Abstract
Carbon dioxide (CO2) concentration in greenhouses is sub-optimal for vegetable production. Many techniques have been used to increase CO2 concentration in greenhouses but most of them are expensive with certain limitations and drawbacks. We adopted a new strategy to elevate CO2 concentration in the greenhouse throughout the day via crop residues and animal manure composting (CRAM). During the whole cultivation period, CRAM-treated greenhouse had doubled CO2 concentration which significantly increased the yield of cherry tomatoes (Lycopersiconesculentum L.) i.e. up to 38%. The influence of CRAM procedure on cherry tomato quality was also investigated and the concentrations of total soluble solids (TSS) and soluble sugar were found to be significantly higher in cherry tomatoes grown under composting greenhouse than that of non-composting greenhouse. Additionally, CRAM-CO2 enrichment also resulted in increased concentrations of ascorbic acid (Vitamin C) and titrate acid as compared with the control. In contrast, the concentration of nitrate was considerably decreased in cherry tomato grown under CO2 enriched condition than that of control. The increase in active oxygen metabolisms such as POD, CAT and SOD while a decrease in MDA, as well as APX was observed for cherry tomatoes grown under CO2 enriched condition. Hence, CO2 fertilization by using CRAM in greenhouse significantly improved quality and increased the yield of cherry tomatoes.
Keywords: Cherry tomato, Crop residues, Animal manure, CO2 enrichment, Quality, Yield
1. Introduction
Tomato is one of the most commonly used vegetables for cooking and food industry condiment around the globe. Tomato enriches a lot of nutrients and provides our body antioxidants, fibre and some vitamins (Petronia et al., 2019). Red colour of tomato is due to the accumulation of beta carotene that contains ascorbic acid and a great amount of phenolic contents (Stewart et al., 2000). Cherry tomato (Solanum Lycopersicum) is an ancestor of the domesticated form of the cultivated tomato and generally contains higher bio-active compound as compared to large ones (Ranc et al., 2008; Choi et al., 2011). Greenhouses are being used for the production of cherry tomato, as it has many advantages over field production including higher quality, enhanced yield and extended growing season for vegetable production. In China alone, the land utilized for the production of vegetables is almost 17.8 million hectares out of which 14.6% is consumed by greenhouse (National Bureau of Statistics of China, 2005). However, tomato's poor quality complaints have been reported in the past few years in China and the better flavour is demanded by the consumers (Baldwin et al., 2000, Causse et al., 2003). One of the major factor for the low quality of vegetables was probably due to the low concentration of CO2 in the greenhouse (Chongwei et al., 2009). Furthermore many studies revealed, that the ideal concentration of CO2 for the production of vegetables in the greenhouse is 800–1000 µLL-1 (Kläring et al., 2007). However, the recorded concentration of CO2 in the greenhouse during day time, due to hermetic conditions is about 110–260 µL L-1(Kläringet al., 2007). Additionally, photosynthetic activity, water usability and the yield of vegetable depend upon the elevation of CO2 in the greenhouse (Kauret al., 2013). There are many well-known benefits of CO2 enrichment in greenhouses such as an increase in biomass and size of the canopy, photosynthetic rate etc. (Ziska, 2008) which ultimately increase the growth and development of the plant. Moreover, many studies revealed that CO2 enrichment in greenhouse improves water use efficiency and product yield (Arena et al., 2011, Klaiber et al., 2013, Brestic et al., 2018). Other reported that by applying a high concentration of CO2 and the presence of enough heat in the greenhouse will make the greenhouse environment optimum for plant growth (Halmann and Steinberg, 1998).
Mostly, commercial growers prefer to use supplemental CO2 to improve crop yield and quality (Li and Wang, 2013). In greenhouses, various techniques have been developed to elevate CO2 concentrations like the direct injection of gas inside the greenhouse, ventilation as well as chemical production by combining ammonium bicarbonate with sulfuric acid (Klaring et al., 2007, Linker et al., 1999). But they are all very expensive and are difficult to operate. Hence, CO2 insufficiency in the greenhouse is still a limiting factor which leads to an undesirable taste of vegetables and ultimately gives lower profit to the farmers.
