Abstract
Glucose isomerase (GI), an enzyme with deserved high potential in the world market. GI plays a major role in high Fructose Corn Syrup Production (HFCS). HFCS is used as a sweetener in food and pharmaceutical industries. Streptomyces are well-known producers of various industrially valuable enzymes, including Glucose isomerase. Currently, recombinant strains have been available for the production of various enzymes, but it has limitation in the large scale production. Therefore, identifying effective streptomyces strains have emerged. The current study, the novel S. lividans RSU26 was isolated from a marine source and optimized its potential to produce glucose isomerase at different physical and chemical conditions. The optimum pH and temperature for GI and biomass production were 7.5 and 35 °C, respectively at 96 h. Characterization study revealed that the approximate molar mass of GI was 43 kDa for monomeric and 170 kDa for tetrameric forms. Kinetic behavior exhibits Km, and Vmax values for the conversion of fructose to glucose conversion were 48.8 mM and 2.54 U mg−1 at 50 °C and glucose to fructose were 29.4 mM and 2.38 U mg−1 at 65 °C protein, respectively. Therefore, the present study suggested that the wild–type S. lividans RSU26 has strong potential to produce glucose isomerase for various industrial applications.
Keywords: Streptomyces, Glucose isomerase, Optimization, Purification, Kinetics
1. Introduction
d-Glucose/xylose isomerase (EC: 5.3.1.5) is an essential enzyme in various sectors such as food, fuel and medical field. GI involves isomerization of d-glucose into d-fructose (EFSA Panel on Food Contact Materials, 2019, Jin et al., 2017, Al-Dhabi et al., 2016). Particularly, the conversion of d-glucose into d-fructose has high commercial values for the production of High Fructose Corn Syrup (HFCS). The more attention in the reversible isomerization of glucose (G) into fructose (F) in the presence of GI has high profitable strategies in the HFCS production (Xu et al., 2014). The recent marketplace value of GI is approximately one billion US dollar (Vandamme et al., 2006, Al-Dhabi et al., 2019, Al-Dhabi et al., 2020). Generally, GI present in the various prokaryotes and fungi. Especially, Streptomyces are largely involved in GI production (Al-Dhabi et al., 2019c, Al-Dhabi et al., 2019d, Al-Dhabi et al., 2019e, Al-Dhabi et al., 2019f). The most commercial GI producers are streptomyces and Bacillus species (Al-Dhabi et al., 2019g). Recent findings of GI from various sources are widely studied and detected a few technical difficulties in the isomerization process. The wild-type GI producers exhibited very low specific activity, thermo-stability and high substrate specificity than for recombinant strains. Temperatures at 55–65 °C and pH at 6.5–7.5 are the most suitable conditions for the fructose production industries (Rozanov et al., 2009). Therefore, we planned to identify novel GI producing Streptomyces from marine sources and optimize its potential to produce GI at various physical and chemical conditions andkinetic behavior also studied.
2. Materials and methods
2.1. Isolation and identification of Streptomyces
The soil samples were collected from different regions of Muthupet mangrove forest, Thanjavur, Tamil Nadu, India. The Streptomyces isolation was carried out by soil dilution plate techniques(Kuester and Williams, 1964, Al-Dhabi et al., 2018a, Al-Dhabi et al., 2018b)). GI production by isolated strains was screened (Sapunova et al., 2006). Based on the preliminary screening, a single strain was selected for further investigation. Physiochemical (Supplementary Tables 1 and 2) and 16srRNA sequences revealed that this strain belonged to the S. lividans and the sequence was deposited at NCBI gene bank (GenBank ID: KP698743.1).
2.2. Quantification of GI
The fresh S. lividans was cultured in peptone yeast extract broth with 50% of seawater (Uyar and Baysal, 2004). After fermentation, the biomass and supernatant were separated. The biomass was disrupted by ultrasonication and supernatant was separated by centrifugation at 12,000 rpm for 15 min (4 °C). The enzyme assay was determined towards d-fructose as a substrate for d-glucose production at OD 540 nm (Miller, 1959). Further, the inter-conversion reaction of d-glucose as a substrate for d-fructose production was read at OD 480 nm (Takasaki, 1966).
