Abstract
Background
Incontinence after prostatectomy for benign or malignant disease is a well‐known and often a feared outcome. Although small degrees of incidental incontinence may go virtually unnoticed, larger degrees of incontinence can have a major impact on a man's quality of life.
Conceptually, post‐prostatectomy incontinence may be caused by sphincter malfunction or bladder dysfunction, or both. Most men with post‐prostatectomy incontinence (60% to 100%) have stress urinary incontinence, which is involuntary urinary leakage on effort or exertion, or on sneezing or coughing. This may be due to intrinsic sphincter deficiency and may be treated with surgery for optimal management of incontinence. Detrusor dysfunction is more common after surgery for benign prostatic disease.
Objectives
To determine the effects of surgical treatment for urinary incontinence related to presumed sphincter deficiency after prostate surgery for:
‐ men with lower urinary tract symptoms (LUTS) secondary to benign prostatic hyperplasia (BPH) ‐ transurethral resection of prostate (TURP), photo vaporisation of the prostate, laser enucleation of the prostate or open prostatectomy ‐ and
‐ men with prostate cancer ‐ radical prostatectomy (retropubic, perineal, laparoscopic, or robotic).
Search methods
We searched the Cochrane Incontinence Group Specialised Register, which contains trials identified from Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, and handsearching of journals and conference proceedings (searched 31 March 2014); MEDLINE (January 1966 to April 2014); EMBASE (January 1988 to April 2014); and LILACS (January 1982 to April 2014). We handsearched the reference lists of relevant articles and conference proceedings. We contacted investigators to locate studies.
Selection criteria
Randomised or quasi‐randomised trials that include surgical treatments of urinary incontinence after prostate surgery.
Data collection and analysis
Two authors independently screened the trials identified, appraised quality of papers, and extracted data.
Main results
Only one study with 45 participants met the inclusion criteria. Men were divided in two sub‐groups (minimal or total incontinence) and each group was randomised to artificial urethral sphincter (AUS) implantation or Macroplastique injection. Follow‐up ranged from six to 120 months. In the trial as a whole, the men treated with AUS were more likely to be dry (18/20, 82%) than those who had the injectable treatment (11/23, 46%) (odds ratio (OR) 5.67, 95% confidence interval (CI) 1.28 to 25.10). However, this effect was only statistically significant for the men with more severe ('total') incontinence (OR 8.89, 95% CI 1.40 to 56.57) and the CIs were wide. There were more severe complications in the group undergoing AUS, and the costs were higher. AUS implantation was complicated in 5/22 (23%) men: the implant had to be removed from one man because of infection and in one man due to the erosion of the cuff, in one man the pump was changed due to mechanical failure, in one man there was migration to the intraperitoneal region, and one man experienced scrotal erosion. In the injectable group, 3/23 (13%) men had a complication: one man treated with Macroplastique injection had to be catheterised because of urinary retention and two men developed urinary tract infections.
Authors' conclusions
The evidence available at present was of very low quality because we identified only one small randomised clinical trial. Although the result was favourable for the implantation of AUS in the group with severe incontinence, this result should be considered with caution due to the small sample size and uncertain methodological quality of the study found.
Keywords: Humans; Male; Dimethylpolysiloxanes; Dimethylpolysiloxanes/administration & dosage; Prostatectomy; Prostatectomy/adverse effects; Prostatic Neoplasms; Prostatic Neoplasms/surgery; Randomized Controlled Trials as Topic; Transurethral Resection of Prostate; Transurethral Resection of Prostate/adverse effects; Urinary Incontinence, Stress; Urinary Incontinence, Stress/etiology; Urinary Incontinence, Stress/surgery; Urinary Sphincter, Artificial; Urinary Sphincter, Artificial/adverse effects; Urinary Sphincter, Artificial/economics
Plain language summary
Surgery for urinary incontinence due to presumed sphincter deficiency after prostate surgery
Background
Urinary leakage (incontinence) after surgery to remove the prostate (prostatectomy) for benign or malignant disease is a well‐known and often feared outcome. Although a small amount of incontinence may not cause a problem, larger degrees of incontinence can have a major impact on a man's quality of life. Improvement in urinary leakage may occur six to 12 months after the prostatic surgery, but for men with persistent bothersome leakage despite conservative therapy such as pelvic floor exercises, surgery may be offered.
