Abstract
The grape is a matrix rich in bioactive compounds and its production generates large quantities of by-products, such as grape stems, which, to date, present low commercial value. However, there is a growing interest in the application of this material as a source of phenolic compounds. Therefore, the present study aims at assessing the phytochemical profile of (poly)phenolic extracts of white Portuguese grape stem varieties produced in the Região Demarcada do Douro (Portugal). The antioxidant activity determined by several assays, as well as the antimicrobial activity using the disc diffusion method against human gastrointestinal pathogenic bacteria of the hydromethanolic extracts, were evaluated. This work presents very positive results as the rich composition in phenolic compounds (94.71–123.09 mg GA−1 and 0.02–73.79 mg g−1 for the total phenol content and for individual phenolics, respectively) presented by grape stems can explain the high antioxidant (0.37–1.17 mmol Trolox g−1) and antimicrobial activities against, essentially, Gram-positive bacteria, and in some cases with higher efficacy than commercial antibiotics. Thus, demonstrating that this wine by-product should deserve greater attention from the pharmaceutical industries due to its excellent biological properties and characteristics not yet applied.
Keywords: Grape stems, Pathogenic bacteria, Antimicrobial activity, Antioxidant activity, Phenolic compounds
1. Introduction
The production of grapes is one of the main economic activities of the agri-food sector, with more than 79 million tons produced worldwide in 2018 (FAOSTAT, 2018). Of these, approximately 47% are grapes destined for the wine industry (“The International Organisation of Vine and Wine”, 2017), where large quantities of by-products are generated as organic waste (grape marc, containing seeds, pulp and skins, stems, and grape leaves), greenhouse gas wastes (CO2, volatile organic compounds, etc), and inorganic wastes (diatomaceous earth, bentonite and perlite clay) (Gouvinhas et al., 2019, Mateo and Maicas, 2015).
The grape stems correspond to almost 25% of the total by-products resulting from the winemaking process and grape production, being the material less characterized and still undervalued even though their chemical composition reveals huge potential as a source of natural compounds (Domínguez-Perles et al., 2016). To date, this by-product has a low commercial value, reflecting its use mainly in the production of spirit drinks, dietary fibres, and vegetable protein concentrates or fertilizers (Anastasiadi et al., 2009, Domínguez-Perles et al., 2014, Sahpazidou et al., 2014).
Currently, there is an increasing interest by the scientific community in the application of this matrix as a source of phenolic compounds, for use as food supplements and/or active ingredients for the cosmetic and pharmaceutical industries (Anastasiadi et al., 2012), once they present biological activities such as antioxidant, antimicrobial, anti-inflammatory, among others (Gouvinhas et al., 2020, Teixeira et al., 2014a). These compounds can affect bacterial growth and metabolism, due to the capacity to stimulate or inhibit their growth according to its constitution and concentration (Vaquero et al., 2007). In the last years, studies involving the use of natural compounds, able to inhibit the development of microorganisms, are of special interest, especially when related with microorganisms responsible for the deterioration of food and their association with the increasing number of microbial infections (Oliveira et al., 2013, Singh et al., 2020). Furthermore, nowadays, the resistance to antibiotics is one of the greatest threats to public health and food safety, so measures must be taken in society to reduce the impact and to limit the spread of this resistance (Liao et al., 2020). Some of these measures include the reduction of the use of antibiotics, and the search for new compounds that can be applied in replacement and/or synergy with drugs used as first choice, being these new applications not harmful to the environment (WHO, 2017).
The aim of this study is to assess the phytochemical profile of (poly)phenolic extracts of grape stems of five Portuguese white varieties, to verify the potential antioxidant activity of these extracts by the ABTS and DPPH assays, and to determine their antimicrobial activity against clinical isolates, namely pathogenic bacteria, to verify if this matrix can be pointed as a good bet and strategy in this scientific area in the future.
2. Material and methods
2.1. Chemicals
The compounds 2,2-diphenyl-1-picrylhidrazyl radical (DPPH•), 2,2-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)diammonium salt (ABTS•+), potassium phosphate, dimethyl sulfoxide, and the standard compounds caftaric acid, resveratrol, quercetin and kaempferol for the chromatographic separation, were obtained from Sigma-Aldrich (Steinheim, Germany). Folin-Ciocalteu’s reagent, 3,4,5-trihydroxybenzoic acid (gallic acid), acetic acid, both extra pure (>99%), potassium hydroxide, and sodium hydroxide were acquired from Panreac (Panreac Química S.L.U., Barcelona, Spain). Sodium nitrate, aluminium chloride, and sodium carbonate, all extra pure (>99%), and saline water (0.9% NaCl) were purchased from Merck (Merck, Darmstadt, Germany). Sodium molybdate (99.5%) was obtained from Chem-Lab (Chem-Lab N.V., Zedelgem, Belgium). The 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) was acquired from Fluka Chemika (Neu-Ulm, Switzerland). All culture media and antibiotics were purchased from Oxoid (Oxoid Limited, Thermo Fisher Scientific Inc.). Ultrapure water was obtained using a Millipore water purification system.
2.2. Plant material and sample processing
The grape (Vitis vinifera L.) stems from the white varieties ‘Rabigato’, ‘Malvasia Fina’, ‘Fernão Pires’, ‘Viosinho’ and ‘Moscatel’ were obtained in the Spring-Autumn season (2017), in Baixo Corgo (Lower Corgo) sub-region of the Região Demarcada do Douro (northern Portugal). Plant material came from three farms of this region, all belonging to the same wine company and near located to the Douro River, at low altitude region (Average altitude: 200 m). No irrigation was applied in all the field trials of this investigation. The productivity of the vineyards are about 8 tons per hectare and all the plots are normal for the fungal diseases (downy mildew and powdery mildew), but controlled with recommended products for organic farming.