2.3. Scale-up process
S. lividans RSU26 with essential parameters were optimized by flask-scale fermentation. Initial GI optimizations were determined specific duration at 24–120 h. To standardize the optimum pH (pH 6.0–9.0) and temperature (20–50 °C); and impact of different carbon, nitrogen, metal ions also studied.
2.4. Purification of GI
Ammonium sulfate precipitation method was used to precipitate GI initially. Then the cell-free extract was dialyzed by 110 kDa membrane (HiMedia-Mumbai, India). Dialyzed fraction was then subjected to the purification of GI by gel filtration chromatography using a Sephacryl S-200 column (Chen and Anderson, 1979) (Sigma Aldrich- Mumbai, India). The protein content of samples was quantified (Bradford, 1976). Purified GI molecular mass was determined by SDS and native PAGE gel electrophoresis (Laemmli, 1970).
2.5. Characterization of GI
The effects of pH on the activity of GI were determined and indicate pH range between 6 and 9 were considered as most suitable for the activity of GI. The different pH was prepared in acetate and phosphate buffers. The pH 6–6.5 was adjusted in acetate buffer; pH 7–7.5 in phosphate buffer; pH 8–9 in Tris buffer at 45 °C for glucose and 60 °C for fructose conversion. Changes in the GI activity by different temperatures (35–80 °C at pH 7.5) were determined. The isomerization reaction was performed at 35–65 °C glucose and 50–80 °C for fructose.
The pH stabilities of GI were ranged from 7 to 8. The enzyme pre-incubated with different pH in phosphate buffer (pH 7–7.5); and Tris buffer (pH 8) at a different phase of incubation time (0–30; 30–60; 60–90 min) at 4 °C. The thermal stabilities of GI were determined in the range between 45–75 °C. The purified GI was pre-incubated with a different phase of incubation time (0–10; 10–20; 20–30 min), isomerization at 50 °C and 60 °C. The effects of various metal ions were determined by adding different concentration Na+, Mg2+, Co2+, Mn2+, Fe2+, Ca2+, Ag2+, Hg2+, Cu2+ and Zn2+ under the standard condition at 50 °C for glucose and 65 °C for fructose production (Wang et al., 2011). The kinetic characterizations of GI were determined with d-glucose and d-fructose as a substrate (10–1000 mM). The affinity of both substrates such as glucose and fructose were calculated during the isomerization process.
3. Results
3.1. Scale-up process
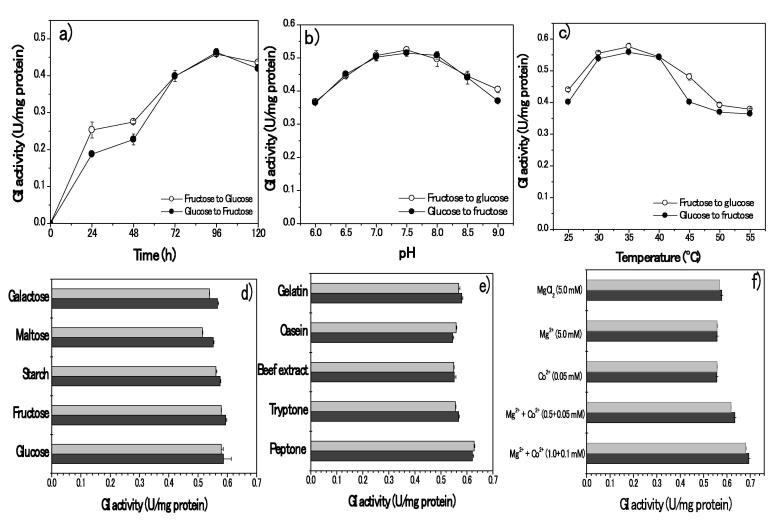
S. lividans RSU26 was produced significant amounts of glucose isomerase (GI) in crude form (0.42 U/mg), and the cell dry weight was 0.64 g/L. The optimum conditions such as incubation periods, pH and temperature for the GI production were 96 h, pH7.5 and 35 °C, respectively. The cell dry weight was reached 1.05 g/L and specific activity 0.57 U/mg (G) and 0.55 U/mg (F) proteins. GI production has strictly adhered with substrates used in the medium. The final step of the scale-up process of GI has reached maximum dry cell weight 3.0 g/L and specific activity 0.69 and 0.68 U/mg (t = 96 h) protein (Fig. 1a–f).