Study characteristics
We searched scientific databases for trials that had considered the effectiveness of the surgical treatments of urinary incontinence after prostate surgery in men. The trials had to compare surgical treatment versus no treatment, non‐surgical treatment, or another surgical treatment. The evidence is current to April 2014.
Key results and quality of the evidence
There are five main types of surgery and, despite some of them being in use since the 1990s, we found only one trial that met the inclusion criteria. There was very low quality evidence that the implantation of an artificial urinary sphincter (a manufactured device to prevent urine leaking out) might be more effective than injectable treatment, but with more adverse effects and higher costs. There was no evidence about the other types of surgery.
Summary of findings
Summary of findings for the main comparison. Artificial urinary sphincter implantation compared with endourethral Macroplastique injection for the treatment of post‐prostatectomy incontinence.
| Artificial urinary sphincter implantation compared with endourethral Macroplastique injection for the treatment of post‐prostatectomy incontinence | |||
|
Patient or population: men with stress urinary incontinence due to presumed sphincter deficiency after prostate surgery Settings: tertiary | |||
| Outcomes | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) |
|
Success rate ‐ dry Follow‐up ‐ AUS ‐ 60 months ‐ Macroplastique injection ‐ 48 months |
OR 5.67 (95% CI 1.28 to 25.1) | 45 (1) | ⊕⊝⊝⊝ Very low1 |
|
Adverse events Follow‐up ‐ AUS ‐ 60 months ‐ Macroplastique injection ‐ 48 months |
AUS ‐ 5/22 (23%) Macroplastique injection ‐ 3/23 (13%) As the types of complications were different for each group there was no statistical comparison |
45 (1) | ⊕⊝⊝⊝ Very low1 |
| AUS: Artificial urinary sphincter; CI: confidence interval; OR: odds ratio | |||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||
1Study with unclear risk of selection bias (random sequence generation and allocation concealment), lack of blinding, and small sample size.
Background
Urinary incontinence, as defined by the International Continence Society, is the involuntary leakage of urine. Stress urinary incontinence (SUI) is the involuntary leakage on effort or exertion, or on sneezing or coughing (Abrams 2003).
Urinary incontinence after prostate surgery is difficult to treat and has a substantial negative impact on men's quality of life (QoL), doctor‐patient relationships, and healthcare costs.
Incontinence after prostatectomy for benign or malignant disease is a well‐known and often feared outcome. Although small degrees of incidental incontinence may go virtually unnoticed, larger degrees of incontinence can have a major impact on a man's QoL (Herr 1994).
Pathophysiology
Conceptually, post‐prostatectomy incontinence may be caused by bladder dysfunction or sphincter malfunction, or both.
Bladder dysfunction includes involuntary detrusor contractions, impaired or absent detrusor contractility, and low bladder compliance. It may be the result of bladder wall injury following long‐standing outflow obstruction or arise de novo after surgery. Surgery would not be appropriate for these problems and may make them worse.
Most men with post‐prostatectomy incontinence (60% to 100%) have SUI due to presumed intrinsic sphincter deficiency, though this may co‐exist with detrusor dysfunction. Men may receive surgery for optimal management of severe SUI (Ullrich 2004).
The precise aetiology of urinary incontinence after prostate surgery is not completely understood. However, bladder dysfunction as well as intraoperative damage to the nerves controlling bladder function and direct trauma to the urethral sphincter may play a causative role. In this regard, damage to the urethral sphincter can result not only from direct muscle damage but also from damage to the neurological innervation (Foote 1991).
