which an effective rational therapy remains elusive. One of the reasons considered as a cause of this disease other than the usual reasons is free radical stress. It is a well-known fact that a reactive free radical produced in our body normally reacts with other non-radicals resulting in a chain reaction of free radicals that eventually leads to the development of fresh free radicals (Tandon et al., 2006). The reactive oxygen species generated may contribute to harm the gastric mucosa by degrading the constituents of epithelial basement membrane, modifying cell metabolism and damaging DNA (Schraufstatter et al. 1988). Our body itself has established several endogenous antioxidant enzymes such as superoxide dismutase, catalase, glutathione peroxidase (GSH-Px), glutathione reductase etc to combat the generation of reactive oxygen species (Ravi et al., 2019). Due to the activity of free radicals the lipids present in the plasma membrane also undergo degradation/breakdown to become hydroperoxides. The lipid hydroperoxides produced, may further degrade to form a range of products such as malondialdehyde (MDA) which is widely considered as an indicator to evaluate oxidative damage of tissues (Bonnes-Taourel et al., 1992). In this work, in order to elucidate the protection mechanism of HAAH and HACR in pylorus ligation induced ulcered rat models, the gastric tissue of the treated and control rats were examined for MDA levels to assess lipid peroxidation as well as catalase and glutathione peroxidase activities to assess endogenous antioxidant efficacy. From the study results it was well clear that there was a very significant rise in lipid peroxidation level and parallel decline in catalase and glutathione peroxidase activities in the pylorus ligation induced gastric ulcer group of animals. The increased levels of MDA in group I rats clearly embody the occurrence of peroxidative damage in the disease process (Venkateshwari et al., 1995). Both the test drugs, HAAH and HACR at their lower as well as higher doses showed significant reduction in lipid peroxidation. The significant effect on lipid peroxidation exhibited by both the drugs signifies the capability of drugs as gastroprotective agents by stabilizing the cell membranes of gastric epithelia ensuring normal physiological functions.
The inactivity of catalase, an important enzyme of the antioxidant defense system might result in certain harmful physiological effects by the accumulation of superoxide radicals and H2O2. Use of higher dose of HAAH and HACR clearly increases the activity of catalase in pylorus ligation induced ulcered rats which prevented further buildup of unnecessary free radicals thereby enhancing gastroprotection.
The activity of another important antioxidant marker enzyme, glutathione peroxidase which acts as a scavenger of lipid peroxides was also studied. The inactivation of this enzyme may result in the accumulation of H2O2, with subsequent oxidation of lipids (Lastra et al., 1998). Results revealed that higher doses of HAAH and HACR significantly elevated GSH-px activity in diseased rats and the effect was comparable to the standard drug pantoprazole.
The drugs HAAH and HACR significantly reduced the ulcerogenic activity induced by pyloric ligation in experimental rats. This gastroprotective activity of drugs is substantiated by the increase in the activity of antioxidant enzyme markers like catalase and GSH-Px as well as reduction in lipid peroxidation. The influence of free radical scavengers on the curing of different ulcer types and also the treatment of therapy resistant ulcers by anti-oxidative therapy was already reported (Salim, 1994).
Histopathological studies further confirmed that pretreatment with higher doses of HAAH as well as HACR prevented pylorus ligation induced structural alterations in the gastric mucosa of rats. The stomach sections from the pyloric ligated control rats showed deep ulceration, severely eroded epithelium, disoriented villi and crypts, oedema of the lamina propria, mucosal and sub-mucosal congestion, a dense inflammatory infiltrate in the lamina propria and hemorrhagic patches. Most of these structural changes were ameliorated by pretreatment with higher dose of HAAH and HACR which indicate their cytoprotective potential.