Grape stem samples were collected randomly at the grape optimum harvest date stipulated by the winemakers during the 2017 growing season, at the wine company, during the grape destemming process to obtain sample homogeneity.
For analytical purposes, plant material (grape stems) was washed in tap water and shopped into small pieces. Then, the grape stem samples were dried in oven (Memmert, Schwabach, Germany) for 72 h at 40 °C, grounded and stored at room temperature protected from light. Each sample (40 mg) was mixed with 1.5 mL of methanol/distilled water (70:30, v/v). Afterwards, samples were vortexed and agitated for 30 min at room temperature, for the extraction of the phenolic compounds, and centrifuged at 10000 rpm for 15 min, at 4 °C (Sigma, Steinheim, Germany), collecting the supernatant (Gouvinhas et al., 2018). This procedure was repeated 3 times. Supernatants were filtered through a 0.45-µm PVDF filter (Millex HV13, Millipore, Bedford, MA, USA) and stored at 4 °C until analysis.
2.3. Phenolic content
Total phenols content was determined by spectrophotometric method of Folin-Ciocalteau, according to the methodology previously exposed by Sousa et al. (2014), adapted to 96-well microplates (Sousa et al., 2014). Ortho-diphenol content was determined colorimetrically by the method adopted by Gouvinhas et al. (2018), also adapted to 96-well microplates. The content in total phenols and ortho-diphenols were expressed in milligrams of gallic acid per gram of sample (dry weight) (mg GA g −1 dw) (Gouvinhas et al., 2018).
Flavonoids content was determined by spectrophotometric method described by Domínguez-Perles et al. (2014), adapted to 96-well microplates. The results were expressed in milligrams of catechin per gram of sample (mg CAT g −1 dw) (Domínguez-Perles et al., 2014a).
2.4. Antioxidant capacity
The free radical scavenging activity was determined by the ABTS and DPPH methods adapted to a microscale according to Mena et al. (2011). The antioxidant activity was evaluated by measuring the variation in absorbance at 734 nm after 30 min of reaction with the radical for ABTṠ and at 520 nm after 15 min for DPPḢ, using 96-well microplates and Multiscan FC microplate reader (Thermo-Fisher Scientific, Oporto, Portugal). Results of the radical scavenging capacity were expressed in millimoles of Trolox per gram of sample (mmol Trolox g −1 dw).
2.5. Qualitative and quantitative analysis of phenolic compounds
The phenolic profile of the grape stem samples was assessed by Reverse Phase - High Performance Liquid Chromatography - Diode Array Detector (RP-HPLC-DAD), with a C18 column (250 × 4.6 mm, 5 µm particle size; ACE, Aberdeen, Scotland), in accordance with the method exposed by Queiroz et al. (2017). The mobile phases administered were distilled water/formic acid (99.9:0.1, v/v) (solvent A) and acetonitrile/formic acid (99.9:0.1, v/v), using the linear gradient scheme (t in min; %B): (0; 5%), (15; 15%), (30; 30%), (40; 50%), (45; 95%), (50; 95%) and (55; 5%). The flow rate was 1.0 mL min−1 and the injection volume was 20 µL. The equipment consisted of a LC pump (SRVYR-LPUMP), an auto-sampler (SRVYR-AS), and a photodiode array detector (SRVYR-PDA5) in series. For the quantification of the compounds at 330 nm, the quercetin and kaempferol standards were used for flavonoids, the standard caftaric acid for hydroxycinnamic acids and the standard resveratrol for stilbenes.
2.6. Bacterial isolates
Bacterial isolates Gram positive, Staphylococcus aureus and Enterococcus faecalis, and Gram negative, Escherichia coli and Klebsiella pneumoniae, were collected from biological samples of the gastrointestinal tract of humans, provided by the Hospital Centre of Trás-os-Montes and Alto Douro (CHTMAD), under the research collaboration protocol established in 2004, belonging to the MJS collection. These bacteria were previously identified by biochemical methods (API 20E, API 20NE, API Staphy (BioMérieux)) and by molecular methods, namely, the partial sequencing of the 16S rRNA gene. Listeria monocytogenes and Pseudomonas aeruginosa bacterial isolates were obtained from the American Type Culture Collection (ATCC) (Table 1). Prior to the antimicrobial activity tests, the isolates were cultured on BHI (Brain Heart Infusion) agar for 24 h at 37 °C.
Table 1.
Bacterial isolates tested.
| Bacterial isolates | Source | Class |
|---|---|---|
| Listeria monocytogenes ATCC 15,313 | American Type Culture Collection | Gram + |
| Staphylococcus aureus MJS241 | Clinical-human gastrointestinal segment | Gram + |
| Enterococcus faecalis MJS257 | Clinical-human gastrointestinal segment | Gram + |
| Pseudomonas aeruginosa ATCC 10,145 | American Type Culture Collection | Gram - |
| Escherichia coli MJS260 | Clinical-human gastrointestinal segment | Gram - |
| Klebsiella pneumoniae MJS281 | Clinical-human gastrointestinal segment | Gram - |
2.7. Antimicrobial activity
For the determination of the antimicrobial activity, the disk diffusion method described by Bauer et al. (1966) was used, with some modifications, as described by Gouvinhas et al., 2018.
