Abstract
The dysregulation of hepatic lipid metabolism is one of the hallmarks in many liver diseases including alcoholic liver diseases (ALD) and non-alcoholic fatty liver diseases (NAFLD). Hepatic inflammation, lipoperoxidative stress as well as the imbalance between lipid availability and lipid disposal, are direct causes of liver steatosis. The application of herbal medicines with anti-oxidative stress and lipid-balancing properties has been extensively attempted as pharmaceutical intervention for liver disorders in experimental and clinical studies. Although the molecular mechanisms underlying their hepatoprotective effects warrant further exploration, increasing evidence demonstrated that many herbal medicines are involved in regulating lipid accumulation processes including hepatic lipolytic and lipogenic pathways, such as mitochondrial and peroxisomal β-oxidation, the secretion of very low density lipoprotein (VLDL), the non-esterified fatty acid (NEFA) uptake, and some vital hepatic lipogenic enzymes. Therefore, in this review, the pathways or crucial mediators participated in the dysregulation of hepatic lipid metabolism are systematically summarized, followed by the current evidences and advances in the positive impacts of herbal medicines and natural products on the lipid metabolism pathways are detailed. Furthermore, several herbal formulas, herbs or herbal derivatives, such as Erchen Dection, Danshen, resveratrol, and berberine, which have been extensively studied for their promising potential in mediating lipid metabolism, are particularly highlighted in this review.
Keywords: herbal medicines, natural products, lipid metabolism, fatty liver, lipolysis, lipogenesis
Introduction
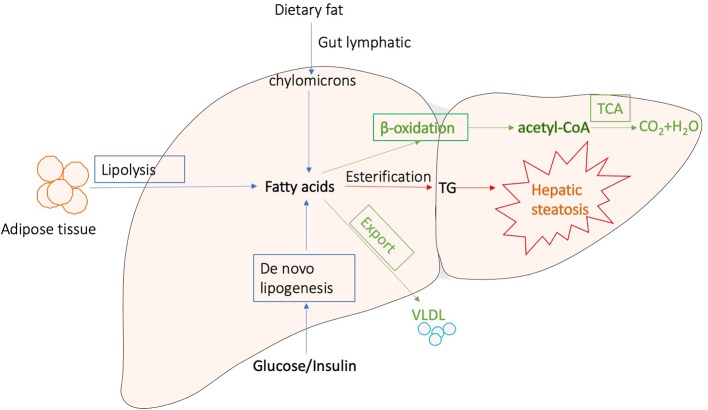
Generally, liver regulates lipid metabolism by three major processes: (1) uptake free fatty acids from circulation, and de novo fatty acid synthesis (FAS); (2) lipid storage, including converting fatty acids into triglyceride (TG) and other lipid droplets, which are subsequently exported to adipose tissue or stored in liver; and (3) lipid consumption, including lipolysis, β-oxidation, and the generation of lipoproteins (Reddy and Rao, 2006; Musso et al., 2009; Ponziani et al., 2015; Mato et al., 2019). These processes are presented in Figure 1. Correct control of lipid level is critical for cellular and organismal homeostasis, while interferences with the lipogenic pathways are accompanied with a variety of metabolic syndromes. The disorders of lipid metabolism, such as decreased β‐oxidation, enhanced lipolysis, and secretion of very low-density lipoprotein (VLDL), as well as altered pathways involved in the FAS, drive the accumulation of lipid droplets into the hepatocytes, eventually leading to the development of hepatic steatosis, which is a common pathological feature in various liver diseases (Reddy and Rao, 2006; Nguyen et al., 2008; Tessari et al., 2009; Perla et al., 2017).
Figure 1.
Major processes involved in hepatic lipid metabolism.
The most prevalent liver diseases resulting from lipid metabolism disorder are alcoholic and non-alcoholic fatty liver diseases. Except difference in alcohol consumption, alcoholic and non-alcoholic fatty liver diseases show similar pathological process, which is characterized by long-term excessive fat accumulation in the liver (Younossi, 2019). They represent a wide range of liver injury, from simple fatty liver through steatosis with necrosis and inflammation to fibrosis and cirrhosis (Lomonaco et al., 2013; Heeboll et al., 2018). In particular, non-alcoholic fatty liver diseases (NAFLD), as the metabolic diseases induced by obesity and type 2 diabetes mellitus, are the second leading causes of death globally, becoming a heavy economic burden in many countries due to the high prevalence (Albhaisi and Sanyal, 2018; Al-Dayyat et al., 2018). Since inordinate lipid metabolism is intensively involved in fatty liver diseases progression, reducing lipid accumulation is a major target of development of pharmaceutical agents for various liver diseases (Ipsen et al., 2018). Simvastatin has been used as lipid-lowing drug in patients with hyperlipidemia (Aronow, 2006). However, it shows side effects, such as constipation headaches, nausea, myopathy, elevated blood sugar, and even liver damage. As a matter of fact, there is currently no satisfying therapeutic drug for fatty liver diseases (Issa et al., 2018; Moctezuma-Velazquez, 2018).
Over the past decades, due to the positive efficacy and minimal side effects, herbal medicines, and natural products have obtained increasing attention as alternative therapeutic agents for liver disorders and dyslipidemia (Xiao et al., 2013; Yao et al., 2016; Liu Q. et al., 2017). Growing evidence from preclinical studies suggests that many herbs and isolated compounds could inhibit the progression of hepatic steatosis (Dong et al., 2012; Liu Z. L. et al., 2013). A variety of mechanisms have been demonstrated to be implicated in preventing hepatic steatosis, including reducing lipogenesis, enhancing β-oxidation, increasing insulin sensitivity, suppressing oxidative stress, and inhibiting activation of inflammatory pathways (Dong et al., 2012; Yao et al., 2016). In recent studies, sterol regulatory elementbinding protein 1c (SREBP-1c), peroxisome proliferator activated receptor α (PPARα), AMP-activated protein kinase (AMPK) and sirtuin 1 (SIRT1) signaling pathways have been highlighted as crucial molecular targets of action mechanisms by which herbal medicines regulate hepatic lipid metabolism (Liu Z. L. et al., 2013). In this review, herbal medicines involved in regulating hepatic lipolytic and lipogenic pathways, such as mitochondrial and peroxisomal β-oxidation, the secretion of very low-density lipoprotein (VLDL), the non-esterified fatty acid (NEFA) uptake, and some vital hepatic lipogenic enzymes are summarized. Current clinical evidences and meta-analysis in the positive impacts of herbal medicines on the hepatic lipid metabolism pathways have also been reviewed. Furthermore, several herbal formulae, herbs or herbal derivatives, such as Erchen Dection, Danshen, resveratrol, and berberine which have been extensively studied for their promising potential in mediating lipid metabolism, are particularly highlighted in this review. This review aims to update and summarize current evidence from laboratory and clinic studies to provide alternative and complementary medical therapies with the regulatory property of hepatic lipid metabolism to current pharmaceuticals for the treatment of liver diseases.
Herbal Medicines and Natural Products Regulate on the Hepatic Lipid Metabolism Pathways
Increasing evidence indicated that many herbs, natural products, and their derived compounds could inhibit the progression of hepatic steatosis. A variety of mechanisms have been demonstrated to be implicated in preventing hepatic steatosis and modulating lipid metabolism by herbs, including anti-oxidative stress, anti-inflammation, reducing hepatocyte fatty acid uptake and trafficking, reducing hepatic de novo lipogenesis, increasing lipolysis, induction of lipophagy, enhancing fatty acid β-oxidation. In particular, SREBP-1c, PPARα, AMPK, and SIRT1 signaling pathways have been highlighted as crucial molecular targets of action mechanisms by which herbal medicines regulate hepatic lipid metabolism. In Table 1, we reviewed the effects and mechanisms of herbs and some natural products on fatty liver diseases from recent studies. In the following section, we will discuss herbs that attenuate hepatic steatosis via reducing hepatocyte fatty acid uptake and trafficking, reducing hepatic de novo lipogenesis, increasing lipolysis, induction of lipophagy, and enhancing fatty acid β-oxidation in detail.
Table 1.
The effects and mechanisms of herbs and some natural products on fatty liver diseases.
| Herbs or Natural products | Model | Effects | Mechanisms | References |
|---|---|---|---|---|
| Rosmarinus officinalis Linn. | Orotic acid induced NAFLD model in rats | Reduced the levels of hepatic TG, TC, FFA and improved cell hypertrophy, vacuolation, and cell necrosis in the liver | ↑Phosphorylation of AMPK and ↓SREBP-1c cracking into the nucleus, following ↓FAS | (Wang et al., 2019) |
| Chinese Herbal Formula (CHF03, composition confidentiality) | HFD induced NAFLD model in mice; AML12 cells treated with palmitic acid in vitro | Reduced hepatic steatosis | ↓lipogenesis via down-regulating the expression of SREBF1, Fasn, and Acaca, ↓ lipid accumulation | (Cui et al., 2019) |
| Dachaihu Decoction (Bupleuri Radix, Scutellaria baicalensis Georgi, Pinellia ternate, Paeonia lactiflora, Citrus trifoliata, Rheum rhabarbarum, Zingiber officinale, Ziziphus jujuba Mill) | High-fat high-fructose diet induced NAFLD model in rats | Reduced the levels of elevated liver coefficient, serum TG, TC, LDL, AST, and ALT, blood glucose, plasma endotoxin, reduced TG, TNF-α, TGF-β, NF-κB, and TLR4 in liver tissues | ↓oxidative stress and inflammation | (Yang J.M. et al., 2019) |
| Leaves of Aloysia citrodora Paláu (syn. Lippia triphylla) | KK‐Ay mice | Improved hepatic lipid metabolism | via activating AMPK | (Zhang Y. et al., 2019) |
| Polygonatum kingianum | HFD induced NAFLD model in rats | ↓ALT, AST, TC, LDL in serum, and hepatic TC and TG | ↑mRNA expression of carnitine palmitoyl transferase-1 and ↓uncoupling protein-2 respectively, ↓caspase 9, caspase 3 and Bax expression in hepatocytes, ↑expression of Bcl-2 in hepatocytes and cytchrome c in mitochondria | (Yang X. X. et al., 2019) |
| Bangpungtongseong-san (Bofutsushosan) | HFD induced NAFLD model in C57BL/6J mice | Ameliorated dyslipidemia and hepatic steatosis, reduced body weight gain | Altered transcriptional changes in the liver, ↓mitochondrial oxidative phosphorylation-related genes in the liver, ↓hepatic fibrosis-related transcriptome. | (Choi et al., 2019) |
| Thymbra spicata L. extracts | endothelial cells in vitro | Ameliorated lipid accumulation, oxidative stress and inflammation, reduced hepatic steatosis | Preventing endothelium dysfunction | (Khalil et al., 2019) |
| Swertiamarin | fructose-fed mice | Lowed levels of serum glucose, TG, uric acid, ALT, AST, alleviation of hepatic ballooning degeneration and steatosis | ↓SREBP-1, FAS and acetyl-CoA carboxylase 1 (ACC1) in liver | (Yang Y. et al., 2019) |
| Si He Decoction (Zingiber officinale., Cyperus rotundus L., Lilium, Lindera aggregate, Salvia miltiorrhiza, Santalum album, Amomum villosum, Typha angustifolia L., Trogopterus xanthipes Milne) | HFD induced NAFLD model in rats | Improved liver pathological conditions | ↓expression level of TNF-alpha and IL-6, ↑visfatin, adiponectin, leptin and resistin, targeting adipokines | (Sun et al., 2019) |
| Modified Longdan Xiegan Tang (composed of Scutellaria baicalnsis Geprgi, Gardenia jasminoides, Adenophora capillaris, Akebia quinate, Plantago asiatica, Angelica sinensis, Rehmannia glutinosa, Alisma plantago-aquatica, Bupleurum gibraltaicum, and Glycyrrhiza uralensis) | Olanzapine-induced fatty liver in rats | ↓TG, cell vacuolar degeneration and Oil Red O-stained area | Regulating hepatic de novo lipogenesis and fatty acid β-oxidation-associated Gene expression mediated by SREBP-1c, PPAR-α and AMPK-α | (Ren et al., 2019) |
| LongShengZhi Capsule | apoE-Deficient Mice | Reduced atherosclerosis | ↓lipogenic and cholesterol synthetic genes while activating expression of triglyceride catabolism genes | (Ma et al., 2019) |
| Thymoquinone | Hypothyroidism with NAFLD rats | Reduced steatosis and lobular inflammation | ↑antioxidant CAT gene | (Ayuob et al., 2019) |
