Abstract
Background
Colonoscopy is considered the gold‐standard investigation for screening and diagnosis of colorectal cancer. It is also becoming increasingly desirable for assessment, management, diagnosis and follow‐up of other colorectal diseases, such as inflammatory bowel diseases and acute diverticulitis. Hence, due to the increasing demand for colonoscopy, devices to advance examination techniques are highly sought‐after and the colonoscope with the transparent cap could be one of these.
Objectives
To identify and review all relevant data in order to determine whether colonoscopy with a transparent cap is a more effective diagnostic tool than colonoscopy.
Search methods
We searched the MEDLINE, EMBASE and CINAHL databases, and the Cochrane Central Register of Controlled Trials for all randomised controlled trials (RCTs) comparing the use of colonoscopy with a transparent cap with standard colonoscopy.
Selection criteria
Studies were included if they were randomised controlled trials which compared the use of colonoscopy with a transparent cap with standard colonoscopy.
Data collection and analysis
Data on study methods, participants, interventions used and outcomes measured was extracted from each study. Data was entered into the Cochrane Review Manager software (RevMan 5.0, 2008) and analysed using Cochrane MetaView.
Main results
In the present meta‐analysis, we considered 14 randomised controlled trials so far published. The findings of our work indicate that colonoscopy with transparent cap has a faster caecal intubation time when compared with standard colonoscopy. Reviewing studies individually would also seem to favour colonoscopy with transparent cap for polyp detection rate and pain during procedure but due to lack of comparable data meta‐analysis was not feasible.
Authors' conclusions
This review suggests that a transparent cap on the end of the colonoscope may give a marginally faster caecal intubation time compared with standard colonoscopy. It also suggests that there is a better polyp detection rate and less pain with the cap. However, the authors feel that further randomised controlled trials in this area would provide more clinically significant information on this adjunct to colonoscopy.
Plain language summary
[Transparent Cap Colonoscopy versus Standard Colonoscopy to Improve Caecal Intubation]
Fourteen randomised controlled trials were included in the review comparing Colonoscopy with the Transparent Cap with Standard Colonoscopy in the investigation of gastrointestinal tract conditions. The findings of our work suggest that there is improvement in time to caecal intubation (which indicates that the colonoscopy is complete) when using the transparent cap compared with standard colonoscopy, although this is not statistically significant. We conclude that further research is required to assess the clinical significance of this result, especially considering that there have been no adverse events noted.]
Summary of findings
Summary of findings for the main comparison. Caecal Intubation Rate for.
| Caecal Intubation Rate for | ||||||
| Patient or population: patients with Settings: Intervention: Caecal Intubation Rate | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Caecal Intubation Rate | |||||
| Total Successful Intubation Rate | Study population | OR 1.36 (0.95 to 1.93) | 5932 (12 studies) | ⊕⊕⊕⊝ moderate1 | ||
| 975 per 1000 | 981 per 1000 (974 to 987) | |||||
| Medium risk population | ||||||
| 982 per 1000 | 987 per 1000 (981 to 991) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 related to blinding of the patients and endoscopists ‐ some trials did attempt to overcome this difficulty, whilst others did not. With regards randomisation, some studies chose an inadequate randomisation method.
Background
Description of the condition
Colorectal Cancer is one of the commonest malignancies worldwide and in the UK, colorectal cancer is the 2nd leading cause of death from malignancy (Quinn 2001). Up to 90% of colorectal cancers are thought to arise from adenomas (Benson 2007) via the adenoma‐carcinoma sequence (Muto 1975). Small cancers with invasive properties are becoming increasingly recognised and account for up to 22% of early colorectal cancer (Ishii 1992; Kudo 1995). Many of these small cancers and adenomas are flat or depressed (Matsumoto 1995; Kanamori 1995; Stolte 1995; Axelrad 1996; Goto 2006), and can be difficult to detect because of their lack of protrusion (Rex 1997a; Bressler 2004). They can be detected as advanced cancers after a few years.
Evidence suggests that mortality from colorectal cancer can be reduced with early diagnosis. This is achieved by screening for early‐stage cancers or pre‐cancerous adenomas (Mandel 1993; Thiis‐Evensen 1999; Newcomb 2003). Faecal Occult Blood test (FOBt) has been used to detect colorectal cancer at an early stage (Mandel 1993; Hardcastle 1996; Kronborg 1996), and screening colonoscopy has been recommended to detect both early cancers and pre‐cancerous adenomas (Imperiale 2000; Leiberman 2000; Rex 2000; Baxter 2009). At present colonoscopy is considered the gold‐standard investigation for screening and diagnosis (Benson 2007) of colorectal cancer. It is valuable in removal of potentially pre‐cancerous lesions by polypectomy (Wolfe 1975; Winawer 1993b; Consolo 2008), which has been shown to be the most effective way of preventing colorectal cancer (Muto 1975; Winawer 1993a; Winawer 1993b; Rex 1997b).
It is not only used in the context of screening for malignant disease, but also assessment, diagnosis and follow‐up of inflammatory bowel diseases (Friedman 2001; Hurlstone 2007; Ando 2008), and evaluating the bowel after an attack of acute diverticulitis (Lahat 2008).
Description of the intervention
Colonoscopy is becoming increasingly desirable for management of colorectal disease (Matsushita 1998). Despite colonoscopy being introduced over four decades ago and many progressions in equipment and techniques, there remain limitations with the procedure. International screening programmes and improved therapeutic abilities has resulted in increasing demand for colonoscopy, and therefore the need for development of methods to improve patient acceptance and diagnostic yield. Rates of patient acceptance of the investigation vary, with problems including pre‐procedure bowel preparation (Lichenstein 2006) and discomfort during procedure (Svensson 2002). Regarding diagnostic yield, even experienced endoscopists leave a small percentage of the mucosa unexamined (Matsushita 1998; Dafnis 2000). Adenoma detection rates vary greatly between countries, from between 5% and 37.5% in diagnostic and screening procedures (Leiberman 2000; Rainis 2007). Using current technology, miss rates for polyps less than 1cm in diameter are reported to be up to 26% (Rex 1997a; Hixson 1990; van Rijn 2006) especially for de novo cancers in the right colon due to their inconspicuous protrusion (Rex 1997a; Bressler 2004). In spite of scrupulous inspection of colonic mucosa, due to the semilunar folds, small lesions can be located behind these in blind spots, where they are easily overlooked (Matsushita 1998). Techniques that aim to improve polyp detection rates include high resolution colonoscopy (Le Rhun 2006), wide angle colonoscopy (Deenadayalu 2004), chromo‐endoscopy (Hurlstone 2004; Lapalus 2004), narrowband imaging colonoscopy (Chiu 2007) and FUJI intelligent chromo‐endoscopy (Rex 2006), but unfortunately mostly these techniques increase the length of the procedure, decreasing patient acceptance. Techniques that aim to improve patient acceptance by decreasing pain and the time taken to caecal intubation, include using a variable stiffness (Yoshikawa 2004), or narrower scopes (Bat 1991; Marshall 1996; Han 2000), carbon dioxide insufflation (Church 2003) and using scope guides Suzuki 2004, but these have little effect on polyp detection. Transparent colonoscopy caps aim to improve both these aspects (Tada 1997; Matsushita 1998; Kondon 2007; Horiuchi 2008; Shida 2008).
A transparent cap attached to the tip of the colonoscope has been reported to shorten time to caecal intubation (Tada 1997; Matsushita 1998; Dafnis 2000; Kondon 2007; Horiuchi 2008; Shida 2008; Choi 2009; De Wijkerslooth 2011; Lee 2009; Rastogi 2012), reduce pain (Shida 2008), improve polyp detection and removal (Tada 1997; Matsushita 1998; Dafnis 2000; Kondon 2007; Horiuchi 2008; Shida 2008).
How the intervention might work
One of the reported main advantages of colonoscopy with the cap is the continuous good visual fields and easy recognition of the luminal continuity at bends (Matsushita 1997; Matsushita 2007). It is stated that the colonic lumen is always clearly seen since the mucosa is never in direct contact with the lens and this helps to reduce loop formation (Lee 2006). Also, it is described that with use of pressure from the cap, depressing the semilunar folds, blind areas behind the folds can be well observed (Tada 1997; Kobayashi 1998; Matsushita 1998; Cotton 1990; Dafnis 2000; Lee 2006), assisting in finding lesions hidden behind colonic folds (Tada 1997), and also increasing the number of flat adenomas/de novo carcinomas found (Kobayashi 1998). The cap is reputed to reduce the percentage of unexamined colonic mucosal surface, in spite of a colonoscopy being deemed “complete” (Cotton 1990).
Some studies reported a significantly higher detection rate of colorectal polyps when using a transparent hood than when using no hood (Tada 1997; Matsushita 1998; Kondon 2007).
The colonoscope with hood is well tolerated by patients (Tada 1997; Dafnis 2000). The transparent hood is commonly available, reusable, and inexpensive as it fits standard colonoscopies (Lee 2006).
Hood‐assissted colonoscopy has also been used as a rescue method to improve the success rate of colonoscopy when failure is encountered (Lee 2006).
Why it is important to do this review
Due to the increasing demand for colonoscopy, devices to advance examination techniques are highly sought‐after. The colonoscope with the transparent cap could be one of these.
Objectives
To identify and review all relevant data in order to determine whether colonoscopy with a transparent hood is a more effective diagnostic tool than colonoscopy without as measured by:
successful caecal intubation rate
caecal intubation time
polyp detection rate
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) comparing the use of colonoscopy with a transparent hood against standard colonoscopy.
Types of participants
Both adults and children investigated using colonoscopy as a diagnostic tool were included.
Types of interventions
Colonoscopy with a transparent hood as compared to standard colonoscopy.
Types of outcome measures
Primary outcomes
successful caecal intubation rate
caecal intubation time
polyp detection rate
Secondary outcomes
pain during procedure
analgesia/sedation used
adverse events
Search methods for identification of studies
We searched the MEDLINE, EMBASE and CINAHL databases, and the Cochrane Central Register of Controlled Trials. There was no limitation based on language or date of publication. Bibilographies of all retrieved and relevant publications identified by these strategies were searched for further studies.
Electronic searches
We searched the MEDLINE, EMBASE and CINAHL databases, and the Cochrane Central Register of Controlled Trials as above.
For comprehensive search strategies, see Appendix 1 (Medline); Appendix 2 (EMBASE).
Searching other resources
In addition, we searched additional trials through scanning of reference lists in relevant papers and conference proceedings and through correspondence with experts and pharmaceutical companies. The customized search strategy for systematic reviews was used to identify relevant articles.
Data collection and analysis
Data from the selected studies was extracted using a paper data extraction form (see attached table). Data was entered into the Cochrane Review Manager software (RevMan 5) and analysed using Cochrane MetaView.
Selection of studies
The reviewers (JM, KT, SB, HLR) independently assessed titles and abstracts of the references identified by the search strategy according to the selection criteria. Full text copies of those articles and studies that appeared to satisfy these criteria were obtained. When it was unclear from the title or abstract whether the paper fulfilled the criteria, or when there was disparity between reviewers, a full text copy was obtained. Obtained studies were individually assessed by two of the three reviewers (JM, KT, SB, HLR) and then agreement obtained as to whether include or exclude a study. Any dispute was resolved by requesting a third independent review (JM, KT, SB, HLR, RLN).
Studies were assessed for quality, with respect to methods of randomisation, allocation concealment, blinding of outcomes and drop‐out rate.
Data extraction and management
Reviewers used a piloted data extraction sheet to summarise details of the studies. Data extraction was undertaken independently by the two reviewers and compared, with any dispute being resolved by the third independent reviewer.
The following data was extracted from each study:
-
Study methods
Definition and diagnostic criteria
-
Participants
Number, source, age, gender, inclusion and exclusion criteria, duration of symptoms, previous investigations and treatments, underlying conditions
-
Interventions
Type of colonoscopy
-
Outcomes
Completion rate
Time to caecal intubation
Polyp detection rate
Associated complications (e.g. abdominal pain ‐ e.g. use of pain medication, sedation)
Unit of analysis issues ‐ Completion rate and polyp detection rate are dichotomous variables. Time to caecal intubation and associated complications were continuous variables.
Assessment of risk of bias in included studies
See risk of bias tables in Characteristics of included studies section.
