Abstract
Gold nanoparticles (AuNPs) possess considerable biocompatibility and therefore gaining more attention for their biomedical applications. Previous studies have shown the transient increase in pro-inflammatory cytokines expression in different organs of rats and mice exposed to AuNPs. Structural changes in the spleen of mice treated with AuNPs have also been reported. This investigation was aimed to study the immunostaining of IL-1β, IL-6 and TNF-α in mice treated with different sizes of AuNPs. The animals were divided into 7 groups of 4 animals in each group. One group received saline and served as control. Two sets of three groups were treated with 5 nm, 20 nm and 50 nm diameter AuNPs. One set was sacrificed on day 1 and the other on day 7 following the AuNPs injections. Spleens were dissected out and promptly fixed in formalin for 3 days and then processed for IL-1β, IL-6 and TNF-α immunostaining using target-specific antibodies. The immunoreactivities of IL-1β and IL-6 were increased with the increase of AuNP size. The immunostaining of IL-1β in spleen of 20 nm AuNP treated mice was subsequently decreased on day 7 whereas it persisted in 50 nm AuNP group. The increase in the immunoreactivity of IL-6 on day 1 was decreased on day 7 in the spleens of mice treated with 20 nm or 50 nm AuNPs. The immunostaining of TNF-α was found to be negative in all the treatment groups. In conclusion, the size of AuNPs plays an important role in the expression of proinflammatory cytokines in mouse spleen; small size (5 nm) AuNPs caused minimal effect, whereas larger (50 nm) AuNPs produced intense immunostaining.
Keywords: Gold nanoparticles, Spleen, Immunohistochemistry, Cytokines, Inflammation
1. Introduction
During this decade, the field of nanotechnology has flourished with the synthesis of novel nanomaterials and their enormous scope in areas like medicine, engineering, environment and industry (Ashraf et al., 2020, Zhang et al., 2016, Nurunnabi et al., 2015, Nafiujjaman et al., 2015, Khatun et al., 2015, Kang et al., 2015, Eswar et al., 2014). Besides the continued research on exploring the novel avenues for biomedical and other applications of nanoparticles (Lopes et al., 2019, Ibrahim et al., 2018a, Ibrahim et al., 2018b, Ibrahim et al., 2018c, Khan et al., 2018) voices have also emerged about studying the side effects and toxicities of different kinds of nanomaterials (Adewale et al., 2019, Khan and Shanker, 2005, Arif et al., 2012). Among various types of metal nanoparticles, gold nanoparticles (AuNPs) have gained higher interest for biomedical usage such as targeted drug delivery (Mioc et al., 2019, Perni and Prokopovich, 2014, Craig et al., 2012) and imaging (Han et al., 2019, Depannemaecker et al., 2019). By virtue of its completely filled d-subshells, gold exhibits chemical inertness and regarded as a noble element due to its resistance to corrosion and oxidation. Because of their chemical inertness, AuNPs possess considerable degree of biocompatibility as compared to other metal nanoparticles (Bailly et al., 2019).
Although metallic gold is inert in reactivity, its conversion into nano-scale size imparts unique properties, particularly the activation of immune response because the body considers AuNP as a foreign antigen. Exposure to AuNPs has been shown to cause some degree of toxicity in different organs of experimental animals (Patel et al., 2019, Bahamonde et al., 2018, Ibrahim et al., 2018a, Ibrahim et al., 2018b, Ibrahim et al., 2018c, Khan et al., 2012) as well as cell lines (Enea et al., 2019, Lee et al., 2019). Several reports have suggested an acute phase expression of proinflammatory cytokines in different organs of rats (Khan et al., 2017, Khan et al., 2016, Khan et al., 2013a, Khan et al., 2013b, Al-Harbi et al., 2019) and mice (Khan et al., 2019, Ibrahim et al., 2018a, Ibrahim et al., 2018b, Ibrahim et al., 2018c). It was also observed that AuNP-induced expression of proinflammatory cytokines at mRNA level was transient and normalized after one week. However, the time-course expression pattern of these cytokines at protein level was not reported and therefore investigated in this study. We chose spleen as the preferred organ because of its immense role in activation and control of immune response (Lewis et al., 2019, Bronte and Pittet, 2013). Previous studies have shown the accumulation (Buzulukov et al., 2014) and structural changes (Ibrahim et al., 2018a, Ibrahim et al., 2018b, Ibrahim et al., 2018c) in the spleen of animals treated with AuNPs. In this study, we conducted the immunohistochemistry for IL-1β, IL-6 and TNF-α expression in spleens of mice at day 1 and day7 following the exposure of 5 nm, 20 nm and 50 nm AuNPs.