| Monomer Hairy Calycosin | NAFLD rats | Control the lipid peroxidation, and reduce the levels of serum TNF-alpha, IL-6, MDA and FFA, improve the steatosis and inflammation of liver tissue | ↓CYP2E1, ↓apoptosis of hepatocytes. | (Liu X. et al., 2019) |
| Hongqi Jiangzhi Formula (Astragali Radix, Red yeast rice, Nelumbinis Folium, Curcumae Longae Rhizoma, Lych Fructus, Magnoliae Officinals Cortex, Artemisiae Scopariae Herba) | HFD induced NAFLD model in rats | Reduced lipid accumulation | ↓the expression of NF-kappa B through TLR4 downstream signalling pathways | (Liang et al., 2019) |
| Jiang Zhi Granule (Herba Gynostemmatis, Folium Nelumbinis, Radix Salviae, Rhizoma Polygoni Cuspidati, and Herba Artemisiae Scopariae) | NAFLD in animal and PA-treated hepatocytes in vitro | Showed anti-steatotic effects | droplet degradation via autophagy though the mTOR signalling | (Zheng et al., 2018) |
| Curcumin | Steatotic hepatocyte model in vitro and NAFLD rat models | Improved lipid accumulation | Reversed the DNA methylation at the PPAR-alpha gene | (Li Y. Y. et al., 2018) |
| Samjunghwan Herbal Formula (Mori Fructus, Lycium chinensis Miller, Atractylodis Rhizoma) | HepG2 Cells and OLETF Rats | ↓Body weights, and visceral adipose tissue (VAT) weights, AST and ALT levels, | ↑HMGCOR, SREBP, and ACC, and ↓AMPK and LDLR gene expressions levels. | (Ansari et al., 2018) |
| Oxyresveratrol | NAFLD in mice | Ameliorated NAFLD | ↓LXR alpha agonists-mediated SREBP-1c induction and expression of the lipogenic genes, ↑mRNA of fatty acid beta-oxidation-related genes in hepatocytes; induced AMPK activation, helped inhibit SREBP-1c using compound C. | (Lee et al., 2018) |
| Sedum sarmentosum Bunge extract | Tilapia fatty liver model | Restored the changes to feed coefficient, immune capacity, and pathological characters | Altered expression of genes in the lipid metabolic process, metabolic process, and oxidation-reduction process. Our results suggest that disorders of the PPAR and p53 signaling pathways | (Huang et al., 2018) |
| Berberine and curcumin | HFD induced NAFLD model in rats | ↓LDL-c, ALT, AST, ALP, MDA, LSP | ↓SREBP-1c, pERK, TNF-alpha, and pJNK | (Feng et al., 2018) |
| Gegen Qinlian decoction (Pueraria lacei Craib, Scutellaria baicalensis Georgi, Coptis chinensis Franch., and Glycyrrhiza uralensis Fisch.) and resveratrol | Rat model of HFD-induced NAFLD | Restored lipid metabolism and inflammatory and histological abnormalities | Triggering the Sirt1 pathway | (Guo et al., 2017) |
| Gegenqinlian Decoction | Rat model of HFD-induced NAFLD and HepG2 | Suppress inflammation and regulate lipid | Improving PPAR-γ | (Wang Y. L. et al., 2015) |
| Lingguizhugan Decoction (Poria, Ramulus Cinnamomi, Rhizoma Atractylodis Macrocephalae, and Radix Glycyrrhizae) | Rat model of HFD-induced NAFLD | Attenuated phenotypic characteristics of NAFLD | By affecting insulin resistance and lipid metabolism related pathways (e.g., PI3K-Akt, AMPK); activating cholesterol secretio; increasing serum thyroid hormone levels, improving beta-oxidation (via modulation of TR beta 1 and CPT1A expression), metabolism and transport (through modulation of SREBP-1c, ACSL and ApoB100 expression) of fatty acid. | (Liu X. et al., 2017; Yang et al., 2017; Zhu et al., 2017) |
| Chinese herb extract, QSHX (Bupleurum falcatum, Salvia miltiorrhiza, rhubarb, lotus leaf, capillary Artemisia, rhizome polygoni cuspidate and gynostemma pentaphyllum) | High-fat and high-sugar diet-induced NAFLD in rat | ↓Body weight, liver index, and serum levels of AST, ALT and TG; and increased the serum level of adiponectin | Promoting the expression of HMW APN and DsbA-L, which may have been induced by inhibiting the activation and expression of FOXO1 in adipocytes | (Liu X. et al., 2017) |
| Qushi Huayu Decoction (Herba Artemisiae capillaris, Polygonum cuspidatum, Hypericum japonicum Thunb, Gardenia, and Rhizoma Curcumae Longae) | NAFLD rats | Attenuated phenotypic characteristics of NAFLD | ↑Hepatic anti-oxidative mechanism, ↓hepatic lipid synthesis, and promoted the regulatory T cell inducing microbiota in the gut. | (Feng et al., 2017) |
| Rhododendron oldhamii Maxim. leaf extract | HepG2 cells and HFD-fed mice | Improves fatty liver syndrome | Increasing lipid oxidation and decreasing the lipogenesis pathway | (Liu Y. L. et al., 2017) |
| Herbal Formula HT048 (Crataegus pinnatifida leaf and Citrus unshiu peel extracts.) | HFD-fed rats | Attenuates Diet-Induced Obesity | ↓Genes involved in lipogenesis, gluconeogenesis, and adipogenesis, ↑β–oxidation genes | (Lee Y. H. et al., 2016) |
| Angelica dahurica (Hoffm.) Benth. & Hook.f. ex Franch. & Sav. | HFD-induced hyperlipidemic mice | ↓TC and TG in the livers | ↓CAT and sterol carrier protein2 (SCP2), ↑ the expression of lipid metabolism related genes-lipase member C (LIPC) and PPAR-γ | (Lu et al., 2016) |
| Daisaikoto (Bupleuri Radix, Scutellaria baicalensis Georgi, Pinellia ternate, Paeonia lactiflora, Citrus trifoliata, Rheum rhabarbarum, Zingiber officinale, Ziziphus jujuba Mill) | Diabetic fatty liver rats induced by a high-fat diet and streptozotocin (STZ) | Reversing dyslipidemia and insulin resistance | Regulating expressions of SIRT1 and NF-κB | (Qian et al., 2016) |
| Herb Formula KIOM2012H (Arctium lappa Linne, Glycyrrhiza uralensis Fischer, Magnolia officinalis Rehder & Wilson, Zingiber officinale Roscoe) | HFD-fed mice | Inhibited lipid accumulation | Gene expressions involved in lipogenesis and related regulators | (Park et al., 2015) |
| Hawthorn (Crataegus) leaf flavonoids | HFD-fed rats | Alleviated NAFLD | Enhancing the adiponectin/AMPK pathway | (Li et al., 2015) |
| Herbal SGR Formula (Semen Hoveniae extract, Ginkgo biloba extract, and Rosa roxburghii Tratt extract) | Acute ethanol-induced liver steatosis in mice | Inhibited acute ethanol-induced liver steatosis, ↓serum and hepatic TG level, and improved classic histopathological changes | ↓Protein expression of hepatic SREBP-1c and TNF-α and increased adiponectin, PPAR-α, and AMPK phosphorylation in the liver | (Qiu et al., 2015) |
| Nitraria retusa (Forssk.) Asch. ethanolic extract | db/db mice model | ↓Increases in body and fat mass weight, ↓TG and LDL-c levels | ↑Gene expression related to lipid homeostasis in liver, modulating the lipolysis-lipogenesis balance | (Zar Kalai et al., 2014) |
| 14-Deoxyandrographolide | Ethanol-induced hepatosteatosis in rats | Alleviate hepatosteatosis | ↑AMPK, ↓SREBP-1c, ACC, and FAS, ↑sirtuin I and depletion of malonyl-CoA, ↑fatty acid oxidation | (Mandal et al., 2014) |
| Total Alkaloids in Rubus aleaefolius Poir | Modified HFD-fed rats | ↓TG, TC, and LDL-C levels and ↑HDL-C level | ↓Expression of FAS, ACC, ↑carnitine palmitoyltransferase (CPT) | (Li Y. et al., 2014) |
| Lycium barbarum L. polysaccharide | HFD-fed mice | Improved body compositions and lipid metabolic profiles, ↓hepatic intracellular TG | ↓SREBP-1c, ↑AMPK activation | (Li W. et al., 2014) |
| Salacia oblonga Wall. ex Wight & Arn. root | fructose-induced fatty liver in rats | Diminished fructose-induced fatty liver | ↓SREBP-1/1c mRNA and nuclear protein | (Liu L. et al., 2013) |
| Chunggan extract (Artemisia capillaries Thunberg, Trionyx sinensis Wiegmann, Raphanus sativus Linne, tractylodes macrocephala Koidz, Poria cocos Wolf, Alisma orientalis (Sam.) Juzepczuk, Atractylodes chinensis Koidzumi, Salvia miltiorrhiza Bunge, Polyporus umbellatus Fries, Poncirus trifoliate Rafin, Amomum villosum Lour, Glycyrrhiza uralensis Fisch., Aucklandia lappa Decne.) | methionine- and choline-deficient (MCD) diet | ↓TG, AST, ALT, ALP, and total bilirubin | Anti-oxidative stress | (Park et al., 2013) |
| Celastrus orbiculatus Thunb. | HFD-induced NAFLD in guinea pigs | ↓TC, free cholesterol (FC), cholesterol ester (CE) and TG in liver | ↑mRNA abundance of cholesterol 7 alpha-hydroxylase A1 (CYP7A1) and 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR). | (Zhang et al., 2013) |
| Oxymatrine | NAFLD rats fed with high fructose diet | ↓Body weight gain, liver weight, liver index, dyslipidemia, and TG, ↓liver lipid accumulation. | ↓ SREBF1 and ↑PPAR-α | (Shi et al., 2013) |
| Rhein | HFD-induced obese mice | ↓Body weight, particularly body fat content, improved insulin resistance, and ↓circulating cholesterol levels, ↓TG, reversed hepatic steatosis, and normalized ALT | Mediated negative energy balance, metabolic regulatory pathways, and immunomodulatory activities involved in hepatic steatosis | (Sheng et al., 2011) |
| Osthol | Alcohol-induced fatty liver in mice | Inhibit alcohol-induced fatty liver | Anti-oxidation and suppression of TNF-α production | (Sun et al., 2009) |
↑ means increase and up-regulate and ↓ means decrease and down-regulate.
Reducing Hepatocyte Fatty Acid Uptake and Trafficking
Nonesterified fatty acids (NEFAs) and glycerol are generated and released from adipose tissue via lipolysis (Kawano and Cohen, 2013). Then NEFAs enter into hepatocytes principally through CD36, and fatty acid transports (FATPs)(Kawano and Cohen, 2013). Several mediators have been demonstrated to play a role in regulating CD36 and FATPs, such as pregnane X receptor (PXR), which impact the hepatocyte fatty acid uptake. Increasing evidence has shown that a variety of herbs and natural compounds attenuate hepatic steatosis via modulating genes for fatty acid uptake.
Scutellarin, one of the Traditional Chinese Medicines (TCM) used for liver diseases and diabetes, was found to reduce insulin-dependent lipid accumulation and the mRNA expression of CD36 in HepG2 cells-treated with palmitic acid (Luan et al., 2019). Several other TCM and isolated compounds, babaodan, licorice extract, polyphenol-enriched fraction from Herba Erigerontis, and magnesium lithospermate B, reduced hepatic CD36 expression in mice fed with High Fat Diet (HFD) (Wu and Wang, 2012; Wang et al., 2016; Sheng et al., 2019). Dansameum reduced the expression level of CD36 in liver of apolipoprotein E-Knockout mice with NAFLD (Ahn et al., 2019). In another mice model of NAFLD induced by high-fat and high-cholesterol diet, gypenosides which are a type of TCM extracted from plants downregulated CD36 level in the liver, alleviating the progression of hepatic steatosis (Huang et al., 2019). Berberine attenuated fat accumulation in the liver partially via suppressing the expression of FATP gene in HFD-fed mice (Zhou et al., 2019).
Reducing Hepatic De Novo Lipogenesis
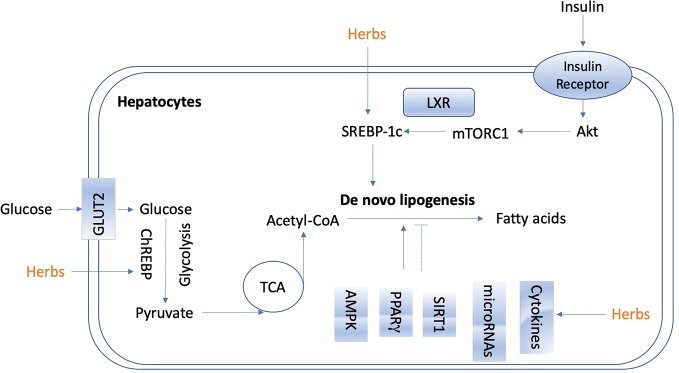
De novo lipogenesis in the liver is tightly controlled by metabolic hormones such as insulin, and glucose level (Wang Y. et al., 2015). In the normal physiological status, high level of glucose promotes the secretion of insulin, activates carbohydrate-responsive element-binding protein (ChREBP), and meanwhile, provides substrate to facilitate lipogenesis in the liver (Wang Y. et al., 2015). In terms of insulin, it activates sterol regulatory element-binding protein 1c (SREBP-1c) to up-regulate lipogenic enzymes, and then promotes de novo lipogenesis (Eissing et al., 2013; Chao et al., 2019). Figure 2 shows the overview of lipogenesis in hepatocytes. Herbs and isolated natural compounds have been demonstrated by animal studies and in vitro studies to alleviate hepatic steatosis by ChREBP pathway and insulin-SREBP-1c pathway, as well as other factors, such as AMPK, PPARγ, SIRT1, inflammatory cytokines, immuno-modulation, and microRNAs. We summarized medicinal herbs and isolated natural compounds from recent literatures with the effects of reducing hepatic lipogenesis in Table 2 and discussed some representative studies in detail as following.
Figure 2.
The overview of lipogenesis in hepatocytes.
Table 2.
