Abstract
Although Eating Disorders (ED) are known to affect bone health and development, little is known about the longitudinal effect of ED and ED behaviours on bone health in community dwelling adult women.
Women (n = 3507) enrolled in the Avon Longitudinal Study of Parents and Children (ALSPAC) participated in a two-phase prevalence study to assess lifetime ED and ED behaviours (fasting, restrictive eating, vomiting and misuse of medication). Crude and adjusted linear regression methods investigated the association between ED diagnoses and behaviours, and total body, hip, leg and arm bone mineral density (BMD) DXA scans at mean ages of 48 and 52 years.
Lifetime occurrence of Anorexia Nervosa (AN) was associated with lower BMD Z-scores for the whole body (mean difference (MD) = −0.28; 95% CI: −0.49, −0.05), hip (MD = −0.45; 95% CI −0.74, −0.16), leg (MD = −0.28; 95% CI −0.52, −0.03) and arm (MD = −0.44; 95% CI −0.68, −0.19) compared to no ED. This effect was mostly accounted for by lowest ever BMI. In post-hoc analyses, Restrictive AN, but not Binge-Purge AN was associated with a lower total body BMD Z-scores (MD = −0.37; 95% CI −0.62, −0.12). Lifetime Fasting and Restrictive Eating were associated with low BMD of the total body, hip, arm and leg in adjusted analyses, all p < 0.05.
Both lifetime ED diagnoses and ED behaviours in a large community sample were predictive of low BMD in mid-life. This study confirms that the effects of AN, fasting and restrictive eating, and low BMI on bone health seen in clinical samples also occur in community samples.
Keywords: Eating Disorders, Anorecia Nervosa, Bulimia Nervosa, Osteoporosis, Bone Mineral Density, ALSPAC, Women
1. Introduction
Eating Disorders (ED) are severe psychiatric disorders associated with disturbed eating behaviours including excessive food restriction, consumption of large quantities of food with loss of control, and compensatory behaviours aimed at weight loss; as well as distorted body image [1]. ED, particularly Anorexia Nervosa (AN), can lead to several medical complications, including low bone mineral density (BMD), bone mineral content (BMC) and bone area (BA) [2–4]. Both adults [5,6] and adolescents [7–9] with an ED, and particularly those with AN, are at increased risk for low BMD and BA, and fractures in comparison to healthy women [10]. Studies to date have almost exclusively investigated clinical samples to identify the effects of ED on bone health and outcomes. As the majority of individuals in the community may never receive either a diagnosis or treatment for an ED, population-based studies can shed light on the consequences of ED and related behaviours and their burden on individuals across the population [11].
The vast majority of studies investigating bone outcomes in EDs have focused on AN, with a recent meta-analysis uncovering only six studies comparing BMD in individuals with Bulimia Nervosa (BN) to healthy control women [12]. Research to date investigating bone health in AN has revealed that approximately 85% of women with AN have BMD 1 standard deviation (SD) below the population mean [2], with approximately 30% of women with a history of AN reporting a fracture at one or more anatomical sites over their lifetime [13]. Moreover, the onset of AN during adolescence can impair the acquisition of peak bone mass, resulting in lower BMD throughout adult life [6] and may limit increases in BMD following recovery [14]. A long-term follow-up study of women recovered from AN found that full clinical recovery did not always result in normalization of BMD [15]. Similarly, recovery from AN during adolescence did not lead to bone parameters comparable to healthy controls [16]. A previous population-based retrospective study investigating fracture outcomes in EDs found that the cumulative incidence of any fracture at 40 years after the diagnosis of anorexia nervosa was 57%. Fractures of the hip, spine, and forearm were late complications, occurring on average 38, 25, and 24 years after diagnosis, respectively [17].
Previous research on the effect of BN on bone health has utilized either mixed ED samples, or samples with a high proportion of individuals with a history of AN, resulting in ambiguous findings regarding the impact of BN on BMD [18–20]. A meta-analysis investigating the Standardised Mean Difference (SMD) in spinal BMD between women with BN and healthy women revealed that women with BN were 30–50% more likely to endure a fracture than healthy women [12], warranting further study into bone health parameters in this group. Given the evidence that not only AN, but also BN is associated with deficits in BMD [12,21], it is timely to investigate the effect of these EDs on long term BMD. However it remains to be determined which ED behaviours might be more strongly related to low BMD in adulthood.
The current study aims to investigate the long-term effects of lifetime AN and BN diagnoses on BMD at various bone sites in mid-life. It also aims to determine the long-term effects of individual ED behaviours, including fasting, restrictive eating, self-induced vomiting and misuse of laxatives/diuretics, on BMD in mid-life in women from a population sample from a a prospective study. This is the first study to investigate the effect of EDs and individual ED behaviours on bone health parameters using a population-based sample.
2. Methods
2.1. Sample
The Avon Longitudinal Study of Children and Parents (ALSPAC) is a longitudinal cohort study of women and their offspring recruited from South West England by Bristol University [22]. ALSPAC recruited 14,541 pregnant women resident in Avon, UK, with expected dates of delivery between 1st April 1991 and 31st December 1992. Of these initial pregnancies, there were 14,062 live births, and 13,988 children were alive at 1 year of age with 13,761 women were included in long-term follow-up. Women and their children have been followed over the last 22–24 years; mothers have completed up to 20 questionnaires, and have had detailed data abstracted from their medical records [23,24]. The study website contains details of all available data through a fully searchable data dictionary (http://www.bris.ac.uk/alspac/researchers/data-access/data-dictionary/). Fig. 1 shows a flow diagram of recruitment and attrition in the ALSPAC mothers sample.
Fig. 1.