Acknowledgements
This work was supported by the Researchers Supporting Project number (RSP-2019/11), King Saud University, Riyadh, Saudi Arabia. The author Hak-Jae Kim thank the support received from Soochunhyang University research fund for this research work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Klein-Junior L.C., Gandolfi R.B., Santin J.R., Lemos M., Cechinel F.V., Andrade S.F. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire & Moquin (Polygalaceae) Naunym- Schmiedberg’s Arch. Pharmacol. 2010;381:121–126. doi: 10.1007/s00210-009-0485-x. [DOI] [PubMed] [Google Scholar]
- Tarnawski A., Szabo I.L., Husain S.S., Soreghan B. Regeneration of gastric mucosa during ulcer healing is triggered by growth factors and signal transduction pathways. J. Physiol. 2001;95:337–344. doi: 10.1016/s0928-4257(01)00046-8. [DOI] [PubMed] [Google Scholar]
- Tulassay Z., Hersnzenyi L. Gastric mucosal defense and cytoprotection. Best Pract. Res. Clin. Gastroenterol. 2010;24:99–108. doi: 10.1016/j.bpg.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Oliveira A.P., Santin J.R., Lemos M., Klein-Junior L.C., Couto A.G., Bittencourt C., Cechinel F.V., Andrade S. Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L. (Lamiaceae) J. Pharm. Pharmacol. 2011;63:1230–1237. doi: 10.1111/j.2042-7158.2011.01321.x. [DOI] [PubMed] [Google Scholar]
- Bighetti A.E., Antonio M.A., Kohn L.K., Rehder V.L.G., Fogli M.A., Possenti A., Vilela L., Carvalho J.E. Antiulcerogenic activity of a crude hydroalcoholic extract and coumarin isolated from Mikania laevigata Schultz Bip. Phytomedicine. 2005;12:72–77. doi: 10.1016/j.phymed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Santin J.R., Lemos M., Klein-Junior L.C., Niero R., de Andrade S.F. Antiulcer effects of Achyrocline satureoides (Lam.) DC (Asteraceae) (Marcela) a folk medicine plant, in different experimental models. J. Ethnopharmaco. 2010;130:334–339. doi: 10.1016/j.jep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Vaghela B., Soni H., Shukla L. A concept of herbal pratinidhi dravyas (substitute drugs) in ayurveda. Pharmagene. 2013;1:85–88. [Google Scholar]
- Venkatasubramanian P., Kumar S.K., Nair V.S. Cyperus rotundus, a substitute for Aconitum heterophyllum: Studies on the ayurvedic concept of Abhava Pratinidhi Dravya (drug substitution) J. Ayurveda Integr. Med. 2010;1:33–39. doi: 10.4103/0975-9476.59825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seethapathy G.S., Balasubramani S.P., Venkatasubramanian P. nrDNA ITS sequence based SCAR marker to authenticate Aconitum heterophyllum and Cyperus rotundus in ayurvedic raw drug source and prepared herbal products. Food Chem. 2014;145:1015–1020. doi: 10.1016/j.foodchem.2013.09.027. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Met. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Shay H., Komarov S.A., Fels S.S., Meranze D., Gruenstein M., Siplet H. A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology. 1945;5:43–61. [Google Scholar]
- Hawk S. MacGraw-Hill Book Company; 1947. Hawk’s Physiological Chemistry; p. 147. [Google Scholar]
- Lowry O.H., Roseborough N.I., Farr A.L., Randall R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265. [PubMed] [Google Scholar]
- Nair, B.R., 1976. Estimation of total carbohydrate contents in investigation of the venom of the south Indian scorpion Heterometrus scaber. Ph.D. Thesis, (University of Kerala, Trivandrum, India), 36.
- Sinha K.A. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1997;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Bancroft J.D., Stevens A. Theory and Practice of histological techniques. J. Am. Med. Assoc. 1977;238 2730 2730. [Google Scholar]
- Bharti S., Wahane V.D., Kumar V.L. Protective effect of Calotropis procera latex extracts on experimentally induced gastric ulcers in rat. J. Ethnopharmacol. 2010;127:440–444. doi: 10.1016/j.jep.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Kaur M., Singh A., Kumar B. Comparative antidiarrheal and antiulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats. J. Adv. Pharm. Technol. Res. 2014;5:122–128. doi: 10.4103/2231-4040.137417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ifeoma, O., Oluwakanyinsola, S., 2013. Screening of herbal medicines for potential toxicities. In: Sivakumar, G. (ed). New Insights into Toxicity and Drug Testing, 63–88, InTech, ISBN: 95351094649789535109464. http://dx.doi.org/10.5772/54493.