Since the pre industrial times, with the involvement of human activities the atmospheric (CO2) has been elevated about 40% and the current (CO2) of 400 ppm is continuously increasing and will reach to 650 ppm by the year 2050 (IPCC, 2007). Day by day increase in CO2 in an open environment is also due to mismanagement of wastages (crops and animal residues) and anti-ecosystem activities of the human being. According to the (National Bureau of Statistics of China, 2005), about 23% of entire Chinese agriculture waste was burnt in the field which results in Carbon (C) emissions of 5.5 × 107 t y−1(Cao et al., 2011). This C emission in the atmosphere can be significantly reduced by using composting. Consequently, in China alone, about 2.75 billion tons of farmyard manure (FYD) is produced every year and about 220 (million ton) animal waste (Ding et al., 2006) is released in water bodies. The massive discharge of animal manures produced by high-density livestock results in P and N to flow into waterbodies which ultimately results in eutrophication (Zvomuya et al., 2006). Composting of crop residues is a way to handle waste efficiently and reducing the CO2 concentration in the environment. In contrast, the mismanagement of agricultural wastages (such as crop-residues and animal-manure) and anti-ecosystem activities of human beings are increasing CO2 in the environment. Composting encourages our farmers to utilize presently unused manure properly and this practice also diminishes dangerous releases of manures into the waterbodies. Furthermore, optimum fermentation conditions for composting to increase CO2 concentration in the greenhouse were reported by (ChongweiJin et al., 2009). According to which the C:N ratio which is most crucial factor must be 40:1. The pH, water content, and temperature for rice straw and pig manure biodegradation should be 6.5–7.0, 71% (w/w) and 50C, respectively. In the present study, we adopted the most simple and efficient unit of composting CRAM (crop residues and animal manure) to elevate CO2 concentration in greenhouses by placing CRAM unit directly in the greenhouse. This strategy is very economical, the increase in yield and higher quality of cherry tomato will encourage farmers to adopt this technique.
2. Materials and methods
2.1. Plant materials and experimental design
The cherry tomato variety used in this experiment was “Huangfei”, treated with two treatments: Control i.e normal condition (local conventional cultivation), and CO2 enrichment, CRAM, by fermentation of organic wastes. In order to conduct the experiment, two neighbouring greenhouses were used (only 40 cm apart) in Huzhou, China. Each greenhouse had the same dimensions (42 m × 6 m × 3 m) and had same soil condition. There were 5 composting units placed in the greenhouse for CRAM treatment. The composting units were made of timber with 1.2 m height and 0.6 diameters. To maintain sufficient aeration in a unit, bars of 6 cm (width) at the bottom with 2 cm intervals were used. The 25 kg of composting material (equivalent to a dry weight of wheat straw) and moist manure (8 kg) were added. At the initial stage, about 90 L of water was added to maintain the moisture level at 70% throughout the experiment (Du et al., 2004). Three fungal species Panusconclmtw zj3, Trichoderma viride zj2, and Aspergillas niger zj1 were inoculated with the CRAM mixture to enhance the production of CO2 via fermentation. The optimum pH (6.5–7) of CRAM mixture was maintained by using pickled vegetable juice (after every 15 days) (Jin et al., 2009). Before sowing, fertilizers were applied as: organic fertilizer 2000 kg, manure fertilizer 25 kg, potassium fertilizer was added in two splits 20 kg and 75 kg, respectively. Each greenhouse had 3 ridges, the distance between the plants was 45 cm, the distance between ridges was 75 cm, the width of the ridge was 40 cm, and there were about 1300 plants in the greenhouse. During the experiment, the water and fertilizer applications were kept the same in all greenhouses.
2.2. CO2 determination in greenhouse
To estimate the CO2 concentration, CO2/ temperature monitor (Telaire 7001, USA) was installed in the greenhouse.
2.3. Determination of plant growth parameters and yield characteristics
From each treatment plant growth parameters were examined by taking five plants. Plant height (at the peak flowering stage), leaf area (using portable leaf area meter; LI-3000C, LI-COR, USA), number of flowers per plant, the total fruit mass per plant and no. of fruits per plant were measured. Physico-chemical parameters of the soil used for this experiment were calculated according to (Bao, 2000) (Table 1). Table 2.
Table1.
Basic Physico-chemical parameters of experimental soil.
| Soil physiological index | Control | +CO2 (CRAM) |
|---|---|---|
| OM (%) | 5.2 | 7.04 |
| Total N (gkg−1) | 0.26 | 0.35 |
| NH4+-N (mgkg−1) | 4.36 | 5.91 |
| NO3–-N (mgkg−1) | 3.54 | 4.8 |
| Available P (mgkg−1) | 2.3 | 3.12 |
| Available K (mgkg−1) | 42.26 | 55.06 |
| TOC (%) | 3.01 | 4.08 |
| PH | 6.86 | 6.98 |
| EC (mgL−1) | 6.15 | 6.54 |
Table 2.
Effects of CO2 enrichment by fermentation of CRAM on growth of cherry tomato.
| Treat. | Seedling | Transplant | CRAM | First flower | First harvest | End harvest |
|---|---|---|---|---|---|---|
| Year. Month. Day | ||||||
| Control | 2016.11.05 | 2016.11.20 | 2017.01.15 | 2017.03.02 | 2017.03.20 | 2017.06.20 |
| +CO2 | 2016.11.05 | 2016.11.20 | 2017.01.15 | 2017.02.25 | 2017.03.12 | 2017.06.15 |
Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM.
2.4. Evaluation of quality traits
By using anthrone method, total soluble sugars concentration was determined from spectrophotometric determination (Fales, 1951) following by (Buysse and Merckx, 1993). By using the titration method (2, 6-dichlorophenol indophenol sodium salt dehydrate) contents of ascorbic acid were measured (Horwitz, 1980). Total soluble solids (TSS) were obtained according to Mamatha et al. (2014). Nitrate concentration in cherry tomato fruits was analyzed by following the similar procedure of (Cataldo et al., 1975). Nitrate concentration in fruits was calculated as mg kg−1. Acidity was calculated by following AOAC method (942.15) (AOAC 2000).