2. Materials and methods
2.1. Animals
Adult female Swiss albino mice, weighting 28–32 g were divided into 7 groups of 4 animals in each group. The animals were kept in polycarbonate cages with sawdust bedding and maintained at 12 h light/dark cycles. All the animals had free access to standard laboratory food and drinking water and were acclimatized for 3 days before starting the AuNPs treatment. The experimental study was approved by the Institutional Review Board.
2.2. Dosing of AuNPs
All the AuNPs used in this study were purchased from MK Nano, Canada. The commercial preparations of all the AuNPs were in the form of aqueous suspension with Au concentration of 0.01%. The commercial solutions of different sized AuNPs were vortexed and sonicated before diluting with sterile normal saline. The control group received a single intraperitoneal (IP) injection of normal saline (100 µl). The remaining 6 groups were assigned into two sets. Mice in both the sets received a single IP injection of 5 nm, 20 nm or 50 nm diameter AuNPs, respectively. The AuNPs dose was adjusted so as each animal received a single injection of 5 µg Au/100 µl. Although all the mice in treatment groups (except control) received the same amount of Au, they were exposed to different counts of AuNPs depending on the size of AuNPs. The mice in one set were sacrificed on day 1, whereas the animals in another set were sacrificed on day 7, following AuNPs injection. Spleen samples were removed and kept immersed for fixation in 10% neutral buffered formalin for at least 3 days.
2.3. Immunohistochemistry
The pre-fixed spleen samples were processed overnight using an automated tissue processor (Sakura, Japan). An embedding station (Sakura, Japan) was used for embedding the processed spleen samples in paraffin blocks. We used a rotary microtome (Leica-RM2245, Germany) for cutting 4 µm thick sections of spleen specimens. The sections were then mounted on super frosted glass slides.
The paraffinized sections were passed through xylene, ethanol (graded concentrations) and distilled water for de-paraffinization. Microwave heating of the sections for 5 min in citrate buffer was used to prime them for efficient antigen retrieval. We used a HRP/DAB Detection kit (Abcam) for immunohistochemistry. The proinflammatory cytokines’ primary antibodies were used in the following dilutions: IL-1β 1:500, IL-6 1:400, and TNF-α 1:100. The sections were incubated with primary antibodies, followed by their incubation with horseradish peroxidase (HRP) conjugate and then with the DAB substrate. Finally, the sections were subjected to counterstaining with Mayer Hematoxylin for light microscopy examination. The immunostaining color intensity of was graded from 0 (no staining) to 5 (highest intensity and larger area) scale. The detailed protocol of immunohistochemistry has been reported earlier (Khan et al., 2017).
2.4. Statistics
The results of semi-quantitative grading of immunostaining in different treatment groups were analyzed by one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test using SPSS software. P values less than 0.05 were considered as statistically significant.