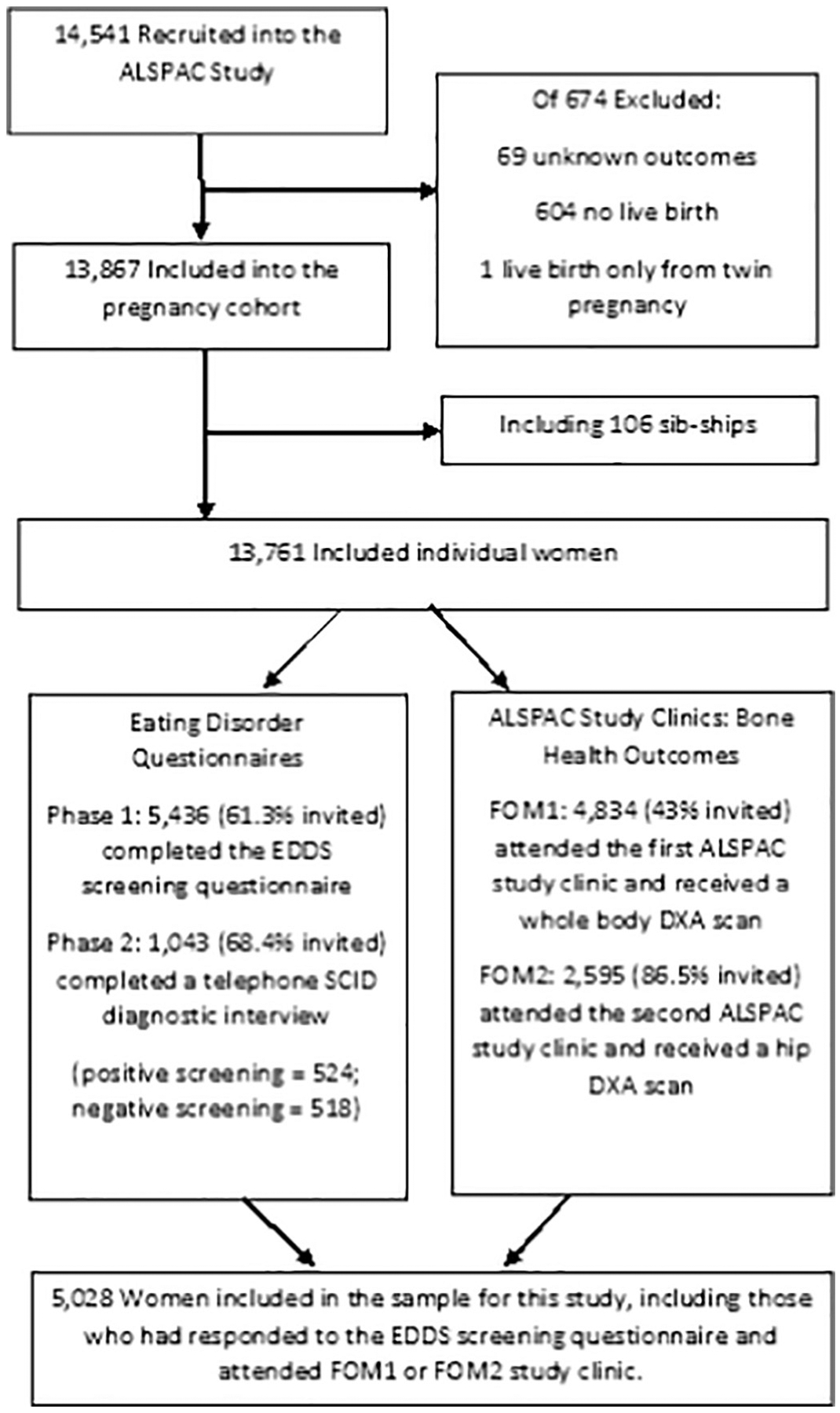
Participant Recruitment and Attrition in the ALSPAC Study.
Between 2009 and 2012 a total of 9233 women who were still alive, enrolled in the study and participating in assessment waves, and were the primary carers for their ALSPAC child, were enrolled in a sub-study focusing on EDs (for details about study procedures see Micali et al., 2017 [25]). Among 5655 respondents who completed a validated questionnaire (Eating Disorders Diagnostic Schedule adapted to cover the whole lifespan) [16], 1524 women were selected for interview using the ED section of the Structured Clinical Interview for DSM-IV-TR disorders (SCID-I) (with no skip rules) [17], supplemented with a version of the LIFE interview [18], adapted to EDs [19], aimed at investigating presence, frequency, and duration of ED behaviours (restriction, fasting, excessive exercise, binge eating, and purging). Each ED behaviour was recorded over the lifetime from its first occurrence to the time of the interview, with both frequency and duration recorded. For details about this two-phase study see reference.
2.2. Outcomes: bone health
Two face-to-face assessment clinics were conducted between 2009 and 2013: Focus on Mothers 1 (FOM1), and Focus on Mothers 2 (FOM2).
Women who were still enrolled in ALSPAC were invited to the FOM1 assessment. As shown in Figs. 1, 4834 (43% of invited) attended, at a mean age of 49 (SD =4.5) years. A subset of these women who were pre-menopausal or peri-menopausal at the time of FOM1 were invited to attend the FOM2 study clinic, with n = 2595 attending (86.5% of those invited) assessment at a mean age of 51 (SD = 4.4) years.
Participants underwent a DXA Scan using a Lunar Prodigy DXA scanner (GE Medical Systems Lunar, Madison, WI, USA) which provided measures of BMD, BMC, BA and also fat mass and lean mass, among other measures. Each scan was manually screened for anomalies, motion, and material artefacts. Total body, total leg and total arm DXA measures were collected in both FOM1 and FOM2 assessments. In addition, hip DXA measures were collected in FOM2 [26]. As total body, leg and arm DXA measurements were highly correlated between FOM1 and FOM2 and larger number of women were included in FOM1, we assessed total body, total leg and total arm DXA measures at FOM1 and only hip DXA measurements from the FOM2 assessment.
Total body BMD and BA were our primary outcome measures, indicating bone density and size respectively. Secondary outcomes were hip BMD and BA, leg BMD and BA, and arm BMD and BA. Spinal BMD measures were not investigated in this study due to the lack of availability of clean data for the spine at the time of data analysis.
2.3. Exposures
2.3.1. ED diagnoses
Lifetime diagnoses of DSM-5 AN and BN were obtained using DSM-5 criteria [26]. ED diagnoses and ED behaviours were not investigated as mutually exclusive categories.
Of 5320 women included in this study, 59 received a lifetime diagnosis of AN and 55 received a BN diagnosis. Of the women who received a diagnosis of AN, 35 were of the Restricting AN subtype (AN-R) and 31 were of the binge-purge AN subtype (AN-BP). Women who participated in the ED sub-study and screened negative acted as controls (n = 4708).
Information regarding presence and frequency of lifetime ED behaviours was obtained from the SCID and the LIFE interview. Restrictive eating, fasting, vomiting and misuse of medication were coded as binary variables and defined as follows:
Restrictive Eating:
women who had, at any time in their life, engaged in dietary restriction to lose weight or to avoid gaining weight, at a frequency of at least one whole day of severe dietary restriction a week for at least 3 months.
Fasting:
women who had, at any time in their life, engaged in fasting behaviours including skipping meals to lose weight or avoid gaining weight, at a frequency of missing a minimum of three meals a week for a period of at least 3 months.