Medicinal herbs and isolated natural compounds with the effect of hepatic lipogenesis reduction.
| Herbs or compounds | Model | Effect | Mechanism | References |
|---|---|---|---|---|
| Dansameum (Salvia miltiorrhiza root) | Apolipoprotein E-Knockout mice | Reduced hepatic lipogenesis and inflammation | Regulating LXR-α, PPAR-γ, SREBP-1, FAS, ACC1, and CD36 | (Ahn et al., 2019) |
| Alisol A | HFD-induced obese mice | Reduced hepatic steatosis and improved liver function | AMPK/ACC/SREBP-1c pathway | (Ho et al., 2019) |
| Ling-gui-zhu-gan decoction (Poria cocos, Ramulus cinnamomi, Atractylodis macrocephalae Rhizoma and Radix glycyrrhizae ) | HFD-fed rats | Reduced hepatic glycogen | Inhibited the activity of ACC, SREBP-1c and HMGCR, via inhibiting PPP1R3C targeting pathways | (Dang et al., 2019) |
| Salvianolic acids | Ovariectomized rats | Reduced body weight gain and attenuated | Blocking STAT-3/SREBP1 signaling | (Dang et al., 2019) |
| Gyeongshingangjeehwan 18 (Laminaria japonica, Rheum palmatum, and Ephedra sinica) | HFD-induced obese mice | Attenuated visceral obesity and NAFLD | Down-regulated lipogenesis-related genes | (Lim et al., 2018) |
| Cordycepin | Oleic acid-induced mouse FL83B hepatocytes | Attenuated lipid accumulation | Activating AMPK and regulating mitochondrial function | (Uen et al., 2018) |
| Oxyresveratrol | HFD-fed mice | Ameliorated NAFLD | AMPK/SREBP-1c pathway | (Lee et al., 2018) |
| Berberine | MIHA and HepG2 cells | Reduced hepatosteatosis | Up-regulation of miR-373 decreased mRNA level target gene AKT1, leading to inhibition of AKT-mTOR-S6K signaling pathway in hepatocytes | (Li C. H. et al., 2018) |
| Genipin | HFD-fed mice | Reduced HFD-induced hyperlipidemia and hepatic lipid accumulation | Increased the expression levels of miR-142a-5p, which bound to 3 untranslated region of SREBP-1c | (Zhong et al., 2018) |
| Gangjihwan (Ephedra intermedia Schrenk & C.A.Mey., Lithospermum erythrorhizon Siebold & Zucc., and Rheum palmatum L.) | HFD-induced obese mice | Inhibited fat accumulation | Modulation of lipogenic transcription factors SREBP-1c, PPAR-γand ChREBP-α | (Jang et al., 2018) |
| Gangjihwan (Ephedra intermedia Schrenk & C.A.Mey., Lithospermum erythrorhizon Siebold & Zucc., and Rheum palmatum L.) | HFD-fed C57BL/6 J mice and HepG2 cells | Anti-obesity and anti-nonalcoholic steatohepatosis | Increased mRNA levels of fatty acid oxidation genes and decreased mRNA levels of genes for lipogenesis | (Roh et al., 2017) |
| Dangguiliuhuang Decoction (root of Rehmannia Glutinosa, Angelica acutiloba Siebold et Zucc., Coptis chinensis Franch., Radix Rehmanniae Praeparata, Astragalus propinquus, Scutellaria baicalensis, and Phellodendron chinense Schneid.) | ob/ob mice | Normalized glucose and insulin level, increased the expression of adiponectin, diminished fat accumulation and lipogenesis, and promoted glucose uptake | ↓T cells, ↑ Tregs differentiation, ↓DCs maturation, ↓ DCs-stimulated T cells proliferation and secretion of IL-12p70 cytokine, promoted the interaction of DCs with Tregs, changed PI3K/Akt signaling pathway and↑ PPAR-γ. | (Cao et al., 2017) |
| Glycycoumarin | MCD diet mice | Prevented hepatic steatosis | Activation of AMPK signaling pathway | (Zhang et al., 2016) |
| Gambigyeongsinhwan (Curcuma longa, Alnus japonica, and Massa Medicata Fermentata) | Otsuka Long-Evans Tokushima fatty rats and HepG2 cells | Suppressed hepatic steatosis and obesity-related hepatic inflammation | ↓mRNA levels of FAS, ACC1, ChREBP alpha, and SREBP-1c | (Yoon et al., 2017) |
| Alisol B 23-acetate | MCD diet-fed mice | ↓ALT, AST, TG | FXR-dependent, ↓hepatic lipogenesis through decreasing hepatic levels of SREBP-1c, FAS, ACC1 and SCD1 and ↑lipid metabolism via inducing PPAR α, CPT1 α, ACADS and LPL | (Meng et al., 2017) |
| Herbal Formula HT048 (Crataegus pinnatifida leaf and Citrus unshiu peel extracts) | HFD-fed obese rats | Decreased obesity and insulin resistance | ↓Genes involved in lipogenesis | (Lee Y. H. et al., 2016) |
| Jatrorrhizine hydrochloride | HFD-induced obesity mouse model | Attenuated hyperlipidemia | ↓ SREBP-1c and FAS, and induced PPAR- and CPT1A | (Yang et al., 2016) |
| Puerarin | Oleic acid (OA)-treated HepG2 cells | Ameliorated hepatic steatosis | ↑PPAR-α and AMPK signaling pathways, ↓SREBP-1 and FAS expression | (Kang et al., 2015) |
| Protopanaxatriol | HFD-induced obesity (DIO) mice | Alleviated steatosis | Inhibition of PPAR-γ activity | (Zhang et al., 2014) |
| Magnolia officinalis Rehder & E.H.Wilson | HepG2 cells and mouse normal FL83B hepatocytes | Attenuated TG biosynthesis | Inhibition of SREBP-1c via AMPK phosphorylation | (Seo et al., 2014) |
| Lycium barbarum polysaccharide | HFD-fed mice | Attenuate liver steatosis | ↓SREBP-1c expression via AMPK activation | (Li W. et al., 2014) |
| Houttuynia cordata Thunb. | HepG2 | Attenuates Lipid Accumulation | AMPK signaling | (Kang and Koppula, 2014) |
| Berberine metabolites | HepG2 | TG-lowering effects | ↓Lipogenesis gene expressions through activation of the AMPK signaling pathway | (Cao et al., 2013) |
| 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea Salicornia europaea L. | HepG2 | Attenuated high glucose-induced hepatic lipogenesis | Prevented lipid accumulation by blocking the expression of SREBP-1c and FAS through LKB1/SIRT1 and AMPK activation | (Pil Hwang et al., 2013) |
| Fructus Xanthii (Xanthium strumarium) | HFD-fed rats | Attenuated hepatic steatosis | ↓The expression of lipogenic genes | (Li et al., 2013) |
↑ means increase and up-regulate and ↓ means decrease and down-regulate.
Magnolia officinalis Rehder & E.H.Wilson, Houttuynia cordata Thunb., 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia europaea L., puerarin and four kinds metabolites of berberine attenuated lipid accumulation in HepG2 cells in vitro via down-regulation of lipogenesis gene expressions through activation of the AMPK signaling pathway (Cao et al., 2013) (Pil Hwang et al., 2013; Kang and Koppula, 2014). Gyeongshingangjeehwan 18 (an herbal drug composed of Laminaria japonica, Rheum palmatum, and Ephedra sinica), Herbal Formula HT048 (Citrus unshiu and Crataegus pinnatifida), Fructus Xanthii (Xanthium sibiricum Patr.), Lycium barbarum polysaccharide, Jatrorrhizine hydrochloride, oxyresveratrol, and alisol A isolated from Rhizoma alismatis (Oriental Waterplantain Tuber.) attenuated liver steatosis in HFD-fed animals via regulating lipogenic genes, predominantly relating with downregulation of SREBP-1c expression via AMPK activation (Li et al., 2013; Li W. et al., 2014; Lee Y. H. et al., 2016; Yang et al., 2016; Lee et al., 2018; Lim et al., 2018; Ho et al., 2019). Gangjihwan, a polyherbal composition of Ephedra intermedia Schrenk & C.A.Mey., Lithospermum erythrorhizon Siebold & Zucc., and Rheum palmatum L., showed anti-obesity and anti-nonalcoholic steatohepatosis effects in HFD-fed mice. Lipogenic transcription factors, SREBP-1c, PPAR-γ, and ChREBP alpha were involved in the action mechanism (Jang et al., 2018); (Roh et al., 2017). Molecular targets of FAS, ACC1, ChREBP alpha, and SREBP-1c were also found to be involved in the underlying mechanism of anti-hepatic steatosis and anti-obesity-related hepatic inflammation effect of Gambigyeongsinhwan in Otsuka Long-Evans Tokushima fatty rats and HepG2 cells (Yoon et al., 2017).
Glycycoumarin, a representative of coumarin compounds isolated from licorice, and Alisol B 23-acetate exert ability of reducing hepatic lipogenesis in methionine-choline-deficient (MCD) diet-fed mice (Meng et al., 2017; Zhang E. et al., 2019). MCD diet is a classical dietary model of non-alcoholic steato-hepatitis. With the lack of methionine and choline and high sucrose (40%) and fat (10%), impaired hepatic mitochondrial β-oxidation and very low-density lipoprotein (VLDL) synthesis are observed in mice (Ibrahim et al., 2016). Glycycoumarin activated AMPK signaling pathway to reduce lipogenesis. Alisol B 23-acetate, a natural triterpenoid derived from TCM Rhizoma alismatis (Oriental Waterplantain Tuber.), decreased hepatic lipogenesis via FXR-dependent pathway. It decreased hepatic levels of SREBP-1c, FAS, ACC1 and SCD1, and promoted lipid metabolism via inducing PPAR α, CPT1 α, ACADS, and LPL (Meng et al., 2017). In an apolipoprotein E-knockout mice model, Dansameum (Salvia miltiorrhiza root), a kind of Korean polyherbal medicine, reduced hepatic lipogenesis, and inflammation via regulating PPAR-γ, SREBP-1c, FAS, ACC1, and CD36 (Ahn et al., 2019).
Dangguiliuhuang Decoction, a TCM formula composed of radix rehmanniae (root of Rehmannia Glutinosa), angelica (Angelica acutiloba Siebold et Zucc.), Coptis chinensis Franch., Radix Rehmanniae Praeparata (Rehmannia root), Astragalus propinquus (the root of astragalus membranaceus), Chinese skullcap (Scutellaria baicalensis) and Phellodendron amurense (Phellodendron chinense Schneid.), is used for the treatment of autoimmune diseases and diabetes (Cao et al., 2017; Cao et al., 2018). In a study of ob/ob mice model, it normalized glucose and insulin level, diminished fat accumulation and lipogenesis, increased the expression of adiponectin, and promoted glucose uptake (Cao et al., 2017). It showed modulation abilities on inflammation and immune response. Dangguiliuhuang Decoction (composition as listed above) promoted the shift of pro-inflammatory to anti-inflammatory cytokines. Furthermore, it decreased T cells proliferation while increased regulatory T cells (Tregs) differentiation, reduced dendritic cells (DCs) maturation and secretion of IL-12p70 cytokine, decreased DCs-stimulated T cells proliferation, and promoted, the interaction of DCs with Tregs. In adipocytes and hepatocytes as well as DCs and T cells, Dangguiliuhuang Decoction treatment altered PI3K/Akt signaling pathway and increased PPAR-γ expression, indicating the ameliorated glucose and lipid metabolism (Cao et al., 2017).
MicroRNA (miR), a small non-coding RNA molecule, has been recently demonstrated to play a role in mediating the anti-hepatic steatosis effects of natural compounds derived from herbs. Berberine reduced steatosis in MIHA and HepG2 cells by mechanism associating with up-regulation of miR-373, which decreased its mRNA level target gene AKT serine/threonine kinase 1 (AKT1), resulting in the suppression of AKT-mTOR-S6K signaling pathway in hepatocytes (Cao et al., 2018). Genipin reduced HFD-induced hyperlipidemia and hepatic lipid accumulation in mice via increasing the expression levels of miR-142a-5p, which bound to 3’-untranslated region of SREBP-1c, thus leading to the inhibition of lipogenesis (Zhong et al., 2018).
Increasing Lipolysis
Lipolysis is the catabolic process of hydrolytic cleavage of ester bonds in TG, leading to the production of fatty acids and glycerol, which could be further utilized for β-oxidation and subsequent ATP generation (Lass et al., 2011). It predominantly occurs in adipose tissues, but also in the liver, with different physiological functions. Dietary fat is digested into the gut lymphatic system as chylomicrons, which arrives at the liver through the circulation and release NEFAs through lipolysis which mediated mainly by lipoprotein lipase (LPL) (Rui, 2014). Other lipolytic enzymes contributing to hepatic TG metabolism include adiponutrin/patatin-like phospholipase domain containing 3 (PNPLA3) (Kumashiro et al., 2013), lysosomal acid lipase (LAL) (Quiroga and Lehner, 2018), arylacetamide deacetylase (Lo et al., 2010), hepatic lipase (HL)(Chatterjee and Sparks, 2011) and some members of the carboxylesterase family. In adipose tissue, inhibition of lipolysis improves glucose metabolism and insulin sensitivity, whereas in liver tissue, increasing lipolysis facilitates the attenuation of hepatic steatosis.
As far from now, limited herbs were found to show regulatory effect on hepatic lipolysis. Lavatera critica (Cornish mallow), a green leafy vegetable, attenuated hepatic lipid accumulation induced by HFD via reversing lipolysis genes acetyl-CoA carboxylase (Veeramani et al., 2017). Nitraria retusa (Forssk.) Asch. ethanolic extract modulated the lipolysis-lipogenesis balance in the liver of db/db mice (Veeramani et al., 2017). Caffeic acid upregulated the phosphorylation of AMPK and its primary downstream targeting enzyme, acetyl-CoA carboxylase, to promote the lipolysis in HepG2 cells with oleic acid administration (Liao et al., 2014). Polygonatum stenophyllum (PS) Maxim. rhizome showed efficacy on menopausal obesity by activating lipolysis-related genes including hormone-sensitive lipase (HSL) and adipose triglyceride lipase (ATGL) (Lee J. E. et al., 2016). Mulberry (Fructus Mori) water extracts promoted hepatic lipolysis and protected liver from steatosis in obesity (Peng et al., 2011). More herbs or natural compounds exerted effects on lipolysis in adipose tissues and attenuated hepatic steatosis via liver-adipose tissue crosstalk, which are not going to be discussed in detail here.