2.5. Measurement of photosynthetic parameters
The photosynthetic parameters e.g. intercellular concentration, transpiration and stomatal conductance in cherry tomato plants were measured using portable photosynthesis system LI-6400 (Li-COR, Lincoln, NE, USA) and Chlorophyll content was measured by using SPAD.
2.6. Measurements of antioxidant enzyme activities and MDA content
The antioxidant enzyme activities were determined by following the methodology of (Chen et al., 2010, Wu et al., 2003). Briefly, 0.5 g of plant leaf sample was supplemented with 8 ml of 50 mM PBS buffer (pH 7.8) and homogenized in pestle and mortar. Then centrifugation of homogenate was followed for 20 min (10000 rpm, 4 °C). The resulted supernatant was used for the measurement of superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) as well as malondialdehyde (MDA) content.
2.7. Statistical analysis
Mean values of each treatment were the presented data. The measurements were done with three replicates. DPS (Data processing system) software package was used for all Statistical analysis by using ANOVA followed by the Duncan’s Multiple Range Test (DMRT) to evaluate the effects of different treatments (P < 0.05, 0.01).
3. Results and analysis
3.1. Effects of CO2 enrichment by fermentation of CRAM on growth and yield of cherry tomato
While evaluating the difference in CO2 concentration in both greenhouses, the results indicated that CO2 level in control was recorded between 480 and 570 µL L-1, while in the CRAM-CO2 greenhouse the recorded concentration of CO2 ranged between 1000 and 1500 µL L-1 during the entire cultivation period. CRAM fermentation resulted in elevated CO2 level more than 100% as compared to the control greenhouse. Graphical abstract for the production of CO2 in the greenhouse is shown in (Fig. 1).
Fig. 1.
Graphical abstract of CRAM procedure in the greenhouse for the production of CO2.
3.2. Growth morphological traits
CO2 enrichment via using CRAM procedure in the greenhouse had a significant impact on cherry tomato growth and morphology i.e. plant height (37%), stem diameter (17%), leaf width (14%) and chlorophyll content increased by (11.4%) than that of the control greenhouse (Table 3). Moreover, the result of the conducted study revealed significant alteration in size, weight, number of fruits and duration of ripening in cherry tomatoes grown under CO2 enriched conditions as compared to control. Furthermore, it can be seen from (Fig. 2) that under the CO2 enriched greenhouse, almost all the cherry tomato fruits were turned yellow, while in control condition, only the fruit at the bottom of the plant changed to yellow. The cherry tomatoes grown in CRAM-treatment greenhouse changed colour 10d earlier, as compared to the control greenhouse.
Table3.
Effects of CO2 enrichment by fermentation of CRAM on morphological traits of cherry tomato.
| Treat. | Plant height (cm) | Stem diameter (cm) | Leaf width (cm) | Chlorophyll |
|---|---|---|---|---|
| Control | 76.7 | 1.12 | 49.7 | 42.1 |
| +CO2 | 105.0** | 1.31** | 56.7** | 46.9** |
Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM. *and **, indicate significant differences between +CO2 and control at significant levels of 0.05 and 0.01, respectively.
Fig. 2.
Morphology of cherry tomato plants at the flowering stage under control (A) and CO2 enrichment (B) condition. Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM.
In addition, yields of cherry tomato grown in control greenhouse and CRAM-CO2 enriched greenhouse was 1300 (kg/hm2) and 1800 (kg/hm2) respectively, indicating a massive increase in yield of CO2 treated cherry tomato plants by 500 kg/hm2 (Table 4). Influences in physiology and morphology directly associated with the yield and quality related traits. A visible phenotypic difference was observed between mature cherry tomato fruit grown in CRAM-CO2 treated greenhouse and control greenhouse (Fig. 3).
Table 4.
Effects of CO2 enrichment by fermentation of CRAM on yield traits of cherry tomato.
| Treatment | Single fruit weight (g) | Fruit diameter (cm) | Fruit number | Yield (kg/hm2) |
|---|---|---|---|---|
| Control | 13.8 | 3 | 12.2 | 1300 |
| +CO2 | 19.0* | 3.6* | 27.4** | 1800* |
Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM. *and **, indicate significant differences between +CO2 and control at significant levels of 0.05 and 0.01, respectively.
Fig. 3.

Difference of mature cherry tomato fruit in control greenhouse and CRAM-CO2 treatment greenhouse. (Scale bar is 1 cm).
3.3. Effect of CO2 enrichment by CRAM on thequality of cherry tomato
CO2 enrichment via CRAM procedure significantly influenced soluble sugar (6%), soluble solids (7.2%), titrate acid (0.4%) and Vitamin C (Ascorbic Acid) up to (5%), while nitrate concentration decreased by (2%) in cherry tomato as compared to control (Fig. 4). The fruit diameter and individual fruit weight for CRAM-CO2 enriched cherry tomato showed significant difference of 25% and 37.7%, respectively. Overall, cherry tomato grown under CRAM-CO2 enriched condition exhibited a significant increase in the quality trait, as compared to the control greenhouse.
