Abstract
Exploring innovative solutions to improve the healthcare of the aging and diseased population continues to be a global challenge. Among a number of strategies toward this goal, tissue engineering and regenerative medicine (TERM) has gradually evolved into a promising approach to meet future needs of patients. TERM has recently received increasing attention in Asia, as evidenced by the markedly increased number of researchers, publications, clinical trials, and translational products. This review aims to give a brief overview of TERM development in Asia over the last decade by highlighting some of the important advances in this field and featuring major achievements of representative research groups. The development of novel biomaterials and enabling technologies, identification of new cell sources, and applications of TERM in various tissues are briefly introduced. Finally, the achievement of TERM in Asia, including important publications, representative discoveries, clinical trials, and examples of commercial products will be introduced. Discussion on current limitations and future directions in this hot topic will also be provided.
Keywords: tissue engineering, regenerative medicine, Asia, biomaterials, cell sources, biomechanics
Introduction
Fully repairing or regenerating damaged tissues or organs and restoring their functions have been a dream of human beings. The advent of tissue engineering and regenerative medicine (TERM) appears to make it possible. Tissue engineering combines cells, scaffolds, and growth factors to regenerate tissues or replace damaged or diseased tissues, while regenerative medicine combines tissue engineering with other strategies, including cell-based therapy, gene therapy, and immunomodulation, to induce in vivo tissue/organ regeneration (Lysaght and Crager, 2009; Lindroos et al., 2011; Salgado et al., 2013; Porada et al., 2016). TERM is a multidisciplinary science and combines basic sciences such as materials science, biomechanics, cell biology, and medical sciences to realize functional tissue/organ repair or reconstruction. With the aging of world population trend intensifying, there is an increasing demand of organ replacements. TERM holds the potential to meet the future needs of patients (Frey et al., 2016).
The aim of TERM is to establish a three-dimensional (3D) cell/biomaterial complex, which has similar function as a living tissue/organ and may be used to repair or regenerate injured tissue/organ. The basic requirement for the complex is that it can support cell growth, transportation of nutrition and waste, and gas exchange. TERM usually uses the following three strategies: (1) cell/biomaterial complex system, in which cell-seeded biomaterials are implanted into the body to repair and regenerate tissues/organs; (2) cell systems, such as stem cell transplantation; and (3) biomaterial systems, which will be implanted into body and undergo the process of tissue integration.
Tissue engineering and regenerative medicine has been proposed and developed for more than 30 years. While several successful attempts in tissue regeneration have been achieved, TERM is still in its infancy and there are many fundamental questions that remain to be answered, including selection of cell sources, development of tissue-specific materials, development of specialized bioreactors, and construction of complex organs. More importantly, the processes and mechanisms of new tissue/organ formed using these tissue-engineered materials in vivo, similarity and difference between these processes with nature tissue/organ development and healing, and transformation and final destination of these materials continue to be the critical concerns in this dynamically developing field. Addressing these questions is the key to the effectiveness, stability, and security of the clinical application of tissue-engineered materials.
The aim of this review is to summarize recent major events in the broad discipline of TERM in Asia. In constructing this review, we retrieved a large number of literature and selected some representative works that are presented in this review. Indeed, we recognized that we are limited by our own small view and specialization, and that this might limit our ability to capture the comprehensiveness and diversity of TERM. Therefore, this review may not be comprehensive, but we will try our best to list some important advances in TERM in Asia.
The population of TERM research community has been rapidly expanding in Asia. In this review, a brief overview of recent development in the broad discipline of TERM in Asia will be summarized. To this end, we retrieved a large number of literature and selected representative achievements to showcase in this review. Due to the page limit, this review is far from being comprehensive; instead, we highlight here only a small fraction of important advances of TERM in Asia as examples.
Achievement of Term in Asia
Biomaterials
Polymer
Native polymers, such as acellular matrix, collagen, gelatin, hyaluronic acid (HA), silk, alginate, and chitosan, are well-known sources for preparing scaffold in TERM. There are many works to explore the interrelationship between the composition, structure, and functions of these native polymer-based scaffolds. Zeng’s group found that peripheral nerve-derived matrix hydrogel promoted remyelination and inhibited synapse formation (Zou et al., 2018). Peng and Wang’s groups used a neurotrophic peptide-functionalized, self-assembling nanofiber hydrogel to produce a neurotrophic microenvironment (Lu et al., 2018). Dai’s group developed a lot of collagen-based scaffolds for nerve regeneration (Chen et al., 2018c; Li X. R. et al., 2018). A collagen scaffold with microchannel and loading paclitaxel liposome-induced neuronal differentiation of neural stem cells (NSCs) through Wnt/β-catenin signaling could be used in spinal cord injury (SCI) repair (Li X. R. et al., 2018). In a recent work, a multichannel poly(propylene fumarate) scaffold modifying with collagen-binding neurotrophic factor 3 promoted neural regeneration of SCI (Chen et al., 2018c). They also found that controlled release of collagen-binding stromal cell-derived factor-1α (SDF-1α) from the scaffold promoted tendon regeneration in a rat Achilles tendon defect model (Sun J. et al., 2018). Chen’s group also attempted to prepare various porous collagen and gelatin scaffolds for application in TERM (Chen et al., 2014; Zhang Q. et al., 2014; Chen S. et al., 2015; Chen S. W. et al., 2017). They found that the pore structure and mechanical property also regulate cartilage regeneration (Chen S. et al., 2015). In another work, they also developed collagen porous scaffolds with parallel and concave microgrooves to regulate vascular endothelial cells and muscle cells (Chen S. W. et al., 2017). Esaki et al.’s (2019) group reported that transplantation of olfactory stem cells with biodegradable gelatin sponge could accelerate facial nerve regeneration after crush injury. They also fabricated human ear- and nose-shaped scaffolds used in gelatin and HA by integrating photocuring 3D printing and lyophilization techniques (Xia et al., 2018). The natural hydrogel such as gelatin methacryloyl (GelMA) could also be electrospun into 3D fibrous scaffolds (Sun et al., 2017). After GelMA fibrous scaffold implantation below the skin flap of rat, more microvascular formation is found. Zhang X. et al.’s (2019) group developed aligned all-cellulose nanofibers coated with bone morphogenetic protein-2 (BMP-2) via electrospinning. Aligned nanofibers instructed cell growth along fiber direction. Gao’s group reported that a HA hydrogel modified by laminin-derived peptide could promote neurite outgrowth of PC12 cells (Xing et al., 2017). In another work, they found that cell-responsive hydrogel could be used to develop a co-culture system of immune cells and smooth muscle cells (Yu S. et al., 2018). Goh’s group prepared different silk-based scaffolds for TERM (Shi et al., 2013; Teh et al., 2013). They prepared aligned fibrous scaffolds using silk fibroin, and found that it could enhance mechanoresponse and tenogenesis of mesenchymal stem cells (MSCs). Deng’s group found enhanced osteogenesis of bone marrow stromal cells (BMSCs) by a functionalized silk fibroin hydrogel (Yan et al., 2019). Cho’s group prepared an injectable microgel composed of arginine–glycine–aspartic acid (RGD)-conjugated alginate that encapsulated endothelial cells and growth factors to enhance vascularization (Kim et al., 2014). Inspired by mussel chemistry, Lu’s group developed many polyacrylamide-based polymers with good tissue adhesiveness that can be used in cartilage and skin repair (Han L. et al., 2018; Gan et al., 2019). Wang’s group used a photo-cross-linkable, injectable sericin hydrogel for minimally invasive repairing cartilage (Qi et al., 2018). Gu’s group found that chitosan/silk fibroin scaffold combined with allogeneic bone marrow mononuclear cells could facilitate nerve regeneration, and the scaffold can improve macrophage-constructed microenvironments (Yao et al., 2016; Zhao Y. H. et al., 2017).
Although nature polymers usually have good bioactivity, their mechanical properties are often weak. Compared with other materials, the properties of synthetic polymer could be easily tailored by changing the molecular structure and processing parameters. Li’s group prepared poly(ether carbonate urethane)urea scaffolds with tunable elasticity and topography, and these properties of scaffolds could regulate the fate of AF-derived stem cells through a YAP-dependent mechanotransduction mechanism (Liu et al., 2015; Zhu et al., 2016; Chu et al., 2019). Yeong et al. (2010) presented highly porous polycaprolactone (PCL) scaffold by sintering PCL powder for application in cardiac tissue engineering. The mechanical property of this scaffold could be controlled by changing porosity. Kim’s group developed a PCL-based scaffold with uniaxially aligned surface topography by stretching a 3D-printed scaffold (Yang G. H. et al., 2019). To modify the bioactivity of polymer, polydopamine (PDA)-assisted surface modification is an easy approach (Jia et al., 2019a). Poly(ether ether ketone) (PEEK) has good mechanical property and has been used as orthopedic implants. Many researchers aim to improve the biofunction of this material by endowing its antibacterial property and osseointegration property. Gao C. et al. (2017) developed a biocompatible and antibacterial coating for PEEK using PDA-based surface modification technology and subsequent deposition of silver nanoparticles, which permits its potential use as an orthopedic material. There are also many studies to design hydrogels with tunable mechanical property to mimic natural tissues. Some groups tried to prepare hydrogel with high mechanical strength. In a study, the hydrophobic, π–π interactions and chemical bonds between the polymer binders and substrate surfaces could improve mechanical performance (Shin et al., 2012). What’s more, the structure of protein could affect the strength of protein hydrogel (Fang et al., 2013). Liu group developed a lot of high-strength hydrogels (Dai et al., 2015; Xu et al., 2017; Gao F. et al., 2018). Among them, a high-strength poly(N-acryloyl glycinamide) hydrogel with additional function of reverse transfection and cell detachment (Dai et al., 2015). Recently, they also reported a 3D-printing high-strength gradient hydrogel synthesized by N-acryloyl glycinamide and N-(tris(hydroxymethyl)methyl) acrylamide for efficient repair of osteochondral defects (Gao F. et al., 2018). Guo’s group developed various tough hydrogels using non-covalent interaction (Guo et al., 2014; Zhao T. et al., 2014; Cui et al., 2015). A multi-responsive supramolecular hydrogel, which could be induced by temperature, light, and redox, was prepared by the formation of host–guest complexes between the polyethylene ethanol-polyhedral oligomeric silsesquioxane-(CD)7 polymer and azo-SS-azo dimer (Wang X. et al., 2017). In another study, Chen et al. (2018a) also prepared supramolecular hydrogels based on host–guest interaction; this hydrogel could protect myoblasts from deleterious mechanical stimulus. Recently, Wang Z. et al. (2018) fabricated robust and biocompatible hydrogels via host–guest interaction. When the hydrogels were damaged, the host and guest could recognize each other immediately to recombine the damaged area.
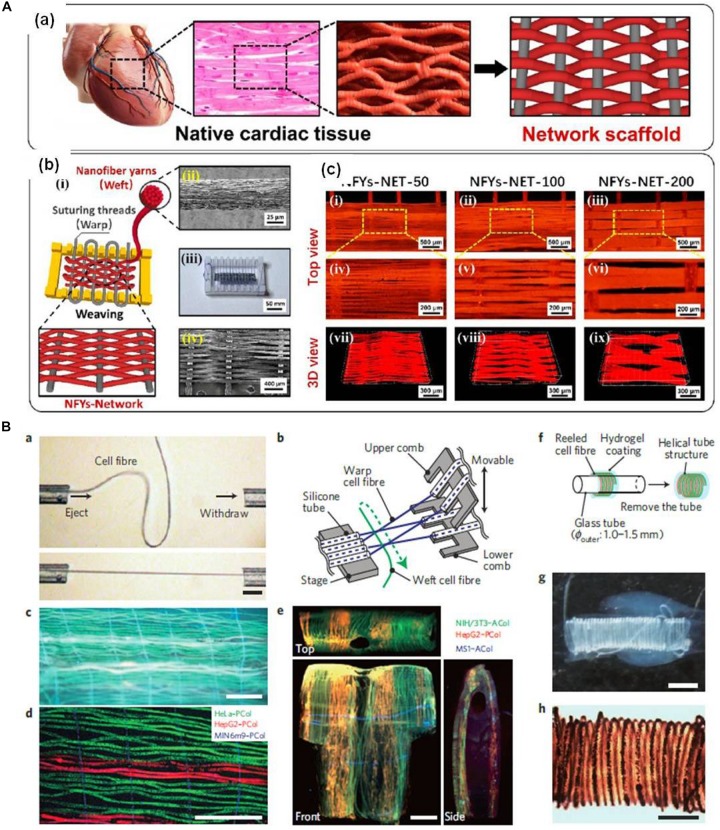
Up to now, many strategies were proposed for the fabrication of polymer fibers, such as electrospinning, microfluidic spinning, and 3D bioprinting. Mo’s group explores the application of electrospun fibers in TERM. They reported that modified PCL or other polymer electrospun fibers can be used in nerve (Yang G. H. et al., 2019), vascular (Liu P. X. et al., 2018), bone (Ye et al., 2019b), and other tissue regeneration in recent work. Yuan’s group also developed different electrospun fibers for drug delivery and vascular regeneration (Han et al., 2013; Zhang et al., 2013). Recently, they also encapsulated live microorganisms in electrospun fibers (Han J. P. et al., 2018) or prepared antibacterial PCL electrospun membranes containing synthetic polypeptides (Liu B. et al., 2018). Many other scholars have also done some interesting work. Yi et al. (2019) prepared aligned electrospun fibers of poly(L-lactide-co-caprolactone)/poly(L-lactic acid) (PLCL/PLLA) shell–core fibers via coaxial electrospinning and found that the stiffness of aligned fibers could regulate the phenotypic expression of vascular smooth muscle cells (VSMCs) (Yi et al., 2019). In another study, via a novel stable jet electrospinning, highly aligned hyaluronan/PLLA nanofibers with core–shell structure were also prepared for vessel regeneration (Yuan H. et al., 2016). Combining the aligned nanofibers and hydrogel, Guo’s group developed a 3D hybrid scaffold based on an aligned conductive nanofiber yarn network composed of silk fibroin, PCL, and carbon nanotubes within a hydrogel to mimic the structure of native cardiac tissue (Figure 1A; Wu et al., 2017). Microfluidic technology is also used to prepare fibrous scaffold for TERM. Matsunaga’s group prepared a gel fiber using a microfluidic device system to manufacture a phase separation polymer blend solution containing hydroxypropyl cellulose and sodium alginate (Tachizawa et al., 2017). This fiber could be used as a scaffold for in vitro neuronal induction. Ying’s group developed hydrodynamic spinning of gelatin-hydroxyphenylpropionic acid, alginate, poly(N-isopropylacrylamide), and polysulfone hydrogel fibers (Hu et al., 2010). Takeuchi’s group also described a long and finely handleable cell alginate fiber, by using a microfluidic device (Figure 1B; Onoe et al., 2013). This cell fiber could encapsulate extracellular matrix (ECM) proteins and somatic stem cells or differentiated cells and could mimic muscle fibers, nerve networks, or blood vessels in vivo. Gu and Zhao’s groups explored cell-laden alginate microfibers with the tunable structures through microfluidic technology (Cheng et al., 2016). Further, they demonstrated that these cell-laden fibers could be further constructed into microfiber-like tissues by stacking the microfibers. Fan’s group also co-developed a simple and reliable way to create cell-laden alginate fibers with hierarchical architecture through a microfluidic-based strategy (Wei et al., 2017). Jiang’s group constructed an artificial blood vessel with simulating autologous vascular structures from PCL and poly(lactide-co-glycolide) (PLGA), combining electrospinning and microfluidic technology (Cheng et al., 2017).
FIGURE 1.
(A) An interwoven aligned conductive nanofiber yarn/hydrogel hybrid scaffolds for mimicking the native cardiac tissue structure (reproduced with permission from Wu et al., 2017). (B) A thin, long, and finely handleable cell fiber fabricated by using a microfluidic device (reproduced with permission from Onoe et al., 2013).
In situ forming hydrogels from polymers have also been widely used for TERM because of the ease of encapsulating proteins, drugs, genes, and cells (Yang et al., 2014; Park and Park, 2018). Various cross-linking strategies, including physical interactions (ionotropic interaction, thermo-sensitivity, and host–guest interaction) and chemical cross-linking reactions (enzyme-mediated or light-controlled cross-linking and click chemistry), have been utilized to create in situ forming hydrogels (Park and Park, 2018). For example, Harada’s group developed redox-responsive self-healing supramolecular hydrogel formed from host–guest polymers. A supermolecular hydrogel could quickly be formed by mixing β-CD modified poly(acrylic acid) (pAA) with ferrocene modified pAA (Nakahata et al., 2011). Photo-cross-linking hydrogels are also widely investigated. Park’s group prepared a variety of in situ forming hydrogels (Le Thi et al., 2017; Lee et al., 2017). An in situ forming gelatin hydrogel by horseradish peroxidase–tyrosinase cross-linking resulted in strong tissue adhesion (Le Thi et al., 2017). In another work, they fabricated in situ forming H2O2-releasing gelatin-hydroxyphenyl propionic acid hydrogels, which could be used in treatment of drug-resistant bacterial infections (Lee et al., 2017). Recently, they reported an injectable gelatin-based hydrogels that could release nitric oxide and show good antibacterial property due to the in situ formation of peroxynitrite (Hoang Thi et al., 2018). Hwang’s group fabricated tissue adhesive hydrogels from tyramine conjugated HA and gelatin for meniscus repair (Kim S. H. et al., 2018). This tissue adhesive hydrogel was obtained by tyrosinase-mediated cross-linking.
Ceramics
Being one of the important components in bone and teeth, calcium phosphate-based materials have attracted substantial attention in TERM (Wu et al., 2011). Porous calcium phosphate-based scaffolds with various compositions and controlled pore size and porosity are designed to achieve the desired biological functions. Zhao N. et al. (2017) have studied hydroxyapatite (HAp)/β-tricalcium phosphate (β-TCP) scaffolds with different weight ratios and macropore percentages, showing that scaffolds with 40% HAp and 50% macropores are optimum for cell proliferation, while 60% HAp and 30% macropores are the best for osteogenic differentiation. Another study conducted by Chen Y. et al. (2015) have revealed that porous calcium phosphate ceramics could promote angiogenic induction ability, and a higher amount of β-TCP is favorable for neovascularization of the ceramics. However, the mechanical insufficiency of calcium phosphate biomaterials limits their further applications in tissue regeneration. High-temperature sintering could strengthen their mechanical performance, but the crystallinity increases during the sintering process, which significantly reduces the degradability of the scaffold. An alternative way is to use polymers to strengthen the calcium phosphate matrix. Kang et al. (2017) applied Ca2+ as “ion glue” to improve the bonding between calcium phosphate minerals and organic polymers. Ma Y. et al. (2016) used PEGylated poly(glycerol sebacate) to enhance the calcium phosphate matrix, resulting in scaffold with enhanced mechanical behavior and osteogenic differentiation of BMSCs. Calcium phosphate nanoparticles with various sizes and shapes are also synthesized and applied in the area of tissue engineering (Lin K. et al., 2014). Cai et al. have demonstrated that the dimension of HAp nanoparticles has great influence on the biological activities of bone-related cells. In comparison with particles in diameters of 40 and 80 nm, 20-nm-sized HAp has shown the best stimulating effect to bone marrow MSCs (Cai et al., 2007).
Calcium silicate materials, particularly tricalcium silicate (Ca3SiO5, C3S), have shown great potential in bone regeneration area (Zhao et al., 2005; Lin Q. et al., 2014). Deng et al. (2018b) prepared 3D-printed bioactive ceramic scaffolds composed of Sr5(PO4)2SiO4 and showed that Sr and Si ions can stimulate cartilage regeneration by activating the hypoxia-inducible factor pathway. These ceramics are efficient to reconstruct the cartilage–bone interface, making them promising materials for the regeneration of osteochondral defect (Deng et al., 2018b). Another study has shown that Li2Ca2Si2O7 bioactive scaffold can promote the maturation of chondrocytes and stimulate the osteogenic differentiation of rabbit MSCs by continuous release of Li and Si ions, suggesting the bi-lineage bioactivities of these scaffold for osteochondral defect regeneration (Deng et al., 2018a). The effect of nanotopography surface on bone regeneration was investigated by Yang C. et al. They demonstrated that the needle-like structure can better enhance the bone regeneration in vitro and in vivo in comparison with pure tricalcium silicate scaffolds (Yang C. et al., 2017). Chang and Wu’s groups prepared porous akermanite (AKT, Ca2MgSi2O7) scaffold with lotus root-like structures via a modified 3D-printing strategy, and these scaffolds promote bone regeneration (Feng et al., 2017).
Silicon dioxide (SiO2) was also widely applied in TERM. For example, Bian’s group coated a photocleavable linker and Arg–Gly–Asp (RGD) peptide-bearing molecular cap on the surface of mesoporous silica by host–guest complexation. Remote control of calcium release and cell fate could be realized using these particles (Kang et al., 2018a, b). Bioactive glass, with a typical composition of 46.1% SiO2, 26.9% CaO, 24.4% Na2O, and 2.6% P2O5, is the first biomaterial that is able to bond to bone (Hench, 2006). The ions such as Si, Ca, and P released from the bioactive glass have the effect of stimulating osteogenesis and bone metabolism (Maeno et al., 2005). By doping with elements such as copper (Cu) (Zhao et al., 2015a), strontium (Sr) (Pan et al., 2010), zinc (Zn) (Wang H. et al., 2015), and boron (B) (Li X. et al., 2016), the promotion and stimulation effects on osteoblast activity and angiogenesis are greatly enhanced. More recently, mesoporous bioactive glass with ordered porosity and pore structures has been demonstrated, promising biomaterials for bone regeneration (Zhou et al., 2017). Zhao et al. (2015b) fabricated Sr-containing mesoporous bioactive glass scaffolds with high porosity and interconnected pores through 3D-printing technique. The mesoporous scaffolds have exhibited good ability to stimulate the proliferation and differentiation of MC3T3-E1 cells, excellent osteoinductive activity to enhance bone formation, and ability to promote new blood vessel formation (Zhao et al., 2015b). Xie et al. dispersed the mesoporous bioactive glass nanorods in bioactive glass sol to form scaffolds with hierarchical pore structures. The scaffolds are able to promote the biomineralization process and to stimulate the proliferation of rat BMSCs (Xie et al., 2019).
Metal
Metal, with high compressive strength, can satisfy the demand of load bearing in orthopedics such as replacement of hip. However, metallic scaffolds lack bioactivity and have problems such as degradability and possible release of toxic metallic ions. Non-biodegradable titanium (Ti) alloys, specifically Ti-6Al-4V, are widely used in orthopedics; they have superior biocompatibility and mechanical property to stainless steel, Co-based alloys, and pure Ti (Alvarez and Nakajima, 2009; Li Y. et al., 2009). However, Ti-6Al-4V contains vanadium, which is cytotoxic. Some researchers tried to develop new Ti alloys containing nontoxic elements such as niobium (Nb) and tantalum (Ta) (Alvarez and Nakajima, 2009). Due to the fact that the porous metallic scaffolds are more suitable for tissue ingrowth, a porous Ti-6Ta-4Sn alloy scaffold was fabricated with a space holder sintering method by Li Y. et al. which showed excellent biomechanical properties as an orthopedic implant (Li Y. et al., 2009). Surface modification is used to further improve the bioactivity of Ti alloys. Jang’s group fabricated an antibacterial Ag nanostructure on a Ti surface by a two-step process involving target-ion-induced plasma sputtering and Ag sputtering (Kim S. et al., 2018). Yamamoto’s group prepared carbon layer coating Ti and carbon-coated oxygen-diffused Ti for TERM (Yamamoto et al., 2011a, b). Liu’s group used graphene oxide films loaded with minocycline hydrochloride to modify the surface of Ti, to enhance osteogenic activity in the presence of bacteria (Qiu et al., 2019). They also found that the topography and elastic modulus could affect cell adhesion and spread on Ti surface (Chen L. et al., 2019). Kim and Cha’s groups assembled silica nanoparticle nanostructures on the surface of Ti to enhance in vitro osteogenesis and in vivo bone formation (Jo et al., 2017). Bioactive molecules are also used to modify the Ti and Ti alloys. Cai’s group fabricated a functional molybdenum disulfide/PDA–RGD coating on Ti implant to inhibit bacterial infection and improve osseointegration (Yuan et al., 2019a). Recently, a synergistic photothermal/photodynamic therapy (PTT/PDT) strategy aiming for remotely controlling the eradication of biofilm was developed (Yuan et al., 2019b). Huh’s group immobilized recombinant human platelet-derived growth factor-BB plus recombinant human BMP-2 on heparinized-Ti implants, and the results showed more new bone formation around implants (Lee S. H. et al., 2018). Nishimura’s group revealed that neuronal PAS domain 2 modified on the rough surface of Ti implant could facilitate osseointegration through neuroskeletal regulatory pathways (Morinaga et al., 2019). Like Ti, Ta possesses a low modulus similar with natural subchondral and cancellous bone that could facilitate load transfer and reduce stress shielding. In addition, Ta could still show unique mechanical properties even at high porosity (>80%), which allows rapid bone ingrowth (Alvarez and Nakajima, 2009). In a comparison study by Wang H. et al. (2019), porous Ta showed an equivalent biological performance with porous Ti in repairing bone. Zhang and Lei’s groups reported that Ta-coated porous Ti-6A1-4V scaffolds loading rabbit BMSCs exhibited better fusion efficacy than Ti-coated Ti-6A1-4V scaffolds in the lumbar vertebral defects of rabbits (Wang F. Q. et al., 2018). In another study by Kim’s group, the introduction of Ta on the silicone surface could reduce fibrous capsule formation (Park C. et al., 2019).
Biodegradable metals such as magnesium (Mg) and its alloys have been attracting great attention in TERM due to their satisfactory mechanical property, biodegradation property, and bioactive effect. Okuzu et al.’s (2017) group investigated the effect of Mg ions released from Mg–Ti alloy on MC3T3-E1 cell proliferation and osteogenic differentiation, and the results showed that Mg–Ti could promote early bone healing. In another study, the Mg2+ and Si4+ ions released from Mg-smectite can also promote skin regeneration (Sasaki et al., 2017). However, Mg implant has too fast degradation rate to support completely healing the tissue before its degradation. In addition, the H2 produced during the corrosion is also a problem. Therefore, many studies began to find ways to delay its degradation, including coating ceramic coating and Ti or using Mg alloys as an alternative. Hiromoto’s group used octacalcium phosphate and HAp coatings to control the degradation speed and to improve the biocompatibility of biodegradable Mg alloys (Hiromoto and Yamazaki, 2017). Yeung’s group prepared a Ti dioxide (TiO2) nano-layer on the surface of ZK60 Mg substrate through Ti and O dual plasma ion immersion implantation technique (Lin Z. et al., 2019). They also constructed a TiO2/Mg2TiO4 nano-layer on the surface of WE43 Mg implant. With these methods, the modified Mg or Ma alloy implants showed enhanced corrosion resistance, osteoconductivity, and antimicrobial activity (Lin Z. et al., 2019). Park and Kim’s groups coated a Zn oxide/polylactic acid (PLA) nanocomposite layer on Mg alloys; this method could not only control the degradation rate but also control the surface topography of Mg alloys substrate (Mousa et al., 2018). Moreover, this coated layer also exerted antibacterial properties. In a study, Mg was also incorporated into PLGA/TCP porous scaffold by 3D printing to promote osteogenic and angiogenic properties of the scaffold (Lai et al., 2019). Iron (Fe) exhibits high mechanical strength and slow corrosion rate, which has the potential as higher load-bearing implants. Zhang and Zheng’s groups reported that the Fe-based scaffold showed good long-term biocompatibility in both rabbit and porcine model. Its corrosion products were biosafe and could be cleared away by the macrophages. Pandey’s group found that the corrosion rate of Fe scaffold could be affected by the property of interconnected micropores (Lin et al., 2017). Sun and Wang’s groups generated engineered human ventricular heart tissue based on Fe oxide scaffolds (Yang H. et al., 2019).
Composite Materials
The composite materials that can keep the advantages from polymer, inorganic material, and metal are developed for TERM. Collagen and HAp are the most common organic and inorganic compounds in bone. Ma X. et al. (2016) prepared biomimetic composite hydrogel for bone repair composed of collagen and HAp; alendronate (ALN), an anti-osteoporosis drug, was also incorporated into this composite hydrogel. Chen et al.’s (2018d) group prepared biphasic calcium phosphate/collagen porous composite scaffolds and incorporated dexamethasone in this composite scaffold for bone tissue engineering. Han F. et al. (2019) prepared silk fibroin/octacalcium phosphate composite scaffold for bone tissue engineering; the addition of silk fibroin could improve the weak mechanical property and poor processability of octacalcium phosphate. Tanaka’s group found that the proliferation and alkaline phosphatase (ALP) activity of MC3T3-E1 cells was enhanced when 10 wt% Zn calcium phosphate was added to calcium phosphate cement (Horiuchi et al., 2014). The combination of organic and inorganic materials can enhance the mechanical properties. Kinsuk’s group dispersed nHA particles into an electrospun solution containing polyurethane/polydimethylsiloxane to prepare a series of composite nanofibers with different mechanical properties (Drupitha et al., 2018). Ao et al. (2017) obtained electrospun cellulose/nano-HAp nanofibers, whose Young’s modulus and tensile strength were higher than commonly used polymer fibers such as PLA. In other work, calcium silicate and Mg silicate were also doped into electrospun fibers and enhanced the mechanical properties of these fibers (Wu Z. et al., 2016; Su et al., 2017). The combination of organic and inorganic materials can also improve the bioactivity. Ruszymah’s group incorporated Zn oxide nanoparticles into chitosan–collagen porous scaffolds, and the results showed that the incorporation of Zn oxide nanoparticles could both enhance the mechanical property and biocompatibility of porous scaffold (Ullah et al., 2017). Prabhakaran and Ramakrishna’s groups also found that the incorporation of inorganic nanoparticles into polymer fiber could improve the bioactivity such as protein absorption (Esfahani et al., 2015; Zhang S. et al., 2017). Nguyen Thi’s group coated electrospun PCL membrane with gelatin–silver nanoparticles; this composite membrane showed good antibacterial ability (Tra Thanh et al., 2018). Sun’s group also incorporated Ag nanowires into polyvinyl alcohol nanofibers for their antibacterial properties (Zhang Z. J. et al., 2017). Jiang’s group coated gold nanoparticles with 6-aminopenicillanic acid, and then these particles were added into electrospun fibers of PCL/gelatin fibers against bacteria (Yang X. L. et al., 2017). Tang’s group prepared nano-TiO2/collagen–chitosan porous scaffold, with the addition of TiO2, and the mechanical properties, resistance to degradation, and antibacterial ability were all improved (Fan et al., 2016). Jayakumar’s group fabricated chitosan hydrogel/nano-ZnO composites; these nanocomposite bandages enhanced wound healing by promoting faster re-epithelialization and collagen deposition (Sudheesh Kumar et al., 2012).
Cell Sources
Cell sources are one of three important factors in TERM. Until now, identifying and acquiring sufficient numbers of cells for application in therapeutics remain a challenge (Mao and Mooney, 2015). Various stem cells, progenitor cells, and adult tissue-derived cells are widely being investigated in TERM, among them adult tissue-derived cells are the dominant cell type used in clinic because of their ready availability and perceived safety (Fisher and Mauck, 2013).
Stem cells are still the frontline in TERM, because they have indefinite cell division potential and multiple differentiation potential (Mahla, 2016). MSCs isolated from various tissues including bone marrow, adipose tissue, blood, and amniotic fluid have potential uses in TERM (Fontaine et al., 2016; Schäfer et al., 2016; Bertheuil et al., 2019). The summary of stem cell used in TERM is shown in Table 1. Except for the above stem cells, several new stem cells also show potential application in TERM. Menstrual blood-derived stem cells (MenSCs) were discovered by Meng et al. (2007) and Cui et al. (2007) a decade ago. Since its discovery, more studies have been focused on MenSCs. The representative advantages of MenSCs compared with other six sources of MSCs are the higher proliferation rates, painless procedures, and almost no ethical issues (Khoury et al., 2014; Zhao et al., 2018). Moreover, Xiang’s group proved that MenSC-derived exosomes could inhibit hepatocyte apoptosis in D-galactosamine and lipopolysaccharide-induced FHF in mice (Chen L. et al., 2017). They also found that MenSC can treat Alzheimer’s disease and acute lung injury (Xiang et al., 2017; Zhao et al., 2018). Urinary stem cells discovered by Bharadwaj et al. (2013) also showed potential application in TERM. The urinary stem cells possess robust proliferative potential and multi-potent differentiation potential (Zhang D. et al., 2014). Recently, they reported the potential use of urinary stem cells in urinary tract reconstruction (Chen L. et al., 2017). Lee and Kwon groups reported that the neuronal differentiation of human urine-derived stem cells could be promoted by the presence of laminin and platelet-derived growth factor-BB (Kim J. Y. et al., 2018). Wang and Wu’s groups found that the exosomes secreted by urine-derived stem cells could repair pubococcygeus muscle injury and improve stress urinary incontinence in rats (Wu R. et al., 2019).