Fig. 1.
Effects of different physiochemical and substrates on GI productions: Influences of physiochemical effects (a-Incubation periods; b- pH; c- temperatures) on GI production and different substrates carbon (d); Nitrogen (e); metals (f) on GI production Carbon sources (1%, w/v of glucose, xylose, starch, fructose and maltose); nitrogen sources (1.2%, w/v of peptone, beef extract, gelatin, casein and tryptone); metal ions, MgCl2 (5.0 mM), Mg2+ (5.0 mM), Co2+ (0.05 mM), Mg2++Co2+ (10.0 and 0.05 mM), Mg2++Co2+ (10.0 and 0.1 mM) (magnesium and cobalt ions were added in the form of sulfate and chloride salts).
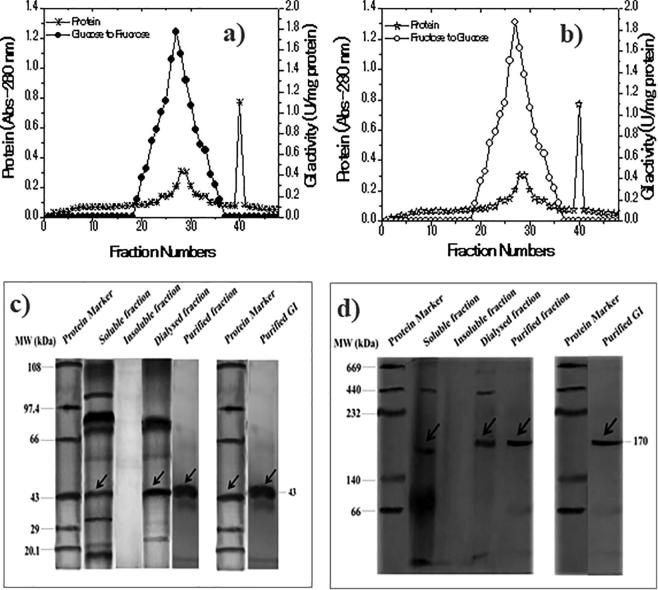
3.2. Purification of GI
The S. lividans RSU26 produced GI was purified successfully by the multistep process (Table 1). The initial precipitation and dialysis of GI protein were expressed to be 1.5–1.56 U/mg proteins. The purity was improved greater than two-fold and 67% of protein. The final step of gel filtration chromatography eluted fractions was measured at OD 280 nm. The GI was expressed total protein 54% yield, and purity increased higher than threefold (Fig. 2a and b). The SDS and Native PAGE analysis of purified GI molar mass were expressed to be 43 kDa (monomer) and 170 kDa (tetramer) (Fig. 2c and d).
Table 1.
GI activity in different forms, extracted from S. lividans RSU26.
| Enzyme in different forms | Total Activity |
Protein | Specific Activity1 |
Purified | Yield | ||
|---|---|---|---|---|---|---|---|
| (µM)2 | (µM)3 | (mg/mL) | F → G | G → F | Fold | (%) | |
| CEE | 1.053 ± 0.002 | 1.064 ± 0.003 | 1.46 ± 0.004 | 0.718 ± 0.008 | 0.726 ± 0.010 | – | 100 |
| ASP | 1.332 ± 0.004 | 1.397 ± 0.019 | 1.23 ± 0.002 | 1.080 ± 0.011 | 1.133 ± 0.041 | 1.5 | 84.1 |
| EED | 1.503 ± 0.009 | 1.556 ± 0.004 | 0.99 ± 0.001 | 1.509 ± 0.028 | 1.562 ± 0.009 | 2.1 | 67.9 |
| PES | 1.550 ± 0.003 | 1.685 ± 0.022 | 0.79 ± 0.014 | 1.943 ± 0.083 | 2.112 ± 0.171 | 2.7 | 54.4 |
CEE-Crude enzyme extract; ASP-Ammonium Sulfate precipitation; EED -Enzyme extract after dialysis; PES-Purified enzyme by Sephacryl S-200.
U/ mg protein.
fru/ml/min.
glu/ml/min.
Fig. 2.
Determination of purified GI activity and molar mass: Eluted fractions of purified GI protein (a) and its specific activity (b); SDS and Native Page profile of purified GI protein mass (c and d).