Fig. 4.
Effects of CO2 enrichment on quality of mature cherry tomato fruits. Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM. *, indicates significant differences between +CO2 and control at the level of 0.05.
3.4. Effects of CO2 enrichment by fermentation of CRAM on physiological traits of cherry tomato
3.4.1. Photosynthetic characteristics
Significant enhancement of photosynthetic rate (Pn) (c.f. by 20.22% higher than the control), stomatal conductance (Gs) (75%), intercellular CO2 (Ci) (9%), and transpiration rate of (Tr) (43%) were observed in cherry tomato under CRAM-CO2 enriched greenhouse as compared to the control (Table 5).
Table 5.
Effects of CO2 enrichment by fermentation of CRAM on photosynthetic characteristics of cherry tomato.
| Treatment | Pn | Gs | Ci | Tr |
|---|---|---|---|---|
| Control | 17.8 | 0.4 | 313.8 | 3.5 |
| +CO2 | 21.4* | 0.7* | 341.2* | 5.0* |
Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM. *, indicate significant differences between +CO2 and control at 0.05.
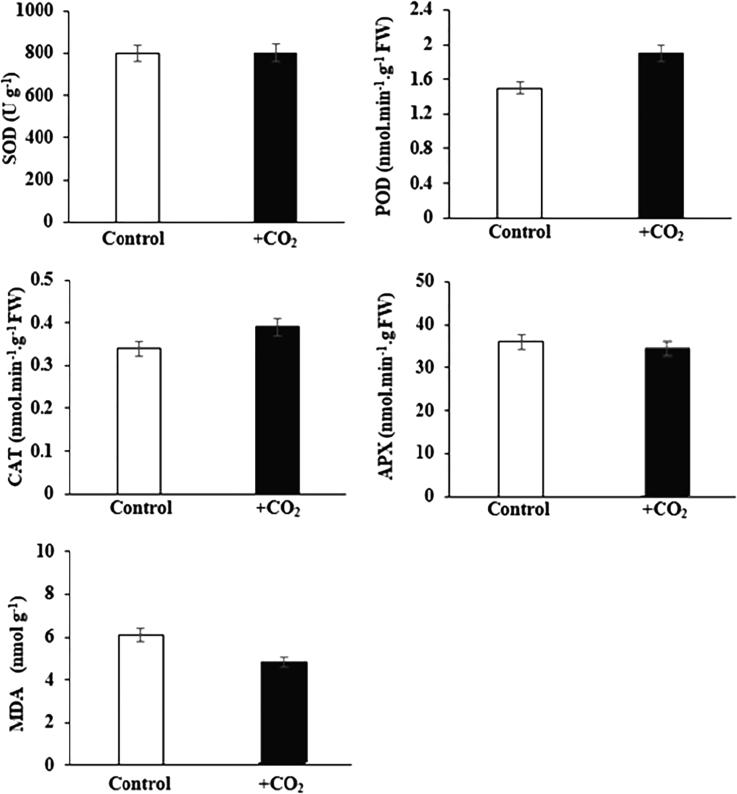
4. Active oxygen metabolism
Activities of ascorbate peroxide (APX), peroxidase (POD), catalase (CAT), superoxide dismutase (SOD) and malondialdehyde (MDA) contents were determined in leaves of cherry tomato Fig. 5. The SOD and APX activities were observed as almost similar between control (CK) and +CO2 enriched cherry tomato. The POD and CAT activities in leaves of cherry tomato under CRAM-CO2 treatment were increased by 26.66% and 14.70% respectively, MDA contents were decreased by 18.03% in CRAM-CO2 enrichment as compared to the control. (Fig. 5).
Fig. 5.
Effect of CO2 enrichment on anti-oxidant enzymes activities of cherry tomato. Control and +CO2 correspond to normal condition and CO2 enrichment by fermentation of CRAM. *, indicates significant differences between +CO2 and control at 0.05.
5. Discussion
Cherry tomatoes are used in many ornamental dishes and are mostly grown in greenhouses (Kauret al., 2013). However, low concentration of CO2 in the greenhouse is the main factor that deteriorates the quality of cherry tomatoes (Jin et al., 2009). So, fertigation of CO2 is essential in the greenhouse in order to obtain massive yield and quality. To fulfill the required amount of CO2 concentration in the greenhouse, CRAM procedure (crop residues as well animal manures) is the most economical and efficient procedure as compared to others strategies such as direct gas injection, ventilation and chemical production (Leakey et al., 2009). It can maintain optimum concentration of CO2 (for the greater time period) required for the successful growth of plants in the greenhouse, as compared to other procedure like chemical production (1–2 h). In addition, the CRAM procedure is easy and simple to operate in the greenhouse. It can be made from common material like bamboo, plastic and timber etc. Therefore, the cost of this technique is very low and economical for farmers. This study revealed that the CRAM-CO2 enrichment increased the production of cherry tomatoes in greenhouse notably. However, it has also been studied that too high concentration of CO2 may have adverse effects on plants (Yuming et al., 2015). This possible negative effect can be controlled in the greenhouse, by altering the number or the size of the composting unit (CRAM). For 100 m3 greenhouse, almost 4.5 kg of moist manure and approximately 14 kg of agriculture wastes are required for optimal production of CO2 in the greenhouse (Yu, 2005).