TABLE 1.
Examples of MSC used in tissue engineering and regenerative medicine in Asia.
| Cells | Disease | Mode of stem cell application | References |
| Bone marrow stem cell (BMSC) | Bone injury | 1. BMSC and endothelial progenitor cell mixture for large segment of bone defect 2. Calcitonin-related gene-modified rat BMSCs for skull defect 3. Repair of oligo(poly(ethylene glycol)fumarate) hydrogels seeded with BMSCs for repairing osteochondral defects |
Peng et al., 2019 Yu et al., 2019 Lim et al., 2013 |
| Cartilage injury | 1. Reconstruction of human-ear-shaped cartilage by co-culturing chondrocytes with BMSCs 2. In vitro engineered cartilage based on autologous BMSC |
Zhang L. et al., 2014 He et al., 2017 |
|
| Spinal cord injury | 1. BMSC-derived extracellular vesicles to repair spinal cord injury 2. Exosomes derived from BMSCs repair traumatic spinal cord injury |
Lu Y. et al., 2019 Liu W. et al., 2019 |
|
| Lung injury | 1. Mice BMSCs were injected into mice via the tail vein to treat acute lung 2. BMSC transplantation to treat smoke inhalation injury |
Feng et al., 2019 Liang et al., 2019 |
|
| Liver cirrhosis | Autologous BMSCs transplantation in human | Rajaram et al., 2017 | |
| Severe Asherman syndrome | GFP-labeled BMSCs was injected systemically through the tail vein in rat | Gao L. et al., 2018 | |
| Intrauterine adhesion | BMSC-loaded elastic poly(glycerol sebacate) scaffold | Xiao et al., 2019 | |
| Hearing loss | BMSC transplantation recovery functional restoration of mouse cochleae | Kada et al., 2019 | |
| Peripheral nerve injury | BMSC-derived acellular matrix-coated chitosan/silk scaffold | Xue et al., 2017 | |
| Type 2 diabetes | Autologous BMSCs transplantation in human | Wang et al., 2011; Bhansali et al., 2014 | |
| Adipose mesenchymal stromal cell (ADSC) | Osteoarthritis | ADSCs with hyaluronic acid were intra-articularly injected into the knee | Kuroda et al., 2015 |
| Cartilage injury | 1. ADSC/hyaluronic acid (HA)-modified thermoresponsive poly(N-isopropylacrylamide) hydrogel 2. Controlled chondrogenesis from ADSC by recombinant transforming growth factor-beta 3 fusion protein in peptide scaffolds |
Wang C. Z. et al., 2018 Zheng et al., 2015 |
|
| Bone injury | MiR-135-modified ADSC | Xie et al., 2016 | |
| Intervertebral disc injury | ADSC-derived tissue-engineered construct | Ishiguro et al., 2019 | |
| Stricture after extended esophageal endoscopic submucosal dissection | ADSC-sheet transplantation | Perrod et al., 2017 | |
| Urethral defect | Polylactid acid fibrous membrane seeded with ADSC | Wang D. J. et al., 2015 | |
| Atrophied muscle | Autologous ADSCs transplantation | Park and Kwon, 2017 | |
| Type 2 diabetes | Autologous ADSCs transplantation | Qi et al., 2019 |
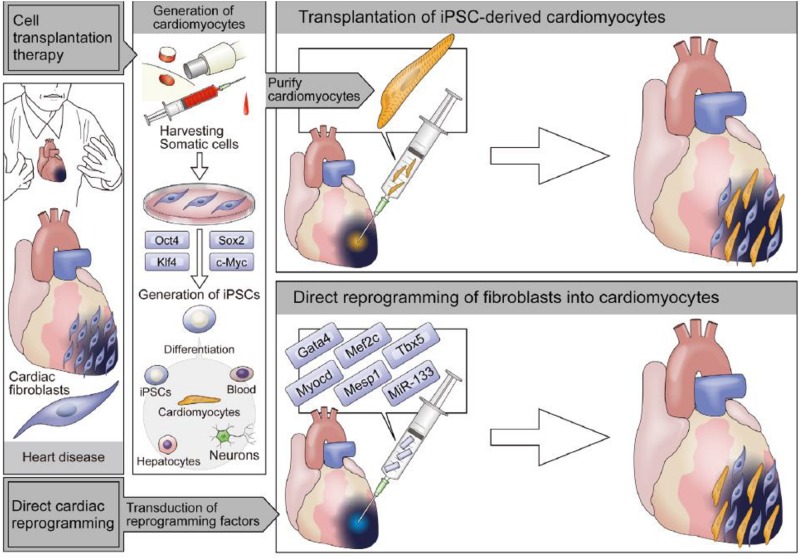
Takahashi and Yamanaka’s (2006) groups reported an induced pluripotent stem (iPS) cell, which could express the marker genes of embryonic stem cells and exhibit properties similar to embryonic stem cells (Okita et al., 2007; Hamanaka et al., 2011). With this method, Zeng and Zhou’s labs first generated several iPS cell lines that could meet the most stringent criteria for pluripotent stem cells and maintained a pluripotent potential close to embryonic stem cells (Zhao et al., 2009). In another study, when the somatic cells were exposed to transcription factors (Oct3/4, Sox2, Klf4, and c-Myc), iPS cells were formed with pluripotency (Takahashi and Yamanaka, 2006). However, reactivation of the c-Myc retrovirus with this method can increase tumorigenicity and hinder its clinical applications. In their further study, they developed a modified approach for the generation of iPS cells that avoid the use of Myc retrovirus (Nakagawa et al., 2007). Other strategies to induce reprogramming with quicker action or reducing risk of tumor formation are also developed. Subsequently, iPSCs have been successfully obtained from different species, including humans, rats, and rhesus monkeys (Okita et al., 2007; Liu et al., 2008; Kang et al., 2009; Hamanaka et al., 2011).
In addition, compared with in vitro reprogramming, direct in vivo reprogramming by retroviral injection has greater efficiency, due to avoiding the steps of in vitro culture and transplantation (Sadahiro et al., 2015). In vivo, the induced efficiency is different with in vitro, because transcription factors, epigenetic factors, microRNAs, secreted molecules, and cellular microenvironment are all important for cell fate specification. A work done by Deng and Ding’s labs reported that appropriate chemical cocktails were needed to add into the medium of pluripotent rat cells to maintain their characteristics (Li W. et al., 2009). This work also underscores the combined importance of the fibroblast growth factor (FGF), WNT, and transforming growth factor-β (TGF-β) pathways in regulating pluripotency in different species. To improve direct reprogramming, the strategies that combine reprogramming with morphogens could potentially be adapted for inducing cells into target cells. A variety of cells, including cardiomyocytes, vascular cells, hepatocytes, neural cells, and pancreatic cells, have been obtained from both direct reprogramming and iPS cell differentiation methods (Table 2).
TABLE 2.
Summary of direct reprogramming by lineage-specific transcription factors.
| Original cells | Reprogramming factors | Induced cells |
| Oct4/Sox2/Klf4/c-Myc (mouse and human) | Pluripotent stem cell | |
| 1. Brn2/Ascl1/Myt1l (BAM) (mouse) | Excitatory neuron | |
| 2. BAM/NeuroD1 (human) | ||
| BAM/Lhx3/Hb9/lsl1/Ngn2 (mouse) | Motor neuron | |
| Ascl1/Nurr1/Lmx1a (mouse and human) | Dopaminergic neuron | |
| Fibroblast | Sox2 (mouse and human) | Neural stem cell |
| 1. Hnf4a/Foxa1 (mouse) | Hepatocyte | |
| 2. Hnf1a/Hnf4a/Hnf6/Atf5/Prox1/Cebpa (human) | ||
| Erg/Gata2/Lmo2/Runx1c/Scl (mouse) | Hematopoietic progenitor cell | |
| Foxo1/Er71/Klf2/Tal/Lmo2 (mouse) | Endothelial cell | |
| Gata4/Mef2c/Tbx5 (mouse) | Cardiomyocyte | |
| MyoD | Skeletal myocyte | |
| Exocrine cell | 1. Neurogenin3/Pdx1/Mafa (mouse) | Pancreatic β-cell |
| 2. Epidermal growth factor/ciliary neurotrophic factor (mouse) | ||
| Myeloid cell | Run1t1/Jlf/Lmo2/Prdm5/Pbx1/Zfp37 | Hematopoietic stem cell |
BAM indicates Brn2, Ascl1, and Myt1l.
Enabling Technologies for TERM
The rapid development of TERM is inseparable from research and development of new technologies. As tissue engineering advances, new technologies, such as 3D printing and microfluidics, have attracted a lot of attention for preparing materials or scaffolds. To obtain suitable cells for TERM, cell sheet and genome engineering have also been widely explored. To construct mature tissues/organs for in vivo implantation, a variety of bioreactors have been developed. In addition, microfluidics and organ-on-a-chip were also effective technologies for TERM.
3D Printing
One of the developments for preparing tissue-engineered constructs is bioprinting. Bioprinting is an effective method to fabricate tissue-mimicking constructs through patterning cells, biomaterials, and biomolecules (Arslan-Yildiz et al., 2016; Tasnim et al., 2018; Matai et al., 2020). The 3D bioprinting of cells, growth factors, and hydrogels is mainly based on laser (Ali et al., 2014), droplet (Cui et al., 2014), and extrusion (Iwami et al., 2010) bioprinting. Bioprinting greatly promoted the development of skin tissue engineering. Fu’s group prepared 3D-ECM through 3D bioprinting (Huang et al., 2016). The created 3D-ECM could direct cell differentiation and promote functional skin regeneration (Huang et al., 2016). They also developed different bioinks to regulate the property of stem cell (Li Z. et al., 2018; Wang R. et al., 2019). Recently, Park’s group prepared a hybrid bioink composed of bioactive peptide-immobilized acrylated HA and tyramine-conjugated HAs (Lee J. et al., 2018). Results demonstrated that MSCs in these hybrid bioinks had high angiogenic and osteogenic activity. Bioprinting technology is also used to prepare a skin model in vitro. Kim et al. (2017) printed a 3D human skin model with a functional transwell system. Maturation of the skin model could be achieved with the support of printed PCL constructs (Kim et al., 2017). Kim’s group and Cho’s group also used skin-derived ECM as a bioink to fabricate a full thickness 3D skin, and the matured bioprinted skin tissue was significantly contracted during in vitro culture (Kim B. S. et al., 2018).
With this new method, a series of studies in which different materials (alginate, fibrin, gelatin, etc.) were performed to construct various tissues/organs (liver, adipose tissue, nerve, and so on) (Yang et al., 2013; Jung et al., 2016; Mishra, 2016; Igami et al., 2018; Liu F. et al., 2018). Other researchers also fabricated many kinds of cell/material complexes. Patterning of Sf-9 cell tissue was achieved using a mixture of cells and thermoreversible hydrogel (Iwami et al., 2010). Engineered human cartilage was prepared using an inkjet printer by printing poly(ethylene glycol) diacrylate (PEGDA) containing human chondrocytes (Cui et al., 2014). Gao G. et al. (2017) developed a 3D cartilage using bioprinting and simultaneous photopolymerization. This printed cartilage showed excellent production of glycosaminoglycan and collagen type II. In the ear reconstruction field, bioprinting is also widely used. For example, a tissue-engineered ear was prepared by printing PCL, cell-laden hydrogel, and sacrificial polyethylene glycol (PEG) layer (Lee et al., 2014a). The chondrocytes and adipocytes differentiated from ADSCs were encapsulated in hydrogel and distributed to the regions of cartilage or fat, respectively. The hydrogel could promote both chondrogenesis and adipogenesis of encapsulated ADSCs.
Functional vascular with perfusion performance could also be prepared using bioprinting cells and biological matrices (Lee et al., 2014b). This perfusable vascular can support the viability of tissue up to 5 mm in distance. Du et al. (2015) prepared BMSC-laden microfibers immobilized with BMP-2 by bioprinting. These prepared CBD-BMP-2-collagen microfibers induced osteogenic differentiation of BMSCs within 14 days more efficiently than the osteogenic medium. Jia et al. (2016) directly printed perfusable vascular constructs using a mixed bioink, composed of GelMA, sodium alginate, and 4-arm poly(ethylene glycol)-tetra-acrylate. These constructs could support the growth of encapsulated endothelial and stem cells. Finally, highly organized and perfusable vessels could be obtained. Jang et al. (2017) prepared a stem cell patch with pre-vascularized and functional multi-material structures by 3D printing using bioink consisting of cell-laden decellularized ECM (dECM), which promoted rapid vascularization after transplantation and enhanced the therapeutic efficacy for cardiac repair. This method was applied in many tissues including adipose, cartilage, and heart. The use of tissue-specific dECM in this process promised high cell viability and functionality (Pati et al., 2014).
Chang et al. (2010) fabricated a 3D liver micro-organ by the combining direct cell writing bioprinting process with micro-patterning techniques; this liver model could act as an in vitro model for drug metabolism. Scientists from Japan fabricated a liver model using the system “PLUTO.” The 3D-printed liver assisted the minor hepatectomy following liver partition (Igami et al., 2018). In hepatectomy for a small tumor that is invisible to intraoperative ultrasonography, the application of 3D-printed liver makes the procedure easy and feasible (Igami et al., 2014). Besides, 3D printing can also be used in fabricating other tissues. Scientists from South Korea and India printed a kidney, heart, and tooth, which presented the applications of 3D printing in regenerating heterogeneous organs and tissues to treat specific defects or injuries (Jung et al., 2016; Mishra, 2016).
Cell Sheet Technology
Cell sheet technology is a scaffold-free approach that has great potential in TERM (Kobayashi et al., 2019; Li M. et al., 2019). Compared with traditional cell therapies (cell suspension injection or cells/scaffold construct), cell sheet technology enables transplanted cells to stay completely in the target sites and fully maintain their viability. Since Okano’s group first devised a scaffold-free method of constructing cell sheets using thermoresponsive poly(N-isopropylacrylamide)-grafted surfaces, this technology has been applied to the regeneration of a variety of tissues (Takezawa et al., 1990; Sekine et al., 2013). In addition, it has also been used in cell micropatterning, cell co-culture, and drug discovery (Hannachi et al., 2009; Takahashi and Okano, 2019). Compared to other tissue engineering techniques, one of the major advantages of cell sheet is the absence of potential material degradation-associated cytotoxicity. Different methods have been developed to prepare cell sheets, including thermo-responsive systems, electro-responsive systems, photo-responsive systems, pH responsive systems, magnetic responsive systems, and so on (Inaba et al., 2009; Chen et al., 2012; Hong et al., 2013; Pan et al., 2013; Zhang W. et al., 2017; Koo et al., 2018; Li M. et al., 2019). Li’s group reported several new methods for cell sheet preparation (Pan et al., 2013; Guo B. et al., 2015). Compared with physical absorption and covalent immobilization approaches, the method through thermo-responsive specific binding RGD in the imprinted hydrogel not only promoted cell adhesion during cell culture but also facilitated cell detachment during cell sheet harvest (Pan et al., 2013). In the electricity-induced method and magnetic method, electricity-responsive thiol layers and pH-responsive layers have been designed to harvest cell sheets, respectively (Hong et al., 2013; Koo et al., 2018).
Cell sheet technology has been applied to treat damaged tissues including bone, articular cartilage, corneas, periodontal ligaments, blood vessels, and the myocardium (Takezawa et al., 1990; Sekine et al., 2013). Yang et al. (2007a) focused on using temperature-responsive surfaces to harvest cell sheets, and their applications in corneal dysfunction, tracheal resection, esophageal cancer, and cardiac failure. They have also created thick vascularized organ-like systems for the heart and liver based on cell sheet. To construct 3D tissues, some studies tried to prepare multilayered cell sheets. Most multilayered cell sheets were fabricated by layer-by-layer stacking individual cell sheet using a gelatin gel plunger or forceps (Haraguchi et al., 2012; Ren et al., 2014). Furthermore, 3D tissues can also be constructed via this protocol. At present, the cell sheet technique has already been applied to human clinical trials for regenerating heart, cornea, blood vessels, periodontal membrane, esophagus, cartilage, functional tendons, and middle ear (Egami et al., 2014; Yamamoto et al., 2017). For example, Yamamoto et al. (2017) developed a novel treatment method to regenerate the middle ear mucosa by tympanoplasty and autologous nasal mucosal epithelial cell sheet transplantation. All patients showed good regeneration result with no adverse complications and adverse effect on the patients’ hearing ability. Cell sheets composed of corneal epithelial cells or autologous oral mucosal cells could also repair severe corneal injury in patients (Nishida et al., 2004). In another study, transplantation of multilayered cell sheets composed of patients’ periodontal ligament derived MSCs was proved to promote regeneration of the periodontal and bone (Iwata et al., 2015).
Genome Editing
Recent advances in genomic technology and genome engineering make it possible to modify the genome in cells. A great progress of genome editing technologies, the CRISPR/Cas system (Komor et al., 2017; Kim, 2018), can directly modify the genome. Therefore, this technology can be used to systematically dissect the functional effect of genetic variants. Recently, there is much work done by Asian researchers (Duan et al., 2014; Liang et al., 2015; Kang et al., 2016; Xue et al., 2016). Gao et al. (2016) determined the mechanism of CRISPR-Cpf1 system, by analyzing the structure of Acidaminococcus sp. Cpf1 in complex with crRNA and target DNA. Recently, Zhou et al. (2018) improved the Cas9 system to be much precise and could simultaneous activate multiple genes and long noncoding RNAs in the nervous system.
The safety of technology is the primary issue to be considered (Pineda et al., 2019). One of the safety concerns for CRISPR technology is unforeseen genetic, which may bring unpredictable physiological changes or even death to patients. The off-target genomic and cellular events are also scientists’ concerns. In addition, mutations in vital genes could cause cancer or the damage of organs. Hence, the safety evaluation for CRISPR technology needs to advance in parallel with CRISPR technologies. To improve the safety and efficiency of CRISPR/Cas9 gene-editing elements, Leong’s groupreported an efficient CRISPR/Cas9 delivery system comprising PEGylated nanoparticles based on poly (γ-4-((2-(piperidin-1-yl)ethyl)aminomethyl)benzyl-L-glutamate) for delivering Cas9 expression plasmid and sgRNA (Wang H. X. et al., 2018). This CRISPR/Cas9 delivery system could reach 47.3% gene editing efficiency in single or multiplex gene editing in vitro, achieve 35% gene deletion in HeLa tumor tissue, suppress the tumor growth by >71%, and prolong the animal survival rate to 60% within 60 days. Peng’s group analyzed the function and structure of two archaeal anti-CRISPR proteins (Acrs) from the lytic rudiviruses, SIRV2 and SIRV3 (He et al., 2018). Tan’s group developed a Cas9 variant whose activity can be switched on or off in cells by 4-hydroxytamoxifen; this Cas9 variant showed high gene editing efficiency and could reduce 437 off-target cleavage (Liu et al., 2016). Dong et al. (2017) revealed the mechanism of SpyCas9 inhibition by AcrIIA4, which make it possible to develop “off-switch” SpyCas9 systems to avoid unwanted genome edits within cells and tissues.
Microfluidics
In the past two decades, various cell microencapsulation technologies have been developed (Alkayyali et al., 2019). Cells encapsulated in microbeads or microfibers could be prepared by changing microfluidic devices or materials. The flexibility of microfluidic technology allows the preparation of multi-structured fibers such as hollow, multilayer, grooves, etc. Yu et al. (2017) prepared alginate helical microfiber with complex structures such as Janus, triplex, core–shell, and even double-helix based on rapid ion cross-linking through microfluidic devices. In recent years, the application of microfluidics in cell-laden droplet production and single-cell encapsulation has been reviewed (Zhu and Yang, 2017; Alam et al., 2018; Alkayyali et al., 2019; Shen et al., 2019). Zhao’s group prepared uniform microfibers by simple injection capillary microfluidics, core–shell or spindle-knot structured microfibers by hierarchical injection capillary microfluidics, and microfibers with multiple components by multi-barrel injection capillary microfluidics (Wang J. et al., 2017; Yu et al., 2017; Yu Y. et al., 2018). A cell microcarrier with controllable macropores and heterogeneous microstructures could also be fabricated by a capillary array microfluidic technology (Wang J. et al., 2017). These microcarriers with spatially heterogeneous cell encapsulations may be used for mimicking physiological structures of nature tissues or organs. Lee’s group encapsulated Escherichia coli (E. coli) in PEGDA microdroplets using a microfluidic device (Lee K. G. et al., 2010). This encapsulated E. coli may be used in biotransformation, bioremediation, biosensing, and artificial cells. Jia et al. (2019b) prepared biomimetic cell-laden helical hydrogel microfibers as blood-vessel-on-chip using alginate based on microfluidics methodology. This hollow microfiber had decent perfusability and could generate swirling blood flow to provide a platform for mimicking nature swirling blood flow. Except cell encapsulation, the applications of microfluidics in single molecule/cell culture and analysis (Zhu and Yang, 2017), cell sorting (Shen et al., 2019), continuous cell separation (Yan et al., 2017), and orthopedics (Wang L. et al., 2019) were also reported in some reviews.
Organ-on-a-Chip
Bridging the gap between findings in 2D cell culture in vitro and 3D tissue culture condition in vivo has been a challenge. 3D printing and microfluidic devices also offer great development of an in vitro model in TERM, organ-on-a-chip (Kim et al., 2013; Bang et al., 2017; Lee and Jun, 2019; Mobini et al., 2019; Ranjith Kumar et al., 2019; Sun W. et al., 2019). Jeon’s group reported several work in this field (Kim et al., 2013; Bang et al., 2017). They constructed a low-permeability blood–brain barrier platform by microfluidics (Bang et al., 2017). Using this platform, co-culturing human umbilical vein endothelial cells and neural cells with independent culture medium could be easily realized. In another work, they modeled natural cellular programs through a microfluidic-based platform, and using this platform, they could spatially control co-culture of endothelial cells with stromal fibroblasts, pericytes, or cancer cells (Kim et al., 2013). The lab-on-a-chip devices were also used to construct ex vivo models for neural tissue engineering (Mobini et al., 2019). The group of He fabricated 3D hydrogel-based vascular structures with macrochannels (Gao Q. et al., 2017). These 3D hydrogel-based vascular structures could be further integrated into organ-on-chip devices to better simulate the microenvironment of blood vessels. In addition, the organ-on-a-chip also has great potential in preclinical testing of drugs (Ranjith Kumar et al., 2019).
Mechano-Regulation in TERM
The organism in a complex mechanical system consists of external and internal forces. Cells, tissues, and even organs are stimulated by mechanical stimuli of different intensity, frequency, and direction. Mechanical stimuli regulate cell function by affecting gene expression and protein synthesis in cells and then regulate cell differentiation, growth, and development of organisms. Therefore, mechanical stimuli also play an important role in TERM. This unique form of mechanical force is regulated in real time by a variety of factors, such as exogenous force, motor proteins, osmotic pressure, mechanosensitive ion channels, intracellular mechanosensors, and actin assembly. Traditional approaches in cellular mechanobiology include loading tissues or organ culture in vivo, or loading cells in monolayer and scaffolds in vitro at various modes, magnitudes, and frequencies of mechanical stimuli. In the past decades, the field of mechanoregulation of tissue engineering and regeneration medicine has witnessed tremendous progress in Asia.
Bone
Mechanical stimulus is one of the most important physical stimuli for bone, which is known to regulate the osteogenic differentiation of MSCs and other osteogenic precursor cells. However, the various modes of stimuli showed different effects on osteogenesis. For example, cyclic mechanical tension inhibited Runx2 expression in MSCs through the RhoA-ERK1/2 pathway (Shi et al., 2011). In a study, 1 Hz cyclic mechanical stretch for 30 min (twice a day) could enhance osteogenic differentiation of BMSCs and restrain osteoclastogenesis of RAW264.7 cells in vitro (Guo Y. et al., 2015). In another study, 2 h of cyclic stretch of 2.5% elongation at 1 Hz on 3 days significantly decreased osteogenesis of adult MSCs by the increased reactive oxygen species (Tan et al., 2015). The mechanical stretch could also affect antioxidant responses and osteogenic differentiation in human MSCs through activation of the adenosine 5′-monophosphate-activated protein kinase–Silent mating type information regulation 2 homolog-1 (AMPK-SIRT1) signaling pathway (Chen et al., 2018b). Additionally, exercise can strengthen bones (Niinimaki, 2012). Exercise was proved to inhibit bone loss in ovariectomized rats (Bu et al., 2012). The expression of Runx2 was reported to gradually increase under strain stimulation (Li C. J. et al., 2015; Li R. et al., 2015). The same result was also found in another study in rats (Notomi et al., 2014). In conclusion, suitable exercise may promote osteogenic differentiation of MSCs by upregulating the expression of key molecules in Wnt signaling pathway.
Tendon and Ligaments
Mechanical stimuli play an important role in regenerating or repairing tendons and ligaments. Mechanical stimuli can enhance cell proliferation and tenogenic differentiation of tendon-derived stem cells (TDSCs). In a study, the TDSCs-poly(LLA-CL)/Col scaffolds were stretched at 4% elongation, 0.5 Hz, and 2 h per day for 14 days, the mechanical stimuli could promote formation of tendon tissues after the implantation of TDSCs-poly(LLA-CL)/Col scaffolds in nude mice (Xu et al., 2014). This result showed that dynamic mechanical stimulus was helpful for the maturation of tissue-engineered tendon in vivo. In another study, the mechanical stretch (10% elongation for 48 h, 10 cycles/min, each cycle containing 2 s of stretch, and 2 s of relaxation) accelerated the healing of tendon–bone by promoting proliferation and matrix production of MSCs and tendon cells (Song et al., 2017). However, there was also a report that the cyclic stretch could induce apoptosis of human periodontal ligament cells via a caspase-dependent pathway (Chen J. et al., 2016; Wu Y. et al., 2016; Zhao D. et al., 2017). In another study, the cyclic tensile promoted BMP-9 synthesis and in vitro mineralization in human periodontal ligament cells (Tantilertanant et al., 2019). The study of mechanical stimuli on tendon/ligament repair has been extensively studied with variable degrees of efficacy. In the future, synergistic therapies for tendon/ligament repair and regeneration may be created by combining mechanical loading with biochemical cues.
Cartilage
Due to its avascular nature and limited proliferative potential of mature chondrocytes, cartilage only has limited intrinsic regeneration ability. Mechanical stimuli have been shown to affect the gradient of nutrient and ion, pH, cell deformation, bioactivity, and synthesis of ECM components or growth factor (Bleuel et al., 2015). Different levels of shear stress could affect the expression of inflammatory stimuli-induced genes in chondrocytes through macrophage-induced c-Jun N-terminal kinase and Akt phosphorylation, nuclear factor κ-B activation, and urokinase plasminogen activator expression (Yeh et al., 2013). The compressive stress may promote chondrogenic differentiation by increasing the activity of TGF-β1 and regulating the downstream targets of TGF-β signaling. It was reported that Smad pathways would be involved in the conversion of mechanical stimuli into biological responses (Li J. et al., 2012). While the physiological level of mechanical stimuli helps the maintenance of healthy tissues, excess mechanical stimuli result in damage of tissues. Koike et al. (2015) demonstrated that excess mechanical stimuli promoted mitochondrial superoxide generation and caused imbalance of superoxide dismutase 2 in chondrocytes, which would result in cartilage degeneration. Recently, Iran researchers constructed a new microdevice used to study chondrogenesis under unidirectional compressive stimulus of cells in a 3D cell culture condition (Kowsari-Esfahan et al., 2018). The results showed that the dynamic mechanical compression enhanced cell viability and upregulated the expression of Sox-9, collagen II, and aggrecan in the absence of exogenous growth factors. Moreover, 10% strain was considered as optimal mechanical stimulus for chondrogenic differentiation of ADSCs.
Cardiac Tissue
Regenerating the beating heart is still a formidable bioengineering challenge. The pumping action of the heart requires mechanical forces to compress a blood-filled chamber with a defined in- and outlet. It is widely accepted that mechanical loads are important in the development and morphogenesis of cardiac tissue. In a study, it was demonstrated that fluid shear stress would affect cardiomyogenic differentiation of rat BMSCs (Huang Y. et al., 2010). Zhang W. et al. (2017) prepared cardiac muscle strips by encapsulating cardiomyocytes derived from human embryonic stem cells in collagen-based biomaterials. The results demonstrated that the mechanical stretch could promote the maturation of human embryonic stem cell-derived cardiomyocytes. Cyclic biaxial tensile strain promoted the differentiation of BMSCs into cardiomyocyte-like cells by miRNA-27a (Cao et al., 2018).
Intervertebral Disc
Intervertebral disc (IVD) not only absorbs the mechanical load but also maintains multi-axial flexibility of the spine. The mechanical disorder of IVD will ultimately cause tissue dehydration, fibrosus, nerve, and vessel ingrowth, and disc degeneration (Chu et al., 2018; Li and Chen, 2019). For instance, the increasing frequency and amplitude of dynamic compress would contribute to higher cell density, because dynamic compression facilitated the diffusion of nutrients around IVD cells (Zhu et al., 2012). But another study showed that static compression significantly decreased the density of nucleus pulposus (NP) cell, but increased TUNEL-positive cells and apoptosis index (Kuo et al., 2014). Moreover, this effect is in a dose-dependent manner. It was thought that the intrinsic (mitochondrial) apoptotic pathway played an important role in compression-induced NP cells apoptosis. Further research showed that the dynamic compressive load reduced the density and viability of NP cells. The localization and mRNA expression of integrin α5β1 were increased in both NP and annulus fibrosus (AF) under dynamic mechanical stimuli. Dynamic compression load (1.3 MPa, 1.0 Hz) also showed a catabolic effect, and the expression of matrix metalloproteinase-3 and metalloproteinase-13 in NP and AF cells would be regulated by dynamic mechanical stimuli (Kurakawa et al., 2015).
Blood Vessels
The mechanical stimuli also play an important role in blood vessels. The suitable cyclic stretch also induced vascular remodeling by promoting VSMC proliferation. In a study, the physiological cyclic stretch promoted the contractile differentiation of VSMCs via the SIRT1/FOXO pathways and thus maintained vascular homeostasis (Huang et al., 2015). Mechanical stretch-induced endoplasmic reticulum stress could also promote the apoptosis, inflammation, and degeneration of VSMCs (Jia et al., 2015).
Applications of Term in Different Tissues
Cartilage
The repair and regeneration of cartilage, such as ear and joint, are still associated with various limitations and degrees of success (Bauer, 2009; Lee T. S. et al., 2010; Tang et al., 2017). TERM provides us a new way to construct engineered cartilage substitutes, which could match both the function and appearance of native ears (Peer, 1954; Watson, 2009). By combining suitable cell source with scaffolds, engineered cartilage constructs could replace injured tissues. Since 1997, Cao and colleagues have used engineered cartilage to treat cartilage diseases. They found that both component and structure of the scaffolds would affect cartilage regeneration. Cao and Zhou’s groups successfully regenerated ear-shaped cartilage combining electrospun gelatin/PCL membranes and cells (Zheng R. et al., 2014). Further, they successfully regenerated subcutaneous cartilage using BMSCs directed by chondrocyte sheet (Li et al., 2017). Liu and Zhou’s groups created a human-ear-shaped cartilage tissue by seeding the microtia chondrocytes and BMSCs into the ear-shaped biodegradable scaffold and subcutaneously implanting them into nude mouse, which provides a promising strategy to construct stable ectopic cartilage (Zhang L. et al., 2014). He and Fu’s groups fabricated the ear tissue using a method named scanning printing polishing casting. They designed a mold according to the 3D scanner of ear and generated ear tissue by casting medical grade silicone to the mold. This strategy provided a method that has a lower cost than the current soft prostheses fabrication methods (He et al., 2014). Cho’s group generated an artificial ear containing auricular cartilage and fat tissue using 3D printing technology. In this process, ADSCs were differentiated to chondrocytes and adipocytes, and then encapsulated in hydrogel, respectively. The ear tissue was printed with PCL and cell-laden hydrogel to ear-shaped structures (Lee et al., 2014a). Hwang et al. (2014) spray-coated a mixture of chondrocytes and fibrin hydrogel on a human ear-shaped implant and implanted it into the dorsal subcutaneous space of athymic mice. The formation of neocartilage covered the implants after 12 weeks.