Self-induced Vomiting:
women who had, at any time in their life, engaged in self-induced vomiting with the aim of losing weight or avoiding gaining weight at a frequency of at least once a week for a period of at least 3 months.
Misuse of medication:
women who had, at any time in their life, misused medication, including the abuse of laxatives, diuretics, or slimming pills, with the aim of losing weight or avoiding gaining weight.
Among this sample, 132 women engaged in fasting behaviours, 54 women reported restricting food intake, 74 women reported self-induced vomiting and 74 women reported misuse of medication in order to control their shape or weight.
2.4. Anthropometry
Height, and current weight were obtained objectively contemporaneous to the DXA assessments at the ALSPAC base at both FOM1 and FOM2 study clincs; BMI was derived as height (cm) / (weight (kg)2). Information regarding the lowest ever reported BMI was obtained by questionnaire as part of the ED sub-study. Anthropomorphic measures for AN (AN-R and AN-BP, BN and healthy control participants are presented in Table 2 (FOM1 measures) and Table 3 (FOM2 measures).
Table 2.
Participant characteristics (mean (SD)) in ED groups versus Healthy Controls from the ALSPAC FOM1 assessment.
| AN | BN | HC | ||
|---|---|---|---|---|
| AN-R | AN-BP | |||
| N = 36 | N = 31 | N = 54 | N = 3006 (ref) | |
| Age (years) | 47.5 (4.08) | 47.83 (4.81) | 47.85 (4.95) | 48.17 (4.29). |
| Height (cm) | 163.86 (5.76) | 162.5 (6.08) | 162.71 (5.7) | 164.19 (6.22). |
| Weight (kg) | 63.05 (10.51)** | 59.23 (10.09)** | 70.94 (18.78) | 70.16 (13.51). |
| Fat Mass (kg) | 19.77 (8.99)** | 17.42 (5.5)** | 25.31 (13.12) | 26.1 (10.04). |
| Lean Mass (kg) | 40.14 (3.79) | 39.32 (4.14) | 42.54 (6.55)* | 40.93 (40.87). |
| Current BMI (kg/m2) | 23.5 (3.87)** | 22.41 (3.43)** | 26.77 (6.87) | 26.03 (4.85). |
| N = 48 | N = 42 | N = 68 | N = 4148 | |
| Lowest Ever BMI (kg/m2) EDDS Questionnaire | 16.86 (2.14)** | 16.92 (2.45)** | 20.52 (4.31) | 20.91 (3.11). |
AN = Anorexia Nervosa; BN = Bulimia Nervosa; HC = Healthy Controls; AN-R = Anorexia Nervosa Restricting sub-type; AN-BP = Anorexia Nervosa Binge-Purge sub-type; kg = kilograms; cm = centimeters; m = meters.
p < 0.05.
p < 0.001.
Table 3.
Participant characteristics (mean (SD)) in ED groups versus Healthy Controls from the ALSPAC FOM2 assessment.
| AN | BN | HC | ||
|---|---|---|---|---|
| AN-R | AN-BP | |||
| N = 23 | N = 22 | N = 42 | N = 2012 | |
| Age (years) | 50.99 (3.81) | 50.75 (3.63) | 50.12 (4.54) | 50.98 (4.36) Ref. |
| Height (cm) | 163.4 (5.91) | 161.72 (6.02) | 162.81 (5.91) | 164.25 (6.31) Ref. |
| Weight (kg) | 61.35 (10.28)* | 57.33 (9.89)** | 69.11 (18.47) | 69.78 (12.75)Ref. |
| Fat Mass (kg) | 19.86 (8.46)** | 15.47 (7.76)** | 23.16 (11.16)* | 26.29 (9.75) Ref. |
| Lean Mass (kg) | 38.59 (3.48) | 38.49 (3.61) | 40.52 (3.98) | 40.29 (4.55) Ref. |
| Current BMI (kg/m2) | 22.93 (3.41)* | 21.89 (3.35)** | 26.08 (6.91) | 25.88 (4.68) Ref. |
| Lowest Ever BMI (kg/m2) | N = 48 | N = 42 | N = 68 | N = 4148 |
| EDDS Questionnaire | 16.86 (2.14)** | 16.92 (2.45)** | 20.52 (4.31) | 20.91 (3.11) Ref. |
AN = Anorexia Nervosa; BN = Bulimia Nervosa; HC = Healthy Controls; AN-R = Anorexia Nervosa Restricting sub-type; AN-BP = Anorexia Nervosa Binge-Purge sub-type; kg = kilograms; cm = centimeters; m = meters.
Bold numbers which are statistically significant.
p < 0.05.
p < 0.001.
2.5. Socio-demographic data
Age was obtained at the FOM1 and FOM2 visits. Women’s ethnicity and educational status were obtained by combining data provided at various time-points between enrollment and child age 18 years. [25]
2.6. Statistical analyses
2.6.1. Primary analyses
Multivariable linear regression models were used to assess the effect of either ED diagnoses or ED behaviours on DXA measures of BMD and BA. All models were initially run unadjusted and then adjusted for covariates in three steps. Covariates were defined as a priori confounders, including maternal age, education, and ethnicity; with a second set of analyses additionally including height2 (used as a measure of current body size), and BA in the case of BMD analyses, in order to control for current bone size. Height2 was chosen as a measure of current body size because BMI is strongly associated with ED diagnoses and behaviours. The effect of lowest ever BMI as a mediator on outcomes was investigated by including it into multivariable analyses as a third step.
2.6.2. Post-hoc analyses
Due to the overlap across ED diagnostic categories and behavioural groups, post hoc analyses were run with mutually exclusive categories for ED diagnoses and ED behaviours to investigate each diagnostic or behavioural group in isolation. All models were initially run unadjusted and then adjusted for covariates in the three steps described above.
2.6.3. Statistical methods
All analyses used sampling weights (generated from the ED sub-study sampling strategy) [25]. The sampling weight provides an indication of the ratio of Phase 1 to Phase 2 participants, and thus how many Phase 1 participants each Phase 2 participant represents. All analyses were performed in Stata (version 13) [27]. The SVYSET command was used in Stata 13 to carry out regression analyses allowing for stratified sampling techniques and providing a robust estimation of 95% confidence intervals (CI). All statistical tests presented are two sided, with p < 0.05 used to define statistical significance.
2.7. Attrition and missing data
The final sample for this study included N = 5028 women, who had participated in both Phase 1 of this study (with a sub-set also participating in Phase 2), and had also attended at least one of the FOM1 or FOM2 ALSPAC clinics. Attrition and drop-out from the study were predicted by sociodemographic factors: specifically parity (multiparous), and education (> A level) significantly predicted attrition.