The quality of the included trials was evaluated independently by the reviewers. It was assessed following the four types of bias: Selection bias; Performance bias; Attrition bias and Detection bias. Criteria for quality assessment included:
(1) Selection bias:
Allocation concealment:
A. Adequate: Use of randomisation method that did not allow investigator and participant to know or influence the allocation of treatment before eligible participants entered the studies.
B. Unclear: Randomization stated but not information on method used is available.
C. Inadequate: Use of alternate medical record numbers or unsealed envelopes as randomisation method, and/or there is information in the study indicating that investigators or participants could have influenced the allocation of treatment.
(2) Performance bias:
Blinding of care providers: Due to the nature of the intervention, the studies in this review cannot be blinded to the endoscopists.
Blinding of participants: Yes/No/Unclear
Care providers and participants are considered not blinded if the intervention group can be identified in >20% of participants because of the side effects of treatment.
(3) Detection bias: Blinding of outcome assessors: again, due to the nature of the intervention, the studies in this review cannot be blinded to the endoscopists.
(4) Attrition bias:
Intention‐to‐treat analysis:
A: Yes: All participants are analysed in the treatment group to which they were allocated, regardless of whether of not they received the allocated intervention.
B: No: Some participants (<5%, 5‐10%, 10‐20 %,> 20%) are not analysed in the treatment group to which they were randomised because they did not receive study intervention; they withdraw from the study, or because of protocol violation.
C: Unclear: Inability to determine if patients were analysed according to the intention‐to‐treat principle after contract with the authors.
Clarification from the author was sought if the published data provided inadequate information for the review. Discrepancies were resolved by consensus. From the quality assessment of the trials the potential risk of bias were summarized into three categories as described in the Cochrane handbook:
Risk of bias interpretation relationships to individual criteria
A:Low risk of bias: plausible bias unlikely. All of the criteria met therefore unlikely to seriously alter the results.
B: Moderate risk of bias: plausible bias. One or more criteria partly met, or one not met, which therefore raise some doubt about the results.
C:High risk of bias: plausible bias. Two or more criteria not met. Seriously weakens confidence in the results.
Measures of treatment effect
The outcomes with continuous variables were assessed using weighted mean difference with 95% confidence intervals. The outcomes with dichotomous variables were assessed by calculating the odds ratios (OR) with 95% confidence intervals. OR greater than 1.0 favoured the intervention group, indicating that colonoscopy with the cap is superior to colonoscopy without the cap in the measured outcomes.
Unit of analysis issues
The primary outcome of caecal intubation time is a continuous outcome (measured as the time taken to achieve caecal intubation in minutes). The primary outcome of polyp detection rate is also a continuous outcome (measured as the number of polyps detected per patient or patient sample). The analysis of continuous data used the weighted mean difference + 95% confidence intervals. The primary outcome of successful caecal intubation rate is dichotomous (yes or no). The secondary outcomes of pain during the procedure used the amount of sedation/analgesia required and was continuous and analysed as for the continuous outcomes above.
Dealing with missing data
The authors of included published studies were contacted to supply missing data for two studies, we received a reply from Yutaka Yamanji, co‐author of Kondo 2007 but received no reply from Shida 2008. Additionally, there were several conference abstracts (Hyun 2010; Hyun 2010a; Jeong 2010; Jung 2011; Lee 2011; Moon 2008; Park SH 2011; Sato 2009; Takano 2008) and an attempt was made to contact all authors to obtain data for inclusion in this review, however no responses were received. Missing data and drop‐outs/attrition was assessed for each included study, and the extent to which the result/conclusion of the review could be altered by the missing data was assessed and discussed.
Assessment of heterogeneity
As trials are conducted by different groups of investigators at different periods of time, they may be heterogeneous. We explored heterogeneity between trail results using multi‐step process including: (1) Forest plots were examined and the presence or absence of overlap in the confidence intervals noted. Lack of overlap of confidence intervals indicates heterogeneity; (2) We looked at the I2 statistic to describe the proportion of the variability in the results that was due to heterogeneity (Higgins 2008); (3) Chi‐Squared test for heterogeneity was performed and data considered heterogenous if P<0.1; (4) If significant heterogeneity was detected, possible explanations were sought.
Assessment of reporting biases
All studies reported appropriate outcomes and only two studies (Dai 2010; Tada 1997) didn't report on caecal intubation rate, however both reported on caecal intubation time.
Data synthesis
The analyses was performed in RevMan version 5.1 . Results were shown using the approach recommended in the Cochrane Handbook (Higgins 2008). Dichotomous data was presented as odds ratios (OR) with 95% confidence intervals. All randomised patients included were analysed using the intention‐to‐treat principle. We assessed heterogeneity between the trials using I2. Where the interventions were the same or similar enough, we synthesized results in meta‐analysis if there was not important clinical heterogeneity. In the case of the absence of heterogeneity, data was analysed using fixed‐effects model. Random‐effects models was used where there was unexplained heterogeneity.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analyses.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies
Results of the search
We assessed 1200 references from the primary search and additional search methods described, until 20/12/2011. From their abstracts, 29 of these were felt to potentially meet our inclusion criteria and the full papers were obtained. Meta‐analyses that were identified were also obtained and a further two randomised controlled trials were identified from hand‐searching the references. After reviewing each of the full papers, we excluded 17 of these trials (see Characteristics of excluded studies), which included conference abstracts that were lacking substantial data to warrant inclusion. A total of fourteen trials were included in the review. The fourteen trials enrolled a total of 6713 participants (range 24 ‐ 1339), of which all were adults. Thirteen of the fourteen trials were reported in English, with one study being published in Korean and translated with the help of Kim Je Young, Jo Youngjin and Professor Roman Lach (Department of German Language and Literature, Keimyung University, Daegu, Korea). The trials included in the review were published between 1997 and 2012.
Location:
Six trials were conducted in Japan, two in Korea, two in China, two in the USA, one in Netherlands and one in Australia. The trials were all conducted in a hospital setting.
Funding:
Two trials stated funding, De Wijkerslooth 2011 who received funding from the Netherlands Organisation for Health Research and Development and the Center for Translational Molecular Medicine; and Rastogi 2012 who received funding via an Endoscopic Research Center for Development Award (A. Rastogi) from the American Society for Gastrointestinal Endoscopy. The remaining trials did not state their source of funding.
Inclusion Criteria:
Trials used different inclusion criteria for the participants. Twelve studies included patients referred for colonoscopy. One study (Matsushita 1998) recruited patients that had colorectal polyps detected on barium enema and one (Park 2011) recruited those who were scheduled to undergo Endoscopic Mucosal Resection (EMR).
Participant Age:
The mean participant age ranged from 46.1 to 64.6 years. Ages of included participants ranged from 18 to 88 years.
Included studies
From 1997 to 2011, fourteen randomised controlled trials were conducted that examined the use of colonoscopy with a transparent cap compared with colonoscopy without the cap and allowed data analysis.
Excluded studies
Five trials were excluded as they were not randomised controlled trials (Dafnis 2000; Inoue 2008; Mamula 2011; Nakamura 2011; Yeung 2011), one of these (Dafnis 2000) was a pilot study. Twelve trials were excluded as they were conference abstracts and did not contain enough data for analysis and quality assessment (Horiuchi 2010; Hyun 2010; Hyun 2010a; Jeong 2010; Jung 2011; Lee 2011; Moon 2008; Park SH 2011; Sato 2009; Takano 2008; Tee 2009; Thomas 2011).
Risk of bias in included studies
1.
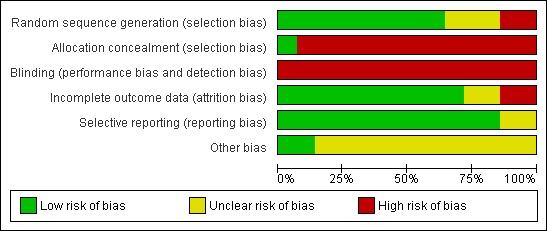
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
2.
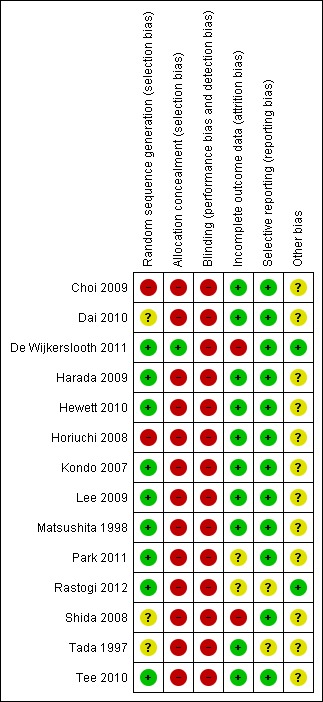
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation
Eleven of the fourteen trials reported on the generation of their allocation sequence. De Wijkerslooth 2011, Hewett 2010, Kondo 2007, Park 2011 and Tee 2010 all used computer‐generated random numbers. Matsushita 1998, Horiuchi 2008, Harada 2009, Lee 2009 and Rastogi 2012 all used sealed envelopes.Choi 2009 used the date of presentation which was deemed as inadequate. Three trials, Dai 2010, Tada 1997 and Shida 2008 didn't specify their randomisation process but did state they "randomly allocated".
Blinding
Due to the nature of the study, it is not possible to blind the endoscopists to the interventions. However, two trials, Shida 2008 and Tada 1997, reported single blinded (of the patients).
Incomplete outcome data
The following studies reported the outcomes below, however due to the use of incomparable scales or incomplete raw data, they could not be included in the meta‐analysis.
Caecal intubation rate
Two of the trials (Dai 2010 and Tada 1997) do not specifically report on caecal intubation rate, however they do report on caecal intubation time and make no comment about unsuccessful intubations or exclusions due to this. It is, therefore, appropriate to assume that their caecal intubation rate was 100% in both groups which has no impact on the meta‐analysis.
Caecal intubation time
Kondo 2007 reported caecal intubation time as an average in minutes with a range but no standard deviation or raw data. We contacted the authors and they have provided us with this raw data to use in the meta‐analysis.
Shida 2008 reported caecal intubation time as a mean average in minutes with a range but no standard deviation or raw data. We were unable to obtain the raw data.
Polyp detection rate
Twelve of the studies reported polyp detection, however there was no standardised presentation of data between studies and so we were unable to use this outcome in the meta‐analysis.
Horiuchi 2008 reported polyp detection as total of patients with at least one polyp.
Kondo 2007 reported polyp detection as total number of polyps found for the total number of patients in each category.
Matsushita 1998 was a tandem study and reported polyp detection as a percentage miss rate.
Tada 1997 reported polyp detection as a mean +/‐ standard deviation.
Choi 2009 reported polyp detection as total number of polyps detected, number (and percentage) of patients with 1 or more polyps, number of patients (and percentage) with one or more adenoma and the total number of adenomas (no./patient).
De Wijkerslooth 2011 reported polyp detection as total number of polyps detected, the total number of adenomas detected and the number of subjects with one or more adenoma.
Harada 2009 reported polyp detection as total number of patients with polyps and the total number of polyps.
Hewett 2010 was a tandems study and reported polyp detection as the number of polyps found on first and second examinations, as well as a percentage miss rate.
Lee 2009 reported polyp detection as subjects with adenomas as a percentage, as well as the number of adenomas/screened subject. They also analysed the number of subjects with advanced lesions and those with colorectal cancer.
Park 2011 was a tandem study and reported polyp detection as the mean number of polyps found (per patient) on the first examination, as well as the mean number of missed polyps per patient.
Rastogi 2012 reported polyp detection as the total number of subjects (and percentage) with adenomas, the total number of adenomas found and the mean number of adenomas per subject.
Tee 2010 reported polyp detection as the number of subjects with polyps, the total number of polyps and the total number of adenomas.
Pain during procedure
Shida 2008 reported pain using a visual analogue scale (between 1 and 100).
Tada 1997 reported pain during the procedure using a scale of 1‐4.
Choi 2009 reported pain using a visual analogue scale (between 1 and 10).
De Wijkerslooth 2011 reported pain using Gloucester Comfort Scores.
Harada 2009 reported pain using a questionnaire with the following responses: comfortable, acceptable, and intolerable.
Lee 2009 reported pain using a visual analogue scale (between 1 and 10).