Successful regeneration of critical-sized cartilage defects in weight-bearing region remains a major challenge in clinic. In the past decades, biomaterials aiming to repair cartilage have been rapidly developed (Zhang Y. B. et al., 2019). Zhang’s group developed injectable carboxymethylated pullulan/chondroitin sulfate hydrogel and collagen I/II composite hydrogel for cartilage tissue engineering (Chen F. et al., 2016; Yuan L. et al., 2016). Yu’s group created chitosan microspheres to carry cells, and this system could be used for cartilage tissue engineering (Zhou et al., 2016). The chitosan microspheres showed an ECM-mimicking nanofibrous structure and tunable size could be constructed into 3D-shaped cartilage-like composite in vitro. Collagen is a widely used material in cartilage tissue engineering. To improve the unmatched stiffness and rapid degradation rate of collagen, Lu Z. et al. (2019) conjugated biocompatible carbon dot nanoparticles onto collagen to prepare an injectable hydrogel. This injectable composite hydrogel combined with PDT enhanced chondrogenesis. To encapsulate chondrocytes in scaffolds to promote cartilage regeneration, Wang C. Y. et al. (2019) prepared an injectable cholesterol-enhanced stereocomplex polylactide thermogel; the modification of cholesterol on the 4-arm PEG-PLA could not only promote the preservation of morphology and biomechanical property but also promote cartilaginous gene expressions. Ding’s group found that nanoscale spatial arrangement of RGD peptides could affect the dedifferentiation of chondrocytes (Li S. Y. et al., 2015). To realize the controlled chondrogenic differentiation, Wu’s group successfully regulated the anti-apoptotic gene and chondrogenic regulator using a double duration inducible gene expression system (Ma Y. et al., 2016). Ouyang’s group successfully used chondrocyte-derived progenitor cells to repair large knee cartilage defects of patients (Jiang et al., 2016). In another work by Ouyang’s group and Wu’s group, they used a silicate-based bioceramic scaffold with the ability to regulate cartilage and bone dual-lineage regeneration and repaired the osteochondral defect (Bunpetch et al., 2019). Recently, Fan and Wang’s groups constructed an acellular matrix microsphere as a scaffold for engineering cartilage (Liu J. et al., 2019). Park’s group and Min’s group used decellularized porcine cartilage-derived ECM to fabricate scaffolds (Oh et al., 2018). This ECM-derived scaffold could provide suitable environments for chondrocyte growth. In another work, they repaired human partial thickness cartilage defects using a cartilage ECM membrane that can deliver chondrocytes (Park et al., 2016). Ha’s group used human umbilical cord blood-derived MSCs to repair cartilage of rats and obtain favorable results (Park Y. B. et al., 2019). In a clinical trial, they successfully regenerated articular cartilage in the defects of osteoarthritis patients, using a composite of allogeneic umbilical cord blood-derived MSCs and hyaluronate hydrogel (Park Y. B. et al., 2017).
Skin
The development of artificial skin has attracted a lot of Asian researchers. Various skin wounds will be caused by burn, freezing, surgical procedures, radiation, and chronic skin ulcers. The skin substitutes prepared by tissue engineering could be used to promote acute and chronic skin wound healing (Tang et al., 2017). Several Asian research groups have made great efforts in skin tissue engineering. Jin’s group developed several multilayered skin substitutes (Zhu et al., 2008; Wang et al., 2009; Huang S. et al., 2010). For instance, they prepared a tricopolymer scaffold consisting of collagen, chondroitin sulfate, and HA to mimic ECM of the skin (Wang et al., 2009). Further, they also attempted different cell sources for skin repair, including BMSCs, melanocytes, keratinocytes, dermal fibroblasts, human amniotic epithelial cells, human amniotic mesenchymal cells, and adipose tissue-derived stem cells (Liu et al., 2007, 2014; Li H. et al., 2012; Lu et al., 2012; An et al., 2015). Based on these works, Jin’s group first developed tissue-engineered artificial skin in China. Fu’s group has constructed different skin substitutes by tissue engineering. A collagen-based and cell-seed skin construct was prepared by culturing sweat gland cells on gelatin microspheres and then combining them with collagen (Huang S. et al., 2010). This artificial skin construct promoted skin repair and wound healing. Further, they designed a skin scaffold with modifying spatial inductive cues by bioprinting, which could promote differentiation of epidermal lineages into sweat glands (Huang et al., 2016). In addition, they also tried various cells for skin tissue engineering (Li H.-H. et al., 2008, 2009). Ruszymah’s group did some studies on skin tissue engineering (Chowdhury et al., 2015; Law et al., 2016, 2017; Fauzi et al., 2019). They found that skin cells, keratinocytes, and fibroblasts showed different cellular activities within collagen and fibrin constructs (Law et al., 2016). Co-culturing keratinocytes with fibroblasts in the collagen scaffold could reduce the proliferation of fibroblast and collagen contraction. In another study, they demonstrated that dermal fibroblast conditioned medium promoted skin wound healing (Chowdhury et al., 2015). In addition, they found that plasma-derived fibrin could promote wound healing (Law et al., 2017). Recently, they reported rapid repair of full-thickness skin injury using ovine tendon collagen I scaffold combining with skin cells (Fauzi et al., 2019).
Until now, different types of scaffolds, including the nanofibers (Xu et al., 2019; Yao et al., 2019; Zhang K. X. et al., 2019), hydrogels (Hsu et al., 2019; Muthuramalingam et al., 2019; Patel et al., 2019), porous scaffolds (Wang et al., 2009), particles (Shi M. et al., 2019), decellularized bioscaffolds (Cui et al., 2019), and bioadhesives (Deng et al., 2019; Park E. et al., 2019) have all been applied in skin repair. The antibacterial conductive hydrogels fabricated by supramolecular assembly of PDA-modified silver nanoparticles, polyaniline, and polyvinyl alcohol could be used for epidermal sensors and diabetic foot wound dressings (Zhao Y. et al., 2019). A β-glucan-based hydrogel significantly not only accelerated wound healing but also promoted the regeneration of skin appendages (Muthuramalingam et al., 2019). To eliminate the undesired immune responses in practical use of chitosan–catechol in the clinic, a catechol-conjugated glycol chitosan adhesive hydrogel was proposed as an alternative and exhibited negligible immune responses (Park E. et al., 2019). Another bioinspired adhesive derived from skin secretion of Andrias davidianus showed stronger tissue adhesion strength than fibrin glue, which may be used for wound healing. Moreover, the elasticity and biocompatibility of this adhesive were better than those of cyanoacrylate glue (Deng et al., 2019). The addition of bioactive substances, such as the grape-seed extracts containing rich flavonoids with oligomeric proanthocyanidins, could promote wound healing (Ma et al., 2019). The multifunctional Zn-doped hollow mesoporous silica/PCL electrospun membranes with good antibacterial activity were shown to enhance hair follicle regeneration and wound healing (Zhang Y. et al., 2019). The electrospun poly(gamma-glutamic acid) fibrous scaffolds decorated with ginsenoside Rg3 could accelerate fibroblasts to sprout and grow, promote scar-free wound healing in vivo, and prevent hypertrophic scars (Xu et al., 2019). In a recent study, researchers combined 3D printing of poly (L-lactide-co-caprolactone) scaffold with bioactive peptide hydrogel and showed a good treatment modality in treating skin defects (Im et al., 2018). In a recent study, Koo et al. (2019) transplanted a three-layered cell sheet stacked by ROS-induced method which took only a short time. The results showed that the stacked cell sheet promoted angiogenesis and skin regeneration.
Bone
The aim of bone tissue engineering is to develop ideal bone substitutes (Shrivats et al., 2014). Generally, these substitutes contain cells and should support cellular behaviors and guide bone regeneration. Yanaga et al. (2006) made an attempt to use a composite of autologous human auricular chondrocytes with autologous serum for craniofacial augmentation in a pilot clinical study. Otsu’s group revealed that neural crest-like cells from iPS cells could differentiate into osteoblasts and contribute to craniofacial bone regeneration without tumor formation in vivo (Kikuchi et al., 2018). This method could resolve the limitation of insufficient source of reconstructive material. Cui’s group developed various scaffolds to mimic the natural bone ECM niche. They developed a nano-HAp/collagen/PLA composite (Liao et al., 2004) or constructed a porous bone scaffold (Li X. et al., 2006). They also added the mineralized collagen into poly(methyl methacrylate) (PMMA) bone cement (Li T. et al., 2015). Now, they have developed a variety of artificial bone repair materials, which acquired the product registration certificate from the Chinese government. Cho’s group and Lee’s group prepared a synthetic scaffold with patient-customized structure and mechanical properties for bone repair (Lee et al., 2019). Aiming to repair bone defects with irregular shapes, Wang L. H. et al. (2019) recently developed 3D super-elastic scaffolds based on electrospun SiO2 nanofibers with self-fitting and tailorable gradient capability. Lin’s group used a HAp–calcium sulfate–HA composite carrying collagenase to repair alveolar bone (Subramaniam et al., 2016). Dewi et al. (2015, 2017) added calcium carbonate into plaster of Paris to improve the mechanical and degradable property of the bone substitutes. In addition, Wu’s group also prepared lithium-containing bioactive glass ceramic, which promoted angiogenesis for bone tissue engineering (Liu L. et al., 2019), and an injectable sodium alginate/bioglass composite hydrogel containing BMSCs for subchondral bone regeneration (Zhu et al., 2019). Kim and Yoo’s groups first reported that immobilizing BMP-2 into hydrogel promoted osteogenic differentiation of human periodontal ligament stem cells (Park S. H. et al., 2017). Liu’s group promoted rapid bone regeneration by spatiotemporal delivery of BMP-2 or interleukin-8 from scaffolds (Chai et al., 2017; Lin D. et al., 2019). Qin’s group designed a targeted delivery system that could release osteogenic phytomolecule icaritin to prevent osteoporosis in a bone-targeting manner (Huang et al., 2018). Wang’s group used allogeneic mesenchymal stromal cell with low immunogenicity to construct bone substitutes and to repair bone defects in pigs (Chen Q. et al., 2010; Ren et al., 2012). In addition, they also found the function of modulating immunity and inducing immune tolerance of allogeneic mesenchymal stromal cells (Peng et al., 2012). Likewise, growth factors such as BMP-2 and TGF-β1 and vascular endothelial growth factor (VEGF) are also introduced to scaffolds to enhance bone repair (Qu et al., 2015; Yan et al., 2016). Yang’s group developed PLGA/PCL scaffold modified by silver impregnation, collagen coating, and electrospinning; this scaffold showed osteogenic and antimicrobial properties for orofacial tissue regeneration (Qian et al., 2019). Chua et al.’s (2004) group prepared polyvinyl alcohol/HAp biocomposite by selective laser sintering; this material showed good potential for craniofacial repair. Due to their cortical bone-like mechanical properties, biometals may become potent options for bone scaffolds. Among them, Mg has attracted a lot of attention of researchers (Zhang et al., 2009; Lai et al., 2019). Dai’s group and Chang’s group have prepared calcium Mg silicate bioceramic scaffolds and found that the scaffolds enhanced angiogenesis in bone regeneration (Huang Y. et al., 2009; Zhai et al., 2012). Yang’s group evaluated the in vivo degradation of Mg alloy implant and showed more new bone tissues around the implant after 10 and 26 weeks post-implantation, while only causing little change to blood composition (Zhang et al., 2009). They also developed various metal implants for bone tissue engineering (Li Y. et al., 2016; Zhang X. Z. et al., 2017). Qin’s group investigated the osteogenic capacity of Mg and found that PLGA/TCP porous scaffold incorporated Mg by 3D printing and had better repair result in challenging bone defect (a 12-mm segment of ulna in rabbit) (Lai et al., 2019).
Cornea
The development of corneal repair and regeneration is a pressing issue in TERM. Zhao X. et al. (2019) introduced gold nanoparticle-loaded microRNA-133b into collagen membrane. These collagen membranes could rapidly repair corneas and effectively inhibit scar formation. Hashimoto et al. (2019) realized the re-epithelialization and remodeling of rabbit decellularized corneal matrix, suggesting that this material may be a useful graft for corneal tissue regeneration. Developing a synthetic scaffold with similar properties to native cornea remains a challenge. In a study, the researchers developed elastomeric and biodegradable poly(glycerol sebacate)-PCL nanofibrous scaffolds and they show similar optical and mechanical properties with cornea (Salehi et al., 2017). Choi et al. (2018) also used biofunctionalized lysophosphatidic acid/silk fibroin film for corneal regeneration; this film showed higher specific gene and protein expression of cornea endothelial cells. Lee H. J. et al. (2018) used an in situ forming corneal stromal substitute based on collagen type I, after encapsulating keratocytes; this substitute exhibited surface epithelialization with multilayered morphology when implanted to rabbit corneas in an organ culture model after keratectomy. The researcher also tried to use short collagen-like peptides (CLPs) to replace collagen in corneal tissue engineering. They found that CLP-modified implants promoted stable regeneration of corneal and nerve tissues in a mini-pig model (Jangamreddy et al., 2018). Cells were used to promote corneal regeneration. For example, Yamashita et al. (2018) found that MSCs derived from human umbilical cord transplanted into a rabbit could maintain the thickness and transparency of cornea. Cell sheets are also used to repair cornea. Okano’s group successfully regenerated cornea in rabbit by transplanting corneal epithelial cell sheets prepared with automated cell culture system (Kobayashi et al., 2013). Later, they reported a novel cell culture device for preparation of corneal epithelial cell sheets (Nakajima et al., 2015). Fujita’s group transplanted an autologous corneal epithelial cell sheet in corneal injury of canine and demonstrated that the cell sheet may restore corneal transparency and prevent irreversible opacity caused by severe injury (Nam et al., 2018).
Corneal stroma provides the principal functions of the cornea. Tissue-engineered corneal stroma will be a promising strategy to overcome donor shortage in cornea replacement. Jin’s group attempted to implant a corneal stroma by seeding rabbit stromal keratocytes onto decellularized porcine cornea (DPC) into a model of corneal ulcer (Zhang et al., 2007). Recently, they used an artificial cornea composed of amniotic epithelial cells and acellular porcine cornea to treat corneal alkali burn (Luo et al., 2013). Cao’s group demonstrated that a polyglycolic acid (PGA) scaffold loading corneal stromal cells successfully repaired cornea (Hu et al., 2005). Moreover, they also reported a composite corneal scaffold containing acellular corneal stroma sheets and keratocytes (Ma et al., 2015). Wang’s group fabricated engineered lamellar cornea by seeding genetically modified embryonic stem cells and corneal epithelial cells on decellular porcine corneal stroma and amniotic membrane (Zhou Q. et al., 2014). The results showed that this substitute promoted wound healing and showed epithelial barrier functions. Recently, Xie and Shi’s groups developed a new method to prepare DPC grafts, which could efficiently remove the major xenoantigen but preserve corneal original structural and transparency (Shi W. Y. et al., 2019). These DPC grafts showed similar epithelial regeneration rate, transparency restoration, and visual acuity improvement when using human donor cornea grafts. Cells were also introduced into developing tissue-engineered corneal stroma. A cell-laden and orthogonal-multilayer tissue-engineered corneal stroma facilitated the construction of physiological feature tissue-engineered corneal stroma and helped to reverse fibrosis pathologies in general (Cui et al., 2018).
Nerve
The regeneration of traumatic and degenerative central or peripheral nervous system (CNS or PNS) injuries is still a big challenge. The CNS of adult mammalian is difficult to regenerate by itself, while the PNS only has limited axonal regeneration ability (Gu et al., 2011). Tissue-engineered neural scaffold may help to repair SCI or brain injury. Li’s group developed a neurotrophin-3 (NT-3)-containing chitosan-based scaffold, which could promote axon regeneration and functional recovery of SCI or brain injury (Li et al., 2009; Mo et al., 2010). In another work, NT-3-containing chitosan-based scaffold could elicit activation of endogenous NSCs, enhance neurogenesis and vascularization, and reduce inflammatory responses, thus improving the recovery of sensory and motor behavior (Duan et al., 2015; Yang Z. et al., 2015). Recently, they have obtained good repair results using the NT-3-containing chitosan scaffold in monkeys after SCI (Rao et al., 2018). Dai’s group developed collagen-based neural scaffold to promote axonal and spinal cord regeneration in canine and rodent models (Duan et al., 2015; Li X. et al., 2015). They also combined scaffold with BMSCs to construct a collagen/BMSCs construct, which showed good repair result for severe uterine injury in rats (Ding et al., 2014). In a recent study, Dai’s group implanted linear-ordered collagen scaffold (LOCS) and LOCS combined with collagen binding NT-3 (CBD-NT-3) into an acute thoracic complete transaction model in rhesus monkeys, which is closer to humans (Han S. et al., 2019). Ten months after surgery, both the LOCS and LOCS + CBD-NT-3 groups facilitated the ingrowth of axonal fibers at the lesion site. Moreover, the LOCS + CBD-NT-3 displayed a significant locomotor and electrophysiological recovery effect compared to other groups. Zeng’s group also loaded NT-3 in gelatin sponge scaffold, which facilitated the regeneration of spinal cord in dogs or rats (Li G. et al., 2016). In other studies, they attempted to combine BMSCs with scaffolds (Zeng et al., 2011, 2016). For instance, they found that the BMSC-seeded gelatin scaffold could attenuate inflammation, promote vascularization, and reduce the formation of cavity in the injured spinal cord of rat (Zeng et al., 2011). In another study, BMSCs and Schwann cells combined with gelatin scaffold could induce neurite elongation and promote the regeneration of nerve fiber in SCI (Zeng et al., 2016). Cui’s group and Xu’s group have made an attempt to use HA-based scaffolds for repair or regeneration of CNS. They found that the HA-poly-L-lysine/nogo-66 receptor antibody composite scaffold could enhance axonal regrowth (Wei et al., 2010). Further, they demonstrated that the modification of laminin or Ile–Lys–Val–Ala–Val peptide in HA hydrogel could promote the regeneration of nerve (Hou et al., 2005; Wei et al., 2007). In addition, the scaffolds with different structures were also prepared for SCI repair. In a work, multi-channel PLLA conduits with nano-fibrous channel walls promoted directional growth of nerve fiber within the channels compared to multi-channel PLLA conduits with ladder-like porous channel wall (Sun X. et al., 2019). Other composite hydrogels consisting of polyacrylamide, graphene oxide, gelatin, and sodium alginate were also developed for repairing SCI (Chen S. et al., 2019). The photoimmobilization of protein on the inner surface of poly(2-hydroxyethyl methacrylate) hydrogel could further improve the adhesion and survival of NSCs in the conduit, and exhibited the sustainable release of basic FGF (bFGF) (Cai et al., 2019). This hydrogel created favorable biologic niches to promote SCI repair. A variety of fibrous scaffolds were also used in neural tissue engineering (Li Y. et al., 2019; Ye et al., 2019a). Chew’s group used poly(ε-caprolactone-co-ethyl ethylene phosphate) electrospun nanofibers–collagen hybrid scaffold to repair SCI (Nguyen et al., 2017). They reported that the aligned nanofibers could enhance axonal regeneration in SCI (Chew et al., 2015). Further, they used a hybrid scaffold composed of aligned nanofibers and hydrogel, which could deliver drug or gene to direct axon regeneration in SCI (Nguyen et al., 2017). In addition, Kwon’s group introduced PLA nanorods to improve the mechanical property of agarose hydrogel-based integrated neuro-electrodes, making it a useful material in neural tissue engineering (Heo et al., 2017). Okano’s group has done some clinical trials in human for treating SCI using iPSC-derived neural precursor cells (iPSC-NPCs) (Isoda et al., 2016; Nagoshi and Okano, 2018; Tsuji et al., 2019). They have successfully generated NPCs from iPSCs and proved their function and safety (Isoda et al., 2016; Nagoshi and Okano, 2018). At present, 2 × 106 iPSC-NPCs was transplanted into the SCI patients at 14–28 days after injury and followed-up for 1 year.
There are also many efforts to promote the repair of peripheral nerve and clinical translation of tissue-engineered products (Gu, 2015). Gus’s group has long contributed to use biomaterials for peripheral nerve regeneration (Gu X. et al., 2014). For example, they reported work using silk fibroin and chitosan for peripheral neural tissue engineering (Wu et al., 2005; Yang et al., 2007b). They further engineered chitosan- and silk fibroin-based neural scaffolds by seeding BMSCs and implanted them in rats (Yang et al., 2011), dogs (Xue et al., 2011), and rhesus monkeys (Hu et al., 2013). Successful nerve repair and functional recovery indicated the therapeutic effect of combining scaffolds and BMSCs. In addition, they identified a bioactive molecule (achyranthes bidentata polypeptides) from a traditional Chinese medicine (Cheng et al., 2014). This molecule could promote repair of peripheral nerve like as growth factors. In recent studies, Gu Y. et al.’s (2014) group proposed that it was important to improve and proved to be regenerative within a neural scaffold. For example, the degradation products from chitosan scaffold may facilitate the regeneration of peripheral nerve by improving macrophage-constructed microenvironments (Zhao Y. H. et al., 2017). Gu’s group also promoted the clinical translation of neural scaffolds in China (Hvistendahl, 2012). In a report, they repaired two patients by chitosan-based scaffolds, and the 3-year follow-up displayed good functional recovery of injured nerves (Fan et al., 2008; Gu et al., 2012). Many other nerve grafts were also developed (Tao et al., 2019; Wang J. et al., 2019). Mo’s group fabricated a composite nanomaterial by coating bioactive graphene oxide on nanofibrous scaffold for successfully repairing a sciatic nerve defect (10 mm) in rats (Wang J. et al., 2019). A drug-loaded PEG–PCL nanoparticle in GelMA hydrogels prepared by 3D printing showed effective nerve repair (Tao et al., 2019). Other acellular nerve grafts were also prepared. A joint research by Lu and Wang’s groups reported novel 3D helix-flexible nerve guide conduits used for nerve repair (Quan et al., 2019). This style of helix-flexible nerve guide conduits could be used to repair peripheral nerve in a cross-joint region due to the lower tension during operation and easy rehabilitation after operation. Lu and Peng’s groups developed acellular nerve grafts transfected with hepatocyte growth factor to repair rat sciatic nerves (Li Z. et al., 2008). Recently, they combined acellular cauda equina allograft with chitin to repair long-distance sciatic nerve defect in rats (Zhao Y. H. et al., 2017). They also combined different cells, including BMSCs (Zhao et al., 2011), BMSC-containing fibrin glue (Zhao Z. et al., 2014), and Schwann cell-like cells derived from ADSCs or BMSCs (Wang Y. et al., 2012), with acellular nerve grafts to repair nerve injury. Other researchers also focused on the application of cells in repairing peripheral nerve. In an interesting work, researchers found that gingiva-derived MSC-extracellular vesicles could activate the repair phenotype of Schwann cells and thereby promote nerve regeneration (Mao et al., 2018). In another work, conduits with spatially neurotrophic factors improved peripheral nerve repair (Sun A. X. et al., 2019). Liu’s group found that the acellular nerve allografts loaded with etifoxine or platelet-rich plasma can achieve a similar effect with autografts for nerve regeneration and functional recovery (Zheng C. et al., 2014; Zhou X. et al., 2014). Furthermore, Liu’s group also attempted to use these nerve grafts in clinic. The results of clinical trial from 72 patients showed that the human acellular nerve grafts could repair digital nerve injury in human (1–5 cm length) (He et al., 2015). They also used this human acellular nerve allograft to repair the injured nerves in the hand and upper extremity of patients (Zhu et al., 2017).
Tendon/Ligaments
Tissue engineering strategy is also being used to treat tendon/ligament injury. Ouyang’s group has prepared nano-microfibrous PLGA or collagen/silk scaffold (Ouyang et al., 2003; Chen et al., 2008; Chen J. L. et al., 2010), or supplemented different cells, including MSCs (Ouyang et al., 2005), human embryonic stem cells (Chen et al., 2009), and human embryonic stem cell-derived MSCs (Chen J. L. et al., 2010) for tendon healing. Later, they found that the combination of histone deacetylase with well-aligned PLLA nanofibers may be more efficient to promote tenogenic differentiation of stem cells (Zhang et al., 2018a). Recently, they also attempted to use histone deacetylase inhibitor-treated tendon stem/progenitor cell-derived cell sheet to promote tendon repair (Zhang et al., 2018b). In another report, they modified the tendon ECM into a random-aligned-random composite, and the results showed that the biomimetic tendon ECM composite enhanced the bone and fibrocartilage formation in the interface (Liu H. H. et al., 2017). It was also reported that modifying the PLGA scaffold with fibronectin and type I collagen can promote the adhesion of human embryonic tenocytes (Qin et al., 2005). Xie’s group demonstrated that tissue-engineered tendons combined with cells and scaffolds could enhance the repair result (Yang et al., 2000). Cao and Liu’s groups used PGA unwoven fibrous scaffold seeded autologous dermal fibroblasts and tenocytes to repair Achilles tendon defect in a rabbit model (Deng et al., 2014). Later, they found that aligned nanofibers could facilitate in vitro tenogenic differentiation and in vivo tendon regeneration (Wang et al., 2016). They also found that tendon-like tissue was generated in a parthenogenetic stem cell-derived tenocytes-PLGA scaffold after in vivo heterotopic implantation (Liu W. et al., 2017). Recently, Lin’s group developed an oxidized HA/adipic acid dihydrazide hydrogel, the regimen of which could be used as a drug carrier and a mitigating agent of symptoms (Hsiao et al., 2019). This study provided a platform for exploring new therapeutics against tendinopathy.
Cardiovascular Tissues
Cardiac disease remains one of the leading causes of death worldwide (Investigators et al., 1991). The goal of cardiovascular tissue engineering is to construct arteries, heart valves, and myocardium substitutes (Rabkin and Schoen, 2002). Similar to other fields of TERM, cardiovascular tissue engineering also consists of scaffolds, cells, and soluble mediators. Wang’s group constructed cardiac tissue, which could beat synchronously and similarly to native cardiac muscle by mixing embryonic stem cell-derived cardiomyocytes with collagen I (Guo et al., 2006). They also tried to use an electrically conductive double-network hydrogel consisting of poly(thiophene-3-acetic acid) and methacrylated aminated gelatin for cardiac tissue engineering (Yang et al., 2016). The injectable HA hydrogel, fullerenol/alginate hydrogel, and oligo(poly(ethylene glycol)fumarate) hydrogel, which could deliver stem cells or some therapeutic drugs, helped the treatment of myocardial infarction and were also developed (Lü et al., 2009; Wang H. et al., 2012; Hao et al., 2017). Ge’s group explored bioabsorbable sirolimus-loaded PLLA stents (Xinsorb) for coronary angioplasty. The preclinical and clinical outcomes of Xinsorb were further studied (Shen et al., 2017; Lv et al., 2018; Wu Y. Z. et al., 2019). Recently, they reported 5 years of serial intravascular imaging outcomes of Xinsorb scaffold (Wu Y. Z. et al., 2019). Other biomimetic vascular substitutes were also developed. Kong and Zhao’s groups developed many small-diameter vascular grafts based on PCL electrospun fibers (Wang Z. H. et al., 2015, 2017; Wang K. et al., 2017; Gong et al., 2016; Dong et al., 2018), for example, grafted fusion protein VEGF-HGFI on PCL to enhance vascular regeneration (Wang K. et al., 2017). A bilayered vascular graft aiming to improve hemocompatibility and endothelial cell monolayer formation was also constructed using smooth PCL surface as internal layer and PCL microfibers as external layer (Dong et al., 2018). In a recent work, they found that MSC-derived small extracellular vesicles functionalized PCL electrospun grafts could enhance patency and inhibit calcification by immunomodulation in a rat model of hyperlipidemia (Wei et al., 2019). Han’s group reported that a dual functionalized vascular stent prepared by spatio-temporal coating of HA conjugated with dopamine showed improved neointimal area and inflammation score in a porcine restenosis model (Kim et al., 2016). 3D printing is also a promising method to construct biomimetic vasculature for tissue regeneration. For instance, Lei et al. (2019) developed perfusable and hierarchical networks with microchannels (PHMs) via the combination of 3D-printed sacrificial caramel templates, polymer coating, and phase separation. The PHMs could support the metabolic functions of heart cells in vitro and efficiently treat myocardial infarction.
Current heart transplantation therapy is still limited by lacking donors, suffering from chronic immunosuppressant therapy, and taking risks of subsequent development of graft vasculopathy (Stehlik et al., 2011). TERM provides several potential strategies for heart regeneration. The mammalian myocardium of human only has a limited regenerative capability. In situ tissue regeneration through mobilizing endogenous cell has been widely investigated. In a study, the phosphorylated form of 7A was loaded in collagen hydrogel to promote regeneration of infarcted myocardium and improve heart function in a rodent model (Zhang Y. et al., 2019). Decellularized matrix is also used for heart regeneration. Dai et al. (2019) fabricated a composite scaffold by combining a porous matrix metalloproteinase degradable PEG hydrogel with SDF-1α. The findings suggested that the SDF-1α-loaded hydrogel was efficient to develop functional decellularized heart valve. MicroRNA was also used to promote cardiac regeneration. For example, Chen’s group proved that miR-19a/19b had a therapeutic effect in cardiac regeneration and protection (Gao et al., 2019). In addition, Sun’s group and Wang’s group successfully generated human ventricular-specific heart tissue (EhVHT) based on 3D Fe oxide scaffolds (Yang H. et al., 2019). This EhVHT could not only meet the specific requirements for ventricular damages in most myocardial infarction but also be used to screen drugs targeting ventricular myocardium. Another way of TERM is to induce differentiation of cardiac progenitor cells into cardiomyocytes in damaged hearts (Garbern and Lee, 2013; Senyo et al., 2013). Exogenous sources, such as embryonic stem cells and iPSCs, are also used to repair the heart (Shiba et al., 2012). In a study, mouse cardiac fibroblasts (CFs) were reprogrammed into induced cardiomyocyte-like cells (iCMs) in the presence of Mef2c, Gata4, and Tbx5 (Ieda et al., 2010). Human fibroblasts were also reported to be induced into cardiomyocyte-like cells (Fu et al., 2013; Wada et al., 2013). To improve the survival of transplanted cells and reprogramming efficiency of iPSCs in the injured heart, in vivo reprogramming could generate more mature iCMs. Therefore, directly injecting the reprogramming factors into the damaged heart may convert the endogenous CF population into new functional iCMs. Compared with in vitro reprogramming method, this in vivo reprogramming approach, which is simple, has lower risk of tumor formation, and avoids cell transplantation, may be a future cardiac regenerative therapy (Figure 2; Sadahiro et al., 2015). With this method, the diseased myocardial tissue covered by fibrous tissue consisting of CFs and ECM can be converted into cardiomyocytes by defined factors in situ by direct cardiac reprogramming approach using defined factors.
FIGURE 2.
Future cardiac regenerative therapy by cell transplantation-based approach using iPS cell-derived cardiomyocytes (Upper) and direct cardiac reprogramming approach (Lower) (reproduced with permission from Sadahiro et al., 2015).
Dental Tissues
A vast array of metals including gold, silver, Cu, palladium, platinum, nickel, and Ti have been widely used for dental restorations. However, the oral environment will cause corrosion of metallic restorations and generation of metal ions. Ti is a common material used in dentistry. In particular, the modulus of Ti alloys could be regulated similarly to human cortical bone by Yang’s group to reduce the stress-shielding effect after implantation (Hao et al., 2007a, b). However, the surface of Ti alloys is basically bioinert, which means it is not conducive to osseointegration. Huang’s group attempted to use nano/submicron-scale TiO2 network (Yang et al., 2009), oxygen plasma immersion ion implantation treatment (Yang C. H. et al., 2015), and genipin (Sun Y. S. et al., 2018) for improving cell response on Ti surface. Recently, they developed a multiform nano-network of TiO2 on Ti surface. This multiform nanostructure was shown to enhance proliferation and differentiation of human BMSCs and regulate the formation of focal adhesion complex (Yang and Huang, 2019). Hwang’s group found that UV treatment of large grit sand-blasting and acid-etching implants followed by wet storage could enhance bioactivity (Choi et al., 2019). Different from other methods using bone matrix components to promote cell adhesion on the surface of implant, Wang’s group developed a polysaccharide coating that could improve osseointegration by regulating macrophages into a pro-regenerative phenotype and producing abundant osteogenic/angiogenic cytokines to enhance osteogenesis locally (Shi et al., 2018). Vijayalakshmi’s group fabricated Ag HAp/functionalized multiwall carbon nanotube (Ag-HA/f-MWCNT) T coating on passivated 316L stainless steel as a novel alternative to dental implant. The results showed that 3 wt% Ag-substituted HA/f-MWCN coating is nonhemolytic and most suited (Sivaraj and Vijayalakshmi, 2019). Cho and Kim’s groups found that 3D-printed cobalt–chrome (Co–Cr) alloys were more biocompatible than nickel–chrome (Ni–Cr) alloys (Ganbold et al., 2019).