Epidemiology
Benign prostatic enlargement
Benign prostatic enlargement (BPE) is the main cause of lower urinary tract symptoms (LUTS) in men, with a prevalence of about 30% in men aged over 60 years, amounting to about 1.8 million men in the United Kingdom. Endoscopic removal of prostate tissue (e.g. transurethral resection of the prostate, TURP) is usually recommended in men who have not benefited from behavioural or drug treatment. About 25,000 such procedures are carried out annually in England at a cost of GBP 53 million (Armstrong 2009).
Based on US Census estimates (July 2003) there were 37.7 million US men aged 50 years or more. Using the assumption that a minimum of 50% of these men have LUTS secondary to benign prostatic hyperplasia (BPH) and 50% of men with BPH will seek treatment, 9.4 million men are estimated to be eligible for treatment of LUTS secondary to BPH annually in the United States (Black 2006).
Rates of incontinence after surgery for benign disease were similar across the various treatment modalities, but were slightly higher after open prostatectomy for benign disease, ranging from 0% to 8.4% according to International Consultation on Incontinence (ICI) (ICI 2008). However, the number of these operations has decreased since about 1986, because alternative treatments for the symptoms secondary to BPE have been developed.
Prostate cancer
Other than skin cancer, prostate cancer is the most common cancer in American men. The American Cancer Society estimates that in the US about 233,000 new cases of prostate cancer will be diagnosed in 2014.
Conversely, the incidence of incontinence after radical prostatectomy has been a source of controversy because reported rates have varied greatly from 0.8% to 87.0% (Augustin 2002; Burkhard 2006; Haab 1996; Penson 2005; Sacco 2006; Veenema 1977; Walsh 1980). This wide range is most likely due to the lack of standardised definition of incontinence, the difference between surgeon and patient's assessment, the time of the assessment (the earlier rates are worse), the unknown effect of the operating physician or type of operation (Eastham 1996), and the age of the man (Wei 2000). In high‐volume centres with small numbers of surgeons, post‐prostatectomy continence rates are reported to be high, between 91% and 98% (Hammerer 1997; Walsh 1994; Walsh 2000). Incontinence that persists for more than one year postoperatively may decrease in these centres to less than 5% and may even reach 1% to 2% (Peyromaure 2002). Some reports suggest that men aged less than 50 years show a significantly better rate of return to continence than men older than 70 years (Kundu 2004).
Risk factors
After TURP, urinary incontinence (UI, the symptom; see Description of the condition) is most likely be due to pre‐existing abnormalities of bladder function such as poor compliance or detrusor overactivity, rather than direct sphincter injury (Abrams 1991).
The risk of incontinence following radical prostatectomy includes preoperative, intraoperative, and postoperative factors (Catalona 1999; Eastham 1996). Risk factors for urinary incontinence after radical prostatectomy include pre‐existing abnormalities of detrusor contractility, older age, previous TURP, preoperative radiotherapy, trauma, spinal cord lesion, new obstruction due to recurrence, bladder neck contracture, urethral stricture, Parkinson's disease, dementia, and medications (Campbell 2012). A surgeon's inadequate skill and expertise (Eastham 1996), and having surgery in a hospital that performs fewer than 20 radical prostatectomies per year, may also be a factor (Albertsen 1997). A better understanding of the male pelvic anatomy has decreased the postoperative incontinence rate (Eastham 1996; Steiner 1991; van der Horst 2007).
Diagnosis
To make the diagnosis of urinary incontinence after prostate surgery, an initial clinical assessment may include:
medical history, co‐morbidities and medication in use;
physical examination;
urinary diary;
an incontinence questionnaire such as the ICI Questionnaire‐Short Form (ICIQ‐SF for Urinary Incontinence), recommended by the European Association of Urology (Seckiner 2007);
ultrasound for residual urine;
urine analysis;
QoL assessment;
pad tests.
