Abstract
Although the broad role of fear and hypervigilance in conditions of the gut-brain axis like irritable bowel syndrome is supported by converging evidence, the underlying mechanisms remain incompletely understood. Even in healthy individuals, it remains unclear how pain-related fear may contribute to pain-related attentional biases for acute visceral pain. Building on our classical fear conditioning work in a clinically relevant model of visceral pain, we herein elucidated pain-related attentional biases shaped by associative learning in healthy women and men, aiming to elucidate possible sex differences and the role of psychological traits. To this end, we compared the impact of differentially conditioned pain-predictive cues on attentional biases in healthy women and men. Sixty-four volunteers accomplished a visual dot-probe task and subsequently underwent pain-related fear conditioning where one visual cue (CS+) was contingently paired with a painful rectal distention (US) while another cue remained unpaired (CS−). During the following test phase, the dot-probe task was repeated to investigate changes in attentional biases in response to differentially valenced cues. While pain-related learning was comparable between groups, men revealed more pronounced attentional engagement with the CS+ and CS− whereas women demonstrated stronger difficulties to disengage from the CS+ when presented with a neutral cue. However, when both CS+ and CS− were presented together, women revealed stronger difficulties to disengage from the CS−. Regression analyses revealed an interaction of sex, with negative affect predicting stronger avoidance of the CS+ and stronger difficulties to disengage attention from the CS− in men. These results provide first evidence that pain-related fear conditioning may induce attentional biases differentially in healthy women and men. Hence, sex differences may play a role in attentional mechanisms underlying hypervigilance, and may be modulated by psychological vulnerability factors relevant to chronic visceral pain.
Keywords: gut-brain axis, visceral pain, attentional bias, hypervigilance, pain-related fear, anxiety, sex differences
Introduction
Fear is a potent motivator that drives learning and behavior. It serves to rapidly shift attention toward signals of threat in order to avoid bodily harm, engage in self-protection and seek safety. Although adaptive by nature, fear and ensuing avoidance behaviors can also become maladaptive, with broad implications for the pathophysiology and treatment of chronic pain (1, 2). The fear-avoidance model of pain proposes a vicious cycle of pain-related fear, hypervigilance and avoidance, maintained, and modulated by psychological vulnerability factors like anxiety, catastrophizing, and negative affect (3, 4). Pain-related fear is essentially governed by the principles of classical and instrumental conditioning, engaging attentional resources that are relevant to hypervigilance, and avoidance. As an inherently fear-evoking stimulus, pain attracts strong attentional responses leading to increased sensitivity for negative information and aberrant attentional orienting toward threat (5, 6). These responses involve opposing, yet inextricably linked mechanisms of facilitated engagement toward threat, avoidance and difficulties to disengage with individuals orienting toward or away from threat early during attentional processing and subsequent avoidance at later stages (7, 8). While these processes are broadly established in the context of anxiety and posttraumatic stress (9, 10), evidence in the context of pain-related fear remains scarce and inconsistent (6, 11), especially with regard to interoceptive, visceral pain. Visceral pain is of high clinical relevance, specifically in conditions of the gut-brain axis like irritable bowel syndrome (IBS). Compared to other types of exteroceptive pain, visceral pain is considered to be characterized by a unique biological salience, as supported by greater visceral pain-related fear and enhanced pain-related learning (12, 13), making this a suitable preclinical model to study pain-related attentional biases. While numerous studies addressed attentional biases toward threat only, safety cues signaling the absence of an aversive event were also found to induce attentional biases (14) and are prioritized under threatening conditions (15). Specifically in visceral pain, patients (16, 17) and healthy individuals (18) demonstrated enhanced awareness of safety cues suggesting that attentional biases may also pertain to safety cues.
Building on our conditioning work in a clinically relevant model of visceral pain (19–21), we herein aimed to elucidate attentional biases induced by conditioned pain-related fear and safety signals in healthy volunteers. Inspired by broad knowledge about higher prevalence and incidence of chronic visceral pain in women (22), initial evidence suggesting sex differences in pain-related fear conditioning (23) and attentional coping strategies (24), we a priori committed to elucidating sex differences. Moreover, pain-related attentional biases seem to vary as a function of inter-individual differences in anxiety sensitivity and catastrophizing, but findings are heterogeneous (22, 25–27).
We herein employed a visual dot-probe task to address whether conditioning results in attentional biases related to attentional avoidance, facilitated engagement and/ or difficulties to disengage from pain-related threat and safety cues. We hypothesized that women would demonstrate more pronounced attentional biases toward threat cues, reflected by stronger engagement at short stimulus durations, as well as stronger difficulties to disengage at longer stimulus durations. Moreover, we assumed that women would demonstrate more pronounced biases to safety cues as conditioned inhibitors of fear responses and a clinically relevant phenotype in anxiety-related disorders (28). Finally, we explored the sex-specific role of psychological vulnerability factors as predictors, specifically aiming to assess a greater contribution of anxiety, negative affect as well as coping and catastrophizing to pain-related attentional biases in women.
Materials and Methods
Recruitment
The study protocol followed the rules stated in the Declaration of Helsinki and was approved by the local Ethics Committee of the University Hospital Essen, Germany (approval #10-4493). Recruitment and data collection were conducted between August 2016 and December 2017. The recruitment and screening process followed our group's established procedures in place for all visceral pain studies involving rectal distensions, and included a structured telephone screening and personal interview with a medical examination conducted by a physician.
To calculate the required sample size, we performed a power analysis using G*Power (version 3.1.9.2) (29). To detect between-groups effects of sex, we specified two groups, i.e., women and men, and eight measurements, i.e., four conditions of the dot-probe task at baseline and during the test phase presenting CS+ and CS− each with a neutral cue, CS+ and CS− together and one condition only presenting neutral cues. Based on the meta-analysis by (11) reporting attentional biases toward signals of impending experimental pain in healthy individuals, we assumed a medium effect size of Cohen's d = 0.676 (conforming to Cohen's f = 0.338). With an actual power of 0.95, the required sample size included 66 participants with a critical F-value of 3.99.
Based on local advertisement, we were initially contacted via telephone or email by a total of N = 143 interested individuals. After providing more detailed study information, N = 102 individuals agreed to participate in our structured telephone screening. Exclusion criteria were age <18 or >45 years, body mass index (BMI) <18 or >30, any known medical or psychological health condition, chronic medication use except occasional use of over-the-counter allergy or pain medications, and prior participation in any other conditioning study conducted by our group. Moreover, we excluded free-cycling women based on self-report to avoid confounding effects of cyclical fluctuations of sex hormones. During telephone screening, a total of N = 26 individuals were excluded. All others (N = 76) were then scheduled for a personal interview at the University Hospital Essen. During the interview, participants were screened for current anxiety or depression symptoms using the German version of the Hospital Anxiety and Depression Scale (HADS; Cronbach's alpha α = 0.80 for the anxiety subscale and α = 0.81 for the depression subscale) (30) and for symptoms suggestive of any functional or organic gastrointestinal condition based on a standardized in-house questionnaire (31). All participants were further evaluated digitally for perianal tissue damage (e.g., painful hemorrhoids). Based on these screening criteria, N = 5 individuals were excluded due to HADS scores ≥8, N = 3 due to increased gastrointestinal symptoms ≥13, and N = 1 due to anal tissue damage. Further, on the day of the experimental study, two participants missed the appointment and one participant terminated the study during the second run of the dot-probe task. The final sample that we herein report on thus includes 64 healthy volunteers (32 women, 32 men).
Study Design and Procedures
Prior to study participation, all participants were instructed not to eat, drink, or exercise within 2 h before arrival to the laboratory. Participants completed the study protocol within ~2 h between 09:00 and 16:00 h. Upon arrival, participants gave written informed consent and completed the questionnaire battery and then underwent the TMT and Stroop test (see below). For women, pregnancy was routinely excluded by commercially available urinary test upon arrival. All participants were tested in a medically-equipped, sound-shielded, and dimly lit room and were positioned in a hospital bed. The rectal balloon was subsequently placed, sensory thresholds were determined, and distension pressure for implementation during conditioning was individually calibrated based on the rectal pain threshold (for details, see below). All instructions and tasks were presented on a 22-inch widescreen monitor with 60 Hz refresh rate and a display resolution of 1,680 ×1,050 pixels at a viewing distance of ~140 cm. All measurements were performed by the same female tester.