In addition, other materials and their functions were also determined. Tsuji group reported a fully functional tooth replacement in mouse through the transplantation of bioengineered tooth germ into the alveolar bone (Ikeda et al., 2009). For the purpose of better esthetics, zirconia ceramics may be an alternative to Ti (Miyazaki et al., 2013). The zirconia ceramics could be fabricated into fixed dental prostheses, by computer-aided/computer-aided manufacturing system. Nakamura et al.’s (2019) group evaluated the mechanical properties of some zirconia-based materials (Itakura et al., 2019). Lee group proved that zirconia implants with acid-etched showed comparable osseointegration with Ti implants (Kuo et al., 2017). Triethylene glycol dimethacrylate (TEGDMA) and mineral trioxide aggregate (MTA) are also common materials used in dentistry. Peng’s group found that the autophagy was activated by TEGDMA via the AMPK/mTOR pathway, but this effect could be abrogated by N-acetyl cysteine pretreatment (Zhu et al., 2015). Shie group evaluated the feasibility of MTA powder coated with PDA and MTA/PCL scaffold by 3D printing in dental tissue regeneration, and found that these materials showed potential application in dental tissue engineering (Chiu et al., 2017; Tu et al., 2018). Lim group found that modified MTA with propolis could promote odontogenic differentiation and mineralization of human dentalpulp stem cells (DPSCs) through ERK pathway (Kim J. H. et al., 2019). However, Lee’s group thought that it should be careful consideration of the concentration of MTA extracts, especially when applying MTA to the elderly patients to maintain the viability of human MSC (Kim S. Y. et al., 2019). Shie group prepared mesoporous calcium silicate nanoparticles as endodontic materials, which could have osteogenic, drug delivery, and antibacterial characteristics (Huang et al., 2017). iRoot BP Plus, a promising MTA alternative, were proved to promote in vitro recruitment of DPSCs and facilitate dentin bridge formation in a pulp repair model in vivo (Zhu et al., 2014). Honda group prepared protamine-loaded dicalcium phosphate anhydride (DCPA), which showed excellent antimicrobial activity against Streptococcus mutans (Fujiki et al., 2019). Lee and Kim’s groups developed a Sr ion-releasing nanobiocement (Sr-NBC) based on sol–gel method. This Sr-NBC showed excellent biocompatibility and high odontogenic potential in vitro and more new dentin formation in vivo (Mandakhbayar et al., 2019). Anastasiou group incorporated Sr2+ or Ce3+ ions doped fluorapatites into chitosan scaffold, the results displayed that Sr2+ presented high osteoconductivity while Ce3+ could retain the good antibacterial property and osteoconductivity (Anastasiou et al., 2019). Lee group used chlorhexidine has been incorporated into the composition of PMMA dental restorations to enhance their antimicrobial performance (Mai et al., 2019). Bindal et al.’s (2019) group determined that 20% human platelet lysate could be optimum for maintaining the in vitro proliferative and angiogenic potential of inflamed dental pulp stem cells. Venkatasubbu group developed polyvinyl alcohol/alginate/HAp films loaded with amoxicillin, and the loading amoxicillin helped in healing the infection while HAP nanoparticles helps in periodontal regeneration (Prakash et al., 2019).
Concluding Remarks
We used the Wed Web of Science database to search for original articles on the topics of “Bone,” “cartilage,” “tendon/ligament,” “cardiovascular,” “nerve,” “skin,” “intervertebral disc,” “urethra,” “bladder,” “cornea,” and “regeneration” published between 2009 and August 2019 inclusively. In Asia, China is one of the leading countries in regenerative medicine research, as shown in Figure 3. Based on the quantity of publications, China, Japan, South Korea, India, and Singapore have paid more attention on TERM.
FIGURE 3.
The number of publications in different Asian countries (A), and different fields in China (B), Japan (C), and South Korea (D) from 2009 to 2019.
First of all, we must admit that this review of recent advances in TERM in Asia is far from comprehensive and a large number of excellent research teams and their work might be missed due to limited space. To date, great progress in TERM has been made by Asian researchers, not only by their publications, but also by their active efforts to promote the clinical transformation of their research results. We believe that TERM will experience a rapid development, because Asian governments and society are increasingly concerned with TERM. For example, in China, regenerative medicine is regarded as one of the five biotechnology fields in the National Medium to Long-term Plan for Scientific and Technological Development (2006–2020). There are also many foundations to support the basic research and clinical transformation of TERM.
Biomaterials, various cell sources, and bioactive factors are essential to TERM. Asian scholars have studied polymer, ceramic, and metal for TERM with the aim of having different applications. There are various preparation methods to construct biomaterials-based scaffold. Among them, 3D bioprinting and microfluidics technology excel due to their precision and controllability in preparing personalized materials and complex tissues/organs. In particular, cell sheet is a novel technique first proposed by Asian scholars. Except for the preparation method, the development in chemistry and materials also promotes the rapid development of biomaterials-based scaffold. Through the chemistry modification, we could improve the properties of the existing materials. A variety of new materials are also created and used in TERM. Furthermore, the materials could be processed into hydrogels, porous scaffolds, and fibers, to meet the different needs of TERM. In the future, researchers will continue to develop numerous functional biomaterials to engineer tissue-specific scaffolds.
The extension of cell sources also promotes the development of TERM. To date, the seed cells are no longer confined to autologous adult cells. Various multipotent cells, including embryonic stem cells, bone marrow stem cells, tissue-specific progenitor stem cells, umbilical cord stem cells, and iPS cells, are proved to be effective in TERM. In particular, the iPSC cell is a novel discovery by Asian scholars. Furthermore, the generation of iPS cell lines by Asian scholars, which could generate fertile progeny by tetraploid complementation, is also a breakthrough. In addition, the direct in vivo reprogramming approach, which is reported simple, has a lower risk of tumor formation and avoids cell transplantation as proposed by Asian scholars. The future direction of stem cells is how to induce stem cell into specific cells showing similar functions with adult cells (Tabar and Studer, 2014). It is predictable that the future of TERM may be updated with stem cell technologies (Tang et al., 2017). Here, we provide some of the recent preclinical and clinical studies using stem cell therapy. In the clinic, allogeneic MSCs from bone marrow or umbilical cord have been evaluated in cartilage defect, SCI, corneal injuries, cardiomyopathy, IVD degeneration, and bone defect with an excellent safety profile. The available clinical studies in cell transplantation indicate that some autologous and allogeneic stem cell therapy from different sources is safe (Table 3).
TABLE 3.
Examples of clinical trials on MSC cell therapy.
| NCT | Disease | MSC source | Objectives | Country | Status |
| NCT01302015 | Buerger’s disease | Autologous ADSCs | Treat patient with Buerger’s disease | South Korea | Completed Estimated study Completion Date: June 2019 |
| NCT01309061 | Romberg’s disease | Human ADSCs | Treat patient with progressive hemifacial atrophy | South Korea | Completed Estimated study Completion Date: June 2019 |
| NCT00979758 | Myocardial infarction | Bone marrow mononuclear cells | Improve the outcome of patients with impaired left ventricle function after myocardial infarction | China | Completed Estimated study Completion Date: January 2018 |
| NCT02948023 | Corneal injuries | Ex vivo cultivated limbal stem cells | Treat different superficial corneal pathologies in human | India | Recruiting Estimated study Completion Date: March 2018 |
| NCT02260713 | Acute spinal cord injury | Autologous bone marrow cells | Treat acute complete spinal cord injury | India | Completed Estimated study Completion Date: March 2018 |
| NCT02669199 | Large area skin wound injury | Allogeneic umbilical cord derived MSCs | Treat large area skin lesions of the subjects | China | Recruiting Estimated study Completion Date: February 2016 |
| NCT00981006 | Ischemic cardiomyopathy | Autologous human cardiac-derived stem cell | Treat severe refractory heart failure patients with chronic ischemic cardiomyopathy concordance with reduced left ventricular dysfunction | Japan | Completed Estimated study Completion Date: April 2015 |
| NCT02338271 | Intervertebral disc degeneration | Autologous ADSCs | Treat chronic low back pain patients with lumbar intervertebral disc degeneration | South Korea | Completed Estimated study Completion Date: January 2015 |
In order to promote the development of TERM, it is also important to better understand and replicate the microenvironment around tissue/organ. The improvement of microenvironment may be realized by applying bioactive factors, including growth factors, chemokines, cytokines, or other signals, such as electric, mechanical, and magnetic stimulation.
The ultimate goal of TERM is to translate the findings of basic research into the clinic. The mineralized collagen, which mimics the natural bone ECM, has acquired the product registration certificate from China government. Some nerve scaffolds, including human acellular nerve grafts and chitosan-based grafts, have been promoted the clinical translation in China (Fan et al., 2008; Gu et al., 2012; Hvistendahl, 2012; He et al., 2015; Zhu et al., 2017). Using a composite of allogeneic umbilical cord blood-derived MSCs and hyaluronate hydrogel, regenerated articular cartilage in the defects of patients was observed (Park Y. B. et al., 2017). Bioabsorbable sirolimus-loaded PLLA stents (Xinsorb) as an alternative to metallic stents for coronary angioplasty showed 5 years of serial intravascular imaging in patients (Wu Y. Z. et al., 2019). There have been many commercial technologies and products such as 3D printing. Two well-known 3D bioprinters, “Life-Printer ‘X”’ and “CellJet Cell Printer,” are developed in Singapore and Japan, respectively (Arslan-Yildiz et al., 2016). Several examples of 3D bioprinting companies and their products and projects are shown in Table 4. In a review on 3D-printing clinical trials for the world, 3D-printing trials were registered in 20 different countries, most commonly in China with 42 (45.65%) trials (Witowski et al., 2018). The application of cell sheet in the clinic includes heart, cornea, blood vessels, periodontal membrane, esophagus, cartilage, functional tendons, and middle ear (Nishida et al., 2004; Egami et al., 2014; Iwata et al., 2015; Yamamoto et al., 2017). Okano’s group has done some clinical trials in humans for treating SCI using iPSC-NPCs (Isoda et al., 2016; Nagoshi and Okano, 2018; Tsuji et al., 2019). In the past decades, part of the tissue-engineered products has been applied to the clinic (Tang et al., 2017). However, they make up only a very small percentage. Although the therapeutic effect of cell–materials composite is better than materials, most successful products are still biomaterial-based scaffolds. The clinical application of stem cells is mostly involved in hematological diseases. In the future, we still have a long way to go in combining TERM with the clinic.
TABLE 4.
Examples of 3D bioprinting companies and products in Asia.
| Company | Project/products | Founding year | Country | Website |
| Next 21 | Custom-made artificial bone implants | 2000 | Japan | http://next21.info |
| Osteopore International | Osteoplug; Osteomesh | 2003 | Singapore | http://osteopore.com.sg |
| Cyfuse Biomedical | Bone, cartilage, blood vessel, neural tissue, liver | 2010 | Japan | http://cyfusebio.com |
| Regenovo Biotechnology | Bone, blood vessel, cartilage | 2013 | China | http://regenovo.com |
| SUNP BIOTECH | Custom-made products, bioink, bioprinter | 2014 | China | http://sunpbiotech.com |
| Rokit | Skin, bioink, bioprinter | South Korea | https://onsbio.com/bioprinting | |
| Pandorum Technologies | Liver, cornea | 2011 | India | http://www.pandorumtechnologies.com |
Author Contributions
BL and FH conceived and designed the review. FH wrote the manuscript. JW, LD, YH, ZY, QG, CZ, LY, HW, ZZ, LJ, JL, YY, WZ, and GC all participated in the data search and analysis. WL and SC assisted with the revision of this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We apologize to anyone whose work was not mentioned due to limited space.
Footnotes
Funding. The authors are grateful to the funding support from the National Natural Science Foundation of China (31872748, 31500779, 81471790, 31530024, and 81672213), the Jiangsu Provincial Special Program of Medical Science (BL2012004), the China Postdoctoral Science Foundation (2016M590500, 2017T100398), the Jiangsu Planned Projects for Postdoctoral Research Funds (1601269C), and the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
References
- Alam M. K., Koomson E., Zou H., Yi C., Li C. W., Xu T., et al. (2018). Recent advances in microfluidic technology for manipulation and analysis of biological cells (2007-2017). Anal. Chim. Acta 1044 29–65. 10.1016/j.aca.2018.06.054 [DOI] [PubMed] [Google Scholar]
- Ali M., Pages E., Ducom A., Fontaine A., Guillemot F. (2014). Controlling laser-induced jet formation for bioprinting mesenchymal stem cells with high viability and high resolution. Biofabrication 6:045001. 10.1088/1758-5082/6/4/045001 [DOI] [PubMed] [Google Scholar]
- Alkayyali T., Cameron T., Haltli B., Kerr R. G., Ahmadi A. (2019). Microfluidic and cross-linking methods for encapsulation of living cells and bacteria - a review. Anal. Chim. Acta 1053 1–21. 10.1016/j.aca.2018.12.056 [DOI] [PubMed] [Google Scholar]
- Alvarez K., Nakajima H. (2009). Metallic scaffolds for bone regeneration. Materials 2 790–832. 10.3390/ma2030790 [DOI] [Google Scholar]
- An Y., Wei W., Jing H., Ming L., Liu S., Jin Y. (2015). Bone marrow mesenchymal stem cell aggregate: an optimal cell therapy for full-layer cutaneous wound vascularization and regeneration. Sci. Rep. 5:17036. 10.1038/srep17036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou A. D., Nerantzaki M., Gounari E., Duggal M. S., Giannoudis P. V., Jha A., et al. (2019). Antibacterial properties and regenerative potential of Sr2+ and Ce3+ doped fluorapatites; a potential solution for peri-implantitis. Sci. Rep. 9:11. 10.1038/s41598-019-50916-50914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ao C., Niu Y., Zhang X., He X., Zhang W., Lu C. (2017). Fabrication and characterization of electrospun cellulose/nano-hydroxyapatite nanofibers for bone tissue engineering. Int. J. Biol. Macromol. 97 568–573. 10.1016/j.ijbiomac.2016.12.091 [DOI] [PubMed] [Google Scholar]
- Arslan-Yildiz A., Assal R. E., Chen P., Guven S., Inci F., Demirci U. (2016). Towards artificial tissue models: past, present, and future of 3D bioprinting. Biofabrication 8:014103. 10.1088/1758-5090/8/1/014103 [DOI] [PubMed] [Google Scholar]
- Bang S., Lee S. R., Ko J., Son K., Tahk D., Ahn J., et al. (2017). A low permeability microfluidic blood-brain barrier platform with direct contact between perfusable vascular network and astrocytes. Sci. Rep. 7:8083. 10.1038/s41598-017-07416-7410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer B. S. (2009). Reconstruction of microtia. Plast. Reconstr. Surg. 124 14e–26e. 10.1097/PRS.0b013e3181aa0e79 [DOI] [PubMed] [Google Scholar]
- Bertheuil N., Chaput B., Ménard C., Varin A., Laloze J., Watier E., et al. (2019). Adipose mesenchymal stromal cells: definition, immunomodulatory properties, mechanical isolation and interest for plastic surgery. Ann. Chir. Plast. Esthét. 64 1–10. 10.1016/j.anplas.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Bhansali A., Asokumar P., Walia R., Bhansali S., Gupta V., Jain A., et al. (2014). Efficacy and safety of autologous bone Marrow-derived Stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transpl. 23 1075–1085. 10.3727/096368913X665576 [DOI] [PubMed] [Google Scholar]
- Bharadwaj S., Liu G., Shi Y., Wu R., Yang B., He T., et al. (2013). Multipotential differentiation of human urine-derived stem cells: potential for therapeutic applications in urology. Stem Cells 31 1840–1856. 10.1002/stem.1424 [DOI] [PubMed] [Google Scholar]
- Bindal P., Gnanasegaran N., Bindal U., Haque N., Ramasamy T. S., Chai W. L., et al. (2019). Angiogenic effect of platelet-rich concentrates on dental pulp stem cells in inflamed microenvironment. Clin. Oral Investig 23 3821–3831. 10.1007/s00784-019-02811-2815 [DOI] [PubMed] [Google Scholar]
- Bleuel J., Zaucke F., Bruggemann G. P., Niehoff A. (2015). Effects of cyclic tensile strain on chondrocyte metabolism: a systematic review. PLoS One 10:e0119816. 10.1371/journal.pone.0119816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu S., Chen Y., Wang S., Zhang F., Ji G. (2012). Treadmill training regulates beta-catenin signaling through phosphorylation of GSK-3beta in lumbar vertebrae of ovariectomized rats. Eur. J. Appl. Physiol. 112 3295–3304. 10.1007/s00421-011-2306-2304 [DOI] [PubMed] [Google Scholar]
- Bunpetch V., Zhang X., Li T., Lin J., Maswikiti E. P., Wu Y., et al. (2019). Silicate-based bioceramic scaffolds for dual-lineage regeneration of osteochondral defect. Biomaterials 192 323–333. 10.1016/j.biomaterials.2018.11.025 [DOI] [PubMed] [Google Scholar]
- Cai Y., Liu Y., Yan W., Hu Q., Tao J., Zhang M., et al. (2007). Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 17:3780 10.1039/b705129h [DOI] [Google Scholar]
- Cai Z., Gan Y., Bao C., Wu W., Wang X., Zhang Z., et al. (2019). Photosensitive hydrogel creates favorable biologic niches to promotespinal cord injury repair. Adv. Healthc. Mater. 8:1900013. 10.1002/adhm.201900013 [DOI] [PubMed] [Google Scholar]
- Cao C., Li L., Li H., He X., Wu G., Yu X. (2018). Cyclic biaxial tensile strain promotes bone marrow-derived mesenchymal stem cells to differentiate into cardiomyocyte-like cells by miRNA-27a. Int. J. Biochem. Cell Biol. 99 125–132. 10.1016/j.biocel.2018.04.004 [DOI] [PubMed] [Google Scholar]
- Chai Y. J., Lin D., Ma Y. F., Yuan Y., Liu C. S. (2017). RhBMP-2 loaded MBG/PEGylated poly(glycerol sebacate) composite scaffolds for rapid bone regeneration. J. Mater. Chem. B 5 4633–4647. 10.1039/c7tb00505a [DOI] [PubMed] [Google Scholar]
- Chang R., Emami K., Wu H., Sun W. (2010). Biofabrication of a three-dimensional liver micro-organ as anin vitrodrug metabolism model. Biofabrication 2:045004. 10.1088/1758-5082/2/4/045004 [DOI] [PubMed] [Google Scholar]
- Chen F., Yu S., Liu B., Ni Y., Yu C., Su Y., et al. (2016). An injectable enzymatically crosslinked carboxymethylated pullulan/chondroitin sulfate hydrogel for cartilage tissue engineering. Sci. Rep. 6:20014. 10.1038/srep20014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Liu Z., Zhong G., Li Z., Qian L., Li X., et al. (2016). Cyclic stretch enhances apoptosis in human lumbar ligamentum fl avum cells via the induction of reactive oxygen species generation. J. Spinal Cord Med. 39 450–454. 10.1080/10790268.2016.1141470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J. L., Yin Z., Shen W. L., Chen X., Heng B. C., Zou X. H., et al. (2010). Efficacy of hESC-MSCs in knitted silk-collagen scaffold for tendon tissue engineering and their roles. Biomaterials 31 9438–9451. 10.1016/j.biomaterials.2010.08.011 [DOI] [PubMed] [Google Scholar]
- Chen L., Wang D. H., Peng F., Qiu J. J., Ouyang L. P., Qjao Y. Q., et al. (2019). Nanostructural surfaces with different elastic moduli regulate the immune response by stretching macrophages. Nano Lett. 19 3480–3489. 10.1021/acs.nanolett.9b00237 [DOI] [PubMed] [Google Scholar]
- Chen L., Xiang B., Wang X., Xiang C. (2017). Exosomes derived from human menstrual blood-derived stem cells alleviate fulminant hepatic failure. Stem Cell Res. Ther. 8:9. 10.1186/s13287-016-0453-456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Yang Z., Sun S., Huang H., Sun X., Wang Z., et al. (2010). Adipose-derived stem cells modified genetically in vivo promote reconstruction of bone defects. Cytotherapy 12 831–840. 10.3109/14653249.2010.495980 [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang Q., Nakamoto T., Kawazoe N., Chen G. (2014). Highly active porous scaffolds of collagen and hyaluronic acid prepared by suppression of polyion complex formation. J. Mater. Chem. B 2 5612–5619. 10.1039/C4TB00780H [DOI] [PubMed] [Google Scholar]
- Chen S., Zhang Q., Nakamoto T., Kawazoe N., Chen G. (2015). Gelatin scaffolds with controlled pore structure and mechanical property for cartilage tissue engineering. Tissue Eng. Part C Methods 22 189–198. 10.1089/ten.tec.2015.0281 [DOI] [PubMed] [Google Scholar]
- Chen S., Zhao Y., Yan X., Zhang L., Li G., Yang Y. (2019). PAM/GO/gel/SA composite hydrogel conduit with bioactivity for repairing peripheral nerve injury. J. Biomed. Mater. Res. Part A 107 1273–1283. 10.1002/jbm.a.36637 [DOI] [PubMed] [Google Scholar]
- Chen S. W., Kawazoe N., Chen G. P. (2017). Biomimetic assembly of vascular endothelial Ccells and muscle cells in microgrooved collagen porous scaffolds. Tissue Eng. Part C Methods 23 367–376. 10.1089/ten.tec.2017.0088 [DOI] [PubMed] [Google Scholar]
- Chen X., Dong C., Wei K., Yao Y., Feng Q., Zhang K., et al. (2018a). Supramolecular hydrogels cross-linked by preassembled host–guest PEG cross-linkers resist excessive, ultrafast, and non-resting cyclic compression. NPG Asia Mater. 10 788–799. 10.1038/s41427-018-0071-0 [DOI] [Google Scholar]
- Chen X., Qi Y. Y., Wang L. L., Yin Z., Yin G. L., Zou X. H., et al. (2008). Ligament regeneration using a knitted silk scaffold combined with collagen matrix. Biomaterials 29 3683–3692. 10.1016/j.biomaterials.2008.05.017 [DOI] [PubMed] [Google Scholar]
- Chen X., Song X. H., Yin Z., Zou X. H., Wang L. L., Hu H., et al. (2009). Stepwise differentiation of Hhuman embryonic stem cells promotes tendon regeneration by secreting fetal tendon matrix and differentiation factors. Stem Cells 27 1276–1287. 10.1002/stem.61 [DOI] [PubMed] [Google Scholar]
- Chen X., Yan J., He F., Zhong D., Yang H., Pei M., et al. (2018b). Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic. Biol. Med. 126 187–201. 10.1016/j.freeradbiomed.2018.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zhao Y. N., Li X., Xiao Z. F., Yao Y. J., Chu Y., et al. (2018c). Functional multichannel poly(propylene fumarate)-collagen scaffold with collagen-binding neurotrophic factor 3 promotes neural regeneration after transected spinal cord injury. Adv. Healthc. Mater. 7:13. 10.1002/adhm.201800315 [DOI] [PubMed] [Google Scholar]
- Chen Y. H., Chung Y. C., Wang I. J., Young T. H. (2012). Control of cell attachment on pH-responsive chitosan surface by precise adjustment of medium pH. Biomaterials 33 1336–1342. 10.1016/j.biomaterials.2011.10.048 [DOI] [PubMed] [Google Scholar]
- Chen Y., Kawazoe N., Chen G. (2018d). Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 67 341–353. 10.1016/j.actbio.2017.12.004 [DOI] [PubMed] [Google Scholar]
- Chen Y., Wang J., Zhu X. D., Tang Z. R., Yang X., Tan Y. F., et al. (2015). Enhanced effect of beta-tricalcium phosphate phase on neovascularization of porous calcium phosphate ceramics: in vitro and in vivo evidence. Acta Biomater. 11 435–448. 10.1016/j.actbio.2014.09.028 [DOI] [PubMed] [Google Scholar]
- Cheng Q., Yuan Y., Sun C., Gu X., Cao Z., Ding F. (2014). Neurotrophic and neuroprotective actions of Achyranthes bidentata polypeptides on cultured dorsal root ganglia of rats and on crushed common peroneal nerve of rabbits. Neurosci. Lett. 562 7–12. 10.1016/j.neulet.2013.12.015 [DOI] [PubMed] [Google Scholar]
- Cheng S., Jin Y., Wang N., Cao F., Zhang W., Bai W., et al. (2017). Self-adjusting, polymeric multilayered roll that can keep the shapes of the blood vessel scaffolds during biodegradation. Adv. Mater 29 1700171. 10.1002/adma.201700171 [DOI] [PubMed] [Google Scholar]
- Cheng Y., Yu Y., Fu F., Wang J., Shang L., Gu Z., et al. (2016). Controlled fabrication of bioactive microfibers for creating tissue constructs using microfluidic techniques. ACS Appl. Mater. Interfaces 8 1080–1086. 10.1021/acsami.5b11445 [DOI] [PubMed] [Google Scholar]
- Chew S., Nguyen L., Milbreta U., Diao H., Wu W., Sun C., et al. (2015). Aligned nanofibers direct and enhance axonal regeneration after spinal cord injury. Tissue Eng. Part A 21 S405–S405. [DOI] [PubMed] [Google Scholar]
- Chiu Y. C., Fang H. Y., Hsu T. T., Lin C. Y., Shie M. Y. (2017). The characteristics of mineral trioxide aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 43 923–929. 10.1016/j.joen.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Choi J. H., Jeon H., Song J. E., Oliveira J. M., Reis R. L., Khang G. (2018). Biofunctionalized lysophosphatidic acid/silk fibroin film for cornea endothelial cell regeneration. Nanomaterials 8:14. 10.3390/nano8050290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S. H., Ryu J. H., Kwon J. S., Kim J. E., Cha J. Y., Lee K. J., et al. (2019). Effect of wet storage on the bioactivity of ultraviolet light- and non-thermal atmospheric pressure plasma-treated titanium and zirconia implant surfaces. Mater. Sci. Eng. C Mater. Biol. Appl. 105:12. 10.1016/j.msec.2019.110049 [DOI] [PubMed] [Google Scholar]
- Chowdhury S. R., Izzah A. G. N., Manira M., Lokanathan Y., Aminuddin B. S., Ruszymah B. H. I. (2015). Dermal fibroblast conditioned medium (DFCM) for skin wound healing. Tissue Eng. Part A 21 S158–S158. [Google Scholar]
- Chu G., Shi C., Wang H., Zhang W., Yang H., Li B. (2018). Strategies for annulus fibrosus regeneration: from biological therapies to tissue engineering. Front. Bioeng. Biotechnol. 6:90. 10.3389/fbioe.2018.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Yuan Z., Zhu C., Zhou P., Wang H., Zhang W., et al. (2019). Substrate stiffness- and topography-dependent differentiation of annulus fibrosus-derived stem cells is regulated by Yes-associated protein. Acta Biomater. 92 254–264. 10.1016/j.actbio.2019.05.013 [DOI] [PubMed] [Google Scholar]
- Chua C. K., Leong K. F., Tan K. H., Wiria F. E., Cheah C. M. (2004). Development of tissue scaffolds using selective laser sintering of polyvinyl alcohol/hydroxyapatite biocomposite for craniofacial and joint defects. J. Mater. Sci.-Mater. Med. 15 1113–1121. 10.1023/B:JMSM.0000046393.81449.a5 [DOI] [PubMed] [Google Scholar]
- Cui C. H., Uyama T., Miyado K., Terai M., Kyo S., Kiyono T., et al. (2007). Menstrual blood-derived cells confer human dystrophin expression in the murine model of Duchenne muscular dystrophy via cell fusion and myogenic transdifferentiation. Mol. Biol. Cell 18 1586–1594. 10.1091/mbc.e06-09-0872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui H. M., Chai Y. M., Yu Y. L. (2019). Progress in developing decellularized bioscaffolds for enhancing skin construction. J. Biomed. Mater. Res. Part A 107 1849–1859. 10.1002/jbm.a.36688 [DOI] [PubMed] [Google Scholar]
- Cui X., Gao G., Yonezawa T., Dai G. (2014). Human cartilage tissue fabrication using three-dimensional inkjet printing technology. JoVE 88:e51294. 10.3791/51294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Tan M., Zhu A., Guo M. (2015). Non-covalent interaction cooperatively induced stretchy, tough and stimuli-responsive polyurethane–urea supramolecular (PUUS) hydrogels. J. Mater. Chem. B 3 2834–2841. 10.1039/C5TB00095E [DOI] [PubMed] [Google Scholar]
- Cui Z. K., Zeng Q. L., Liu S. W., Zhang Y. N., Zhu D. L., Guo Y. L., et al. (2018). Cell-laden and orthogonal-multilayer tissue-engineered corneal stroma induced by a mechanical collagen microenvironment and transplantation in a rabbit model. Acta Biomater. 75 183–199. 10.1016/j.actbio.2018.06.005 [DOI] [PubMed] [Google Scholar]
- Dai J. C., Qiao W. H., Shi J. W., Liu C. G., Hu X. J., Dong N. G. (2019). Modifying decellularized aortic valve scaffolds with stromal cell-derived factor-1 alpha loaded proteolytically degradable hydrogel for recellularization and remodeling. Acta Biomater. 88 280–292. 10.1016/j.actbio.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Dai X. Y., Zhang Y. Y., Gao L. N., Bai T., Wang W., Cui Y. L., et al. (2015). A mechanically strong, highly stable, thermoplastic, and self-healable supramolecular polymer hydrogel. Adv. Mater. 27 3566–3571. 10.1002/adma.201500534 [DOI] [PubMed] [Google Scholar]
- Deng C., Yang Q., Sun X., Chen L., Feng C., Chang J., et al. (2018a). Bioactive scaffolds with Li and Si ions-synergistic effects for osteochondral defects regeneration. Appl. Mater. Today 10 203–216. 10.1016/j.apmt.2017.12.010 29556366 [DOI] [Google Scholar]
- Deng C., Zhu H., Li J., Feng C., Yao Q., Wang L., et al. (2018b). Bioactive Scaffolds for Regeneration of cartilage and subchondral bone interface. Theranostics 8 1940–1955. 10.7150/thno.23674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng D., Wang W. B., Wang B., Zhang P. H., Zhou G. D., Zhang W. J., et al. (2014). Repair of Achilles tendon defect with autologous ASCs engineered tendon in a rabbit model. Biomaterials 35 8801–8809. 10.1016/j.biomaterials.2014.06.058 [DOI] [PubMed] [Google Scholar]
