Abstract
Purpose
To investigate macular microvasculature changes using optical coherence tomography angiography (OCTA) and analyze their correlation with the structural parameters in highly myopic eyes.
Methods
We measured the area of the foveal avascular zone (FAZ) and the parafoveal vessel density in the superficial and deep retinal plexuses using OCTA. The magnification effect of the FAZ area was corrected using Bennett's formula. Retinal thickness measured at each corresponding area of the OCTA parameters, subfoveal choroidal thickness, and ocular characteristics were reviewed, and the relationships between the microvasculature measurements and the ocular structural characteristics were explored.
Results
Fifty-two eyes with high myopia and 52 normal sex- and age-matched controls were included in the analysis. The FAZ area was significantly larger in the myopic eyes (p = 0.023). The superficial parafoveal vascular density was significantly decreased (p = 0.007) in the myopic eyes compared with the normal eyes, whereas there was no significant difference in the deep parafoveal vascular density (p = 0.226). Regarding the retinal thickness, only the parafoveal inner retinal thickness was significantly smaller in the myopic eyes than in the normal eyes (p = 0.023). The FAZ and subfoveal choroidal thickness were significantly correlated with the axial length, and the parafoveal inner retinal thickness was significantly correlated with the superficial parafoveal vascular density (all p < 0.05).
Conclusions
The FAZ was enlarged and the parafoveal vascular density was reduced in the highly myopic eyes. The decrease was prominent in the superficial capillary plexuses and well-correlated with the retinal thickness profiles. The macular microvascular network alteration may be attributed to the ocular axial elongation that occurs with myopia.
Keywords: Foveal avascular zone, Macular microvasculature, Myopia, Optical coherence tomography angiography
Myopia is the leading cause of vision impairment, affecting more than 1.4 billion people worldwide [1,2,3,4,5,6,7,8,9,10]. Its prevalence has been increasing in Asia [1,2,3,5,7,9,10,11,12,13,14]. Myopia with a refractive error >−6 diopters (D) or an axial length >26 mm is considered pathologic. It can lead to myopic retinopathy, characterized by lacquer crack formation, choroidal neovascularization, and chorioretinal atrophy [1,2,3,5,6,8,10,12,15,16]. In pathologic myopia, reduced retinal vessel density and blood flow are evident by fundus photography, and decreased choroidal blood flow has been noticed with increased axial length [5,15]. With decreased retinal microvasculature supplies, the retina may be more susceptible in pathologic myopia [5,10]. In this context, studying the changes in the microvasculature and their interaction may reveal the underlying pathophysiology of the disorder and enable an effective treatment or prevention [5].
The use of optical coherence tomography angiography (OCTA) has allowed researchers to noninvasively measure the retinal microvasculature [1,5,10,11,17,18,19]. The purpose of this study was to evaluate the retinal and choriocapillaris microvasculature using OCTA in highly myopic eyes and to investigate the associations between the OCTA parameters and the structural parameters.
Materials and Methods
Study subjects
The study was approved by the institutional review board of the Asan Medical Center (2019-1083) before initiation and was performed in compliance with the tenets of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study. We retrospectively recruited patients with high myopia and their age- and sex-matched controls who visited a university-based retina clinic (Asan Medical Center, Seoul, South Korea) between January 2016 and December 2017. Eyes with an axial length greater than 26.5 mm were included in the high myopia group. The exclusion criteria for the high myopia group were as follows: (1) evidence of ocular disease other than myopia; (2) advanced myopic maculopathy more severe than “diffuse chorioretinal atrophy” (C2) as per the Meta Analysis for Pathologic Myopia study group classification: “patchy chorioretinal atrophy” (C3), “macular atrophy” (C4), or any of the “plus” lesions (lacquer crack, myopic choroidal neovascularization, or Fuch's spot); (3) eyes with obvious staphylomatous outpouching; (4) history of prior vitreoretinal surgery; (5) presence of a systemic illness that might affect the chorioretinal vasculature, such as diabetes mellitus and hypertension; and (6) poor-quality OCTA scan images with a signal strength less than 55. If both eyes met the criteria, one eye was randomly selected. In addition, sex- and age-matched controls with an axial length less than 26.5 mm were included in the analysis for comparison with the highly myopic eyes.