Induction of Lipophagy
In addition to lipolysis, lipid breakdown can also be accessed via lipophagy, a special kind of autophagy to degrade lipid droplets (Singh and Cuervo, 2012; Kounakis et al., 2019). It is a process that the double membrane wraps lipid droplets and sends them to lysosomes to form autolysosomes for degradation of excessive lipid droplets deposited in cells (Liu and Czaja, 2013; Ward et al., 2016). It plays a vital role in maintaining the cellular steady state. During the early stage of NAFLD, lipophagy is activated in response to acute increase in lipid availability, thus reduce lipid deposition (Czaja, 2016; Ipsen et al., 2018). However, in the condition of such as long-lasting high fat dieting, hepatic lipophagy is impaired when lipids are sustained overwhelmed (Kwanten et al., 2014; Czaja, 2016; Ipsen et al., 2018). Growing evidence raised from recent studies indicate that lipophagy is partially suppressed in patients and animal models of NAFLD and restoring lipophagy may slow the progression of hepatic steatosis. Lipophagy could be activated by various approaches, such as mTOR and AMPK-targeting agents. Glycycoumarin, a representative of coumarin compounds isolated from licorice, mitigated hepatic steatosis partially through AMPK-mediated lipophagy in a murine model of NAFLD induced by MCD diet (Zhang et al., 2016). Dioscin is a saponin extracted and isolated from Polygonatum zanlanscianense Pamp. It has been proposed as a healthcare product against hepatic fibrosis with remarkable ability to inhibit the expression of p-mTOR/mTOR level and sequentially promote autophagy (Xu et al., 2017). In another study, Bergamot polyphenol fraction prevents NAFLD via stimulation of lipophagy in cafeteria dietinduced rat model of metabolic syndrome. The increased levels of LC3 and Beclin 1, and concomitant reduction of SQSTM1/p62 proved the promoted lipophagy with the treatment of Bergamot polyphenol fraction (Parafati et al., 2015). Increasing number of herbs or natural products have been demonstrated to exert significant effects on regulating lipophagy in the liver. Current understanding of mechanisms associated with autophagy/lipophagy of herbal medicines and natural products in preventing and treating NAFLD has been well reviewed in Zhang et al. (2018), which could be referred for further reading.
In alcoholic liver diseases (ALD), upon acute consumption of alcohol, lipophagy is activated in hepatocytes, serving as a defensive mechanism against injury to steatosis (Yan et al., 2019). However, it is impaired by chronic alcohol exposure, which is likely due to the activation of mTOR signaling and decreased lysosomal biogenesis in hepatocytes (Kounakis et al., 2019; Yang L. et al., 2019). There are growing number of herbs and natural products have been found to protect liver from injury induced by alcohol by mechanism of lipophagy stimulation. Corosolic acid, a compound derived from the leaves of Langertroemia speciosa L Pers., protected the liver from alcoholic-induced liver injury partially via restoring hepatic lipophagy due to mTORC1 suppression after AMPK activation (Guo et al., 2016). Another natural compound, quercitin, which is extensively found in many fruits and herbal plants, remarkably reversed the alcohol-induced blockade of TFEB nuclear localization, via restoring lysosome function and autophagic flux in livers of ethanol-fed C57BL6 mice (Li et al., 2019). Salvianolic acid A, a phenolic carboxylic acid extracted from Salvia miltiorrhiza Bunge, reduced hepatic steatosis induced by alcohol administration in rats. The action mechanism is attributed to enhanced autophagosome-lysosome fusion after restoring lysosomal cathepsin activities (Shi et al., 2018).
As a matter of fact, the field of lipophagy in liver diseases has yet to be fully developed. Its pathological role in different stages and circumstances of various liver disorders still needs to be revealed. Nevertheless, current studies concerning lipophagy have already provided new insights on lipid metabolism and energy homeostasis in the liver. It represents a promising path forward to the therapeutic of hepatic steatosis. Pharmaceutic agents including herbs, natural products or compounds targeting lipophagy in the liver deserve to be further investigated in future basic and clinic researches.
Enhancing Fatty Acid β-Oxidation
Fatty acid could be oxidized by β-oxidation, α-oxidation, omega-oxidation, and peroxisomal oxidation, among which β-oxidation is the major type occurring in the mitochondria matrix (Wanders et al., 2015). In β-oxidation, two carbon subunits from fatty acids are removed repeatedly until the fatty acid carbon chain is fully degraded to form acetyl-CoA, which is further oxidized to carbon dioxide and H2O in the tricarboxylic acid cycle (TCA) (Canbay et al., 2007). β-oxidation plays a vital role in hepatic lipid consumption. A variety of proteins and enzymes are involved in the process of mitochondrial fatty acid β-oxidation, such as plasma membrane fatty acid binding protein (FABPpm) (Furuhashi and Hotamisligil, 2008), fatty acid transport protein (FATP) (Ouali et al., 2000), carnitine acylcarnitine translocase (CACT) (Pierre et al., 2007), carnitine palmitoyltransferases 1 and 2 (CPT1/2), etc. (Bonnefont et al., 2004; Houten and Wanders, 2010). More importantly, mitochondrial fatty acid β-oxidation is regulated by both transcriptional and posttranscriptional mechanisms. Peroxisome proliferator-activated receptors (PPARs) are activated by fatty acids, having specific roles in physiology of different tissues (Yu et al., 2003; Lamichane et al., 2018). In liver, PPARα controls many genes involved in mitochondrial fatty acid β-oxidation (Lamichane et al., 2018). In terms of posttranscriptional mechanism, the inhibition of CPT1 by malonyl-CoA is a vital regulatory step. The levels of malonyl-CoA in hepatocytes are regulated via degradation induced by malonyl-CoA decarboxylase and via production by acetyl-CoA carboxylase (ACC)(Park et al., 2002). PPARs-mediated activation persuades transcription of malonyl-CoA decarboxylase, and phosphorylated AMPK inactivated ACC (Saha and Ruderman, 2003). They stimulate mitochondrial fatty acid β-oxidation by reducing malonyl-CoA levels. Additionally, peroxisome proliferator activated receptor gamma coactivator 1-alpha (PGC-1α) has also been regarded as a factor of posttranscriptional regulation of β-oxidation (Fernandez-Marcos and Auwerx, 2011). The activation of PGC-1α is mediated by AMPK via SIRT1-mediated deacetylation (Canto and Auwerx, 2009).
Many herbs and active compounds protect liver from steatosis via regulation of fatty acid β-oxidation. Herbacetin is a dietary flavonoid with plenty of pharmacological activities. Its anti-hyperglycemic and anti-hyperlipidemic properties was associated with up-regulation of CPT to enhanced β-oxidation and hepatic lipid metabolism (Veeramani et al., 2018). Acteoside, a major compound isolated from leaves of Aloysia citriodora Palau (syn. Lippia triphylla), promoted lipolysis and fatty acid oxidation by enhancing mRNA expression level of adipose triglyceride lipase (ATGL) and CPT-1, and thus improved hepatic lipid metabolism (Zhang Y. et al., 2019). Cordycepin enhanced β-oxidation and suppressed lipid accumulation via regulating AMPK pathway and mitochondrial fusion in hepatocytes (Uen et al., 2018).
In China, the modified Longdan Xiegan Tang (mLXT, composed of Scutellaria baicalnsis Geprgi, Gardenia jasminoides, Adenophora capillaris, Akebia quinate, Plantago asiatica, Angelica sinensis, Rehmannia glutinosa, Alisma plantago-aquatica, Bupleurum gibraltaicum, and Glycyrrhiza uralensis) has been used clinically for various liver diseases such as NAFLD. It was found to activate hepatic expression of PPAR α and its target genes associated with fatty acid β-oxidation (Ren et al., 2019). Babaodan, a TCM, up-regulated the expression of CPT-1 and PPAR α in liver of HFD-fed mice with NAFLD, leading to the enhanced β-oxidation (Sheng et al., 2019). Rosa rugosa Thunb., another TCM, is used for treatment of cardiovascular diseases and diabetes, hypertension, hyperlipidemia, and inflammation. R. rugosa flavonoids, the major components in R. rugosa Thunb., were observed to up-regulate the mRNA expression of PPAR α and its downstream gene of acyl-coenzyme A oxidas X (ACOX) in a mouse model of hypertriglyceridemia (Baiyisaiti et al., 2019). Thereby, R. rugosa flavonoids could reduce TG in hepatocytes via rising β-oxidation. Gynura procumbens Merr., one of precious medicinal herbs of Asterceaes, up-regulated the mRNA expression of genes involved in β-oxidation, including PPAR α, CPT1 α, ACOX, fatty acid-binding proteins 5 (FABP5), stearoyl-coenzyme A desaturase-1 (SCD-1), glycerol-3-phosphate acyltransferase (mGPAT), microsomal triglyceride transfer protein (MTTP), to increase β-oxidation and efflux of fatty acids in liver of mice fed with MCD diet, and consequently decreased hepatic lipid accumulation (Liu Y. Y. et al., 2019). An herbal formula Gyeongshingangjeehwan 18 (GGEx18), composed of Laminaria japonica Aresch (Laminariaceae), Rheum palmatum L. (Polygonaceae) and Ephedra sinica Stapf (Ephedraceae), has traditionally been described to against obesity and related metabolic disease such as dyslipidemia. In HFD-fed mice receiving GGEx18, genes related to hepatic fatty acid β-oxidation was higher compared to mice fed with only HFD (Lim et al., 2018).
Evidence from recent studies has also indicated that some natural compounds promoted fatty acid oxidation by regulating the AMPK/PGC-1α signaling pathway. Yellow pigments, monascin, and ankaflavin, as secondary metabolites derived from monascus-fermented products, could reduce fatty acid accumulation partly mediated by the AMPK signaling activation and enhancement of β-oxidation by PGC-1α (Hsu et al., 2014). Myricetin, a natural flavonol with many biological activities, decreased PGC-1α acetylation through SIRT1 activation, and thus enhanced mitochondrial activity, suggesting its potential role in regulating hepatic lipid metabolism (Jung et al., 2017).
Clinical Trials
Given to the encouraging effects of herbal medicines on liver diseases, plenty of clinical trials have been extensively performed. The potential therapeutic benefits of herbal medicines in patients with NAFLD have been reviewed in several papers in recent years (Xiao et al., 2013; Bedi et al., 2016; Perumpail et al., 2018). In present review, we focused on the efficacy of herbal medicines to mediate lipid metabolism and attenuate hepatic steatosis.
Dava Al-Balgham, as one of the traditional medicine products composed of Nigella sativa L., Pistacia lentiscus L., Zataria multiflora Boiss. (ZM), and Trachyspermum ammi, was tested for its effect on NAFLD by a randomized, double-blinded, placebo-controlled trial with 76 NAFLD patients. Placebo or Dava Al-Balgham were consumed with each meal for three months. The results showed that Dava Al-Balgham could cause weight loss and have anti-hypolipidemic effect (Hormati et al., 2019).
The effect of Z. multiflora supplementation on NAFLD was studied by a randomized double-blind placebo-controlled clinical trial. Total 85 patients with NAFLD were treated with ZM powder (700 mg) or placebo twice daily for 3 months. However, no significant difference between two ZM-treated groups and placebo groups regarding ALT, TNF-α, grade of fatty liver in ultrasonography, lipid profiles, and high sensitive C-reactive protein (hs-CRP), while it could improve insulin resistance in patients with NAFLD. Further studies with larger sample size and longer duration are recommended (Zamani et al., 2018).
A 12-weeks randomized, controlled, double-blind trial included with was 44 NAFLD patients, was performed to evaluate the efficacy of Capparis spinosa L. on disease regression of NAFLD. Patients are randomly divided into control (n=22) or caper (n=22) group. The caper group was treated with 40-50 g caper fruit pickles with meals every day. Results obtained after treatment of 12 weeks indicated that the grade of fatty liver and serum lipoproteins were improved by C. spinosa administration (Khavasi et al., 2017).
We further checked the registered clinical trials about testing effects of the herbs and natural products on fatty liver via the website of www.clinialtrial.gov. The intensively studied herbs and derived compounds are resveratrol, ginseng, and ginger, which were discussed in detail in following. Other herbs and some natural products that are undergoing or were performed clinical trials on fatty liver diseases are listed in Table 3.
Table 3.
Registered clinical trials of herbs and natural products on fatty liver diseases (Referred to http://www.ClinicalTrials.gov website).