- Deng J., Tang Y. Y., Zhang Q., Wang C., Liao M., Ji P., et al. (2019). A bioinspired medical adhesive derived from skin secretion of andrias davidianus for wound healing. Adv. Funct. Mater. 29:13 10.1002/adfm.201809110 [DOI] [Google Scholar]
- Dewi A. H., Ana I. D., Jansen J. (2017). Preparation of a calcium carbonate-based bone substitute with cinnamaldehyde crosslinking agent with potential anti-inflammatory properties. J. Biomed. Mater. Res. Part A 105 1055–1062. 10.1002/jbm.a.35990 [DOI] [PubMed] [Google Scholar]
- Dewi A. H., Ana I. D., Wolke J., Jansen J. (2015). Behavior of POP-calcium carbonate hydrogel as bone substitute with controlled release capability: a study in rat. J. Biomed. Mater. Res. Part A 103 3273–3283. 10.1002/jbm.a.35460 [DOI] [PubMed] [Google Scholar]
- Ding L., Li X. A., Sun H., Su J., Lin N., Péault B., et al. (2014). Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials 35 4888–4900. 10.1016/j.biomaterials.2014.02.046 [DOI] [PubMed] [Google Scholar]
- Dong D., Guo M., Wang S., Zhu Y., Wang S., Xiong Z., et al. (2017). Structural basis of CRISPR–SpyCas9 inhibition by an anti-CRISPR protein. Nature 546:436. 10.1038/nature22377 [DOI] [PubMed] [Google Scholar]
- Dong X. H., Yuan X. Y., Wang L. N., Liu J. L., Midgley A. C., Wang Z. H., et al. (2018). Construction of a bilayered vascular graft with smooth internal surface for improved hemocompatibility and endothelial cell monolayer formation. Biomaterials 181 1–14. 10.1016/j.biomaterials.2018.07.027 [DOI] [PubMed] [Google Scholar]
- Drupitha M. P., Das B., Parameswaran R., Dhara S., Nando G. B., Naskar K. (2018). Hybrid electrospun fibers based on TPU-PDMS and spherical nanohydroxyapatite for bone tissue engineering. Mater. Today Commun. 16 264–273. 10.1016/j.mtcomm.2018.06.013 [DOI] [Google Scholar]
- Du M., Chen B., Meng Q., Liu S., Zheng X., Zhang C., et al. (2015). 3D bioprinting of BMSC-laden methacrylamide gelatin scaffolds with CBD-BMP2-collagen microfibers. Biofabrication 7:044104. 10.1088/1758-5090/7/4/044104 [DOI] [PubMed] [Google Scholar]
- Duan H., Ge W., Zhang A., Xi Y., Chen Z., Luo D., et al. (2015). Transcriptome analyses reveal molecular mechanisms underlying functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. U.S.A. 112:13360. 10.1073/pnas.1510176112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J., Lu G., Xie Z., Lou M., Luo J., Guo L., et al. (2014). Genome-wide identification of CRISPR/Cas9 off-targets in human genome. Cell Res. 24:1009. 10.1038/cr.2014.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egami M., Haraguchi Y., Shimizu T., Yamato M., Okano T. (2014). Latest status of the clinical and industrial applications of cell sheet engineering and regenerative medicine. Arch. Pharm. Res. 37 96–106. 10.1007/s12272-013-0299-298 [DOI] [PubMed] [Google Scholar]
- Esaki S., Katsumi S., Hamajima Y., Nakamura Y., Murakami S. (2019). Transplantation of olfactory stem cells with biodegradable hydrogel accelerates facial nerve regeneration after crush injury. Stem Cells Transl. Med. 8 169–178. 10.1002/sctm.15-0399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esfahani H., Prabhakaran M. P., Salahi E., Tayebifard A., Keyanpour-Rad M., Rahimipour M. R., et al. (2015). Protein adsorption on electrospun zinc doped hydroxyapatite containing nylon 6 membrane: kinetics and isotherm. J. Coll. Interface Sci. 443 143–152. 10.1016/j.jcis.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Fan W., Gu J., Hu W., Deng A., Ma Y., Liu J., et al. (2008). Repairing a 35-mm-long median nerve defect with a chitosan/PGA artificial nerve graft in the human: a case study. Microsurgery 28 238–242. 10.1002/micr.20488 [DOI] [PubMed] [Google Scholar]
- Fan X., Chen K., He X., Li N., Huang J., Tang K., et al. (2016). Nano-TiO2/collagen-chitosan porous scaffold for wound repairing. Int. J. Biol. Macromol. 91 15–22. 10.1016/j.ijbiomac.2016.05.094 [DOI] [PubMed] [Google Scholar]
- Fang J., Mehlich A., Koga N., Huang J., Koga R., Gao X., et al. (2013). Forced protein unfolding leads to highly elastic and tough protein hydrogels. Nat. Commun. 4:2974. 10.1038/ncomms3974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauzi M. B., Rajab N. F., Tabata Y., Aminuddin B. S., Ruszymah B. H. I., Chowdhury S. R. (2019). Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 13 874–891. 10.1002/term.2842 [DOI] [PubMed] [Google Scholar]
- Feng C., Zhang W., Deng C., Li G., Chang J., Zhang Z., et al. (2017). 3D printing of lotus root-like biomimetic materials for cell delivery and tissue regeneration. Adv. Sci. 4:1700401. 10.1002/advs.201700401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng Y., Xu Q., Yang Y., Shi W., Meng W., Zhang H., et al. (2019). The therapeutic effects of bone marrow-derived mesenchymal stromal cells in the acute lung injury induced by sulfur mustard. Stem Cell Res. Ther. 10:90. 10.1186/s13287-019-1189-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M. B., Mauck R. L. (2013). Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue Eng. Part B Rev. 19 1–13. 10.1089/ten.TEB.2012.0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine M. J., Shih H., Schäfer R., Pittenger M. F. (2016). Unraveling the mesenchymal stromal cells’ paracrine immunomodulatory effects. Transfus. Med. Rev. 30 37–43. 10.1016/j.tmrv.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Frey B. M., Zeisberger S. M., Hoerstrup S. P. (2016). Tissue engineering and regenerative medicine - new initiatives for individual treatment offers. Transfus. Med. Hemother. 43 318–319. 10.1159/000450716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J. D., Stone N. R., Liu L., Spencer C. I., Qian L., Hayashi Y., et al. (2013). Direct reprogramming of human fibroblasts toward a cardiomyocyte-like state. Stem Cell Rep. 1 235–247. 10.1016/j.stemcr.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki M., Abe K., Hayakawa T., Yamamoto T., Torii M., Iohara K., et al. (2019). Antimicrobial activity of protamine-loaded calcium phosphates against oral bacteria. Materials 12:E2816. 10.3390/ma12172816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan D., Xu T., Xing W., Wang M., Fang J., Wang K., et al. (2019). Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J. Mater. Chem. B 7 1716–1725. 10.1039/C8TB01664J [DOI] [PubMed] [Google Scholar]
- Ganbold B., Heo S. J., Koak J. Y., Kim S. K., Cho J. (2019). Human stem cell responses and surface characteristics of 3D printing Co-Cr dental material. Materials 12:E3419. 10.3390/ma12203419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C., Wang Y., Han F., Yuan Z., Li Q., Shi C., et al. (2017). Antibacterial activity and osseointegration of silver-coated poly(ether ether ketone) prepared using the polydopamine-assisted deposition technique. J. Mater. Chem. B 5 9326–9336. 10.1039/C7TB02436C [DOI] [PubMed] [Google Scholar]
- Gao F., Kataoka M., Liu N., Liang T., Huang Z. P., Gu F., et al. (2019). Therapeutic role of miR-19a/19b in cardiac regeneration and protection from myocardial infarction. Nat. Commun. 10:15. 10.1038/s41467-019-09530-9531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Xu Z. Y., Liang Q. F., Liu B., Li H. F., Wu Y. H., et al. (2018). Direct 3D printing of high strength biohybrid gradient hydrogel scaffolds for efficient repair of osteochondral defect. Adv. Funct. Mater. 28 13 10.1002/adfm.201706644 [DOI] [Google Scholar]
- Gao G., Hubbell K., Schilling A. F., Dai G., Cui X. (2017). “Bioprinting cartilage tissue from mesenchymal stem cells and PEG hydrogel,” in 3D cell Culture: Methods and Protocols, ed. Koledova Z. (New York, NY: Springer New York; ), 391–398. [DOI] [PubMed] [Google Scholar]
- Gao L., Huang Z., Lin H., Tian Y., Li P., Lin S. (2018). Bone marrow mesenchymal stem cells (BMSCs) restore functional endometrium in the rat model for severe asherman syndrome. Reprod. Sci. 26 436–444. 10.1177/1933719118799201 [DOI] [PubMed] [Google Scholar]
- Gao P., Yang H., Rajashankar K. R., Huang Z., Patel D. J. (2016). Type V CRISPR-Cas Cpf1 endonuclease employs a unique mechanism for crRNA-mediated target DNA recognition. Cell Res. 26:901. 10.1038/cr.2016.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Q., Liu Z., Lin Z., Qiu J., Liu Y., Liu A., et al. (2017). 3D Bioprinting of vessel-like structures with multilevel fluidic channels. ACS Biomater. Sci. Eng. 3 399–408. 10.1021/acsbiomaterials.6b00643 [DOI] [PubMed] [Google Scholar]
- Garbern J. C., Lee R. T. (2013). Cardiac stem cell therapy and the promise of heart regeneration. Cell Stem Cell 12 689–698. 10.1016/j.stem.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong W. H., Lei D., Li S., Huang P., Qi Q., Sun Y. J., et al. (2016). Hybrid small-diameter vascular grafts: anti-expansion effect of electrospun poly epsilon-caprolactone on heparin-coated decellularized matrices. Biomaterials 76 359–370. 10.1016/j.biomaterials.2015.10.066 [DOI] [PubMed] [Google Scholar]
- Gu J., Hu W., Deng A., Zhao Q., Lu S., Gu X. (2012). Surgical repair of a 30 mm long human median nerve defect in the distal forearm by implantation of a chitosan–PGA nerve guidance conduit. J. Tissue Eng. Regen. Med. 6 163–168. 10.1002/term.407 [DOI] [PubMed] [Google Scholar]
- Gu X. (2015). Progress and perspectives of neural tissue engineering. Front. Med. 9:401–411. 10.1007/s11684-015-0415-x [DOI] [PubMed] [Google Scholar]
- Gu X., Ding F., Williams D. F. (2014). Neural tissue engineering options for peripheral nerve regeneration. Biomaterials 35 6143–6156. 10.1016/j.biomaterials.2014.04.064 [DOI] [PubMed] [Google Scholar]
- Gu X., Ding F., Yang Y., Liu J. (2011). Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog. Neurobiol. 93 204–230. 10.1016/j.pneurobio.2010.11.002 [DOI] [PubMed] [Google Scholar]
- Gu Y., Zhu J., Xue C., Li Z., Ding F., Yang Y., et al. (2014). Chitosan/silk fibroin-based, Schwann cell-derived extracellular matrix-modified scaffolds for bridging rat sciatic nerve gaps. Biomaterials 35 2253–2263. 10.1016/j.biomaterials.2013.11.087 [DOI] [PubMed] [Google Scholar]
- Guo B., Pan G., Guo Q., Zhu C., Cui W., Li B., et al. (2015). Crides and temperature dual-responsive hydrogel layers for harvesting cell sheets. Chem. Commun. 51 644–647. 10.1039/c4cc08183h [DOI] [PubMed] [Google Scholar]
- Guo M., Pitet L. M., Wyss H. M., Vos M., Dankers P. Y., Meijer E. W. (2014). Tough stimuli-responsive supramolecular hydrogels with hydrogen-bonding network junctions. J. Am. Chem. Soc. 136 6969–6977. 10.1021/ja500205v [DOI] [PubMed] [Google Scholar]
- Guo X.-M., Zhao Y. S., Chang H. X., Wang C. Y., Ling-Ling E., Zhang X. A., et al. (2006). Creation of engineered cardiac tissue in vitro from mouse embryonic stem cells. Circulation 113 2229–2237. 10.1161/CIRCULATIONAHA.105.583039 [DOI] [PubMed] [Google Scholar]
- Guo Y., Wang Y., Liu Y., Wang H., Guo C., Zhang X. (2015). Effect of the same mechanical loading on osteogenesis and osteoclastogenesis in vitro. Chin. J. Traumatol. 18 150–156. [DOI] [PubMed] [Google Scholar]
- Hamanaka S., Yamaguchi T., Kobayashi T., Kato-Itoh M., Yamazaki S., Sato H., et al. (2011). Generation of germline-competent rat induced pluripotent stem cells. PLoS One 6:e22008. 10.1371/journal.pone.0022008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han F., Hu Y., Li J., Gong J., Guo Q., Zhu C., et al. (2019). In situ silk fibroin-mediated crystal formation of octacalcium phosphate and its application in bone repair. Mater. Sci. Eng. C 95 1–10. 10.1016/j.msec.2018.10.041 [DOI] [PubMed] [Google Scholar]
- Han F. X., Jia X. L., Dai D. D., Yang X. L., Zhao J., Zhao Y. H., et al. (2013). Performance of a multilayered small-diameter vascular scaffold dual-loaded with VEGF and PDGF. Biomaterials 34 7302–7313. 10.1016/j.biomaterials.2013.06.006 [DOI] [PubMed] [Google Scholar]
- Han J. P., Liang C. Y., Cui Y. C., Xiong L. K., Guo X. C., Yuan X. Y., et al. (2018). Encapsulating microorganisms inside electrospun microfibers as a living material enables room-temperature storage of microorganisms. ACS Appl. Mater. Interfaces 10 38799–38806. 10.1021/acsami.8b14978 [DOI] [PubMed] [Google Scholar]
- Han L., Wang M. H., Li P. F., Gan D. L., Yan L. W., Xu J. L., et al. (2018). Mussel-inspired tissue-adhesive hydrogel based on the polydopamine-condroitin sulfate complex for growth-factor-free cartilage regeneration. ACS Appl. Mater. Interfaces 10 28015–28026. 10.1021/acsami.8b05314 [DOI] [PubMed] [Google Scholar]
- Han S., Yin W., Li X., Wu S., Cao Y., Tan J., et al. (2019). Pre-clinical evaluation of CBD-NT3 modified collagen scaffolds in completely spinal cord transected non-human primates. J. Neurotrauma 36 2316–2324. 10.1089/neu.2018.6078 [DOI] [PubMed] [Google Scholar]
- Hannachi I. E., Yamato M., Okano T. (2009). Cell sheet technology and cell patterning for biofabrication. Biofabrication 1:022002. 10.1088/1758-5082/1/2/022002 [DOI] [PubMed] [Google Scholar]
- Hao T., Li J. J., Yao F. L., Dong D. Y., Wang Y., Yang B. G., et al. (2017). Injectable fullerenol/alginate hydrogel for suppression of oxidative stress damage in brown adipose-derived stem cells and cardiac repair. ACS Nano 11 5474–5488. 10.1021/acsnano.7b00221 [DOI] [PubMed] [Google Scholar]
- Hao Y. L., Li S. J., Sun B. B., Sui M. L., Yang R. (2007a). Ductile titanium alloy with low poisson’s ratio. Phys. Rev. Lett. 98:216405. 10.1103/PhysRevLett.98.216405 [DOI] [PubMed] [Google Scholar]
- Hao Y. L., Li S. J., Sun S. Y., Zheng C. Y., Yang R. (2007b). Elastic deformation behaviour of Ti–24Nb–4Zr–7.9Sn for biomedical applications. Acta Biomater. 3 277–286. 10.1016/j.actbio.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Haraguchi Y., Shimizu T., Sasagawa T., Sekine H., Sakaguchi K., Kikuchi T., et al. (2012). Fabrication of functional three-dimensional tissues by stacking cell sheets in vitro. Nat. Protoc. 7:850. 10.1038/nprot.2012.027 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Funamoto S., Sasaki S., Negishi J., Hattori S., Honda T., et al. (2019). Re-epithelialization and remodeling of decellularized corneal matrix in a rabbit corneal epithelial wound model. Mater. Sci. Eng. C-Mater. Biol. Appl. 102 238–246. 10.1016/j.msec.2019.04.024 [DOI] [PubMed] [Google Scholar]
- He A. J., Liu L. N., Luo X. S., Liu Y., Liu Y., Liu F. J., et al. (2017). Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci. Rep. 7:12. 10.1038/srep40489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B., Zhu Q., Chai Y., Ding X., Tang J., Gu L., et al. (2015). Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: a prospective, multicentre controlled clinical trial. J. Tissue Eng. Regen. Med. 9 286–295. 10.1002/term.1707 [DOI] [PubMed] [Google Scholar]
- He F., Bhoobalan-Chitty Y., Van L. B., Kjeldsen A. L., Dedola M., Makarova K. S., et al. (2018). Anti-CRISPR proteins encoded by archaeal lytic viruses inhibit subtype I-D immunity. Nat. Microbiol. 3 461–469. 10.1038/s41564-018-0120-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Xue G. H., Fu J. Z. (2014). Fabrication of low cost soft tissue prostheses with the desktop 3D printer. Sci. Rep. 4:6973. 10.1038/srep06973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench L. L. (2006). The story of bioglass. J. Mater. Sci. Mater. Med. 17 967–978. 10.1007/s10856-006-0432-z [DOI] [PubMed] [Google Scholar]
- Heo M., Lee S. J., Lee D., Heo D. N., Lee J. S., Youn Y. H., et al. (2017). Preparation of mechanically enhanced hydrogel scaffolds by incorporating interfacial polymer nanorods for nerve electrode application. Fibers Polym. 18 2248–2254. 10.1007/s12221-017-7456-7455 [DOI] [Google Scholar]
- Hiromoto S., Yamazaki T. (2017). Micromorphological effect of calcium phosphate coating on compatibility of magnesium alloy with osteoblast. Sci. Technol. Adv. Mater. 18 96–109. 10.1080/14686996.2016.1266238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang Thi T. T., Lee Y., Le Thi P., Park K. D. (2018). Nitric oxide-releasing injectable hydrogels with high antibacterial activity through in situ formation of peroxynitrite. Acta Biomater. 67 66–78. 10.1016/j.actbio.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Hong Y., Yu M., Weng W., Cheng K., Wang H., Lin J. (2013). Light-induced cell detachment for cell sheet technology. Biomaterials 34 11–18. 10.1016/j.biomaterials.2012.09.043 [DOI] [PubMed] [Google Scholar]
- Horiuchi S., Hiasa M., Yasue A., Sekine K., Hamada K., Asaoka K., et al. (2014). Fabrications of zinc-releasing biocement combining zinc calcium phosphate to calcium phosphate cement. J. Mech. Behav. Biomed. Mater. 29 151–160. 10.1016/j.jmbbm.2013.09.005 [DOI] [PubMed] [Google Scholar]
- Hou S., Xu Q., Tian W., Cui F., Cai Q., Ma J., et al. (2005). The repair of brain lesion by implantation of hyaluronic acid hydrogels modified with laminin. J. Neurosci. Methods 148 60–70. 10.1016/j.jneumeth.2005.04.016 [DOI] [PubMed] [Google Scholar]
- Hsiao M. Y., Lin A. C., Liao W. H., Wang T. G., Hsu C. H., Chen W. S., et al. (2019). Drug-loaded hyaluronic acid hydrogel as a sustained-release regimen with dual effects in early intervention of tendinopathy. Sci. Rep. 9:4784. 10.1038/s41598-019-41410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu L. C., Peng B. Y., Chen M. S., Thalib B., Ruslin M., Tung T. D. X., et al. (2019). The potential of the stem cells composite hydrogel wound dressings for promoting wound healing and skin regeneration: in vitro and in vivo evaluation. J. Biomed. Mater. Res. Part B-Appl. Biomater. 107 278–285. 10.1002/jbm.b.34118 [DOI] [PubMed] [Google Scholar]
- Hu M., Deng R., Schumacher K. M., Kurisawa M., Ye H., Purnamawati K., et al. (2010). Hydrodynamic spinning of hydrogel fibers. Biomaterials 31 863–869. 10.1016/j.biomaterials.2009.10.002 [DOI] [PubMed] [Google Scholar]
- Hu N., Wu H., Xue C., Gong Y., Wu J., Xiao Z., et al. (2013). Long-term outcome of the repair of 50 mm long median nerve defects in rhesus monkeys with marrow mesenchymal stem cells-containing, chitosan-based tissue engineered nerve grafts. Biomaterials 34 100–111. 10.1016/j.biomaterials.2012.09.020 [DOI] [PubMed] [Google Scholar]
- Hu X., Lui W., Cui L., Wang M., Cao Y. (2005). Tissue engineering of nearly transparent corneal stroma. Tissue Eng. 11 1710–1717. 10.1089/ten.2005.11.1710 [DOI] [PubMed] [Google Scholar]
- Huang C. Y., Huang T. H., Kao C. T., Wu Y. H., Chen W. C., Shie M. Y. (2017). Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J. Endod. 43 69–76. 10.1016/j.joen.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Huang K., Yan Z. Q., Zhao D., Chen S. G., Gao L. Z., Zhang P., et al. (2015). SIRT1 and FOXO mediate contractile differentiation of vascular smooth muscle cells under cyclic stretch. Cell. Physiol. Biochem. 37 1817–1829. 10.1159/000438544 [DOI] [PubMed] [Google Scholar]
- Huang L., Wang X., Cao H., Li L., Chow D. H. K., Tian L., et al. (2018). A bone-targeting delivery system carrying osteogenic phytomolecule icaritin prevents osteoporosis in mice. Biomaterials 182 58–71. 10.1016/j.biomaterials.2018.07.046 [DOI] [PubMed] [Google Scholar]
- Huang S., Xu Y., Wu C., Sha D., Fu X. (2010). In vitro constitution and in vivo implantation of engineered skin constructs with sweat glands. Biomaterials 31 5520–5525. 10.1016/j.biomaterials.2010.03.060 [DOI] [PubMed] [Google Scholar]
- Huang S., Yao B., Xie J., Fu X. (2016). 3D bioprinted extracellular matrix mimics facilitate directed differentiation of epithelial progenitors for sweat gland regeneration. Acta Biomater. 32 170–177. 10.1016/j.actbio.2015.12.039 [DOI] [PubMed] [Google Scholar]
- Huang S., Zhang Y., Tang L., Deng Z., Lu W., Feng F., et al. (2009). Functional bilayered skin substitute constructed by tissue-engineered extracellular matrix and microsphere-incorporated gelatin hydrogel for wound repair. Tissue Eng. Part A 15 2617–2624. 10.1089/ten.tea.2008.0505 [DOI] [PubMed] [Google Scholar]
- Huang Y., Jia X., Bai K., Gong X., Fan Y. (2010). Effect of fluid shear stress on cardiomyogenic differentiation of rat bone marrow mesenchymal stem cells. Arch. Med. Res. 41 497–505. 10.1016/j.arcmed.2010.10.002 [DOI] [PubMed] [Google Scholar]
- Huang Y., Jin X., Zhang X., Sun H., Tu J., Tang T., et al. (2009). In vitro and in vivo evaluation of akermanite bioceramics for bone regeneration. Biomaterials 30 5041–5048. 10.1016/j.biomaterials.2009.05.077 [DOI] [PubMed] [Google Scholar]
- Hvistendahl M. (2012). China’s push in tissue engineering. Science 338:900. 10.1126/science.338.6109.900 [DOI] [PubMed] [Google Scholar]
- Hwang C. M., Lee B. K., Green D., Jeong S. Y., Khang G., Jackson J. D., et al. (2014). Auricular reconstruction using tissue- engineered alloplastic implants for improved clinical outcomes. Plast. Reconstr. Surg 133 360E–369E. 10.1097/01.prs.0000438460.68098.4b [DOI] [PubMed] [Google Scholar]
- Ieda M., Fu J. D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B. G., et al. (2010). Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 142 375–386. 10.1016/j.cell.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igami T., Nakamura Y., Hirose T., Ebata T., Yokoyama Y., Sugawara G., et al. (2014). Application of a three-dimensional print of a liver in hepatectomy for small tumors invisible by intraoperative ultrasonography: preliminary experience. World J. Surg. 38 3163–3166. 10.1007/s00268-014-2740-2747 [DOI] [PubMed] [Google Scholar]
- Igami T., Nakamura Y., Oda M., Tanaka H., Nojiri M., Ebata T., et al. (2018). Application of three-dimensional print in minor hepatectomy following liver partition between anterior and posterior sectors. ANZ J. Surg. 88 882–885. 10.1111/ans.14331 [DOI] [PubMed] [Google Scholar]
- Ikeda E., Morita R., Nakao K., Ishida K., Nakamura T., Takano-Yamamoto T., et al. (2009). Fully functional bioengineered tooth replacement as an organ replacement therapy. Proc. of the Natl. Acad. Sci. U. S. A. 106 13475–13480. 10.1073/pnas.0902944106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im H., Kim S. H., Kim S. H., Jung Y. (2018). Skin regeneration with a scaffold of predefined shape and bioactive peptide hydrogels. Tissue Eng. Part A 24 1518–1530. 10.1089/ten.tea.2017.0489 [DOI] [PubMed] [Google Scholar]
- Inaba R., Khademhosseini A., Suzuki H., Fukuda J. (2009). Electrochemical desorption of self-assembled monolayers for engineering cellular tissues. Biomaterials 30 3573–3579. 10.1016/j.biomaterials.2009.03.045 [DOI] [PubMed] [Google Scholar]
- Investigators S., Yusuf S., Pitt B., Davis C. E., Hood W. B., Cohn J. N. (1991). Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N. Engl. J. Med. 325 293–302. 10.1056/NEJM199108013250501 [DOI] [PubMed] [Google Scholar]
- Ishiguro H., Kaito T., Yarimitsu S., Hashimoto K., Okada R., Kushioka J., et al. (2019). Intervertebral disc regeneration with an adipose mesenchymal stem cell-derived tissue-engineered construct in a rat nucleotomy model. Acta Biomater. 87 118–129. 10.1016/j.actbio.2019.01.050 [DOI] [PubMed] [Google Scholar]
- Isoda M., Kohyama J., Iwanami A., Sanosaka T., Sugai K., Yamaguchi R., et al. (2016). Robust production of human neural cells by establishing neuroepithelial-like stem cells from peripheral blood mononuclear cell-derived feeder-free iPSCs under xeno-free conditions. Neurosci. Res. 110 18–28. 10.1016/j.neures.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Itakura M., Nakamura H., Kitagaki T., Hoshino T., Machida M. (2019). First-principles calculation of mechanical properties of simulated debris ZrxU1-xO2. J. Nucl. Sci. Technol. 56 915–921. 10.1080/00223131.2019.1604271 [DOI] [Google Scholar]
- Iwami K., Noda T., Ishida K., Morishima K., Nakamura M., Umeda N. (2010). Bio rapid prototyping by extruding/aspirating/refilling thermoreversible hydrogel. Biofabrication 2:014108. 10.1088/1758-5082/2/1/014108 [DOI] [PubMed] [Google Scholar]
- Iwata T., Washio K., Yoshida T., Ishikawa I., Ando T., Yamato M., et al. (2015). Cell sheet engineering and its application for periodontal regeneration. J. Tissue Eng. Regen. Med. 9 343–356. 10.1002/term.1785 [DOI] [PubMed] [Google Scholar]
- Jang J., Park H. J., Kim S. W., Kim H., Park J. Y., Na S. J., et al. (2017). 3D printed complex tissue construct using stem cell-laden decellularized extracellular matrix bioinks for cardiac repair. Biomaterials 112 264–274. 10.1016/j.biomaterials.2016.10.026 [DOI] [PubMed] [Google Scholar]
- Jangamreddy J. R., Haagdorens M. K. C., Islam M. M., Lewis P., Samanta A., Fagerholm P., et al. (2018). Short peptide analogs as alternatives to collagen in pro-regenerative corneal implants. Acta Biomater. 69 120–130. 10.1016/j.actbio.2018.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Han F., Wang H., Zhu C., Guo Q., Li J., et al. (2019a). Polydopamine-assisted surface modification for orthopaedic implants. J. Orthop. Translat. 17 82–95. 10.1016/j.jot.2019.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L., Han F., Yang H., Turnbull G., Wang J., Clarke J., et al. (2019b). Microfluidic fabrication of biomimetic helical hydrogel microfibers for blood-vessel-on-a-chip applications. Adv. Healthc. Mater. 8:1900435. 10.1002/adhm.201900435 [DOI] [PubMed] [Google Scholar]
- Jia L. X., Zhang W. M., Zhang H. J., Li T. T., Wang Y. L., Qin Y. W., et al. (2015). Mechanical stretch-induced endoplasmic reticulum stress, apoptosis and inflammation contribute to thoracic aortic aneurysm and dissection. J. Pathol. 236 373–383. 10.1002/path.4534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Gungor-Ozkerim P. S., Zhang Y. S., Yue K., Zhu K., Liu W., et al. (2016). Direct 3D bioprinting of perfusable vascular constructs using a blend bioink. Biomaterials 106 58–68. 10.1016/j.biomaterials.2016.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y., Cai Y., Zhang W., Yin Z., Hu C., Tong T., et al. (2016). Human cartilage-derived progenitor cells from committed chondrocytes for efficient cartilage repair and regeneration. Stem Cell Transl. Med. 5 733–744. 10.5966/sctm.2015-2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo Y. K., Choi B. H., Kim C. S., Cha H. J. (2017). Diatom-inspired silica nanostructure coatings with controllable microroughness using an engineered mussel protein glue to accelerate bone growth on titanium-based implants. Adv. Mater. 29:1704906. 10.1002/adma.201704906 [DOI] [PubMed] [Google Scholar]
- Jung J. W., Lee J. S., Cho D. W. (2016). Computer-aided multiple-head 3D printing system for printing of heterogeneous organ/tissue constructs. Sci. Rep. 6:21685. 10.1038/srep21685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kada S., Hamaguchi K., Ito J., Omori K., Nakagawa T. (2019). Bone marrow stromal cells accelerate hearing recovery via regeneration or maintenance of cochlear fibrocytes in mouse Spiral Ligaments. Anat. Rec. 303 478–486. 10.1002/ar.24063 [DOI] [PubMed] [Google Scholar]
- Kang H., Zhang K., Pan Q., Lin S., Wong D. S. H., Li J., et al. (2018a). Remote control of intracellular calcium using upconversion nanotransducers regulates stem cell differentiation in vivo. Adv. Funct. Mater. 28:1802642 10.1002/adfm.201802642 [DOI] [Google Scholar]
- Kang H., Zhang K., Wong D. S. H., Han F., Li B., Bian L. (2018b). Near-infrared light-controlled regulation of intracellular calcium to modulate macrophage polarization. Biomaterials 178 681–696. 10.1016/j.biomaterials.2018.03.007 [DOI] [PubMed] [Google Scholar]
- Kang L., Wang J., Zhang Y., Kou Z., Gao S. (2009). iPS cells can support full-term development of tetraploid blastocyst-complemented embryos. Cell Stem Cell 5 135–138. 10.1016/j.stem.2009.07.001 [DOI] [PubMed] [Google Scholar]
- Kang X., He W., Huang Y., Yu Q., Chen Y., Gao X., et al. (2016). Introducing precise genetic modifications into human 3PN embryos by CRISPR/Cas-mediated genome editing. J. Assist. Reprod. Genet. 33 581–588. 10.1007/s10815-016-0710-718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Z., Zhang X., Chen Y., Akram M. Y., Nie J., Zhu X. (2017). Preparation of polymer/calcium phosphate porous composite as bone tissue scaffolds. Mater. Sci. Eng C 70 1125–1131. 10.1016/j.msec.2016.04.008 [DOI] [PubMed] [Google Scholar]
- Khoury M., Alcayaga-Miranda F., Illanes S. E., Figueroa F. E. (2014). The promising potential of menstrual stem cells for antenatal diagnosis and cell therapy. Front. Immunol. 5:205. 10.3389/fimmu.2014.00205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K., Masuda T., Fujiwara N., Kuji A., Miura H., Jung H. S., et al. (2018). Craniofacial bone regeneration using iPS cell-derived neural crest like cells. J. Hard Tissue Biol. 27 1–10. 10.2485/jhtb.27.1 [DOI] [Google Scholar]
- Kim B. S., Kwon Y. W., Kong J. S., Park G. T., Gao G., Han W., et al. (2018). 3D cell printing of in vitro stabilized skin model and in vivo pre-vascularized skin patch using tissue-specific extracellular matrix bioink: a step towards advanced skin tissue engineering. Biomaterials 168 38–53. 10.1016/j.biomaterials.2018.03.040 [DOI] [PubMed] [Google Scholar]
- Kim B. S., Lee J. S., Gao G., Cho D. W. (2017). Direct 3D cell-printing of human skin with functional transwell system. Biofabrication 9:025034. 10.1088/1758-5090/aa71c8 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Kim S. Y., Woo S. M., Jeong H. N., Jung J. Y., Kim S. M., et al. (2019). Combination of mineral trioxide aggregate and propolis promotes odontoblastic differentiation of human dental pulp stem cells through ERK signaling pathway. Food Sci. Biotechnol. 28 1801–1809. 10.1007/s10068-019-00609-605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. Y., Chun S. Y., Park J. S., Chung J. W., Ha Y. S., Lee J. N., et al. (2018). Laminin and platelet-derived growth factor-BB promote neuronal differentiation of human urine-derived stem cells. Tissue Eng. Regen. Med. 15 195–209. 10.1007/s13770-017-0102-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P. H., Yim H. G., Choi Y. J., Kang B. J., Kim J., Kwon S. M., et al. (2014). Injectable multifunctional microgel encapsulating outgrowth endothelial cells and growth factors for enhanced neovascularization. J. Control. Release 187 1–13. 10.1016/j.jconrel.2014.05.010 [DOI] [PubMed] [Google Scholar]