Selective reporting
Our primary outcome of caecal intubation rate was reported by all authors except for Tada 1997 and Dai 2010. Both report caecal intubation time and do not comment on failed caecal intubation or exclusions, therefore it could be surmised that as they reported caecal intubation time that all colonoscopies reached the caecum, however this is not stated.
Other potential sources of bias
Twelve of the fourteen of the trials did not state whether they received funding ‐ this could be considered a potential source of bias but the authors are unable to comment further.
Effects of interventions
See: Table 1
All trials compared the used of transparent cap colonoscopy and standard colonoscopy using a combination of outcomes to assess this.
Caecal Intubation Rate:
Twelve of the fourteen trials, Choi 2009; De Wijkerslooth 2011; Harada 2009; Hewett 2010; Horiuchi 2008; Kondo 2007; Lee 2009; Matsushita 1998; Park 2011; Rastogi 2012; Shida 2008; Tee 2010, reported caecal intubation rate and all were assessed in the metanalysis. Singularly taken, five trials, De Wijkerslooth 2011; Harada 2009; Kondo 2007; Lee 2009; and Rastogi 2012, showed colonoscopy with the transparent cap to have a better caecal intubation rate than standard colonoscopy. Two trials, Shida 2008 and Tee 2010, showed standard colonoscopy to have a better caecal intubation rate than colonoscopy with the transparent cap. Five trials, Choi 2009; Hewett 2010; Horiuchi 2008; Matsushita 1998; Park 2011, showed no difference between the standard colonoscopy and colonoscopy with the transparent cap, all having a 100% caecal intubation rate. On meta‐analysis there was no significant difference between the two Analysis 1.1, however our results favoured colonoscopy with the transparent cap. We found no significant heterogeneity (Heterogeneity: Chi² = 3.25, df = 6 (P = 0.78); I² = 0%)
1.1. Analysis.
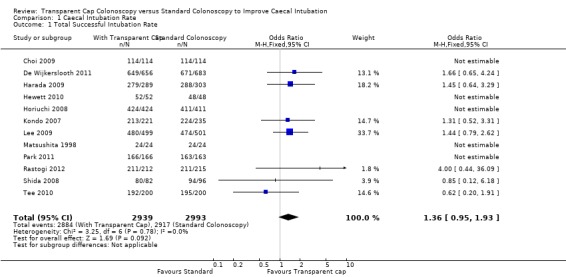
Comparison 1 Caecal Intubation Rate, Outcome 1 Total Successful Intubation Rate.
Shida 2008 also compared caecal intubation rate using a paediatric scope with a transparent cap versus a paediatric scope without. However they describe no statistical difference in this instance.
Kondo 2007 also compared the use of a "short hood" versus standard colonoscopy, however this demonstrated no significant difference in caecal intubation rate.
Caecal Intubation Time:
All trials reported on this outcome, however only thirteen of the fourteen trials, Choi 2009; Dai 2010; De Wijkerslooth 2011; Harada 2009; Hewett 2010; Horiuchi 2008; Kondo 2007; Lee 2009; Matsushita 1998; Park 2011; Rastogi 2012; Tada 1997; Tee 2010, reported caecal intubation time using comparable data for meta‐analysis. Singularly taken, nine trials, Choi 2009; De Wijkerslooth 2011; Harada 2009; Horiuchi 2008; Kondo 2007; Lee 2009; Park 2011; Rastogi 2012; Tee 2010 showed that colonoscopy with the transparent cap had a faster caecal intubation time than standard colonoscopy, with the data from Choi 2009; De Wijkerslooth 2011; Harada 2009; Horiuchi 2008; Kondo 2007; Lee 2009; and Park 2011 being statistically significant. Three trials, Dai 2010; Hewett 2010 and Tada 1997, showed that standard colonoscopy had a faster caecal intubation time than colonoscopy with the transparent cap. One trial, Matsushita 1998, showed absolutely no difference in time taken for caecal intubation between standard colonoscopy and colonoscopy with the transparent cap. Metanalysis of this data favours colonoscopy with transparent cap (‐0.80; CI ‐1.31, ‐0.30), although only by a mean of 48 seconds, Analysis 2.1. There was significant heterogeneity between this data: Tau² = 0.60; Chi² = 93.09, df = 12 (P < 0.00001); I² = 87%.
2.1. Analysis.
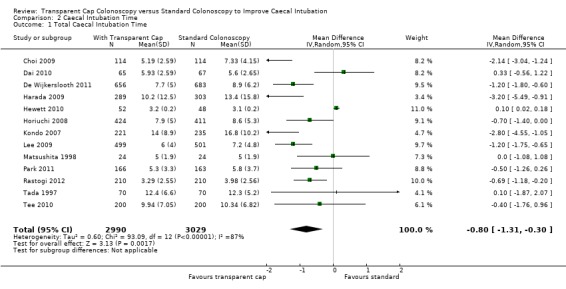
Comparison 2 Caecal Intubation Time, Outcome 1 Total Caecal Intubation Time.
Shida 2008 also reported caecal intubation time, however they used non‐comparable data, but showed that colonoscopy with the transparent cap had a significantly faster time to caecal intubation than standard colonoscopy. They also compared caecal intubation time using a paediatric scope with a transparent cap versus a paediatric scope without, which again demonstrated that colonoscopy with the transparent cap had a significantly faster time to caecal intubation than standard colonoscopy.
Kondo 2007 also compared the use of a "short hood" versus standard colonoscopy, however this demonstrated no significant difference in caecal intubation time.
Polyp Detection Rate:
Twelve of the fourteen trials, Choi 2009; De Wijkerslooth 2011; Harada 2009; Hewett 2010; Horiuchi 2008; Kondo 2007; Lee 2009; Matsushita 1998; Park 2011; Rastogi 2012; Tada 1997; and Tee 2010, reported polyp detection, however due to the variable reporting methods we were unable to use this outcome in the meta‐analysis. Singularly taken, seven of these twelve trials Hewett 2010; Horiuchi 2008; Kondo 2007; Lee 2009; Matsushita 1998; Park 2011; and Rastogi 2012 showed a significantly improved polyp detection rate using the transparent cap.
Horiuchi 2008 reported polyp detection as total number of patients with at least one polyp CAC 123 (29%) vs RC 99 (24.1%) P = 0.11 as well as the total number of adenomas CAC 205 vs RC 150 and this was significantly higher in the colonoscopy with the cap compared with standard colonoscopy (P = 0.04).
Kondo 2007 reported polyp detection as total number of polyps found for the total number of patients in each category CAC 49.3% vs RC 39.1% and this was significantly higher in the colonoscopy with the cap compared to standard colonoscopy (P = 0.04).
Matsushita 1998 was a tandem study and reported polyp detection as a percentage miss rate (with‐without 0% vs without‐with 15%). They reported that standard colonoscopy had a higher polyp miss rate than colonoscopy with the transparent cap (P=0.0125).
Tada 1997 reported polyp detection as a mean +/‐ standard deviation CAC 0.86 +/‐ 0.96 vs RC 0.58 +/‐ 0.81 (P<0.05) demonstrating a significant difference in the average number of lesions found between standard colonoscopy and colonoscopy with the transparent cap.
Choi 2009 reported polyp detection as total number of polyps detected CAC 75 vs RC 71 (P = not significant), number (and percentage) of patients with 1 or more polyps CAC 44 (38.6%) vs RC 38 (33.3%) (not significant), number of patients (and percentage) with one or more adenoma CAC 32 (28.1) vs RC 29 (25.4) (P = not significant) and the total number of adenomas (no./patient) 51 (0.45) vs 49 (0.43) (P = not significant).
De Wijkerslooth 2011 reported polyp detection as total number of polyps detected CAC 665 vs RC 682 (P = 0.71), the total number of adenomas detected CAC 339 vs RC 341 (P = 0.92) and the number of subjects with one or more adenoma CAC 196 (29) vs RC 189 (29) (P = 0.96).
Harada 2009 reported polyp detection as total number of patients with polyps CAC 120 vs RC 122 and the total number of polyps CAC 432 vs RC 456 (P = 0.8757).
Hewett 2010 was a tandems study and reported polyp detection as a percentage miss rate CAC 21% vs RC 33% (P = 0.037).
Lee 2009 reported polyp detection as subjects with adenomas as a percentage CAC 30.5% vs RC 37.5% (P = 0.018), as well as the number of adenomas/screened subject CAC 0.63 +/‐ 1.47 vs RC 0.96 +/‐ 2.86 (P = 0.023). They also analysed the number of subjects with advanced lesions and those with colorectal cancer.
Park 2011 was a tandem study and reported polyp detection as the mean number of polyps found (per patient) on the first examination CAC 2.2 +/‐ 1.7 vs RC 2.0 +/‐ 1.8 (P = 0.221), as well as the mean number of missed polyps per patient CAC 1.1 +/‐ 1.5 vs RC 0.8 +/‐ 0.9 (P = 0.024).
Rastogi 2012 reported polyp detection as the total number of subjects (and percentage) with adenomas CAC 144/210 vs RC 117/210 (P = 0.009), the total number of adenomas found CAC 474 vs RC 298 (P < 0.001) and the mean number of adenomas per subject CAC 2.3 vs RC 1.4 (P < 0.001).
Tee 2010 reported polyp detection as the number of subjects with polyps CAC 63/192 vs RC 61/195 (P = 0.75), the total number of polyps CAC 147 vs RC 107 (P = 0.59) and the total number of adenomas CAC 75 vs RC 55 (P = 0.26).
Pain During Procedure:
Six of the trials, Choi 2009; De Wijkerslooth 2011; Harada 2009; Lee 2009; Shida 2008; and Tada 1997, reported patient pain levels. However there was no standard method of presenting the results and so it was not included in the meta‐analysis. Singularly taken, three of these six De Wijkerslooth 2011; Harada 2009;and Shida 2008 showed a significantly reduced reported pain level in the colonoscopy with the transparent cap.
Shida 2008 reported pain using a visual analogue scale (between 1 and 100). Colonoscopy with the transparent cap had a significantly lower mean visual analogue score for pain than standard colonoscopy (P=0.01).
Tada 1997 reported pain during the procedure using a scale of 1‐4. They reported no significant difference in patients' discomfort when using the transparent cap compared to without it.
Choi 2009 reported pain using a visual analogue scale (between 1 and 10). CAC 2.48 vs RC 2.74 P = 0.353
De Wijkerslooth 2011 reported pain using Gloucester Comfort Scores. Overall scores were lower in the CAC group 2.0 +/‐ 1.0 vs 2.2 +/‐ 1.0 (P = 0.03).
Harada 2009 reported pain using a questionnaire with the following responses: comfortable, acceptable, and intolerable. They reported that the number of patients answering comfortable was significantly higher in the hood group (P = 0.0398) and the number of patients answering intolerable was significantly lower in the hood group (P = 0.0369).
Lee 2009 reported pain using a visual analogue scale (between 1 and 10). No significant differences in scores (CAC 3.5 +/‐ 2.9 vs 3.6 +/‐ 2.9; P = 0.71)
Discussion
Due to the increasing demand for colonoscopy, devices to advance examination techniques are highly sought‐after. The colonoscope with the transparent cap could be one of these. Fourteen randomised studies have been conducted comparing the use of a transparent cap compared with conventional colonoscopy, Choi 2009; Dai 2010; De Wijkerslooth 2011; Harada 2009; Hewett 2010; Horiuchi 2008; Kondo 2007; Lee 2009; Matsushita 1998; Park 2011; Rastogi 2012; Shida 2008; Tada 1997; Tee 2010 providing enough information for a systematic review comparing these for efficacy.
Summary of main results
In the present meta‐analysis, we considered for the all fourteen randomised controlled trials so far performed. The findings of our work indicate that colonoscopy with transparent cap has a significantly faster caecal intubation time when compared with standard colonoscopy. Reviewing studies individually would also seem to favour colonoscopy with transparent cap for polyp detection rate and pain during procedure but due to lack of comparable data meta‐analysis was not feasible.
Overall completeness and applicability of evidence
In the light of the faster caecal intubation time when using colonoscopy with transparent cap, the findings of the present meta‐analysis must be taken into consideration in the investigation of colonic pathology. We would recommend that this adjunct to colonoscopy is further investigated as to its clinical significance.
Quality of the evidence
There was no significant heterogeneity. We assessed each study individually for quality.