3. Results
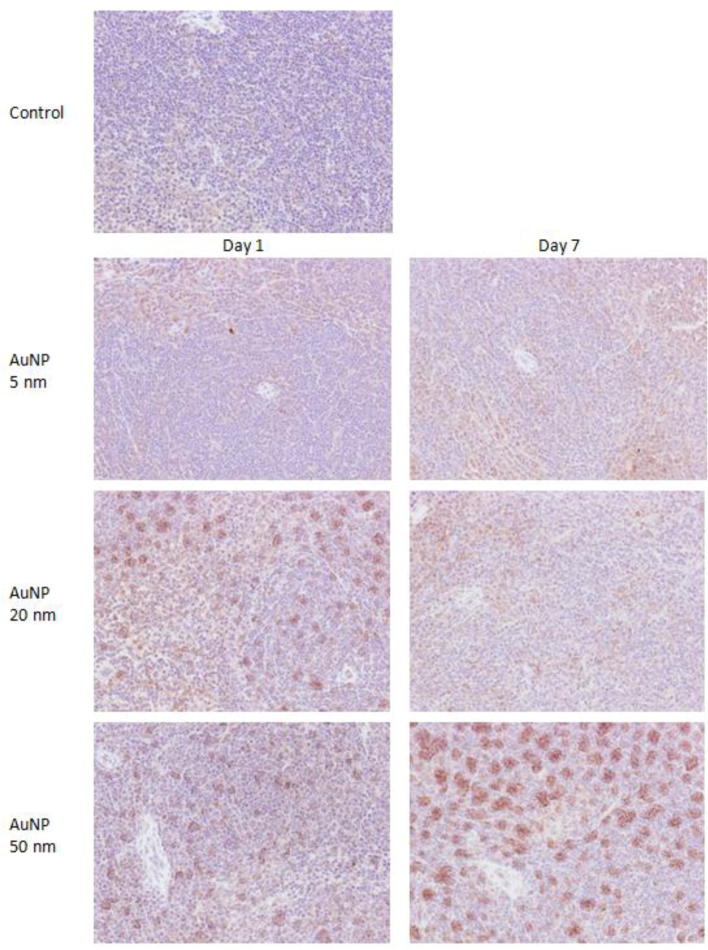
The immunohistopathology of control spleen showed almost negligible immunostaining for IL-1β (Fig. 1). A single injection of 5 nm AuNPs caused mild immunostaining for IL-1β on day 1 that persisted with similar intensity on day 7. Administration of 20 nm AuNPs caused intense immunostaining for IL-1β on day 1 that subsided after one week. The spleen of mice treated with 50 nm AuNPs showed moderate immunostaining for IL-1β on day 1 that turned to highly intense on day 7 (Fig. 1).
Fig. 1.
Immunostaining of IL-1β in spleen of mice treated with AuNPs. Original magnification ×200.
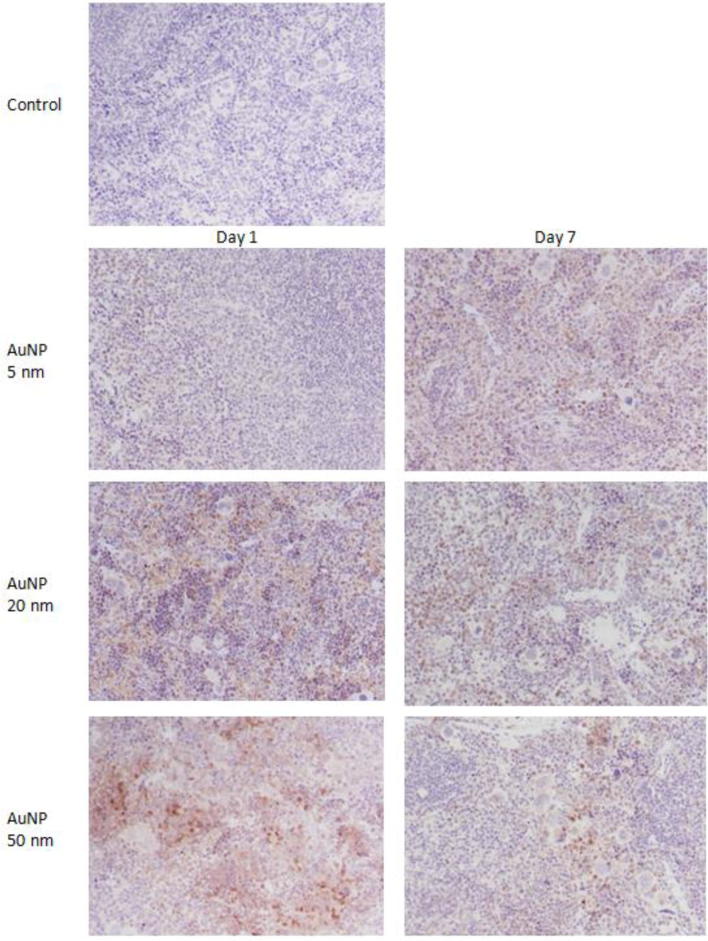
There was no immunostaining for IL-6 in the spleen of control mice (Fig. 2). Administration of the 5 nm, 20 nm, and 50 nm AuNPs showed a size-dependent increase in IL-6 specific staining in the spleens of mice on day 1. Although the intensity of IL-6 immunostaining in the spleens of mice treated with the 5 nm AuNPs was slightly increased on day 7, mice treated with the 20 nm and 50 nm AuNPs showed a gradual decrease in IL-6 immunostaining on day 7 (Fig. 2).