- Kim S. H., An Y. H., Kim H. D., Kim K., Lee S. H., Yim H. G., et al. (2018). Enzyme-mediated tissue adhesive hydrogels for meniscus repair. Int. J. Biol. Macromol. 110 479–487. 10.1016/j.ijbiomac.2017.12.053 [DOI] [PubMed] [Google Scholar]
- Kim S., Lee H., Chung M., Jeon N. L. (2013). Engineering of functional, perfusable 3D microvascular networks on a chip. Lab Chip 13 1489–1500. 10.1039/C3LC41320A [DOI] [PubMed] [Google Scholar]
- Kim S., Park C., Cheon K. H., Jung H. D., Song J., Kim H. E., et al. (2018). Antibacterial and bioactive properties of stabilized silver on titanium with a nanostructured surface for dental applications. Appl. Surf. Sci. 451 232–240. 10.1016/j.apsusc.2018.04.270 [DOI] [Google Scholar]
- Kim S. M., Park K. S., Lih E., Hong Y. J., Kang J. H., Kim I. H., et al. (2016). Fabrication and characteristics of dual functionalized vascular stent by spatio-temporal coating. Acta Biomater. 38 143–152. 10.1016/j.actbio.2016.04.029 [DOI] [PubMed] [Google Scholar]
- Kim S. Y., Lee S. M., Lee J. H. (2019). Initial cytotoxicity of mineral trioxide aggregate (MTA) during setting on human mesenchymal stem cells. Adv. Mater. Sci. Eng. 2019:7 10.1155/2019/2365104 [DOI] [Google Scholar]
- Kim V. N. (2018). RNA-targeting CRISPR comes of age. Nat. Biotechnol. 36 44–45. 10.1038/nbt.4054 [DOI] [PubMed] [Google Scholar]
- Kobayashi J., Kikuchi A., Aoyagi T., Okano T. (2019). Cell sheet tissue engineering: cell sheet preparation, harvesting/manipulation, and transplantation. J. Biomed.Mater. Res. Part A 107 955–967. 10.1002/jbm.a.36627 [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Kan K., Nishida K., Yamato M., Okano T. (2013). Corneal regeneration by transplantation of corneal epithelial cell sheets fabricated with automated cell culture system in rabbit model. Biomaterials 34 9010–9017. 10.1016/j.biomaterials.2013.07.065 [DOI] [PubMed] [Google Scholar]
- Koike M., Nojiri H., Ozawa Y., Watanabe K., Muramatsu Y., Kaneko H., et al. (2015). Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 5:11722. 10.1038/srep11722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor A. C., Badran A. H., Liu D. R. (2017). CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 168 20–36. 10.1016/j.cell.2016.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo M. A., Lee M. H., Kwon B. J., Seon G. M., Kim M. S., Kim D., et al. (2018). Exogenous ROS-induced cell sheet transfer based on hematoporphyrin-polyketone film via a one-step process. Biomaterials 161 47–56. 10.1016/j.biomaterials.2018.01.030 [DOI] [PubMed] [Google Scholar]
- Koo M. A., Hong S. H., Lee M. H., Kwon B. J., Seon G. M., Kim M. S., et al. (2019). Effective stacking and transplantation of stem cell sheets using exogenous ROS-producing film for accelerated wound healing. Acta Biomater. 95 418–426. 10.1016/j.actbio.2019.01.019 [DOI] [PubMed] [Google Scholar]
- Kowsari-Esfahan R., Jahanbakhsh A., Saidi M. S., Bonakdar S. (2018). A microfabricated platform for the study of chondrogenesis under different compressive loads. J. Mech. Behav. Biomed. Mater. 78 404–413. 10.1016/j.jmbbm.2017.12.002 [DOI] [PubMed] [Google Scholar]
- Kuo T. F., Lu H. C., Tseng C. F., Yang J. C., Wang S. F., Yang T. C. K., et al. (2017). Evaluation of osseointegration in titanium and zirconia-based dental implants with surface modification in a miniature pig model. J. Med. Biol. Eng. 37 313–320. 10.1007/s40846-017-0230-238 [DOI] [Google Scholar]
- Kuo Y. J., Wu L. C., Sun J. S., Chen M. H., Sun M. G., Tsuang Y. H. (2014). Mechanical stress-induced apoptosis of nucleus pulposus cells: an in vitro and in vivo rat model. J. Orthop. Sci. 19 313–322. 10.1007/s00776-013-0510-512 [DOI] [PubMed] [Google Scholar]
- Kurakawa T., Kakutani K., Morita Y., Kato Y., Yurube T., Hirata H., et al. (2015). Functional impact of integrin alpha5beta1 on the homeostasis of intervertebral discs: a study of mechanotransduction pathways using a novel dynamic loading organ culture system. Spine J. 15 417–426. 10.1016/j.spinee.2014.12.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Kabata T., Hayashi K., Maeda T., Kajino Y., Iwai S., et al. (2015). The paracrine effect of adipose-derived stem cells inhibits osteoarthritis progression. BMC Musculoskelet. Disord. 16:236 10.1186/s12891-015-0701-704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lü S., Wang H., Lu W., Liu S., Lin Q., Li D., et al. (2009). Both the transplantation of somatic cell nuclear transfer- and fertilization-derived mouse embryonic stem cells with temperature-responsive chitosan hydrogel improve myocardial performance in infarcted rat hearts. Tissue Eng. Part A 16 1303–1315. 10.1089/ten.tea.2009.0434 [DOI] [PubMed] [Google Scholar]
- Lai Y. X., Li Y., Cao H. J., Long J., Wang X. L., Li L., et al. (2019). Osteogenic magnesium incorporated into PLGA/TCP porous scaffold by 3D printing for repairing challenging bone defect. Biomaterials 197 207–219. 10.1016/j.biomaterials.2019.01.013 [DOI] [PubMed] [Google Scholar]
- Law J. X., Chowdhury S. R., Aminuddin B. S., Ruszymah B. H. I. (2017). Role of plasma-derived fibrin on keratinocyte and fibroblast wound healing. Cell Tissue Bank. 18 585–595. 10.1007/s10561-017-9645-9642 [DOI] [PubMed] [Google Scholar]
- Law J. X., Musa F., Ruszymah B. H. I., El Haj A. J., Yang Y. (2016). A comparative study of skin cell activities in collagen and fibrin constructs. Med. Eng. Phys. 38 854–861. 10.1016/j.medengphy.2016.05.017 [DOI] [PubMed] [Google Scholar]
- Le Thi P., Lee Y., Nguyen D. H., Park K. D. (2017). In situ forming gelatin hydrogels by dual-enzymatic cross-linking for enhanced tissue adhesiveness. J. Mater. Chem. B 5 757–764. 10.1039/C6TB02179D [DOI] [PubMed] [Google Scholar]
- Lee H. J., Fernandes-Cunha G. M., Na K. S., Hull S. M., Myung D. (2018). Bio-orthogonally crosslinked, in situ forming corneal stromal tissue substitute. Adv. Healthc. Mater. 7:e1800560. 10.1002/adhm.201800560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Hong J. M., Jung J. W., Shim J. H., Oh J. H., Cho D. W. (2014a). 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 6:024103. 10.1088/1758-5082/6/2/024103 [DOI] [PubMed] [Google Scholar]
- Lee J., Lee S. H., Kim B. S., Cho Y. S., Park Y. (2018). Development and evaluation of hyaluronic acid-based hybrid bio-ink for tissue regeneration. Tissue Eng. Regen. Med. 15 761–769. 10.1007/s13770-018-0144-148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. G., Park T. J., Soo S. Y., Wang K. W., Kim B. I. I., Park J. H., et al. (2010). Synthesis and utilization of E. coli-encapsulated PEG-based microdroplet using a microfluidic chip for biological application. Biotechnol. Bioeng 107 747–751. 10.1002/bit.22861 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Lee K. G., Hwang J. H., Cho Y. S., Lee K. S., Jeong H. J., et al. (2019). Evaluation of mechanical strength and bone regeneration ability of 3D printed kagome-structure scaffold using rabbit calvarial defect model. Mater. Sci. Eng. C 98 949–959. 10.1016/j.msec.2019.01.050 [DOI] [PubMed] [Google Scholar]
- Lee S. H., Bae E. B., Kim S. E., Yun Y. P., Kim H. J., Choi J. W., et al. (2018). Effects of immobilizations of rhBMP-2 and/or rhPDGF-BB on titanium iilant surfaces on osseointegration and bone regeneration. Coatings 8:17 10.3390/coatings8010017 [DOI] [Google Scholar]
- Lee S. H., Jun B. H. (2019). Advances in dynamic microphysiological organ-on-a-chip: design principle and its biomedical application. J. Ind. Eng. Chem. 71 65–77. 10.1016/j.jiec.2018.11.041 [DOI] [Google Scholar]
- Lee T. S., Lim S. Y., Pyon J. K., Mun G. H., Bang S. I., Oh K. S. (2010). Secondary revisions due to unfavourable results after microtia reconstruction. J. Plast. Reconstr. Aesthet. Surg. 63 940–946. 10.1016/j.bjps.2009.04.016 [DOI] [PubMed] [Google Scholar]
- Lee V. K., Kim D. Y., Ngo H., Lee Y., Seo L., Yoo S. S., et al. (2014b). Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 35 8092–8102. 10.1016/j.biomaterials.2014.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Choi K. H., Park K. M., Lee J. M., Park B. J., Park K. D. (2017). In situ forming and H2O2-releasing hydrogels for treatment of drug-resistant bacterial infections. ACS Appl. Mater. Interfaces 9 16890–16899. 10.1021/acsami.7b03870 [DOI] [PubMed] [Google Scholar]
- Lei D., Yang Y., Liu Z. H., Yang B. Q., Gong W. H., Chen S., et al. (2019). 3D printing of biomimetic vasculature for tissue regeneration. Mater. Horiz. 6 1197–1206. 10.1039/c9mh00174c [DOI] [Google Scholar]
- Li B., Chen D. (2019). Degenerative musculoskeletal diseases: pathology and treatments. J. Orthop. Transl. 17 1–2. 10.1016/j.jot.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. J., Cheng P., Liang M. K., Chen Y. S., Lu Q., Wang J. Y., et al. (2015). MicroRNA-188 regulates age-related switch between osteoblast and adipocyte differentiation. J. Clin. Invest. 125 1509–1522. 10.1172/JCI77716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Zhu L., Liu Y., Yin Z. Q., Liu Y., Liu F. J., et al. (2017). Stable subcutaneous cartilage regeneration of bone marrow stromal cells directed by chondrocyte sheet. Acta Biomater. 54 321–332. 10.1016/j.actbio.2017.03.031 [DOI] [PubMed] [Google Scholar]
- Li G., Che M. T., Zhang K., Qin L. N., Zhang Y.-T., Chen R. Q., et al. (2016). Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials 83 233–248. 10.1016/j.biomaterials.2015.11.059 [DOI] [PubMed] [Google Scholar]
- Li H.-H., Fu X.-B., Zhang L., Zhou G. (2008). Comparison of proliferating cells between human adult and fetal eccrine sweat glands. Arch. Dermato. Res. 300 173–176. 10.1007/s00403-007-0823-820 [DOI] [PubMed] [Google Scholar]
- Li H.-H., Zhou G., Fu X. B., Zhang L. (2009). Antigen expression of human eccrine sweat glands. J. Cutan. Pathol. 36 318–324. 10.1111/j.1600-0560.2008.01020.x [DOI] [PubMed] [Google Scholar]
- Li H., Chu Y., Zhang Z., Zhang G., Jiang L., Wu H., et al. (2012). Construction of bilayered tissue-engineered skin with human amniotic mesenchymal cells and human amniotic epithelial cells. Artif. Organs 36 911–919. 10.1111/j.1525-1594.2012.01461.x [DOI] [PubMed] [Google Scholar]
- Li J., Wang J., Zou Y., Zhang Y., Long D., Lei L., et al. (2012). The influence of delayed compressive stress on TGF-beta1-induced chondrogenic differentiation of rat BMSCs through Smad-dependent and Smad-independent pathways. Biomaterials 33 8395–8405. 10.1016/j.biomaterials.2012.08.019 [DOI] [PubMed] [Google Scholar]
- Li M., Ma J., Gao Y., Yang L. (2019). Cell sheet technology: a promising strategy in regenerative medicine. Cytotherapy 21 3–16. 10.1016/j.jcyt.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Li R., Liang L., Dou Y., Huang Z., Mo H., Wang Y., et al. (2015). Mechanical strain regulates osteogenic and adipogenic differentiation of bone marrow mesenchymal stem cells. Biomed. Res. Int. 2015 873251. 10.1155/2015/873251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S. Y., Wang X., Cao B., Ye K., Li Z. H., Ding J. D. (2015). Effects of nanoscale spatial arrangement of arginine-glycine-aspartate peptides on dedifferentiation of chondrocytes. Nano Lett. 15 7755–7765. 10.1021/acs.nanolett.5b04043 [DOI] [PubMed] [Google Scholar]
- Li T., Weng X. S., Bian Y. Y., Zhou L., Cui F. Z., Qiu Z. Y. (2015). Influence of nano-HA coated bone collagen to acrylic (polymethylmethacrylate) bone cement on mechanical properties and bioactivity. PLos One 10:e0129018. 10.1371/journal.pone.0129018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Wei W., Zhu S., Zhu J., Shi Y., Lin T., et al. (2009). Generation of rat and human induced pluripotent stem cells by combining genetic reprogramming and chemical inhibitors. Cell Stem Cell 4 16–19. 10.1016/j.stem.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Li X., Feng Q., Liu X., Dong W., Cui F. (2006). Collagen-based implants reinforced by chitin fibres in a goat shank bone defect model. Biomaterials 27 1917–1923. 10.1016/j.biomaterials.2005.11.013 [DOI] [PubMed] [Google Scholar]
- Li X., Han J., Zhao Y., Ding W., Wei J., Han S., et al. (2015). Functionalized collagen scaffold neutralizing the myelin-inhibitory molecules promoted neurites outgrowth in vitro and facilitated spinal cord regeneration in vivo. ACS Appl. Mater. Interfaces 7 13960–13971. 10.1021/acsami.5b03879 [DOI] [PubMed] [Google Scholar]
- Li X., Wang X., Jiang X., Yamaguchi M., Ito A., Bando Y., et al. (2016). Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 104 323–329. 10.1002/jbm.b.33391 [DOI] [PubMed] [Google Scholar]
- Li X., Yang Z., Zhang A., Wang T., Chen W. (2009). Repair of thoracic spinal cord injury by chitosan tube implantation in adult rats. Biomaterials 30 1121–1132. 10.1016/j.biomaterials.2008.10.063 [DOI] [PubMed] [Google Scholar]
- Li X. R., Fan C. X., Xiao Z. F., Zhao Y. N., Zhang H. M., Sun J., et al. (2018). A collagen microchannel scaffold carrying paclitaxel-liposomes induces neuronal differentiation of neural stem cells through Wnt/beta-catenin signaling for spinal cord injury repair. Biomaterials 183 114–127. 10.1016/j.biomaterials.2018.08.037 [DOI] [PubMed] [Google Scholar]
- Li Y., Liao C., Tjong C. S. (2019). Electrospun polyvinylidene fluoride-based fibrous scaffolds with piezoelectric characteristics for bone and neural tissue engineering. Nanomaterials 9 :E952. 10.3390/nano9070952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu L. N., Wan P., Zhai Z. J., Mao Z. Y., Ouyang Z. X., et al. (2016). Biodegradable Mg-Cu alloy implants with antibacterial activity for the treatment of osteomyelitis: in vitro and in vivo evaluations. Biomaterials 106 250–263. 10.1016/j.biomaterials.2016.08.031 [DOI] [PubMed] [Google Scholar]
- Li Y., Xiong J., Wong C. S., Hodgson P. D., Wen C. E. (2009). Ti6Ta4Sn alloy and subsequent scaffolding for bone tissue engineering. Tissue Eng. Part A 15 3151–3159. 10.1089/ten.tea.2009.0150 [DOI] [PubMed] [Google Scholar]
- Li Z., Huang S., Liu Y., Yao B., Hu T., Shi H., et al. (2018). Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci. Rep. 8:8020. 10.1038/s41598-018-26407-26403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Peng J., Wang G., Yang Q., Yu H., Guo Q., et al. (2008). Effects of local release of hepatocyte growth factor on peripheral nerve regeneration in acellular nerve grafts. Exp. Neurol. 214 47–54. 10.1016/j.expneurol.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Liang P., Xu Y., Zhang X., Ding C., Huang R., Zhang Z., et al. (2015). CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein Cell 6 363–372. 10.1007/s13238-015-0153-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Yin C., Lu X. I., Jiang H., Jin F. (2019). Bone marrow mesenchymal stem cells protect lungs from smoke inhalation injury by differentiating into alveolar epithelial cells via Notch signaling. J. Biosci. 44:2. 10.1007/s12038-018-9824-9828 [DOI] [PubMed] [Google Scholar]
- Liao S. S., Cui F. Z., Zhang W., Feng Q. L. (2004). Hierarchically biomimetic bone scaffold materials: nano-HA/collagen/PLA composite. J. Biomed. Mater. Res. Part B: Appl. Biomater. 69B 158–165. 10.1002/jbm.b.20035 [DOI] [PubMed] [Google Scholar]
- Lim C. T., Ren X., Afizah M. H., Tarigan-Panjaitan S., Yang Z., Wu Y., et al. (2013). Repair of osteochondral defects with rehydrated freeze-dried oligo[poly(ethylene glycol) fumarate] hydrogels seeded with bone marrow mesenchymal stem cells in a porcine model. Tissue Eng. Part A 19 1852–1861. 10.1089/ten.TEA.2012.0621 [DOI] [PubMed] [Google Scholar]
- Lin D., Chai Y. J., Ma Y. F., Duan B., Yuan Y., Liu C. S. (2019). Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials 196 122–137. 10.1016/j.biomaterials.2017.11.011 [DOI] [PubMed] [Google Scholar]
- Lin K., Wu C., Chang J. (2014). Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 10 4071–4102. 10.1016/j.actbio.2014.06.017 [DOI] [PubMed] [Google Scholar]
- Lin Q., Zhang W., Lu C., Hou G., Xu Z. (2014). In vivo investigation of biological responses to tricalcium silicate pastes in muscle tissue. Ceram. Int. 40 1879–1883. 10.1016/j.ceramint.2013.07.092 [DOI] [Google Scholar]
- Lin W. J., Qin L., Qi H. P., Zhang D. Y., Zhang G., Gao R. L., et al. (2017). Long-term in vivo corrosion behavior, biocompatibility and bioresorption mechanism of a bioresorbable nitrided iron scaffold. Acta Biomater. 54 454–468. 10.1016/j.actbio.2017.03.020 [DOI] [PubMed] [Google Scholar]
- Lin Z., Wu S., Liu X., Qian S., Chu P. K., Zheng Y., et al. (2019). A surface-engineered multifunctional TiO2 based nano-layer simultaneously elevates the corrosion resistance, osteoconductivity and antimicrobial property of a magnesium alloy. Acta Biomater. 99 495–513. 10.1016/j.actbio.2019.09.008 [DOI] [PubMed] [Google Scholar]
- Lin Z., Zhao Y., Chu P. K., Wang L., Pan H., Zheng Y., et al. (2019). A functionalized TiO2/Mg2TiO4 nano-layer on biodegradable magnesium implant enables superior bone-implant integration and bacterial disinfection. Biomaterials 219:119372. 10.1016/j.biomaterials.2019.119372 [DOI] [PubMed] [Google Scholar]
- Lindroos B., Suuronen R., Miettinen S. (2011). The potential of adipose stem cells in regenerative medicine. Stem Cell Rev. 7 269–291. 10.1007/s12015-010-9193-9197 [DOI] [PubMed] [Google Scholar]
- Liu B., Yao T. T., Ren L. X., Zhao Y. H., Yuan X. Y. (2018). Antibacterial PCL electrospun membranes containing synthetic polypeptides for biomedical purposes. Colloids Surf. B-Biointerfaces 172 330–337. 10.1016/j.colsurfb.2018.08.055 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhu C. H., Li J., Zhou P. H., Chen M., Yang H. L., et al. (2015). The effect of the fibre orientation of electrospun scaffolds on the matrix production of rabbit annulus fibrosus-derived stem cells. Bone Res. 3 15012. 10.1038/Boneres.2015.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F., Chen Q. H., Liu C., Ao Q., Tian X. H., Fan J., et al. (2018). Natural polymers for organ 3D bioprinting. Polymers 10:26. 10.3390/polym10111278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H., Zhu F., Yong J., Zhang P., Hou P., Li H., et al. (2008). Generation of induced pluripotent stem cells from adult rhesus monkey fibroblasts. Cell Stem Cell 3 587–590. 10.1016/j.stem.2008.10.014 [DOI] [PubMed] [Google Scholar]
- Liu H. H., Yang L., Zhang E. C., Zhang R., Cai D. D., Zhu S. A., et al. (2017). Biomimetic tendon extracellular matrix composite gradient scaffold enhances ligament-to-bone junction reconstruction. Acta Biomater. 56 129–140. 10.1016/j.actbio.2017.05.027 [DOI] [PubMed] [Google Scholar]
- Liu J., Wang X., Lu G., Tang J. Z., Wang Y., Zhang B., et al. (2019). Bionic cartilage acellular matrix microspheres as a scaffold for engineering cartilage. J. Mater. Chem. B 7 640–650. 10.1039/C8TB02999G [DOI] [PubMed] [Google Scholar]
- Liu K. I., Ramli M. N. B., Woo C. W. A., Wang Y., Zhao T., Zhang X., et al. (2016). A chemical-inducible CRISPR–Cas9 system for rapid control of genome editing. Nat. Chem. Biol. 12:980. 10.1038/nchembio.2179 [DOI] [PubMed] [Google Scholar]
- Liu L., Liu Y. Q., Feng C., Chang J., Fu R. Q., Wu T. T., et al. (2019). Lithium-containing biomaterials stimulate bone marrow stromal cell-derived exosomal miR-130a secretion to promote angiogenesis. Biomaterials 192 523–536. 10.1016/j.biomaterials.2018.11.007 [DOI] [PubMed] [Google Scholar]
- Liu P. X., Liu Y. J., Li P. L., Zhou Y. J., Song Y. Y., Shi Y., et al. (2018). Rosuvastatin- and heparin-loaded poly(L-lactide-co-caprolactone) nanofiber aneurysm stent promotes endothelialization via vascular endothelial growth factor type A modulation. ACS Appl. Mater. Interfaces 10 41012–41018. 10.1021/acsami.8b11714 [DOI] [PubMed] [Google Scholar]
- Liu S., Jiang L., Li H., Shi H., Luo H., Zhang Y., et al. (2014). Mesenchymal stem cells prevent hypertrophic scar formation via inflammatory regulation when undergoing apoptosis. J. Invest. Dermatol. 134 2648–2657. 10.1038/jid.2014.169 [DOI] [PubMed] [Google Scholar]
- Liu W., Wang Y., Gong F., Rong Y., Luo Y., Tang P., et al. (2019). Exosomes derived from bone mesenchymal stem cells repair traumatic spinal cord injury by suppressing the activation of A1 neurotoxic reactive astrocytes. J. Neurotrauma 36 469–484. 10.1089/neu.2018.5835 [DOI] [PubMed] [Google Scholar]
- Liu W., Yin L., Yan X. R., Cui J. H., Liu W. G., Rao Y., et al. (2017). Directing the differentiation of parthenogenetic stem cells into tenocytes for tissue-engineered tendon regeneration. Stem Cells Transl. Med. 6 196–208. 10.5966/sctm.2015-2334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Suwa F., Wang X., Takemura A., Fang Y. R., Li Y., et al. (2007). Reconstruction of a tissue-engineered skin containing melanocytes. Cell Biol. Int. 31 985–990. 10.1016/j.cellbi.2007.03.009 [DOI] [PubMed] [Google Scholar]
- Lu J., Sun X., Yin H., Shen X., Yang S., Wang Y., et al. (2018). A neurotrophic peptide-functionalized self-assembling peptide nanofiber hydrogel enhances rat sciatic nerve regeneration. Nano Res. 11 4599–4613. 10.1007/s12274-018-2041-2049 [DOI] [Google Scholar]
- Lu W., Yu J., Zhang Y., Ji K., Zhou Y., Li Y., et al. (2012). Mixture of fibroblasts and adipose tissue-derived stem cells can improve epidermal morphogenesis of tissue-engineered skin. Cells Tissues Organs 195 197–206. 10.1159/000324921 [DOI] [PubMed] [Google Scholar]
- Lu Y., Zhou Y., Zhang R., Wen L., Wu K., Li Y., et al. (2019). Bone mesenchymal stem cell-derived extracellular vesicles promote recovery following spinal cord injury via improvement of the integrity of the blood-spinal cord barrier. Front. Neurosci. 13:209. 10.3389/fnins.2019.break00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Z., Liu S., Le Y., Qin Z., He M., Xu F., et al. (2019). An injectable collagen-genipin-carbon dot hydrogel combined with photodynamic therapy to enhance chondrogenesis. Biomaterials 218:119190. 10.1016/j.biomaterials.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Luo H. L., Lu Y. B., Wu T. T., Zhang M., Zhang Y. J., Jin Y. (2013). Construction of tissue-engineered cornea composed of amniotic epithelial cells and acellular porcine cornea for treating corneal alkali burn. Biomaterials 34 6748–6759. 10.1016/j.biomaterials.2013.05.045 [DOI] [PubMed] [Google Scholar]
- Lv X. C., Shen L., Wu Y. Z., Ge L., Chen J. H., Yin J. S., et al. (2018). Healing score of the Xinsorb scaffold in the treatment of de novo lesions: 6-month imaging outcomes. Int. J. Cardiovasc. Imaging 34 1009–1016. 10.1007/s10554-018-1326-1320 [DOI] [PubMed] [Google Scholar]
- Lysaght M. J., Crager J. (2009). Origins. Tissue Eng. Part A 15 1449–1450. 10.1089/ten.tea.2007.0412 [DOI] [PubMed] [Google Scholar]
- Ma H. S., Zhou Q., Chang J., Wu C. T. (2019). Grape seed-inspired smart hydrogel scaffolds for melanoma therapy and wound healing. ACS Nano 13 4302–4311. 10.1021/acsnano.8b09496 [DOI] [PubMed] [Google Scholar]
- Ma X., He Z., Han F., Zhong Z., Chen L., Li B. (2016). Preparation of collagen/hydroxyapatite/alendronate hybrid hydrogels as potential scaffolds for bone regeneration. Colloids Surf. B: Biointerfaces 143 81–87. 10.1016/j.colsurfb.2016.03.025 [DOI] [PubMed] [Google Scholar]
- Ma X. Y., Zhang Y., Zhu D., Lu Y., Zhou G., Liu W., et al. (2015). Corneal stroma regeneration with acellular corneal stroma sheets and keratocytes in a rabbit model. PLoS One 10:e0132705. 10.1371/journal.pone.0132705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Li J., Yao Y., Wei D., Wang R., Wu Q. (2016). A controlled double-duration inducible gene expression system for cartilage tissue engineering. Sci. Rep. 6:26617. 10.1038/srep26617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y., Zhang W., Wang Z., Wang Z., Xie Q., Niu H., et al. (2016). PEGylated poly(glycerol sebacate)-modified calcium phosphate scaffolds with desirable mechanical behavior and enhanced osteogenic capacity. Acta Biomater. 44 110–124. 10.1016/j.actbio.2016.08.023 [DOI] [PubMed] [Google Scholar]
- Maeno S., Niki Y., Matsumoto H., Morioka H., Yatabe T., Funayama A., et al. (2005). The effect of calcium ion concentration on osteoblast viability, proliferation and differentiation in monolayer and 3D culture. Biomaterials 26 4847–4855. 10.1016/j.biomaterials.2005.01.006 [DOI] [PubMed] [Google Scholar]
- Mahla R. S. (2016). Stem cells applications in regenerative medicine and disease therapeutics. Int. J. Cell Biol. 2016:6940283. 10.1155/2016/6940283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai H. N., Kim D. Y., Hyun D. C., Park J. H., Lee S. M., Lee D. H. (2019). A new antibacterial agent-releasing polydimethylsiloxane coating for polymethyl methacrylate dental restorations. J. Clin. Med 8:E1831. 10.3390/jcm8111831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandakhbayar N., El-Fiqi A., Lee J. H., Kim H. W. (2019). Evaluation of strontium-doped nanobioactive glass cement for dentin-pulp complex regeneration therapy. ACS Biomater. Sci. Eng. 5 6117–6126. 10.1021/acsbiomaterials.9b01018 [DOI] [PubMed] [Google Scholar]
- Mao A. S., Mooney D. J. (2015). Regenerative medicine: current therapies and future directions. Proc. Natl. Acad. Sci. U.S.A. 112 14452–14459. 10.1073/pnas.1508520112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao Q., Nguyen P. D., Shanti R. M., Shi S., Shakoori P., Zhang Q., et al. (2018). Gingiva-derived mesenchymal stem cell-extracellular vesicles activate schwann cell repair phenotype and promote nerve regeneration. Tissue Eng. Part A 25 887–900. 10.1089/ten.tea.2018.0176 [DOI] [PubMed] [Google Scholar]
- Matai I., Kaur G., Seyedsalehi A., McClinton A., Laurencin C. T. (2020). Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 226:119536. 10.1016/j.biomaterials.2019.119536 [DOI] [PubMed] [Google Scholar]
- Meng X., Ichim T. E., Zhong J., Rogers A., Yin Z., Jackson J., et al. (2007). Endometrial regenerative cells: a novel stem cell population. J. Transl. Med. 5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S. (2016). Application of 3D printing in medicine. Indian Heart J. 68 108–109. 10.1016/j.ihj.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T., Nakamura T., Matsumura H., Ban S., Kobayashi T. (2013). Current status of zirconia restoration. J. Prosthodont. Res. 57 236–261. 10.1016/j.jpor.2013.09.001 [DOI] [PubMed] [Google Scholar]
- Mo L., Yang Z., Zhang A., Li X. (2010). The repair of the injured adult rat hippocampus with NT-3-chitosan carriers. Biomaterials 31 2184–2192. 10.1016/j.biomaterials.2009.11.078 [DOI] [PubMed] [Google Scholar]
- Mobini S., Song Y. H., McCrary M. W., Schmidt C. E. (2019). Advances in ex vivo models and lab-on-a-chip devices for neural tissue engineering. Biomaterials 198 146–166. 10.1016/j.biomaterials.2018.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinaga K., Sasaki H., Park S., Hokugo A., Okawa H., Tahara Y., et al. (2019). Neuronal PAS domain 2 (Npas2) facilitated osseointegration of titanium implant with rough surface through a neuroskeletal mechanism. Biomaterials 192 62–74. 10.1016/j.biomaterials.2018.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousa H. M., Abdal-hay A., Bartnikowski M., Mohamed I. M. A., Yasin A. S., Ivanovski S., et al. (2018). A multifunctional zinc oxide/poly(lactic acid) nanocomposite layer coated on magnesium alloys for controlled degradation and antibacterial function. ACS Biomater. Sci. Eng. 4 2169–2180. 10.1021/acsbiomaterials.8b00277 [DOI] [PubMed] [Google Scholar]
- Muthuramalingam K., Choi S. I., Hyun C., Kim Y. M., Cho M. (2019). beta-glucan-based wet dressing for cutaneous wound healing. Adv. Wound Care 8 125–135. 10.1089/wound.2018.0843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagoshi N., Okano H. (2018). iPSC-derived neural precursor cells: potential for cell transplantation therapy in spinal cord injury. Cell. Mol. Life Sci. 75 989–1000. 10.1007/s00018-017-2676-2679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., et al. (2007). Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 26 101–106. 10.1038/nbt1374 [DOI] [PubMed] [Google Scholar]
- Nakahata M., Takashima Y., Yamaguchi H., Harada A. (2011). Redox-responsive self-healing materials formed from host–guest polymers. Nat. Commun. 2:511. 10.1038/ncomms1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima R., Kobayashi T., Moriya N., Mizutani M., Kan K., Nozaki T., et al. (2015). A novel closed cell culture device for fabrication of corneal epithelial cell sheets. J. Tissue Eng. And Regen. Med. 9 1259–1267. 10.1002/term.1639 [DOI] [PubMed] [Google Scholar]
- Nakamura T., Nakano Y., Usami H., Okamura S., Wakabayashi K., Yatani H. (2019). In vitro investigation of fracture load and aging resistance of high-speed sintered monolithic tooth-borne zirconia crowns. J. Prosthodont. ResS1883-1958, 30344–X. 10.1016/j.jpor.2019.07.003 [DOI] [PubMed] [Google Scholar]
- Nam E., Fujita N., Morita M., Lin H. Y., Endo K., Nakagawa T., et al. (2018). A pilot study of transplantation of an autologous corneal epithelial cell sheet in a canine model of corneal injury. Jpn J. Vet. Res. 66 83–92. 10.14943/jjvr.66.2.83 [DOI] [Google Scholar]
- Nguyen L. H., Gao M., Lin J., Wu W., Wang J., Chew S. Y. (2017). Three-dimensional aligned nanofibers-hydrogel scaffold for controlled non-viral drug/gene delivery to direct axon regeneration in spinal cord injury treatment. Sci. Rep. 7 42212–42212. 10.1038/srep42212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinimaki S. (2012). The relationship between musculoskeletal stress markers and biomechanical properties of the humeral diaphysis. Am. J. Phys. Anthropol. 147 618–628. 10.1002/ajpa.22023 [DOI] [PubMed] [Google Scholar]