The grade of stress incontinence can be classified to mild (incontinence only with severe stress, such as coughing or sneezing), moderate (incontinence with minimal stress, including standing from sitting or walking), or severe (incontinence during bed rest, continuous leakage) (Stamey 1981).
If incontinence persists six to 12 months after the surgery, a specialised clinical assessment is recommended, with urodynamic assessment and urethrocystoscopy (Herschorn 2010).
Conservative treatments
Conservative treatments are the first‐line treatment for early incontinence that follows prostatectomy within the first six months to 12 months. These include pelvic floor muscle training with or without biofeedback, electrical stimulation delivered via surface electrodes, lifestyle adjustment, and external penile compression devices (Campbell 2012).
For men whose incontinence does not improve adequately, containment using absorbent pads (Fader 2008), or indwelling or sheath catheters remain the mainstay of conservative treatment.
Pharmacological treatment
Currently, there is no approved medical therapy for post‐prostatectomy urinary incontinence. Nevertheless, there are reports of the use of duloxetine, a serotonin‐noradrenaline reuptake inhibitor, used for female stress incontinence, which is commonly used off‐label to treat male stress incontinence (Filocamo 2007; Schlenker 2006; Zahariou 2006).
In early post‐prostatectomy incontinence, de novo urgency with or without detrusor overactivity may play a role (Walsh 1980). For these men, additional anticholinergic treatment could be pursued. Currently there are no evidence‐based recommendations in the existing guidelines for this treatment.
Description of the condition
Urinary incontinence (UI, the symptom) is defined as the complaint of any involuntary leakage of urine. In this review, it will be taken to mean UI that occurs after prostate surgery.
Urinary incontinence (the sign) is defined as urine leakage seen during examination.
SUI is the report (symptom) or observation (sign) of involuntary leakage from the urethra, synchronous with exertion/effort, or sneezing or coughing.
Urodynamic stress incontinence (USI) is diagnosed during filling cystometry, and is defined as the involuntary leakage of urine during increased abdominal pressure, in the absence of a detrusor contraction (Abrams 2003).
Description of the intervention
Surgical treatment
Improvement in urinary continence may occur six to 12 months after prostatic surgery. In one study, urinary incontinence was at its worst in the first two months after surgery but then improved in most men. By one year after prostatectomy, 6% of men had a moderate problem and 2% a significant problem judged on QoL scores (Sanda 2008).
Some 2% to 5% of the men with incontinence after radical prostatectomy report persistent incontinence for greater than one year postoperatively despite conservative therapy attempts. For these men, surgical treatment is recommended (Bauer 2009). Surgery includes: injection therapy, male slings, an implanted device to co‐apt the urethral walls using inflatable balloons, an implanted artificial urinary sphincter (AUS), and stem cell therapy.
1. Injection therapy
A variety of substances (e.g. collagen, Teflon, silicone, autologous fat, autologous chondrocytes, dextranomer/hyaluronic acid co‐polymer) have been used as bulking agents for many years. The procedure can be performed in the doctor's office or in an outpatient clinic or hospital in approximately 30 minutes, with local anaesthesia in the tissues near the bladder neck to reduce discomfort. The bulking agent is injected into the submucosa the urethral tissue surrounding of the bladder neck using a cystoscope. The injection creates increased tissue bulk and subsequent coaptation (closure) of the bladder neck or urethra or both.
Injection therapy has limited efficacy in the treatment of SUI after radical prostatectomy (Imamoglu 2005; Secin 2005).
After the detection of Teflon (Polytef™, DuPont) in lymph nodes, spleen, lung, and brain following its injection in the external sphincter in research using animals, the use of Teflon for medical therapies was discontinued. Agents currently used include dextranomer/hyaluronic acid copolymer (Deflux™, Galderma), pyrolytic carbon microspheres (Durasphere™, Carbon Medical Technologies, Inc.), and polydimethylsiloxane (Macroplastique™, Uroplasty, Inc.).
