Short abstract
Objective
To investigate the prevalence, risk factors and mortality rate for acute respiratory distress syndrome (ARDS) in Chinese patients with sepsis.
Methods
This prospective study was based on data from consecutive patients with sepsis who attended Cangzhou Central Hospital between January 2017 and May 2019 and who developed ARDS. Multivariate logistic regression was used to identify risk factors associated independently with ARDS development.
Results
Of the 150 sepsis patients, 41 (27%) developed ARDS. Smoking history, presence of chronic obstructive pulmonary disease (COPD), the C-reactive protein (CRP) levels and the Acute Physiology and Chronic Health Evaluation II (APACHE II) scores were associated with developing ARDS. Moreover, combination of the four factors had an even better predictive value for risk of ARDS than each factor alone. 28-day mortality was higher in sepsis patients with ARDS compared with those without ARDS.
Conclusions
In Chinese patients with sepsis, ARDS is relatively common and is associated with increased mortality. Smoking, COPD, CRP levels and APACHE II scores may be useful in predicting sepsis patients who may be at risk of developing ARDS.
Keywords: Sepsis, acute respiratory distress syndrome, risk factors
Introduction
Worldwide, an estimated 19 million people each year develop sepsis, a life-threatening organ dysfunction caused by an aberrant immune response to an infection.1,2 Acute respiratory distress syndrome (ARDS) is a common complication of sepsis.3–6 It develops because the systemic infection produces general inflammation and unrestrained release of inflammatory mediators which result in severe lung injury. ARDS is responsible for a high mortality in patients with sepsis and is associated with a reduced quality of life for survivors.6 Despite understanding the pathophysiology of the condition and advances in therapies, few treatments have been effective in improving outcomes in patients with sepsis and ARDS.4–6 For those patients who do survive, both conditions are associated with significant psychological and financial burdens to families and society as a whole.6
Previous studies have shown that several sociodemographic and clinical characteristics of sepsis patients were associated with a risk of developing ARDS.7–10 For example, one study found that pneumonia, coagulation score and the central nervous system (CNS) score were independent risk factors for ARDS in Korean patients with sepsis.10 In addition ARDS is correlated with poor prognosis in sepsis patients.8–11 For example, one study showed that the 28-day mortality rate was higher in Korean patients with sepsis and ARDS (64%) compared with sepsis patients without ARDS (9%).10 These data were confirmed by another study performed in Switzerland that found patients with sepsis and ARDS had shorter survival rates than those without ARDS (58% vs. 31%, respectively).11
Data on the prevalence, mortality and risk factors for patients with sepsis who develop ARDS in China is sparse. One study focused on patients in intensive care units (ICUs) irrespective of the presence of sepsis.12 Therefore, the aim of this present, prospective study was to evaluate the prevalence, potential risk factors and mortality rates of Chinese patients with sepsis who developed ARDS.
Methods
This prospective study included consecutive patients admitted to Cangzhou Central Hospital with sepsis between January 2017 and May 2019. Patients considered eligible for the study were ≥18 years of age, diagnosed with sepsis according to Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3)13 and had no history of cancer or haematological malignancy. Exclusion criteria included: death within 24 hours of admission; positive for human immunodeficiency virus; had received immunosuppressive therapy within previous six months; pregnant or lactating women.