- Nishida K., Yamato M., Hayashida Y., Watanabe K., Maeda N., Watanabe H., et al. (2004). Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation 77 379–385. 10.1097/01.TP.0000110320.45678.30 [DOI] [PubMed] [Google Scholar]
- Notomi T., Karasaki I., Okazaki Y., Okimoto N., Kato Y., Ohura K., et al. (2014). Insulinogenic sucrose+amino acid mixture ingestion immediately after resistance exercise has an anabolic effect on bone compared with non-insulinogenic fructose+amino acid mixture in growing rats. Bone 65 42–48. 10.1016/j.bone.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Oh H. J., Kim S. H., Cho J. H., Park S. H., Min B. H. (2018). Mechanically reinforced extracellular matrix scaffold for application of cartilage tissue engineering. Tissue Eng. Regen. Med. 15 287–299. 10.1007/s13770-018-0114-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okita K., Ichisaka T., Yamanaka S. (2007). Generation of germline-competent induced pluripotent stem cells. Nature 448 313–317. 10.1038/nature05934 [DOI] [PubMed] [Google Scholar]
- Okuzu Y., Fujibayashi S., Yamaguchi S., Yamamoto K., Shimizu T., Sono T., et al. (2017). Strontium and magnesium ions released from bioactive titanium metal promote early bone bonding in a rabbit implant model. Acta Biomater. 63 383–392. 10.1016/j.actbio.2017.09.019 [DOI] [PubMed] [Google Scholar]
- Onoe H., Okitsu T., Itou A., Kato-Negishi M., Gojo R., Kiriya D., et al. (2013). Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Mater. 12 584–590. 10.1038/nmat3606 [DOI] [PubMed] [Google Scholar]
- Ouyang H. W., Goh J. C. H., Thambyah A., Teoh S. H., Lee E. H. (2003). Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 9 431–439. 10.1089/107632703322066615 [DOI] [PubMed] [Google Scholar]
- Ouyang H. W., Toh S. L., Goh J., Tay T. E., Moe K. (2005). Assembly of bone marrow stromal cell sheets with knitted poly (L-lactide) scaffold for engineering ligament analogs. J. Biomed. Mater. Res. Part B: Appl. Biomater. 75B 264–271. 10.1002/jbm.b.30281 [DOI] [PubMed] [Google Scholar]
- Pan G., Guo Q., Ma Y., Yang H., Li B. (2013). Thermo-responsive hydrogel layers imprinted with RGDS peptide: a system for harvesting cell sheets. Angew. Chem. Int. Ed. Engl. 52 6907–6911. 10.1002/anie.201300733 [DOI] [PubMed] [Google Scholar]
- Pan H. B., Zhao X. L., Zhang X., Zhang K. B., Li L. C., Li Z. Y., et al. (2010). Strontium borate glass: potential biomaterial for bone regeneration. J. R. Soc. Interface 7 1025–1031. 10.1098/rsif.2009.0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park C., Lee S. W., Kim J., Song E. H., Jung H. D., Park J. U., et al. (2019). Reduced fibrous capsule formation at nano-engineered silicone surfaces via tantalum ion implantation. Biomater. Sci. 7 2907–2919. 10.1039/c9bm00427k [DOI] [PubMed] [Google Scholar]
- Park D. Y., Min B. H., Lee H. J., Kim Y. J., Choi B. H. (2016). Repair of partial thickness cartilage defects using cartilage extracellular matrix membrane-based chondrocyte delivery system in human Ex Vivo model. Tissue Eng. Regen. Med. 13 182–190. 10.1007/s13770-016-9043-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park E., Lee J., Huh K. M., Lee S. H., Lee H. (2019). Toxicity-attenuated glycol chitosan adhesive inspired by mussel adhesion mechanisms. Adv. Healthc. Mater. 8:1900275. 10.1002/adhm.201900275 [DOI] [PubMed] [Google Scholar]
- Park J. U., Kwon S. T. (2017). Potential of autologous adipose-derived stem cells to regenerate atrophied muscle in a rat model. Wound Repair Regen. 25 944–955. 10.1111/wrr.12598 [DOI] [PubMed] [Google Scholar]
- Park K. M., Park K. D. (2018). In situ cross-linkable hydrogels as a dynamic matrix for tissue regenerative medicine. Tissue Eng. Regen. Med. 15 547–557. 10.1007/s13770-018-0155-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S. H., Kwon J. S., Lee B. S., Park J. H., Lee B. K., Yun J. H., et al. (2017). BMP2-modified injectable hydrogel for osteogenic differentiation of human periodontal ligament stem cells. Sci. Rep. 7:6603. 10.1038/s41598-017-06911-6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. B., Ha C. W., Kim J. A., Kim S., Park Y. G. (2019). Comparison of undifferentiated versus chondrogenic predifferentiated mesenchymal stem cells derived from human umbilical cord blood for cartilage repair in a rat model. Am. J. Sports Med. 47 451–461. 10.1177/0363546518815151 [DOI] [PubMed] [Google Scholar]
- Park Y. B., Ha C. W., Lee C. H., Yoon Y. C., Park Y. G. (2017). Cartilage regeneration in osteoarthritic patients by a composite of allogeneic umbilical cord blood-derived mesenchymal stem cells and hyaluronate hydrogel: results from a clinical trial for safety and proof-of-concept with 7 years of extended follow-up. Stem Cells Transl. Med. 6 613–621. 10.5966/sctm.2016-2157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel M., Nakaji-Hirabayashi T., Matsumura K. (2019). Effect of dual-drug-releasing micelle–hydrogel composite on wound healing in vivo in full-thickness excision wound rat model. J. Biomed. Mater. Res. Part A 107 1094–1106. 10.1002/jbm.a.36639 [DOI] [PubMed] [Google Scholar]
- Pati F., Jang J., Ha D. H., Won Kim S., Rhie J. W., Shim J. H., et al. (2014). Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink. Nat. Commun. 5:3935. 10.1038/ncomms4935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer L. A. (1954). Extended use of diced cartilage grafts. Plast Reconstr Surg 14 178–185. [DOI] [PubMed] [Google Scholar]
- Peng J., Chen L., Peng K., Chen X., Wu J., He Z., et al. (2019). Bone marrow mesenchymal stem cells and endothelial progenitor cells co-culture enhances large segment bone defect repair. J. Biomed. Nanotechnol. 15 742–755. 10.1166/jbn.2019.2735 [DOI] [PubMed] [Google Scholar]
- Peng W., Gao T., Yang Z. L., Zhang S. C., Ren M. L., Wang Z. G., et al. (2012). Adipose-derived stem cells induced dendritic cells undergo tolerance and inhibit Th1 polarization. Cell. Immunol. 278 152–157. 10.1016/j.cellimm.2012.07.008 [DOI] [PubMed] [Google Scholar]
- Perrod G., Pidial L., Camilleri S., Bellucci A., Casanova A., Viel T., et al. (2017). ADSC-sheet transplantation to prevent stricture after extended esophageal endoscopic submucosal dissection. J. Vis. Exp. 120:55018. 10.3791/55018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda M., Lear A., Collins J. P., Kiani S. (2019). Safe CRISPR: challenges and possible solutions. Trends Biotechnol. 37 389–401. 10.1016/j.tibtech.2018.09.010 [DOI] [PubMed] [Google Scholar]
- Porada C. D., Atala A. J., Almeida-Porada G. (2016). The hematopoietic system in the context of regenerative medicine. Methods 99 44–61. 10.1016/j.ymeth.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash J., Kumar T. S., Venkataprasanna K. S., Niranjan R., Kaushik M., Samal D. B., et al. (2019). PVA/alginate/hydroxyapatite films for controlled release of amoxicillin for the treatment of periodontal defects. Appl. Surf. Sci. 495:9 10.1016/j.apsusc.2019.143543 [DOI] [Google Scholar]
- Qi C., Liu J., Jin Y., Xu L. M., Wang G. B., Wang Z., et al. (2018). Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 163 89–104. 10.1016/j.biomaterials.2018.02.016 [DOI] [PubMed] [Google Scholar]
- Qi Y., Ma J., Li S., Liu W. (2019). Applicability of adipose-derived mesenchymal stem cells in treatment of patients with type 2 diabetes. Stem Cell Res. Ther. 10 274. 10.1186/s13287-019-1362-1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y. Z., Zhou X. F., Zhang F. M., Diekwisch T. G. H., Luan X. H., Yang J. X. (2019). Triple PLGA/PCL scaffold modification including silver impregnation, collagen coating, and electrospinning significantly improve biocompatibility, antimicrobial, and osteogenic properties for orofacial tissue regeneration. ACS Appl. Mater. Interfaces 11 37381–37396. 10.1021/acsami.9b07053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin T. W., Yang Z. M., Wu Z. Z., Xie H. Q., Qin J., Cai S. X. (2005). Adhesion strength of human tenocytes to extracellular matrix component-modified poly(dl-lactide-co-glycolide) substrates. Biomaterials 26 6635–6642. 10.1016/j.biomaterials.2005.04.023 [DOI] [PubMed] [Google Scholar]
- Qiu J. J., Qian W. H., Zhang J. K., Chen D. F., Yeung K. W. K., Liu X. Y. (2019). Minocycline hydrochloride loaded graphene oxide enables enhanced osteogenic activity in the presence of Gram-positive bacteria. Staphylococcus aureus. J. Mater. Chem. B 7 3590–3598. 10.1039/c9tb00405j [DOI] [Google Scholar]
- Qu X., Cao Y., Chen C., Die X., Kang Q. (2015). A poly(lactide-co-glycolide) film loaded with abundant bone morphogenetic protein-2: a substrate-promoting osteoblast attachment, proliferation, and differentiation in bone tissue engineering. J. Biomed. Mater. Res. Part A 103 2786–2796. 10.1002/jbm.a.35379 [DOI] [PubMed] [Google Scholar]
- Quan Q., Meng H., Chang B., Hong L., Li R., Liu G., et al. (2019). Novel 3-D helix-flexible nerve guide conduits repair nerve defects. Biomaterials 207 49–60. 10.1016/j.biomaterials.2019.03.040 [DOI] [PubMed] [Google Scholar]
- Rabkin E., Schoen F. J. (2002). Cardiovascular tissue engineering. Cardiovasc. Patho. 11 305–317. 10.1016/S1054-8807(02)00130-138 [DOI] [PubMed] [Google Scholar]
- Rajaram R., Subramani B., Abdullah B. J. J., Mahadeva S. (2017). Mesenchymal stem cell therapy for advanced liver cirrhosis: a case report. JGH Open 1 153–155. 10.1002/jgh3.12027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjith Kumar K., Shi-Bin W., Ai-Zheng C. (2019). Microengineered organ-on-a-chip platforms towards personalized medicine. Curr. Pharm.l Des. 25 1–13. 10.2174/1381612825666190222143542 [DOI] [PubMed] [Google Scholar]
- Rao J. S., Zhao C., Zhang A. F., Duan H. M., Hao P., Wei R. H., et al. (2018). NT3-chitosan enables de novo regeneration and functional recovery in monkeys after spinal cord injury. Proc. Natl. Acad. Sci. U.S. A. 115 E5595–E5604. 10.1073/pnas.1804735115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L., Ma D., Liu B., Li J., Chen J., Yang D., et al. (2014). Preparation of three-dimensional vascularized MSC cell sheet constructs for tissue regeneration. BioMed Res. Int. 2014 301279–301279. 10.1155/2014/301279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M. L., Peng W., Yang Z. L., Sun X. J., Zhang S. C., Wang Z. G., et al. (2012). Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transpl. 21 2711–2721. 10.3727/096368912X654966 [DOI] [PubMed] [Google Scholar]
- Sadahiro T., Yamanaka S., Ieda M. (2015). Direct cardiac reprogramming: progress and challenges in basic biology and clinical applications. Circ. Res. 116 1378–1391. 10.1161/CIRCRESAHA.116.305374 [DOI] [PubMed] [Google Scholar]
- Salehi S., Czugala M., Stafiej P., Fathi M., Bahners T., Gutmann J. S., et al. (2017). Poly (glycerol sebacate)-poly (epsilon-caprolactone) blend nanofibrous scaffold as intrinsic bio- and immunocompatible system for corneal repair. Acta Biomater. 50 370–380. 10.1016/j.actbio.2017.01.013 [DOI] [PubMed] [Google Scholar]
- Salgado A. J., Oliveira J. M., Martins A., Teixeira F. G., Silva N. A., Neves N. M., et al. (2013). Tissue engineering and regenerative medicine: past, present, and future. Int. Rev. Neurobiol. 108 1–33. 10.1016/B978-0-12-410499-0.00001-0 [DOI] [PubMed] [Google Scholar]
- Sasaki Y., Sathi G. A., Yamamoto O. (2017). Wound healing effect of bioactive ion released from Mg-smectite. Mater. Sci. Eng. C Mater. Biol. Appl. 77 52–57. 10.1016/j.msec.2017.03.236 [DOI] [PubMed] [Google Scholar]
- Schäfer R., Spohn G., Baer P. C. (2016). Mesenchymal stem/stromal cells in regenerative medicine: can preconditioning strategies improve therapeutic efficacy? Transfus. Med. Hemother. 43 256–267. 10.1159/000447458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine H., Shimizu T., Sakaguchi K., Dobashi I., Wada M., Yamato M., et al. (2013). In vitro fabrication of functional three-dimensional tissues with perfusable blood vessels. Nat. Commun. 4 1399. 10.1038/ncomms2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo S. E., Steinhauser M. L., Pizzimenti C. L., Yang V. K., Cai L., Wang M., et al. (2013). Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493 433–436. 10.1038/nature11682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L., Wu Y. Z., Ge L., Zhang Y. J., Wang Q. B., Qian J. Y., et al. (2017). A head to head comparison of XINSORB bioresorbable sirolimus-eluting scaffold versus metallic sirolimus-eluting stent: 180 days follow-up in a porcine model. International J. Cardiovasc. Imaging 33 1473–1481. 10.1007/s10554-017-1148-1145 [DOI] [PubMed] [Google Scholar]
- Shen Y., Yalikun Y., Tanaka Y. (2019). Recent advances in microfluidic cell sorting systems. Senso. Actuators B: Chem. 282 268–281. 10.1016/j.snb.2018.11.025 [DOI] [Google Scholar]
- Shi M., Zhang H., Song T., Liu X. F., Gao Y. F., Zhou J. H., et al. (2019). Sustainable dual release of antibiotic and growth factor from pH-responsive uniform alginate composite microparticles to enhance wound healing. ACS Appl. Mater. Interfaces 11 22730–22744. 10.1021/acsami.9b04750 [DOI] [PubMed] [Google Scholar]
- Shi P. J., Teh T. K. H., Toh S. L., Goh J. C. H. (2013). Variation of the effect of calcium phosphate enhancement of implanted silk fibroin ligament bone integration. Biomaterials 34 5947–5957. 10.1016/j.biomaterials.2013.04.046 [DOI] [PubMed] [Google Scholar]
- Shi W. Y., Zhou Q. J., Gao H., Li S. X., Dong M. C., Wang T., et al. (2019). Protectively decellularized porcine cornea versus human donor cornea for lamellar transplantation. Adv. Funct. Mater. 29:1902491 10.1002/adfm.201902491 [DOI] [Google Scholar]
- Shi Y., Li H., Zhang X., Fu Y., Huang Y., Lui P. P., et al. (2011). Continuous cyclic mechanical tension inhibited Runx2 expression in mesenchymal stem cells through RhoA-ERK1/2 pathway. J. Cell. Physiol. 226 2159–2169. 10.1002/jcp.22551 [DOI] [PubMed] [Google Scholar]
- Shi Y. C., Wang L. T., Niu Y. M., Yu N., Xing P. F., Dong L., et al. (2018). Fungal component coating enhances titanium implant-bone integration. Adv. Funct. Mater. 28:1804483 10.1002/adfm.201804483 [DOI] [Google Scholar]
- Shiba Y., Fernandes S., Zhu W. Z., Filice D., Muskheli V., Kim J., et al. (2012). Human ES-cell-derived cardiomyocytes electrically couple and suppress arrhythmias in injured hearts. Nature 489 322–325. 10.1038/nature11317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin S. R., Bae H., Cha J. M., Mun J. Y., Chen Y. C., Tekin H., et al. (2012). Carbon nanotube reinforced hybrid microgels as scaffold materials for cell encapsulation. ACS Nano 6 362–372. 10.1021/nn203711s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivats A. R., McDermott M. C., Hollinger J. O. (2014). Bone tissue engineering: state of the union. Drug Discov. Today 19 781–786. 10.1016/j.drudis.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Sivaraj D., Vijayalakshmi K. (2019). Enhanced antibacterial and corrosion resistance properties of Ag substituted hydroxyapatite/functionalized multiwall carbon nanotube nanocomposite coating on 316L stainless steel for biomedical application. Ultrason. Sonochem. 59 104730. 10.1016/j.ultsonch.2019.104730 [DOI] [PubMed] [Google Scholar]
- Song F., Jiang D., Wang T., Wang Y., Chen F., Xu G., et al. (2017). Mechanical loading improves tendon-bone healing in a rabbit anterior cruciate ligament reconstruction model by promoting proliferation and matrix formation of mesenchymal stem cells and tendon cells. Cell. Physiol. Biochem. 41 875–889. 10.1159/000460005 [DOI] [PubMed] [Google Scholar]
- Stehlik J., Edwards L. B., Kucheryavaya A. Y., Benden C., Christie J. D., Dobbels F., et al. (2011). The registry of the international society for heart and lung transplantation: twenty-eighth adult heart transplant report–2011. J. Heart Lung Transpl. 30 1078–1094. 10.1016/j.healun.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Su C. J., Tu M. G., Wei L. J., Hsu T. T., Kao C. T., Chen T. H., et al. (2017). Calcium silicate/chitosan-coated electrospun poly (lactic acid) fibers for bone tissue engineering. Materials 10:501. 10.3390/ma10050501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam S., Fang Y. H., Sivasubramanian S., Lin F. H., Lin C. P. (2016). Hydroxyapatite-calcium sulfate-hyaluronic acid composite encapsulated with collagenase as bone substitute for alveolar bone regeneration. Biomaterials 74 99–108. 10.1016/j.biomaterials.2015.09.044 [DOI] [PubMed] [Google Scholar]
- Sudheesh Kumar P. T., Lakshmanan V. K., Anilkumar T. V., Ramya C., Reshmi P., Unnikrishnan A. G., et al. (2012). Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: in vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 4 2618–2629. 10.1021/am300292v [DOI] [PubMed] [Google Scholar]
- Sun A. X., Prest T. A., Fowler J. R., Brick R. M., Gloss K. M., Li X., et al. (2019). Conduits harnessing spatially controlled cell-secreted neurotrophic factors improve peripheral nerve regeneration. Biomaterials 203 86–95. 10.1016/j.biomaterials.2019.01.038 [DOI] [PubMed] [Google Scholar]
- Sun J., Mou C. C., Shi Q., Chen B., Hou X. L., Zhang W., et al. (2018). Controlled release of collagen-binding SDF-1 alpha from the collagen scaffold promoted tendon regeneration in a rat Achilles tendon defect model. Biomaterials 162 22–33. 10.1016/j.biomaterials.2018.02.008 [DOI] [PubMed] [Google Scholar]
- Sun W., Luo Z., Lee J., Kim H. J., Lee K., Tebon P., et al. (2019). Organ-on-a-chip for cancer and immune organs modeling. Adv. Healthc. Mater. 8:1801363. 10.1002/adhm.201801363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Bai Y., Zhai H., Liu S., Zhang C., Xu Y., et al. (2019). Devising micro/nano-architectures in multi-channel nerve conduits towards a pro-regenerative matrix for the repair of spinal cord injury. Acta Biomater. 86 194–206. 10.1016/j.actbio.2018.12.032 [DOI] [PubMed] [Google Scholar]
- Sun X., Lang Q., Zhang H., Cheng L., Zhang Y., Pan G., et al. (2017). Electrospun photocrosslinkable hydrogel fibrous scaffolds for rapid in vivo vascularized skin flap regeneration. Adv. Funct. Mater. 27:1604617 10.1002/adfm.201604617 [DOI] [Google Scholar]
- Sun Y. S., Huang C. Y., Chen C. S., Chang J. H., Hou W. T., Li S. J., et al. (2018). Bone cell responses to a low elastic modulus titanium alloy surface immobilized with the natural cross-linker genipin. Surf. Coat. Technol. 350 918–924. 10.1016/j.surfcoat.2018.03.069 [DOI] [Google Scholar]
- Tabar V., Studer L. (2014). Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat. Rev. Genet. 15 82–92. 10.1038/nrg3563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachizawa S., Takahashi H., Kim Y. J., Odawara A., Pauty J., Ikeuchi Y., et al. (2017). Bundle gel fibers with a tunable microenvironment for in vitro neuron cell guiding. ACS Appl. Mater. Interfaces 9 43250–43257. 10.1021/acsami.7b14585 [DOI] [PubMed] [Google Scholar]
- Takahashi H., Okano T. (2019). Thermally-triggered fabrication of cell sheets for tissue engineering and regenerative medicine. Adv. Drug Deliv. Rev. 138 276–292. 10.1016/j.addr.2019.01.004 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126 663–676. 10.1016/j.cell.2006.07.024 [DOI] [PubMed] [Google Scholar]
- Takezawa T., Mori Y., Yoshizato K. (1990). Cell culture on a thermo-responsive polymer surface. Biotechnology 8 854–856. [DOI] [PubMed] [Google Scholar]
- Tan J., Xu X., Tong Z., Lin J., Yu Q., Lin Y., et al. (2015). Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res. 3:15003. 10.1038/boneres.2015.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Qin H., Gu X., Fu X. (2017). China’s landscape in regenerative medicine. Biomaterials 124 78–94. 10.1016/j.biomaterials.2017.01.044 [DOI] [PubMed] [Google Scholar]
- Tantilertanant Y., Niyompanich J., Everts V., Supaphol P., Pavasant P., Sanchavanakit N. (2019). Cyclic tensile force stimulates BMP9 synthesis and in vitro mineralization by human periodontal ligament cells. J. Cell. Physiol. 234 4528–4539. 10.1002/jcp.27257 [DOI] [PubMed] [Google Scholar]
- Tao J., Zhang J., Du T., Xu X., Deng X., Chen S., et al. (2019). Rapid 3D printing of functional nanoparticle-enhanced conduits for effective nerve repair. Acta Biomater. 90 49–59. 10.1016/j.actbio.2019.03.047 [DOI] [PubMed] [Google Scholar]
- Tasnim N., De la Vega L., Anil Kumar S., Abelseth L., Alonzo M., Amereh M., et al. (2018). 3D bioprinting stem cell derived tissues. Cell. Mol. Bioeng 11 219–240. 10.1007/s12195-018-0530-532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teh T. K. H., Toh S. L., Goh J. C. H. (2013). Aligned fibrous scaffolds for enhanced mechanoresponse and tenogenesis of mesenchymal stem cells. Tissue Eng. Part A 19 1360–1372. 10.1089/ten.tea.2012.0279 [DOI] [PubMed] [Google Scholar]
- Tra Thanh N., Ho Hieu M., Tran Minh Phuong N., Do Bui, Thuan T., Nguyen Thi, et al. (2018). Optimization and characterization of electrospun polycaprolactone coated with gelatin-silver nanoparticles for wound healing application. Mater. Sci. Eng. C 91 318–329. 10.1016/j.msec.2018.05.039 [DOI] [PubMed] [Google Scholar]
- Tsuji O., Sugai K., Yamaguchi R., Tashiro S., Nagoshi N., Kohyama J., et al. (2019). Concise review: laying the groundwork for a first-in-human study of an induced pluripotent stem cell-based intervention for spinal cord injury. Stem Cells 37 6–13. 10.1002/stem.2926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu M. G., Ho C. C., Hsu T. T., Huang T. H., Lin M. J., Shie M. Y. (2018). Mineral trioxide aggregate with mussel-inspired surface nanolayers for stimulating odontogenic differentiation of dental pulcp Cells. J. Endod. 44 963–970. 10.1016/j.joen.2018.02.018 [DOI] [PubMed] [Google Scholar]
- Ullah S., Zainol I., Idrus R. H. (2017). Incorporation of zinc oxide nanoparticles into chitosan-collagen 3D porous scaffolds: effect on morphology, mechanical properties and cytocompatibility of 3D porous scaffolds. Int. J. Biol. Macromol. 104 1020–1029. 10.1016/j.ijbiomac.2017.06.080 [DOI] [PubMed] [Google Scholar]
- Wada R., Muraoka N., Inagawa K., Yamakawa H., Miyamoto K., Sadahiro T., et al. (2013). Induction of human cardiomyocyte-like cells from fibroblasts by defined factors. Proc. Natl. Acad. Sci. U. S. A. 110 12667–12672. 10.1073/pnas.1304053110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Y., Feng N. B., Chang F., Wang J. C., Yuan B. M., Cheng Y. L., et al. (2019). Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv. Healthc. Mater. 8:1900312. 10.1002/adhm.201900312 [DOI] [PubMed] [Google Scholar]
- Wang C. Z., Eswaramoorthy R., Lin T. H., Chen C. H., Fu Y. C., Wang C. K., et al. (2018). Enhancement of chondrogenesis of adipose-derived stem cells in HA-PNIPAAm-CL hydrogel for cartilage regeneration in rabbits. Sci. Rep. 8:10526. 10.1038/s41598-018-28893-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. J., Li M. Y., Huang W. T., Lu M. H., Hu C., Li K., et al. (2015). Repair of urethral defects with polylactid acid fibrous membrane seeded with adipose-derived stem cells in a rabbit model. Connect. Tissue Res. 56 434–439. 10.3109/03008207.2015.1035376 [DOI] [PubMed] [Google Scholar]
- Wang F. Q., Wang L., Feng Y. F., Yang X. J., Ma Z. S., Shi L., et al. (2018). Evaluation of an artificial vertebral body fabricated by a tantalum-coated porous titanium scaffold for lumbar vertebral defect repair in rabbits. Sci. Rep. 8:8927. 10.1038/s41598-018-27182-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Liu Z., Li D., Guo X., Kasper F. K., Duan C., et al. (2012). Injectable biodegradable hydrogels for embryonic stem cell transplantation: improved cardiac remodelling and function of myocardial infarction. J. Cell. Mol. Med. 16 1310–1320. 10.1111/j.1582-4934.2011.01409.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Su K. X., Su L. Z., Liang P. P., Ji P., Wang C. (2019). Comparison of 3D-printed porous tantalum and titanium scaffolds on osteointegration and osteogenesis. Mater. Sci. Eng. C-Mater. Biol. Appl. 104:109908. 10.1016/j.msec.2019.109908 [DOI] [PubMed] [Google Scholar]
- Wang H., Zhao S., Xiao W., Cui X., Huang W., Rahaman M. N., et al. (2015). Three-dimensional zinc incorporated borosilicate bioactive glass scaffolds for rodent critical-sized calvarial defects repair and regeneration. Colloids Surf. B Biointerfaces 130 149–156. 10.1016/j.colsurfb.2015.03.053 [DOI] [PubMed] [Google Scholar]
- Wang H. X., Song Z., Lao Y. H., Xu X., Gong J., Cheng D., et al. (2018). Nonviral gene editing via CRISPR/Cas9 delivery by membrane-disruptive and endosomolytic helical polypeptide. Proc. Natl. Acad. Sci. U.S. A. 115 4903–4908. 10.1073/pnas.1712963115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Zheng W., Chen L., Zhu T., Shen W., Fan C., et al. (2019). Enhancement of schwann cells function using graphene-oxide-modified nanofiber scaffolds for peripheral nerve regeneration. ACS Biomater. Sci. Eng. 5 2444–2456. 10.1021/acsbiomaterials.8b01564 [DOI] [PubMed] [Google Scholar]
- Wang J., Zou M., Sun L., Cheng Y., Shang L., Fu F., et al. (2017). Microfluidic generation of Buddha beads-like microcarriers for cell culture. Sci. China Mater. 60 857–865. 10.1007/s40843-017-9081-9085 [DOI] [Google Scholar]
- Wang K., Zhang Q. Y., Zhao L. Q., Pan Y. W., Wang T., Zhi D. K., et al. (2017). Functional modification of electrospun poly(epsilon-caprolactone) vascular grafts with the fusion protein VEGF-HGFI Enhanced Vascular Regeneration. ACS Appl. Mater. Interfaces 9 11415–11427. 10.1021/acsami.6b16713 [DOI] [PubMed] [Google Scholar]
- Wang L., Jiang D., Wang Q., Wang Q., Jia W., Hu H. (2019). The Application of Microfluidic Techniques on Tissue Engineering in Orthopaedics. Curr. Pharm. Des. 24 5397–5460. 10.2174/1381612825666190301142833 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhao S., Mao H., Zhou L., Wang Z. J., Wang H. X. (2011). Autologous bone marrow stem cell transplantation for the treatment of type 2 diabetes mellitus. Chin. Med. J. 124 3622–3628. 10.3760/cma.j.issn.0366-6999.2011.22.005 [DOI] [PubMed] [Google Scholar]
- Wang L. H., Qiu Y. Y., Lv H. J., Si Y., Liu L. F., Zhang Q., et al. (2019). 3D superelastic scaffolds constructed from flexible inorganic nanofibers with self-fitting capability and tailorable gradient for bone regeneration. Adv. Funct. Mater. 29:1901407 10.1002/adfm.201901407 [DOI] [Google Scholar]
- Wang R., Wang Y., Yao B., Hu T., Li Z., Huang S., et al. (2019). Beyond 2D: 3D bioprinting for skin regeneration. Int. Wound J. 16 134–138. 10.1111/iwj.13003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Zhang M., Lu W., Zhang X., Ma D., Rong X., et al. (2009). Cross-linked Collagen–chondroitin sulfate–hyaluronic acid imitating extracellular matrix as scaffold for dermal tissue engineering. Tissue Eng. Part C Methods 16 269–279. 10.1089/ten.tec.2009.0161 [DOI] [PubMed] [Google Scholar]
- Wang W. B., He J., Feng B., Zhang Z. Y., Zhang W. J., Zhou G. D., et al. (2016). Aligned nanofibers direct human dermal fibroblasts to tenogenic phenotype in vitro and enhance tendon regeneration in vivo. Nanomedicine 11 1055–1072. 10.2217/nnm.16.24 [DOI] [PubMed] [Google Scholar]
- Wang X., Wang J., Yang Y., Yang F., Wu D. (2017). Fabrication of multi-stimuli responsive supramolecular hydrogels based on host–guest inclusion complexation of a tadpole-shaped cyclodextrin derivative with the azobenzene dimer. Polym. Chem. 8 3901–3909. 10.1039/C7PY00698E [DOI] [Google Scholar]
- Wang Y., Zhao Z., Ren Z., Zhao B., Zhang L., Chen J., et al. (2012). Recellularized nerve allografts with differentiated mesenchymal stem cells promote peripheral nerve regeneration. Neurosci. Lett. 514 96–101. 10.1016/j.neulet.2012.02.066 [DOI] [PubMed] [Google Scholar]
- Wang Z., Ren Y., Zhu Y., Hao L., Chen Y., An G., et al. (2018). A rapidly self-healing host–guest supramolecular hydrogel with high mechanical strength and excellent biocompatibility. Angew. Chem. Int. Ed. 57 9008–9012. 10.1002/anie.201804400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z. H., Lu Y. X., Qin K., Wu Y. F., Tian Y. P., Wang J. N., et al. (2015). Enzyme-functionalized vascular grafts catalyze in-situ release of nitric oxide from exogenous NO prodrug. J. Control. Release 210 179–188. 10.1016/j.jconrel.2015.05.283 [DOI] [PubMed] [Google Scholar]
- Wang Z. H., Wu Y. F., Wang J. N., Zhang C. N., Yan H. Y., Zhu M. F., et al. (2017). Effect of resveratrol on modulation of endothelial cells and macrophages for rapid vascular regeneration from electrospun poly(epsilon-caprolactone) scaffolds. ACS Appl. Mater. Interfaces 9 19541–19551. 10.1021/acsami.6b16573 [DOI] [PubMed] [Google Scholar]
- Watson D. (2009). Tissue engineering for rhinoplasty. Facial Plast. Surg. Clin. North Am. 17 157–165. 10.1016/j.fsc.2008.09.010 [DOI] [PubMed] [Google Scholar]
- Wei D., Sun J., Bolderson J., Zhong M., Dalby M. J., Cusack M., et al. (2017). Continuous fabrication and assembly of spatial cell-laden fibers for a tissue-like construct via a photolithographic-based microfluidic chip. ACS Appl. Mater. Interfaces 9 14606–14617. 10.1021/acsami.7b00078 [DOI] [PubMed] [Google Scholar]
- Wei Y. T., He Y., Xu C. L., Wang Y., Liu B. F., Wang X. M., et al. (2010). Hyaluronic acid hydrogel modified with nogo-66 receptor antibody and poly-L-lysine to promote axon regrowth after spinal cord injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 95B 110–117. 10.1002/jbm.b.31689 [DOI] [PubMed] [Google Scholar]
- Wei Y. T., Tian W. M., Yu X., Cui F. Z., Hou S. P., Xu Q. Y., et al. (2007). Hyaluronic acid hydrogels with IKVAV peptides for tissue repair and axonal regeneration in an injured rat brain. Biomed. Mater. 2 S142–S146. 10.1088/1748-6041/2/3/s11 [DOI] [PubMed] [Google Scholar]