3.3. Characteristics of GI
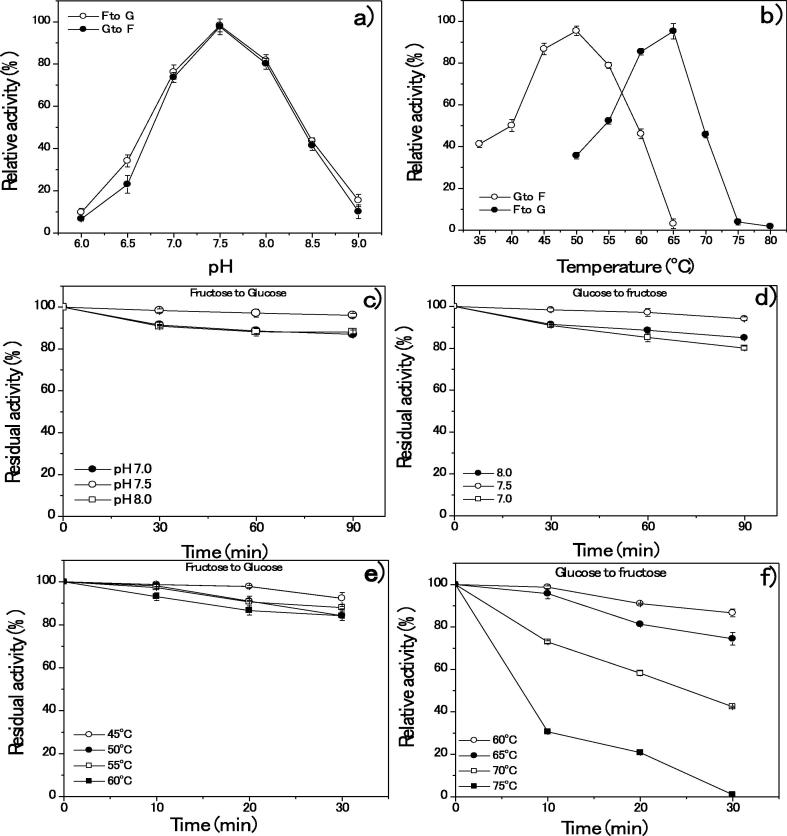
The optimum pH and temperature for the activity of purified GI were noted at 7.5 (98%), temperatures 50 °C for glucose and 65 °C for fructose (≥98%) yield (Fig. 3a and b). The pH stabilities of purified GI protein residual activity ranged from 98 to 92%. The GI protein half-life stability was maintained at 90 min. The stability was reduced by ≥20% when pH was increased (pH 8) during 30–90 min (Fig. 3c and d). Thermal stabilities of purified GI protein activities were exhibited at ranges between 40 and 50 °C at 10–30 min. The GI half-life was stabled until temperatures between 60 and 65 °C for 10 min. The purified GI activity was started to denature at higher than 65 °C (20–30 min), which denotes the GI relatively thermostable. The GI was completely denatured at 75–80 °C (Fig. 3e and f). The relative activity of S. lividans RSU26 GI was predicted in the presence of different metal ions added in the final isomerization. The combination of Mg2+ and Co2+ at the concentrations of 10.0 mM-Mg2+ and 1.0 mM-Co2+ respectively increased maximum relative activity (98%). The combination of Mg2+ Co2+ significantly enhanced the activity (Table 2).
Fig. 3.
Relative and residual activity of purified GI from S. lividans RSU26: Relative activity of pH (a) and Temperature (b); Residual activity of pH (c and d) and Temperature (e and f).
Table 2.
Effects of different metal ions on purified GI activity.