The antimicrobial activity was calculated according to the following equation:
‘% RIZD = (IZD sample – IZD negative control) / IZD antibiotic × 100%’, in which IZD represents the inhibition halos (mm) and RIZD the percentage of relative inhibition halo diameter (Aires et al., 2009), comparing the antimicrobial activity of each sample relatively to each antibiotic Additionally, the antimicrobial activity of each extract was also classified according to the following:
-
i.
No effect (−): inhibition halo = 0;
-
ii.
Moderate efficacy (+): 0 < inhibition halo < antibiotic inhibition halo;
-
iii.
Good efficacy (++): antibiotic inhibition halo < inhibition halo < 2 × antibiotic inhibition halo;
-
iv.
High efficacy (+++): inhibition halo > 2 × antibiotic inhibition halo.
2.8. Statistical analysis
All data were subjected to the IBM SPSS 22.0 statistical software (SPSS Inc., Chicago, IL, USA), using variance analysis (ANOVA) and a multiple range test (Tukey’s test), for a p value < 0.05. The results of the samples are presented as mean values ± standard deviation (n = 3).
3. Results and discussion
3.1. Phenolic content of grape stem extracts
The results of the total phenol content, ortho-diphenols and flavonoids of the analysed stem extracts are shown in Table 2. For all the analysed parameters, there are significant differences among the five varieties. The total phenol content varied between 94.71 ± 4.65 (Rabigato) and 123.09 ± 5.02 (Malvasia Fina) mg GA g −1 dw. When comparing these results with those described in the literature, Domínguez-Perles et al., (2014) presented values of 48 mg GA g −1 dw for the Viosinho variety, also used in the present study, where higher values were obtained (96.99 ± 4.53 mg GA g −1 dw). Also, Anastasiadi et al. (2012) reported a lower content of total phenols for the white varieties Asyrtiko, Aidani and Athiri, with 11146, 7220 and 4808 mg GA kg −1 dw, respectively. Furthermore, high values were shown by Sahpazidou et al. (2014), with 372 mg GA g −1 dw for the white variety Assyrtiko. Concerning the ortho-diphenols, this content varied between 80.62 ± 3.69 (Viosinho) and 116.18 ± 2.67 (Malvasia Fina) mg GA g−1 dw. Once again, the study carried out by Domínguez-Perles et al. (2014) showed values of 36.54 mg GA g−1 dw for the Viosinho variety, much lower than those presented in this study. Regarding the flavonoids content, this varied from 51.37 ± 2.39 (Fernão Pires) to 106.42 ± 4.63 (Malvasia Fina) mg CAT g−1 dw. Domínguez-Perles et al. (2014) reported, for Viosinho variety, 24.65 mg CAT g−1 dw, much lower than those found in this study (61.75 ± 3.94 CAT g−1 dw) (Domínguez-Perles et al., 2014b). Makris, Boskou, & Andrikopoulos (2007) presented, for varieties Roditis (white) and Agiorgitiko (red), values of 53.99 mg CAT g −1 dw (Makris et al., 2007). These differences found on the phenolic composition in this work, as well as in the others studies found in the literature, can be explained by the specific characteristics of each variety, by the climate and biotic factors, as well as by the viticultural practices (Portu et al., 2018). In fact, the growing conditions has a great effect on the phenolic composition of plants. For instance, a recent work demonstrated the impact of the geographical region on this parameter, in which samples of grape stems revealed higher content of total phenols, ortho-diphenols and flavonoids in lower altitude sites (Lower Corgo sub-region), where the samples of the present work came from (Gouvinhas et al., 2020). This low altitude region is characterized to present some stress factors, such as thermal and water stress once in this region the climate has an Atlantic influence, translated, for example, by abundant rains. This situation can explain the induction of production of these secondary metabolites, as it happened on the concentration of these parameters from 2017 to 2018 due to the high thermal stress felt in this last year (Gouvinhas et al., 2020), whereby, higher concentration of phenolics would be also expected in the present work. However, in the present study, the concentration of phenolics were higher than those demonstrated by Gouvinhas et al., 2020 in 2017, which can be due to the different cultivars studied.
Table 2.
Phenolic content of grape stem extracts.
| Samples | Total phenols (mg GA g −1 dw) | Ortho-diphenols (mg GA g −1 dw) | Flavonoids (mg CAT g −1 dw) |
|---|---|---|---|
| Rabigato | X94,71 ± 4,65a | 82,12 ± 5,48a | 86,22 ± 5,42b |
| Malvasia Fina | 123,09 ± 5,02b | 116,18 ± 2,67c | 106,42 ± 4,63c |
| Fernão Pires | 110,15 ± 8,47ab | 91,55 ± 7,04ab | 51,37 ± 2,39a |
| Viosinho | 96,99 ± 4,53a | 80,62 ± 3,69a | 61,75 ± 3,94a |
| Moscatel | 108,71 ± 9,41ab | 99,38 ± 8,13b | 59,43 ± 3,17a |
| P-values | Y** | *** | *** |
Values are presented as mean ± standard deviation (n = 3). Different letters indicate significantly different results (ANOVA, p < 0.05).
Significance: non-significant, N.S. (p > 0.05); * significant at p < 0.05; ** significant at p < 0.01; *** significant at p < 0.001.
Despite these differences found, this wine industry by-product presents a high concentration of phenolics compounds, in some cases, higher than the fruits (grapes) or the other by-products (M. Teixeira et al., 2014), revealing the importance of this waste as a rich source of phenolic compounds (M. Teixeira et al., 2014).