Clinical characteristics collected after enrolment, included: age; sex; body mass index (BMI); smoking status; chronic comorbidities (e.g., chronic obstructive pulmonary disease [COPD], cardiomyopathy, chronic kidney failure (glomerular filtration rate persistently <15 ml/min/1.73 m2)14 and cirrhosis). Laboratory indexes measured immediately after admission included: serum creatinine, albumin, white blood cell count (WBC); C-reactive protein (CRP). In addition, within 24 hours after admission, the severity of the patients’ condition was assessed by independent clinicians using the Acute Physiology and Chronic Health Evaluation (APACHE) II score and the Sequential Organ Failure Assessment (SOFA) score.15,16
Treatments and resuscitation were administered to patients based on recommendations of the International Guidelines for Management of Sepsis and Septic Shock.17 All patients were followed-up for 28 days and for those who were discharged within that period, follow-up was by telephone or clinic visit. During hospitalization all patients were closely monitored especially those with pulmonary risk factors such as pneumonia, inhalational injury and pulmonary contusion. ARDS was defined according to established criteria18 on the basis of onset, chest imaging (i.e., Chest X-ray or computed tomography [CT] scan) and origin of oedema. Patients with ARDS received mechanical ventilation with a tidal volume of 6 ml/kg predicted body weight and a positive end-expiratory pressure of 12-15 cm H2O. The plateau pressure was maintained below 30 cm H2O. Patients with severe ARDS received prone positioning for more than 12h/day.
According to previous studies,8,19–21 the following variables were selected as risk factors for ARDS development in sepsis patients: baseline demographic characteristics (i.e., age, sex, BMI and smoking status); chronic comorbidities; laboratory indices (i.e., WBC, CRP); APACHE II scores; SOFA scores. The study was approved by the Ethics Committee of Cangzhou Central Hospital and all patients provided written informed consent.
Statistical analyses
Data were analysed using the Statistical Package for Social Sciences (SPSS®) for Windows® release 24.0 (IBM, Chicago, IL, USA) and figures were plotted using GraphPad Prism 7.01 software (GraphPad Software, San Diego, California, USA). A P-value <0.05 was considered to indicate statistical significance.
Continuous variables were checked for normality using a quantile-quantile plot (Q-Q plot). Normally or approximately normally distributed variables were presented as mean ± standard deviation (SD) and skewed or unknown distributed variables were expressed as median and interquartile range (IQR). Categorical variables were presented as frequency (percentage). Groups were compared using χ-square, Student’s t test or Wilcoxon rank sum test as appropriate.
Univariate logistic regression analysis model was used to examine factors predicting ARDS risk, and a forward stepwise multivariate logistic regression model was used to screen independent predictors for ARDS risk. The predictive performance of single independent predictors and combined independent predictors of risk for ARDS was further assessed by plotting receiver operating characteristic (ROC) curves and calculating sensitivity, specificity and area under the curve (AUC)s with 95% confidence intervals (CIs).
Results
In total, 150 patients with sepsis were enrolled into this study and followed-up for 28 days. Baseline characteristics of the patients are shown in Table 1. The mean age (±SD) was 56.9±10.3 years, 39% of the population were smokers and 65% were male. Overall, 15% of the population had COPD and lung infection was the primary cause of sepsis for 41% patients.
Table 1.
Demographic baseline characteristics of the 150 patients with sepsis.
| Characteristic | Study populationn = 150 |
|---|---|
| Age, years | 56.9 ± 10.3 |
| Sex, male | 98 (65) |
| Sex, female | 52 (35) |
| BMI, kg/m2 | 22.8 ± 4.8 |
| Smoker | 58 (39) |
| Chronic comorbidities | |
| COPD | 23 (15) |
| Cardiomyopathy | 49 (33) |
| Chronic kidney failure | 18 (12) |
| Cirrhosis | 28 (19) |
| Primary infection | |
| Lung infection | 61 (41) |
| Urinary tract infection | 44 (29) |
| Intra-abdomen infection | 23 (15) |
| Skin and soft tissue infection | 10 (7) |
| Bone and joints infection | 3 (2) |
| Other infection | 9 (6) |
| Laboratory indexes | |
| Serum creatinine, mg/dl | 1.7 (1.2-2.4) |
| Albumin (g/l) | 27.2 (21.6-36.8) |
| White blood cells (x109/l) | 14.0 (2.9-28.1) |
| C-reactive protein (mg/l) | 98.5 (52.7-128.8) |
| APACHE II score | 16.3 ± 6.3 |
| SOFA score | 7.3 ± 3.2 |
Data are presented as mean ± standard deviation or median (interquartile range) or n (%)
BMI, body mass index; COPD, chronic obstructive pulmonary disease; APACHE II score, acute physiology and chronic health evaluation II score; SOFA score, sequence organ failure assessment score.