Fig. 2.
Immunostaining of IL-6 in spleen of mice treated with AuNPs. Original magnification ×200.
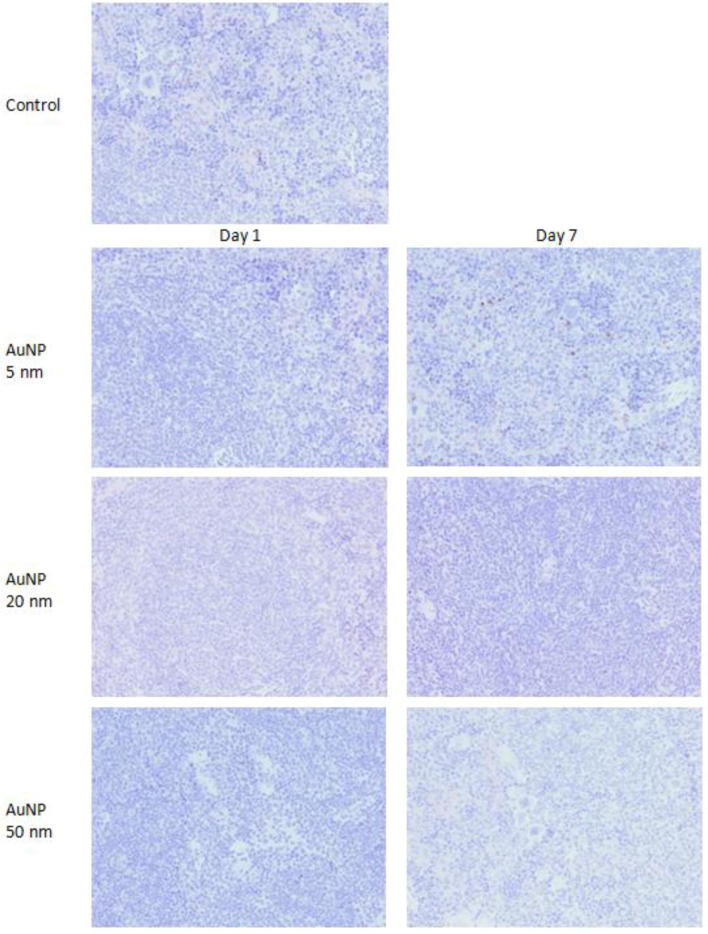
The immunostaining for TNF-α was absent in the spleens of control as well as AuNPs treated groups irrespective of AuNPs sizes and exposure times, except one animal in 5 nm AuNP group showed very light immunostaining at few points (Fig. 3). The results of semi-quantitative analysis of immunostaining for different proinflammatory cytokines are summarized in Table 1.
Fig. 3.
Immunostaining of TNF-α in spleen of mice treated with AuNPs. Original magnification ×200.
Table 1.