3.2. Qualitative and quantitative analysis of phenolic compounds by RP-HPLC-DAD
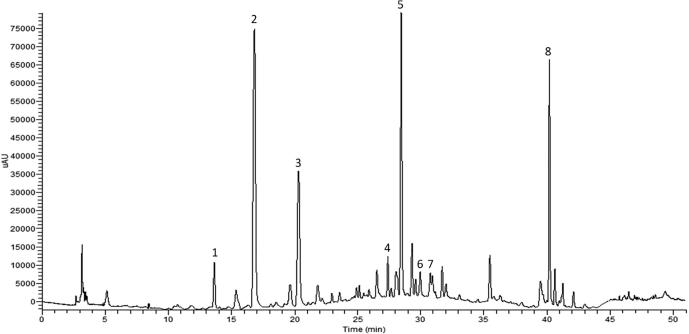
The RP-HPLC-DAD analysis revealed the presence of several phenolic compounds with retention time, molecular masses, fragmentation and UV spectra characteristic of hydroxycinnamic acids, flavonoids and stilbenes (Fig. 1) (Barros et al., 2014, Dias et al., 2015). These identified compounds were also quantified, being the results presented in Table 3. The extracts of the varieties studied presented similar phenolic profiles, however, the concentrations of each individual compound showed significant differences between the samples, except for kaempferol-3-O-glucoside (Table 3). Regarding hydroxycinnamic acids, the extracts of Malvasia Fina variety showed higher concentration, namely 12.82 ± 0.15 mg g−1 dw for caftaric acid and 3.36 ± 0.09 mg g−1 dw for the unidentified hydroxycinnamic acid. Concerning flavonoids, the Rabigato variety extracts presented higher concentration of isorhamnetin-3-O-(6-O-feruloyl)-glucoside (0.80 ± 0.01 mg g−1 dw). For the compound quercetin-3-O-rutinoside, Moscatel was the variety that presented the higher content (0.23 ± 0.02 mg g−1 dw), however, in the case of quercetin-3-O-glucuronide, Malvasia Fina variety showed higher concentration. Furthermore, this flavonol was the major compound found for all varieties with contents ranging between 29.27 ± 1.39 and 73.79 ± 0.79 mg g−1 dw. With respect to the compound kaempferol-3-O-rutinoside, this one showed to be more abundant in the Malvasia Fina variety (0.40 ± 0.02 mg g−1 dw). Finally, regarding the case of stilbenes, the Fernão Pires variety revealed higher concentration of Ɛ-viniferin (0.90 ± 0.03 mg g−1 dw).
Fig. 1.
Representative RP-HPLC-DAD chromatogram of white grape stems and identified compounds at 330 nm corresponding to the variety 'Moscatel'.
Table 3.
Phenolic compounds present in grape stem extracts.
| Phenolic compounds | Rabigato (mg g −1 dw) | Malvasia Fina (mg g −1dw) | Fernão Pires (mg g −1 dw) | Viosinho (mg g −1 dw) | Moscatel (mg g −1 dw) | P-values |
|---|---|---|---|---|---|---|
|
X0,80 ± 0,01b | 0,60 ± 0,00a | 0,57 ± 0,04a | 0,60 ± 0,00a | 0,60 ± 0,02a | Y*** |
|
2,28 ± 0,05b | 12,82 ± 0,15d | 1,71 ± 0,02a | 1,51 ± 0,04a | 5,01 ± 0,19c | *** |
|
0,59 ± 0,04a | 3,36 ± 0,09c | 0,45 ± 0,01a | 0,63 ± 0,11a | 2,58 ± 0,09b | *** |
|
0,15 ± 0,01bc | 0,19 ± 0,01c | 0,14 ± 0,01b | 0,05 ± 0,00a | 0,23 ± 0,02d | *** |
|
37,56 ± 2,09ab | 73,79 ± 0,79c | 40,27 ± 0,04b | 34,63 ± 4,72ab | 29,27 ± 1,39a | *** |
|
0,04 ± 0,00a | 0,40 ± 0,02c | 0,14 ± 0,01b | 0,10 ± 0,01b | 0,14 ± 0,01b | *** |
|
0,02 ± 0,01a | 0,02 ± 0,01a | 0,04 ± 0,00a | 0,04 ± 0,01a | 0,04 ± 0,01a | N. S. |
|
0,31 ± 0,01a | 0,17 ± 0,00a | 0,90 ± 0,03b | 0,83 ± 0,15b | 0,76 ± 0,05b | *** |
Glc, glucoside; Gluc, glucuronide; K, kaempferol; Q, quercetin; Rut, rutinoside.
The values are presented as mean ± standard deviation (n = 3). Different letters indicate significantly different results (ANOVA, p < 0.05).
Significance: non-significant, N.S. (p > 0.05); * significant at p < 0.05; ** significant at p < 0.01; *** significant at p < 0.001.