Many studies reported that optimum CO2 concentrations are important in order to obtain massive yield and higher quality of vegetables in the greenhouse (Buddendorf and woltering, 1994). In general, elevated CO2 can enhance the production of vegetables (Long et al., 2006, Parajuli et al., 2019). It has been frequently observed that elevated CO2 increased the fruit set percentage of tomato 226 (Mamatha et al., 2014). Current study reveals significant alteration in size, weight, number of fruits, quality, yield and duration of ripening under CO2 enriched cherry tomato as compared to control. For instance, in CO2 enriched greenhouse, almost all fruits of cherry tomato had changed to yellow colour 10 days earlier than that in control greenhouse. In the present study, plant growth parameters such as leaf width, plant height and plant biomass were found significantly higher for cherry tomatoes grown under CRAM-CO2 enriched condition. Consequently, under enriched CO2 condition (Kadam et al., 2012, Conroy, 2012) increased leaf area was observed as well as plant height was increased for gladiolus plants and Pinus radiate, respectively. The concentration of CO2 was significantly higher in composting greenhouse as compared to the non-composting greenhouse which results in increased biomass of cherry tomato. In previous literature, it was reported that higher level of CO2 in the greenhouse, significantly enhanced the growth of the plant, this was probably due to the increased photosynthetic rate of the plant (Heineke et al., 1999, Porter, 1984). Besides morphological traits, beneficial substances i.e. ascorbic acid and soluble sugars were also significantly increased in cherry tomato under elevated CO2. (Islam et al., 1996) also reported higher ascorbic acid and sugar contents for tomatoes grown under CO2 enriched condition as compared to ambient concentration. (Idsoet al., 2002) also found that the vitamin C content of sour orange (Citrus aurantiumL.) increased when grown under elevated CO2 condition and the same scenario was observed for strawberry by (Wang et al., 2003). Many other studies revealed that increased plant growth by increased CO2 was often correlated with the increased concentration of ascorbic acid and soluble sugars (Springer et al., 2008). In the present study, the concentrations of titrate and soluble solid in cherry tomato were increased in CO2 enriched greenhouse than that of control and cherry tomatoes quality increases in response to CO2 enrichment; these results are in consistence with (Mamatha et al., 2014). Furthermore, the concentration of nitrate decreased considerably in cherry tomatoes grown under CO2 enriched condition as compared to the control. This might be due to increase in nitrate reductase activity under increased CO2 condition (Fonseca et al., 1997) or due to the dilution effect of vigorous growth (Vorneet al., 2002). As we know, nitrate is non-toxic itself but its conversion into metabolites like nitrite and N-nitroso compounds (NOCs) has an adverse effect on human health, among metabolites NOCs are highly carcinogenic (Hill, 1999, L’Hirondel and L’Hirondel, 2002). The enhanced (Pn) at higher CO2 concentration increased plant growth as well as yield in both C3 and C4 crops (Reddy et al., 2010). In the present study, we found that the Photosynthetic parameters such as Pn, Gs, Ci, Tr increased significantly for cherry tomato plants grown under elevated CO2 enriched condition as compared to the control. (Kimball et al., 2002) also observed a significant increase in Pn along with Gs, Ci ad Tr for tomatoes which were exposed to CO2 enriched condition. Moreover higher chlorophyll content was observed for cherry tomatoes grown under high CO2. In previous studies, it was reported that the constraint of plants/crops for resourceful photosynthesis was satisfied by generated CO2 during the growing period (Springer et al., 2008). In contrast, the MDA concentration was substantially higher in cherry tomato plants growth in control greenhouse as compared to cherry tomatoes grown under CO2 enriched condition. This suggests that cherry tomato plants grown under CO2 enriched condition developed an efficient antioxidant defensive system for ROS. Moreover, under CO2 enriched condition POD and CAT remarkably increased for cherry tomatoes growth under CO2 enriched condition but SOD remains unchanged in both treatments. These results indicated that the CRAM-CO2 enrichment in greenhouse triggered an increase in POD and CAT antioxidant enzymes allowing cherry tomato plant to perform better under CO2 enriched circumstances. These results are inconsistence with the previous finding, reporting increased antioxidative-enzymes activities in response to CO2 enrichment for tomato plants (Wang et al, 2004, Lentheric et al., 2003). Further, the higher ascorbate levels observed in the CO2 treated fruits were probably related to the decrease in APX activity. These specific changes in activity may explain the increase in the overall production of cherry tomato grown under CO2 enriched condition.