Optical coherence tomography and OCTA measurements
All patients underwent comprehensive ophthalmologic examinations, including best-corrected visual acuity, manifest refraction, and axial length (IOL Master; CarlZeiss, Jena, Germany) and were thoroughly examined using spectral-domain optical coherence tomography (OCT) and OCTA (Optovue, Fremont, CA, USA). All OCT and OCTA scans were reviewed to ensure correct segmentation and sufficient image quality. The built-in software (version 3.0) allows automated segmentation not only of the whole retinal thickness, but also of the inner retinal layer (the vitreoretinal interface to the outer border of the inner plexiform layer) and the outer retinal layer (the outer border of the inner plexiform layer to the outer border of the retinal pigment epithelium). We analyzed the thickness of each layer measured at the fovea and parafoveal region of the standard 9-area Early Treatment of Diabetic Retinopathy Study grid sectors [20]. The subfoveal choroidal thickness (SFCT) was defined as the vertical distance from the hyper-reflective line of Bruch's membrane to the hyper-reflective line of the inner surface of the sclera.
For the OCTA, AngioVue (Optovue) was used to obtain split-spectrum amplitude-decorrelation angiography as previously described [20]. The OCTA scan area was centered on the fovea with a field view of 3 × 3 mm2, which corresponded to 10 degrees. OCTA images of the superficial, deep, and whole layers were used after segmentation. Automatic segmentation was performed by the viewing software to generate en face projection images of the retinal superficial capillary plexus (SCP), deep capillary plexus (DCP), and the choriocapillaris. The SCP en face OCTA image was segmented with an inner boundary 3 µm below the internal limiting membrane and an outer boundary 15 µm below the inner plexiform layer. The DCP image was segmented with an inner boundary 15 µm below the inner plexiform layer and an outer boundary 70 µm below the inner plexiform layer. Foveal avascular zone (FAZ) area and parafoveal vascular density (VD) of SCP and DCP were used to represent macular vascular integrity.
Statistical analysis
Descriptive statistics (the number and percentage of each categorical variable and the mean ± standard deviation of each continuous variable) were initially evaluated in order to determine subject baseline characteristics. The Wilk-Shapiro test was used to explore the distribution of the numerical data. To demonstrate the characteristics of the subjects with myopia compared with those of the controls, the Student's t-test or Mann-Whitney U-test were used, depending on the normality of the data distribution. To compare the categorical data, the chi-squared test was used. To assess the relationship between the parameters of the macular microvasculature and the structural profiles, Pearson's correlations were used. All statistical analyses were performed using PASW Statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA).
Results
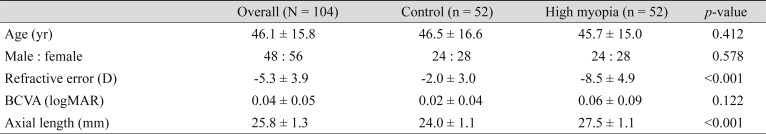
Fifty-two eyes of 52 patients with high myopia and 52 normal sex- and age-matched controls were included in the analysis. The demographic and clinical information of the patients is listed in Table 1. There were no significant differences in age or sex (p > 0.05) between patients with myopia and healthy controls. The mean age was 46.1 ± 15.8 years for all subjects (46.5 ± 16.6 for controls and 45.7 ± 15.0 for patients with myopia). Significant differences between the groups were noted in the refractive error and axial length (p < 0.05). The mean refractive error, best-corrected visual acuity (logarithm of the minimum angle of resolution), and axial length were −2.0 ± 3.0 D, 0.02 ± 0.04, and 24.0 ± 1.1 mm in the control group, and −8.5 ± 4.9 D, 0.06 ± 0.09, and 27.5 ± 1.1 mm in the myopia group, respectively. All OCT images of myopic eyes across the fovea and the optic disc nerve showed no outpouching, suggesting posterior staphyloma.