Semi-quantitative analysis of immunostaining for IL-1β, IL-6 and TNF-α in spleen of mice treated with AuNPs.
| Groups | IL-1β |
IL-6 |
TNF-α |
|||
|---|---|---|---|---|---|---|
| Day 1 | Day 7 | Day 1 | Day 7 | Day 1 | Day 7 | |
| Control | 0.75 ± 0.25 | 0.25 ± 0.25 | 0.00 ± 0.00 | |||
| AuNP-5 nm | 1.50 ± 0.28 | 2.25 ± 0.25* | 1.25 ± 0.25 | 2.75 ± 0.25* | 0.00 ± 0.00 | 0.25 ± 0.25 |
| AuNP-20 nm | 3.75 ± 0.25** | 2.50 ± 0.28* | 2.75 ± 0.25* | 2.25 ± 0.25* | 0.00 ± 0.00 | 0.00 ± 0.00 |
| AuNP-50 nm | 3.50 ± 0.28** | 4.50 ± 0.28** | 3.50 ± 0.28** | 2.25 ± 0.25* | 0.00 ± 0.00 | 0.00 ± 0.00 |
*P<0.05 and **P<0.01 versus control group using Dunnett’s test.
4. Discussion
The results of this study showed that the spleens of control mice did not indicate any staining specific to IL-6 and TNF-α, while very light immunostaining was observed for IL-1β in control spleens. In a previous study, we compared the sensitivity of immunohistochemistry and real-time PCR for the expression analysis of these cytokines at protein and mRNA level, respectively. We noticed that real-time PCR is comparatively more sensitive because it successfully detected all the cytokines mRNA expression in controls (Khan et al., 2016). The high sensitivity of real-time PCR was attributed to the exponential amplification of the target gene as well as to the variations in the half-life of mRNA and the translated protein.
The small size AuNPs (5 nm diameter) did not affect the expression of proinflammatory cytokines in mice spleen whereas both medium (20 nm) and large (50 nm) AuNPs caused intense immunostaining of IL-1β and IL-6. The inability of 5 nm AuNPs to induce the expression of proinflammatory cytokines in spleen can be associated to their fast clearance of smaller AuNPs through renal filtration as the pore size of glomerular basement membrane is around 8 nm (Balasubramanian et al., 2010). Naz et al. (2016) showed that smaller AuNPs (2–10 nm) were slowly excreted through urine without causing any systemic toxicity in mice after a single intravenous injection of a high dose (1250 µg/kg). An important role of size of AuNPs in induction of proinflammatory cytokines expression in different organs such as liver, kidney and brain of rodents has been reported earlier (Khan et al., 2013a, Khan et al., 2018, Khan et al., 2019). In a multi-organ histopathological study, spleen was found to be the preferential target of both 20 nm and 50 nm AuNPs, as they significantly affected the cellular architecture of spleen and produced pathological alterations after 24 h that also persisted after one week (Ibrahim et al., 2018a, Ibrahim et al., 2018b, Ibrahim et al., 2018c).
The immune response is greatly influenced by the nanoparticle size due to variation in the deposition of complement proteins according to the size of nanoparticles. The complement system is known to play a key role in modulation of proinflammatory immune response besides its active involvement in the uptake and removal of nanoparticles (Pondman et al., 2015). Not only the size of AuNPs but their surface charge also affected the biodistribution and clearance of AuNPs in mice, as the distribution of positively charged AuNPs was comparatively higher in spleen, heart and lung whereas negatively charged AuNPs mainly targeted liver (Lee et al., 2015). Shape is another important determinant of AuNPs biodistribution, accumulation, persistence and toxicity in mice. Intravenous injections (0.5 mg/kg) of gold nanomaterials of different shapes such as nanoclusters, nanorods and nanospheres showed wide variations in their biodistribution and toxicity profile in mice (Zhang et al., 2014).