Overall, the increase in fruit set percentage along with a number of fruits per plant resulted in higher fruit yield and quality per plant at CRAM-CO2 enriched greenhouse (Table 4) was probably because of increase in sink strength as compared to source strength in cherry tomatoes. Furthermore, more carbohydrate might be partitioned to the tomato fruit, during the fruit development which leads to higher yields. Our findings are consistent with the previous reports (Yelle et al., 1990, Reinert et al., 1997) which also observed an increase in yield for tomato plants grown under CO2 enriched condition. Similarly (Islam et al., 2006) found significantly developed fruits for tomato plants grown under CO2 enriched condition. Thus control on climate in the greenhouse could lead to the significantly higher yield and quality of cherry tomato.
6. Conclusion
In the current study, our findings suggest that CO2 enrichment in greenhouse via using CRAM technique is an efficient approach to increase CO2 concentration. More importantly, this technique is an economical and easily adaptable management tool for farmers to use in the greenhouse for cherry tomato production. Consequently, the implementation of this technique would readily be accepted by the farmers as it is economical as well as increases the quality and quantity of cherry tomato production. Furthermore, by using this strategy the environmental pollution problems caused by improper disposal of agricultural bi-products and by burning of crop residues can be reduced significantly.
Acknowledgements
This research work was financially supported by the National Natural Science Foundation of China, International (Regional) Cooperation and Exchange Program, Research fund for International young scientists (517102-N11808ZJ), PSF-NSFC grant 517102-N11909ZJ and Jiangsu Collaborative Innovation Center for Modern Crop Production (JCICMCP) China.
Footnotes
Peer review under responsibility of King Saud University.
References
- AOAC: Titratable acidity of fruit products. – In: Official Methods of Analysis (17th ed.). 942.15. AOAC International, Gaithersburg 2000.
- Arena C., Vitale L., Desanto A.V. Influence of irradiance on photosynthesis and PSII photochemical efficiency in maize during short-term exposure at high CO2 concentration. Photosynthetica. 2011;49:267–274. [Google Scholar]
- Baldwin E.A., Scott J.W., Shewmaker C.K., Schuch W. Flavor trivia and tomato aroma: biochemistry and possible mechanisms for control of important aroma components. HortScience. 2000;35:1013–1021. [Google Scholar]
- Bao S.D., 2000. Soil and agricultural chemistry analysis. Agricul. Publ. 355-356.
- Brestic M., Zivcak M., Hauptvogel P., Misheva S., Kocheva K., Yang X., Li X., Allakhverdiev S.I. Wheat plant selection for high yields entailed improvement of leaf anatomical and biochemical traits including tolerance to non-optimal temperature conditions. Photosynth. Res. 2018;136:245–255. doi: 10.1007/s11120-018-0486-z. [DOI] [PubMed] [Google Scholar]
- Buddendorf-Joosten J., Woltering E. Components of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul. 1994;15:1–16. [Google Scholar]
- Buysse J., Merckx R. An improved colorimetric method to quantify sugar content of plant tissue. J Exp. Bot. 1993;44:1627–1629. [Google Scholar]
- Cao G., Zhang X., Gong S., An X., Wang Y. Emission inventories of primary particles and pollutant gases for China. Chin. Sci. Bull. 2011;56:781–788. [Google Scholar]
- Cataldo D.A., Haroon M., Schrader L.E., Youngs V.L. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Comm. Soil Sci. Plant Anal. 1975;6:71–80. [Google Scholar]
- Causse M., Buret M., Robini K., Verschave P. Inheritance of nutritional and sensory quality traits in fresh market tomato and relation to consumer preferences. J. Food Sci. 2003;68:2342–2350. [Google Scholar]
- Chen F., Wang F., Wu F.B., Mao W.H., Zhang G.P., Zhou M.X. Modulation of exogenous glutathione in antioxidant defense system against Cd stress in the two barley genotypes differing in Cd tolerance.Plant. PhysiolBiochem. 2010;48:663–672. doi: 10.1016/j.plaphy.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Chongwei J., Shaoting D., Yue W., Jason C., Xianyong L., Yongsong Z. Carbon dioxide enrichment by composting in greenhouses and its effect on vegetable production. J. Plant Nutr. Soil Sci. 2009;172:418–424. [Google Scholar]
- Conroy J.P. Influence of elevated atmospheric CO2 concentrations on plant nutrition. Aust. J. Bot. 2012;40:445–456. [Google Scholar]