- Abdel-Hameed E.S., Salih A., Bazaid S.A., Shohayeb M.M., El- Sayed M.M., El-Wakil E.A. Phytochemical studies and evaluation of antioxidant, anticancer and antimicrobial properties of Conocarpus erectus L. growing in Taif. Saudi Arabia. Eur. J. Med. Plants. 2012;2:93–112. [Google Scholar]
- Baggio C.H., Freitas C.S., Rieck L., Marques M.C. Gastroprotective effects of a crude extract of Baccharis illinita DC in rats. Pharmacol. Res. 2001;47:93–98. doi: 10.1016/s1043-6618(02)00253-0. [DOI] [PubMed] [Google Scholar]
- Rao P.S., Murthy K.D., Nayak B.S., Sathiamoorthy S.S. Prostaglandin mediated gastric acid secretion inhibitory effect as a possible mechanism for the antiulcer effect of angiotensin converting enzyme inhibitor (Captopril) in pylorus ligated rats. Ind. J. Pharma. 1998;30:385–389. [Google Scholar]
- Grossman M.I. Saunders; Philadelphia, W.B: 1978. Control of gastric secretion, gastro-intestinal disease, patho-physiology, diagnosis and management; p. 27. [Google Scholar]
- Sanyal A.K., Debnath P.K., Bhattacharya S.K., Gode K.D. Protective effect of mucous on gastric mucosa. J. Gastroenterol. 1971;8:774. [Google Scholar]
- Jain S.M., Parmar N.S., Santani D.D. Gastric antiulcer activity of calcium channel blockers in rats. Ind. J. Pharma. 1994;26:29–34. [Google Scholar]
- Tandon R., Mukherjee N., Dixit V.K., Khanna R., Khanna H.D. Lipid peroxidation levels in peptic ulcer and gastric carcinoma. Ind. J. Physiol. Pharmac. 2006;50:83–86. [PubMed] [Google Scholar]
- Schraufstatter I., Hyslop P.A., Jackson J.H., Cochrane C.G. Oxidant induced DNA damage of target cells. J. Clin. Invest. 1988;82:1040–1050. doi: 10.1172/JCI113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi M., Padmaja U., Sunil Kumar K.N., Shivanada N., Alfarhan A.H., Thomas J., Rajakrishnan R. Protective effect of Garcinia pedunculata fruit rind in acetic acid induced ulcerative colitis. Farmacia. 2019;67(1):160–166. [Google Scholar]
- Bonnes-Taourel D., Guerin M.C., Torreilles J. Is malondialdehyde a valuable indicator in lipid peroxidation? Biochem. Pharmacol. 1992;44:985–988. doi: 10.1016/0006-2952(92)90132-3. [DOI] [PubMed] [Google Scholar]
- Venkateshwari A., Prathibha N., Vidyasagar A., Pratap B., Murty J.S. Malondialdehyde levels in patients with duodenal ulcer. Ind. J. Gastroenterol. 1995;14:57–58. [PubMed] [Google Scholar]
- Lastra C.A., Casa C.L., Martin M.J., Motilva V. Effects of cinitapride on gastric ulceration and secretion in rats. Inflamm. Res. 1998;47:131–136. doi: 10.1007/s000110050301. [DOI] [PubMed] [Google Scholar]
- Salim A.S. Role of free radical scavengers in the management of refractory duodenal ulceration. A new approach. J. Surg. Res. 1994;56:45–52. doi: 10.1006/jsre.1994.1008. [DOI] [PubMed] [Google Scholar]