In Barros et al. (2014) study, the same compounds were identified for the varieties Fernão Pires, Rabigato and Viosinho. However, the concentrations were lower than in the present study, with the exception for the compound kaempferol-3-O-glucoside, that presented higher contents for these three varieties, and the compound Ɛ-viniferin with higher concentration for Rabigato variety (approximately 0.35 mg g−1 dw). These compounds were also identified by Dias et al. (2015), for the same three varieties (Dias et al., 2015). Regarding the flavonoids group, the major compound was quercetin-3-O-glucuronide, ranging from 0.04 to 0.08 mg g−1 dw. In contrast, in the present work, the concentrations were much higher for these varieties (between 34.63 and 40.27 mg g−1 dw). Regarding the hydroxycinnamic acids identified, as in the present work, caftaric acid is the most abundant in these three varieties (between 0.04 and 0.07 mg g−1 dw), but with higher concentrations in our study, oscillating between 1.51 and 2.28 mg g−1 dw. Regarding stilbenes, comparatively to the present work, the Ɛ-viniferin contents were lower, varying between 0.02 and 0.03 mg g−1 dw, being in the current study among 0.31 and 0.90 mg g−1 dw, for the varieties Fernão Pires, Viosinho and Rabigato. These differences between the samples can be also explained by the factors mentioned previously, however, there is not a clear evidence on the high concentration of phenolics in white or red varieties. In fact, and contrary to what happens with grape fruits, white varieties of grape stems demonstrated to possess equal or higher concentration of some phenolic compounds than red varieties (Anastasiadi et al., 2012, Apostolou et al., 2013, Llobera and Cañellas, 2007, Piñeiro et al., 2013, Sahpazidou et al., 2014), which is of great importance and it will allow white varieties to be also valued in this kind of wine by-products.
3.3. Antioxidant activity
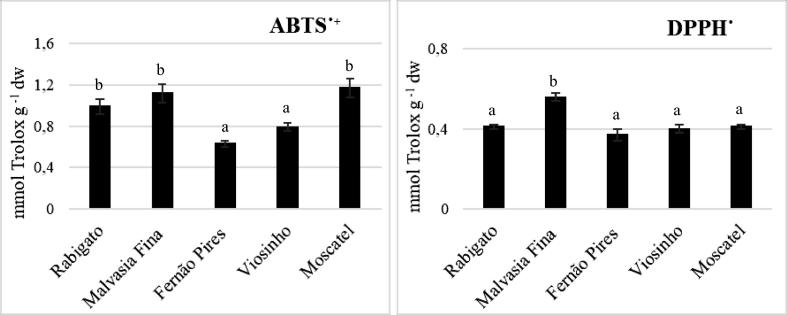
For the determination of the antioxidant activity of grape stems, two methods were used, ABTS and DPPH. The results obtained are shown in Fig. 2. For both methods, we can verify that there are significant differences between the samples. More precisely, the variety Fernão Pires exhibited the lower antioxidant activity for the both assays (0.63 ± 0.03 and 0.37 ± 0.03 mmol Trolox g−1 dw, for ABTS and DPPH, respectively), while the variety Moscatel presented the higher antioxidant activity for the ABTS method (1.17 ± 0.09 mmol Trolox g−1 dw) and the Malvasia Fina for the DPPH method (0.56 ± 0.02 mmol Trolox g−1). Domínguez-Perles et al. (2014), presented, for the variety Viosinho, antiradicalar activities of 0.049 mmol Trolox g−1 dw regarding the ABTS method (Domínguez-Perles et al., 2014b). Comparing with our results (0.79 ± 0.04 mmol Trolox g−1 dw), their values are very low, which is expected, since the antioxidant activity is positively correlated with the polyphenolic content (Anastasiadi et al., 2012).
Fig. 2.
Antioxidant activity of grape stem extracts by ABTS and DPPH methods. Columns with different letters indicate significantly different results (ANOVA, p < 0.05).
Lower values were obtained in Gouvinhas et al. (2020) study, except for the samples from Penajóia, belonging to the same region of the present stem samples, and the samples of the 2018 harvest season, which antioxidant activity increased reaching the values of the present study, due to the extreme heat verified this year. The same has been observed for the DPPH method, with similar values between the samples of this work and those of Gouvinhas et al. (2020) work, namely of lower altitude sites and of 2018 season. As well as we know, there are no more data comparable to those obtained for this method, essentially due to different extraction methods, extraction solvents or protocols, as in the case of the study of Anastasiadi et al., 2012, Barros et al., 2014, despite the use of the same white varieties. In fact, as it was possible to verify, there are several factors that contribute to the differences found between the samples, such as the vintage of the harvested stems and the edaphoclimatic conditions of the vineyards, whereby these variables should always be taken into account throughout the investigations.
3.4. Antimicrobial activity
The antimicrobial activity of the white grape stem extracts was measured by the determination of the % RIZD, in which antimicrobial activity of each sample was compared relatively to each antibiotic, being the results presented in Table 4. The stem extracts presented no effect on the microbial inhibition of E. coli MJS260 and K. pneumoniae MJS281 (% RIZD = 0), independently of the antibiotic tested. Regarding the % RIZD with no values presented (#), it was impossible to determine this percentage, since antibiotics didn't show any type of inhibition, unlike the extracts, considering that these extracts have a very high % RIZD, indicating its potential for posterior use.
Table 4.