Choi 2009 had a poor method of randomisation, using alternate days of study period to allocate participants to each group. There was also no description as to whether all randomised patients were included in the analysis. However, appropriate outcomes were reported (including caecal intubation rate and time, polyp detection rate, pain during procedure, adverse events) and the study size was reasonable. Groups were comparable at baseline. Endoscopists had experience of more than 3000 colonoscopies.
Dai 2010 did not state the method of allocation or whether all randomised patients were included in the analysis. However appropriate outcomes were reported (including caecal intubation time, pain during procedure, distension, total procedure time) and the study size was reasonable. Groups were comparable at baseline. Two subgroups of endoscopists were included: those with at least 5 years experience and those with at least 1 year experience.
De Wijkerslooth 2011 was a large study that compared standard colonoscopy with transparent cap colonoscopy in a screening population. It used computer‐generated randomisation methods and appeared to be a well‐designed and carried‐out study. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection rate, pain during procedure, perceived burden of colonoscopy, size of adenoma analysis). Patients who did not undergo the procedure were excluded and exclusion reasons were documented. Groups were comparable at baseline. Endoscopists were considered experienced, having performed more than 1000 colonoscopies.
Harada 2009 was a large study in a screening/surveillance population. It used sealed envelope randomisation and appeared to be a well‐designed and well carried‐out study. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection rate, pain during procedure, withdrawal time, sedation/analgesia requirements). All patients were included in the study analysis. There were nearly twice as many men compared with women in the study, however groups were comparable at baseline. Six endoscopists were involved in the study 3 with >5000 caecal intubations, 1 with >3000 caecal intubations and 2 with <1000 caecal intubations.
Hewett 2010 was a fairly small study with 100 participants. It used computer‐generated randomisation and all patients were included in data analysis. Groups were comparable at baseline. The study was not blinded. Appropriate outcomes were reported (including caecal intubation time, polyp detection rate, propofol dose, missed adenomas). Endoscopists were described as experienced.
Horiuchi 2008 had an unnecessarily complex design with a small proportion of patients being allocated to a second colonoscopy. We wonder if the RCT protocol was altered during the 17 months of recruitment. The outcome of the first colonoscopy was blinded to the second investigator. The generation of their allocation sequence was inadequate, using date of presentation for randomisation, or sealed envelopes. The study was not blinded. They estimated, using historical data, the number of patients required to be at least 800 and exceeded this. Both groups were comparable at baseline. All procedures were carried out by a single endoscopist. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time and polyp detection rate), although patients factors, such as pain during procedure, were not studied. We felt the design of this study was weak.
Kondo 2007 appeared to be a well‐designed and carried‐out study. Generation of allocation sequence was adequate but the study was not blinded. A calculation of study size was carried out requiring 200 patients in each arm, which was exceeded. Recruitment to the study was open for eleven months. Groups were comparable at baseline. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection rate, trainee intubation rate, complications). We do note however that patient tolerance was not discussed, even though this may have been a reason for the attending Colonoscopist replacing the trainee.
Lee 2009 was a large study across two regional centres. A priori calculation was performed and met. Generation of allocation sequence was adequate with sealed envelopes and randomisation blocks of 10. All participants were included in the analysis and groups were comparable at baseline. There were two groups of endoscopists: 8 with at least 5 years experience and >3000 cases. 3 with at least 2 years experience and >1000 cases. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection, pain during procedure, analgesia/sedation requirements).
Matsushita 1998 was a small study. Tandem colonoscopies were carried out and recruitment and ethics for such a design may be a reason for the small numbers, particularly as these patients had already undergone barium enema which originally diagnosed their polyps. Generation of allocation sequence was adequate but the study was not blinded. No priori calculation of sample size was described. This was a cross‐over design, therefore groups were comparable. All procedures were carried out by a single endoscopist. Appropriate outcomes were reported (included caecal intubation rate, caecal intubation time, polyp detection rate, terminal ileal intubation rate, rectal retroflexion rate, polyp miss rate).
Park 2011 used a computer generated allocation sequence and was single blinded (to the patient) with allocation concealment using sealed envelopes. Patients were scheduled for elective colonoscopic EMR. There were more than twice as many men than women in the study but groups were comparable at baseline. A priori calculation was performed and met. 25 patients were excluded from analysis due to poor bowel prep. Endoscopists were described as experienced with more than 3000 colonoscopies between them. Appropriate outcomes were reported (including caecal intubation time, polyp detection rate, total procedure time, withdrawal time, polyp miss rate, time required for EMR).
Rastogi 2012 was a fairly large study in a screening/surveillance population and participants were stratified according to indication. Generation of allocation sequence was acceptable, using sealed envelopes. Patient groups were comparable at baseline. Patients were excluded from analysis when there was poor bowel prep or when the endoscopist did not reach the caecum for whatever reason. All endoscopists were considered experienced with more than 3000 colonoscopies each. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection rate, use of sedation/analgesia, polyp analysis, withdrawal time).
Shida 2008 compared two different colonoscope types and therefore multiple different caps. Generation of allocation sequence was unclear and the study was unblinded. A priori calculation of sample size was not described. We feel that comparing four interventions in a relatively small study (372 patients) may mean that this was underpowered. Groups were comparable at baseline. All procedures were carried out by a single experienced endoscopist. Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, level of pain during procedure). Difficulty of insertion was also reported, despite being operator‐dependent in an unblinded study.
Tada 1997 compared standard colonoscopy with colonoscopy with a transparent cap in a screening population. Generation of the allocation sequence was unclear and the study was unblinded. A priori calculation of sample size was not described. This was a small study (140 patients) we question why when this was recruited from a "screening population". Groups were comparable at baseline. All procedures were carried out by a single experienced endoscopist. Appropriate outcomes were reported (including caecal intubation time, level of pain during procedure and polyp detection rate). However, caecal intubation rate was not reported.
Tee 2010 used computer‐generated randomisation and patients were blinded to allocation. This was a moderate sized study and a priori calculation was performed and met.Groups were comparable at baseline and the withdrawal rate was less than 10%. Procedures were carried out by a mixture of consultant gastroenterologists (five) and trainees (ten). Appropriate outcomes were reported (including caecal intubation rate, caecal intubation time, polyp detection and complications).
Potential biases in the review process
The difficulty with blinding the endoscopist may have lead to bias, however we do not see a way to overcome this due the nature of the intervention.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis performed by Westwood 2012 et al, did demonstrate a significant difference in favour of the cap, although not in time. Ng 2011 et al have shown results comparable to our own.
Authors' conclusions
Implications for practice.
We have a demonstrated statistically significant difference between colonoscopy with the transparent cap and standard colonoscopy with regards caecal intubation time, with the time to caecal intubation being shorter by approximately 48 seconds. The clinical significance of these results remains debatable as it reduces the overall procedure time by less than a minute. We have not demonstrated any worse outcomes when using the transparent cap. We do not feel that this can recommend the introduction of transparent caps into regular clinical practice but suggest that further research into their use in routine practice and in training Colonoscopists may be beneficial and does not appear to be harmful.
Implications for research.
For the outcomes of caecal intubation rate and time, further studies looking at the effects on both experienced endoscopists and trainees would be helpful in the evaluation of this intervention. In these cases a large‐scale randomised controlled trial, with patients randomised simply to one arm or the other, would best assess this. By undertaking this type of study, overall clinical implications, such as patient satisfaction and complication rates, could be looked at if the study numbers were large enough.
To assess polyp detection, a cross‐over design may be more appropriate but careful ethical consideration would be required. Such a study may enable investigation of whether the cap has wider application as an adjunct to therapeutic procedures, such as EMR.
It has been suggested that the outcomes assessed within this review are surrogate markers for the cap having a clinical rather than a procedural advantage. A study looking at this, with outcomes such as improved survival, would need to be large with very lengthy follow‐up in the context of an internationally funded study. However, in view of the fact the basic science of this has already been thoroughly investigated and widely accepted (adenoma‐carcinoma sequence) and as such this may be deemed unnecessary.
What's new
| Date | Event | Description |
|---|---|---|
| 2 November 2012 | New citation required but conclusions have not changed | Nine more studies added this update of the review first published in 2011 issue 2 |
| 1 October 2012 | New search has been performed | Nine new RCTs were included in this updated review. |
Notes
None
Acknowledgements
Dr Tea Monk‐Hansen for initial registration of this review title.
For their contribution regarding translation of the Korean publications, the authors would like to thank:
Kim Je Young, Jo Youngjin and Professor Roman Lach, Department of German Language and Literature, Keimyung University, Daegu, Korea.
Appendices
Appendix 1. Medline search strategy
JMO 078 Medline 26.11.08
1. randomized controlled trial.pt.
2. controlled clinical trial.pt.
3. randomized.ab.
4. placebo.ab.
5. drug therapy.fs.
6. randomly.ab.
7. trial.ab.
8. groups.ab.
9. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
10. humans.sh.
11. 10 and 9
12. ((colorectal or colonic or colon or rectal or anus) adj3 (cancer or neoplasm* or carcinom* or tumor* or tumour* or polyp* or adenom*)).tw.
13. (bowel condition* or polyp*).tw.
14. exp Colonic Neoplasms/ or exp Colorectal Neoplasms/ or exp Adenoma/ or exp Colonoscopy/ or exp Rectal Neoplasms/
15. 13 or 12 or 14
16. colorectal cancer screening.tw.
17. (video capsule endoscopy or transparent cap‐fitted colonoscopy or Push enteroscopy).mp. or wireless capsule endoscopy.tw. [mp=title, original title, abstract, name of substance word, subject heading word]
18. (standard colonoscopy or conventional colonoscopy).mp. or traditional colonoscopy.tw. [mp=title, original title, abstract, name of substance word, subject heading word]
19. 18 or 16 or 17
20. 11 and 19 and 15
21. from 20 keep 1‐457
MEDLINE (Ovid) 20.12.11 – 58 hits (2008‐2011)
1. exp Colonoscopy/
2. exp Colonoscopes/
3. colonoscop*.mp.
4. 1 or 2 or 3
5. exp Endoscopy, Gastrointestinal/
6. exp Colon/
7. 5 and 6
8. (endoscop* and colon*).mp.
9. 4 or 7 or 8
10. (transparent or hood* or cap*).mp.
11. 9 and 10
12. randomized controlled trial.pt.
13. controlled clinical trial.pt.
14. randomized.ab.
15. placebo.ab.
16. clinical trial.sh.
17. randomly.ab.
18. trial.ti.
19. 12 or 13 or 14 or 15 or 16 or 17 or 18
20. humans.sh.
21. 19 and 20
22. 11 and 21
Appendix 2. EMBASE search strategy
JMO 078 Embase 26.11.08
1. (((((random$ or factorial$ or crossover$ or cross over$ or placebo$ or doubl$) adj blind$) or singl$) adj blind$) or assign$ or allocat$ or volunteer$).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
2. crossover procedure/
3. Randomized Controlled Trial/
4. single blind procedure/
5. Double Blind Procedure/
6. 1 or 2 or 3 or 4 or 5
7. ((colorectal or colonic or colon or rectal or anus) adj3 (cancer or neoplasm* or carcinom* or tumor* or tumour* or polyp* or adenom*)).tw.
8. (bowel condition* or polyp*).tw.
9. exp colorectal cancer/ or exp colorectal carcinoma/ or exp rectum carcinoma/
10. 8 or 7 or 9
11. colorectal cancer screening.tw.
12. (video capsule endoscopy or transparent cap‐fitted colonoscopy or Push enteroscopy).mp. or wireless capsule endoscopy.tw. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
13. (standard colonoscopy or conventional colonoscopy).tw.
14. 11 or 13 or 12
15. 6 and 10 and 14
16. from 15 keep 1‐129
EMBASE (Ovid) 20.12.11 – 235 hits (2008‐2011)
1. exp colonoscopy/
2. exp colonoscope/
3. colonoscop*.mp.
4. 1 or 2 or 3
5. gastrointestinal endoscopy/
6. colon/
7. 5 and 6
8. (endoscop* and (colon* or colorect*)).m_titl.
9. 4 or 7 or 8
10. (transparent or hood* or cap*).mp.
11. 9 and 10
12. randomized controlled trial/
13. randomization/
14. controlled study/
15. multicenter study/
16. phase 3 clinical trial/
17. phase 4 clinical trial/
18. double blind procedure/
19. single blind procedure/
20. ((singl* or doubl* or trebl* or tripl*) adj (blind* or mask*)).ti,ab.