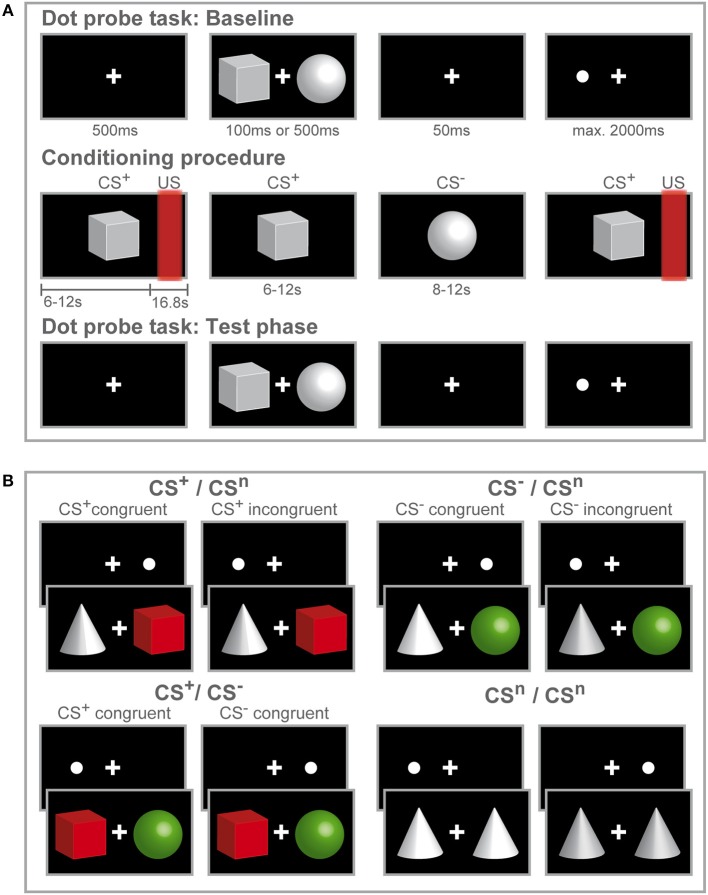
The study design consisted of a mixed-group (women, men) repeated-measures design with three consecutive experimental phases (Figure 1A): A visual dot-probe task (baseline, for details on the task see below) was followed by fear conditioning, after which the same visual dot-probe task was repeated during the test phase. For conditioning, explained in detail below, we implemented an established differential delay conditioning paradigm with individually calibrated painful rectal distensions as interoceptive unconditioned stimuli, carried out with a pressure-controlled barostat system, and visual cues as conditioned stimuli (CS). The total duration of the dot-probe task varied between participants with a notional minimum duration of 4.67 min assuming overall reaction times of 200 ms and a maximum duration of 13.07 min assuming the longest possible reaction time of 2,000 ms with an average of 8.87 min. After completion of the first dot-probe task, instructions were given for the conditioning procedure that commenced immediately afterwards. The total duration of the conditioning procedure was 7.77 min. Subsequently, the study investigator informed the participant that a second run of the dot-probe task using the same procedure as during the first run will immediately start. Together, average duration for all three experimental phases was 25.51 min.
Figure 1.
Study design and experimental procedure. Schematic illustration of the experimental procedure (A) and conditions during the dot-probe task (B). (A) Participants initially accomplished a dot-probe task (baseline) during which three different visual cues were used as stimulus material. Participants were instructed to respond by button press to the dot appearing either on the left or right side of the fixation cross. During the subsequent fear-conditioning procedure, one visual cue (CS+) was repeatedly paired with a rectal distension (US) while a second visual cue (CS−) was presented without US. Only two out of three visual cues were presented during conditioning. Afterwards participants completed a second run of the dot-probe task (test phase) with the same visual stimuli presented during the baseline task. After each phase, visual analog scale (VAS) ratings of CS valence and CS-US contingency (only after fear conditioning) were accomplished. (B) In the dot-probe task, counterbalanced and randomized order of the three different visual cues yielded four conditions. The cues are color-coded for visual purposes only to illustrate the different CS valences acquired after conditioning. For the CS+ /CSn and CS− /CSn conditions, trials were considered congruent if the dot-probe appeared at the location previously occupied by the CS+ (shown in red) or CS− (shown in green) and incongruent, if the dot appeared at the location previously occupied by the neutral cue (shown in gray). When CS+ and CS− where presented together, the dot-probe was considered CS+-congruent when it appeared at the location previously occupied by the CS+ and CS−-congruent when the dot appeared at the location previously occupied by the CS−. During the neutral condition, only neutral cues were presented.
Questionnaire Battery and Neuropsychological Testing
On the study day, participants initially completed a questionnaire battery assessing sociodemographic variables as well as several psychological characteristics. Trait anxiety was measured with the State Trait Anxiety Inventory (STAI-T; α between 0.88 and 0.94) (32) as a self-report of stable aspects of anxiety proneness. To assess situation-specific aspects of cognitive coping and catastrophizing with pain, we used the Pain-Related Self Statements Scale (PRSS; α = 0.92) (33). In addition, we measured current emotional states and fluctuations using the Positive and Negative Affect Schedule (PANAS; for both subscales α = 0.86) (34).
Moreover, the Trail Making Test (TMT) and Stroop Color Word Test were administered as control parameters for possible differences between women and men in attentional and executive functioning. The TMT is a neuropsychological task proposed to measure selective attention and processing speed in version A and set shifting in version B (35, 36), with first evidence suggesting that healthy women perform worse on version B (37). The Stroop test is thought to provide a measure of cognitive inhibition (38, 39). However, evidence regarding sex differences remains scarce and has so far yielded inconclusive results (40, 41).
Visual Dot-Probe Task and Attentional Bias Assessment
The visual dot-probe task employed in the present study was used to assess the phenomenological characteristics of attentional biases that have previously been determined in various empirical investigations (7): attentional avoidance, engagement and disengagement. Most studies on attentional biases in chronic pain so far utilized words or varying categories of pictures or facial expressions that however raised concerns about their ecological validity and that may not be sufficiently intense to induce attentional biases (42). Therefore, it has been proposed that stimuli need to be sufficiently matched to the individual qualities of pain-related fear (26), for instance through instructed fear stimuli (43) or differential conditioning (44, 45). We herein modified the original dot-probe task (46) by utilizing differentially valenced visual stimuli induced through differential, pain-related fear conditioning.
Moreover, many studies utilizing the dot-probe task have encountered methodological and analytical problems due to the absence of baseline measures. Herein, the implementation of neutral stimuli within conditions creates a neutral baseline against which responses toward threat and safety cues can be compared and therefore allows to specifically determine the type of attentional bias observed (47, 48). Likewise, the implementation of a baseline measurement of the dot-probe task before pain-related fear conditioning allows assessing intra-individual changes in attentional biases in response to experimentally induced pain-related fear and safety cues in a within-subject design.
Finally, while common dot-probe tasks often employ static stimulus durations, we furthermore aimed to elucidate the time course of attentional biases by varying the time between stimulus onset and appearance of the probe. According to a two-stage model proposing that initial attentional vigilance is followed by attentional avoidance, some studies yet revealed that the utilization of short and long stimulus durations captures attentional features occurring prior to attentional avoidance (49, 50). Moreover, Koster et al. (8) have emphasized the potential interaction of stimulus duration and threat value, indicating that attentional biases may show different phenomenological characteristics which has been corroborated by multiple studies demonstrating stimulus duration to exert a moderating effect (8, 51, 52). Specifically, stimuli of high biological significance like pain can immediately draw attention to the potential source of threat even before conscious perception and evaluation (53), suggesting that acquired signals of imminent threat as well as safety cues signaling the absence of pain may likewise occupy different phenomenological and temporal characteristics. We herein adopted presentation times from (8) who demonstrated the impact of stimulus duration and threat value on the time course of attentional biases using pictorial stimuli.
Each trial consisted of the following sequence (Figure 1A): First, a fixation cross was displayed in the center of the screen for 500 ms. Then, a stimulus pair of geometric symbols was presented with one stimulus presented left and one stimulus presented right from the fixation cross with a variable duration of either 100 or 500 ms. After a blank screen depicting the fixation cross for 50 ms, a dot appeared either on the left or right side from the fixation cross at the same location occupied by one of the stimuli before. Participants were asked to respond as fast as possible to the dot with button press of the corresponding arrow key on a keyboard. If a response was registered, the dot disappeared or if no response was registered, the dot lasted until a maximum response time set at 2,000 ms. Inter-trial intervals (ITI) were 750 ms. Randomizing and counterbalancing stimulus presentation across trials and stimuli across the left and right side of the fixation cross resulted in four conditions (Figure 1B), presenting the CS+ with a neutral cue (CS+ + CSn; CSn + CS+), presenting the CS− with a neutral cue (CS− + CSn; CSn + CS−), conditions presenting the CS+ and CS− together (CS+ + CS−; CS− + CS+) and neutral trials (CSn + CSn). For each run of the dot-probe task, 280 trials were presented, 80 per each condition presenting a CS+ and/ or CS− and 40 trials presenting only neutral cues.
As main outcome, reaction time (RT) was recorded on each trial to assess the speed of response to the location of the dot-probe. Before statistical analyses, premature responses <100 ms, missing and false responses were removed from RT data according to empirical recommendations for analyses of the dot-probe task (50). Mean RT was calculated for each condition, type of congruency (congruent, incongruent) and stimulus duration (100, 500 ms) for the dot-probe task at baseline and during the test phase. Type of congruency was determined using target t and dot-probe d appearing either on the left l or right r side. A trial is considered congruent when the dot appears at the same location as the previously displayed target stimulus, calculating the mean RT as RTcongruent = (RTtldl + RTtrdr)/2. On incongruent trials, the dot appears at the location of the neutral stimulus and is calculated as RTincongruent = (RTtldr + RTtrdl)/2. Trials presenting both CS+ and CS− represent a special case where trials can be determined as CS+-congruent if the dot appears at the location of the CS+, and CS−-congruent if the dot appears at the location of the CS−. Likewise, mean RT for the neutral condition presenting only neutral CS that were not subjected to fear conditioning were calculated as RTneutral = (RTtldl+ RTtrdr + RTtldr + RTtrdl)/4. Outlying RT values were then identified based on the interquartile range (IQR) with a lower threshold of Q1−1.5 × IQR and the upper threshold of Q3−1.5 × IQR.
Indices of attentional avoidance, engagement and disengagement were calculated for each condition following previous studies (8, 54). Attentional avoidance was calculated as RTincongruent–RTcongruent with negative scores indicating stronger attentional avoidance. Attentional engagement was calculated as RTneutral–RTcongruent with positive scores indicating enhanced attentional capture and negative scores indicating slower attentional engagement. Attentional disengagement was calculated as RTneutral–RTincongruent with negative scores indicating stronger attentional vigilance with difficulty to divert onto another stimulus and positive scores indicating faster attention away from the cue. For all these indices, a score of zero implies no differences either in attentional avoidance, engagement or disengagement.