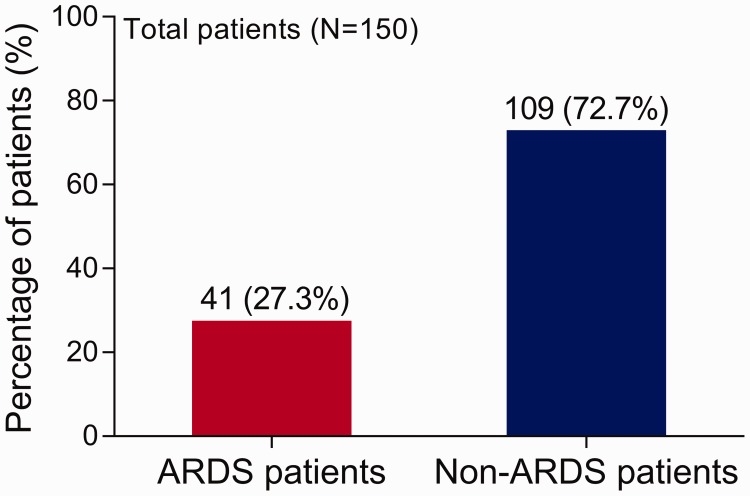
During hospitalization, 41 (27%) of sepsis patients developed ARDS (Figure 1). By comparison with sepsis patients without ARDS, sepsis patients with ARDS were statistically significantly older (P = 0.006), had a higher smoking prevalence (P = 0.021), higher COPD prevalence (P = 0.004), greater CRP level (P = 0.001) and higher APACHE II and SOFA scores (P < 0.001 and P = 0.038, respectively) (Table 2). There were also statistically significant (P < 0.001) differences between groups in the primary site of infection. For the ARDS group, lung infection was the most common primary infection site whereas for the non-ARDS group, intra-abdominal and urinary tract infections were more common as the primary sites. There were no differences between groups in sex, BMI, other comorbidities or any other laboratory indices.
Figure 1.
The percentage of sepsis patients with and without acute respiratory distress syndrome (ARDS) (n=150).
Table 2.
Comparison of clinical characteristics of sepsis patients with and without acute respiratory distress syndrome (ARDS).
| Characteristic | ARDSn = 41 | Non-ARDSn = 109 | Statistical significance |
|---|---|---|---|
| Age, years | 60.6 ± 10.4 | 55.5 ± 10.0 | P = 0.006 |
| Sex | ns | ||
| Male | 27 (66) | 71 (65) | |
| Female | 14 (34) | 38 (35) | |
| BMI, kg/m2 | 23.5 ± 4.9 | 22.6 ± 4.8 | ns |
| Smoker | 22 (54) | 36 (33) | P = 0.021 |
| Chronic comorbidities | |||
| COPD | 12 (29.3) | 11 (10) | P = 0.004 |
| Cardiomyopathy | 14 (34) | 35 (32) | ns |
| Chronic kidney failure | 7 (17) | 11 (10) | ns |
| Cirrhosis | 6 (15) | 22 (20) | ns |
| Primary infection | P<0.001 | ||
| Lung infection | 30 (73) | 31 (28) | |
| Urinary tract infection | 5 (12) | 39 (36) | |
| Intra-abdomen infection | 3 (7) | 20 (18) | |
| Skin and soft tissue infection | 2 (5) | 8 (7) | |
| Bone and joints infection | 0 (0) | 3 (3) | |
| Other infection | 1 (2) | 8 (7) | |
| Laboratory indexes | |||
| Serum creatinine, mg/dl | 1.8 (1.3-3.1) | 1.6 (1.1-2.2) | ns |
| Albumin (g/l) | 27.8 (20.6-38.3) | 27.2 (21.7-36.7) | ns |
| White blood cells (x109/l) | 19.2 (3.2-30.1) | 12.5 (2.9-26.9) | ns |
| C-reactive protein (mg/l) | 131.2 (72.1-217.1) | 83.7 (52.1-121.0) | P = 0.001 |
| APACHE II score | 19.3 ± 5.9 | 15.2 ± 6.1 | P < 0.001 |
| SOFA score | 8.2 ± 3.0 | 6.9 ± 3.2 | P = 0.038 |
Data are presented as mean ± standard deviation or median (interquartile range) or n (%).