| Metals and other Compound | Fructose → glucose | Glucose → fructose |
|---|---|---|
| Relative activity (%) | Relative activity (%) | |
| Without metals | 5.80 ± 0.546 | 5.70 ± 0.824 |
| Mg2+ (5.0 mM) | 54.9 ± 0.376 | 51.2 ± 0.468 |
| Ca2+ (0.5 mM) | 6.40 ± 4.584 | 7.20 ± 4.929 |
| Co2+ (0.1 mM) | 5.00 ± 4.742 | 4.80 ± 4.755 |
| Co2+ (0.5 mM) | 49.0 ± 3.257 | 47.2 ± 2.670 |
| Co2+ (1.0 mM) | 61.6 ± 3.818 | 61.3 ± 3.902 |
| Mg2+ (5.0 mM) + Co2+ (0.1 mM) | 75.3 ± 1.355 | 78.8 ± 0.125 |
| Mg2+ (10.0 mM) + Co2+ (0.5 mM) | 90.0 ± 3.110 | 86.5 ± 0.785 |
| Mg2+ (10.0 mM) + Co2+ (1.0 mM) | 98.7 ± 0.947 | 96.3 ± 3.108 |
| MgCl2 (0.5 mM) | 28.3 ± 0.310 | 18.1 ± 2.459 |
| MgSO4 (0.5 mM) | 38.2 ± 0.100 | 41.3 ± 0.451 |
3.4. Kinetic behavior
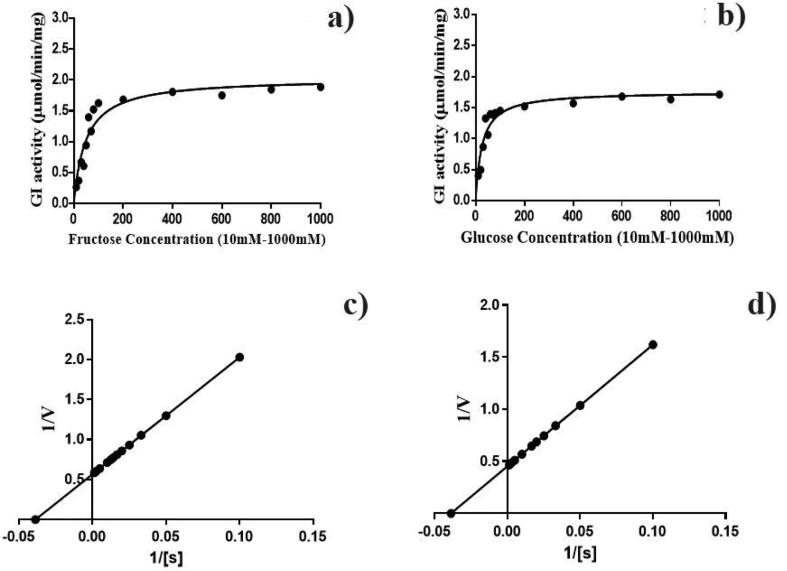
The GI reaction was performed in the presence of two different substrates, such as d-glucose and d-fructose at various concentrations individually (Table 3). The double reciprocal plots of the GI reaction rate were estimated against the two substrates. The Km (48.8 and 29.5 mM) and Vmax (2.5 and 2.3 U/mg protein) value were calculated. The Kcat/Km value was expressed as 0.9 fold for fructose production (Fig. 4a–d). The S. lividans RSU26 producing GI had high affinity with glucose than the fructose.
Table 3.
Kinetic behavior of purified GI from S. lividans RSU26.
| Substrates | Products | Vmax (U/mg protein) | K m (mM) | Kcat (s−1) | Kcat/Km × 103 (M−1 s−1) |
|---|---|---|---|---|---|
| d-Fructose | d-Glucose | 2.544 ± 0.10 | 48.85 ± 9.50 | 12.72 ± 0.70 | 0.260 ± 0.07 |
| d-Glucose | d-Fructose | 2.388 ± 0.09 | 29.43 ± 4.59 | 11.94 ± 0.45 | 0.405 ± 0.09 |
Fig. 4.
Michaelis-Menten kineticsof purified GI from S. lividans RSU26: the reaction rate of substrate and productconversion (a and b); Lineweaver- Burk double reciprocal plots (c and d).