Data on BMD and BA measures from the FOM1 clinic were less likely to be missing in those with a diagnosis of BN or reporting Fasting (BN: odds ratio [OR] = 0.45; Fasting: OR: 0.56). Missing data on Hip BMD and BA at the FOM2 clinic were negatively predicted by a BN diagnosis and Fasting and Restricting ED behaviours (BN: OR: 0.48; Fasting: OR: 0.72; Restricting: OR: 0.54; all p < 0.05).
Multiple imputation was used to deal with missing covariate data. All predictor and outcome variables were used as predictors in the imputation model, which was set for 10 imputations. Analyses were run on complete case and imputed datasets, and a comparison of results showed that differences were negligible. Only results based on imputed datasets are presented here as complete case analysis is thought to suffer from more chance variation, and multiple random imputation is assumed to correct any bias. The rules of Rubin et al. [28] were used to combine the estimates to obtain valid overall estimates.
2.8. Ethical approval
Ethical approval for this study was granted by the ALSPAC Law and Ethics Committee and Local Ethics Committees.
3. Results
3.1. Descriptive characteristics
The descriptive characteristics of all participants attending FOM1 and FOM2 clinics are described in Table 1. A comparison of descriptive characteristics in ED and non-ED participants in the FOM1 and FOM2 ALSPAC study clinics is presented in Table 2 and Table 3. Current BMI and lowest ever BMI were both lower in women who had received a lifetime diagnosis of AN (AN-R or AN-BP) compared to non-ED women, however women with a lifetime diagnosis of BN had a comparable current and lowest ever BMI to control women.
Table 1.
Whole sample participant characteristics in the ALSPAC FOM1 and FOM2 assessments.
| FOM1 | FOM2 | |||||
|---|---|---|---|---|---|---|
| N | Mean | SD | N | Mean | SD | |
| Age (years) | 4717 | 48 | 4.48 | 2771 | 50.82 | 4.41 |
| Height (cm) | 4699 | 164.01 | 6.13 | 2762 | 164.14 | 6.24 |
| Weight (kg) | 4696 | 71.7 | 14.88 | 2763 | 70.92 | 14.26 |
| Fat Mass (kg) | 4550 | 27.19 | 10.95 | 2716 | 27.03 | 10.91 |
| Lean Mass (kg) | 4550 | 41.28 | 5.16 | 1829 | 40.51 | 5.21 |
| TBLH BMC (g) | 4550 | 2167.63 | 366.18 | 2716 | 2612.82 | 420.39 |
| TBLH BA (cm2) | 4550 | 1976.15 | 232.26 | 2716 | 2161.77 | 240.15 |
| TBLH BMD (g/cm2) | 4550 | 1.091 | 0.08 | 2716 | 1.21 | 0.08 |
TBLH = Total Body Less Head; BMC = Bone Mineral Content; BMD = Bone Mineral Density; BA = Bone Area.
3.2. BMD in women with ED diagnoses
A lifetime diagnosis of AN, but not BN, was associated with significantly lower BMD z-scores in crude analyses at the total body (mean difference = −0.28; 95% CI −0.49, −0.05), legs (mean difference = −0.28; 95% CI −0.53, −0.04), arms (mean difference = −0.31; 95% CI −0.55, −0.08) and hip (mean difference = −0.46; 95% CI −0.77, −0.17) (all p < 0.05). Conversely, in analyses adjusted for confounders, women with AN had a significantly lower BMD Z-scores for the total body (mean difference = −0.28; 95% CI −0.49, 0.05), legs (mean difference = −0.28; 95% CI: −0.53, −0.04), and arms (mean difference = −0.31; 95% CI: −0.55, −0.08). Women with BN had significantly higher total body (mean difference = 0.22; 95% CI 0.01, 0.44) and leg (mean difference = 0.41; 95% CI 0.18, 0.64) BA (see Table 4 and Supplement Table 2) BMD. Finally, in the final model, when lowest ever BMI was included as a covariate, AN and BN were no longer significantly associated with BMD or BA (see Table 4 and Supplemental Table 2).
Table 4.
Multivariate analyses from FOM1 and FOM2 Clinic. Mean Difference in Z-Scores between BMD and BA Measures in ALSPAC Mothers with AN or BN.
| FOM1 clinic | AN (59) | BN (54) | HC (2942) | |||
|---|---|---|---|---|---|---|
| Total body minus head | ß (95% CI) | p | ß (95% CI) | p | Ref. | |
| Crude analysis | BMD | −0.39 (−0.67; −0.12) | 0.007 | 0.28 (−0.01; 0.58) | 0.059 | |
| BA | −0.32 (−0.61; −0.02) | 0.025 | 0.09 (−0.19; 0.36) | 0.539 | ||
| Adjusted | BMD | −0.28 (−0.49; −0.05) | 0.003 | 0.19 (−0.07; 0.47) | 0.143 | |
| BA | −0.19 (−0.41; 0.03) | 0.087 | 0.22 (0.01; 0.44) | 0.048 | ||
| Adjusted + Lowest ever BMI | BMD | 0.06 (−0.31; 0.18) | 0.627 | 0.19 (−0.05; 0.42) | 0.120 | |
| BA | 0.12 (−0.14; 0.35) | 0.390 | 0.18 (−0.05; 0.41) | 0.123 | ||
| FOM2 Clinic | AN (41) | p | BN (39) | HC (1948) | ||
| Total Hip | ß (95% CI) | ß (95% CI) | p | Ref. | ||
| Crude analysis | BMD | −0.42 (−0.69; −0.15) | 0.003 | 0.13 (−0.16; 0.41) | 0.374 | |
| BA | −0.29 (−0.64; 0.05) | 0.091 | −0.05 (−0.41; 0.31) | 0.77 | ||
| Adjusted | BMD | −0.46 (−0.75; −0.17) | 0.002 | 0.07 (−0.18; 0.34) | 0.449 | |
| BA | −0.07 (−0.37; 0.21) | 0.59 | 0.11 (−0.18; 0.41) | 0.45 | ||
| Adjusted + lowest ever BMI | BMD | −0.02 (−0.36; 0.32) | 0.876 | 0.11 (−0.14; 0.35) | 0.332 | |
| BA | −0.04 (−0.39; 0.31) | 0.808 | 0.12 (−0.18; 0.43) | 0.43 | ||
BMD = Bone Mineral Density; BA = Bone Area; FOM1 = Focus on Mothers Clinic 1; FOM2 = Focus on Mothers Clinic Adjusted = Adjusted for Confounders including: Ethnicity, Educational Level, Height2, BA *In Analysis of BA, BA was excluded from the Confounding set.