2. Slings
Analogous to the successful use of sling surgery in women (e.g. tension‐free vaginal tape; Ogah 2009), slings have been developed for use in men with urinary incontinence. A variety of types are available:
bone‐anchored sling system (Invance™, AMS) consists of a silicon‐coated polyester mesh sling positioned under the bulbar urethra via a perineal incision. It is attached to both ischiopubic rami by three titanium screws. It is recommended for low and intermediate levels of incontinence (Onur 2004).
re‐adjustable sling systems are suburethral sling systems that allow implant adjustment and regulation of the desired tension. The REMEEX™ (Reemex Vertriebs GmbH) is a re‐adjustable suburethral sling composed of a monofilament sling connected via two monofilament traction threads to a suprapubic mechanical regulator. The regulator is a permanent subcutaneous implant over the abdominal rectum fascia 2 cm above the pubis. In addition, implant adjustment is possible via an external manipulator. The Argus™ system (Promedon), first described by Sierra et al. in 2006 (Sierra 2006), is composed of a radio‐opaque cushioned system with a silicone foam pad (42 mm x 26 mm x 9 mm thick) for soft bulbar urethral compression, two silicone columns formed by multiple conical elements, which are attached to the pad to allow system readjustment, and two radio‐opaque silicone washers which allow regulation of the desired tension.
The trans‐obturator sling, Advance™ (AMS), is a new sling suspension that offers a non‐obstructive functional therapeutic approach. The sling adjusts the anatomy after radical prostatectomy by re‐positioning the lax and descended supporting structures of the sphincter to their former preoperative position. Thus, continence can again be achieved. This sling was first described by Rehder and Gozzi (Rehder 2007). Other implantable devices, such as the Pro‐ACT™ system and the AUS, achieve continence mainly by compression of the urethra.
3. Adjustable continence therapy (Pro‐ACT™, Uromedica) for male post‐prostatectomy stress urinary incontinence
The Pro‐ACT™ system is an adjustable therapy option. It uses the principle of augmenting titration for optimal urethral coaptation (closure). Two balloons are placed bilaterally at the bladder neck. Titanium ports are placed in the scrotum for volume adjustment. Postoperative re‐adjustment is very simple and only local anaesthesia is necessary. This system was first introduced in 2000.
4. Artificial urinary sphincter
The AMS 800™ (AMS) is composed of a pressure regulating balloon placed in the pre‐vesical space using an abdominal suprapubic incision; an inflatable cuff is placed around the urethra using a perineal incision; and a control pump is placed in the scrotum via the abdominal incision. The intervention is expensive and requires invasive surgery and experienced surgeons, but is generally considered the gold standard for treatment of severe or persistent incontinence in men.
The FlowSecure™ (Reinhard Becker Medical Devices) is a new AUS with conditional occlusion for stress incontinence. The new prototype was conceived and designed by Craggs M. and Mundy A.R (García Montes 2007). The FlowSecure™ sphincter consists of an adjustable pressure‐regulating balloon, a stress relief reservoir, a control pump and valve assembly unit with self sealing port, and a urethral cuff. The pressure regulating balloon determines the operating pressure of the device: the pressure is adjustable within the range 0 to 80 cmH2O and can be altered by injection or removal of normal saline through the self sealing port. The stress relief balloon transmits transient intra‐abdominal pressure to the cuff during periods of stress. An adjustable circular urethral cuff minimises creasing and possible stress fractures.
5. Stem cell therapy
Stem cell treatments are a type of therapy that introduces new cells into damaged tissue in order to treat a disease or injury.
Self regenerating, pluripotent processed lipoaspirate (PLA) cells can be isolated from human adipose tissue. These PLA cells may provide a feasible and cost‐effective cell source for urinary tract reconstruction (Jack 2005). A combined application of these cells may restore normal morphology and function of urethral submucosa and rhabdosphincter in incontinent people.