4. Discussion
The current study, we isolated novel S. lividans RSU26 from marine soil and scaled the GI productions. This study suggested that S. lividans RSU26 is the highly valuable strain for the production of GI. S. lividans RSU26 significantly produced the desired quantity of GI in the medium than the other isolates. The result was concurrent with previous reports (Lama et al., 2001, Arasu et al., 2013, Balachandran et al., 2015, Arasu et al., 2017). Also, the flask scale parameters such as incubation time (96 h), pH (7.5) and temperature (35 °C) report coincided with earlier findings of GI (Givry and Duchiro, 2008). Different source of carbon, nitrogen and minerals could influence GI production. The dependence of nutrient sources such as glucose, peptone and metal ions supplement could be improved activity of GI greater than 10%, our current result coincided with previous reports (Chanitnun and Pinphanichakarn, 2012, Kaneko et al., 2001). The overall fermentation of GI yield 0.69 U/mg proteins and the cell dry weight 3 g/L was noted. The scale-up process could increase the GI production up to ≥4 fold (Sapunova et al., 2006). Therefore, the production medium constituents with peptone, 12 g; Yeast extract, 5g; glucose 10 g, K2HPO4, 3 g; MgSO4, 1 g; CoCl2, 0.1 g was recommended for enhancing the production of GI yield by S. lividans RSU26. The result was slightly comparable activity with earlier fermentation process (Yassien, 2012).
S. lividans RSU26 produced GI expressed desired molar mass indicated that the protein bands in gel were denoted as 43 kDa and ~170 kDa. The similar molar mass was observed in previous reports (Akan, 2018, Joo et al., 2005). The relative pH for purified GI was noted at pH 7.5. The relative temperature for GI activity exhibited at 50 °C and 65 °C for both product conversions individually. The residual activity of GI noted at pH 7.5 for 90 min, and thermal stability retained, after incubation at 65 °C for 10 min. The GI active up to 60 °C, the result strongly associated with the previous report (Staudigl et al., 2014).
The purified GI was active in tetrameric forms and required Mg2+, Mn2+ or Co2+ for catalytic activity and thermostability. While, Ca2+ as a solid competitive inhibitor and Cu2+, Zn2+, Hg2+ were inhibited GI activity. The S. lividansRSU26 was required only a trace amount of Co2+ for activity (Bhosale et al., 1996, Karaoglu et al., 2013). The kinetic behavior of GI was tiny similar and dissimilar with previous work (Akan, 2018, Sriprapundh et al., 2000).
5. Conclusion
In this study, the wild-type S. lividans RSU26 was isolated and optimized its GI production. Furthermore, GI was purified by Sephacryl S-200 column and characterized its kinetic behavior. The strain was grown in limited nutrients with suitable physical and chemical conditions. The S. lividans produced GI without any raw materials and inducer (xylose). Hence, the production has been an economically feasible and inexpensive process. The kinetics of GI effectively indicated both conversions (glucose and fructose) have a reversible and irreversible mode. In conclusion, S. lividans RSU26 is highly recommended for GI production. Hence, the upcoming work bonds with the heterologous system will be used for GI production.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No (RG-1440-056). We thank PRIST University, Thanjavur, Tamilnadu, India, for their technical support and thank Prof. Ki Choon Choi, National Institute of Animal Science, Korea, for his assistance in molecular identification of Streptomyces species.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2019.12.024.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Akan O. Production, characterization and optimal performance studies of glucose isomerase by achromobacter xylosoxidans mck-4 isolated from starch milling wastes. Microbiol. Res. J. Int. 2018:25. [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Duraipandiyan V., Valan Arasu M., Salem-Bekhit M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring Saudi Arabia. Extremophiles. 2016;20:79–90. doi: 10.1007/s00792-015-0799-1. [DOI] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Duraipandiyan V., Valan Arasu M. Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J. Biol. Sci. 2019;26:758–766. doi: 10.1016/j.sjbs.2019.