21. (random* or cross* over* or factorial* or placebo* or volunteer*).ti,ab.
22. 17 or 14 or 18 or 20 or 13 or 19 or 15 or 12 or 21 or 16
23. "human*".ti,ab.
24. (animal* or nonhuman*).ti,ab.
25. 24 and 23
26. 24 not 25
27. 22 not 26
28. 11 and 27
Data and analyses
Comparison 1. Caecal Intubation Rate.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Successful Intubation Rate | 12 | 5932 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.95, 1.93] |
Comparison 2. Caecal Intubation Time.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total Caecal Intubation Time | 13 | 6019 | Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.31, ‐0.30] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Choi 2009.
| Methods | Generation of allocation sequence: Randomised according to date of endoscopy.Allocation concealment: Not stated.Blinding: Not applicable.Inclusion of all randomised patients: Not stated. | |
| Participants | Number: 228 (108M:1206F) Age: Cap group mean 46.1 +/‐ 11.1 years. Standard group mean 48.5 +/‐ 10.9 years. Source: Yang Hospital, Seoul. Inclusion criteria: All patients undergoing colonoscopy. Exclusion criteria: Prior colonic surgery, colonic obstruction or obstructing tumour. |
|
| Interventions | Colonoscope: not stated. Bowel Preparation: 4L polyethylene glycol/ or 50mg sodium phosphate. Intra‐porcedure medication: Midazolam, Propofol, Oxygen. Colonoscopists: Experienced colonoscopists having performed >3000 colonoscopies. Cap: D‐201‐13404 (Olympus, Tokyo, Japan) transparent plastic cap: 13.7mm outer diameter, 11.7mm inner diameter, depth 8mm, protruding 4mm ahead of scope. |
|
| Outcomes | Included in review: Caecal intubation rate and time, polyp detection rate, pain during procedure, adverse events. | |
| Notes | Location: Korea. Source of funding: Not stated. Attempts to clarify information: Translation from Korean. Language of Publication: Korean. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate method ‐ randomised by alternate days. |
| Allocation concealment (selection bias) | High risk | Not blinded. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data appears complete. |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
Dai 2010.
| Methods | Generation of allocation sequence: Not stated. Allocation concealment: Not stated. Blinding: Not stated. Inclusion of all randomised patients: Not stated. |
|
| Participants | Number: 250 (134M:116F) Age: Cap group mean 49.4 years (range 22‐77). Regular group mean 53.3 years (range 18‐78). Source: Renji Hospital, Shanghai. Inclusion criteria: Patients undergoing outpatient colonoscopy. Exclusion criteria: Prior colonic surgery, IBD, colonic stricture or tumour, poor bowel preparation. |
|
| Interventions | Colonoscope: CF‐240 (Olympus, Tokyo, Japan). Bowel Preparation: 2L polyethylene glycol/electrolyte lavage solution (Shenzhen Wanhe, Shenzhen, China) 6hours before. Intra‐porcedure medication: 10mg Scopolamine, 10mg Diazepam I.M. Colonoscopists: 2 subgroups ‐ experienced (at least 5 years experience) and inexperienced (1 year experience). Caps: D‐201‐13404 (Olympus, Tokyo, Japan) transparent plastic cap: 13.7mm outer diameter, 11.7mm inner diameter, depth 8mm, protruding 4mm ahead of scope. |
|
| Outcomes | Included in review: Caecal intubation time, pain during procedure Excluded from review: Distension, total procedure time. |
|
| Notes | Location: China Source of funding: Not stated Attempts to clarify information: Not required. Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | High risk | Not blinded to endoscopist |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated but not blinded to endoscopist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data appears complete. |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
De Wijkerslooth 2011.
| Methods | Generation of allocation sequence: Computer randomisation program. Patients stratified by age, sex and screening center using random block sizes of maximum 6 per block. Allocation concealment: Unclear. Blinding: Single blinded. Inclusion of all randomised patients: No. Did not include data for patients who did not undergo the intervention (reasons included withdrawal (26), absence of trained endoscopist (13) and technical problem (2). |
|
| Participants | Number: 1339 (685M:654F) Age: 60 (50‐65) in CC group; 60 (55‐65) in CAC group (years; median (IQR)). Source: Academic Medical Center, Amsterdam & Erasmus Medical Center, Roterdam. Inclusion criteria: All patients undergoing screening colonoscopy. Exclusion criteria: Full colonoscopic examination within the previous 5 years, surveillance colonoscopy, subjects with end‐stage disease and previous colonic resection. |
|
| Interventions | Colonoscopes used: CF‐Q160, CF‐Q180, PCF‐Q180 variable stiffness (Olympus Medical Systems, Tokyo, Japan). Bowel Preparation: low fibre diet, 2litres transparent fluid, 2litred polyethylene glycol (Moviprep; Norgine, Amsterdam). Intra‐procedure medications: IV midazolam, IV fentanyl as required. Endoscopist: "Experienced" (at least 1000 colonoscopies and at least 20 CAC procedures). Caps: Transparent cap protruding 4mm: D‐201‐12704 (13.4mm diameter) or D‐201‐14304 (15mm diameter) (Olympus Medical Systems, Tokyo, Japan). |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, Polyp detection rate, Pain during procedure Excluded from review: perceived burden of colonoscopy, Size of adenoma analysis. |
|
| Notes | Location: Netherlands. Source of funding: The Netherlands Organisation for Health Research and Development; Center for Translational Molecular Medicine. Attempts to clarify information: Not required. Language of Publication: English. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Participants and Endoscopists were blinded to randomisation until start of procedure. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded to endoscopist. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Patients excluded from data analysis if did not have procedure. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None found. |
Harada 2009.
| Methods | Generation of allocation sequence: Sealed envelopes Allocation concealment: Not applicable as not blinded. Blinding: Not stated but unblinded to endoscopists Inclusion of all randomised patients: Yes |
|
| Participants | Number: 592 (391M:201F) Age: Hood group ‐ 62.6 years +/‐ 63.7. No Hood ‐ 62.7 years +/‐ 64.0 (mean) Source: A single Japanese tertiary referral centre Inclusion criteria: Screening/surveillance total colonoscopy. Exclusion criteria: Emergency, therapeutic or EUS procedures. Poor bowel preparation. |
|
| Interventions | Colonoscope: PCF‐Q240ZI, PCF‐Q260AI, PCF‐P240AI (Olympus Optical Co, Ltd, Japan). Bowel Preparation: Colonic lavage with polyethylene glycol. Intra‐procedure medication: 2‐5mg midazolam as required for pain IV, Scopolamine butyl bromide or glucagon as antispasmodic. Endoscopists: 3 with >5000 caecal intubations, 1 with >3000 caecal intubations and 2 with <1000 caecal intubations. Cap: D‐201‐13404, D‐201‐012704, D‐201‐11804 (Olympus Medical Systems, Tokyo, Japan). |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, polyp detection rate, Pain during procedure Excluded from review: Withdrawal time (?sedation/analgesia requirements) |
|
| Notes | Location: Japan Source of funding: Not stated Attempts to clarify information: Not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes. |
| Allocation concealment (selection bias) | High risk | Not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded to endoscopist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in data analysis. |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
Hewett 2010.
| Methods | Generation of allocation sequence: computer generated. Allocation concealment: Not applicable as not blinded Blinding: Not stated but not blinded to endoscopist Inclusion of all randomised patients: Yes |
|
| Participants | Number: 100 (57M:43F) Age: group 1: mean age 61.0 years +/‐ 1.0. Group 2: 62.9 +/‐1.2. Source:Indiana University Hospital, USA. Inclusion criteria: Patients 50 years of age or older and able to give informed consent, scheduled for elective colonoscopy. Exclusion criteria: ASA III or more, previous surgical resection of colon or rectum, IBD, current anticoagulant use. |
|
| Interventions | Colonoscope: High‐definition videocolonoscopes CF‐H180AL (Olympus, America). Bowel preparation: not stated. Intra‐procedure medication: propofol sedation "endoscopist‐directed". Endoscopist: "experienced". Caps: Soft, transparent plastic cap 13.4mm outer diameter, 4mm protrusion. (D‐201‐12704, Olympus). |
|
| Outcomes | Included in review: Caecal intubation time, Polyp detection rate Excluded from review: propofol dose, missed adenomas |
|
| Notes | Location: USA Source of funding: Not stated Attempts to clarify information: Not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded to endoscopist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data used for analysis |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes measured |
| Other bias | Unclear risk | Source of funding not stated. |
Horiuchi 2008.
| Methods | Generation of allocation sequence: For first colonoscopy randomised by date of presentation, for second colonoscopy randomised by sealed opaque envelopes. Allocation concealment: not applicable as not blinded. Blinding: the endoscopist was not blinded to the intervention itself (transparent retractable extension) but the endoscopist in the second colonoscopy was blinded to the findings from the first colonoscopy. Inclusion of all randomised patients: All randomised patients were included in data analysis. |
|
| Participants | Number: 835 Age: 63.7 +/‐ 11 in group 1 and 64.6 +/‐13 in group 2 Source: Showa Inan General Hospital, Komagane, Japan. Inclusion criteria: consecutive patients undergoing screening colonoscopy in whom the endoscopist evaluated greater that 90% of mucosa seen during endoscopy. Exclusion criteria: previous colorectal surgical resection, less than 20 years of age, pregnancy, ASA class 3 or 4, overweight (body weight >100kg) and allergy to the drugs used. |
|
| Interventions | 7mm Olympus PCF Q260A1 transparent retractable extension (TRE) device fitted onto a paediatric, variable stiffness, colonoscope. Bowel preparation using polyethylene glycol electrolyte solution. All procedures we conducted under nurse‐administered propofol sedation. Between august 2005 and December 2006, consecutive patients were enrolled. Colonoscopy with or without transparent retractable extension was then undertaken by one endoscopist. A subset of patients with colonic adenomas were randomised to a repeat colonoscopy with or without the TRE. Adenoma removal was performed at the second colonoscopy. | |
| Outcomes | Included in review: Caecal intubation time, caecal intubation rate, polyp detection rate Excluded from review: none |
|
| Notes | Location: Showa Inan General Hospital, Komagane, Japan. Source of funding: Not stated Attempts to clarify information: not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | For first colonoscopy randomised by date of presentation, for second colonoscopy randomised by sealed opaque envelopes. |
| Allocation concealment (selection bias) | High risk | Not concealed to endoscopist. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded to endoscopist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients were included in data analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
Kondo 2007.
| Methods | Generation of allocation sequence: Patients were randomly assigned to transparent hood, short hood or no hood on the basis of computer‐generated random numbers. Allocation concealment: Although the allocation was concealed until each examination, the Colonoscopists and possibly the patients, noticed the allocation. Blinding: Unblinded Inclusion of all randomised patients: All randomised patients were included in data analysis. |
|
| Participants | Number: 684 Age: 62.0 (19‐88) years Source: Patients referred for total colonoscopy at University of Tokyo Hospital between July 2004 and May 2005. Inclusion criteria: Consecutive patients referred for total colonoscopy giving written, informed consent. Exclusion criteria: Prior colonic resection, known fulminant colitis, severe hematochezia. |
|
| Interventions | All patients underwent bowel preparation with sodium pico sulphate the day before the examination and two litres of polyethylene glycol‐containing lavage solution on the day of examination. Scopolamine butyl bromide 20mg or glucagon 1IU, was administered intra‐muscularly. No sedatives were used. Either Olympus PCF 230 or PCF 240I colonoscopes were used for all examinations. Each colonoscopic examination was started by one of fourteen trainees with less than 1000 cases of colonoscopy experience. They performed colonoscopy under supervision of attending Colonoscopist. If the trainee failed to reach the caecum within 15 minutes, or the patient complained of intolerable pain, the attending Colonoscopist replaced the trainee and continued the examination. In all cases examinations during withdrawal of the scope were performed by trainees. The patients were randomly assigned to the: Transparent Hood (Olympus D‐201‐12704) made of thermoplastic elastomer. The hood has an outer diameter of 12.6mm and an inner diameter of 10.6mm. The portion of the hood that sticks out from the tip is 4mm. The edge of this hood comes into the field of vision of the colonoscope but objects can be seen through the transparent wall. Short Hood (Olympus MB46) made of fluorine rubber. The hood has a distal end with an outer diameter of 12mm and an inner diameter of 10.6mm. The length is 2mm. The hood does not come into the field of vision of the colonoscope. No hood. |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time and polyp detection rate Excluded from review: Complications, trainee intubation rate |
|
| Notes | Location: University of Tokyo Hospital, Japan Source of funding: Not stated Attempts to clarify information: attempts made to contact authors for raw data on caecal intubation time. Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned to transparent hood, short hood or no hood on the basis of computer‐generated random numbers. |
| Allocation concealment (selection bias) | High risk | Not concealed as unblinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias | Unclear risk | Source of funding not stated |
Lee 2009.