BMI, body mass index; COPD, chronic obstructive pulmonary disease; APACHE II score, acute physiology and chronic health evaluation II score; SOFA score, sequence organ failure assessment score.
Univariate logistics analysis showed that age (P = 0.008), smoking status (P = 0.022), presence of COPD (P = 0.005), the CRP level (P < 0.001), the APACHE II scores (P = 0.001) and the SOFA scores (P = 0.040) were significantly correlated with a high risk of ARDS in sepsis patients (Table 3). In a forward stepwise multivariate logistic regression, smoking status (P =0.037), presence of COPD (P = 0.002), the CRP level (P = 0.002) and the APACHE II score (P = 0.008) were significant independent predictive factors for increased risk of ARDS in sepsis patients (Table 4).
Table 3.
Univariate logistic regression model analysis of factors predicting risk of acute respiratory distress syndrome (ARDS) in patients with sepsis.
| Factor | Odds ratio (95% CI) | Statistical significance |
|---|---|---|
| Age | 1.05 (1.01, 1.09) | P = 0.008 |
| Male | 1.03 (0.49, 2.20) | ns |
| BMI | 1.04 (0.97, 1.12) | ns |
| Smoker | 2.35 (1.13, 4.88) | P = 0.022 |
| COPD | 3.69 (1.47, 9.22) | P = 0.005 |
| Cardiomyopathy | 1.10 (0.51,2.35) | ns |
| Chronic kidney failure | 1.83 (0.66, 5.11) | ns |
| Cirrhosis | 0.68 (0.25, 1.81) | ns |
| Serum creatinine | 1.21 (0.93,1.57) | ns |
| Albumin | 0.99 (0.95,1.03) | ns |
| White blood cells | 1.02 (0.99,1.04) | ns |
| C-reactive protein | 1.01 (1.01, 1.02) | P = 0.001 |
| APACHE II score | 1.11 (1.05, 1.18) | P = 0.001 |
| SOFA score | 1.13 (1.01, 1.26) | P = 0.040 |
BMI, body mass index; COPD, chronic obstructive pulmonary disease; APACHE II score, acute physiology and chronic health evaluation II score; SOFA score, sequence organ failure assessment score.
Table 4.
Forward stepwise multivariate logistic regression model analysis of factors predicting risk of acute respiratory distress syndrome (ARDS) in patients with sepsis.
| Factor | Odds ratio (95% CI) | Statistical significance |
|---|---|---|
| Smoker | 2.49 (1.06, 5.86) | P = 0.037 |
| COPD | 4.94 (1.75, 13.91) | P = 0.002 |
| C-reactive protein | 1.01 (1.00, 1.02) | P = 0.002 |
| APACHE II score | 1.10 (1.03, 1.18) | P = 0.008 |
COPD, chronic obstructive pulmonary disease; APACHE II score, acute physiology and chronic health evaluation II score.