% RIZD relative to antibiotics tested.
| Antibiotics | CN10 |
CN30 |
CIP10 |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial isolates | RG | MF | FP | VS | MP | P-value | RG | MF | FP | VS | MP | P-value | RG | MF | FP | VS | MP | P-value |
| L. monocytogenes ATCC 15,313 | X47ab | 51b | 44a | 47ab | 49ab | * | 44a | 47a | 40a | 44a | 45a | N.S. | 44ab | 47b | 40a | 44ab | 45ab | Y* |
| S. aureus MJS241 | # | # | # | # | # | – | # | # | # | # | # | – | # | # | # | # | # | – |
| E. faecalis MJS257 | # | # | # | # | # | – | # | # | # | # | # | – | # | # | # | # | # | – |
| P. aeruginosa ATCC 10,145 | 100ab | 110b | 100ab | 93a | 100ab | * | 78ab | 87b | 78ab | 73a | 78ab | N.S. | 48a | 53a | 48a | 45a | 48a | N. S. |
| E. coli MJS260 | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | – |
| K. pneunomiae MJS281 | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | – | 0 | 0 | 0 | 0 | 0 | – |
CN10: Gentamicin (10 µg per disc); CN30: Gentamicin (30 µg per disc); CIP10: Ciprofloxacin (10 µg per disc); RG: Rabigato; MF: Malvasia Fina; VS: Viosinho; FP: Fernão Pires; MP: Moscatel.
# Extract effective and antibiotic without effect.
Values in the same row for each clinical antibiotic followed by different superscript lowercase letters are significantly different at p < 0.05.
Significance: non-significant, N.S. (p > 0.05); * significant at p < 0.05; ** significant at p < 0.01; *** significant at p < 0.001.
Additionally, the antimicrobial activity of each extract was classified according to: (i) no effect; (ii) moderate efficacy; (iii) good efficacy; and (iv) high efficacy, being the results presented in Table 5. Through this classification, it was verified once again the potential of stem extracts, mainly in inhibiting the growth of the bacteria S. aureus MJS241 and E. faecalis MJS257.
Table 5.
Antimicrobial classification of the tested grape stem extracts.
| Antibiotics | CN10 |
CN30 |
CIP10 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial isolates | RG | MF | VS | FP | MP | RG | MF | VS | FP | MP | RG | MF | VS | FP | MP |
| L. monocytogenes ATCC 15,313 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| S. aureus MJS241 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| E. faecalis MJS257 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| P. aeruginosa ATCC 10,145 | + | ++ | + | + | + | + | + | + | + | + | + | + | + | + | + |
| E. coli MJS260 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| K. pneunomiae MJS281 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
CN10: Gentamicin (10 µg per disc); CN30: Gentamicin (30 µg per disc); CIP10: Ciprofloxacin (10 µg per disc); RG: Rabigato; MF: Malvasia Fina; VS: Viosinho; FP: Fernão Pires; MP: Moscatel; no effect: -; moderate efficacy: +; good efficacy: ++; high efficacy: +++.
The study performed by Dias et al. (2015) also showed that the extracts of grape stems presented antimicrobial activity, however with a different methodology used to evaluate this activity (minimum inhibitory concentration (MIC) vs. disc diffusion) (Dias et al., 2015). In fact, some studies already revealed some antibacterial activity of grape stems against gastrointestinal bacteria. However, in this work, through the disc diffusion method employed, it was investigated not only one class of antibiotics (quinolones: ciprofloxacin), but also another class (Aminoglycoside: Gentamicin) as a positive control at two different concentrations. It was possible to verify in this method that these antibiotics had no effect on the inhibition of bacterial growth, unlike the stem samples which proved to be effective against S. aureus and E. faecalis, contrary to the work developed by (Barros et al., 2015, Barros et al., 2014) and Dias et al., 2015 where the ciprofloxacin revealed to be always effective.
Oliveira et al. (2013) also exhibited antimicrobial activities of another wine by-product, namely grape pomace of red varieties (Syrah and Merlot) (Oliveira et al., 2013). In this study, the authors also used Gram-positive and Gram-negative bacteria, which demonstrated to be resistant to several groups of antibiotics, namely, carbapenems, antibiotics used at the hospital level. Additionally, it should be noted that S. aureus, B. cereus and E. coli are bacteria associated with foodborne diseases, and two of them are used in the present work. Also, Katalinić et al. (2010) showed antimicrobial activity of another by-product, namely grape skins (Katalinić et al., 2010).
Studies reported that the bactericidal action of grape phenolics compounds is due to several factors, namely: (i) changes in cellular morphology; (ii) in the DNA content; (iii) interaction with enzymes and substrates; (iv) deprivation of metallic ions (Paulo et al., 2010, Teixeira et al., 2014a, Vaquero et al., 2007). Moreover, the results obtained in the present work showed a greater efficacy in inhibiting the growth of Gram-positive bacteria, and in some cases, higher than the commercial antibiotics used as control. These differences found between the Gram positive and Gram negative bacteria can be explained by the cell membrane of the last one which has two layers, where the outer membrane is a strong barrier due to its high hydrophilicity. In contrast, Gram-positive bacteria have a simple cellular membrane, which allows the entry of lipophilic compounds (Oliveira et al., 2013).
In this way, and after this preliminary investigation, grape stems seem to be an important candidate to be used as raw material, and thus, to find an alternative to recycle them with a minimum environmental impact, once its rich content in biological compounds can allow the creation of innovative products, for example, by the use of these extracts as active ingredient for several industrial sectors.
4. Conclusion
In this study, white grape stems varieties from the Região Demarcada do Douro revealed to be a matrix rich in useful compounds, namely phenolics, with proven antioxidant properties and revealing a good scavenging capacity, Malvasia Fina being the white cultivar which showed the most promising since it presented higher concentrations and antioxidant activity. Furthermore, stems showed antimicrobial activity with high efficacy against Gram-positive bacteria, especially S. aureus and E. faecalis, contrary to the positive controls which were unable to inhibit this bacterial growth. However, the concentration of these bioactive compounds and their biological properties are dependent on several factors, such as vintage, whereby this factor has to be considered in further studies.