Visceral Pain Model and Conditioning
Rectal distensions were carried out with a pressure-controlled barostat system (modified ISOBAR 3 device, G & J Electronics, Toronto, ON, Canada). Sensory and pain thresholds were determined using the ascending method of limits delivering distensions with random pressure increments of 5 mmHg and a maximal distension pressure of 50 mmHg as previously described (31, 55, 56). During conditioning, individually calibrated rectal distensions were implemented as interoceptive painful US. To this end, prior to the baseline dot-probe task, individual US intensity was carefully titrated. Participants rated selected pressures (i.e., starting with pressures just below pain threshold) on a visual analog scale (VAS) ranging from 0 to 100 with endpoints labeled “not painful at all” and “very painful.” When participants rated pain intensities between 60 and 70, the corresponding pressure was chosen for US presentation during the conditioning procedure.
During conditioning, one geometric visual symbol (CS+) was consistently paired with a painful rectal distension (US) while a second visual cue (CS−) was never followed by the US (differential delay conditioning) (Figure 1A). Overall, 32 CS were presented (16 CS+ and 16 CS−) in pseudo-randomized order with a 75% reinforcement schedule, i.e., 12 out of 16 CS+ were followed by a US and 4 CS+ remained unpaired. Duration of distensions was 16 s and US onset varied randomly between 6 and 12 s after CS+ onset with both stimuli co-terminating. Please note that reinforced CS+ were presented longer than non-reinforced CS+. Inter-trial intervals (ITI) were 20 s. In our previous conditioning studies, this conditioning paradigm revealed successful pain-related learning to threat and safety cues on behavioral and neural measures in healthy individuals (19, 56) and in patients with chronic visceral pain (16, 17).
Behavioral Measures of Emotional Learning and Contingency Awareness
Online visual analog scales (VAS) were used to assess learning-induced changes in CS valence along with expected changes in cognitive aspects that together verify the efficacy of conditioning herein (57). Prior pain-related conditioning studies from our own group [reviewed in (58, 59)] and in the broader fear conditioning literature support the notion that conditioned changes in cue valence constitutes a sensitive and relevant behavioral measure (60, 61). CS valence was assessed at three time points: (1) after the first dot-probe task and prior to conditioning (baseline), (2) after conditioning and (3) following the second dot-probe task. Participants were prompted to indicate how they perceived each of the visual cues by presenting a digitized vertical 200 mm scale with end points labeled “very unpleasant” and “very pleasant,” indicating “neutral” in the middle of the scale. These values were then transformed into a scale with end points −100 indicating “very pleasant,” 100 indicating “very unpleasant” and “neutral” at 0 as previously accomplished in all of our prior conditioning work with this visceral pain model (13, 19–21). After conditioning, contingency awareness was assessed with ratings on how often each of the visual cues was followed by a rectal distension with corresponding scale end points labeled “never” (0%) and “always” (100%).
Statistical Analyses
Analyses of all data were carried out with the Statistical Package for the Social Sciences (SPSS, IBM Corp. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp.). Initially, we analyzed whether women and men were comparable with respect to social demographics, psychological state and trait variables as well as perceptual and pain thresholds. All variables were normally distributed and analyzed by means of two-sample t-tests.
For CS valence ratings, we conducted a repeated measures ANCOVA with within-subject factors CS (CS+, CS−, CSn) and phase (baseline, conditioning, test phase), between-subject factor sex (women, men), and BMI as covariate due to a significant difference between women and men for each condition separately. For CS-US contingency, paired t-tests were calculated with values assessed after conditioning for women and men separately.
For attentional bias indices, we carried out repeated measures ANCOVAs with the within-subject factors phase (baseline, test phase), stimulus duration (100, 500 ms), the between-subject factor sex (women, men), and BMI as covariate. To address the specificity of attentional bias to threat and safety cues, we further compared trials presenting a CS with a neutral cue with trials presenting both CS+ and CS− using repeated measures ANCOVAs with within-subject factors condition (CS + CSn, CS+ + CS−), phase (baseline, test phase), the between-subject factor sex (women, men), and BMI as covariate. Results are reported for significant interactions with Greenhouse-Geisser correction and post-hoc testing was accomplished with Bonferroni correction to adjust for multiple comparisons. To test the effects of response slowing as a potential confound in analyses of attentional biases, we furthermore performed ANCOVAS using reaction time data. For these analyses, conditions presenting the CS+ with the neutral cue were compared with conditions presenting only neutral cues to test for cue (CS+ + CSn; CSn + CSn) × congruency (congruent trials; incongruent trials) interactions.
Finally, we carried out multiple regression analyses to predict changes in indices of attentional avoidance, engagement and disengagement from baseline to test phase for each condition separately. Predictors included sex, psychological vulnerability factors of trait anxiety (STAI-T), positive and negative affect (PANAS-P, PANAS-N), cognitive coping and catastrophizing (PRSS) and CS valence changes. Moreover, interaction terms with sex were calculated by multiplying sex by each predictor. For all regression models, sex was entered into the first block and always maintained. Into the second block, a pair including the main effect and interaction with sex for one predictor was entered. If the regression model did not yield significance, the interaction term was removed first and the subsequent regression model was set up with sex in the first block and the main effect of the predictor in the second block. If the main effect did not yield significance, it was removed from the model and the subsequent regression model was set up with sex only. Results are reported with F- and p-values, adjusted R2 and for the coefficients, standardized beta-, t-, and p-values are given. If the main effect and/ or interaction term was found as significant predictor, subsequent regression analyses were carried out with the main effect of the predictor for women and men separately, reporting F- and p-values and adjusted R2 for the model and for the coefficient the beta- and t-values. Detailed results of the regression analyses are given in the Supplementary Material.
All data are given as mean ± standard error of the mean (SEM), unless indicated otherwise.
Results
Sample Characteristics
In total, 64 healthy volunteers (32 women, 32 men) completed the study. Sociodemographic and psychological characteristics were comparable between men and women, except for a lower BMI in women (Table 1). Positive and negative affective states (PANAS), trait anxiety (STAI-T) and pain-related cognitions including catastrophizing and active coping (PRSS), all assessed upon arrival to the laboratory on the study day, did not reveal differences between women and men. Likewise, neuropsychological attention tests including the TMT and Stroop task as well as rectal and pain thresholds were comparable between groups (Table 1).
Table 1.
Sample characterization.
| Full sample | Women | Men | Statistics | |
|---|---|---|---|---|
| (A) Measured during screening | ||||
| Age | 28.22 ± 1.00 | 28.53 ± 1.71 | 27.91 ± 1.06 | t = 0.31, p = .757 |
| Body mass index | 22.79 ± 0.33 | 21.92 ± 0.41 | 23.66 ± 0.47 | t = 2.80, p = .007 |
| Gastrointestinal symptoms | 2.70 ± 0.36 | 3.13 ± 0.55 | 2.28 ± 0.46 | t = 1.18, p = .241 |
| HADS anxiety | 3.70 ± 0.32 | 4.16 ± 0.48 | 3.25 ± 0.42 | t = 1.44, p = .156 |
| HADS depression | 1.76 ± 0.28 | 1.29 ± 0.26 | 2.22 ± 0.48 | t = 1.68, p = .096 |
| (B) Measured on study day | ||||
| STAI Trait | 35.67 ± 0.89 | 36.75 ± 1.35 | 34.59 ± 1.16 | t = 1.21, p = .230 |
| PANAS positive | 3.25 ± 0.08 | 3.27 ± 0.12 | 3.24 ± 0.11 | t = 0.15, p = .885 |
| PANAS negative | 1.39 ± 0.06 | 1.41 ± 0.09 | 1.37 ± 0.08 | t = 0.37, p = .715 |
| PRSS catastrophizing | 0.80 ± 0.10 | 0.77 ± 0.17 | 0.83 ± 0.12 | t = 0.31, p = .762 |
| PRSS coping | 3.54 ± 0.10 | 3.57 ± 0.14 | 3.52 ± 0.15 | t = 0.24, p = .811 |
| TMT version A | 26.30 ± 1.03 | 26.59 ± 1.60 | 26.00 ± 1.33 | t = 0.29, p = .776 |
| TMT version B | 53.70 ± 2.36 | 54.72 ± 2.34 | 52.69 ± 4.13 | t = 0.43, p = .670 |
| SCWT word reading | 30.65 ± 0.64 | 30.97 ± 1.09 | 30.34 ± 0.69 | t = 0.49, p = .628 |
| SCWT color naming | 47.29 ± 1.10 | 48.32 ± 1.72 | 46.28 ± 1.39 | t = 0.93, p = .358 |
| SCWT color-word naming | 69.25 ± 1.66 | 70.61 ± 2.68 | 67.94 ± 2.00 | t = 0.80, p = .425 |
| Perception threshold | 15.75 ± 0.82 | 16.33 ± 1.22 | 15.17 ± 1.11 | t = 0.71, p = .483 |
| Pain threshold | 39.21 ± 1.29 | 38.04 ± 1.90 | 40.34 ± 1.75 | t = 0.90, p = .375 |
Overview of sociodemographic and psychological variables as well neuropsychological attention test results obtained during screening procedure (A) and on study day (B). Psychological variables were assessed with the Hospital Anxiety and Depression Scale (HADS), State-Trait Anxiety Inventory (STAI) including only the trait subscale, Positive, and Negative Affective Schedule (PANAS) including positive and negative subscales and Pain-Related Self Statements Scale (PRSS) including subscales of pain catastrophizing and coping. Neuropsychological attention tests included the Trail Making Test (TMT) with versions A and B as well as the Stroop color word test (Stroop) with conditions word reading, color naming and the interference condition of color-word naming, all results given in seconds. During the individual rectal distention calibration procedure, perception, and pain thresholds were measured in mmHG. All values are given as mean ± standard error of the mean (M ± SEM). Differences between women and men were calculated by means of independent sample t-tests.