| NCT number | Status | Conditions | Interventions | Outcome Measures | Population | Dates |
|---|---|---|---|---|---|---|
| NCT02030977 | Completed | NAFLD | Resveratrol | ALT | Enrollment: 50 Age: 18 Years to 80 Years (Adult, Older Adult) Sex: All | Study Start: June 2012 Study Completion: March 2013 |
| NCT01464801 | Completed | Fatty liver | Resveratrol |
|
Enrollment: 28 Age: 18 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: September 2011 Study Completion: June 2015 |
| NCT01446276 | Completed | Obesity
|
Resveratrol |
|
Enrollment: 26 Age: 25 Years to 65 Years (Adult, Older Adult) Sex: Male | Study Start: November 2011 Study Completion: April 2014 |
| NCT04130321 | Not yet recruiting |
|
Camu camu (Myrciaria dubia) |
|
Enrollment: 32 Age: 18 Years to 75 Years (Adult, Older Adult) Sex: All | Study Start: January 6, 2020 Study Completion: June 30, 2022 |
| NCT0394512 | Completed | Liver Dysfunction | Red ginseng | Liver enzyme | Enrollment: 94 Age: 37 Years to 63 Years (Adult) Sex: All | Study Start: January 1, 2018 Study Completion: December 31, 2018 |
| NCT03260543 | Completed | NAFLD | Fermented ginseng powder | Changes of ALT
|
Enrollment: 90 Age: 19 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: July 2016 Study Completion: August 2017 |
| NCT04049396 | Completed | NAFLD | Berberine | ALT; AST; ALP; fasting blood sugar; total cholesterol; LDL-Cholesterol; HDL - Cholesterol; TG | Enrollment: 50 Age: 18 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: October 1, 2018 Study Completion: June 15, 2019 |
| NCT02535195 | Completed |
|
Ginger |
|
Enrollment: 60 Age: 18 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: March 2013 Study Completion: August 2015 |
| NCT02289235 | Enrolling by invitation |
|
Ginger |
|
Enrollment: 90 Age: 20 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: November 1, 2018 Study Completion: December 1, 2019 |
| NCT03864783 | Recruiting |
|
Curcumin (Meriva®) |
|
Enrollment: 40 Age: 20 Years and older (Adult, Older Adult) Sex: Male | Study Start: March 5, 2019 Study Completion: October 2020 |
| NCT03073343 | Recruiting | Non-Alcoholic Fatty Liver Disease
|
Betaine |
|
Enrollment: 48 Age: 18 Years to 75 Years (Adult, Older Adult) Sex: All | Study Start: November 12, 2013 Study Completion: June 30, 2020 |
| NCT02973295 | Recruiting | NAFLD | Silymarin |
|
Enrollment: 400 Age: 18 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: September 20, 2019 Study Completion: June 30, 2021 |
| NCT02929901 | Completed | Type 2 Diabetes Nonalcoholic Fatty Liver | Caffeine and chlorogenic acid |
|
Enrollment: 200 Age: 30 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: December 2016 Study Completion: March 2019 |
| NCT02908152 | Unknown status |
|
Curcumin |
|
Enrollment: 50 Age: 30 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: February 2017 Study Completion: October 2017 |
| NCT02006498 | Completed | NAFLD | Sillymarin |
|
Enrollment: 99 Age: 18 Years and older (Adult, Older Adult) Sex: All | Study Start: June 2012 Study Completion: December 2015 |
| NCT01940263 | Completed | NAFLD | Anthocyanin |
|
Enrollment: 63 Age: 18 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: June 2013 Study Completion: June 2014 |
| NCT02307344 | Unknown status |
|
Nigella sativa L. |
|
Enrollment: 100 Age: 18 Years and older (Adult, Older Adult) Sex: All | Study Start: January 2015 Study Completion: January 2017 |
| NCT02303314 | Completed | NAFLD | Trigonella Foenum-graecum Seed Extract | Liver stiffness change | Enrollment: 35 Age: 18 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: November 2014 Study Completion: September 2017 |
| NCT01707914 | Completed | NAFLD | Chinese bayberry juice (Myrica rubra) | Plasma lipids profile | Enrollment: 44 Age: 18 Years to 25 Years (Adult) Sex: All | Study Start: June 2012 |
| NCT01677325 | Completed | NAFLD | Drug: Chinese herb (YiQiSanJu) (Angelica sinensis, Rehmannia, Cinnamomum cassia, Glycyrrhiza uralensis, Eucommia ulmoides, Achyranthes bidentate, Lycium chinense) |
|
Enrollment: 40 Age: 18 Years to 65 Years (Adult, Older Adult) Sex: Male | Study Start: January 2007 Study Completion: January 2008 |
| NCT01210989 | Completed | NAFLD | Phyllanthus urinaria L. |
|
Enrollment: 60 Age: 18 Years to 70 Years (Adult, Older Adult) Sex: All | Study Start: May 2010 Study Completion: May 2012 |
| NCT00816465 | Completed | NAFLD | Hoodia gordonii (Masson) Sweet ex Decne. |
|
Enrollment: 20 Age: 18 Years to 65 Years (Adult, Older Adult) Sex: All | Study Start: May 2009 Study Completion: August 2010 |
Resveratrol is a stilbenoid and a phytoalexin generated by several plants, such as red grapes in response to stimuli (Hasan and Bae, 2017). It is an activator of AMPK and SIRT1, and thus has a critical role in promoting fat breakdown and removal from the liver, preventing liver damage and inhibiting the progression of NAFLD (Shang et al., 2008; Charytoniuk et al., 2017; Theodotou et al., 2019). Resveratrol has been involved in three trials (NCT01446276; NCT01464801; NCT02030977) included patients of fatty liver, NAFLD, and obesity.
Another herb, ginseng, has been traditionally used for more than 2,000 years with various biological effects. A great deal of preclinical studies have demonstrated the protective effects of ginseng on liver diseases, including ALD and NAFLD. Korean Red Ginseng (Panax ginseng) (Park et al., 2017) enhanced the decreased phosphorylation of AMPK induced by ethanol consumption. Notably, it reduced the accumulation of fat in hepatocytes caused by ethanol via regulation of SREBP-1, SIRT-1 and PPAR-α (Huu Tung et al., 2012; Park et al., 2017). Clinical trial (NCT0394512) has been performed to study the effect of red ginseng on liver dysfunction. Fermented ginseng powder has also been tested to study its efficacy on NAFLD (NCT03260543).
Ginger is the root of Zingiber officinale Roscoe and is one of the most used spices in many countries (Huu Tung et al., 2012). It contains active compounds, such as shogaol, gingerol, zingerone, and β-bisabolene. It has been shown that ginger can reduce insulin resistance and serum TG level in patients with Type II diabetes and hyperlipidemia (Arablou et al., 2014). In a randomized, double-blind, placebo-controlled clinical trial with 44 patients of NAFLD, ginger supplementation significantly reduced the levels of ALT, inflammatory cytokines, γ-glutamyl transferase, as well as hepatic steatosis grade and the insulin resistance index in comparison to the control group. Another clinical trial of ginger supplement on fatty liver or Type 2 Diabetes Mellitus is still undergoing (NCT02289235).
Meta-Analysis Studies
HuoXueHuaYu (HXHY), a TCM formula, has been widely used in clinic for patients with NAFLD. Cai et al. performed a meta-analysis of randomized controlled trial of HXHY in NAFLD. There are 13 studies involving 1429 patients which 654 patients receiving conventional treatment group and 775 patients belonged to HXHY group. HXHY showed better ability on lowing TC and TG levels than that of conversational treatment. HXHY might be an effective and safe therapy for NAFLD, and trials with rigorous design, multicenter, large-scale, and high-quality worldwide are still expected (Cai et al., 2019).
Erchen Decoction (ECD), a TCM formula, is often used in the therapy of various diseases. A meta-analysis of the efficacy of ECD for the treatment of NAFLD by PRISMA systematic review standard has been performed. Seven randomized controlled trial with a total of 1951 participants were included in this study. The analysis results showed that patients with ECD treatment showed an improved status compared to the conventional treatment. Longer follow-up periods and larger-scale randomized controlled trial are still required to evaluate the efficacy of ECD in NAFLD (Li et al., 2017).
The efficiency and safety of a famous TCM Danshen in the treatment of NAFLD has also been analyzed by a meta-analysis study. Eight randomized controlled trials with 800 patients of NAFLD were identified. The results indicated that Danshen had improved total effectiveness rate, lower level of TC, TG, LDL, ALT, and AST, suggesting that Danshen may have potential effects on NAFLD, while multicenter large-sample randomized clinical trials are still expected to confirm the efficacy and safety of Danshen (Peng et al., 2016).
Another study performed by Narjes et al. on 2017 has evaluated the efficiency of all kinds of TCM on the treatment of NAFLD. Literature were searched on China National Knowledge and PubMed from 1995 to 2010. Total 5904 patients from 62 randomized controlled trials were included for meta-analysis. Results showed that TCM had a better effect on the normalization of ALT level and disappearance of radiological steatosis for the patients of NAFLD. Finally, authors concluded that TCM is of modest benefit to the therapy of NAFLD (Shi et al., 2012).
Conclusions and Perspectives
Due to the positive efficacy and minimal side effects, herbal medicines have obtained increasing attention as alternative therapeutic agents for liver disorders and dyslipidemia. Increasing evidence from laboratory studies suggests that many herbs, natural products, and derived compounds could inhibit the progression of hepatic steatosis. A variety of mechanisms have been demonstrated to be implicated in preventing hepatic steatosis and modulating lipid metabolism by herbs, including reducing hepatocyte fatty acid uptake and trafficking, reducing hepatic de novo lipogenesis, increasing lipolysis, inducing lipophagy, enhancing fatty acid β-oxidation. In particular, SREBP-1c, PPARα, AMPK, and SIRT1 signaling pathways have been highlighted as crucial molecular targets of action mechanisms by which herbal medicines regulate hepatic lipid metabolism. Current clinical evidences and meta-analysis showing the positive impacts of herbal medicines on the hepatic lipid metabolism pathways are still not strong enough. Further multicenter large-sample randomized clinical trials are still required to confirm the efficacy and safety of herbal medicines on hepatic lipid metabolism. Herbs mix and single medical plants as well as their components have been widely applied in the treatment of NAFLD. We consider the main actor should be the active components. For both herbs mix and single medical plants, they are containing many compounds, which may act synergistically in ways to enhance the therapeutic effects. Identifying the active components in herbs is a crucial and significant subject for the development of TCM. Currently, network pharmacology-based strategy has been extensively used for the prediction of the active components from herbs. Network pharmacology is an approach based on systems biology, poly-pharmacology, and molecular networks, to analyze relationships between drugs and diseases in recent decade, which has attracted considerable attention among Chinese medicine researchers for its ability in predicting and illustrating interactive relationships between numerous components and targets of herbal medicines. Network-based pharmacological analysis is a desirable approach as well as a good tool of in silico prediction for investigating the mechanisms of action for herbs and formulae and their potential bioactive components at molecular and systematic levels, which renders more effective subsequent exploration with experimental approaches. With the promising and effective prediction, subsequently validation experiments in laboratory and bench would be performed to confirm their pivotal role. In conclusion, herbal medicines have the potency to be alternative and complementary medical therapies to current pharmaceuticals for the treatment of liver diseases with lipid metabolism disorder.
Author Contributions
YF designed and conceived the study. SL and YF retrieved and analyzed the data, and drafted the manuscript. SL, YX, WG, FC, CZ, HT, and NW discussed and revised the manuscript. All authors confirmed final version of the manuscript.
Funding
This research was partially supported by the Research Council of the University of Hong Kong (project codes: 104004092 and 104004460), Wong’s donation (project code: 200006276), a donation from the Gaia Family Trust of New Zealand (project code: 200007008), the Research Grants Committee (RGC) of Hong Kong, HKSAR (project codes: 740608, 766211, 17152116, and 17121419), and Health and Medical Research Fund (project codes: 15162961, 16171511, and 16172751).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahn S. H., Lee K. P., Kim K., Choi J. Y., Park S. Y., Cheon J. H. (2019). Dansameum regulates hepatic lipogenesis and inflammation in vitro and in vivo. Food Sci. Biotechnol. 28 (5), 1543–1551. 10.1007/s10068-019-00579-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albhaisi S., Sanyal A. (2018). Recent advances in understanding and managing non-alcoholic fatty liver disease. F1000Res 7. 10.12688/f1000research.14421.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Dayyat H. M., Rayyan Y. M., Tayyem R. F. (2018). Non-alcoholic fatty liver disease and associated dietary and lifestyle risk factors. Diabetes Metab. Syndr. 12 (4), 569–575. 10.1016/j.dsx.2018.03.016 [DOI] [PubMed] [Google Scholar]