Table 1. Baseline characteristics of the participants.
Values are presented as mean ± standard deviation or number.
D = diopters; BCVA = best-corrected visual acuity; logMAR = logarithm of the minimum angle of resolution.
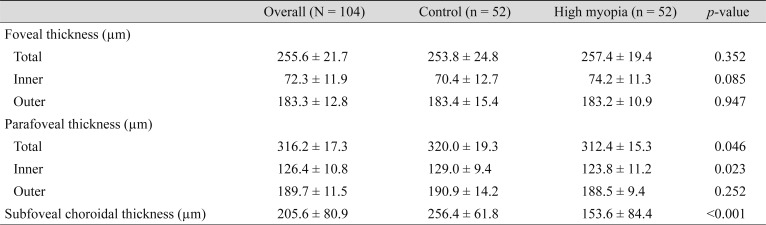
With respect to the retinal and choroidal thickness parameters, the mean foveal retinal thickness showed no differences between the two groups; however, there was a significant difference in total thickness in the parafoveal area (312.4 ± 15.3 µm in the myopia group and 320.0 ± 19.3 µm in the control group, p = 0.046). The mean SFCT was 153.6 ± 84.4 µm in the myopia group and 256.4±61.8 µm in the control group (p < 0.001) (Table 2).
Table 2. Retinal and choroidal thickness parameters.
Values are presented as mean ± standard deviation.
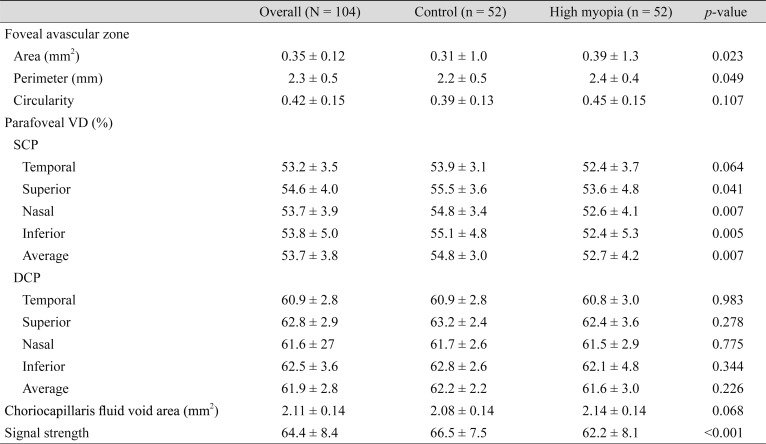
With respect to the retinal and choriocapillaris microvasculature parameters, the FAZ area, FAZ perimeter, and the average of the parafoveal VD in the SCP showed significant differences. When regional studies were performed, significance was found in the nasal and inferior sectors for the parafoveal VD in the SCP. The FAZ area was 0.31 ± 1.0 mm2 in the control group and 0.39 ± 1.3 mm2 in the myopia group (p = 0.023). The FAZ perimeter was 2.2 ± 0.5 mm in the control group and 2.4 ± 0.4 mm in the myopia group (p = 0.049). The average parafoveal VD in the SCP was 54.8 ± 3.0% and 52.7 ± 4.2% for the control and the myopia groups, respectively (p = 0.007). In the DCP, they were 62.2 ± 2.2% and 61.6 ± 3.0%, respectively (p = 0.426). The mean parafoveal VD in the SCP was 54.8 ± 3.4% and 52.6 ± 4.1% in the nasal sector (p = 0.007), and 55.1 ± 4.8% and 52.4 ± 5.3% in the inferior sector (p = 0.005) in the control and myopia groups, respectively. The choriocapillaris fluid void area showed no significant differences, with 2.08 ± 0.14 and 2.14 ± 0.14 mm2 in the control and myopia groups, respectively (p = 0.068) (Table 3).