CS Valence and CS-US Contingency
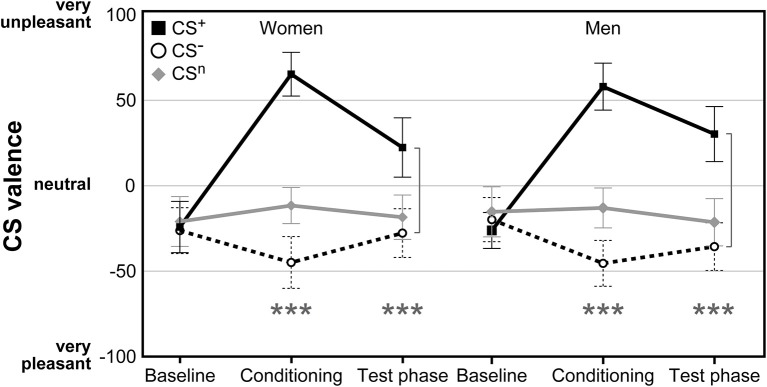
For CS valence (Figure 2; for single subject data see Figure S1), analysis revealed a significant time × CS interaction (F = 2.79, p = .038, η2 = .05), supporting the efficacy of the conditioning procedure without any effect of sex (F = 0.01, p = .943). While ratings of all CS were expectedly comparable and neutral at baseline (all p > .598), after conditioning both women and men perceived the CS+ as significantly more unpleasant and the CS− as more pleasant compared to the neutral cue (both p < .001). The difference between CS+ and CS− was still evident after the test phase (p < .001), indicating persisting effects of conditioning. Likewise, both men and women were similarly aware of conditioning contingencies for both CS+ and CS−, as supported by changes in CS-US contingency ratings assessed after conditioning with higher perceived contingency for CS+-US pairings compared to CS−-US pairings in women (CS+-US contingency M ± SEM: 77.41% ± 2.46; CS−-US contingency: 15.69% ± 3.63; t = 11.00, p < .001), and men (CS+-US contingency: 81.63% ± 2.18; CS−-US contingency: 11.41% ± 3.46; t = 15.42, p < .001).
Figure 2.
Ratings of CS valence assessed on visual analog scales (VAS) before and after conditioning as well as after the test phase. Both women and men demonstrated significant increases in CS+ aversiveness and increases in CS− pleasantness following acquisition without differences between groups. ***p < 0.001.
Sex Differences in Indices of Attentional Bias
Attentional Avoidance
For attentional avoidance indices (Table 2; for single subject data see Figure S2), analyses yielded no significant interactions between phase and sex (all F < 0.45, all p > .507), and no significant interactions between phase, sex and stimulus duration (all F < 0.53, all p > .470), indicating no differences in attentional avoidance between women and men.
Table 2.
Indices of attentional avoidance.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Test phase | Baseline | Test phase | Interaction effect | F | p | η2 | |
| CS+/CSn | 2.73 ± 2.20 | 5.55 ± 2.96 | 3.28 ± 3.67 | 2.88 ± 2.59 | Phase × sex | 0.13 | .717 | .00 |
| Phase × duration × sex | 0.53 | .470 | .01 | |||||
| CS−/CSn | 5.99 ± 3.53 | 1.66 ± 3.51 | 10.87 ± 2.98 | 5.96 ± 2.71 | Phase × sex | 0.45 | .507 | .01 |
| Phase × duration × sex | 0.16 | .691 | .00 | |||||
| CS+/CS− | −5.89 ± 3.34 | −2.78 ± 3.10 | −6.72 ± 3.40 | −6.17 ± 3.50 | Phase × sex | 0.18 | .671 | .00 |
| Phase × duration × sex | 0.38 | .542 | .01 | |||||
Descriptive statistics and results from ANCOVAs comparing indices of attentional avoidance across phase (baseline vs. test phase), stimulus duration (100 ms, 500 ms) and sex (women vs. men). Conditions include presentation of CS+ and CS− each with a neutral cue and presentation of CS+ and CS− together. Descriptive statistics are given as mean values across 100 and 500 ms stimulus duration ± standard error of the mean (M ± SEM).
Attentional Engagement
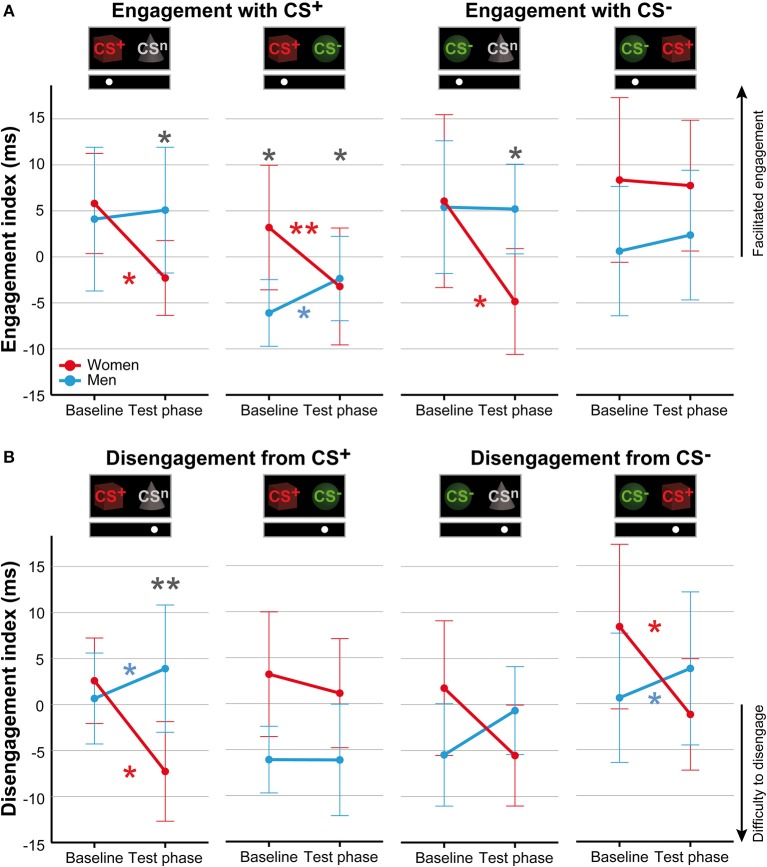
For attentional engagement indices (Table 3, Figure 3A; for single subject data see Figure S3), analyses revealed significant phase × sex interactions for both conditions presenting the CS+ and the CS− with the neutral cue (all F > 7.06, all p < .011, all η2 > .14). However, there was no effect of stimulus duration (all F < 0.61, all p > .441). Post-hoc comparisons revealed during the test phase significantly higher indices for men (all p < .038), indicating that men showed higher attentional engagement after fear conditioning compared to women. In contrast, women revealed significant decreases from baseline to test phase in attentional engagement indices across these conditions (all p < .021), while men revealed a significant increase in response to the CS+ when presented together with the CS− (p = .032).
Table 3.
Indices of attentional engagement.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Test phase | Baseline | Test phase | Interaction effect | F | p | η2 | |
| CS+/CSn | 5.39 ± 2.62 | −1.43 ± 2.19 | 3.93 ± 3.87 | 4.90 ± 3.39 | Phase × sex | 9.20 | .004 | .16 |
| Phase × duration × sex | 0.12 | .731 | .00 | |||||
| CS+/CS− | 3.49 ± 3.30 | −3.47 ± 3.09 | −6.10 ± 1.81 | −2.36 ± 2.29 | Phase × sex | 11.73 | .001 | .18 |
| Phase × duration × sex | 0.36 | .551 | .01 | |||||
| CS−/CSn | 6.48 ± 4.57 | −3.88 ± 2.95 | 5.40 ± 3.61 | 5.20 ± 2.43 | Phase × sex | 7.06 | .011 | .14 |
| Phase × duration × sex | 0.03 | .869 | .00 | |||||
| CS−/CS+ | 9.04 ± 4.39 | 8.89 ± 3.63 | 0.62 ± 3.51 | 2.37 ± 3.52 | Phase × sex | 2.13 | .151 | .05 |
| Phase × duration × sex | 0.61 | .441 | .01 | |||||
Descriptive statistics and results from ANCOVAs comparing indices of attentional engagement across phase (baseline vs. test phase), stimulus duration (100, 500 ms) and sex (women vs. men). Conditions include presentation of CS+ and CS− each with a neutral cue and presentation of CS+ and CS− together. The underscore indicates the appearance of the dot-probe at the location either of the CS+ or CS−. Descriptive statistics are given as mean values across 100 and 500 ms stimulus duration ± standard error of the mean (M ± SEM). Significant interactions are given in bold.
Figure 3.
Sex differences in attentional biases. Mean indices of attentional engagement (A) and disengagement (B) given in ms for women (red) and men (blue) for the dot-probe tasks accomplished at baseline and during the test phase. Please note that mean values depicted in the figure are not corrected for BMI that was used as covariate in statistical analyses. Significant differences between women and men are given with gray asterisks and within-group differences between baseline and test phase are given with the corresponding color code for women and men. Men compared to women showed higher attentional engagement with the CS+ and CS− when presented with the neutral cue and with the CS+ when presented together with the CS−. Women compared to men demonstrated stronger difficulties to disengage attention from the CS+ when presented with the neutral cue. **p < .010; *p < .050.
To further investigate the specificity of attentional engagement, conditions presenting one CS with the neutral cue were compared with conditions presenting both the CS+ and CS−. For the CS−, analyses revealed an interaction between condition, phase and sex that marginally missed to yield significance (F = 3.87, p = .054). Exploratory post-hoc analyses revealed for women significantly higher attentional engagement with the CS− when presented with the CS+ as compared to the concomitant presentation of the neutral cue (p = .012) while for men no effects were observed.