- Ansari A., Bose S., Patra J. K., Shin N. R., Lim D. W., Kim K. W., et al. (2018). A Controlled Fermented Samjunghwan Herbal Formula Ameliorates Non-alcoholic Hepatosteatosis in HepG2 Cells and OLETF Rats. Front. Pharmacol. 9, 596. 10.3389/fphar.2018.00596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arablou T., Aryaeian N., Valizadeh M., Sharifi F., Hosseini A., Djalali M. (2014). The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 65 (4), 515–520. 10.3109/09637486.2014.880671 [DOI] [PubMed] [Google Scholar]
- Aronow W. S. (2006). Management of hyperlipidemia with statins in the older patient. Clin. Interv. Aging 1 (4), 433–438. 10.2147/ciia.2006.1.4.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuob N. N., Abdel-Hamid A., Helal G. M. M., Mubarak W. A. (2019). Thymoquinone reverses nonalcoholic fatty liver disease (NAFLD) associated with experimental hypothyroidism. Rom. J. Morphol. Embryol. 60 (2), 479–486. [PubMed] [Google Scholar]
- Baiyisaiti A., Wang Y., Zhang X., Chen W., Qi R. (2019). Rosa rugosa flavonoids exhibited PPARalpha agonist-like effects on genetic severe hypertriglyceridemia of mice. J. Ethnopharmacol. 240, 111952. 10.1016/j.jep.2019.111952 [DOI] [PubMed] [Google Scholar]
- Bedi O., Bijjem K. R. V., Kumar P., Gauttam V. (2016). Herbal Induced Hepatoprotection and Hepatotoxicity: A Critical Review. Indian J. Physiol. Pharmacol. 60 (1), 6–21. [PubMed] [Google Scholar]
- Bonnefont J. P., Djouadi F., Prip-Buus C., Gobin S., Munnich A., Bastin J. (2004). Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol. Aspects Med. 25 (5-6), 495–520. 10.1016/j.mam.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Cai Y., Liang Q., Chen W., Chen M., Chen R., Zhang Y., et al. (2019). Evaluation of HuoXueHuaYu therapy for nonalcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trial. BMC Complement Altern. Med. 19 (1), 178. 10.1186/s12906-019-2596-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canbay A., Bechmann L., Gerken G. (2007). Lipid metabolism in the liver. Z. Gastroenterol. 45 (1), 35–41. 10.1055/s-2006-927368 [DOI] [PubMed] [Google Scholar]
- Canto C., Auwerx J. (2009). PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 20 (2), 98–105. 10.1097/MOL.0b013e328328d0a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S., Zhou Y., Xu P., Wang Y., Yan J., Bin W., et al. (2013). Berberine metabolites exhibit triglyceride-lowering effects via activation of AMP-activated protein kinase in Hep G2 cells. J. Ethnopharmacol. 149 (2), 576–582. 10.1016/j.jep.2013.07.025 [DOI] [PubMed] [Google Scholar]
- Cao H., Tuo L., Tuo Y., Xia Z., Fu R., Liu Y., et al. (2017). Immune and Metabolic Regulation Mechanism of Dangguiliuhuang Decoction against Insulin Resistance and Hepatic Steatosis. Front. Pharmacol. 8, 445. 10.3389/fphar.2017.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Li S., Xie R., Xu N., Qian Y., Chen H., et al. (2018). Exploring the Mechanism of Dangguiliuhuang Decoction Against Hepatic Fibrosis by Network Pharmacology and Experimental Validation. Front. Pharmacol. 9, 187. 10.3389/fphar.2018.00187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao H. W., Chao S. W., Lin H., Ku H. C., Cheng C. F. (2019). Homeostasis of Glucose and Lipid in Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 20 (2). 10.3390/ijms20020298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytoniuk T., Drygalski K., Konstantynowicz-Nowicka K., Berk K., Chabowski A. (2017). Alternative treatment methods attenuate the development of NAFLD: A review of resveratrol molecular mechanisms and clinical trials. Nutrition 34, 108–117. 10.1016/j.nut.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Chatterjee C., Sparks D. L. (2011). Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am. J. Pathol. 178 (4), 1429–1433. 10.1016/j.ajpath.2010.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J. Y., Kwon E. Y., Choi M. S. (2019). Elucidation of the Metabolic and Transcriptional Responses of an Oriental Herbal Medicine, Bangpungtongseong-san, to Nonalcoholic Fatty Liver Disease in Diet-Induced Obese Mice. J. Med. Food 22 (9), 928–936. 10.1089/jmf.2018.4383 [DOI] [PubMed] [Google Scholar]
- Cui Y., Chang R., Zhang T., Zhou X., Wang Q., Gao H., et al. (2019). Chinese Herbal Formula (CHF03) Attenuates Non-Alcoholic Fatty Liver Disease (NAFLD) Through Inhibiting Lipogenesis and Anti-Oxidation Mechanisms. Front. Pharmacol. 10, 1190. 10.3389/fphar.2019.01190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaja M. J. (2016). Function of Autophagy in Nonalcoholic Fatty Liver Disease. Dig. Dis. Sci. 61 (5), 1304–1313. 10.1007/s10620-015-4025-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang Y., Hao S., Zhou W., Zhang L., Ji G. (2019). The traditional Chinese formulae Ling-gui-zhu-gan decoction alleviated non-alcoholic fatty liver disease via inhibiting PPP1R3C mediated molecules. BMC Complement Altern. Med. 19 (1), 8. 10.1186/s12906-018-2424-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H., Lu F. E., Zhao L. (2012). Chinese herbal medicine in the treatment of nonalcoholic fatty liver disease. Chin. J. Integr. Med. 18 (2), 152–160. 10.1007/s11655-012-0993-2 [DOI] [PubMed] [Google Scholar]
- Eissing L., Scherer T., Todter K., Knippschild U., Greve J. W., Buurman W. A., et al. (2013). De novo lipogenesis in human fat and liver is linked to ChREBP-beta and metabolic health. Nat. Commun. 4, 1528. 10.1038/ncomms2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Q., Liu W., Baker S. S., Li H., Chen C., Liu Q., et al. (2017). Multi-targeting therapeutic mechanisms of the Chinese herbal medicine QHD in the treatment of non-alcoholic fatty liver disease. Oncotarget 8 (17), 27820–27838. 10.18632/oncotarget.15482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W. W., Kuang S. Y., Tu C., Ma Z. J., Pang J. Y., Wang Y. H., et al. (2018). Natural products berberine and curcumin exhibited better ameliorative effects on rats with non-alcohol fatty liver disease than lovastatin. BioMed. Pharmacother. 99, 325–333. 10.1016/j.biopha.2018.01.071 [DOI] [PubMed] [Google Scholar]
- Fernandez-Marcos P. J., Auwerx J. (2011). Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am. J. Clin. Nutr. 93 (4), 884S–8890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuhashi M., Hotamisligil G. S. (2008). Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discovery 7 (6), 489–503. 10.1038/nrd2589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Cui R., Zhao J., Mo R., Peng L., Yan M. (2016). Corosolic acid protects hepatocytes against ethanol-induced damage by modulating mitogen-activated protein kinases and activating autophagy. Eur. J. Pharmacol. 791, 578–588. 10.1016/j.ejphar.2016.09.031 [DOI] [PubMed] [Google Scholar]
- Guo Y., Li J. X., Mao T. Y., Zhao W. H., Liu L. J., Wang Y. L. (2017). Targeting Sirt1 in a rat model of high-fat diet-induced non-alcoholic fatty liver disease: Comparison of Gegen Qinlian decoction and resveratrol. Exp. Ther. Med. 14 (5), 4279–4287. 10.3892/etm.2017.5076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M., Bae H. (2017). “An Overview of Stress-Induced Resveratrol Synthesis in Grapes: Perspectives for Resveratrol-Enriched Grape Products. Molecules 22 (2). 10.3390/molecules22020294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeboll S., Vilstrup H., Gronbaek H. (2018). “[Treatment of non-alcoholic fatty liver disease]. Ugeskr Laeger 180 (31). [PubMed] [Google Scholar]
- Ho C., Gao Y., Zheng D., Liu Y., Shan S., Fang B., et al. (2019). Alisol A attenuates high-fat-diet-induced obesity and metabolic disorders via the AMPK/ACC/SREBP-1c pathway. J. Cell Mol. Med. 23 (8), 5108–5118. 10.1111/jcmm.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormati A., Tooiserkany F., Mohammadbeigi A., Aliasl F., Dehnavi H. M. (2019). Effect of an Herbal Product on the Serum Level of Liver Enzymes in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blinded, Placebo-Controlled Trial. Iranian Red Crescent Med. J. 21 (7), 7. 10.5812/ircmj.91024 [DOI] [Google Scholar]
- Houten S. M., Wanders R. J. (2010). A general introduction to the biochemistry of mitochondrial fatty acid beta-oxidation. J. Inherit. Metab. Dis. 33 (5), 469–477. 10.1007/s10545-010-9061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu W. H., Chen T. H., Lee B. H., Hsu Y. W., Pan T. M. (2014). Monascin and ankaflavin act as natural AMPK activators with PPARalpha agonist activity to down-regulate nonalcoholic steatohepatitis in high-fat diet-fed C57BL/6 mice. Food Chem. Toxicol. 64, 94–103. 10.1016/j.fct.2013.11.015 [DOI] [PubMed] [Google Scholar]
- Huang L., Cheng Y., Huang K., Zhou Y., Ma Y., Zhang M. (2018). Ameliorative effect of Sedum sarmentosum Bunge extract on Tilapia fatty liver via the PPAR and P53 signaling pathway. Sci. Rep. 8 (1), 8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Chen W., Yan C., Yang R., Chen Q., Xu H., et al. (2019). Gypenosides improve the intestinal microbiota of non-alcoholic fatty liver in mice and alleviate its progression. BioMed. Pharmacother. 118, 109258. 10.1016/j.biopha.2019.109258 [DOI] [PubMed] [Google Scholar]
- Huu Tung N., Uto T., Morinaga O., Kim Y. H., Shoyama Y. (2012). Pharmacological effects of ginseng on liver functions and diseases: a minireview. Evid. Based Complement Alternat. Med. 2012, 173297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim S. H., Hirsova P., Malhi H., Gores G. J. (2016). Animal Models of Nonalcoholic Steatohepatitis: Eat, Delete, and Inflame. Dig. Dis. Sci. 61 (5), 1325–1336. 10.1007/s10620-015-3977-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ipsen D. H., Lykkesfeldt J., Tveden-Nyborg P. (2018). Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell Mol. Life Sci. 75 (18), 3313–3327. 10.1007/s00018-018-2860-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa D., Patel V., Sanyal A. J. (2018). Future therapy for non-alcoholic fatty liver disease. Liver Int. 38 Suppl 1, 56–63. 10.1111/liv.13676 [DOI] [PubMed] [Google Scholar]
- Jang J., Jung Y., Chae S., Cho S. H., Yoon M., Yang H., et al. (2018). Gangjihwan, a polyherbal composition, inhibits fat accumulation through the modulation of lipogenic transcription factors SREBP1C, PPARgamma and C/EBPalpha. J. Ethnopharmacol. 210, 10–22. 10.1016/j.jep.2017.08.024 [DOI] [PubMed] [Google Scholar]
- Jung H. Y., Lee D., Ryu H. G., Choi B. H., Go Y., Lee N., et al. (2017). Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1alpha. Sci. Rep. 7 (1), 6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H., Koppula S. (2014). Houttuynia cordata attenuates lipid accumulation via activation of AMP-activated protein kinase signaling pathway in HepG2 cells. Am. J. Chin. Med. 42 (3), 651–664. 10.1142/S0192415X14500426 [DOI] [PubMed] [Google Scholar]
- Kang O. H., Kim S. B., Mun S. H., Seo Y. S., Hwang H. C., Lee Y. M., et al. (2015). Puerarin ameliorates hepatic steatosis by activating the PPARalpha and AMPK signaling pathways in hepatocytes. Int. J. Mol. Med. 35 (3), 803–809. 10.3892/ijmm.2015.2074 [DOI] [PubMed] [Google Scholar]
- Kawano Y., Cohen D. E. (2013). Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 48 (4), 434–441. 10.1007/s00535-013-0758-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Khalifeh H., Baldini F., Salis A., Damonte G., Daher A., et al. (2019). Antisteatotic and antioxidant activities of Thymbra spicata L. extracts in hepatic and endothelial cells as in vitro models of non-alcoholic fatty liver disease. J. Ethnopharmacol. 239, 111919. [DOI] [PubMed] [Google Scholar]
- Khavasi N., Somi M. H., Khadem E., Faramarzi E., Ayati M. H., Fazljou S. M. B., et al. (2017). Effect of Daily Caper Fruit Pickle Consumption on Disease Regression in Patients with Non-Alcoholic Fatty Liver Disease: a Double-Blinded Randomized Clinical Trial. Adv. Pharm. Bull. 7 (4), 645–650. 10.15171/apb.2017.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kounakis K., Chaniotakis M., Markaki M., Tavernarakis N. (2019). Emerging Roles of Lipophagy in Health and Disease. Front. Cell Dev. Biol. 7, 185. 10.3389/fcell.2019.00185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumashiro N., Yoshimura T., Cantley J. L., Majumdar S. K., Guebre-Egziabher F., Kursawe R., et al. (2013). Role of patatin-like phospholipase domain-containing 3 on lipid-induced hepatic steatosis and insulin resistance in rats. Hepatology 57 (5), 1763–1772. 10.1002/hep.26170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwanten W. J., Martinet W., Michielsen P. P., Francque S. M. (2014). Role of autophagy in the pathophysiology of nonalcoholic fatty liver disease: a controversial issue. World J. Gastroenterol. 20 (23), 7325–7338. 10.3748/wjg.v20.i23.7325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichane S., Dahal Lamichane B., Kwon S. M. (2018). Pivotal Roles of Peroxisome Proliferator-Activated Receptors (PPARs) and Their Signal Cascade for Cellular and Whole-Body Energy Homeostasis. Int. J. Mol. Sci. 19 (4). 10.3390/ijms19040949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lass A., Zimmermann R., Oberer M., Zechner R. (2011). Lipolysis - a highly regulated multi-enzyme complex mediates the catabolism of cellular fat stores. Prog. Lipid Res. 50 (1), 14–27. 10.1016/j.plipres.2010.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. E., Kim E. J., Kim M. H., Hong J., Yang W. M. (2016). Polygonatum stenophyllum improves menopausal obesity via regulation of lipolysis-related enzymes. J. Nat. Med. 70 (4), 789–796. 10.1007/s11418-016-1018-9 [DOI] [PubMed] [Google Scholar]