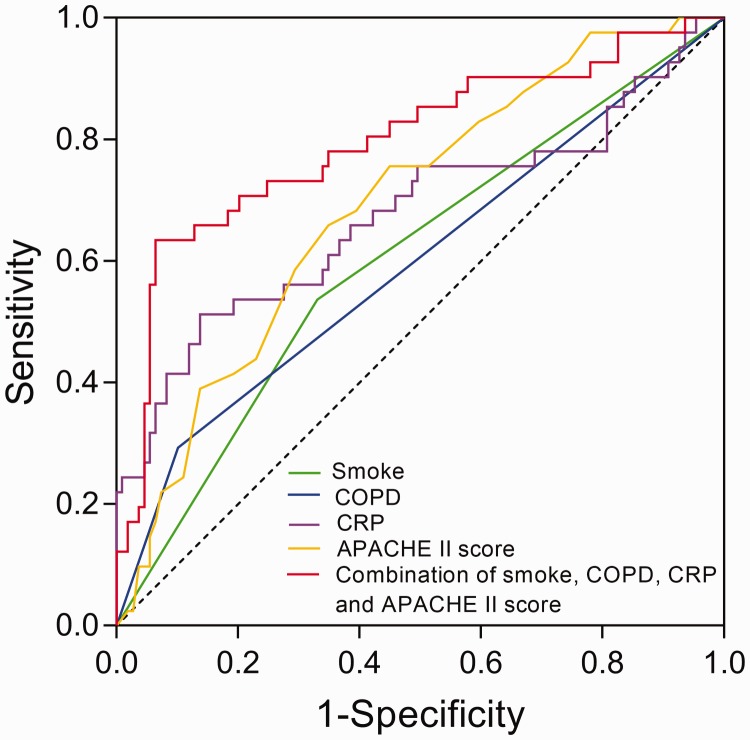
Independent predictive factors (smoking status, presence of COPD, CRP levels and APACHE II scores) for risk of ARDS were included in a ROC curve analysis. In terms of AUC, sensitivity and specificity, ROC curve analysis showed that smoking (AUC: 0.60, 95%CI: 0.50, 0.71), presence of COPD (AUC: 0.60, 95%CI: 0.49, 0.70), the CRP levels (AUC: 0.68, 95%CI: 0.57, 0.79) and the APACHE II scores (AUC: 0.69, 95%CI: 0.60, 0.79) could predict increased risk from ARDS in sepsis patients (Figure 2). In addition, the combination of these four risk factors had a greater predictive value than each individual factor alone (AUC:0.80, 95%CI: 0.71, 0.89).
Figure 2.
Receiver operating characteristic (ROC) curve analysis. The analysis showed that smoking status (area under the curve [AUC]: 0.60, 95%CI: 0.50, 0.71), presence of chronic obstructive pulmonary disease (COPD) (AUC: 0.60, 95%CI: 0.49, 0.70), C-reactive protein (CRP) levels (AUC: 0.68, 95%CI: 0.57, 0.79) and Acute Physiology and Chronic Health Evaluation (APACHE) II scores (AUC: 0.69, 95%CI: 0.60, 0.79) could predict increased risk from acute respiratory distress syndrome (ARDS) in sepsis patients. The combination of the four risk factors had a greater predictive value than each individual factor (AUC: 0.80, 95%CI: 0.71, 0.89).
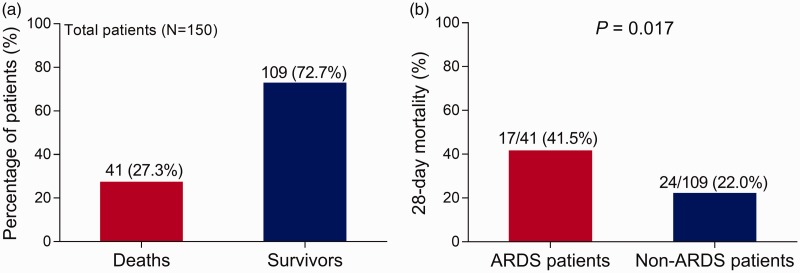
There were 41 (27%) deaths and 109 (73%) survivors in sepsis patients (Figure 3A). The 28-day mortality rates were statistically significantly higher for sepsis patients with ARDS (17/41, 42%) than sepsis patients without ARDS (24/109, 22.0%) (P = 0.017) (Figure 3B). These data suggested that ARDS was associated with increased 28-day mortality in patients with sepsis.
Figure 3.
(a) The 28-day mortality rates in sepsis patients and (b) those with and without acute respiratory distress syndrome (ARDS).