Attentional Disengagement
For disengagement indices (Table 4, Figure 3B; for single subject data see Figure S4), analyses revealed significant phase × sex interactions for conditions when the CS+ and CS− were each presented with the neutral cue as well as for the CS− when presented with the CS+ (all F > 6.44, all p < .014, all η2 > .11). Stimulus duration again had no effect (all F < 0.66, all p > .421). Post-hoc comparisons between women and men during the test phase yielded significance only when the CS+ was presented with the neutral cue (p = .009) indicating that women had stronger difficulties to disengage attention from the CS+. For the remaining conditions, results did not survive Bonferroni correction (CS−/CSn p = .080; CS−/CS+ p = .062). Moreover, comparisons between baseline and test phase revealed that women had stronger difficulties to disengage from the CS+ when presented with the neutral cue (p = .010) and from the CS− when presented with the CS+ (p = .023) while men demonstrated significant increases in these conditions (both p < .039). Further analyses on the specificity of attentional disengagement between conditions did not yield significance.
Table 4.
Indices of attentional disengagement.
| Women | Men | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Test phase | Baseline | Test phase | Interaction effect | F | p | η2 | |
| CS+/CSn | 2.47 ± 2.25 | −6.98 ± 2.64 | 0.65 ± 2.47 | 3.90 ± 3.46 | Phase × sex | 10.36 | .002 | .18 |
| Phase × duration × sex | 0.66 | .421 | .01 | |||||
| CS+/CS− | 9.04 ± 4.39 | −0.69 ± 2.98 | 0.62 ± 3.51 | 3.81 ± 4.16 | Phase × sex | 1.92 | .173 | .04 |
| Phase × duration × sex | 0.47 | .497 | .01 | |||||
| CS−/CSn | 2.21 ± 3.57 | −5.54 ± 2.66 | −5.48 ± 2.78 | −0.67 ± 2.39 | Phase × sex | 6.44 | .014 | .11 |
| Phase × duration × sex | 0.05 | .831 | .00 | |||||
| CS−/CS+ | 3.49 ± 3.30 | 1.31 ± 2.88 | −6.10 ± 1.81 | −6.14 ± 3.03 | Phase × sex | 9.73 | .003 | .15 |
| Phase × duration × sex | 0.06 | .802 | .00 | |||||
Descriptive statistics and results from ANCOVAs comparing indices of attentional disengagement across phase (baseline vs. test phase), stimulus duration (100, 500 ms) and sex (women vs. men). Conditions include presentation of CS+ and CS− each with a neutral cue and presentation of CS+ and CS− together. The underscore indicates the appearance of the dot-probe at the location either of the CS+ or CS−. Descriptive statistics are given as mean values across 100 and 500 ms stimulus duration ± standard error of the mean (M ± SEM). Significant interactions are given in bold.
Response Slowing
Previous research on the allocation of visuospatial attention demonstrated a response slowing effect of threat indicating that differences in reaction times between trials containing a threat cue and trials containing a neutral cue might arise from avoidance or freezing of motor responses [e.g., (57, 58)]. We tested for this effect by comparing reaction times from conditions presenting the CS+ with a neutral cue with conditions presenting the neutral cue only for 100 and 500 ms stimulus duration. For 100 ms stimulus duration, the cue x congruency interaction yielded significance (F = 7.80, p = .007, η2 = .14) with post-hoc comparisons demonstrating higher reaction times for incongruent compared to congruent CS+ trials (p = .017). However, there were no significant differences between the CS+ and the neutral condition for either congruent or incongruent trials (both p > .102). For 500 ms stimulus duration, there was no significant cue x congruency interaction (F = 1.81, p = .184) indicating no effect of response slowing.
Sex-Specific Associations Between Emotional Learning, Psychological Traits and Attentional Biases
Multiple regression analyses with the main effects of sex, psychological vulnerability factors, and changes in CS valence as well as the interaction terms considering sex were calculated to predict changes in attentional biases from baseline to test phase.
For attentional avoidance, the model with the interaction between sex and negative affect as well as the interaction between sex and active coping yielded significance (F = 4.48, p = .007, adj. R2 = .14). Both, the interaction sex*negative affect (β = 0.48, t = 3.04, p = .004) and sex*active coping emerged as significant predictors (β = −0.45, t = 2.26, p = .028) while sex alone did not yield significance (β = 0.06, t = 0.26, p = .797) (for more details, see Table S1). Subsequent analyses revealed a larger slope for men (F = 9.03, p = .005, adj. R2 = .23, β = 0.48, t = 3.01) compared to women (F = 0.02, p = .879, adj. R2 = −.03, β = 0.03, t = 0.15), indicating that only in men higher negative affect was associated with stronger avoidance of the CS+ when presented with the CS−. However, for active coping neither regression model yielded significance although the slope observed for men (F = 2.76, p = .107, adj. R2 = .06, β = −0.29, t = 1.67) was higher compared to women (F = 2.76, p = .107, adj. R2 = .06, β = −0.29, t = 1.67).
For attentional engagement, sex was found as a significant predictor (F = 4.27, p = .043, adj. R2 = .05, β = 0.25, t = 2.07) for the condition presenting the CS+ together with the CS− while regression models including psychological vulnerability factors and changes in CS valence failed to reach significance (F = 3.77, p = .057, adj. R2 = .04) (Table S2).
For attentional disengagement, sex emerged as a significant predictor for conditions presenting the CS+ with the neutral cue (F = 4.95, p = .030, adj. R2 = .06, β = 0.27, t = 2.22) and the CS− with the neutral cue (F = 5.14, p = .027, adj. R2 = .06, β = 0.28, t = 2.27) (Tables S3, S4). Moreover, for the condition presenting the CS− together with the CS+, the model including sex, negative affect and sex*negative affect yielded significance (F = 4.93, p = .004, adj. R2 = .16) (Table S5). Herein, sex (β = 1.13, t = 3.18, p = .002), negative affect (β = 0.84, t = 2.36, p = .022) and the interaction between sex and negative affect (β = 1.54, t = 3.19, p = .002) were found as significant predictors. Subsequent regression analyses for the interaction effect revealed a larger slope for men (F = 16.99, p < .001, adj. R2 = .34, β = −0.60, t = 4.12) compared to women (F = 0.36, p = .555, adj. R2 = −.02, β = 0.11, t = 0.60), indicating that only in men higher negative affect was related to stronger difficulties to disengage from the CS−.
Discussion
Although the broad role of fear and hypervigilance in the transition from acute to chronic pain is widely acknowledged, pain-related attentional biases as putative neurocognitive mechanism remain incompletely understood. In addition, sex differences as they are considered highly relevant to both acute and chronic pain are rarely systematically studied, especially in the context of visceral pain (62). The aim of this study was to elucidate attentional biases induced by pain-related conditioning in healthy women and men. To this end, we implemented a visual dot-probe task before and after differential fear conditioning with visceral pain as highly salient and clinically relevant interoceptive US (12, 13, 63). Conditioning successfully induced emotional pain-related learning to threat and safety cues, as evidenced by differential changes in cue valence. Consistent with behavioral and neural findings in our earlier conditioning studies (12, 19, 21), visual cues that were contingently paired with visceral pain became highly unpleasant predictors of threat, likely reflecting conditioned pain-related fear in anticipation of visceral pain. In contrast, cues that predicted the absence of impending pain acquired positive emotional valence, consistent with their role as conditioned safety signals. There was no evidence of sex differences in emotional pain-related learning, confirming earlier behavioral results in a different, smaller sample of healthy volunteers (23). Similarly, men and women did not differ in contingency awareness of cue-pain relationships, which was fairly accurate in both sexes. Interestingly, comparing men and women with respect to attentional avoidance, facilitated engagement, and difficulties to disengage from visceral pain-related threat and safety cues revealed novel evidence supporting sex differences in attentional biases. Specifically, men showed more facilitated attentional engagement with both threat and safety cues. Women, on the other hand, demonstrated more pronounced difficulties to disengage from threat in the presence of a neutral cue. However, when both threat and safety cues were presented, women showed stronger difficulties to disengage from the safety cue. While these findings do not support our hypothesis of facilitated threat engagement in women, they are consistent with a proposed bias toward safety cues. While the role of conditioned safety signals remains incompletely understood and arguably underappreciated, we previously reported distinctly altered neural processes during safety learning in healthy women (18) as well as in women with IBS (17). Specifically, IBS patients demonstrated a more pronounced positive valence increase to conditioned safety cues, higher awareness of safety cue contingencies, as well as greater neural responses involving regions relevant to reward processing (17) and conditioned autonomic, somatomotor and cognitive fear responses (16). Distinct neural networks engaged during the acquisition and extinction of conditioned threat vs. safety cues have not only been reported in our model, but also more broadly in the fear conditioning literature (64). Together, our findings support that visceral pain-related fear conditioning induces attentional biases differently in healthy women and men, supporting a role of sex or gender in attentional mechanisms underlying hypervigilance.
Attentional biases to signals predicting experimental pain have previously been shown, albeit outside of interoceptive, visceral pain, as summarized in recent meta-analyses (11, 65). Our results complement results gathered in different conditioning paradigms (47, 48, 66–69), and extend knowledge regarding the role of pain-related fear as a putative mediator. Moreover, several psychological state and trait factors demonstrably contribute to inter-individual differences in the modulation of pain (22, 70, 71), which have thus far rarely been considered in experimental studies on pain-related attentional biases. Therefore, we explored sex differences in relationships between attentional biases and positive and negative affect, trait anxiety, pain-related coping and catastrophizing using regression analyses. Our results revealed an influence of sex on negative affect in predicting attentional threat avoidance and difficulties to disengage from the safety cue. This finding is well in line with the fear avoidance model of chronic pain, emphasizing that a higher propensity toward experiencing negative emotions impacts upon pain control and increases pain-related fear, thereby promoting avoidance behaviors (72). Interestingly, these effects were observed in men while no relationships between psychological vulnerability factors and attentional biases were observed in women. While this is at odds with our hypothesis, we cannot with certainty exclude a self-selection bias for participation due to the research setting at a university hospital and the nature of the study, specifically concerning the application of painful stimuli (73). This may have resulted in an overall sample of healthy individuals presenting with rather low anxiety and negative affect and may have specifically discouraged healthy women with higher experience of gastrointestinal symptoms. Therefore, the translation to the general population or patient populations is limited. Moreover, it has previously been suggested that individuals with high fear of pain exhibit a selective attention bias toward pain-related information, supporting that biased attentional processes mediate increased susceptibility to negative pain experience (74, 75). Our conditioning model experimentally induces fear as a state, rather than a trait assessed by questionnaire. This calls attention to the fact that fear of pain and by inference pain-related attentional biases are likely shaped by more permanent, trait-like factors, as well as more state-like learning processes regarding threat and reward. Hence, inter-individual differences in pain-related attentional biases could be explained by pre-existing differences in fear of pain, as previously shown in our visceral pain model (12), as well as by sex differences, as suggested herein.