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Ghilan A.-K.M., Arasu M.V. Isolation and screening of Streptomyces sp.Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi J. Biol. Sci. 2020;27:474–479. doi: 10.1016/j.sjbs.2019.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Ghilan A.-K.M., Arasu M.V., Duraipandiyan V., Ponmurugan K. C Isolation and purification of starch hydrolysing amylase from Streptomyces sp. Al-Dhabi-46 obtained from the Jazan region of Saudi Arabia with industrial applications. J. King Saud Univ.-Sci. 2019 [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Ghilan A.-K.M., Arasu M.V., Duraipandiyan V., Ponmurugan K. Characterization and fermentation optimization of novel thermo stable alkaline protease from Streptomyces sp. Al-Dhabi-82 from the Saudi Arabian environment for eco-friendly and industrial applications. J. King Saud Univ.-Sci. 2019 [Google Scholar]
- Al-Dhabi N.A., Esmail G.A., Ghilan A.K.M., Arasu M.V., Duraipandiyan V., Ponmurugan K. Chemical constituents of Streptomyces sp. strain AlDhabi-97 isolated from the marine region of Saudi Arabia with antibacterial and anticancer properties. J. Infect Public Health. 2019 doi: 10.1016/j.jiph.2019.09.004. [DOI] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Ghilan A.-K.M., Arasu M.V. Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum betalactamase Clinical Pathogens. Nanomater. 2018;8(5) doi: 10.3390/nano8050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Ghilan A.K.M., Arasu M.V., Duraipandiyan V. Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B. 2019:111529. doi: 10.1016/j.jphotobiol.2018.09.012. [DOI] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Ghilan A.K.M., Arasu M.V., Duraipandiyan V., Ponmurugan K. Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol., B. 2018;189:176–184. doi: 10.1016/j.jphotobiol.2019.111529. [DOI] [PubMed] [Google Scholar]
- Al-Dhabi N.A., Mohammed Ghilan A.K., Esmail G.A., Valan Arasu M., Duraipandiyan V., Ponmurugan K. Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum betalactamase clinical bacterial pathogens. J. Infect. Public Health. 2019;12:549–556. doi: 10.1016/j.jiph.2019.01.065. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Duraipandiyan V., Ignacimuthu S. Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479–487. doi: 10.1016/j.chemosphere.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Arasu M.V., Thirumamagal R., Srinivasan M.P., Al-Dhabi N.A., Ayeshamariam A., Saravana Kumar D., Punithavel N., Jayachandran M. Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50–60. doi: 10.1016/j.jphotobiol.2017.05.032. [DOI] [PubMed] [Google Scholar]
- Balachandran C., Duraipandiyan V., Emi N., Ignacimuthu S. Antimicrobial and cytotoxic properties of Streptomyces sp. (ERINLG-51) isolated from SouthernWestern Ghats. South Indian J. Biol. Sci. 2015;1:7–14. [Google Scholar]
- Bhosale S.H., Rao M.B., Deshpande V.V. Molecular and industrial aspects of glucose isomerase. Microbiol. Rev. 1996;60:280–300. doi: 10.1128/mr.60.2.280-300.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chanitnun K., Pinphanichakarn P. Glucose(xylose) isomerase production by Streptomyces sp. CH7 grown on agricultural residues. Brazilian J. Microbiol.: [Publ. Brazilian Soc. Microbiol.] 2012;43:1084–1093. doi: 10.1590/S1517-838220120003000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.P., Anderson A.W. Purification, immobilization, and some properties of glucose isomerase from streptomyces flavogriseus. Appl. Environ. Microbiol. 1979;38:1111–1119. doi: 10.1128/aem.38.6.1111-1119.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Panel on Food Contact Materials, E., Aids, P., Silano, V., Barat Baviera, J.M., Bolognesi, C., Brüschweiler, B.J., Cocconcelli, P.S., Crebelli, R., Gott, D.M., Grob, K., Lampi, E., Mortensen, A., Rivière, G., Steffensen, I.-L., Tlustos, C., Van Loveren, H., Vernis, L., Zorn, H., Glandorf, B., Herman, L., Marcon, F., Penninks, A., Smith, A., Aguilera, J., Kovalkovicova, N., Liu, Y., Maia, J., Engel, K.-H., Chesson, A., 2019. Safety evaluation of the food enzyme glucose isomerase from Streptomyces murinus (strain NZYM-GA). EFSA J. 17, e05547