Table 3. Retinal and choriocapillaris microvasculature parameters.
Values are presented as number or mean ± standard deviation.
VD = vessel density; SCP = superficial capillary plexus; DCP = deep capillary plexus.
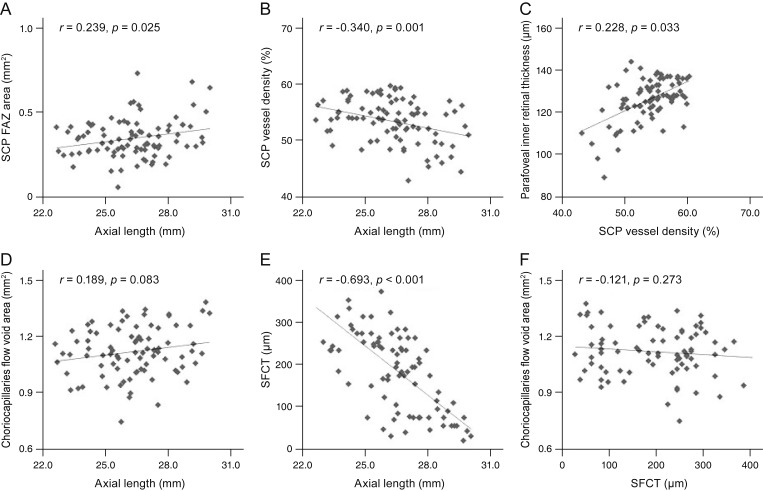
The association of the axial length with the FAZ showed a positive correlation (r = 0.239, p = 0.025), and that with the SCP VD and SFCT showed a negative correlation (r = −0.340, p = 0.001; r = −0.693, p < 0.001), but the difference was not statistically significant for the choriocapillaris fluid void area. The parafoveal inner retinal thickness showed a positive correlation with the SCP VD (r = 0.228, p = 0.033). The regional correlations between the SCP parafoveal VD and the parafoveal inner retinal thicknesses were significant in all four quadrants; temporal (r = 0.391, p < 0.001), superior (r = 0.396, p < 0.001), nasal (r = 0.442, p < 0.001), and inferior sectors (r = 0.460, p < 0.001), respectively. There was no significant correlation between the choriocapillaris fluid void area and the SFCT (r = −0.21, p = 0.273) (Fig. 1A–1F).
Fig. 1. Correlations between the microvasculature and structural profiles. (A) Axial length and the superficial capillary plexus (SCP) foveal avascular zone (FAZ) area showed a positive correlation. (B) Axial length and the SCP vessel density showed a negative correlation. (C) SCP vessel density and the parafoveal inner retinal thickness showed a positive correlation. (D) Axial length and the choriocapillaris flow void area showed a positive correlation. (E) Axial length and subfoveal choroidal thickness (SFCT) showed a negative correlation. (F) SFCT and the choriocapillaris flow void area showed no significant correlation.
Discussion
In our study, the VD in the myopia group was significantly decreased in the SCP, and the SCP VD and the axial length showed a negative correlation. This is consistent with the findings of previous studies and indicates that the decreased VD could be due to mechanical stretching of the eyeball and consequent narrowing of the retinal vessels [1,5,6,10,15]. Interestingly, we found that the VD in the myopia group was not significantly decreased in the DCP. Meanwhile, other studies revealed that both the SCP and DCP showed a significant reduction in the VD [1,5,10,15]. Fan et al. [1] showed that myopia severity might be associated with such differences; significantly decreased SCP VD was found in both moderate and high myopia, but the decreased DCP VD was significant only in the high myopia group. In line with our study, this could mean that the SCP VD is significantly reduced before the DCP in the myopic eye. A possible reason for this could be that the vessels are more densely packed in the DCP of the parafoveal region [18].