Herein, men and women were comparable with respect to pain thresholds and pain ratings, and the intensity of pain stimuli implemented during conditioning was individually calibrated, supporting that sex differences in attentional biases were not attributable to differences in the response to pain or in the strength of emotional learning to either threat or safety cues. Further strengths of this study include the ecological validity and translational qualities of the visceral pain model, as rectal distension-induced pain is highly salient even in healthy individuals (12) and closely resembles clinical pain and related symptoms such as urgency (73) in functional gastrointestinal disorders like IBS (76). In the context of attentional bias research, the utilization of semantic and pictorial threat stimuli has raised concerns about their ecological validity and generalizability (26), resulting in calls for research with paradigms that closely resemble real-life situations (77) or consider motivational context (69). In line with these efforts, our model offers research perspectives in patient populations, especially those with somatic symptom disorder or chronic visceral pain such as in IBS. Utilizing modified Stroop and exogenous cueing tasks, IBS patients revealed alterations in attentional processing for pain and symptom-related words (78, 79) as well as situational threat words (80). This attentional bias was moreover associated with symptom severity, illness behaviors and anxiety (49, 80, 81). Hence, increased attention to interoceptive, visceral sensations may lead to the exacerbation of symptoms and distress (82) in line with the fear avoidance model, which has yet to be more fully tested in the context of chronic visceral pain and the gut-brain axis. Neuroimaging studies support this assumption by demonstrating increased functional connectivity in the salience network in IBS patients during resting state (83), rectal stimulation (84) and contextual threat situations (85). Initial support that the behavioral modification of attentional bias may improve attentional functioning and regulation of brain mechanisms related to anxiety and attention in IBS patients (86, 87), provides a treatment perspective to complement more basic mechanistic research.
Lastly, our findings are relevant toward further elucidating the neurocognitive and emotional mechanisms of associative learning and memory processes in the context of visceral pain. Brain imaging studies revealed conditioning-induced changes in the brain emotional arousal and salience networks, supporting that conditioning processes contribute to hypervigilance, possibly by engaging nocebo mechanisms (58). It has also been proposed that pain-related conditioning may impair perceptual discrimination acuity (88), enhance fear generalization (89) or interfere with normal habituation processes (90). Although our attempts to show hyperalgesia as a result of conditioning provided negative results (21, 63), data from other groups do support sensitization (91, 92) and lowered pain thresholds (93). Regardless of these inconsistencies and a clear need for further study, it is important to emphasize that different yet intricately intertwined mechanisms engaged during associative learning are clearly not mutually exclusive. They may indeed play distinct roles in modulating responses to acute pain, shaping the transition from acute to chronic pain and the maintenance of pain. Importantly, attentional biases arguably play a role in many if not all of these proposed processes, and could thus be viewed as a fundamental neurocognitive mechanism that is shaped by pain-related learning and memory processes (48, 94). This is supported by first evidence that attentional bias to threat signals is still present after extinction (47) and re-emerges during reinstatement (48), consistent with our brain imaging work on the reactivation of previously extinguished responses to conditioned pain and safety cues induced by reinstatement (20, 56) or renewal (19).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the University Hospital Essen, Germany. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
FL and SE planned the study, contributed to data analysis and interpretation and wrote the manuscript. FL and SK-R were involved in conducting the study. All authors: revision of the manuscript for critical intellectual content and approval of the final draft submitted. All authors contributed to manuscript revision, read and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Christoph Ritter and Ann-Kathrin Stock for excellent technical support and Madeleine Hetkamp and Simone Kotulla for excellent support in conducting this project.
Footnotes
Funding. This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) as part of the collaborative research center Extinction Learning (project number 316803389—SFB 1280), and by funding to support gender research provided by the Ministry for Innovation, Science and Research of the State of North Rhine-Westphalia. The funding sources had no role in the conception, analysis or interpretation of this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2020.00197/full#supplementary-material
References
- 1.Flor H. New developments in the understanding and management of persistent pain. Curr Opin Psychiatry. (2012) 25:109–13. 10.1097/YCO.0b013e3283503510 [DOI] [PubMed] [Google Scholar]
- 2.Harvie DS, Moseley GL, Hillier SL, Meulders A. Classical conditioning differences associated with chronic pain: a systematic review. J Pain. (2017) 18:889–98. 10.1016/j.jpain.2017.02.430 [DOI] [PubMed] [Google Scholar]
- 3.Crombez G, Eccleston C, Van Damme S, Vlaeyen JWS, Karoly P. Fear-Avoidance model of chronic pain. Clin J Pain. (2012) 28:475–83. 10.1097/AJP.0b013e3182385392 [DOI] [PubMed] [Google Scholar]
- 4.Hollander M den, de Jong JR, Volders S, Goossens ME, Smeets RJ, Vlaeyen JW. Fear reduction in patients with chronic pain: a learning theory perspective. Expert Rev Neurother. (2010) 10:1733–45. 10.1586/ern.10.115 [DOI] [PubMed] [Google Scholar]
- 5.Van Ryckeghem DML, Crombez G, Goubert L, De Houwer J, Onraedt T, Van Damme S. The predictive value of attentional bias towards pain-related information in chronic pain patients: a diary study. Pain. (2013) 154:468–75. 10.1016/j.pain.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 6.Schoth DE, Nunes VD, Liossi C. Attentional bias towards pain-related information in chronic pain; a meta-analysis of visual-probe investigations. Clin Psychol Rev. (2012) 32:13–25. 10.1016/j.cpr.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 7.Cisler JM, Bacon AK, Williams NL. Phenomenological characteristics of attentional biases towards threat: a critical review. Cognit Ther Res. (2009) 33:221–34. 10.1007/s10608-007-9161-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koster EHW, Crombez G, Verschuere B, Van Damme S, Wiersema JR. Components of attentional bias to threat in high trait anxiety: facilitated engagement, impaired disengagement, and attentional avoidance. Behav Res Ther. (2006) 44:1757–71. 10.1016/j.brat.2005.12.011 [DOI] [PubMed] [Google Scholar]
- 9.Mogg K, Bradley BP. Anxiety and attention to threat: cognitive mechanisms and treatment with attention bias modification. Behav Res Ther. (2016) 87:76–108. 10.1016/j.brat.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 10.Schoorl M, Putman P, Van Der Werff S, Van Der Does AJW. Attentional bias and attentional control in posttraumatic stress disorder. J Anxiety Disord. (2014) 28:203–10. 10.1016/j.janxdis.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 11.Crombez G, Van Ryckeghem DML, Eccleston C, Van Damme S. Attentional bias to pain-related information: a meta-analysis. Pain. (2013) 154:497–510. 10.1016/j.pain.2012.11.013 [DOI] [PubMed] [Google Scholar]
- 12.Koenen LR, Icenhour A, Forkmann K, Pasler A, Theysohn N, Forsting M, et al. Greater fear of visceral pain contributes to differences between visceral and somatic pain in healthy women. Pain. (2017) 158:1599–608. 10.1097/j.pain.0000000000000924 [DOI] [PubMed] [Google Scholar]
- 13.Koenen LR, Icenhour A, Forkmann K, Theysohn N, Forsting M, Bingel U, et al. From anticipation to the experience of pain: the importance of visceral versus somatic pain modality in neural and behavioral responses to pain-predictive cues. Psychosom Med. (2018) 80:826–35. 10.1097/PSY.0000000000000612 [DOI] [PubMed] [Google Scholar]
- 14.Schmidt LJ, Belopolsky AV, Theeuwes J. The time course of attentional bias to cues of threat and safety. Cogn Emot. (2017) 31:845–57. 10.1080/02699931.2016.1169998 [DOI] [PubMed] [Google Scholar]
- 15.Vogt J, Koster EHW, De Houwer J. Safety first: instrumentality for reaching safety determines attention allocation under threat. Emotion. (2017) 17:528–37. 10.1037/emo0000251 [DOI] [PubMed] [Google Scholar]
- 16.Claassen J, Labrenz F, Ernst TM, Icenhour A, Langhorst J, Forsting M, et al. Altered cerebellar activity in visceral pain-related fear conditioning in irritable bowel syndrome. Cerebellum. (2017) 16:508–17. 10.1007/s12311-016-0832-7 [DOI] [PubMed] [Google Scholar]