Discussion
Our study found that the prevalence of ARDS was 27% in Chinese patients hospitalised with sepsis. These findings are in agreement with a previous study also conducted in China that showed 29% of sepsis patients developed ARDS during their hospitalization.22 However, another study conducted in the USA found that ARDS occurred in 6% of all sepsis patients and 9% of sepsis patients admitted to ICU.9 Differences among study findings may result from differences in disease severity. For example, in our study we included patients with severe sepsis. In addition, variations in study populations (e.g., age, sex, comorbidities) may also affect study outcomes. Moreover, the definition of ARDS may have varied between studies.
We observed that smoking, presence of COPD, the CRP levels and the APACHE II scores were independent predictive factors for increased risk of ARDS in sepsis patients. Furthermore, the combination of these four risk factors was an even better predictor of the risk of ARDS than each factor alone. As stated previously, data from Chinese patients with sepsis who develop ARDS are few. However, our findings are compatible with those from two previous studies conducted in the USA that found patient, treatment and disease-related factors correlated with the risk of ARDS in sepsis patients.8,9 In one of these studies, APACHE II scores, age, total fluid infused in the first six hours, shock, pneumonia as a site of infection, pancreatitis and acute abdomen were associated with the development of ARDS in sepsis patients.8 In the other study from the USA, intermediate (2– 3.9 mmol/l) and high (≥4 mmol/l) serum lactate levels, lung injury prediction score (LIPS) and microbiologically-proven infections were independent risk factors for ARDS in sepsis patients.9
Our findings for the possible risk factors for ARDS in sepsis patients are not surprising since those who are smokers and/or have COPD probably already have direct lung injury in addition to indirect lung injury triggered by inflammatory cascades induced by the sepsis. In addition, levels of CRP tend to correlate with levels of systemic inflammation and will be associated with subsequent lung injury in patients with sepsis. Finally, APACHE II scores correlate with disease severity and so will undoubtedly be a useful indicator of the risk for ARDS in sepsis patients.
Consistent with other studies, we found that the 28-day mortality rate was higher in sepsis patients with ARDS (42%) compared with patients without ARDS (22%).9,10,23 For example, in one US study, although the proportion of sepsis patients who went on to develop ARDS was low, mortality was significantly higher in those who developed ARDS compared with those who did not.9 Similarly, in another US study, the in-hospital mortality rate for sepsis patients with ARDS was higher than that in sepsis patients who did not develop ARDS (53% and 22%, respectively).23 Another study from the USA reported that both the 28-day and 60-day mortality was elevated in sepsis patients with ARDS compared with those without ARDS.10 It has been reported that, in sepsis patients, the progression to ARDS is rapid and contributes to increased risk of in-hospital mortality.8,9 A likely explanation is that ARDS causes decreased lung compliance, inefficient gas exchange, increased physiological dead space and hypoxemia so potentiating disease severity and subsequent multiple-system organ failure.1 It is important to note that, irrespective of ARDS diagnosis, we excluded patients from our study who died during the first 24h after hospital admission because their severe symptoms may have skewed the results.24
This study had several limitations. Firstly, this was a single centre study with a small sample size which may have led to unintentional selection bias and poor statistical power. Secondly, the variability in chest x-ray interpretation by independent clinicians may have influenced the reliability of ARDS prevalence in our sepsis patients. Finally, we only assessed 28-day mortality and so the effect of ARDS on long-term prognosis in sepsis patients needs to be studied further.
In conclusion, we found that in this hospital centre in China, ARDS was common in sepsis patients. A smoking history, presence of COPD, the CRP levels and the APACHE II scores in these patients were associated with increased risk of ARDS. Moreover, all four factors combined provided a better risk assessment and may be useful as a prognostic marker for assisting with ARDS management in sepsis patients in clinical practice. Furthermore, our study showed that the presence of ARDS was associated with high rates of 28-day mortality in patients with sepsis.