| Methods | Generation of allocation sequence: Sealed opaque envelopes, randomisation blocks of 10 Allocation concealment: No stated. Blinding: Not stated. Inclusion of all randomised patients: Yes |
|
| Participants | Number: 1000 (460M: 540F) Age: CAC group: 52.6 +/‐ 14.0 years. RC group 52.6 +/‐ 13.8 years Source: 2 regional endoscopy centres. Inclusion criteria: Aged 18 years or over, undergoing first colonoscopy and could provide written consent. Exclusion criteria: Prior colonoscopy or prior colonic surgery (except appendicectomy), having colonic stricture or obstructing tumour. Acute surgical conditions or acute GI bleeding. |
|
| Interventions | Colonoscope: Regular colonoscopes without variable stiffness: CF‐240 or CF‐Q240 (Olympus Medical Systems, Tokyo, Japan). Bowel preparation: 3 day of low‐residue diet. Either 4L polyethylene glycol (Klean‐Pred, Harefield, UK) or 90ml phosphosoda (Fleet, CB Fleet, Virginia, USA). Intra‐procedure medication: Diazepam 0.05mg/kg IV, pethidine 0.25mg/kg IV. Endoscopist experience: 8 had at least 5 years experience and >3000 cases. 3 had at least 2 years experience and >1000 cases. Caps: Mucosectomy cap MAJ‐665 or MH‐596 (Olympus Medical Systems Corp). |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, Polyp detection, Pain during procedure Excluded from review: Analgesia/sedation requirements? Complications. |
|
| Notes | Location: China Source of funding: Not stated Attempts to clarify information: Not necessary Language of Publication: Englilsh |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | High risk | Not concealed as not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated but endoscopist not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised patients included in analysis |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported |
| Other bias | Unclear risk | Source of funding not stated. |
Matsushita 1998.
| Methods | Generation of allocation sequence: randomisation was carried out using closed envelopes. Allocation concealment: not applicable as not blinded. Blinding: Unblinded. Inclusion of all randomised patients: All randomised patients were included in data analysis. |
|
| Participants | Number: 24 Age: 58.6 (42‐77) years Source: 24 patients with colorectal polyps detected at previous barium enema examination in Tenri Hospital, Nara, Japan. Inclusion criteria: Previous polyps detected at barium enema examination. Exclusion criteria: Previous abdominal or pelvic surgery, or if it was considered their general medical condition was poor. |
|
| Interventions | Tandem colonoscopies were carried out, randomly either first without a transparent cap (Obliclear, Top, Tokyo, Japan) and then with the cap (without‐to‐with) or first with the cap and then without (with‐to‐without) in the 24 patients. All patients were initially prepared with Polyethylene glycol solution and they received no pre‐medications. The Olympus CF2001 scope was used for all procedures. All polyps were removed whilst removing the colonoscope, intubation of the terminal ileum was attempted in all cases, as was retroflexion in the rectum. | |
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, polyp detection rate. Excluded from review: polyp miss rate, retroflexion within rectum, and terminal ileal intubation rates. |
|
| Notes | Location: Department of gastroenterology, Tenri Hospital, Nara, Japan Source of funding: Not stated Attempts to clarify information: not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Closed envelopes |
| Allocation concealment (selection bias) | High risk | Not concealed as not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes assessed |
| Other bias | Unclear risk | Source of funding not stated |
Park 2011.
| Methods | Generation of allocation sequence: Computer generated Allocation concealment: Sealed envelopes Blinding: Single blinded (patient only) Inclusion of all randomised patients: No (25 excluded due to poor bowel prep) |
|
| Participants | Number: 329 (235M:94F) Age: CAC group 61.3 +/‐ 10.3 years. RC 59.3 +/‐ 10.2 years. Source: Chonnam National University Hospital Inclusion criteria: Patients 18 years or older who were able to give informed consent and were scheduled for elective colonoscopic EMR. Exclusion criteria: Previous colorectal surgery, colonic stricture, obstructing tumour, other acute surgical conditions (such as severe colitis, toxic megacolon, Ischaemic colitis or acute GI bleed). |
|
| Interventions | Colonoscope: Regular colonoscopes without variable stiffness function: CF‐240 or CF‐Q240 (Olympus Medical Systems, Tokyo, Japan. Bowel Preparation: 3 day low residue diet, followed by 4L polyethylene glycol solution. Intra‐procedure medications: not stated. Endoscopist experience: "Experienced" >3000 procedures between them. Caps: Soft, transparent plastic cap, 14mm outer diameter and protrudes 4mm. D‐201‐12704 (Olympus). |
|
| Outcomes | Included in review: Caecal intubation time, polyp detection rate Excluded from review: Total procedure time, withdrawal time, polyp miss rate, time required for EMR. |
|
| Notes | Location: South Korea Source of funding: Not stated Attempts to clarify information: Not necessary Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Not concealed as not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Endoscopist not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 25 excluded from analysis due to poor bowel prep. |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
Rastogi 2012.
| Methods | Generation of allocation sequence: Opaque, sealed envelopes. Stratified by indication (screening or surveillance). Allocation concealment: Not stated Blinding: Not stated Inclusion of all randomised patients: No ‐ patients with inadequate bowel prep or where they could not reach the caecum were excluded from analysis. |
|
| Participants | Number: 427 randomised, 420 analysed (398M:22F) Age: CAC group 60.7 +/‐ 7.2 years. Standard group 61.3 +/‐ 7.3 Source: Tertiary care Veterans Affairs Medical Center, USA. Inclusion criteria: Referral for screening or surveillance colonoscopy and the ability to provide informed consent. Exclusion criteria: Previous surgical colonic resection, history of colorectal cancer or IBD, use of antiplatelet agents or anti‐coagulants precluding removal of polyps, poor general condition or any other reason to avoid prolonged procedure time, history of polyposis syndrome or HNPCC, inability to give informed consent. |
|
| Interventions | Colonoscope: CF‐H180AL (Olympus America Inc, Centervalley, Pennsylvania, USA). Bowel preparation: not stated. Intra‐procedure medication: IV midazolam +/‐ Meperidine or Fentanyl. Endoscopist: "Experienced" with more than 3000 colonoscopies each and more than 20 CAC procedures. |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, Polyp detection rate Excluded from review: use of sedation/analgesia, polyp analysis, withdrawal time |
|
| Notes | Location: USA Source of funding: Endoscopic Research Center for Development Award (A. Rastogi) from the American Society for Gastrointestinal Endoscopy. Attempts to clarify information: Not required. Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | High risk | Not concealed as not blinded |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated but endoscopist not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Patients excluded from data analysis when caecum not reached or when bowel prep was poor. |
| Selective reporting (reporting bias) | Unclear risk | Appropriate outcomes all reported. |
| Other bias | Low risk | None identified. |
Shida 2008.
| Methods | Generation of allocation sequence: "randomly assigned" Allocation concealment: Not stated. Blinding: Unblinded to endoscopist, blinded to patient ‐ single blinded. Inclusion of all randomised patients: All randomised patients were included in data analysis. |
|
| Participants | Number: 372 Age: range 28‐86 years Source: Patients undergoing colonoscopy between April 2006 and March 2007 at Omigawa Hospital, Chiba, Japan. Inclusion criteria: All patients undergoing colonoscopy in the allocated time frame. Exclusion criteria: Patients with prior abdominal surgery, including hysterectomy, obstructing tumours of the distal colon, and poor bowel preparation. |
|
| Interventions | All patients underwent bowel preparation with 2 litres of polyethylene glycol lavage solution. Scopolamine butyl bromide 20mg or glucagon 1IU was administered intramuscularly. No sedatives were used. Patients were examined by standard colonoscope with transparent hood (range of hoods used, depending on scope), standard colonoscope without transparent hood, small calibre colonoscope with transparent hood, and small calibre colonoscope without transparent hood. | |
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, Pain during procedure Excluded from review: Difficulty of insertion. |
|
| Notes | Location: Omigawa Hospital, Chiba, Japan Source of funding: Not stated Attempts to clarify information: attempts made to contact authors for raw data on caecal intubation time. Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blinded (to patient) but not to endoscopist |
| Incomplete outcome data (attrition bias) All outcomes | High risk | None identified |
| Selective reporting (reporting bias) | Low risk | Appropriate outcomes assessed |
| Other bias | Unclear risk | Source of funding not stated |
Tada 1997.
| Methods | Generation of allocation sequence: "Randomly allocated". Allocation concealment: Not stated. Blinding: Unblinded to endoscopist. Blinded to patient. Inclusion of all randomised patients: All randomised patients were included in data analysis. |
|
| Participants | Number: 140 Age: 61.1 +/‐ 10.5 years in Group 1, 59.1 +/‐ 11.5 years in Group 2 Source: Patients attending for screening colonoscopy at First Department of Surgery, Tokyo Medical and Dental University, Japan. Inclusion criteria: All patients recommended to undergo screening colonoscopy at this unit. Exclusion criteria: No previous abdominal surgery. |
|
| Interventions | Screening colonoscopy was undertaken with an Olympus optical‐core colonoscope or an Olympus optical‐core colonoscope with a 10mm transparent plastic cap (17mm outer diameter, 2mm wall thickness and 10mm in length). | |
| Outcomes | Included in review: Caecal intubation time, Polyp detection rate and Pain during procedure. Excluded from review: Endoscopic Mucosal resection of flat lesions vs strip biopsy. |
|
| Notes | Location: First department of Surgery, Tokyo Medical and Dental University Source of funding: Not stated Attempts to clarify information: not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned" |
| Allocation concealment (selection bias) | High risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blinded (to patient not endoscopist) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None |
| Selective reporting (reporting bias) | Unclear risk | Caecal intubation rate was not reported |
| Other bias | Unclear risk | Source of funding not described |
Tee 2010.