Among the four quadrants, the parafoveal VD reduction in the SCP showed the highest significance in the inferior and nasal regions in our study. This finding was consistent with that of other studies [5,6,10]. Similar findings in the DCP and large vessels were also shown. Although the reason for the greater VD reduction in the inferior and nasal sectors is not well-understood, several previous studies have speculated that it corresponds to the loss of the retinal nerve fiber layer inferiorly in myopic eyes [5,7,10,18].
The FAZ area positively correlated with the axial length in our study, while previous studies showed no significant difference in the FAZ area between myopic and control groups [5,6]. One study reported that a larger FAZ area was associated with a shorter axial length and a hyperopic refractive error [19]. Little is known about the FAZ area in regard to myopia, and the differences in the results are most likely due to individual variation in age and other factors [6]. One of the causes of this discrepancy could be the use of Bennett's formula in our study to correct the magnification factors of OCT en face images of myopic eyes [5,6,18,21].
In our study, the parafoveal retinal thickness was significantly decreased in the myopia group, while the foveal retinal thickness was increased, but showed no significance. This finding was similar to that of a previous study [14]. In other studies, however, the increased foveal retinal thickness was significant [4,6,7,11,12,13,16,22,23,24], which could be due to the foveal elevation secondary to the mechanical stretching and pararetinal thinning in myopic eyes and the foveal retinal pigment epithelium's high permeability [6,10,12,13,14,16,23,24]. Additionally, the absence of large vessels and optic fibers make the peripheral retina less resistant to stretching, while the foveal thickness is preserved [4,7,13,14,16]. In addition, the parafoveal retinal thickness was positively correlated with the SCP VD in our study, and the correlation was the strongest in the nasal and inferior sectors. The association between retinal thickness and VD is scarce in the literature and requires further study [6].
Our study showed a significant decrease in the SFCT in myopic eyes (p < 0.001), in line with the findings of others [2,7,19,25,26,27]. Choroidal thinning is one of the earliest signs of non-pathological myopic change and can lead to pathological changes later, such as lacquer crack formation and choroidal neovascularization, and is associated with a poorer prognosis [2,3,7,8,9,15]. However, choriocapillaris blood flow results are controversial in the literature. This could be due to the extensive choroidal thinning in myopic eyes, which makes the choriocapillaris blood flow difficult to evaluate [6,8,15,19]. Our study showed no significant increase in the choriocapillaris fluid void areas in the myopia group. This may be because larger choroidal vessels are more affected than the choriocapillaris [6,28]. Moreover, studies that did show significant differences in the choriocapillaris perfusion area mentioned that the larger vessels could not be distinguished from the choriocapillaris when imaging studies were performed [15,19,28]. However, in our study, no myopic eyes showed staphyloma. As other studies have found that staphyloma is a representative deformity of pathologic myopia, this might be because we excluded the patients with pathologic changes [29].
We acknowledge that the mono-racial, single-center background and retrospective design limit this study. Our study focused on retinal VD, but changes in retinal blood flow were not explored. Interestingly, despite the decreased VD, the overall retinal perfusion was known to be maintained and this may indicate that reduced VD is related to increased axial length (mechanical stretching), rather than to vessel loss [5,15,30]. Correlative studies between retinal blood flow and VD are required to better understand why there is a decrease in VD, but maintenance of the overall perfusion [5,15,30]. Longitudinal studies are required to determine whether the structural changes or blood flow changes are affected first [10]. The change in the microvasculature was explored in our study, but not in large vessels as in other studies [5,10]. Stratifying patients in terms of degree of myopia could further understanding of the course and progression of myopic changes in relation to the degree of myopia, but this was not done in our study.
In conclusion, our study showed that eyes with long axial length had an enlarged FAZ area and a reduced SCP VD before the occurrence of the pathological changes of myopia. Interestingly, such changes were well correlated with the axial length and the changes in the SCP VD showed good associations with the parafoveal inner retinal thickness. The sequence of these changes and how they are correlated with one another are crucial to better understand the early myopic changes. OCTA provides a way to visualize these changes clearly and noninvasively.