Bold numbers which are statistically significant.
In post-hoc adjusted analyses women with AN-R, but not AN-BP had lower BMD Z-scores at the total body (mean difference = −0.37; 95% CI −0.62, −0.12), hip (mean difference = −0.51; 95% CI −0.82, −0.21) (Table 5), legs (mean difference = −0.42; 95% CI −0.69, −0.15) and arms (mean difference = 0.37; 95% CI −0.65, −0.11) when adjusted for sociodemographic variables and confounders. Women with both AN-R and AN-BP showed a trend towards having a lower BA than women with no ED at all anatomical sites measured.
Table 5.
Multiple Imputation results from FOM1 and FOM2 Clinic. Mean Difference in Z-Scores between BMD and BA Measures in ALSPAC Mothers with lifetime AN-R or AN-BP diagnoses.
| FOM1 Clinic N = 3440 | AN_R (N = 19) | AN_BP (N = 29) | HC (N = 2942) | |||
|---|---|---|---|---|---|---|
| Total Body Minus Head | ß (95% CI) | p | ß (95% CI) | p | Ref. | |
| Crude Analysis | BMD | −0.45 (−0.79; 0.12) | 0.007 | −0.24 (−0.65; 0.18) | 0.260 | |
| BA | −0.32 (−0.82; 0.18) | 0.205 | −0.27 (−0.61; 0.05) | 0.106 | ||
| Adjusted | BMD | −0.37 (−0.62; −0.12) | 0.004 | −0.13 (−0.46; 0.19) | 0.426 | |
| BA | −0.23 (−0.58; 0.12) | 0.197 | −0.13 (−0.41; 0.14) | 0.333 | ||
| Adjusted + Lowest Ever BMI | BMD | −0.15 (−0.42; 0.12) | 0.269 | 0.07(−0.28; 0.43) | 0.700 | |
| BA | 0.12 (−0.28; 0.52) | 0.560 | 0.15 (−0.14; 0.45) | 0.311 | ||
| FOM2 Clinic N = 2256 | AN_R (N = 19) | p | AN_BP (N = 29) | p | HC (N = 1948) | |
| Total Hip | ß (95% CI) | ß (95% CI) | Ref. | |||
| Crude Analysis | BMD | −0.47 (−0.76; −0.19) | 0.001 | −0.35 (−0.79; −0.08) | 0.114 | |
| BA | −0.37 (−0.82; 0.07) | 0.101 | −0.16 (−0.59; 0.26) | 0.450 | ||
| Adjusted | BMD | −0.51 (−0.82; −0.21) | 0.001 | −0.38 (−0.83; 0.06) | 0.093 | |
| BA | −0.21 (−0.54; 0.14) | 0.246 | 0.09 (−0.27; 0.46) | 0.601 | ||
| Adjusted + Lowest Ever BMI | BMD | −0.09 (−0.46; 0.27) | 0.602 | 0.04 (−0.42; 0.52) | 0.836 | |
| BA | −0.03 (−0.41; 0.34) | 0.859 | 0.27 (−0.11; 0.66) | 0.165 | ||
BMD = Bone Mineral Density; BA = Bone Area; FOM1 = Focus on Mothers Clinic 1; FOM2 = Focus on Mothers Clinic.
Adjusted = Adjusted for Confounders including: Ethnicity, Educational Level, Height2, BA *In Analysis of BA, BA was excluded from the Confounding set. Bold numbers which are statistically significant.
3.3. BMD in women with ED behaviours
Lifetime Fasting was predictive of low BMD Z-scores at the legs in crude analyses (mean difference = −0.18; 95% CI −0.36, −0.012), and of the hip in adjusted analyses (mean difference = −0.21; 95% CI −0.43, −0.008). Restrictive eating was predictive of low BMD Z-scores at the arms (mean difference = −0.31; 95% CI −0.58, −0.03), and the hip in crude (mean difference = −0.34; 95% CI −0.64, −0.03) and adjusted analyses (mean difference = −0.33; 95% CI −0.63, −0.04). When lowest ever reported BMI was included in the model neither fasting nor restrictive eating were significantly associated with any BMD measure.
Lifetime self-induced vomiting was associated with lower BA at the total body in both crude (mean difference = −0.31; 95% CI −0.55, −0.08) and adjusted analyses (mean difference = −0.33; 95% CI −0.56, −0.09), and low arm BMD Z-scores in crude analyses only (mean difference = −0.26; 95% CI −0.49, −0.03). Misuse of medication was associated with low BMD Z-scores at the hip in adjusted analyses (mean difference = −0.31; 95% CI −0.58, −0.027). Similar to the results for fasting and restricting ED behaviours, these associations were non-significant when analyses additionally adjusted for lowest ever reported BMI. The associations between ED behaviours and total body and hip BMD and BA are presented in Table 6.
Table 6.
Multiple Imputation Results from FOM1 and FOM2 Clinic. Mean Difference in Z-Scores between BMD and BA Measures in ALSPAC Mothers with lifetime ED Behaviours.