- Lee Y. H., Jin B., Lee S. H., Song M., Bae H., Min B. J., et al. (2016). Herbal Formula HT048 Attenuates Diet-Induced Obesity by Improving Hepatic Lipid Metabolism and Insulin Resistance in Obese Rats”. Molecules 21 (11). 10.3390/molecules21111424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. H., Baek S. Y., Jang E. J., Ku S. K., Kim K. M., Ki S. H., et al. (2018). Oxyresveratrol ameliorates nonalcoholic fatty liver disease by regulating hepatic lipogenesis and fatty acid oxidation through liver kinase B1 and AMP-activated protein kinase. Chem. Biol. Interact. 289, 68–74. 10.1016/j.cbi.2018.04.023 [DOI] [PubMed] [Google Scholar]
- Li X., Li Z., Xue M., Ou Z., Liu M., Yang M., et al. (2013). Fructus Xanthii attenuates hepatic steatosis in rats fed on high-fat diet. PloS One 8 (4), e61499. 10.1371/journal.pone.0061499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Li Y., Wang Q., Yang Y. (2014). Crude extracts from Lycium barbarum suppress SREBP-1c expression and prevent diet-induced fatty liver through AMPK activation. BioMed. Res. Int. 2014, 196198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao J., Zheng H., Zhong X., Zhou J., Hong Z. (2014). Treatment of Nonalcoholic Fatty Liver Disease with Total Alkaloids in Rubus aleaefolius Poir through Regulation of Fat Metabolism. Evid. Based Complement Alternat. Med. 2014, 768540. 10.1155/2014/768540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Xu J., Zheng P., Xing L., Shen H., Yang L., et al. (2015). Hawthorn leaf flavonoids alleviate nonalcoholic fatty liver disease by enhancing the adiponectin/AMPK pathway. Int. J. Clin. Exp. Med. 8 (10), 17295–17307. [PMC free article] [PubMed] [Google Scholar]
- Li W. S., Wu Y., Ge W. Z., Fan L., Sun W. (2017). A herbal formula Erchen decoction for non-alcoholic fatty liver disease: a systematic review and meta-analysis of randomized controlled trials. Int. J. Clin. Exp. Med. 10 (6), 9110–9116. [Google Scholar]
- Li C. H., Tang S. C., Wong C. H., Wang Y., Jiang J. D., Chen Y. (2018). Berberine induces miR-373 expression in hepatocytes to inactivate hepatic steatosis associated AKT-S6 kinase pathway. Eur. J. Pharmacol. 825, 107–118. 10.1016/j.ejphar.2018.02.035 [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Tang D., Du Y. L., Cao C. Y., Nie Y. Q., Cao J., et al. (2018). Fatty liver mediated by peroxisome proliferator-activated receptor-alpha DNA methylation can be reversed by a methylation inhibitor and curcumin. J. Dig. Dis. 19 (7), 421–430. 10.1111/1751-2980.12610 [DOI] [PubMed] [Google Scholar]
- Li Y. Y., Chen M., Wang J., Guo X. P., Xiao L., Liu P. Y., et al. (2019). Quercetin ameliorates autophagy in alcohol liver disease associated with lysosome through mTOR-TFEB pathway. J. Funct. Foods 52, 177–185. 10.1016/j.jff.2018.10.033 [DOI] [Google Scholar]
- Liang Z. E., Zhang Y. P., Tang K. R., Deng Y. J., Liang Y. Q., Liang S., et al. (2019). Anti-inflammation effect via TLR4-mediated MyD88-dependent and -independent signalling pathways in non-alcoholic fatty liver disease rats: Chinese herb formula. Int. J. Clin. Exp. Med. 12 (3), 2265–2277. [Google Scholar]
- Liao C. C., Ou T. T., Huang H. P., Wang C. J. (2014). The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up-regulation of AMP-activated kinase. J. Sci. Food Agric. 94 (6), 1154–1162. 10.1002/jsfa.6386 [DOI] [PubMed] [Google Scholar]
- Lim J., Lee H., Ahn J., Kim J., Jang J., Park Y., et al. (2018). The polyherbal drug GGEx18 from Laminaria japonica, Rheum palmatum, and Ephedra sinica inhibits hepatic steatosis and fibroinflammtion in high-fat diet-induced obese mice. J. Ethnopharmacol. 225, 31–41. 10.1016/j.jep.2018.06.034 [DOI] [PubMed] [Google Scholar]
- Liu K., Czaja M. J. (2013). Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ. 20 (1), 3–11. 10.1038/cdd.2012.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Yang M., Lin X., Li Y., Liu C., Yang Y., et al. (2013). Modulation of hepatic sterol regulatory element-binding protein-1c-mediated gene expression contributes to Salacia oblonga root-elicited improvement of fructose-induced fatty liver in rats. J. Ethnopharmacol. 150 (3), 1045–1052. 10.1016/j.jep.2013.10.020 [DOI] [PubMed] [Google Scholar]
- Liu Z. L., Xie L. Z., Zhu J., Li G. Q., Grant S. J., Liu J. P. (2013). Herbal medicines for fatty liver diseases. Cochrane Database Syst. Rev. (8), CD009059. 10.1002/14651858.CD009059.pub2 [DOI] [PubMed] [Google Scholar]
- Liu Q., Zhu L., Cheng C., Hu Y. Y., Feng Q. (2017). Natural Active Compounds from Plant Food and Chinese Herbal Medicine for Nonalcoholic Fatty Liver Disease. Curr. Pharm. Des. 23 (34), 5136–5162. [DOI] [PubMed] [Google Scholar]
- Liu X., Tong W., Zhao X., Zhang H., Tang Y., Deng X. (2017). Chinese herb extract improves liver steatosis by promoting the expression of high molecular weight adiponectin in NAFLD rats. Mol. Med. Rep. 16 (4), 5580–5586. 10.3892/mmr.2017.7284 [DOI] [PubMed] [Google Scholar]
- Liu Y. L., Lin L. C., Tung Y. T., Ho S. T., Chen Y. L., Lin C. C., et al. (2017). Rhododendron oldhamii leaf extract improves fatty liver syndrome by increasing lipid oxidation and decreasing the lipogenesis pathway in mice. Int. J. Med. Sci. 14 (9), 862–870. 10.7150/ijms.19553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Xie Z. H., Liu C. Y., Zhang Y. (2019). Effect of Chinese Herbal Monomer Hairy Calycosin on Nonalcoholic Fatty Liver Rats and its Mechanism. Comb. Chem. High Throughput Screen 22 (3), 194–200. 10.2174/1386207322666190411112814 [DOI] [PubMed] [Google Scholar]
- Liu Y. Y., You J. J., Xu W., Zhai T., Du C. Y., Chen Y., et al. (2019). Gynura procumbens aqueous extract alleviates nonalcoholic steatohepatitis through CFLAR-JNK pathway in vivo and in vitro. Chin. Herb. Medicines 11 (4), 369–378. 10.1016/j.chmed.2019.09.005 [DOI] [Google Scholar]
- Lo V., Erickson B., Thomason-Hughes M., Ko K. W., Dolinsky V. W., Nelson R., et al. (2010). Arylacetamide deacetylase attenuates fatty-acid-induced triacylglycerol accumulation in rat hepatoma cells. J. Lipid Res. 51 (2), 368–377. 10.1194/jlr.M000596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonaco R., Sunny N. E., Bril F., Cusi K. (2013). Nonalcoholic fatty liver disease: current issues and novel treatment approaches. Drugs 73 (1), 1–14. 10.1007/s40265-012-0004-0 [DOI] [PubMed] [Google Scholar]
- Lu X., Yuan Z. Y., Yan X. J., Lei F., Jiang J. F., Yu X., et al. (2016). Effects of Angelica dahurica on obesity and fatty liver in mice. Chin. J. Nat. Med. 14 (9), 641–652. 10.1016/S1875-5364(16)30076-0 [DOI] [PubMed] [Google Scholar]
- Luan H., Huo Z., Zhao Z., Zhang S., Huang Y., Shen Y., et al. (2019). Scutellarin, a modulator of mTOR, attenuates hepatic insulin resistance by regulating hepatocyte lipid metabolism via SREBP-1c suppression. Phytother. Res. 10.1002/ptr.6582 [DOI] [PubMed]
- Ma J., Zhao D., Wang X., Ma C., Feng K., Zhang S., et al. (2019). LongShengZhi Capsule Reduces Established Atherosclerotic Lesions in apoE-Deficient Mice by Ameliorating Hepatic Lipid Metabolism and Inhibiting Inflammation. J. Cardiovasc. Pharmacol. 73 (2), 105–117. 10.1097/FJC.0000000000000642 [DOI] [PubMed] [Google Scholar]
- Mandal S., Mukhopadhyay S., Bandhopadhyay S., Sen G., Biswas T. (2014). 14-Deoxyandrographolide alleviates ethanol-induced hepatosteatosis through stimulation of AMP-activated protein kinase activity in rats. Alcohol 48 (2), 123–132. 10.1016/j.alcohol.2013.11.005 [DOI] [PubMed] [Google Scholar]
- Mato J. M., Alonso C., Noureddin M., Lu S. C. (2019). Biomarkers and subtypes of deranged lipid metabolism in non-alcoholic fatty liver disease. World J. Gastroenterol. 25 (24), 3009–3020. 10.3748/wjg.v25.i24.3009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng Q., Duan X. P., Wang C. Y., Liu Z. H., Sun P. Y., Huo X. K., et al. (2017). Alisol B 23-acetate protects against non-alcoholic steatohepatitis in mice via farnesoid X receptor activation. Acta Pharmacol. Sin. 38 (1), 69–79. 10.1038/aps.2016.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moctezuma-Velazquez C. (2018). Current treatment for non-alcoholic fatty liver disease. Rev. Gastroenterol. Mex. 83 (2), 125–133. 10.1016/j.rgmxen.2018.05.014 [DOI] [PubMed] [Google Scholar]
- Musso G., Gambino R., Cassader M. (2009). Recent insights into hepatic lipid metabolism in non-alcoholic fatty liver disease (NAFLD). Prog. Lipid Res. 48 (1), 1–26. 10.1016/j.plipres.2008.08.001 [DOI] [PubMed] [Google Scholar]
- Nguyen P., Leray V., Diez M., Serisier S., Le Bloc’h J., Siliart B., et al. (2008). Liver lipid metabolism. J. Anim. Physiol. Anim. Nutr. (Berl.) 92 (3), 272–283. 10.1111/j.1439-0396.2007.00752.x [DOI] [PubMed] [Google Scholar]
- Ouali F., Djouadi F., Merlet-Benichou C., Riveau B., Bastin J. (2000). Regulation of fatty acid transport protein and mitochondrial and peroxisomal beta-oxidation gene expression by fatty acids in developing rats. Pediatr. Res. 48 (5), 691–696. 10.1203/00006450-200011000-00023 [DOI] [PubMed] [Google Scholar]
- Parafati M., Lascala A., Morittu V. M., Trimboli F., Rizzuto A., Brunelli E., et al. (2015). Bergamot polyphenol fraction prevents nonalcoholic fatty liver disease via stimulation of lipophagy in cafeteria diet-induced rat model of metabolic syndrome. J. Nutr. Biochem. 26 (9), 938–948. 10.1016/j.jnutbio.2015.03.008 [DOI] [PubMed] [Google Scholar]
- Park H., Kaushik V. K., Constant S., Prentki M., Przybytkowski E., Ruderman N. B., et al. (2002). Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J. Biol. Chem. 277 (36), 32571–32577. 10.1074/jbc.M201692200 [DOI] [PubMed] [Google Scholar]
- Park H. J., Han J. M., Kim H. G., Choi M. K., Lee J. S., Lee H. W., et al. (2013). Chunggan extract (CGX), methionine-and choline-deficient (MCD) diet-induced hepatosteatosis and oxidative stress in C57BL/6 mice. Hum. Exp. Toxicol. 32 (12), 1258–1269. 10.1177/0960327113485253 [DOI] [PubMed] [Google Scholar]
- Park H., Hwang Y. H., Kim D. G., Jeon J., Ma J. Y. (2015). Hepatoprotective effect of herb formula KIOM2012H against nonalcoholic fatty liver disease. Nutrients 7 (4), 2440–2455. 10.3390/nu7042440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park T. Y., Hong M., Sung H., Kim S., Suk K. T. (2017). Effect of Korean Red Ginseng in chronic liver disease. J. Ginseng Res. 41 (4), 450–455. 10.1016/j.jgr.2016.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. H., Liu L. K., Chuang C. M., Chyau C. C., Huang C. N., Wang C. J. (2011). Mulberry water extracts possess an anti-obesity effect and ability to inhibit hepatic lipogenesis and promote lipolysis. J. Agric. Food Chem. 59 (6), 2663–2671. 10.1021/jf1043508 [DOI] [PubMed] [Google Scholar]
- Peng H., He Y., Zheng G., Zhang W., Yao Z., Xie W. (2016). Meta-analysis of traditional herbal medicine in the treatment of nonalcoholic fatty liver disease. Cell Mol. Biol. (Noisy-le-grand) 62 (4), 88–95. [PubMed] [Google Scholar]
- Perla F. M., Prelati M., Lavorato M., Visicchio D., Anania C. (2017). The Role of Lipid and Lipoprotein Metabolism in Non-Alcoholic Fatty Liver Disease. Children (Basel) 4 (6). 10.3390/children4060046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumpail B. J., Li A. A., Iqbal U., Sallam S., Shah N. D., Kwong W., et al. (2018). Potential Therapeutic Benefits of Herbs and Supplements in Patients with NAFLD. Diseases 6 (3). 10.3390/diseases6030080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre G., Macdonald A., Gray G., Hendriksz C., Preece M. A., Chakrapani A. (2007). Prospective treatment in carnitine-acylcarnitine translocase deficiency. J. Inherit. Metab. Dis. 30 (5), 815. 10.1007/s10545-007-0518-x [DOI] [PubMed] [Google Scholar]
- Pil Hwang Y., Gyun Kim H., Choi J. H., Truong Do M., Tran T. P., Chun H. K., et al. (2013). 3-Caffeoyl, 4-dihydrocaffeoylquinic acid from Salicornia herbacea attenuates high glucose-induced hepatic lipogenesis in human HepG2 cells through activation of the liver kinase B1 and silent information regulator T1/AMPK-dependent pathway. Mol. Nutr. Food Res. 57 (3), 471–482. 10.1002/mnfr.201200529 [DOI] [PubMed] [Google Scholar]
- Ponziani F. R., Pecere S., Gasbarrini A., Ojetti V. (2015). Physiology and pathophysiology of liver lipid metabolism. Expert Rev. Gastroenterol. Hepatol. 9 (8), 1055–1067. 10.1586/17474124.2015.1056156 [DOI] [PubMed] [Google Scholar]
- Qian W., Cai X., Zhang X., Wang Y., Qian Q., Hasegawa J. (2016). Effect of Daisaikoto on Expressions of SIRT1 and NF-kappaB of Diabetic Fatty Liver Rats Induced by High-Fat Diet and Streptozotocin. Yonago Acta Med. 59 (2), 149–158. [PMC free article] [PubMed] [Google Scholar]
- Qiu P., Li X., Kong D. S., Li H. Z., Niu C. C., Pan S. H. (2015). Herbal SGR Formula Prevents Acute Ethanol-Induced Liver Steatosis via Inhibition of Lipogenesis and Enhancement Fatty Acid Oxidation in Mice. Evid. Based Complement Alternat. Med. 2015, 613584. 10.1155/2015/613584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiroga A. D., Lehner R. (2018). Pharmacological intervention of liver triacylglycerol lipolysis: The good, the bad and the ugly. Biochem. Pharmacol. 155, 233–241. 10.1016/j.bcp.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Reddy J. K., Rao M. S. (2006). Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am. J. Physiol. Gastrointest Liver Physiol. 290 (5), G852–G858. [DOI] [PubMed] [Google Scholar]