In conclusion, 5 nm AuNPs did not affect the expression of proinflammatory cytokines in mouse spleen whereas 20 nm and 50 nm AuNPs significantly increased the immunostaining of IL-1β and IL-6. The inflammatory response was evident as early as day 1 following AuNPs exposure and persisted to varying degrees after one week. These findings suggest an important role of nanoparticles size on the acute phase immune response in mice spleen.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding the Research Group No. RGP-1435-066.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adewale O.B., Davids H., Cairncross L., Roux S. Toxicological behavior of gold nanoparticles on various models: Influence of physicochemical properties and other factors. Int. J. Toxicol. 2019;38:357–384. doi: 10.1177/1091581819863130. [DOI] [PubMed] [Google Scholar]
- Al-Harbi N.S., Alrashood S.T., Siddiqi N.J., Arafah M.M., Ekhzaimy A., Khan H.A. Effect of naked and PEG-coated gold nanoparticles on histopathology and cytokines expression in rat liver and kidneys. Nanomedicine (Lond) 2019 doi: 10.2217/nnm-2019-0220. [DOI] [PubMed] [Google Scholar]
- Arif I.A., Khan H.A., Al Rokayan S., Alhomida A.S., Bakir M.A., Khanam F. Toxic Effects of Nanomaterials. Bentham Science Publishers; USA: 2012. Toxicologic and environmental issues related to nanotechnology development; pp. 137–147. [Google Scholar]
- Ashraf R., Sofi H.S., Akram T. Fabrication of multifunctional cellulose/TiO2/Ag composite nanofibers scaffold with antibacterial and bioactivity properties for future tissue engineering applications. J. Biomed. Mater. Res. A. 2020 doi: 10.1002/jbm.a.36872. [DOI] [PubMed] [Google Scholar]
- Bahamonde J., Brenseke B., Chan M.Y., Kent R.D., Vikesland P.J., Prater M.R. Gold nanoparticle toxicity in mice and rats: species differences. Toxicol. Pathol. 2018;46:431–443. doi: 10.1177/0192623318770608. [DOI] [PubMed] [Google Scholar]
- Bailly A.L., Correard F., Popov A. In vivo evaluation of safety, biodistribution and pharmacokinetics of laser-synthesized gold nanoparticles. Sci. Rep. 2019;9:12890. doi: 10.1038/s41598-019-48748-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S.K., Jittiwat J., Manikandan J., Ong C.N., Yu L.E., Ong W.Y. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- Bronte V., Pittet M.J. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–818. doi: 10.1016/j.immuni.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzulukov Iu.P., Arianova E.A., Demin V.F., Safenkova I.V., Gmoshinskiĭ I.V., Tutel'ian V.A. Bioaccumulation of silver and gold nanoparticles in organs and tissues of rats by neutron activation analysis. Izv. Akad. Nauk. Ser. Biol. 2014:286–295. [PubMed] [Google Scholar]
- Craig G.E., Brown S.D., Lamprou D.A., Graham D., Wheate N.J. Cisplatin-tethered gold nanoparticles that exhibit enhanced reproducibility, drug loading, and stability: a step closer to pharmaceutical approval? Inorg. Chem. 2012;51:3490–3497. doi: 10.1021/ic202197g. [DOI] [PubMed] [Google Scholar]
- Depannemaecker D., Santos L.E.C., de Almeida A.G. Gold nanoparticles for X-ray microtomography of neurons. ACS Chem. Neurosci. 2019;10:3404–3408. doi: 10.1021/acschemneuro.9b00290. [DOI] [PubMed] [Google Scholar]
- Enea M., Peixoto de Almeida M., Eaton P. A multiparametric study of gold nanoparticles cytotoxicity, internalization and permeability using an in vitro model of blood-brain barrier. Influence of size, shape and capping agent. Nanotoxicology. 2019;13:990–1004. doi: 10.1080/17435390.2019.1621398. [DOI] [PubMed] [Google Scholar]