| F0M1 Clinic N = 3440 | Fasting (N = 131) | Restricting (N = 53) | Vomiting (N = 71) | Misuse of Medication (N = 72) | HC (N = 2942) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Body | ß (95% CI) | p | ß (95% CI) | p | ß (95% CI) | p | ß (95% CI) | p | Ref. | |
| Crude Analysis | BMD | −0.11 (−0.28; 0.06) | 0.211 | −0.25 (−0.52; 0.02) | 0.072 | −0.15 (−0.39; 0.08) | 0.199 | −0.13 (−0.36; 0.09) | 0.257 | |
| BA | −0.21 (−0.38; −0.03) | 0.021 | −0.38 (−0.65; 0.12) | 0.005 | −0.31 (−0.55; −0.08) | 0.009 | −0.15 (−0.38; 0.07) | 0.197 | ||
| Adjusted | BMD | −0.41 (−0.18; 0.11) | 0.584 | −0.03 (−0.26; 0.19) | 0.774 | −0.02 (0.22; 0.18) | 0.852 | −0.08 (−0.27; 0.11) | 0.414 | |
| BA | −0.12 (−0.26; 0.02) | 0.093 | −0.33 (−0.55; −0.11) | 0.004 | −0.18 (−0.37; 0.06) | 0.059 | −0.08 (−0.27; 0.11) | 0.384 | ||
| Adjusted + Lowest Ever BMI | BMD | 0.02 (−0.12; 0.17) | 0.722 | 0.11 (−0.12; 0.33) | 0.362 | 0.04 (−0.15; 0.24) | 0.647 | −0.01 (−0.21; 0.17) | 0.904 | |
| BA | −0.02 (−0.16; 0.11) | 0.733 | −0.12 (−0.34; 0.92) | 0.263 | −0.08 (−0.26; 0.09) | 0.363 | 0.01 (−0.17; 0.19) | 0.884 | ||
| FOM2 Clinic N = 2256 | Fasting (N = 85) | Restricting (N = 42) | Vomiting (N = 50) | Misuse of Medication (N = 49) | HC (N = 1948) | |||||
| Hip | ß (95% CI) | P | ß (95% CI) | P | ß (95% CI) | P | ß (95% CI) | P | Ref. | |
| Crude Analysis | BMD | −0.18 (−0.39; 0.03) | 0.091 | −0.36 (−0.64; −0.03) | 0.029 | −0.05 (−0.32; 0.23) | 0.748 | −0.25 (−0.54; 0.03) | 0.075 | |
| BA | −0.11 (−0.32; 0.11) | 0.318 | 0.06 (−0.24; 0.37) | 0.668 | −0.12 (−0.39; 0.16) | 0.403 | 0.07 (−0.21; 0.35) | 0.608 | ||
| Adjusted | BMD | −0.22 (−0.43; −0.001) | 0.042 | −0.33 (−0.63; −0.04) | 0.027 | −0.06 (−0.33; 0.21) | 0.668 | −0.29 (−0.58; −0.02) | 0.035 | |
| BA | 0.05 (−0.14; 0.23) | 0.620 | 0.06 (−0.21; 0.32) | 0.650 | 0.03 (−0.22; 0.27) | 0.832 | 0.13 (−0.11; 0.38) | 0.271 | ||
| Adjusted + Lowest Ever BMI | BMD | −0.11 (−0.31; 0.09) | 0.299 | −0.08 (−0.36; 0.21) | 0.606 | 0.11 (−0.16; 0.36) | 0.437 | −0.18 (−0.45; 0.08) | 0.191 | |
| BA | 0.11 (−0.08; 0.029) | 0.280 | 0.19 (−0.08; 0.45) | 0.160 | 0.11 (−0.13; 0.35) | 0.385 | 0.21 (−0.04; 0.45) | 0.106 | ||
BMD = Bone Mineral Density; BA = Bone Area; FOM1 = Focus on Mothers Clinic 1; FOM2 = Focus on Mothers Clinic Adjusted = Adjusted for Confounders including: Ethnicity, Educational Level, Height2, BA *In Analysis of BA, BA was excluded from the Confounding set.
Bold text should be for numbers which are statistically significant.
Post-hoc analyses investigating ED behaviours as mutually exclusive categories revealed that when women with vomiting or misuse of medication were excluded from the analyses fasting, but not restrictive eating, was associated with a significantly low BMD at the arms (mean difference = −0.25; 95% CI: −0.49, −0.01) in adjusted analyses. Restrictive eating remained predictive of a low BA, specifically at total body BA (mean difference = −0.32; 95% CI: −0.64, −0.02). Analyses only including women who exclusively either vomited of misused medication (or engaged in both), showed that neither of these beahviours were predictive of poor bone health in isolation.
4. Discussion
This study found that a lifetime diagnosis of AN, but not BN was associated with low BMD at all sites measured, including total body, the hip, the arms and the legs. When separated post-hoc, AN-R, rather than AN-BP was individually predictive of low total body, hip, arms, and legs BMD. The association between AN, and specifically AN-R, and BMD and BA at all skeletal sites diminished however, when lowest ever BMI was included in the regression model, with the mean difference decreasing by 4–5 fold. ED behaviours including fasting and restricting were individually predictive of poor bone health outcomes at both total body and the hip, with mean differences in BMD similar to those observed between AN-R and healthy women. Vomiting and misuse of medication when studied in isolation, were not significantly associated with either bone health outcomes in this study. As with ED diagnoses, these associations significantly decreased when lowest ever BMI was included in the regression model, with a greatly reduced effect size and wider confidence intervals.
This study supports evidence that AN but not BN is prospectively associated with low BMD and BA [18,20,29], and that this association is mostly driven by restricting (AN-R), rather than binge-purging (AN-BP) AN subtypes. Previous research has found that women with BN have significantly lower BMD at the total body and the spine [12,21,30] than healthy women regardless of BMI. A recent meta-analysis by our group found that women with BN had lower BMD at the spine (SMD −0.47) than healthy control women, indicating an increased fracture risk of between 30 and 50% [12]. However, when separating those with and without a history of AN in post-hoc analyses, it was evident that only women with BN and a history of AN presented with significantly low BMD [18–20], suggesting that a diagnosis of BN alone may not be a risk factor for poor bone health in adulthood.
Fasting and restricting ED behaviours were associated with low total body and hip BMD and BA; with the mean difference approximately halving in the majority of these analyses when including lowest ever BMI in the model. Fasting for prolonged periods of time and restricting food intake are associated with poor nutritional intake [31], with calcium, vitamin D and vitamin K all known to contribute to bone accrual and the maintenance of BMD [32–34]. Furthermore, endocrine changes, including disruption of the HPG-axis resulting in deficiency of the sex hormones, oestrogen and testosterone, have been suggested to contribute to loss of BMD in AN [6,35,36]. Reaching a significantly low body weight in one’s lifetime, as is evident in those who report fasting and restricting ED behaviours, is likely to be closely linked to both malnutrition and also the disruption of endocrine factors known to be associated with bone health. This supports previous research which suggests that a significantly low BMI is an independent risk factor for poor bone health outcomes [16,37].
Vomiting and misuse of medication at any time throughout lifetime were found to be predictive of poor bone health outcomes in this study when present alongside either fasting or restricting behaviours. These ED behaviours are characteristic of BN, AN-BP and also sub-EDs in DSM-5 diagnostic categories [26]. However, this study found that vomiting and misuse of medication ED behaviours were not individually predictive of poor bone health outcomes, suggesting that fasting and restricting (AN-R ‘type’ behaviours) are driving the relationship between EDs and low BMD.