Acknowledgements
This study was supported by grant from Ministry of Science and ICT (Information and Communication Technology), South Korea (NRF-2019R1F1A1063124).
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Fan H, Chen HY, Ma HJ, et al. Reduced macular vascular density in myopic eyes. Chin Med J (Engl) 2017;130:445–451. doi: 10.4103/0366-6999.199844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta P, Saw SM, Cheung CY, et al. Choroidal thickness and high myopia: a case-control study of young Chinese men in Singapore. Acta Ophthalmol. 2015;93:e585–e592. doi: 10.1111/aos.12631. [DOI] [PubMed] [Google Scholar]
- 3.Kumar A, Chawla R, Kumawat D, Pillay G. Insight into high myopia and the macula. Indian J Ophthalmol. 2017;65:85–91. doi: 10.4103/ijo.IJO_863_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lam DS, Leung KS, Mohamed S, et al. Regional variations in the relationship between macular thickness measurements and myopia. Invest Ophthalmol Vis Sci. 2007;48:376–382. doi: 10.1167/iovs.06-0426. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Yang Y, Jiang H, et al. Retinal microvascular network and microcirculation assessments in high myopia. Am J Ophthalmol. 2017;174:56–67. doi: 10.1016/j.ajo.2016.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milani P, Montesano G, Rossetti L, et al. Vessel density, retinal thickness, and choriocapillaris vascular flow in myopic eyes on OCT angiography. Graefes Arch Clin Exp Ophthalmol. 2018;256:1419–1427. doi: 10.1007/s00417-018-4012-y. [DOI] [PubMed] [Google Scholar]
- 7.Ng DS, Cheung CY, Luk FO, et al. Advances of optical coherence tomography in myopia and pathologic myopia. Eye (Lond) 2016;30:901–916. doi: 10.1038/eye.2016.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sayanagi K, Ikuno Y, Uematsu S, Nishida K. Features of the choriocapillaris in myopic maculopathy identified by optical coherence tomography angiography. Br J Ophthalmol. 2017;101:1524–1529. doi: 10.1136/bjophthalmol-2016-309628. [DOI] [PubMed] [Google Scholar]
- 9.Wang NK, Lai CC, Chou CL, et al. Choroidal thickness and biometric markers for the screening of lacquer cracks in patients with high myopia. PLoS One. 2013;8:e53660. doi: 10.1371/journal.pone.0053660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Wang J, Jiang H, et al. Retinal microvasculature alteration in high myopia. Invest Ophthalmol Vis Sci. 2016;57:6020–6030. doi: 10.1167/iovs.16-19542. [DOI] [PubMed] [Google Scholar]
- 11.Lim MC, Hoh ST, Foster PJ, et al. Use of optical coherence tomography to assess variations in macular retinal thickness in myopia. Invest Ophthalmol Vis Sci. 2005;46:974–978. doi: 10.1167/iovs.04-0828. [DOI] [PubMed] [Google Scholar]
- 12.Luo HD, Gazzard G, Fong A, et al. Myopia, axial length, and OCT characteristics of the macula in Singaporean children. Invest Ophthalmol Vis Sci. 2006;47:2773–2781. doi: 10.1167/iovs.05-1380. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Wu Q, Hu P, Jia L. Macular thickness assessed with optical coherence tomography in young Chinese myopic patients. J Ophthalmol. 2015;2015:715798. doi: 10.1155/2015/715798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao Z, Zhou X, Jiang C, Sun X. Effects of myopia on different areas and layers of the macula: a Fourier-domain optical coherence tomography study of a Chinese cohort. BMC Ophthalmol. 2015;15:90. doi: 10.1186/s12886-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Sheikh M, Phasukkijwatana N, Dolz-Marco R, et al. Quantitative OCT angiography of the retinal microvasculature and the choriocapillaris in myopic eyes. Invest Ophthalmol Vis Sci. 2017;58:2063–2069. doi: 10.1167/iovs.16-21289. [DOI] [PubMed] [Google Scholar]