- Eswar K.A., Rouhi J., Husairi F.S., Dalvand R., Alrokayan S.H., Khan H.A., Mahmood R., Abdullah S. Hydrothermal growth of flower-like ZnO nanostructures on porous silicon substrate. J. Mol. Struc. 2014;1074:140–143. [Google Scholar]
- Han S., Bouchard R., Sokolov K.V. Molecular photoacoustic imaging with ultra-small gold nanoparticles. Biomed. Opt. Express. 2019;10:3472–3483. doi: 10.1364/BOE.10.003472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim K.E., Bakhiet A.O., Awadalla M.E., Khan H.A. A priming dose protects against gold nanoparticles-induced proinflammatory cytokines mRNA expression in mice. Nanomedicine (Lond) 2018;13:313–323. doi: 10.2217/nnm-2017-0332. [DOI] [PubMed] [Google Scholar]
- Ibrahim K.E., Bakhiet A.O., Khan A., Khan H.A. Recent trends in biomedical applications of nanomaterials. Biosci. Biotech. Res. Asia. 2018;15:235–243. [Google Scholar]
- Ibrahim K.E., Al Mutary M.G., Bakhiet A.O., Khan H.A. Histopathology of the liver, kidney, and spleen of mice exposed to gold nanoparticles. Molecules. 2018;23:1848. doi: 10.3390/molecules23081848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.H., Nafiujjaman M., Nurunnabi M., Li L., Khan H.A., Cho K.J., Huh K.M., Lee Y. Hybrid photoactive nanomaterial composed of gold nanoparticles, pheophorbide-A and hyaluronic acid as a targeted bimodal phototherapy. Macromol. Res. 2015;23:474–484. [Google Scholar]
- Khan H.A., Al Amery S., Ibrahim K.E., El Nagar D.M., Alharbi N., Rusop M., Alrokayan S.H. Size and time-dependent induction of proinflammatory cytokines expression in brains of mice treated with gold nanoparticles. Saudi J. Biol. Sci. 2019;26:625–631. doi: 10.1016/j.sjbs.2018.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.A., Abdelhalim M.A., Al Ayed M.S., Alhomida A.S. Effect of gold nanoparticles on glutathione and malondialdehyde levels in liver, lung and heart of rats. Saudi J. Biol. Sci. 2012;19:461–464. doi: 10.1016/j.sjbs.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.A., Abdelhalim M.A., Alhomida A.S., Al Ayed M.S. Transient increase in IL-1β, IL-6 and TNF-α gene expression in rat liver exposed to gold nanoparticles. Genet. Mol. Res. 2013;12:5851–5857. doi: 10.4238/2013.November.22.12. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Abdelhalim M.A., Alhomida A.S., Al Ayed M.S. Effects of naked gold nanoparticles on proinflammatory cytokines mRNA expression in rat liver and kidney. Biomed. Res. Int. 2013;2013:590730. doi: 10.1155/2013/590730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H.A., Ibrahim K.E., Khan A., Alrokayan S.H., Alhomida A.S. Immunostaining of proinflammatory cytokines in renal cortex and medulla of rats exposed to gold nanoparticles. Histol. Histopathol. 2017;32:597–607. doi: 10.14670/HH-11-825. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Ibrahim K.E., Khan A., Alrokayan S.H., Alhomida A.S., Lee Y.K. Comparative evaluation of immunohistochemistry and real-time PCR for measuring proinflammatory cytokines gene expression in livers of rats treated with gold nanoparticles. Exp. Toxicol. Pathol. 2016;68:381–390. doi: 10.1016/j.etp.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Khan H.A., Sakharkar M., Nayak A., Kishore U., Khan A. Nanobiomaterials: Nanostructured Materials for Biomedical Applications. Elsevier; USA: 2018. Nanoparticles for biomedical applications; pp. 357–384. [Google Scholar]