This study found that the associations between ED diagnoses and ED behaviours and bone health outcomes diminished when the lowest ever reported BMI was included in the model, indicating that having a low BMI explains to a great extent the poor bone health outcomes in women with ED. Of note, the mean BMI of all ED diagnosis and behaviour groups was > 22.5 kg/m2 at the time of a DXA scan, with only 5 women diagnosed with AN at any time during life remaining clinically underweight (BMI < 18.5 kg/m2). It is thus evident that weight gain in the AN group was not sufficient to fully recover BMD, as reported in previous research [16,32,38,39]. Previous studies investigating endocrine mediators of the relationship between AN and poor bone health have reported that a low BMI is a significant predictor of both endocrine disruption and loss of BMD [7,40]. Similarly, this study found that the lowest ever reported BMI explained a large proportion of variance in all analyses presented here. Our findings support previous reports of low BMI being an independent risk factor for poor bone health outcomes [16].
Although BMD is known to be a key predictor of fracture risk in both children and adults, BA is an indicator of skeletal size and so is particularly relevant when considering those with EDs who may have a small skeleton as well as a low body weight. There was a trend towards a low BA in those with AN (AN-R and AN-BP), and also in those reporting fasting, restricting and vomiting ED behaviours. However, all groups showed larger effect sizes in the analyses of BMD, indicating that these EDs and ED behaviours have a greater effect on BMD than BA at all skeletal sites.
Our findings have to be understood in light of strengths and limitations. The prospective nature of this study allowed us to investigate ED at any time during life and their association with bone health outcomes in mid-life. Furthermore, this is the first study to investigate the association between ED behaviours and bone health outcomes in order to determine which ED behaviours within broader ED phenotypes might affect bone health. Whereas the majority of previoius studies in this field have focused on clinical samples, the use of a population-based sample using a longitudinal study design allows us to widen what we know about the effect of ED on bone health to women who do not necessarily access healthcare for their ED, and also to determine if women with less severe forms of ED are also at risk for poor bone health outcomes.
Although the sample used in this study is large, there are a number of limitations that need to be taken into account. Two ALSPAC study clinics were used in this study, which were conducted at separate time-points. Women in this study thus received a hip DXA scan (FOM2 clinic) on average two years later than the total body DXA scan (FOM1 clinic) [41]. As only pre- or peri-menopausal women who attended the FOM1 clinic were invited to attend FOM2, the findings from the FOM2 clinic (hip bone health measures) are prone to selection bias and thus only generalizable to other pre- or peri-menopausal women.
Participation in the ED substudy was biased towards women with a higher level of education, and those with fewer children, who were more likely to participate [25]. Furthermore, the nature of the ALSPAC study recruitment results in the entirety of our sample being mothers of one or more children. Pregnancy and parity are both known to have an effect on bone health outcomes [42–45]. Women with AN in this study may have either a higher BMI or less severe symptoms than women with AN who fail to become pregnant, as women with acute AN may take longer to become pregnant or have difficulty conceiving in comparison to healthy women [46,47]. Furthermore, attrition was negatively predicted by the presence of ED behaviours and diagnoses, with these women being less likely to drop out of the study. Hence, it is possible that the relationship between ED diagnoses and ED behaviours and BMD is exaggerated in this study due to this slightly skewed sample.
5. Conclusions
For the first time, we show that lifetime ED behaviours (fasting and restrictive eating) as well as AN-R are associated with low BMD in mid-life adult women across four anatomical sites. As poor bone health was common among this sample of ED women, this study has important public health as well as clinical implications for early identification of osteopenia and osteoporosis, before the occurrence of fractures.
Supplementary Material
Acknowledgements
We are extremely grateful to all the families who took part in this study, the midwives for their help in recruiting them, and the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses.
Funding
The UK Medical Research Council and Wellcome (Grant ref.: 102215/2/13/2) and the University of Bristol provide core support for ALSPAC. This publication is the work of the authors and Dr. Lauren Robinson and Dr. Nadia Micali will serve as guarantors for the contents of this paper.
A comprehensive list of grants funding is available on the ALSPAC website (http://www.bristol.ac.uk/alspac/external/documents/grant-acknowledgements.pdf); This research was specifically funded by a PhD studenship grant awarded to Dr. Lauren Robinson by University College London.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2018.12.005.
References
- [1].Fairburn CG, Harrison PJ, Eating disorders, Lancet 361 (9355) (2003) 407–416. [DOI] [PubMed] [Google Scholar]
- [2].Grinspoon S, et al. , Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa, Ann. Intern. Med 133 (10) (2000) 790–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Miller KK, et al. , Determinants of skeletal loss and recovery in anorexia nervosa, J. Clin. Endocrinol. Metab 91 (8) (2006) 2931–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Milos G, et al. , Are patterns of bone loss in anorexic and postmenopausal women similar? Preliminary results using high resolution peripheral computed tomography, Bone 58 (2014) 146–150. [DOI] [PubMed] [Google Scholar]
- [5].Misra M, Klibanski A, Bone health in anorexia nervosa, Curr. Opin. Endocrinol. Diabetes Obesity 18 (6) (2011) 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Seeman E, et al. , Osteoporosis in anorexia nervosa: the influence of peak bone density, bone loss, oral contraceptive use, and exercise, J. Bone Miner. Res 7 (12) (1992) 1467–1474. [DOI] [PubMed] [Google Scholar]
- [7].Munoz M, Argente J, Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances, Eur. J. Endocrinol 147 (3) (2002) 275–286. [DOI] [PubMed] [Google Scholar]
- [8].Misra M, Klibanski A, Bone metabolism in adolescents with anorexia nervosa, J. Endocrinol. Investig 34 (4) (2011) 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Misra M, et al. , Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls, Pediatrics 114 (6) (2004) 1574–1583. [DOI] [PubMed] [Google Scholar]