- Ding G., Tongbin C., Bin L., Yuanming Z., Guodi Z., Yan-xia L. Releases of pollutants from poultry manure in China and recommended strategies for the pollution prevention. 2006;25:311–319. [Google Scholar]
- Du J., Lin X.Y., Zhang Y.S. Affecting factors of CO2 evolution from biodegradation of agricultural organic wastes. Chin. J. Appl. Ecol. 2004;15:501–505. [PubMed] [Google Scholar]
- Fales F.W. The assimilation and degradation of carbohydrates by yeast cells. J. Biol. Chem. 1951;193:113–124. [PubMed] [Google Scholar]
- Fonseca F., Bowsher C.G., Stulen I. Impact of elevated atmospheric CO2 on nitrate reductase transcription and activity in leaves and roots of Plantago major. Physiol. Plantarum. 1997;100:940–948. [Google Scholar]
- Halmann M.M., Steinberg M. CRC Press; 1998. Greenhouse gas carbon dioxide mitigation: science and technology. [Google Scholar]
- Heineke D., Kauder F., Frommer W., Khn C., Gillissen B., Ludewig F., Sonnewald U. Application of transgenic plants in understanding responses to atmospheric change. Plant. 1999;22:623–628. [Google Scholar]
- Hill M.J. Nitrate toxicity: myth or reality? Brit. J. Nutr. 1999;81:343–344. [PubMed] [Google Scholar]
- Horwitz W. 13th ed. Association of Official Analytical Chemists; Washington, DC: 1980. Official Methods of Analysis of the Association of Official Analytical Chemists; p. 476. [Google Scholar]
- Lentheric I., Pintó E., Graell J., Larrigaudiere C. Effects of CO2 pretreatment on oxidative metabolism and core- browning incidence in controlled atmosphere stored pears. J. Hortic. Sci. Biotechnol. 2003;78:177–181. [Google Scholar]
- Idso S.B., Kimball B.A., Shaw P.E., Widmer W., Vanderslice J.T., Higgs D.J., Montanari A., Clark W.D. The effect of elevated atmospheric CO2 on the vitamin C concentration of (sour) orange juice.Agriculture, ecosystems. & environment. 2002;90:1–7. [Google Scholar]
- IPCC: Summary for policymakers. – In: Solomon, S., Qin, D., Manning, M. et al. (ed.): Climate Change (2007): The physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Inter-governmental Panel on Climate Change. Cambridge University press, Cambridge, New York.
- Islam M.S., Matsui T., Yoshida Y. Effect of carbon dioxide enrichment on physico-chemical and enzymatic changes in tomato fruits at various stages of maturity. ScientiaHorticulturae. 1996;65:137–149. [Google Scholar]
- Islam S., Khan S., Garner J.O. Elevated atmospheric CO2 concentration enhances carbohydrate metabolism in developing LycopersiconesculentumMill. cultivars. Int. J. Agr. Biol. 2006;8:157–161. [Google Scholar]
- Jin C., Du S., Wang Y., Condon J., Lin X., Zhang Y. Carbon dioxide enrichment by composting in greenhouses and its effect on vegetable production. J. Plant Nutr. Soil Sci. 2009;172:418–424. [Google Scholar]
- Kadam G.B., Singh K.P., Pal M. Effect of elevated carbon-dioxide levels on morphological and physiological parameters in gladiolus. Indian J. Hortic. 2012;69:379–384. [Google Scholar]
- Kaur, C., Walia, S., Nagal, S., Walia, S., Singh, J., Singh, B.B., Saha, S., Singh, B., Kalia, P., Jaggi, S., 2013. Functional quality and antioxidant composition of selected tomato (Solanumlycopersicon L) cultivars grown in Northern India. LWT-Food Sci. Technol. 50, 139-145.
- Kimball B., Kobayashi K., Bindi M. Responses of agricultural crops to free-air CO2 enrichment. Adv. Agronomy. 2002;77:293–368. [PubMed] [Google Scholar]
- Klaiber J., Najar-Rodriguez A.J., Piskorski R., Dorn S. Plant acclimation to elevated CO2 affects important plant functional traits, and concomitantly reduces plant colonization rates by an herbivorous insect. Planta. 2013;237:29–42. doi: 10.1007/s00425-012-1750-7. [DOI] [PubMed] [Google Scholar]
- Klaring H.P., Hauschild C., Heibner A., Bar-yosef B. Model-based control of CO2 concentration in greenhouses at ambient levels increases cucumber yield. Agric. For. Meteorol. 2007;143:208–216. [Google Scholar]
- Kläring H.P., Hauschild C., Heißner A., Bar-Yosef B. Model-based control of CO2 concentration in greenhouses at ambient levels increases cucumber yield. Agric. For. Meteorol. 2007;143:208–216. [Google Scholar]
- L’Hirondel J., L’Hirondel J.L. CABI publishing; Oxford: 2002. Nitrate and man: Toxic, harmless or beneficial. [Google Scholar]
- Leakey A.D.B., Ainsworth E.A., Bernacchi C.J., Rogers A., Long S.P., Ort D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: six important lessons from FACE. J. Exp. Bot. 2009;60:2859–2876. doi: 10.1093/jxb/erp096. [DOI] [PubMed] [Google Scholar]