- 17.Icenhour A, Langhorst J, Benson S, Schlamann M, Hampel S, Engler H, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil. (2015) 27:114–27. 10.1111/nmo.12489 [DOI] [PubMed] [Google Scholar]
- 18.Labrenz F, Icenhour A, Thürling M, Schlamann M, Forsting M, Timmann D, et al. Sex differences in cerebellar mechanisms involved in pain-related safety learning. Neurobiol Learn Mem. (2015) 123:92–9. 10.1016/j.nlm.2015.05.006 [DOI] [PubMed] [Google Scholar]
- 19.Icenhour A, Kattoor J, Benson S, Boekstegers A, Schlamann M, Merz CJ, et al. Neural circuitry underlying effects of context on human pain-related fear extinction in a renewal paradigm. Hum Brain Mapp. (2015) 36:3179–93. 10.1002/hbm.22837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kattoor J, Gizewski ER, Kotsis V, Benson S, Gramsch C, Theysohn N, et al. Fear conditioning in an abdominal pain model: neural responses during associative learning and extinction in healthy subjects. PLoS ONE. (2013) 8:e51149. 10.1371/journal.pone.0051149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labrenz F, Icenhour A, Schlamann M, Forsting M, Bingel U, Elsenbruch S. From Pavlov to pain: how predictability affects the anticipation and processing of visceral pain in a fear conditioning paradigm. Neuroimage. (2016) 130:104–14. 10.1016/j.neuroimage.2016.01.064 [DOI] [PubMed] [Google Scholar]
- 22.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL. Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. (2009) 10:447–85. 10.1016/j.jpain.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benson S, Kattoor J, Kullmann JS, Hofmann S, Engler H, Forsting M, et al. Towards understanding sex differences in visceral pain: enhanced reactivation of classically-conditioned fear in healthy women. Neurobiol Learn Mem. (2014) 109:113–21. 10.1016/j.nlm.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 24.Keogh E, Hatton K, Ellery D. Avoidance versus focused attention and the perception of pain: differential effects for men and women. Pain. (2000) 85:225–30. 10.1016/S0304-3959(99)00270-5 [DOI] [PubMed] [Google Scholar]
- 25.Brookes M, Sharpe L, Kozlowska K. Attentional and interpretational biases toward pain-related stimuli in children and adolescents: asystematic review of the evidence. J Pain. (2018) 19:1091–101. 10.1016/j.jpain.2018.04.010 [DOI] [PubMed] [Google Scholar]
- 26.Dear BF, Sharpe L, Nicholas MK, Refshauge K. Pain-related attentional biases: the importance of the personal relevance and ecological validity of stimuli. J Pain. (2011) 12:625–32. 10.1016/j.jpain.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 27.MacLeod C, Grafton B. Anxiety-linked attentional bias and its modification: illustrating the importance of distinguishing processes and procedures in experimental psychopathology research. Behav Res Ther. (2016) 86:68–86. 10.1016/j.brat.2016.07.005 [DOI] [PubMed] [Google Scholar]
- 28.Christianson JP, Fernando ABP, Kazama AM, Jovanovic T, Ostroff LE, Sangha S. Inhibition of fear by learned safety signals: a mini-symposium review. J Neurosci. (2012) 32:14118–24. 10.1523/JNEUROSCI.3340-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erdfelder E, Faul F, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods. (2009) 41:1149–60. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- 30.Herrmann-Lingen C, Buss U, Snaith R. Hospital Anxiety and Depression Scale - Deutsche Version. Second. Bern: Hans Huber; (2005). [Google Scholar]
- 31.Lacourt TE, Houtveen JH, Doornen LJP, Benson S, Grigoleit JS, Cesko E, et al. Biological and psychological predictors of visceral pain sensitivity in healthy premenopausal women. Eur J Pain. (2014) 18:567–74. 10.1002/j.1532-2149.2013.00397.x [DOI] [PubMed] [Google Scholar]
- 32.Laux L, Glanzmann P, Schaffner P, Spielberger C. Das State-Trait-Angstinventar. Theoretische Grundlagen und Handanweisung. Weinheim: Beltz; (1981). [Google Scholar]
- 33.Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther. (1993) 31:63–73. 10.1016/0005-7967(93)90044-U [DOI] [PubMed] [Google Scholar]
- 34.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. (1988) 54:1063–70. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- 35.Partington J, Leiter R. Partington's pathway test. Psychol Serv Cent J. (1949) 1:9–20. [Google Scholar]
- 36.Tombaugh TN. Trail making test a and b: normative data stratified by age and education. Arch Clin Neuropsychol. (2004) 19:203–14. 10.1016/S0887-6177(03)00039-8 [DOI] [PubMed] [Google Scholar]
- 37.Foroozandeh E. Gender differences in trail making test performance in a nonclinical sample of adults. Int J Clin Exp Neurol. (2014) 2:1–3. 10.12691/ijcen-2-1-1 [DOI] [Google Scholar]
- 38.Macleod CM. The stroop task: the “gold standard” of attentional measures. J Exp Psychol Gen. (1992) 121:12–4. 10.1037/0096-3445.121.1.12 [DOI] [Google Scholar]
- 39.Stroop JR. Studies of interference. J Exp Psychol. (1935) 18:643–62. 10.1037/h0054651 [DOI] [Google Scholar]
- 40.MacLeod Colin M. Half a century of research on the stroop effect: an integrative review. Psychol Bull. (1991) 109:163–203. 10.1037/0033-2909.109.2.163 [DOI] [PubMed] [Google Scholar]
- 41.Mekarski JE, Cutmore TRH, Suboski W. Gender differences during processing of the stroop task. Percept Mot Skills. (1996) 83:563–8. 10.2466/pms.1996.83.2.563 [DOI] [PubMed] [Google Scholar]
- 42.Frewen PA, Dozois DJA, Joanisse MF, Neufeld RWJ. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clin Psychol Rev. (2008) 28:307–37. 10.1016/j.cpr.2007.05.006 [DOI] [PubMed] [Google Scholar]
- 43.Deltomme B, Mertens G, Tibboel H, Braem S. Instructed fear stimuli bias visual attention. Acta Psychol (Amst). (2018) 184:31–8. 10.1016/j.actpsy.2017.08.010 [DOI] [PubMed] [Google Scholar]
- 44.Koenig S, Uengoer M, Lachnit H. Attentional bias for uncertain cues of shock in human fear conditioning: evidence for attentional learning theory. Front Hum Neurosci. (2017) 11:1–13. 10.3389/fnhum.2017.00266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schrooten MGS, Van Damme S, Crombez G, Peters ML, Vogt J, Vlaeyen JWS. Nonpain goal pursuit inhibits attentional bias to pain. Pain. (2012) 153:1180–6. 10.1016/j.pain.2012.01.025 [DOI] [PubMed] [Google Scholar]
- 46.MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. J Abnorm Psychol. (1986) 95:15–20. 10.1037/0021-843X.95.1.15 [DOI] [PubMed] [Google Scholar]
- 47.Van Damme S, Crombez G, Eccleston C, Koster EHW. Hypervigilance to learned pain signals: a componential analysis. J Pain. (2006) 7:346–57. 10.1016/j.jpain.2005.12.006 [DOI] [PubMed] [Google Scholar]
- 48.Van Damme S, Crombez G, Hermans D, Koster EHW, Eccleston C. The role of extinction and reinstatement in attentional bias to threat: a conditioning approach. Behav Res Ther. (2006) 44:1555–63. 10.1016/j.brat.2005.11.008 [DOI] [PubMed] [Google Scholar]
- 49.Boyer MC, Compas BE, Stanger C, Colletti RB, Konik BS, Morrow SB, et al. Attentional biases to pain and social threat in children with recurrent abdominal pain. J Pediatr Psychol. (2006) 31:209–20. 10.1093/jpepsy/jsj015 [DOI] [PubMed] [Google Scholar]
- 50.Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND, et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychol Assess. (2015) 27:365–76. 10.1037/pas0000036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koster EHW, Verschuere B, Crombez G, Van Damme S. Time-course of attention for threatening pictures in high and low trait anxiety. Behav Res Ther. (2005) 43:1087–98. 10.1016/j.brat.2004.08.004 [DOI] [PubMed] [Google Scholar]
- 52.Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: testing the vigilance-avoidance hyporthesis. Cogn Emot. (2004) 18:689–700. 10.1080/02699930341000158 [DOI] [Google Scholar]
- 53.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, Van Ijzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull. (2007) 133:1–24. 10.1037/0033-2909.133.1.1 [DOI] [PubMed] [Google Scholar]
- 54.Koster EHW, Crombez G, Verschuere B, De Houwer J. Selective attention to threat in the dot probe paradigm: differentiating vigilance and difficulty to disengage. Behav Res Ther. (2004) 42:1183–92. 10.1016/j.brat.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 55.Elsenbruch S, Rosenberger C, Enck P, Forsting M, Schedlowski M, Gizewski ER. Affective disturbances modulate the neural processing of visceral pain stimuli in irritable bowel syndrome: an fMRI study. Gut. (2010) 59:489–95. 10.1136/gut.2008.175000 [DOI] [PubMed] [Google Scholar]
- 56.Gramsch C, Kattoor J, Icenhour A, Forsting M, Schedlowski M, Gizewski ER, et al. Learning pain-related fear: neural mechanisms mediating rapid differential conditioning, extinction and reinstatement processes in human visceral pain. Neurobiol Learn Mem. (2014) 116:36–45. 10.1016/j.nlm.2014.08.003 [DOI] [PubMed] [Google Scholar]
- 57.Lonsdorf TB, Menz MM, Andreatta M, Fullana MA, Golkar A, Haaker J, et al. Don't fear ‘fear conditioning': methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci Biobehav Rev. (2017) 77:247–85. 10.1016/j.neubiorev.2017.02.026 [DOI] [PubMed] [Google Scholar]