- [10].Nagata JM, et al. , Assessment of sex differences in fracture risk among patients with anorexia nervosa: a population-based cohort study using the Health Improvement Network, J. Bone Miner. Res 32 (5) (2017) 1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Swanson SA, et al. , Prevalence and correlates of eating disorders in adolescents: results from the national comorbidity survey replication adolescent supplement, Arch. Gen. Psychiatry 68 (7) (2011) 714–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Robinson L, et al. , A systematic review and meta-analysis of the association between eating disorders and bone density, Osteoporos. Int 27 (6) (2016) 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Miller KK, et al. , Medical findings in outpatients with anorexia nervosa, Arch. Intern. Med 165 (5) (2005) 561–566. [DOI] [PubMed] [Google Scholar]
- [14].Heaney R, et al. , Peak bone mass, Osteoporos. Int 11 (12) (2000) 985–1009. [DOI] [PubMed] [Google Scholar]
- [15].Hartman D, et al. , Bone density of women who have recovered from anorexia nervosa, Int. J. Eat. Disord 28 (1) (2000) 107–112. [DOI] [PubMed] [Google Scholar]
- [16].Misra M, et al. , Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1, J. Clin. Endocrinol. Metab 93 (4) (2008) 1231–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lucas AR, et al. , Long-term fracture risk among women with anorexia nervosa: a population-based cohort study, Mayo Clinic Proceedings, Elsevier, 1999. [DOI] [PubMed] [Google Scholar]
- [18].Davies K, et al. , Reduced bone mineral in patients with eating disorders, Bone 11 (3) (1990) 143–147. [DOI] [PubMed] [Google Scholar]
- [19].Naessén S, et al. , Bone mineral density in bulimic women–influence of endocrine factors and previous anorexia, Eur. J. Endocrinol 155 (2) (2006) 245–251. [DOI] [PubMed] [Google Scholar]
- [20].Sundgot-Borgen J, et al. , Normal bone mass in bulimic women, J. Clin. Endocrinol. Metab 83 (9) (1998) 3144–3149. [DOI] [PubMed] [Google Scholar]
- [21].Newton JR, et al. , Osteoporosis and normal weight bulimia nervosa—which patients are at risk? J. Psychosom. Res 37 (3) (1993) 239–247. [DOI] [PubMed] [Google Scholar]
- [22].Team AS, ALSPAC–the Avon longitudinal study of parents and children, Paediatr. Perinat. Epidemiol 15 (1) (2001) 74–87. [DOI] [PubMed] [Google Scholar]
- [23].Fraser A, et al. , Cohort profile: the Avon Longitudinal Study of parents and Children: ALSPAC mothers cohort, Int. J. Epidemiol 42 (1) (2013) 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Harris PA, et al. , Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support, J. Biomed. Inform 42 (2) (2009) 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Micali N, et al. , Lifetime and 12-month prevalence of eating disorders amongst women in mid-life: a population-based study of diagnoses and risk factors, BMC Med. 15 (1) (2017) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Association, A.P, Diagnostic and Statistical Manual of Mental Disorders (DSM-5®), American Psychiatric Pub, 2013. [Google Scholar]
- [27].L. Statacorp, Stata Data Analysis and Statistical Software, Vol. 13 Special Edition Release, 2013. [Google Scholar]
- [28].Rubin DB, Multiple imputation after 18+ years, J. Am. Stat. Assoc 91 (434) (1996) 473–489. [Google Scholar]
- [29].Mitchell JE, Crow S, Medical complications of anorexia nervosa and bulimia nervosa, Curr. Opin. Psychiatr 19 (4) (2006) 438–443. [DOI] [PubMed] [Google Scholar]
- [30].Iketani T, et al. , Effects of weight gain and resumption of menses on reduced bone density in patients with anorexia nervosa, Biol. Psychiatry 37 (8) (1995) 521–527. [DOI] [PubMed] [Google Scholar]
- [31].Patrick L, Eating disorders: a review of the literature with emphasis on medical complications and clinical nutrition.(Eating Disorders), Altern. Med. Rev 7 (3) (2002) 184–203. [PubMed] [Google Scholar]
- [32].Robinson L, et al. , Pharmacological treatment option for low Bone Mineral Density and secondary osteoporosis in Anorexia Nervosa: a systematic review of the literature, J. Psychosom. Res 98 (2017) 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lehtonen-Veromaa MKM, et al. , Vitamin D and attainment of peak bone mass among peripubertal Finnish girls: a 3-y prospective study, Am. J. Clin. Nutr 76 (6) (2002) 1446–1453. [DOI] [PubMed] [Google Scholar]
- [34].Knapen M, Schurgers L, Vermeer C, Vitamin K2 supplementation improves hip bone geometry and bone strength indices in postmenopausal women, Osteoporos. Int 18 (7) (2007) 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Klibanski A, et al. , The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa, J. Clin. Endocrinol. Metab 80 (3) (1995) 898–904. [DOI] [PubMed] [Google Scholar]
- [36].Misra M, et al. , Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa, J. Bone Miner. Res 26 (10) (2011) 2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].De Laet C, et al. , Body mass index as a predictor of fracture risk: a meta-analysis, Osteoporos. Int 16 (11) (2005) 1330–1338. [DOI] [PubMed] [Google Scholar]
- [38].Jáuregui-Lobera I, Bolaños-Ríos P, Sabaté J, Bone mineral density in anorexia nervosa: only weight and menses recovery? Endocrinol. Nutr 63 (9) (2016) 458–465. [DOI] [PubMed] [Google Scholar]
- [39].El Ghoch M, et al. , The association between weight gain/restoration and bone mineral density in adolescents with anorexia nervosa: a systematic review, Nutrients 8 (12) (2016) 769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Misra M, et al. , Secretory dynamics of leptin in adolescent girls with anorexia nervosa and healthy adolescents, Am. J. Physiol. Endocrinol. Metab 289 (3) (2005) E373–E381. [DOI] [PubMed] [Google Scholar]
- [41].Fraser A, et al. , Cohort profile: the Avon longitudinal Study of parents and Children: ALSPAC mothers cohort, Int. J. Epidemiol 42 (1) (2012) 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Ghannam N, et al. , Bone mineral density of the spine and femur in healthy Saudi females: relation to vitamin D status, pregnancy, and lactation, Calcif. Tissue Int 65 (1) (1999) 23–28. [DOI] [PubMed] [Google Scholar]
- [43].Kritz-Silverstein D, Barrett-Connor E, Hollenbach KA, Pregnancy and lactation as determinants of bone mineral density in postmenopausal women, Am. J. Epidemiol 136 (9) (1992) 1052–1059. [DOI] [PubMed] [Google Scholar]
- [44].Murphy S, et al. , Parity and bone mineral density in middle-aged women, Osteoporos. Int 4 (3) (1994) 162–166. [DOI] [PubMed] [Google Scholar]
- [45].Streeten EA, et al. , The relationship between parity and bone mineral density in women characterized by a homogeneous lifestyle and high parity, J. Clin. Endocrinol. Metab 90 (8) (2005) 4536–4541. [DOI] [PubMed] [Google Scholar]
- [46].Bulik CM, et al. , Unplanned pregnancy in anorexia nervosa, Obstet. Gynecol 116 (5) (2010) 1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Ekeus C, et al. , Birth outcomes and pregnancy complications in women with a history of anorexia nervosa, BJOG Int. J. Obstet. Gynaecol 113 (8) (2006) 925–929. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