- Li F., Wang J. Estimation of carbon emission from burning and carbon sequestration from biochar producing using crop straw in China. Trans. Chin. Soc. Agricul. Eng. 2013;29:1–7. [Google Scholar]
- Linker R., Gutman P.O., Seginer I. Robust controllers for simultaneous control of temperature and CO2 concentration in greenhouses. Control Eng Pract. 1999;7:851–862. [Google Scholar]
- Long S.P., Ainsworth E.A., Leakey A.D.B., Nösberger J., Ort D.R. Food for thought: lower than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312(5782):1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Mamatha H., Rao N.S., Laxman R., Shivashankara K., Bhatt R., Pavithra K. Impact of elevated CO2 on growth, physiology, yield, and quality of tomato (Lycopersiconesculentum Mill) cv. ArkaAshish Photosynthetica. 2014;52:519–528. [Google Scholar]
- National Bureau of Statistics of China . China Statistics Press; Beijing: 2005. China statistical yearbook. [Google Scholar]
- Parajuli R., Thoma G., Matlock M.D. Environmental sustainability of fruit and vegetable production supply chains in the face of climate change: a review. Sci. Total Environ. 2019;650(Pt 2):2863–2879. doi: 10.1016/j.scitotenv.2018.10.019. [DOI] [PubMed] [Google Scholar]
- Petronia C., Marios C.K., Christophe E.N., Antonio P., Emilia A., Luisa A., Giuseppe C., Gianluca C., Stefania P., Youssef R. Sensory and functional quality characterization of protected designation of origin ‘PiennolodelVesuvio’cherry tomato landraces from Campania-Italy. Food Chem. 2019;292:166–175. doi: 10.1016/j.foodchem.2019.04.056. [DOI] [PubMed] [Google Scholar]
- Porter M.A. Acclimation to high CO2: Carbonic anhydrase and ribulosebiphosphate carboxylase. Plant Physiol. 1984;74:413–416. doi: 10.1104/pp.74.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy A.R., Rasineni G.K., Raghavendra A.S. The impact of global elevated CO₂ concentration on photosynthesis and plant productivity. Curr. Sci. 2010:46–57. [Google Scholar]
- Reinert R.A., Eason G., Barton J. Growth and fruiting of tomato as influenced by elevated carbon dioxide and ozone. New Phytol. 1997;137:411–420. doi: 10.1046/j.1469-8137.1997.00846.x. [DOI] [PubMed] [Google Scholar]
- Springer C.J., Orozco R.A., Kelly J.K., Ward J.K. Elevated CO2 influences the expression of floral-initiation genes in Arabidopsis thaliana. New Phytol. 2008;178:63–67. doi: 10.1111/j.1469-8137.2008.02387.x. [DOI] [PubMed] [Google Scholar]
- Stewart A.J., Bozonnet S., Mullen W. Occurrence of flavonols in tomatoes and tomato-based products. J. Agr. Food Chem. 2000;48:2663–2669. doi: 10.1021/jf000070p. [DOI] [PubMed] [Google Scholar]
- Vorne V., Ojanperä K., Detemerman L., Bindi M., Högy P., Jones M., Lawson T., Persson K. Effects of elevated carbon dioxide and ozone on potato tuber quality in the European multiple-site experiment ‘CHIP-project’. Eur. J. Agron. 2002;17:369–381. [Google Scholar]
- Wang, J., LI, F., Zou, Z.R., Wang, X.L., 2004.Study on double CO2 concentration reduce the inhibition of enhanced UV-B radiation on tomato in plastic greenhouse. ActaBotanicaBoreali-OccidentallaSinica. 24, 817-821.
- Wang, S.Y., Bunce J.A., Maas, J., 2003. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J Agric Food Chem. 51, 4315–4320.doi:10.1021/ jf021172d. [DOI] [PubMed]
- Wu F.B., Zhang G.P., Dominy P. Four barley genotypes respond differently to cadmium: lipid peroxidation and activities of antioxidant capacity. Environ. Exp. Bot. 2003;50:67–78. [Google Scholar]
- Yelle S., Beeson R.C., Trudel M.J., Gosselin A. Duration of CO2 enrichment influences growth, yield, and gas exchange of two tomato species. J. Am. Soc. Hortic. Sci. 1990;115:52–57. [Google Scholar]
- Yu C.Y. Zhejiang University, China; 2005. pH regulation for CO2 evolution by bio-degradation of organic wastes from agriculture and effects of CO2 enrichment on vegetable growth in greenhouse, Master Thesis. [Google Scholar]
- Yuming F., Lingzhi S., Hui L., Hongyan Li, Zhiruo Z., Peiliang Y., Pingzhen C., Hong L. Unexpected decrease in yield and antioxidants in vegetable at very high CO2 levels. Environ ChemLett. 2015;13:473–479. [Google Scholar]
- Ziska L.H. Rising atmospheric carbon dioxide and plant biology: the overlooked paradigm. DNA Cell Biol. 2008;27:165–172. doi: 10.1089/dna.2007.0726. [DOI] [PubMed] [Google Scholar]
- Zvomuya F., Helgason B.L., Larney F.J., Janzen H.H., Akinremi O.O., Olson B.M. Predicting phosphorus availability from soil-applied composted and non-composted cattle feedlot manure. J. Environ. Qual. 2006;35:928–937. doi: 10.2134/jeq2005.0409. [DOI] [PubMed] [Google Scholar]