- 58.Elsenbruch S, Labrenz F. Nocebo effects and experimental models in visceral pain. Int Rev Neurobiol. (2018) 138:285–306. 10.1016/bs.irn.2018.01.010 [DOI] [PubMed] [Google Scholar]
- 59.Enck P, Chae Y, Elsenbruch S. Novel designs and paradigms to study the placebo response in gastroenterology. Curr Opin Pharmacol. (2017) 37:72–9. 10.1016/j.coph.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 60.Dirikx T, Hermans D, Vansteenwegen D, Baeyens F, Eelen P. Reinstatement of extinguished conditioned responses and negative stimulus valence as a pathway to return of fear in humans. Learn Mem. (2004) 11:549–54. 10.1101/lm.78004 [DOI] [PubMed] [Google Scholar]
- 61.Dour HJ, Brown LA, Craske MG. Positive valence reduces susceptibility to return of fear and enhances approach behavior. J Behav Ther Exp Psychiatry. (2016) 50:277–82. 10.1016/j.jbtep.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 62.Icenhour A, Labrenz F, Roderigo T, Siebert C, Elsenbruch S, Benson S. Are there sex differences in visceral sensitivity in young healthy men and women? Neurogastroenterol Motil. (2019) 31:e13664. 10.1111/nmo.13664 [DOI] [PubMed] [Google Scholar]
- 63.Icenhour A, Labrenz F, Ritter C, Theysohn N, Forsting M, Bingel U, et al. Learning by experience? visceral pain-related neural and behavioral responses in a classical conditioning paradigm. Neurogastroenterol Motil. (2017) 29:e13026. 10.1111/nmo.13026 [DOI] [PubMed] [Google Scholar]
- 64.Fullana MA, Harrison BJ, Soriano-Mas C, Vervliet B, Cardoner N, Àvila-Parcet A, et al. Neural signatures of human fear conditioning: an updated and extended meta-analysis of fMRI studies. Mol Psychiatry. (2016) 21:500–8. 10.1038/mp.2015.88 [DOI] [PubMed] [Google Scholar]
- 65.Todd J, van Ryckeghem DML, Sharpe L, Crombez G. Attentional bias to pain-related information: a meta-analysis of dot-probe studies. Health Psychol Rev. (2018) 12:419–36. 10.1080/17437199.2018.1521729 [DOI] [PubMed] [Google Scholar]
- 66.Van Damme S, Lorenz J, Eccleston C, Koster EH, De Clercq A, Crombez G. Fear-conditioned cues of impending pain facilitate attentional engagement. Neurophysiol Clin. (2004) 34:33–9. 10.1016/j.neucli.2003.11.001 [DOI] [PubMed] [Google Scholar]
- 67.Van Damme S, Crombez G, Eccleston C, Goubert L. Impaired disengagement from threatening cues of impending pain in a crossmodal cueing paradigm. Eur J Pain. (2004) 8:227–36. 10.1016/j.ejpain.2003.08.005 [DOI] [PubMed] [Google Scholar]
- 68.Van Damme S, Crombez G, Eccleston C. The anticipation of pain modulates spatial attention: evidence for pain-specificity in high-pain catastrophizers. Pain. (2004) 111:392–9. 10.1016/j.pain.2004.07.022 [DOI] [PubMed] [Google Scholar]
- 69.Schrooten MGS, Van Damme S, Crombez G, Kindermans H, Vlaeyen JWS. Winning or not losing? The impact of non-pain goal focus on attentional bias to learned pain signals. Scand J pain. (2018) 18:675–86. 10.1515/sjpain-2018-0055 [DOI] [PubMed] [Google Scholar]
- 70.Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. (2009) 9:745–58. 10.1586/ern.09.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wiech K, Tracey I. The influence of negative emotions on pain: behavioral effects and neural mechanisms. Neuroimage. (2009) 47:987–94. 10.1016/j.neuroimage.2009.05.059 [DOI] [PubMed] [Google Scholar]
- 72.Vlaeyen JWS, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain. (2016) 157:1588–9. 10.1097/j.pain.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 73.Roderigo T, Benson S, Schöls M, Hetkamp M, Schedlowski M, Enck P, et al. Effects of acute psychological stress on placebo and nocebo responses in a clinically relevant model of visceroception. Pain. (2017) 158:1489–98. 10.1097/j.pain.0000000000000940 [DOI] [PubMed] [Google Scholar]
- 74.Keogh E, Ellery D, Hunt C, Hannent I. Selective attentional bias for pain-related stimuli amongst pain fearful individuals. Pain. (2001) 91:91–100. 10.1016/S0304-3959(00)00422-X [DOI] [PubMed] [Google Scholar]
- 75.Todd J, Sharpe L, Colagiuri B. Attentional bias modification and pain: the role of sensory and affective stimuli. Behav Res Ther. (2016) 83:53–61. 10.1016/j.brat.2016.06.002 [DOI] [PubMed] [Google Scholar]
- 76.Keszthelyi D, Troost FJ, Masclee AA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Methods to assess visceral hypersensitivity in irritable bowel syndrome. AJP Gastrointest Liver Physiol. (2012) 303:G141–54. 10.1152/ajpgi.00060.2012 [DOI] [PubMed] [Google Scholar]
- 77.Schoth DE, Ma Y, Liossi C. Exploring attentional bias for real-world, pain-related information in chronic musculoskeletal pain using a novel change detection paradigm. Clin J Pain. (2015) 31:680–8. 10.1097/AJP.0000000000000149 [DOI] [PubMed] [Google Scholar]
- 78.Afzal M, Potokar JP, Probert CSJ, Munafò MR. Selective processing of gastrointestinal symptom-related stimuli in irritable bowel syndrome. Psychosom Med. (2006) 68:758–61. 10.1097/01.psy.0000232270.78071.28 [DOI] [PubMed] [Google Scholar]
- 79.Phillips K, Wright BJ, Kent S. Irritable bowel syndrome and symptom severity: evidence of negative attention bias, diminished vigour, and autonomic dysregulation. J Psychosom Res. (2014) 77:13–9. 10.1016/j.jpsychores.2014.04.009 [DOI] [PubMed] [Google Scholar]
- 80.Tkalcic M, Domijan D, Pletikosic S, Setic M, Hauser G. Attentional biases in irritable bowel syndrome patients. Clin Res Hepatol Gastroenterol. (2014) 38:621–8. 10.1016/j.clinre.2014.02.002 [DOI] [PubMed] [Google Scholar]
- 81.Chapman S, Martin M. Attention to pain words in irritable bowel syndrome: increased orienting and speeded engagement. Br J Heal Psychol. (2011) 16:47–60. 10.1348/135910710X505887 [DOI] [PubMed] [Google Scholar]
- 82.Hauser G, Pletikosic S, Tkalcic M. Cognitive behavioral approach to understanding irritable bowel syndrome. World J Gastroenterol. (2014) 20:6744–58. 10.3748/wjg.v20.i22.6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Icenhour A, Witt ST, Elsenbruch S, Lowén M, Engström M, Tillisch K, et al. Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. NeuroImage Clin. (2017) 15:449–57. 10.1016/j.nicl.2017.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu X, Silverman A, Kern M, Ward BD, Li SJ, Shaker R, et al. Excessive coupling of the salience network with intrinsic neurocognitive brain networks during rectal distension in adolescents with irritable bowel syndrome: a preliminary report. Neurogastroenterol Motil. (2016) 28:43–53. 10.1111/nmo.12695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hong JY, Naliboff B, Labus JS, Gupta A, Kilpatrick LA, Ashe-Mcnalley C, et al. Altered brain responses in subjects with irritable bowel syndrome during cued and uncued pain expectation. Neurogastroenterol Motil. (2016) 28:127–38. 10.1111/nmo.12710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tayama J, Saigo T, Ogawa S, Takeoka A, Hamaguchi T, Hayashida M, et al. Effect of attention bias modification on brain function and anxiety in patients with irritable bowel syndrome: a preliminary electroencephalogram and psycho-behavioral study. Neurogastroenterol Motil. (2017) 29:e13131. 10.1111/nmo.13131 [DOI] [PubMed] [Google Scholar]
- 87.Tayama J, Saigo T, Ogawa S, Takeoka A, Hamaguchi T, Inoue K, et al. Effect of attention bias modification on event-related potentials in patients with irritable bowel syndrome: a preliminary brain function and psycho-behavioral study. Neurogastroenterol Motil. (2018) 29:e13402 10.1111/nmo.13402 [DOI] [PubMed] [Google Scholar]
- 88.Zaman J, Vlaeyen JWS, Van Oudenhove L, Wiech K, Van Diest I. Associative fear learning and perceptual discrimination: a perceptual pathway in the development of chronic pain. Neurosci Biobehav Rev. (2015) 51:118–25. 10.1016/j.neubiorev.2015.01.009 [DOI] [PubMed] [Google Scholar]
- 89.Meulders A, Jans A, Vlaeyen JWS. Differences in pain-related fear acquisition and generalization. Pain. (2015) 156:108–22. 10.1016/j.pain.0000000000000016 [DOI] [PubMed] [Google Scholar]
- 90.Lowén MBO, Mayer E, Tillisch K, Labus J, Naliboff B, Lundberg P, et al. Deficient habituation to repeated rectal distensions in irritable bowel syndrome patients with visceral hypersensitivity. Neurogastroenterol Motil. (2015) 27:646–55. 10.1111/nmo.12537 [DOI] [PubMed] [Google Scholar]
- 91.Jensen K, Kirsch I, Odmalm S, Kaptchuk T, Ingvar M. Classical conditioning of analgesic and hyperalgesic pain responses without conscious awareness. Proc Natl Acad Sci USA. (2015) 112:7863–7. 10.1073/pnas.1504567112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bruce Overmier J. Sensitization, conditioning, and learning: can they help us understand somatization and disability? Scand J Psychol. (2002) 43:105–12. 10.1111/1467-9450.00275 [DOI] [PubMed] [Google Scholar]
- 93.Williams AE, Rhudy JL. The influence of conditioned fear on human pain thresholds: does preparedness play a role? J Pain. (2007) 8:598–606. 10.1016/j.jpain.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 94.Vlaeyen JWS, Morley S, Crombez G. The experimental analysis of the interruptive, interfering, and identity-distorting effects of chronic pain. Behav Res Ther. (2016) 86:23–34. 10.1016/j.brat.2016.08.016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.