- Wei Y. Z., Wu Y. F., Zhao R. X., Zhang K. Y., Midgley A. C., Kong D. L., et al. (2019). MSC-derived sEVs enhance patency and inhibit calcification of synthetic vascular grafts by immunomodulation in a rat model of hyperlipidemia. Biomaterials 204 13–24. 10.1016/j.biomaterials.2019.01.049 [DOI] [PubMed] [Google Scholar]
- Witowski J., Sitkowski M., Zuzak T., Coles-Black J., Chuen J., Major P., et al. (2018). From ideas to long-term studies: 3D printing clinical trials review. Int. J. Comput. Assist. Radiol. Surg. 13 1473–1478. 10.1007/s11548-018-1793-1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Yao J., Hu W., Wang X., Gu X., Cao Y. (2005). Dog sciatic nerve regeneration across a 30-mm defect bridged by a chitosan/PGA artificial nerve graft. Brain 128 1897–1910. 10.1093/brain/awh517 [DOI] [PubMed] [Google Scholar]
- Wu R., Huang C., Wu Q., Jia X., Liu M., Xue Z., et al. (2019). Exosomes secreted by urine-derived stem cells improve stress urinary incontinence by promoting repair of pubococcygeus muscle injury in rats. Stem Cell Res. Ther. 10:80. 10.1186/s13287-019-1182-1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. C., Hsu H. C., Hsu S. K., Wang W. H., Ho W. F. (2011). Preparation and characterization of four different compositions of calcium phosphate scaffolds for bone tissue engineering. Mater. Charact. 62 526–534. 10.1016/j.matchar.2011.03.014 [DOI] [Google Scholar]
- Wu Y., Wang L., Guo B., Ma P. X. (2017). Interwoven aligned conductive nanofiber yarn/hydrogel composite scaffolds for engineered 3D cardiac anisotropy. ACS Nano 11 5646–5659. 10.1021/acsnano.7b01062 [DOI] [PubMed] [Google Scholar]
- Wu Y., Zhao D., Zhuang J., Zhang F., Xu C. (2016). Caspase-8 and Caspase-9 functioned differently at different stages of the cyclic stretch-induced apoptosis in human periodontal ligament cells. PLoS One 11:e0168268. 10.1371/journal.pone.0168268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Z., Shen L., Yin J. S., Chen J. H., Ge L., Ge J. B. (2019). 5 Years of serial intravascular imaging outcomes of XINSORB sirolimus- eluting bioresorbable vascular scaffold. JACC Cardiovasc. Interv. 12 602–603. 10.1016/j.jcin.2018.11.029 [DOI] [PubMed] [Google Scholar]
- Wu Z., Zheng K., Zhang J., Tang T., Guo H., Boccaccini A. R., et al. (2016). Effects of magnesium silicate on the mechanical properties, biocompatibility, bioactivity, degradability, and osteogenesis of poly(butylene succinate)-based composite scaffolds for bone repair. J. Mater. Chem. B 4 7974–7988. 10.1039/C6TB02429G [DOI] [PubMed] [Google Scholar]
- Xia H. T., Zhao D. D., Zhu H. L., Hua Y. J., Xiao K. Y., Xu Y., et al. (2018). Lyophilized scaffolds fabricated from 3D-printed photocurable natural hydrogel for cartilage regeneration. ACS Appl. Mater. Interfaces 10 31704–31715. 10.1021/acsami.8b10926 [DOI] [PubMed] [Google Scholar]
- Xiang B., Chen L., Wang X., Zhao Y., Wang Y., Xiang C. (2017). Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of LPS-induced acute lung injury. Int. J. Mol. Sci. 18:689. 10.3390/ijms18040689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Yang W., Lei D., Huang J., Yin Y., Zhu Y., et al. (2019). PGS scaffolds promote the in vivo survival and directional differentiation of bone marrow mesenchymal stem cells restoring the morphology and function of wounded rat uterus. Adv. Healthc. Mater. 8 1801455. 10.1002/adhm.201801455 [DOI] [PubMed] [Google Scholar]
- Xie P., Du J., Li Y., Wu J., He H., Jiang X., et al. (2019). Robust hierarchical porous MBG scaffolds with promoted biomineralization ability. Colloids Surf. B Biointerfaces 178 22–31. 10.1016/j.colsurfb.2019.02.042 [DOI] [PubMed] [Google Scholar]
- Xie Q., Wang Z., Zhou H. F., Yu Z., Huang Y. Z., Sun H., et al. (2016). The role of miR-135-modified adipose-derived mesenchymal stem cells in bone regeneration. Biomaterials 75 279–294. 10.1016/j.biomaterials.2015.10.042 [DOI] [PubMed] [Google Scholar]
- Xing D., Ma L., Gao C. (2017). A bioactive hyaluronic acid–based hydrogel cross-linked by Diels–Alder reaction for promoting neurite outgrowth of PC12 cells. J. Bioact. Compat. Polym. 32 382–396. 10.1177/0883911516684654 [DOI] [Google Scholar]
- Xu B., Zheng P. B., Gao F., Wang W., Zhang H. T., Zhang X. R., et al. (2017). A mineralized high strength and tough hydrogel for skull bone regeneration. Adv. Funct. Mater. 27:1604327 10.1002/adfm.201604327 [DOI] [Google Scholar]
- Xu T. T., Yang R., Ma X. B., Chen W., Liu S., Liu X., et al. (2019). Bionic poly(gamma-glutamic acid) electrospun fibrous scaffolds for preventing hypertrophic Scars. Adv. Healthc. Mater. 8:12. 10.1002/adhm.201900123 [DOI] [PubMed] [Google Scholar]
- Xu Y., Dong S., Zhou Q., Mo X., Song L., Hou T., et al. (2014). The effect of mechanical stimulation on the maturation of TDSCs-poly(L-lactide-co-e-caprolactone)/collagen scaffold constructs for tendon tissue engineering. Biomaterials 35 2760–2772. 10.1016/j.biomaterials.2013.12.042 [DOI] [PubMed] [Google Scholar]
- Xue C., Hu N., Gu Y., Yang Y., Liu Y., Liu J., et al. (2011). Joint use of a chitosan/PLGA scaffold and MSCs to bridge an extra large gap in dog sciatic nerve. Neurorehabil. Neural Repair 26 96–106. 10.1177/1545968311420444 [DOI] [PubMed] [Google Scholar]
- Xue C. B., Ren H. C., Zhu H., Gu X. K., Guo Q., Zhou Y., et al. (2017). Bone marrow mesenchymal stem cell-derived acellular matrix-coated chitosan/silk scaffolds for neural tissue regeneration. J. Mater. Chem. B 5 1246–1257. 10.1039/c6tb02959k [DOI] [PubMed] [Google Scholar]
- Xue H. Y., Zhang X., Wang Y., Xiaojie L., Dai W. J., Xu Y. (2016). In vivo gene therapy potentials of CRISPR-Cas9. Gene Ther. 23 557–559. 10.1038/gt.2016.25 [DOI] [PubMed] [Google Scholar]
- Yamamoto K., Yamato M., Morino T., Sugiyama H., Takagi R., Yaguchi Y., et al. (2017). Middle ear mucosal regeneration by tissue-engineered cell sheet transplantation. NPJ Regen. Med. 2 6. 10.1038/s41536-017-0010-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto O., Alvarez K., Kashiwaya Y., Fukuda M. (2011a). Significant effect of a carbon layer coating on interfacial bond strength between bone and Ti implant. Carbon 49 1588–1598. 10.1016/j.carbon.2010.12.041 [DOI] [Google Scholar]
- Yamamoto O., Alvarez K., Kashiwaya Y., Fukuda M. (2011b). Surface characterization and biological response of carbon-coated oxygen-diffused titanium having different topographical surfaces. J. Mater. Sci.-Mater. Med. 22 977–987. 10.1007/s10856-011-4267-x [DOI] [PubMed] [Google Scholar]
- Yamashita K., Inagaki E., Hatou S., Higa K., Ogawa A., Miyashita H., et al. (2018). Corneal endothelial regeneration using mesenchymal stem cell derived from human umbilical cord. Stem Cells Dev. 27 1097–1108. 10.1089/scd.2017.0297 [DOI] [PubMed] [Google Scholar]
- Yan H., Liu X., Zhu M., Luo G., Sun T., Peng Q., et al. (2016). Hybrid use of combined and sequential delivery of growth factors and ultrasound stimulation in porous multilayer composite scaffolds to promote both vascularization and bone formation in bone tissue engineering. J. Biomed. Mater. Res. Part A 104 195–208. 10.1002/jbm.a.35556 [DOI] [PubMed] [Google Scholar]
- Yan S., Zhang J., Yuan D., Li W. (2017). Hybrid microfluidics combined with active and passive approaches for continuous cell separation. Electrophoresis 38 238–249. 10.1002/elps.201600386 [DOI] [PubMed] [Google Scholar]
- Yan Y. F., Cheng B. C., Chen K. Z., Cui W. G., Qi J., Li X. M., et al. (2019). Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 8:e1801043. 10.1002/adhm.201801043 [DOI] [PubMed] [Google Scholar]
- Yanaga H., Yanaga K., Imai K., Koga M., Soejima C., Ohmori K. (2006). Clinical application of cultured autologous human auricular chondrocytes with autologous serum for craniofacial or nasal augmentation and repair. Plast. Reconstr. Surg. 117 2019–2030. 10.1097/01.prs.0000210662.12267.de [DOI] [PubMed] [Google Scholar]
- Yang B. G., Yao F. L., Hao T., Fang W. C., Ye L., Zhang Y. B., et al. (2016). Development of electrically conductive double-network hydrogels via one-step facile strategy for cardiac tissue engineering. Adv. Healthc. Mater. 5 474–488. 10.1002/adhm.201500520 [DOI] [PubMed] [Google Scholar]
- Yang C. H., Li Y. C., Tsai W. F., Ai C. F., Huang H. H. (2015). Oxygen plasma immersion ion implantation treatment enhances the human bone marrow mesenchymal stem cells responses to titanium surface for dental implant application. Clin. Oral Implants Res. 26 166–175. 10.1111/clr.12293 [DOI] [PubMed] [Google Scholar]
- Yang C., Wang X., Ma B., Zhu H., Huan Z., Ma N., et al. (2017). 3D-Printed bioactive Ca3SiO5 bone cement scaffolds with nano surface structure for bone regeneration. ACS Appl. Mater. Interfaces 9 5757–5767. 10.1021/acsami.6b14297 [DOI] [PubMed] [Google Scholar]
- Yang G. H., Lee J., Kim G. (2019). The fabrication of uniaxially aligned micro-textured polycaprolactone struts and application for skeletal muscle tissue regeneration. Biofabrication 11:025005. 10.1088/1758-5090/ab0098 [DOI] [PubMed] [Google Scholar]
- Yang H., Wei L., Liu C., Zhong W. Y., Li B., Chen Y. C., et al. (2019). Engineering human ventricular heart tissue based on macroporous iron oxide scaffolds. Acta Biomater. 88 540–553. 10.1016/j.actbio.2019.02.024 [DOI] [PubMed] [Google Scholar]
- Yang J. A., Yeom J., Hwang B. W., Hoffman A. S., Hahn S. K. (2014). In situ-forming injectable hydrogels for regenerative medicine. Prog. Polym. Sci. 39 1973–1986. 10.1016/j.progpolymsci.2014.07.006 [DOI] [Google Scholar]
- Yang J., Yamato M., Shimizu T., Sekine H., Ohashi K., Kanzaki M., et al. (2007a). Reconstruction of functional tissues with cell sheet engineering. Biomaterials 28 5033–5043. 10.1016/j.biomaterials.2007.07.052 [DOI] [PubMed] [Google Scholar]
- Yang L., Ning X., Bai Y., Jia W. (2013). A scalable synthesis of non-agglomerated and low-aspect ratio hydroxyapatite nanocrystals using gelatinized starch matrix. Mater. Lett. 113 142–145. 10.1016/j.matlet.2013.09.059 [DOI] [Google Scholar]
- Yang W. E., Hsu M. L., Lin M. C., Chen Z. H., Chen L. K., Huang H. H. (2009). Nano/submicron-scale TiO2 network on titanium surface for dental implant application. J. Alloys Comp. 479 642–647. 10.1016/j.jallcom.2009.01.021 [DOI] [Google Scholar]
- Yang W. E., Huang H. H. (2019). Multiform TiO2 nano-network enhances biological response to titanium surface for dental implant applications. Appl. Surf. Sci. 471 1041–1052. 10.1016/j.apsusc.2018.11.244 [DOI] [Google Scholar]
- Yang X. L., Yang J. C., Wang L., Ran B., Jia Y. X., Zhang L. M., et al. (2017). Pharmaceutical intermediate-modified gold nanoparticles: against multidrug-resistant bacteria and wound-healing application via an electrospun scaffold. Acs Nano 11 5737–5745. 10.1021/acsnano.7b01240 [DOI] [PubMed] [Google Scholar]
- Yang Y., Ding F., Wu J., Hu W., Liu W., Liu J., et al. (2007b). Development and evaluation of silk fibroin-based nerve grafts used for peripheral nerve regeneration. Biomaterials 28 5526–5535. 10.1016/j.biomaterials.2007.09.001 [DOI] [PubMed] [Google Scholar]
- Yang Y., Yuan X., Ding F., Yao D., Gu Y., Liu J., et al. (2011). Repair of rat sciatic nerve gap by a silk fibroin-based scaffold added with bone marrow mesenchymal stem cells. Tissue Eng. Part A 17 2231–2244. 10.1089/ten.tea.2010.0633 [DOI] [PubMed] [Google Scholar]
- Yang Z., Xie H., Li T. (2000). Tissue engineering of the musculo-skeletal system-basic research and clinical applications. Hand Surg. 05 49–55. 10.1142/S0218810400000132 [DOI] [PubMed] [Google Scholar]
- Yang Z., Zhang A., Duan H., Zhang S., Hao P., Ye K., et al. (2015). NT3-chitosan elicits robust endogenous neurogenesis to enable functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. 112:13354. 10.1073/pnas.1510194112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C. H., Chen K. Y., Chen Y. S., Li S. J., Huang C. H. (2019). Lithospermi radix extract-containing bilayer nanofiber scaffold for promoting wound healing in a rat model. Mater. Sci. Eng. C 96 850–858. 10.1016/j.msec.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Yao M., Zhou Y., Xue C. B., Ren H. C., Wang S. R., Zhu H., et al. (2016). Repair of rat sciatic nerve defects by using allogeneic bone marrow mononuclear cells combined with chitosan/silk fibroin scaffold. Cell Transpl. 25 983–993. 10.3727/096368916x690494 [DOI] [PubMed] [Google Scholar]
- Ye K., Kuang H., You Z., Morsi Y., Mo X. (2019a). Electrospun nanofibers for tissue engineering with drug loading and release. Pharmaceutics 11:182. 10.3390/pharmaceutics11040182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye K. Q., Liu D. H., Kuang H. Z., Cai J. Y., Chen W. M., Sun B. B., et al. (2019b). Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 534 625–636. 10.1016/j.jcis.2018.09.071 [DOI] [PubMed] [Google Scholar]
- Yeh C. C., Chang S. F., Huang T. Y., Chang H. I., Kuo H. C., Wu Y. C., et al. (2013). Shear stress modulates macrophage-induced urokinase plasminogen activator expression in human chondrocytes. Arthritis Res. Ther. 15:R53. 10.1186/ar4215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeong W. Y., Sudarmadji N., Yu H. Y., Chua C. K., Leong K. F., Venkatraman S. S., et al. (2010). Porous polycaprolactone scaffold for cardiac tissue engineering fabricated by selective laser sintering. Acta Biomater. 6 2028–2034. 10.1016/j.actbio.2009.12.033 [DOI] [PubMed] [Google Scholar]
- Yi B., Shen Y., Tang H., Wang X., Li B., Zhang Y. (2019). Stiffness of aligned fibers regulates the phenotypic expression of vascular smooth muscle cells. ACS Appl. Mater. Interfaces 11 6867–6880. 10.1021/acsami.9b00293 [DOI] [PubMed] [Google Scholar]
- Yu S., Duan Y. Y., Zuo X. G., Chen X. Y., Mao Z. W., Gao C. Y. (2018). Mediating the invasion of smooth muscle cells into a cell-responsive hydrogel under the existence of immune cells. Biomaterials 180 193–205. 10.1016/j.biomaterials.2018.07.022 [DOI] [PubMed] [Google Scholar]
- Yu X., Liu S., Chen X., Du Y., Yin X., Du Y., et al. (2019). Calcitonin gene related peptide gene-modified rat bone mesenchymal stem cells are effective seed cells in tissue engineering to repair skull defects. Histol. Histopathol. 34 1229–1241. 10.14670/hh-18-102 [DOI] [PubMed] [Google Scholar]
- Yu Y., Fu F., Shang L., Cheng Y., Gu Z., Zhao Y. (2017). Bioinspired helical microfibers from microfluidics. Adv. Mater. 29 1605765. 10.1002/adma.201605765 [DOI] [PubMed] [Google Scholar]
- Yu Y., Shang L., Guo J., Wang J., Zhao Y. (2018). Design of capillary microfluidics for spinning cell-laden microfibers. Nat. Protoc. 13 2557–2579. 10.1038/s41596-018-0051-54 [DOI] [PubMed] [Google Scholar]
- Yuan H., Qin J., Xie J., Li B., Yu Z., Peng Z., et al. (2016). Highly aligned core-shell structured nanofibers for promoting phenotypic expression of vSMCs for vascular regeneration. Nanoscale 8 16307–16322. 10.1039/c6nr05075a [DOI] [PubMed] [Google Scholar]
- Yuan L., Li B., Yang J., Ni Y., Teng Y., Guo L., et al. (2016). Effects of composition and mechanical property of injectable collagen I/II composite hydrogels on chondrocyte behaviors. Tissue Eng. Part A 22 899–906. 10.1089/ten.TEA.2015.0513 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Tao B. L., He Y., Liu J., Lin C. C., Shen X. K., et al. (2019a). Biocompatible MoS2/PDA-RGD coating on titanium implant with antibacterial property via intrinsic ROS-independent oxidative stress and NIR irradiation. Biomaterials 217:119290. 10.1016/j.biomaterials.2019.119290 [DOI] [PubMed] [Google Scholar]
- Yuan Z., Tao B. L., He Y., Mu C. Y., Liu G. H., Zhang J. X., et al. (2019b). Remote eradication of biofilm on titanium implant via near-infrared light triggered photothermal/photodynamic therapy strategy. Biomaterials 223:119479. 10.1016/j.biomaterials.2019.119479 [DOI] [PubMed] [Google Scholar]
- Zeng X., Ma Y. H., Chen Y. F., Qiu X. C., Wu J. L., Ling E. A., et al. (2016). Autocrine fibronectin from differentiating mesenchymal stem cells induces the neurite elongation in vitro and promotes nerve fiber regeneration in transected spinal cord injury. J. Biomed. Mater. Res. Part A 104 1902–1911. 10.1002/jbm.a.35720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Zeng Y. S., Ma Y. H., Lu L. Y., Du B. L., Zhang W., et al. (2011). Bone marrow mesenchymal stem cells in a three-dimensional gelatin sponge Scaffold attenuate inflammation, promote angiogenesis, and reduce cavity formation in experimental spinal cord Injury. Cell Transpl. 20 1881–1899. 10.3727/096368911X566181 [DOI] [PubMed] [Google Scholar]
- Zhai W., Lu H., Chen L., Lin X., Huang Y., Dai K., et al. (2012). Silicate bioceramics induce angiogenesis during bone regeneration. Acta Biomater. 8 341–349. 10.1016/j.actbio.2011.09.008 [DOI] [PubMed] [Google Scholar]
- Zhang C., Nie X., Hu D., Liu Y., Deng Z., Dong R., et al. (2007). Survival and integration of tissue-engineered corneal stroma in a model of corneal ulcer. Cell Tissue Res. 329 249–257. 10.1007/s00441-007-0419-411 [DOI] [PubMed] [Google Scholar]
- Zhang C., Wang X. L., Zhang E. C., Yang L., Yuan H. H., Tu W. J., et al. (2018a). An epigenetic bioactive composite scaffold with well-aligned nanofibers for functional tendon tissue engineering. Acta Biomater. 66 141–156. 10.1016/j.actbio.2017.09.036 [DOI] [PubMed] [Google Scholar]
- Zhang C., Zhang E. C., Yang L., Tu W. J., Lin J. X., Yuan C. H., et al. (2018b). Histone deacetylase inhibitor treated cell sheet from mouse tendon stem/progenitor cells promotes tendon repair. Biomaterials 172 66–82. 10.1016/j.biomaterials.2018.03.043 [DOI] [PubMed] [Google Scholar]
- Zhang D., Wei G., Li P., Zhou X., Zhang Y. (2014). Urine-derived stem cells: a novel and versatile progenitor source for cell-based therapy and regenerative medicine. Genes Dis. 1 8–17. 10.1016/j.gendis.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang E., Xu L., Yu G., Pan F., Yang K. (2009). In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. J. Biomed. Mater. Res. A 90A 882–893. 10.1002/jbm.a.32132 [DOI] [PubMed] [Google Scholar]
- Zhang H., Jia X. L., Han F. X., Zhao J., Zhao Y. H., Fan Y. B., et al. (2013). Dual-delivery of VEGF and PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 34 2202–2212. 10.1016/j.biomaterials.2012.12.005 [DOI] [PubMed] [Google Scholar]
- Zhang K. X., Bai X. F., Yuan Z. P., Cao X. T., Jiao X. Y., Li Y. S., et al. (2019). Layered nanofiber sponge with an improved capacity for promoting blood coagulation and wound healing. Biomaterials 204 70–79. 10.1016/j.biomaterials.2019.03.008 [DOI] [PubMed] [Google Scholar]
- Zhang L., He A., Yin Z., Yu Z., Luo X., Liu W., et al. (2014). Regeneration of human-ear-shaped cartilage by co-culturing human microtia chondrocytes with BMSCs. Biomaterials 35 4878–4887. 10.1016/j.biomaterials.2014.02.043 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Lu H., Kawazoe N., Chen G. (2014). Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 10 2005–2013. 10.1016/j.actbio.2013.12.042 [DOI] [PubMed] [Google Scholar]
- Zhang S., Jiang G., Prabhakaran M. P., Qin X., Ramakrishna S. (2017). Evaluation of electrospun biomimetic substrate surface-decorated with nanohydroxyapatite precipitation for osteoblasts behavior. Mater. Sci. Eng. C 79 687–696. 10.1016/j.msec.2017.05.113 [DOI] [PubMed] [Google Scholar]
- Zhang W., Kong C. W., Tong M. H., Chooi W. H., Huang N., Li R. A., et al. (2017). Maturation of human embryonic stem cell-derived cardiomyocytes (hESC-CMs) in 3D collagen matrix: effects of niche cell supplementation and mechanical stimulation. Acta Biomater. 49 204–217. 10.1016/j.actbio.2016.11.058 [DOI] [PubMed] [Google Scholar]
- Zhang W., Yang G., Wang X., Jiang L., Jiang F., Li G., et al. (2017). Magnetically controlled growth-factor-immobilized multilayer cell sheets for complex tissue regeneration. Adv. Mater. 29:1703795. 10.1002/adma.201703795 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang C., Liao M., Dai L., Tang Y., Zhang H., et al. (2019). Aligned electrospun cellulose scaffolds coated with rhBMP-2 for both in vitro and in vivo bone tissue engineering. Carbohydr. Polym. 213 27–38. 10.1016/j.carbpol.2019.02.038 [DOI] [PubMed] [Google Scholar]
- Zhang X. Z., Zu H. Y., Zhao D. W., Yang K., Tian S. M., Yu X. M., et al. (2017). Ion channel functional protein kinase TRPM7 regulates Mg ions to promote the osteoinduction of human osteoblast via PI3K pathway: in vitro simulation of the bone-repairing effect of Mg-based alloy implant. Acta Biomater. 63 369–382. 10.1016/j.actbio.2017.08.051 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Chang M. L., Bao F., Xing M., Wang E., Xu Q., et al. (2019). Multifunctional Zn doped hollow mesoporous silica/polycaprolactone electrospun membranes with enhanced hair follicle regeneration and antibacterial activity for wound healing. Nanoscale 11 6315–6333. 10.1039/c8nr09818b [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhu D. S., Wei Y. Z., Wu Y. F., Cui W. L., Liuqin L. F., et al. (2019). A collagen hydrogel loaded with HDAC7-derived peptide promotes the regeneration of infarcted myocardium with functional improvement in a rodent model. Acta Biomater. 86 223–234. 10.1016/j.actbio.2019.01.022 [DOI] [PubMed] [Google Scholar]
- Zhang Y. B., Liu X. C., Zeng L. D., Zhang J., Zuo J. L., Zou J., et al. (2019). Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv. Funct. Mater 29:1903279 10.1002/adfm.201903279 [DOI] [Google Scholar]
- Zhang Z. J., Wu Y. P., Wang Z. H., Zhang X., Zhao Y. B., Sun L. (2017). Electrospinning of Ag Nanowires/polyvinyl alcohol hybrid nanofibers for their antibacterial properties. Mater. Sci. Eng. C-Mater. Biol. Appl. 78 706–714. 10.1016/j.msec.2017.04.138 [DOI] [PubMed] [Google Scholar]
- Zhao D., Wu Y., Xu C., Zhang F. (2017). Cyclic-stretch induces apoptosis in human periodontal ligament cells by activation of caspase-5. Arch. Oral Biol. 73 129–135. 10.1016/j.archoralbio.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Zhao N., Wang Y., Qin L., Guo Z., Li D. (2017). Effect of composition and macropore percentage on mechanical and in vitro cell proliferation and differentiation properties of 3D printed HA/β-TCP scaffolds. RSC Adv. 7 43186–43196. 10.1039/c7ra07204j [DOI] [Google Scholar]
- Zhao S., Li L., Wang H., Zhang Y., Cheng X., Zhou N., et al. (2015a). Wound dressings composed of copper-doped borate bioactive glass microfibers stimulate angiogenesis and heal full-thickness skin defects in a rodent model. Biomaterials 53 379–391. 10.1016/j.biomaterials.2015.02.112 [DOI] [PubMed] [Google Scholar]
- Zhao S., Zhang J., Zhu M., Zhang Y., Liu Z., Tao C., et al. (2015b). Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 12 270–280. 10.1016/j.actbio.2014.10.015 [DOI] [PubMed] [Google Scholar]
- Zhao T., Tan M., Cui Y., Deng C., Huang H., Guo M. (2014). Reactive macromolecular micelle crosslinked highly elastic hydrogel with water-triggered shape-memory behaviour. Polym. Chem. 5 4965–4973. 10.1039/C4PY00554F [DOI] [Google Scholar]
- Zhao W., Wang J., Zhai W., Wang Z., Chang J. (2005). The self-setting properties and in vitro bioactivity of tricalcium silicate. Biomaterials 26 6113–6121. 10.1016/j.biomaterials.2005.04.025 [DOI] [PubMed] [Google Scholar]
- Zhao X. Y., Li W., Lv Z., Liu L., Tong M., Hai T., et al. (2009). iPS cells produce viable mice through tetraploid complementation. Nature 461 86–90. 10.1038/nature08267 [DOI] [PubMed] [Google Scholar]
- Zhao X., Song W. J., Chen Y. W., Liu S., Ren L. (2019). Collagen-based materials combined with microRNA for repairing cornea wounds and inhibiting scar formation. Biomater. Sci. 7 51–62. 10.1039/c8bm01054d [DOI] [PubMed] [Google Scholar]
- Zhao Y., Chen X., Wu Y., Wang Y., Li Y., Xiang C. (2018). Transplantation of human menstrual blood-derived mesenchymal stem cells alleviates alzheimer’s disease-like pathology in APP/PS1 transgenic mice. Front. Mol. Neurosci. 11:140 10.3389/fnmol.2018.00140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Li Z. H., Song S. L., Yang K. R., Liu H., Yang Z., et al. (2019). Skin-inspired antibacterial conductive hydrogels for epidermal sensors and diabetic foot wound dressings. Adv. Funct. Mater. 29:12 10.1002/adfm.201901474 [DOI] [Google Scholar]
- Zhao Y. H., Wang Y. J., Gong J. H., Yang L., Niu C. M., Ni X. J., et al. (2017). Chitosan degradation products facilitate peripheral nerve regeneration by improving macrophage-constructed microenvironments. Biomaterials 134 64–77. 10.1016/j.biomaterials.2017.02.026 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Wang Y., Peng J., Ren Z., Zhan S., Liu Y., et al. (2011). Repair of nerve defect with acellular nerve graft supplemented by bone marrow stromal cells in mice. Microsurgery 31 388–394. 10.1002/micr.20882 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Wang Y., Peng J., Ren Z., Zhang L., Guo Q., et al. (2014). Improvement in nerve regeneration through a decellularized nerve graft by supplementation with bone marrow stromal cells in fibrin. Cell Transpl. 23 97–110. 10.3727/096368912X658845 [DOI] [PubMed] [Google Scholar]
- Zheng C., Zhu Q., Liu X., Huang X., He C., Jiang L., et al. (2014). Improved peripheral nerve regeneration using acellular nerve allografts loaded with platelet-rich plasma. Tissue Eng. Part A 20 3228–3240. 10.1089/ten.tea.2013.0729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng D., Dan Y., Yang S. H., Liu G. H., Shao Z. W., Yang C., et al. (2015). Controlled chondrogenesis from adipose-derived stem cells by recombinant transforming growth factor-beta 3 fusion protein in peptide scaffolds. Acta Biomater. 11 191–203. 10.1016/j.actbio.2014.09.030 [DOI] [PubMed] [Google Scholar]
- Zheng R., Duan H., Xue J., Liu Y., Feng B., Zhao S., et al. (2014). The influence of gelatin/PCL ratio and 3-D construct shape of electrospun membranes on cartilage regeneration. Biomaterials 35 152–164. 10.1016/j.biomaterials.2013.09.082 [DOI] [PubMed] [Google Scholar]
- Zhou H., Liu J., Zhou C., Gao N., Rao Z., Li H., et al. (2018). In vivo simultaneous transcriptional activation of multiple genes in the brain using CRISPR–dCas9-activator transgenic mice. Nat. Neurosci. 21 440–446. 10.1038/s41593-017-0060-66 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Liu Z., Wu Z., Wang X., Wang B., Li C., et al. (2014). Reconstruction of highly proliferative auto-tissue-engineered lamellar cornea enhanced by embryonic stem cell. Tissue Eng. Part C Methods 21 639–648. 10.1089/ten.tec.2014.0481 [DOI] [PubMed] [Google Scholar]
- Zhou X., He B., Zhu Z., He X., Zheng C., Xu J., et al. (2014). Etifoxine provides benefits in nerve repair with acellular nerve grafts. Muscle Nerve 50 235–243. 10.1002/mus.24131 [DOI] [PubMed] [Google Scholar]
- Zhou Y., Gao H. L., Shen L. L., Pan Z., Mao L. B., Wu T., et al. (2016). Chitosan microspheres with an extracellular matrix-mimicking nanofibrous structure as cell-carrier building blocks for bottom-up cartilage tissue engineering. Nanoscale 8 309–317. 10.1039/C5NR06876B [DOI] [PubMed] [Google Scholar]
- Zhou Y., Shi M., Jones J. R., Chen Z., Chang J., Wu C., et al. (2017). Strategies to direct vascularisation using mesoporous bioactive glass-based biomaterials for bone regeneration. Int. Mater. Rev. 62 392–414. 10.1080/09506608.2016.1266744 [DOI] [Google Scholar]
- Zhu C. H., Li J., Liu C., Zhou P. H., Yang H. L., Li B. (2016). Modulation of the gene expression of annulus fibrosus-derived stem cells using poly(ether carbonate urethane)urea scaffolds of tunable elasticity. Acta Biomater. 29 228–238. 10.1016/j.actbio.2015.09.039 [DOI] [PubMed] [Google Scholar]
- Zhu L., Yang J., Zhang J., Lei D., Xiao L., Cheng X., et al. (2014). In vitro and in vivo evaluation of a nanoparticulate bioceramic paste for dental pulp repair. Acta Biomater. 10 5156–5168. 10.1016/j.actbio.2014.08.014 [DOI] [PubMed] [Google Scholar]
- Zhu L., Zhang J., Xiao L., Liu S., Yu J., Chen W., et al. (2015). Autophagy in resin monomer-initiated toxicity of dental mesenchymal cells: a novel therapeutic target of N-acetyl cysteine. J. Mater. Chem. B 3 6820–6836. 10.1039/C5TB00894H [DOI] [PubMed] [Google Scholar]
- Zhu Q., Jackson A. R., Gu W. Y. (2012). Cell viability in intervertebral disc under various nutritional and dynamic loading conditions: 3d Finite element analysis. J. Biomech. 45 2769–2777. 10.1016/j.jbiomech.2012.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu S., Liu J., Zheng C., Gu L., Zhu Q., Xiang J., et al. (2017). Analysis of human acellular nerve allograft reconstruction of 64 injured nerves in the hand and upper extremity: a 3 year follow-up study. J Tissue Eng. Regener. Med. 11 2314–2322. 10.1002/term.2130 [DOI] [PubMed] [Google Scholar]
- Zhu X., Cui W., Li X., Jin Y. (2008). Electrospun fibrous mats with high porosity as potential scaffolds for skin tissue engineering. Biomacromolecules 9 1795–1801. 10.1021/bm800476u [DOI] [PubMed] [Google Scholar]
- Zhu Y. L., Kong L. Z., Farhadi F., Xia W., Chang J., He Y. H., et al. (2019). An injectable continuous stratified structurally and functionally biomimetic construct for enhancing osteochondral regeneration. Biomaterials 192 149–158. 10.1016/j.biomaterials.2018.11.017 [DOI] [PubMed] [Google Scholar]
- Zhu Z., Yang C. J. (2017). Hydrogel droplet microfluidics for high-throughput single molecule/cell analysis. Acc. Chem. Res. 50 22–31. 10.1021/acs.accounts.6b00370 [DOI] [PubMed] [Google Scholar]
- Zou J. L., Liu S., Sun J. H., Yang W. H., Xu Y. W., Rao Z. L., et al. (2018). Peripheral nerve-derived matrix hydrogel promotes remyelination and inhibits synapse formation. Adv. Funct. Mater. 28:1705739 10.1002/adfm.201705739 [DOI] [Google Scholar]