Declaration of conflicting interest
The authors declare that there are no conflicts of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
References
- 1.Englert JA, Bobba C, Baron RM. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight 2019; 4: pii: 124061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prescott HC, Angus DC. Enhancing recovery from sepsis: a review. JAMA 2018; 319: 62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fialkow L, Fochesatto Filho L, Bozzetti MCet al. Neutrophil apoptosis: a marker of disease severity in sepsis and sepsis-induced acute respiratory distress syndrome. Crit Care 2006; 10: R155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillamat-Prats R, Camprubi-Rimblas M, Bringue Jet al. Cell therapy for the treatment of sepsis and acute respiratory distress syndrome. Ann Transl Med 2017; 5: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.National Heart L, Blood Institute ACTN, Truwit JDet al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 2014; 370: 2191–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toner P, McAuley DF, Shyamsundar M. Aspirin as a potential treatment in sepsis or acute respiratory distress syndrome. Crit Care 2015; 19: 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKown AC, McGuinn EM, Ware LBet al. Preadmission oral corticosteroids are associated with reduced risk of acute respiratory distress syndrome in critically Ill adults with sepsis. Crit Care Med 2017; 45: 774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seethala RR, Hou PC, Aisiku IPet al. Early risk factors and the role of fluid administration in developing acute respiratory distress syndrome in septic patients. Ann Intensive Care 2017; 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mikkelsen ME, Shah CV, Meyer NJet al. The epidemiology of acute respiratory distress syndrome in patients presenting to the emergency department with severe sepsis. Shock 2013; 40: 375–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nam H, Jang SH, Hwang YIet al. Nonpulmonary risk factors of acute respiratory distress syndrome in patients with septic bacteraemia. Korean J Intern Med 2019; 34: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eggimann P, Harbarth S, Ricou Bet al. Acute respiratory distress syndrome after bacteremic sepsis does not increase mortality. Am J Respir Crit Care Med 2003; 167: 1210–1214. [DOI] [PubMed] [Google Scholar]
- 12.Song Y, Xu F, Seeley EJet al. Acute respiratory distress syndrome: emerging research in China. Am J Respir Crit Care Med 2014; 190: 1090–1093. [DOI] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CWet al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA 2016; 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ortiz A, Covic A, Fliser Det al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014; 383: 1831–1843. [DOI] [PubMed] [Google Scholar]
- 15.Knaus WA, Draper EA, Wagner DPet al. APACHE II: a severity of disease classification system. Crit Care Med 1985; 13: 818–829. [PubMed] [Google Scholar]
- 16.Vincent JL, Moreno R, Takala Jet al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani Wet al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43: 304–377. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson ND, Fan E, Camporota Let al. The Berlin definition of ARDS: an expanded rationale, justification, and supplementary material. Intensive Care Med 2012; 38: 1573–1582. [DOI] [PubMed] [Google Scholar]
- 19.Bellani G, Laffey JG, Pham Tet al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016; 315: 788–800. [DOI] [PubMed] [Google Scholar]
- 20.Flaatten H, Gjerde S, Guttormsen ABet al. Outcome after acute respiratory failure is more dependent on dysfunction in other vital organs than on the severity of the respiratory failure. Crit Care 2003; 7: R72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monchi M, Bellenfant F, Cariou Aet al. Early predictive factors of survival in the acute respiratory distress syndrome. A multivariate analysis. Am J Respir Crit Care Med 1998; 158: 1076–1081. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y, Fu X, Yu Bet al. Long non-coding RNA THRIL predicts increased acute respiratory distress syndrome risk and positively correlates with disease severity, inflammation, and mortality in sepsis patients. J Clin Lab Anal 2019; 33: e22882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moss M, Parsons PE, Steinberg KPet al. Chronic alcohol abuse is associated with an increased incidence of acute respiratory distress syndrome and severity of multiple organ dysfunction in patients with septic shock. Crit Care Med 2003; 31: 869–877. [DOI] [PubMed] [Google Scholar]
- 24.Javed A, Guirgis FW, Sterling SAet al. Clinical predictors of early death from sepsis. J Crit Care 2017; 42: 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]