- Ren L., Sun D., Zhou X., Yang Y., Huang X., Li Y., et al. (2019). Chronic treatment with the modified Longdan Xiegan Tang attenuates olanzapine-induced fatty liver in rats by regulating hepatic de novo lipogenesis and fatty acid beta-oxidation-associated gene expression mediated by SREBP-1c, PPAR-alpha and AMPK-alpha. J. Ethnopharmacol. 232, 176–187. 10.1016/j.jep.2018.12.034 [DOI] [PubMed] [Google Scholar]
- Roh J. S., Lee H., Lim J., Kim J., Yang H., Yoon Y., et al. (2017). Effect of Gangjihwan on hepatic steatosis and inflammation in high fat diet-fed mice. J. Ethnopharmacol. 206, 315–326. 10.1016/j.jep.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Rui L. (2014). Energy metabolism in the liver. Compr. Physiol. 4 (1), 177–197. 10.1002/cphy.c130024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha A. K., Ruderman N. B. (2003). Malonyl-CoA and AMP-activated protein kinase: an expanding partnership. Mol. Cell Biochem. 253 (1-2), 65–70. 10.1023/A:1026053302036 [DOI] [PubMed] [Google Scholar]
- Seo M. S., Hong S. W., Yeon S. H., Kim Y. M., Um K. A., Kim J. H., et al. (2014). Magnolia officinalis attenuates free fatty acid-induced lipogenesis via AMPK phosphorylation in hepatocytes. J. Ethnopharmacol. 157, 140–148. 10.1016/j.jep.2014.09.031 [DOI] [PubMed] [Google Scholar]
- Shang J., Chen L. L., Xiao F. X., Sun H., Ding H. C., Xiao H. (2008). Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 29 (6), 698–706. 10.1111/j.1745-7254.2008.00807.x [DOI] [PubMed] [Google Scholar]
- Sheng X., Wang M., Lu M., Xi B., Sheng H., Zang Y. Q. (2011). Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 300 (5), E886–E893. 10.1152/ajpendo.00332.2010 [DOI] [PubMed] [Google Scholar]
- Sheng D., Zhao S., Gao L., Zheng H., Liu W., Hou J., et al. (2019). BabaoDan attenuates high-fat diet-induced non-alcoholic fatty liver disease via activation of AMPK signaling. Cell Biosci. 9, 77. 10.1186/s13578-019-0339-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi K. Q., Fan Y. C., Liu W. Y., Li L. F., Chen Y. P., Zheng M. H. (2012). Traditional Chinese medicines benefit to nonalcoholic fatty liver disease: a systematic review and meta-analysis. Mol. Biol. Rep. 39 (10), 9715–9722. 10.1007/s11033-012-1836-0 [DOI] [PubMed] [Google Scholar]
- Shi L. J., Shi L., Song G. Y., Zhang H. F., Hu Z. J., Wang C., et al. (2013). Oxymatrine attenuates hepatic steatosis in non-alcoholic fatty liver disease rats fed with high fructose diet through inhibition of sterol regulatory element binding transcription factor 1 (Srebf1) and activation of peroxisome proliferator activated receptor alpha (Pparalpha). Eur. J. Pharmacol. 714 (1-3), 89–95. 10.1016/j.ejphar.2013.06.013 [DOI] [PubMed] [Google Scholar]
- Shi X., Sun R. M., Zhao Y., Fu R., Wang R. W., Zhao H. Y., et al. (2018). Promotion of autophagosome-lysosome fusion via salvianolic acid A-mediated SIRT1 up-regulation ameliorates alcoholic liver disease. Rsc Adv. 8 (36), 20411–20422. 10.1039/C8RA00798E [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Cuervo A. M. (2012). Lipophagy: connecting autophagy and lipid metabolism. Int. J. Cell Biol. 2012, 282041. 10.1155/2012/282041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F., Xie M. L., Zhu L. J., Xue J., Gu Z. L. (2009). Inhibitory effect of osthole on alcohol-induced fatty liver in mice. Dig. Liver Dis. 41 (2), 127–133. 10.1016/j.dld.2008.01.011 [DOI] [PubMed] [Google Scholar]
- Sun X. H., Zhang L. D., Wei W. (2019). A study on the mechanism of adipokine in non-alcoholic fatty liver in rats treated by four herbs decoction. Eur. J. Inflammation 17, 8. 10.1177/2058739219853970 [DOI] [Google Scholar]
- Tessari P., Coracina A., Cosma A., Tiengo A. (2009). Hepatic lipid metabolism and non-alcoholic fatty liver disease. Nutr. Metab. Cardiovasc. Dis. 19 (4), 291–302. 10.1016/j.numecd.2008.12.015 [DOI] [PubMed] [Google Scholar]
- Theodotou M., Fokianos K., Moniatis D., Kadlenic R., Chrysikou A., Aristotelous A., et al. (2019). Effect of resveratrol on non-alcoholic fatty liver disease. Exp. Ther. Med. 18 (1), 559–565. 10.3892/etm.2019.7607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uen W. C., Shi Y. C., Choong C. Y., Tai C. J. (2018). Cordycepin suppressed lipid accumulation via regulating AMPK activity and mitochondrial fusion in hepatocytes. J. Food Biochem. 42 (5), 7. 10.1111/jfbc.12569 [DOI] [Google Scholar]
- Veeramani C., Alsaif M. A., Al-Numair K. S. (2017). Lavatera critica, a green leafy vegetable, controls high fat diet induced hepatic lipid accumulation and oxidative stress through the regulation of lipogenesis and lipolysis genes. BioMed. Pharmacother. 96, 1349–1357. 10.1016/j.biopha.2017.11.072 [DOI] [PubMed] [Google Scholar]
- Veeramani C., Alsaif M. A., Al-Numair K. S. (2018). Herbacetin, a flaxseed flavonoid, ameliorates high percent dietary fat induced insulin resistance and lipid accumulation through the regulation of hepatic lipid metabolizing and lipid-regulating enzymes. Chem. Biol. Interact. 288, 49–56. 10.1016/j.cbi.2018.04.009 [DOI] [PubMed] [Google Scholar]
- Wanders R. J., Waterham H. R., Ferdinandusse S. (2015). Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 3, 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. L., Liu L. J., Zhao W. H., Li J. X. (2015). Intervening TNF-alpha via PPARgamma with Gegenqinlian Decoction in Experimental Nonalcoholic Fatty Liver Disease. Evid. Based Complement Alternat. Med. 2015, 715638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Viscarra J., Kim S. J., Sul H. S. (2015). Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 16 (11), 678–689. 10.1038/nrm4074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. P., Wat E., Koon C. M., Wong C. W., Cheung D. W., Leung P. C., et al. (2016). The beneficial potential of polyphenol-enriched fraction from Erigerontis Herba on metabolic syndrome. J. Ethnopharmacol. 187, 94–103. 10.1016/j.jep.2016.04.040 [DOI] [PubMed] [Google Scholar]
- Wang S. J., Chen Q., Liu M. Y., Yu H. Y., Xu J. Q., Wu J. Q., et al. (2019). Regulation effects of rosemary (Rosmarinus officinalis Linn.) on hepatic lipid metabolism in OA induced NAFLD rats. Food Funct. 10 (11), 7356–7365. [DOI] [PubMed] [Google Scholar]
- Ward C., Martinez-Lopez N., Otten E. G., Carroll B., Maetzel D., Singh R., et al. (2016). Autophagy, lipophagy and lysosomal lipid storage disorders. Biochim. Biophys. Acta 1861 (4), 269–284. 10.1016/j.bbalip.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Wu W. Y., Wang Y. P. (2012). Pharmacological actions and therapeutic applications of Salvia miltiorrhiza depside salt and its active components. Acta Pharmacol. Sin. 33 (9), 1119–1130. 10.1038/aps.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Fai So K., Liong E. C., Tipoe G. L. (2013). Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J. Tradit. Complement Med. 3 (2), 88–94. 10.4103/2225-4110.110411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Yin L., Tao X., Qi Y., Han X., Xu Y., et al. (2017). Dioscin, a potent ITGA5 inhibitor, reduces the synthesis of collagen against liver fibrosis: Insights from SILAC-based proteomics analysis. Food Chem. Toxicol. 107 (Pt A), 318–328. 10.1016/j.fct.2017.07.014 [DOI] [PubMed] [Google Scholar]
- Yan S., Khambu B., Hong H., Liu G., Huda N., Yin X. M. (2019). Autophagy, Metabolism, and Alcohol-Related Liver Disease: Novel Modulators and Functions. Int. J. Mol. Sci. 20 (20). 10.3390/ijms20205029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W., She L., Yu K., Yan S., Zhang X., Tian X., et al. (2016). Jatrorrhizine hydrochloride attenuates hyperlipidemia in a high-fat diet-induced obesity mouse model. Mol. Med. Rep. 14 (4), 3277–3284. 10.3892/mmr.2016.5634 [DOI] [PubMed] [Google Scholar]
- Yang L., Lin W., Nugent C. A., Hao S., Song H., Liu T., et al. (2017). Lingguizhugan Decoction Protects against High-Fat-Diet-Induced Nonalcoholic Fatty Liver Disease by Alleviating Oxidative Stress and Activating Cholesterol Secretion. Int. J. Genomics 2017, 2790864. 10.1155/2017/2790864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J. M., Sun Y., Wang M., Zhang X. L., Zhang S. J., Gao Y. S., et al. (2019). Regulatory effect of a Chinese herbal medicine formula on non-alcoholic fatty liver disease. World J. Gastroenterol. 25 (34), 5105–5119. 10.3748/wjg.v25.i34.5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Yang C., Thomes P. G., Kharbanda K. K., Casey C. A., McNiven M. A., et al. (2019). Lipophagy and Alcohol-Induced Fatty Liver. Front. Pharmacol. 10, 495. 10.3389/fphar.2019.00495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. X., Wang X., Shi T. T., Dong J. C., Li F. J., Zeng L. X., et al. (2019). Mitochondrial dysfunction in high-fat diet-induced nonalcoholic fatty liver disease: The alleviating effect and its mechanism of Polygonatum kingianum. BioMed. Pharmacother. 117, 109083. 10.1016/j.biopha.2019.109083 [DOI] [PubMed] [Google Scholar]
- Yang Y., Li J., Wei C., He Y., Cao Y., Zhang Y., et al. (2019). Amelioration of nonalcoholic fatty liver disease by swertiamarin in fructose-fed mice. Phytomedicine 59, 152782. 10.1016/j.phymed.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Yao H., Qiao Y. J., Zhao Y. L., Tao X. F., Xu L. N., Yin L. H., et al. (2016). Herbal medicines and nonalcoholic fatty liver disease. World J. Gastroenterol. 22 (30), 6890–6905. 10.3748/wjg.v22.i30.6890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S., Kim J., Lee H., Lee H., Lim J., Yang H., et al. (2017). The effects of herbal composition Gambigyeongsinhwan (4) on hepatic steatosis and inflammation in Otsuka Long-Evans Tokushima fatty rats and HepG2 cells. J. Ethnopharmacol. 195, 204–213. 10.1016/j.jep.2016.11.020 [DOI] [PubMed] [Google Scholar]
- Younossi Z. M. (2019). Non-alcoholic fatty liver disease - A global public health perspective. J. Hepatol. 70 (3), 531–544. 10.1016/j.jhep.2018.10.033 [DOI] [PubMed] [Google Scholar]
- Yu S., Rao S., Reddy J. K. (2003). Peroxisome proliferator-activated receptors, fatty acid oxidation, steatohepatitis and hepatocarcinogenesis. Curr. Mol. Med. 3 (6), 561–572. 10.2174/1566524033479537 [DOI] [PubMed] [Google Scholar]
- Zamani N., Shams M., Nimrouzi M., Zarshenas M. M., Abolhasani Foroughi A., Fallahzadeh Abarghooei E., et al. (2018). The effects of Zataria multiflora Boiss. (Shirazi thyme) on nonalcoholic fatty liver disease and insulin resistance: A randomized double-blind placebo-controlled clinical trial. Complement Ther. Med. 41, 118–123. 10.1016/j.ctim.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Zar Kalai F., Han J., Ksouri R., Abdelly C., Isoda H. (2014). Oral administration of Nitraria retusa ethanolic extract enhances hepatic lipid metabolism in db/db mice model ‘BKS.Cg-Dock7(m)+/+ Lepr(db/)J’ through the modulation of lipogenesis-lipolysis balance. Food Chem. Toxicol. 72, 247–256. 10.1016/j.fct.2014.07.029 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Si Y., Zhai L., Yang N., Yao S., Sang H., et al. (2013). Celastrus orbiculatus Thunb. ameliorates high-fat diet-induced non-alcoholic fatty liver disease in guinea pigs. Pharmazie 68 (10), 850–854. [PubMed] [Google Scholar]
- Zhang Y., Yu L., Cai W., Fan S., Feng L., Ji G., et al. (2014). Protopanaxatriol, a novel PPARgamma antagonist from Panax ginseng, alleviates steatosis in mice. Sci. Rep. 4, 7375. 10.1038/srep07375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E., Yin S., Song X., Fan L., Hu H. (2016). Glycycoumarin inhibits hepatocyte lipoapoptosis through activation of autophagy and inhibition of ER stress/GSK-3-mediated mitochondrial pathway. Sci. Rep. 6, 38138. 10.1038/srep38138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Yao Z., Ji G. (2018). Herbal Extracts and Natural Products in Alleviating Non-alcoholic Fatty Liver Disease via Activating Autophagy. Front. Pharmacol. 9, 1459. 10.3389/fphar.2018.01459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E., Yin S., Zhao S., Zhao C., Yan M., Fan L., et al. (2019). Protective effects of glycycoumarin on liver diseases. Phytother. Res. 10.1002/ptr.6598 [DOI] [PubMed]
- Zhang Y., Liu M., Chen Q., Wang T., Yu H., Xu J., et al. (2019). Leaves of Lippia triphylla improve hepatic lipid metabolism via activating AMPK to regulate lipid synthesis and degradation. J. Nat. Med. 73 (4), 707–716. 10.1007/s11418-019-01316-5 [DOI] [PubMed] [Google Scholar]
- Zheng Y., Wang M., Zheng P., Tang X., Ji G. (2018). Systems pharmacology-based exploration reveals mechanisms of anti-steatotic effects of Jiang Zhi Granule on non-alcoholic fatty liver disease. Sci. Rep. 8 (1), 13681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H., Chen K., Feng M., Shao W., Wu J., Chen K., et al. (2018). Genipin alleviates high-fat diet-induced hyperlipidemia and hepatic lipid accumulation in mice via miR-142a-5p/SREBP-1c axis. FEBS J. 285 (3), 501–517. 10.1111/febs.14349 [DOI] [PubMed] [Google Scholar]
- Zhou W., Rahimnejad S., Lu K., Wang L., Liu W. (2019). Effects of berberine on growth, liver histology, and expression of lipid-related genes in blunt snout bream (Megalobrama amblycephala) fed high-fat diets. Fish Physiol. Biochem. 45 (1), 83–91. 10.1007/s10695-018-0536-7 [DOI] [PubMed] [Google Scholar]
- Zhu M., Hao S., Liu T., Yang L., Zheng P., Zhang L., et al. (2017). Lingguizhugan decoction improves non-alcoholic fatty liver disease by altering insulin resistance and lipid metabolism related genes: a whole trancriptome study by RNA-Seq. Oncotarget 8 (47), 82621–82631. 10.18632/oncotarget.19734 [DOI] [PMC free article] [PubMed] [Google Scholar]