This investigation allowed to identify the potential of grape stems to be applied in several biotechnological sectors, including cosmetic, food and pharmaceutical industries, being a possible approach to develop new proper valorization ways of these by-products and thus, allowing to get even higher value from them in terms of efficacy. In these sense, it is mandatory to stimulate the research and to develop new applications of active agents, whether used as whole or in extract form, to different purposes in order to provide economical advantage to these sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was supported by National Funds by FCT - Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020, and the I&D project Interact - Integrative Research in Environment, Agro-Chains and Technology (NORTE-01-0145-FEDER-000017), regarding the research line “Fostering viticulture sustainability for Douro Valley: multidisciplinary efforts from field to wine (VitalityWINE)”, and the research line “Innovation for Sustainable Agro-food Chains (ISAC)” co-founded by the European Regional Development Fund (FEDER) through NORTE-2020 (Programa Operacional Regional do Norte 2014/2020). The authors also want to acknowledge Interreg Program for the financial support of the Project IBERPHENOL, Project Number 0377_IBERPHENOL_6_E, co-financed by European Regional Development Fund (ERDF) through POCTEP 2014-2020. Santos was supported by the grant SFRH/BD/ 131069/2017. We also acknowledge Eng. Manuel Henriques from Rozés for the samples provided.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aires A., Mota V.R., Saavedra M.J., Rosa E.A.S., Bennett R.N. The antimicrobial effects of glucosinolates and their respective enzymatic hydrolysis products on bacteria isolated from the human intestinal tract. J. Appl. Microbiol. 2009;106:2086–2095. doi: 10.1111/j.1365-2672.2009.04180.x. [DOI] [PubMed] [Google Scholar]
- Anastasiadi M., Chorianopoulos N.G., Nychas G.J.E., Karoutounian S.A. Antilisterial activities of polyphenol-rich extracts of grapes and vinification byproducts. J. Agric. Food Chem. 2009;57:457–463. doi: 10.1021/jf8024979. [DOI] [PubMed] [Google Scholar]
- Anastasiadi M., Pratsinis H., Kletsas D., Skaltsounis A.L., Haroutounian S.A. Grape stem extracts: Polyphenolic content and assessment of their in vitro antioxidant properties. LWT – Food Sci. Technol. 2012;48:316–322. [Google Scholar]
- Apostolou A., Stagos D., Galitsiou E., Spyrou A., Haroutounian S., Portesis N., Trizoglou I., Wallace Hayes A., Tsatsakis A.M., Kouretas D. Assessment of polyphenolic content, antioxidant activity, protection against ROS-induced DNA damage and anticancer activity of Vitis vinifera stem extracts. Food Chem. Toxicol. 2013;61:60–68. doi: 10.1016/j.fct.2013.01.029. [DOI] [PubMed] [Google Scholar]
- Barros A., Gironés-Vilaplana A., Teixeira A., Collado-González J., Moreno D.A., Gil-Izquierdo A., Rosa E., Domínguez-Perles R. Evaluation of grape (Vitis vinifera L.) stems from Portuguese varieties as a resource of (poly)phenolic compounds: A comparative study. Food Res. Int. 2014;65:375–384. [Google Scholar]
- Barros A., Gironés-Vilaplana A., Texeira A., Baenas N., Domínguez-Perles R. Grape stems as a source of bioactive compounds: application towards added-value commodities and significance for human health. Phytochem. Rev. 2015;14:921–931. [Google Scholar]
- Bauer A.W., Kirby W.M., Sherris J.C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45:493–496. [PubMed] [Google Scholar]
- Dias C., Domínguez-Perles R., Aires A., Teixeira A., Rosa E., Barros A., Saavedra M.J. Phytochemistry and activity against digestive pathogens of grape (Vitis vinifera L.) stem’s (poly)phenolic extracts. LWT – Food Sci. Technol. 2015;61:25–32. [Google Scholar]
- Domínguez-Perles R., Guedes A., Queiroz M., Silva A.M., Barros A.I.R.N.A. Oxidative stress prevention and anti-apoptosis activity of grape (Vitis vinifera L.) stems in human keratinocytes. Food Res. Int. 2016;87:92–102. doi: 10.1016/j.foodres.2016.06.030. [DOI] [PubMed] [Google Scholar]
- Domínguez-Perles R., Teixeira A.I., Rosa E., Barros A.I. Assessment of (poly)phenols in grape (Vitis vinifera L.) stems by using food/pharma industry compatible solvents and Response Surface Methodology. Food Chem. 2014;164:339–346. doi: 10.1016/j.foodchem.2014.05.020. [DOI] [PubMed] [Google Scholar]
- FAOSTAT. 2018. FAOSTAT. Food Agric. Organ. United Nations.