- Givry S., Duchiro F. Optimization of culture medium and growth conditions for production of L-arabinose isomerase and D-xylose isomerase by Lactobacillus bifermentans. Mikrobiologiia. 2008;77:324–330. [PubMed] [Google Scholar]
- Jin L.Q., Xu Q., Liu Z.Q., Jia D.X., Liao C.J., Chen D.S., Zheng Y.G. Immobilization of recombinant glucose isomerase for efficient production of high fructose corn syrup. Appl. Biochem. Biotechnol. 2017;183:293–306. doi: 10.1007/s12010-017-2445-0. [DOI] [PubMed] [Google Scholar]
- Joo G.J., Shin J.H., Heo G.Y., Kim Y.M., Rhee I.K. Molecular cloning and expression of a thermostable xylose (glucose) isomerase gene, xylA, from Streptomyces chibaensis J-59. J. Microbiol. (Seoul, Korea) 2005;43:34–37. [PubMed] [Google Scholar]
- Kaneko T., Saito K., Kawamura Y., Takahashi S. Molecular cloning of acid-stable glucose isomerase gene from Streptomyces olivaceoviridis E-86 by a simple two-step PCR method, and its expression in Escherichia coli. Biosci. Biotechnol. Biochem. 2001;65:1054–1062. doi: 10.1271/bbb.65.1054. [DOI] [PubMed] [Google Scholar]
- Karaoglu H., Yanmis D., Sal F.A., Celik A., Canakci S., Belduz A.O. Biochemical characterization of a novel glucose isomerase from Anoxybacillus gonensis G2T that displays a high level of activity and thermal stability. J. Mol. Catal. B Enzym. 2013;97:215–224. [Google Scholar]
- Kuester E., Williams S.T. Production of hydrogen sulfide by streptomycetes and methods for its detection. Appl. Microbiol. 1964;12:46–52. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lama L., Nicolaus B., Calandrelli V., Romano I., Basile R., Gambacorta A. Purification and characterization of thermostable xylose(glucose) isomerase from Bacillus thermoantarcticus. J. Ind. Microbiol. Biotechnol. 2001;27:234–240. doi: 10.1038/sj.jim.7000182. [DOI] [PubMed] [Google Scholar]
- Miller G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959;31:426–428. [Google Scholar]
- Rozanov A.S., Zagrebel'nyi S.N., Beklemishchev A.B. Cloning of Escherichia coli K12 xylose isomerase (glucose isomerase) and studying the enzymatic properties of its expression product. Prikl. Biokhim. Mikrobiol. 2009;45:38–44. [PubMed] [Google Scholar]
- Sapunova L.I., Lobanok A.G., Kazakevich I.O., Shlyakhotko E.A., Evtushenkov A.N. Biosynthetic features and properties of xylose isomerases from Arthrobacter nicotianae, Escherichia coli, and Erwinia carotovora subsp. atroseptica. Appl. Biochem. Microbiol. 2006;42:246–251. [PubMed] [Google Scholar]
- Sriprapundh D., Vieille C., Zeikus J.G. Molecular determinants of xylose isomerase thermal stability and activity: analysis of thermozymes by site-directed mutagenesis. Protein Eng. 2000;13:259–265. doi: 10.1093/protein/13.4.259. [DOI] [PubMed] [Google Scholar]
- Staudigl P., Haltrich D., Peterbauer C.K. L-Arabinose isomerase and D-xylose isomerase from Lactobacillus reuteri: characterization, coexpression in the food grade host Lactobacillus plantarum, and application in the conversion of D-galactose and D-glucose. J. Agric. Food. Chem. 2014;62:1617–1624. doi: 10.1021/jf404785m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takasaki Y. Studies on sugar-isomerizing enzyme. Agric. Biol. Chem. 1966;30:1247–1253. [Google Scholar]
- Uyar F., Baysal Z. Production and optimization of process parameters for alkaline protease production by a newly isolated Bacillus sp. under solid state fermentation. Process Biochem. 2004;39:1893–1898. [Google Scholar]
- Vandamme E., Cerdobbel A., Soetaert W. Biocatalysis on the rise: Part 2 - applications. Chim. Oggi. 2006;24:57–61. [Google Scholar]
- Wang H., Yang R., Hua X., Zhang Z., Zhao W., Zhang W. Expression, enzymatic characterization, and high-level production of glucose isomerase from Actinoplanes missouriensis CICIM B0118(A) in Escherichia coli. Zeitschrift fur Naturforschung. C. J. Biosci. 2011;66:605–613. doi: 10.1515/znc-2011-11-1210. [DOI] [PubMed] [Google Scholar]
- Xu H., Shen D., Wu X.Q., Liu Z.W., Yang Q.H. Characterization of a mutant glucose isomerase from Thermoanaerobacterium saccharolyticum. J. Ind. Microbiol. Biotechnol. 2014;41:1581–1589. doi: 10.1007/s10295-014-1478-4. [DOI] [PubMed] [Google Scholar]
- Yassien M. Optimization of glucose isomerase production by Streptomyces albaduncus. Afric. J. Microbiol. Res. 2012;6 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.