- 16.Samuel NE, Krishnagopal S. Foveal and macular thickness evaluation by spectral OCT SLO and its relation with axial length in various degree of myopia. J Clin Diagn Res. 2015;9:NC01–NC04. doi: 10.7860/JCDR/2015/11780.5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Carlo TE, Romano A, Waheed NK, Duker JS. A review of optical coherence tomography angiography (OCTA) Int J Retina Vitreous. 2015;1:5. doi: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassan M, Sadiq MA, Halim MS, et al. Evaluation of macular and peripapillary vessel flow density in eyes with no known pathology using optical coherence tomography angiography. Int J Retina Vitreous. 2017;3:27. doi: 10.1186/s40942-017-0080-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Q, Chan S, Yang JY, et al. Vascular density in retina and choriocapillaris as measured by optical coherence tomography angiography. Am J Ophthalmol. 2016;168:95–109. doi: 10.1016/j.ajo.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kim YJ, Jo J, Lee JY, et al. Macular capillary plexuses after macular hole surgery: an optical coherence tomography angiography study. Br J Ophthalmol. 2018;102:966–970. doi: 10.1136/bjophthalmol-2017-311132. [DOI] [PubMed] [Google Scholar]
- 21.Iafe NA, Phasukkijwatana N, Chen X, Sarraf D. Retinal capillary density and foveal avascular zone area are age-dependent: quantitative analysis using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2016;57:5780–5787. doi: 10.1167/iovs.16-20045. [DOI] [PubMed] [Google Scholar]
- 22.Huynh SC, Wang XY, Rochtchina E, Mitchell P. Distribution of macular thickness by optical coherence tomography: findings from a population-based study of 6-year-old children. Invest Ophthalmol Vis Sci. 2006;47:2351–2357. doi: 10.1167/iovs.05-1396. [DOI] [PubMed] [Google Scholar]
- 23.Sato A, Fukui E, Ohta K. Retinal thickness of myopic eyes determined by spectralis optical coherence tomography. Br J Ophthalmol. 2010;94:1624–1628. doi: 10.1136/bjo.2009.165472. [DOI] [PubMed] [Google Scholar]
- 24.Wu PC, Chen YJ, Chen CH, et al. Assessment of macular retinal thickness and volume in normal eyes and highly myopic eyes with third-generation optical coherence tomography. Eye (Lond) 2008;22:551–555. doi: 10.1038/sj.eye.6702789. [DOI] [PubMed] [Google Scholar]
- 25.Agawa T, Miura M, Ikuno Y, et al. Choroidal thickness measurement in healthy Japanese subjects by three-dimensional high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011;249:1485–1492. doi: 10.1007/s00417-011-1708-7. [DOI] [PubMed] [Google Scholar]
- 26.Flores-Moreno I, Lugo F, Duker JS, Ruiz-Moreno JM. The relationship between axial length and choroidal thickness in eyes with high myopia. Am J Ophthalmol. 2013;155:314–319. doi: 10.1016/j.ajo.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Wei WB, Xu L, Jonas JB, et al. Subfoveal choroidal thickness: the Beijing Eye Study. Ophthalmology. 2013;120:175–180. doi: 10.1016/j.ophtha.2012.07.048. [DOI] [PubMed] [Google Scholar]
- 28.Mo J, Duan A, Chan S, et al. Vascular flow density in pathological myopia: an optical coherence tomography angiography study. BMJ Open. 2017;7:e013571. doi: 10.1136/bmjopen-2016-013571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohno-Matsui K, Lai TY, Lai CC, Cheung CM. Updates of pathologic myopia. Prog Retin Eye Res. 2016;52:156–187. doi: 10.1016/j.preteyeres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Shimada N, Ohno-Matsui K, Harino S, et al. Reduction of retinal blood flow in high myopia. Graefes Arch Clin Exp Ophthalmol. 2004;242:284–288. doi: 10.1007/s00417-003-0836-0. [DOI] [PubMed] [Google Scholar]