| Methods | Generation of allocation sequence: Computer generated Allocation concealment: Not stated but unblinded to endoscopists Blinding: Single blinding Inclusion of all randomised patients: Yes |
|
| Participants | Number: 400 (190M:210F) Age: CAC group 53.8 +/‐ 15.1 years. Standard group 53.6 +/‐ 14.8 years. Source: A tertiary referral hospital ‐ Royal Prince ALfred Hospital Sydney, Australia. Inclusion criteria: All patients referred to the endoscopy service for colonoscopy, aged over 18 years. Exclusion criteria: Prior colonic resection, pregnancy, severe co‐morbidities, acute surgical conditions, acute GI bleeding, inability to provide consent. |
|
| Interventions | Colonoscopes: CF‐Q160AL, CF‐Q180AL, PCF‐180AL, PCF‐160AL (Olympus Optical Co., Tokyo, Japan). Bowel Preparation: Clear fluid diet the day before with either sodium picosulfate (Pcioprep, Pharmatel Fresenius Kabi Pty Ltd, Hornby, Australia) or sodium‐phospate (Fleet, Ferring Pharmaceuticals, Gordon, Australia) and fasting for 8 hours. Intra‐procedure medication: IV Midazolam (Pfizer, Bentley, Australia), Fentanyl (Mayre Pharma LTd, Mulgrave, Australia) and Propofol (Fresofol 1%; Pharmatel Fresenius Kabi Pty Ltd.) +/‐ antispasmodic with hyoscine butyl bromide. Endoscopist: 5 consultants and 10 trainees. Caps: 3 sizes of cap (different diameters for each scope ‐ 15.3mm, 14.6mm and 13.0mm) all protruding 4mm: D‐201‐15004, D‐201‐14304, D‐201‐12704 (Olympus Medical Systems, Tokyo, Japan). |
|
| Outcomes | Included in review: Caecal intubation rate, caecal intubation time, Polyp detection. Excluded from review: Complications |
|
| Notes | Location: Australia Source of funding: Not stated Attempts to clarify information: Not required Language of Publication: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Not stated but not blinded to endoscopist |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not stated but not blinded to endoscopist |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in analysis |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes reported. |
| Other bias | Unclear risk | Source of funding not stated. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dafnis 2000 | This was a pilot study and not a randomised controlled trial. |
| Horiuchi 2010 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Hyun 2010 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Hyun 2010a | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Inoue 2008 | Not a randomised controlled trial comparing standard colonoscopy with cap‐assisted colonoscopy. |
| Jeong 2010 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Jung 2011 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Lee 2011 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Mamula 2011 | Not a randomised controlled trial. |
| Moon 2008 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Nakamura 2011 | Not a randomised controlled trial comparing standard colonoscopy with transparent‐cap fitted colonoscopy. |
| Park SH 2011 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Sato 2009 | Comparison between an oblique transparent cap colonoscope and magnetic endoscope imaging (MEI): not a randomised controlled trial comparing standard colonoscopy with transparent‐cap fitted colonoscopy. |
| Takano 2008 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Tee 2009 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Thomas 2011 | Conference abstract only. Data not reported in full. No information regarding quality issues available in publication. Efforts made to find contact details in order to obtain further data but not found. Recommendation: await full publication of results. |
| Yeung 2011 | Not a randomised controlled trial. |
Contributions of authors
| Draft the protocol | JM, KT, HLR, RLN |
| Develop a search strategy | JM, KT, HLR, RLN |
| Search for trials | JM, SB, KT, HLR, RLN |
| Select which trials to include | JM, SB, KT, HLR, RLN |
| Extract data from trials | JM, SB, KT, HLR, RLN |
| Enter data into RevMan | JM |
| Carry out the analysis | JM, KT |
| Interpret the analysis | JM, KT |
| Draft the final review | JM, SB, KT, HLR, RLN |
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Choi 2009 {published data only}
- Choi DH, Shin HK, Lee YC, Lim CH, Jeong SK, Lee S‐H, et al. Efficacy of Transparent Cap‐attached Colonoscopy: Does It Improve the Quality of Colonoscopy?. J Korean Soc Coloproctol 2009;26(2):116‐22. [Google Scholar]
Dai 2010 {published data only}
- Dai J, Feng N, Lu H, Li XB, Yang CH, Ge ZZ. Transparent cap improves patients' tolerance of colonoscopy and shortens examination time by inexperienced endoscopists.. Journal of digestive diseases 2010;11:264‐368. [DOI] [PubMed] [Google Scholar]
De Wijkerslooth 2011 {published data only}
Harada 2009 {published data only}
- Harada Y, Hirasawa D, Fuijita N, Noda Y, Kobayashi G, Ishida K, Yonechi M, Ito K, Suzuki T, Sugawara T, Horaguchi J, Takasawa O, Obana T, Oohira T, Onochi K, Kanno Y, Kuroha M, Iwai W. Impact of a transparent hood on the performance of total colonoscopy: a randomised controlled trial.. Gastrointestinal Endoscopy 2009;69:637‐644. [DOI] [PubMed] [Google Scholar]
Hewett 2010 {published data only}
- Hewett DG, Rex DK. Cap‐fitted colonoscopy: a randomized, tandem colonoscopy study of adenoma miss rates. Gastrointestinal Endoscopy 2010;72:775‐781. [DOI] [PubMed] [Google Scholar]
Horiuchi 2008 {published data only}
- Horiuchi A, Nakayama Y. Improved Colorectal Adenoma Detection With a Transparent Retractable Extension Device. American Journal of Gastroenterology 2008;103:341‐345. [DOI] [PubMed] [Google Scholar]
Kondo 2007 {published data only}
- Kondo S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, et al. A Randomized Controlled Trial Evaluating the Usefulness of a Transparent Hood Attached to the Tip of the Colonoscope. American Journal of Gastroenterology 2007;102:75‐81. [DOI] [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee YT, Lai LH, Hui AJ, Wong VW, Ching JY, Wong GL, et al. Efficacy of cap‐assisted colonoscopy in comparison with regular colonoscopy: a randomized controlled trial. American Journal of Gastroenterology 2009;104:41‐46. [DOI] [PubMed] [Google Scholar]
Matsushita 1998 {published data only}
- Matsushita K, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Efficacy of Total Colonoscopy with a Transparent Cap in Comparison with Colonoscopy without the Cap. Endoscopy 1998;30(5):444‐7. [DOI] [PubMed] [Google Scholar]
Park 2011 {published data only}
- Park SY, Kin HS, Yoon KW, Cho SB, Lee WS, Park CH, et al. Usefulness of cap‐assisted colonoscopy during colonoscopic EMR: A randomized controlled trial.. Gastrointestinal Endoscopy 2011;74:869‐875. [DOI] [PubMed] [Google Scholar]
Rastogi 2012 {published data only}
- Rastogi A, Bansal A, Rao DS, et al. Higher adenoma detection rates with cap‐assisted colonoscopy: a randomised controlled trial. Gut 2012;16:402‐408. [DOI] [PubMed] [Google Scholar]
Shida 2008 {published data only}
- Shida T, Katsuura Y, Teramoto O, Kaiho M, Takano S, Yoshidome H, et al. Transparent hood attached to the colonoscope: does it really work for all types of colonoscopes?. Surgical Endoscopy 2008;22(12):2654‐8. [DOI] [PubMed] [Google Scholar]
Tada 1997 {published data only}
- Tada M, Inoue H, Yabata E, Okabe S, Endo M. Feasibility of the Transparent Cap‐Fitted Colonoscope for Screening and Mucosal Resection. Diseases of the Colon and Rectum 1997;40(5):618‐621. [DOI] [PubMed] [Google Scholar]
Tee 2010 {published data only}
- Tee HP, Corte C, Al‐Ghamdi H, Prakoso E, Darke J, Chettiar R, et al. Prospective randomized controlled trial evaluating cap‐assisted colonoscopy vs standard colonoscopy.. World Journal of Gastroenterology 2010;16:3905‐3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Dafnis 2000 {published data only}
- Dafnis G. Technical Considerations and Patient Comfort in Total Colonoscopy with and without a Transparent Cap: Intial Experiences from a Pilot Study.. Endoscopy 2000;32(5):381‐384. [DOI] [PubMed] [Google Scholar]
Horiuchi 2010 {published data only}
- Horiuchi A, Nakayama Y. Is hood‐assisted colonoscopy more effective than narrow band imaging for colorectal adenoma detection?. Gastrointestinal Endoscopy. 2009; Vol. 69:AB130.
Hyun 2010 {published data only}
- Hyun LJ. Clinical usefulness of cap fitted colonoscopy in inexperienced colonoscopist: A randomized Controlled study. Journal of Gastroenterology and Hepatology. 2010; Vol. 25, issue (Suppl 2):A23‐A78.
Hyun 2010a {published data only}
- Hyun YS, Han DS, Bae JH, Park HS, Ahn SB, Kim TY, et al. A randomized tandem colonoscopy trial of transparent hood attached versus conventional examination to compare polyp miss rate.. Gastrointestinal Endoscopy 2010;71(5):AB332. [Google Scholar]
Inoue 2008 {published data only}
- Inoue T, Abe S‐Y, Murano M, Umegaki E, Higuchi K. Recommendation of a newly developed slit‐and‐hole‐type elastic hood for detection of more colon polyps.. Journal of Gastroenterology 2008;43:809‐810. [Google Scholar]
Jeong 2010 {published data only}
- Jeong JY, Han DS, Yu YH, Hyun YS, Bae JH, Park HS, et al. A randomized tandem colonoscopy trial of transparent hood attached versus conventional examination to compare polyp miss rate.. Journal of Gastroenterology and Hepatology 2010;25(suppl 2):A46. [Google Scholar]
Jung 2011 {published data only}
- Jung SH, Park SJ, Park MI, Moon W, Kim HH. Efficacy of transparent cap‐fitted colonoscopy in cecal intubation time and detection of lesions compared with non‐cap fitted colonoscopy.. Journal of Gastroenterology and Hepatology 2011;26(suppl 5):59. [Google Scholar]
Lee 2011 {published data only}
- Lee JS, Choi SH, Choi SW, Park HS, Kwak SD, Hwang S. Efficacy of hood‐cap associated colonoscopy: Comparison with regular colonoscopy, prospective study. . Gastrointestinal Endoscopy.. 2011; Vol. 73, issue 4S:AB410.
Mamula 2011 {published data only}
- Mamula P, Tierney WM, Banerjee S, Desilets D, Diehl DL, Farraye FA, et al. Devices to improve colon polyp detection.. Gastrointestinal Endoscopy 2011;73:1092‐1097. [DOI] [PubMed] [Google Scholar]
Moon 2008 {published data only}
- Moon W, Park SJ, Song JY, Park MI, Kim KJ. The Usefulness of Total Colonoscopy with a Transparent Cap. Gastroenterology 2008;134(suppl 1):A319 –A320. [Google Scholar]
Nakamura 2011 {published data only}
- Nakamura H, Fu K, Yamamura A. A Magnifying gastroscopy using a soft black hood for difficult colonoscopy. Surgical Endoscopy and Other Interventional Techniques 2011;25:3016‐3021. [DOI] [PubMed] [Google Scholar]
Park SH 2011 {published data only}
Sato 2009 {published data only}
- Sato K, Hirahata K, Furuhata T, Kakemura T, Fujinuma S, Maetani I. Efficacy of oblique transparent cap with colonoscope for trainees analyzed with magnetic endoscope imaging (MEI): A preliminary report.. Gastrointestinal Endoscopy 2009;69(5):AB120. [Google Scholar]
Takano 2008 {published data only}
- Takano N, Yamaji Y, Kajiwara H, et al. A randomized controlled trial of the usefulness of cap‐fitted colonoscopy on the polyp detection. Gastrointest Endosc 2008;67:AB303.. [Google Scholar]
Tee 2009 {published data only}
- Tee H‐P, Corte C, Al‐Ghamdi H, Prakoso E, Chettier R, Darke J, et al. A prospective randomized controlled trial evaluating cap‐assisted colonoscopy versus standard colonoscopy ‐ A western experience.. Journal of Gastroenterology and Hepatology 2009;24(suppl 2):A250‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2011 {published data only}
- Thomas RA, Rao DS, Gupta N, Gaddam S, Wani SB, Singh V, et al. Does cap‐assisted colonoscopy (CAC) significantly impact surveillance interval recommendations compared to standard colonoscopy (SC)? Results from a randomized controlled trial.. Gastrointestinal Endoscopy 2011;73(suppl 4):AB287. [Google Scholar]
Yeung 2011 {published data only}
- Yeung TM, Mortensen NJ. Advances in endoscopic visualization of colorectal polyps. Colorectal Disease 2011;13:352‐359. [DOI] [PubMed] [Google Scholar]
Additional references
Ando 2008