- Gouvinhas Irene, Pinto Rosa, Santos Rafaela, Saavedra Maria José, Barros Ana Isabel. Enhanced phytochemical composition and biological activities of grape (Vitis vinifera L.) Stems growing in low altitude regions. Sci. Hortic. 2020;265:109248. [Google Scholar]
- Gouvinhas I., Queiroz M., Rodrigues M., Barros A.I.R.N.A. Polyphenols in Plants. Elsevier; 2019. Evaluation of the phytochemistry and biological activity of grape (Vitis vinifera L.) stems: toward a sustainable winery industry; pp. 381–394. [Google Scholar]
- Gouvinhas I., Santos R.A., Queiroz M., Leal C., José M., Domínguez-perles R., Rodrigues M., Barros A.I.R.N.A. Industrial Crops & Products Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crop. Prod. 2018;126:83–91. [Google Scholar]
- Katalinić V., Možina S.S., Skroza D., Generalić I., Abramovič H., Miloš M., Ljubenkov I., Piskernik S., Pezo I., Terpinc P., Boban M. Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia) Food Chem. 2010;119:715–723. [Google Scholar]
- Liao X., Ma Y., Daliri E.B.M., Koseki S., Wei S., Liu D., Ye X., Chen S., Ding T. Interplay of antibiotic resistance and food-associated stress tolerance in foodborne pathogens. Trends Food Sci. Technol. 2020;95:97–106. [Google Scholar]
- Llobera A., Cañellas J. Dietary fibre content and antioxidant activity of Manto Negro red grape (Vitis vinifera): pomace and stem. Food Chem. 2007;101:659–666. [Google Scholar]
- Makris D.P., Boskou G., Andrikopoulos N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007;20:125–132. [Google Scholar]
- Mateo J.J., Maicas S. Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015;73:13–25. [Google Scholar]
- Mena P., García-Viguera C., Navarro-Rico J., Moreno D.A., Bartual J., Saura D., Martí N. Phytochemical characterisation for industrial use of pomegranate (Punica granatum L.) cultivars grown in Spain. J. Sci. Food Agric. 2011;91:1893–1906. doi: 10.1002/jsfa.4411. [DOI] [PubMed] [Google Scholar]
- Oliveira D.A., Salvador A.A., Smânia A., Smânia E.F.A., Maraschin M., Ferreira S.R.S. Antimicrobial activity and composition profile of grape (Vitis vinifera) pomace extracts obtained by supercritical fluids. J. Biotechnol. 2013;164:423–432. doi: 10.1016/j.jbiotec.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Paulo L., Ferreira S., Gallardo E., Queiroz J.A., Domingues F. Antimicrobial activity and effects of resveratrol on human pathogenic bacteria. World J. Microbiol. Biotechnol. 2010;26:1533–1538. [Google Scholar]
- Piñeiro Z., Guerrero R.F., Fernández-Marin M.I., Cantos-Villar E., Palma M. Ultrasound-assisted extraction of stilbenoids from grape stems. J. Agric. Food Chem. 2013;61:12549–12556. doi: 10.1021/jf4030129. [DOI] [PubMed] [Google Scholar]
- Portu J., López R., Santamaría P., Garde-Cerdán T. Methyl jasmonate treatment to increase grape and wine phenolic content in Tempranillo and Graciano varieties during two growing seasons. Sci. Hortic. (Amsterdam) 2018;240:378–386. [Google Scholar]
- Queiroz M., Oppolzer D., Gouvinhas I., Silva A.M., Barros A.I.R.N.A., Domínguez-Perles R. New grape stems’ isolated phenolic compounds modulate reactive oxygen species, glutathione, and lipid peroxidation in vitro : Combined formulations with vitamins C and E. Fitoterapia. 2017;120:146–157. doi: 10.1016/j.fitote.2017.06.010. [DOI] [PubMed] [Google Scholar]
- Sahpazidou D., Geromichalos G.D., Stagos D., Apostolou A., Haroutounian S.A., Tsatsakis A.M., Tzanakakis G.N., Hayes A.W., Kouretas D. Anticarcinogenic activity of polyphenolic extracts from grape stems against breast, colon, renal and thyroid cancer cells. Toxicol. Lett. 2014;230:218–224. doi: 10.1016/j.toxlet.2014.01.042. [DOI] [PubMed] [Google Scholar]
- Singh A., Gautam P.K., Verma A., Singh V., Shivapriya P.M., Shivalkar S., Sahoo A.K., Samanta S.K. Green synthesis of metallic nanoparticles as effective alternatives to treat antibiotics resistant bacterial infections: A review. Biotechnol. Reports. 2020;25:e00427. doi: 10.1016/j.btre.2020.e00427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa C., Gouvinhas I., Barreira D., Carvalho M.T., Vilela A., Lopes J., Martins-Lopes P., Barros A.I. “Cobrançosa” olive oil and drupe: Chemical composition at two ripening stages. JAOCS. J. Am. Oil Chem. Soc. 2014;91 [Google Scholar]
- Teixeira A., Baenas N., Dominguez-Perles R., Barros A., Rosa E., Moreno D.A., Garcia-Viguera C. Natural bioactive compounds from winery by-products as health promoters: A review. Int. J. Mol. Sci. 2014;15:15638–15678. doi: 10.3390/ijms150915638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira M., Becker P., Gómez-alonso S., Teixeira H., Hermosín-gutiérrez I. Phenolic composition of grape and winemaking by-products of Brazilian hybrid cultivars BRS Violeta and BRS Lorena. Food Chem. 2014;159:95–105. doi: 10.1016/j.foodchem.2014.02.163. [DOI] [PubMed] [Google Scholar]
- The International Organisation of Vine and Wine [WWW Document]. 2017.
- Vaquero M.J.R., Alberto M.R., de Nadra M.C.M. Antibacterial effect of phenolic compounds from different wines. Food Control. 2007;18:93–101. [Google Scholar]
- WHO. 2017. World Health Organization. Global antimicrobial resistance surveillance system (GLASS) report: early implementation 2016-2017. Geneva: World Health Organization; 2017. Licence: CC BY-NC-SA 3.0 IGO., Who. https://doi.org/ISBN 978-92-4-151344-9.