- Khan H.A., Shanker R. Toxicity of nanomaterials. Biomed. Res. Int. 2005;2015:521014. doi: 10.1155/2015/521014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatun Z., Nurunnabi M., Nafiujjaman M., Reeck G.R., Khan H.A., Cho K.J., Lee Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale. 2015;7:10680–10689. doi: 10.1039/c5nr01075f. [DOI] [PubMed] [Google Scholar]
- Lee J.K., Kim T.S., Bae J.Y. Organ-specific distribution of gold nanoparticles by their surface functionalization. J. Appl. Toxicol. 2015;35:573–580. doi: 10.1002/jat.3075. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Ahn E.Y., Park Y. Shape-dependent cytotoxicity and cellular uptake of gold nanoparticles synthesized using green tea extract. Nanoscale Res. Lett. 2019;14:129. doi: 10.1186/s11671-019-2967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S.M., Williams A., Eisenbarth S.C. Structure-function of the immune system in the spleen. Sci. Immunol. 2019;4 doi: 10.1126/sciimmunol.aau6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes T.S., Alves G.G., Pereira M.R., Granjeiro J.M., Leite P.E.C. Advances and potential application of gold nanoparticles in nanomedicine. J. Cell Biochem. 2019;120:16370–16378. doi: 10.1002/jcb.29044. [DOI] [PubMed] [Google Scholar]
- Mioc A., Mioc M., Ghiulai R. Gold nanoparticles as targeted delivery systems and theranostic agents in cancer therapy. Curr. Med. Chem. 2019 doi: 10.2174/0929867326666190506123721. [DOI] [PubMed] [Google Scholar]
- Nafiujjaman M., Nurunnabi M., Kang S.H., Reeck G., Khan H.A., Lee Y. Ternary graphene quantum dot-polydopamine-Mn3O4 nanoparticles for optical imaging guided photodynamic therapy and T1-weighted magnetic resonance imaging. J. Mater. Chem. B. 2015;3:5815–5823. doi: 10.1039/c5tb00479a. [DOI] [PubMed] [Google Scholar]
- Naz F., Koul V., Srivastava A., Gupta Y.K., Dinda A.K. Biokinetics of ultrafine gold nanoparticles (AuNPs) relating to redistribution and urinary excretion: a long-term in vivo study. J. Drug Target. 2016;24:720–729. doi: 10.3109/1061186X.2016.1144758. [DOI] [PubMed] [Google Scholar]
- Nurunnabi M., Parvez K., Nafiujjaman M., Revuri V., Khan H.A., Feng X., Lee Y. Bioapplication of graphene oxide derivatives: drug/gene delivery, imaging, polymeric modification, toxicology, therapeutics and challenges. RSC Adv. 2015;5:42141–42161. [Google Scholar]
- Patel M., Siddiqi N.J., Sharma P., Alhomida A.S., Khan H.A. Reproductive toxicity of pomegranate peel extract synthesized gold nanoparticles: a multi-generation study in C. elegans. J. Nanomaterial. 2019;2019:8767943. [Google Scholar]
- Perni S., Prokopovich P. Continuous release of gentamicin from gold nanocarriers. RSC Adv. 2014;4:51904–51910. doi: 10.1039/c4ra10023a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pondman K.M., Pednekar L., Paudyal B., Tsolaki A.G., Kouser L., Khan H.A., Shamji M.H., Haken B.T., Stenbeck G., Sim R.B., Kishore U. Innate immune humoral factors, C1q and factor H, with differential pattern recognition properties, alter macrophage response to carbon nanotubes. Nanomedicine. 2015;11:2109–2118. doi: 10.1016/j.nano.2015.06.009. [DOI] [PubMed] [Google Scholar]
- Zhang J., Nie X., Ji Y., Liu Y., Wu X., Chen C., Fang X. Quantitative biokinetics and systemic translocation of various gold nanostructures are highly dependent on their size and shape. J. Nanosci. Nanotechnol. 2014;14:4124–4138. doi: 10.1166/jnn.2014.8274. [DOI] [PubMed] [Google Scholar]
- Zhang X., Qian J., Pan B. Fabrication of novel magnetic nanoparticles of multifunctionality for water decontamination. Environ. Sci. Technol. 2016;50:881–889. doi: 10.1021/acs.est.5b04539. [DOI] [PubMed] [Google Scholar]