- Ando T, Nishio Y, Watanabe O, Takahashi H, Maeda O, Ishiguro K, et al. Value of colonoscopy for prediction of prognosis in patients with ulcerative colitis. World Journal of Gastroenterology 2008;14(14):2133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Axelrad 1996
- Axelrad AM, Fleischer DE, Geller AJ, Nguyen CC, Lewis JH, Al‐Kawas FH, et al. High‐resolution chromoendoscopy for the diagnosis of diminutive colon polyps: implications for colon cancer screening. Gastroenterology 1996;110:1253‐8. [DOI] [PubMed] [Google Scholar]
Bat 1991
- Bat L, Williams CB. Usefulness of pediatric colonoscopes in adult colonoscopy. Gastrointestinal Endoscopy 1991;37:152‐4. [DOI] [PubMed] [Google Scholar]
Baxter 2009
- Baxter N, Goldwasser M, Paszat L, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med 2009;150:1‐8. [DOI] [PubMed] [Google Scholar]
Benson 2007
- Benson AB 3rd. Epidemiology, disease progression, and economic burden of colorectal cancer. Journal of Managed Care Pharmacy 2007;13(6 Suppl C):S5‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bressler 2004
- Bressler B, Paszat LF, Vinden C, Li C, He J, Rabeneck L. Colonoscopic miss rates for right‐sided colon cancer: A populations‐based analysis. Gastroenterology 2004;127:452‐6. [DOI] [PubMed] [Google Scholar]
Chiu 2007
- Chui H, Chang CY, Chen CC, Lee YC, Wu MS, Lin JT, et al. A prospective comparative study of narrow‐band imaging, chromoendoscopy and conventional colonoscopy in the diagnosis of colorectal neoplasia. Gut 2007;56:373‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
Church 2003
- Church J, Delany C. Randomized, controlled trial of carbon dioxide insufflation during colonoscopy. Diseases of the Colon and Rectum 2003;46(3):322‐325. [DOI] [PubMed] [Google Scholar]
Consolo 2008
- Consolo P, Luigiano C, Strangio G, Scaffidi MG, Giacobbe G, Giuseppe G, et al. Efficacy, risk factors and complications of endoscopic polypectomy: Ten year experience at a single centre. World Journal of Gastroenterology 2008;14(15):2364‐2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cotton 1990
- Cotton PB, Williams CB. Colonoscopy. In: Cotton PB, Williams CB editor(s). Practical Gastrointestinal Endoscopy. Oxford: Blackwell Scientific, 1990:160‐223. [Google Scholar]
Deenadayalu 2004
- Deenadayalu V, Chadalawada V, Rex D. 170º wide‐angle colonoscope: effect on efficiency and miss rates. American Journal of Gastroenterology 2004;99(11):2138‐2142. [DOI] [PubMed] [Google Scholar]
Friedman 2001
- Friedman S, Rubin PH, Bodian C, Goldstein E, Harpaz N, Present DH. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology 2001;120:820‐826. [DOI] [PubMed] [Google Scholar]
Goto 2006
- Goto H, Oda Y, Murakami Y, Tanaka T, Hasuda K, Goto S, et al. Proportion of de novo cancers among colorectal cancers in Japan. Gastroenterology 2006;131:40‐6. [DOI] [PubMed] [Google Scholar]
Han 2000
- Han Y, Uno Y, Munakata A. Does flexible small‐diameter colonoscope reduce insertion pain during colonoscopy. World Journal of Gastroenterology 2000;6(5):659‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hardcastle 1996
- Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, et al. Randomised controlled trial of faecal‐occult‐blood screening for colorectal cancer. Lancet 1996;348:1472‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0. 5.0.0 edition. The Cochrane Collaboration, 2008, updated February 2008.. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration, 2008. [Google Scholar]
Hixson 1990
- Hixson LJ, Fennerty MB, Sampliner RE, McGee D, Garewal H. Prospective study of the frequency and size distribution of polyps missed by colonoscopy. Journal of the National Cancer Institute 1990;82:1769‐72. [DOI] [PubMed] [Google Scholar]
Hurlstone 2004
- Hurlstone DP, Cross SS, Slater R, Sanders DS, Brown S. Detecting diminutive colorectal lesions at colonoscopy: a randomized controlled trial of pan‐colonic versus targeted chromoscopy. Gut 2004;53:376‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hurlstone 2007
- Hurlstone DP, Brown S. Techniques for targeting screening in ulcerative colitis. Postgraduate Medical Journal 2007;83(981):451‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Imperiale 2000
- Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. The New England Journal of Medicine 2000;343:169‐74. [DOI] [PubMed] [Google Scholar]
Ishii 1992
- Iishi H, Tatsuta M, Tsutsui S, Imanishi K, Otani T, Okuda S, et al. Early depressed adenocarcinomas of the large intestine. Cancer 1992;69:2406‐10. [DOI] [PubMed] [Google Scholar]
Kanamori 1995
- Kanamori T, Itoh M, Yokoyama Y, Takeuchi T. Endoscopic and clinicopathologic evaluation of four cases of minute flat invasive colorectal carcinoma. Gastrointestinal Endoscopy 1995;41:75‐9. [DOI] [PubMed] [Google Scholar]
Kobayashi 1998
- Kobayashi K, Sivak MV. Flat adenoma: are western colonoscopists careful enough?. Endoscopy 1998;30:487‐489. [DOI] [PubMed] [Google Scholar]
Kondon 2007
- Kondon S, Yamaji Y, Watabe H, Yamada A, Sugimoto T, Ohta M, et al. A Randomized Controlled Trial Evaluating the Usefulness of a Transparent Hood Attached to the Tip of the Colonoscope. American Journal of Gastroenterology 2007;102:75‐81. [DOI] [PubMed] [Google Scholar]
Kronborg 1996
- Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomized study of screening for colorectal cancer with faecal‐occult blood test. Lancet 1996;348:1467‐71. [DOI] [PubMed] [Google Scholar]
Kudo 1995
- Kudo S, Tamure S, Nakajima T, Hirota S, Asano M, Ito O, et al. Depressed type of colorectal cancer. Endoscopy 1995;27:54‐7. [DOI] [PubMed] [Google Scholar]
Lahat 2008
- Lahat A, Yanai H, Sakhnini E, Menachem Y, Bar‐Meir S. Role of colonoscopy in patients with persistent acute diverticulitis. World Journal of Gastroenterology 2008;14(17):2763‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lapalus 2004
- Lapalus MG, Helbert T, Napoleon B, Rey JF, Houcke P, Ponchon T, et al. Does chromoendoscopy in association with high‐resolution video endoscopy improve colorectal adenomas diagnosis by colonoscopy?. Gastrointestinal Endoscopy 2004;59(5):147. [Google Scholar]
Le Rhun 2006
- Rhun M, Coron E, Parlier D, Nguyen J, Canard J, Alamdari A, et al. High resolution colonoscopy with chromoscopy versus standard colonoscopy for the detection of colonic neoplasia: a randomized study. Clinical Gastroenterology and Hepatology 2006;4(3):349‐354. [DOI] [PubMed] [Google Scholar]
Lee 2006
- Lee YT, Hui AJ, Wong VW, Hung LC, Sung JJ. Improved colonoscopy success rate with a distally attached mucosectomy cap. Endoscopy 2006;38:739‐42. [DOI] [PubMed] [Google Scholar]
Leiberman 2000
- Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. The New England Journal of Medicine 2000;343:162‐8. [DOI] [PubMed] [Google Scholar]
Lichenstein 2006
- Lichenstein GR, Cohen LB, Uribarri J. Review article: bowel preparation for colonoscopy ‐ the importance of adequate hydration. Alimentary Pharmacology and Therapeutics 2006;26:633‐641. [DOI] [PubMed] [Google Scholar]
Mandel 1993
- Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. The New England Journal of Medicine 1993;328:1365‐1371. [DOI] [PubMed] [Google Scholar]
Marshall 1996
- Marshall JB. Use of paediatric colonoscope improves the success of total colonoscopy in selected adult patients. Gastrointestinal Endoscopy 1996;44:675‐8. [DOI] [PubMed] [Google Scholar]
Matsumoto 1995
- Matsumoto T, Lida M, Kuwano Y, Tada S, Yao T, Fujishima M. Small nonpolypoid neoplastic lesions of the colon: endoscopic features with emphasis on their progression. Gastrointestinal Endoscopy 1995;41:135‐40. [DOI] [PubMed] [Google Scholar]
Matsushita 1997
- Matsushita M, Hajiro K, Okazaki K, Takakuwa H, Tominaga M. Total colonoscopy with a transparent cap. Endoscopy 1997;30:444‐7. [DOI] [PubMed] [Google Scholar]
Matsushita 2007
- Matsushita M, Danbara N, Fukui T, Matsumoto T, Uchida K, Omiya M, et al. Total colonoscopy with a transparent hood for trainees. American Journal of Gastroenterology 2007;102(10):2355‐6. [DOI] [PubMed] [Google Scholar]
Muto 1975
- Muto T, Bussey H, Morson BC. The evaluation of cancer of the colon and rectum. Cancer 1975;36:2251‐70. [DOI] [PubMed] [Google Scholar]
Newcomb 2003
- Newcomb PA, Storer BE, Morimoto LM, Templeton A, Potter JD. Long‐term efficacy of sigmoidoscopy in the reduction of colorectal cancer incidence. Journal of the National Cancer Institute 2003;95:622‐625. [DOI] [PubMed] [Google Scholar]
Ng 2011
- Ng SC, Tsoi KK, Hirai HW, Wu JC, Chan FKL, Sung JJ, et al. CAP‐assisted colonoscopy versus standard colonoscopy in polyp detection and cecal intubation outcomes: A meta‐analysis of randomised‐controlled trials.. Gastrointestinal Endoscopy 2011;73(suppl 4):AB388. [Google Scholar]
Quinn 2001
- Quinn M, Babb P, Brock A, Kirby L, Jones J. Cancer Trends in England and Wales 1950‐1999. Studies on Medical and Population Subjects. Vol. No 66, Stationery Office, 2001. [Google Scholar]
Rainis 2007
- Rainis T, JKeren D, Goldstein O, Stermer E, Lavy A. Diagnostic yield and safety of colonoscopy in Israeli patients in an open access referral system. Journal of Clinical Gastroenterology 2007;41(4):394‐399. [DOI] [PubMed] [Google Scholar]
Rex 1997a
- Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back‐to‐back colonoscopies. Gastroenterology 1997;112:24‐8. [DOI] [PubMed] [Google Scholar]
Rex 1997b
- Rex DK, Rahmani EY, Haseman JH, Lemmel GT, Kaster S, Buckley JS. Relative sensitivity of colonoscopy and barium enema for detection colorectal cancer in clinical practice. Gastroenterology 1997;112:17‐23. [DOI] [PubMed] [Google Scholar]
Rex 2000
- Rex DK, Johnson DA, Lieberman DA, Burt RW, Sonnenberg A. Colorectal cancer prevention 2000: screening recommendations of the American College of Gastroenterology. American College of Gastroenterology. American Journal of Gastroenterology 2000;95:868‐77. [DOI] [PubMed] [Google Scholar]
Rex 2006
- Rex DK. Maximising detection of adenomas and cancer during colonoscopy. American Journal of Gastroenterology 2006;101(12):2866‐2877. [DOI] [PubMed] [Google Scholar]
Stolte 1995
- Stolte M, Bethke B. Colorectal mini‐de novo carcinoma: A reality in Germany too. Endoscopy 1995;27:286‐90. [DOI] [PubMed] [Google Scholar]
Suzuki 2004
- Suzuki T, Matsushima M, Ihara K, Tokiwa K, Kania T, Ito A, et al. Clinical significance of the use of magnetic endoscope imaging for colonoscopy. Digestive Endoscopy 2004;16(4):322‐326. [Google Scholar]
Svensson 2002
- Svensson MH, Svensson E, Lasson A, Hellstrom M. Patient acceptance of CT colonography and conventional colonoscopy: prospective comparative study in patients with or suspected of having colorectal disease. Radiology 2002;222:337‐345. [DOI] [PubMed] [Google Scholar]
Thiis‐Evensen 1999
- Thiis‐Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population‐based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scandinavian Journal of Gastroenterology 1999;34:414‐420. [DOI] [PubMed] [Google Scholar]
van Rijn 2006
- Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. American Journal of Gastroenterology 2006;101:343‐50. [DOI] [PubMed] [Google Scholar]
Westwood 2012
- Westwood DA, Alexakis N, Connor SJ. Transparent Cap‐Assisted Colonoscopy Versus Standard Adult Colonoscopy: A Systematic Review and Meta‐Analysis. Dis Colon and Rectum 2012;55:218‐225. [DOI] [PubMed] [Google Scholar]
Winawer 1993a
- Winawer SJ, Zauber AG, Ho MN, O'Brien MJ, Gottlieb LS, Sternberg SS, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. New England Journal of Medicine 1993;329:1977‐81. [DOI] [PubMed] [Google Scholar]
Winawer 1993b
- Winawer SJ, Zauber AG, O'Brien MJ, Ho MN, Gottlieb L, Sternberg SS, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. New England Journal of Medicine 1993;328:901‐6. [DOI] [PubMed] [Google Scholar]
Wolfe 1975
- Wolfe WI, Shinya H. Endoscopic polypectomy. Therapeutic and clinicopathologic aspects. Cancer 1975;36:683‐690. [DOI] [PubMed] [Google Scholar]
Yoshikawa 2004
- Yoshikawa I, Honda H, Nagata K, Kanda K, Yamasaki T, Kume K, et al. Variable stiffness colonoscopes are associated with less pain during colonoscopy in unsedated patients. American Journal of Gastroenterology 2004;97(12):3052‐3055. [DOI] [PubMed] [Google Scholar]