How the intervention might work
The rationale is that surgery to correct incontinence increases the closure pressure of the proximal urethra and thus reduce the involuntary leakage of urine. The stem cell therapy approach is that the cells will regenerate new muscle cells that will allow the sphincter to function normally again.
Why it is important to do this review
Urinary incontinence after prostate surgical procedures is one of the most feared problems by men and urologists because it can have a major negative impact on morale and QoL. The surgical options use different types of prosthesis such as AUS, male slings, and injectable substances, but many of them are very expensive and invasive. Although the literature has several studies on this subject, the quality of the published evidence on efficacy, safety, complications, and long‐term results is unknown. There are few comparative studies and only two narrative reviews. Therefore, the purpose of this systematic review is to summarise the evidence available from randomised controlled trials to identify which interventions are effective and cost effective, and thus to enable men with urinary incontinence after prostate surgery and their care providers to decide on the best management.
Objectives
To determine the effects of surgical treatment for urinary incontinence related to presumed sphincter deficiency after prostate surgery for:
men with LUTS secondary to BPH ‐ transurethral resection of prostate (TURP), photo vaporisation of the prostate, laser enucleation of the prostate or open prostatectomy ‐ and
men with prostate cancer ‐ radical prostatectomy (retropubic, perineal, laparoscopic, or robotic).
Methods
Criteria for considering studies for this review
Types of studies
Randomised or quasi‐randomised trials that include surgical treatments of urinary incontinence after prostate surgery.
Types of participants
Adult men with urinary incontinence after prostate surgery. We accepted the trialists' definitions for adult and for diagnosis and classification of incontinence.
Types of interventions
At least one arm of the trial included surgical treatment for UI after prostate surgery: male slings, injectable bulking agents, AUS, Pro‐ACT™ system, or stem cell therapy. These interventions were compared with no treatment, non‐surgical treatment, or with each other, alone or in combination.
The following comparisons were made for surgical treatments of UI after any type of prostatectomy.
-
Male sling
Male sling versus no intervention.
Male sling versus other non‐surgical therapy.
Male sling versus other surgical therapy.
-
Injectable bulking agent
Injectable bulking agent versus no intervention.
Injectable bulking agent versus other non‐surgical therapy.
Injectable bulking agent versus other surgical therapy.
-
AUS
AUS versus no intervention.
AUS versus other non‐surgical therapy.
AUS versus other surgical therapy.
One type of AUS versus another type of AUS.
-
Pro‐ACT™ system
Pro‐ACT™ system versus no intervention.
Pro‐ACT™ system versus other non‐surgical therapy.
Pro‐ACT™ system versus other surgical therapy.
-
Stem cell therapy
Stem cell therapy versus no intervention.
Stem cell therapy versus other non‐surgical therapy.
Stem cell therapy versus other surgical therapy.
We excluded surgery for prevention of UI after prostate surgery (i.e. surgical procedures carried out at the same time as the initial prostate surgery).
Types of outcome measures
Primary outcomes
1. Participant‐reported measures
Success rate defined as number of men with no urinary incontinence (cured).
Secondary outcomes
1. Quantification of symptoms
Frequency of pad/clothing changes. Frequency of incontinence episodes from self report or diary. Frequency of micturition, day or night time. Standardised pad tests.
2. Clinician‐reported measures
Cough stress test. Urodynamic measurements/studies.
3. Quality of life
General health status measures (e.g. Short Form 36 (Ware 1993)). Condition‐specific health measures (specific instruments designed to assess incontinence).
4. Socioeconomic measures
Health economic measures.
5. Surgical outcome measures
Length of operating time. Length of inpatient hospital stay. Time to return to normal activity level. Time to catheter removal.
6. Adverse events
Number with perioperative surgical complications (e.g. infection, haemorrhage, etc.). Number with de novo detrusor overactivity (urodynamic diagnosis). Number with voiding dysfunction/voiding difficulty (with or without urodynamic confirmation). Number undergoing repeat incontinence surgery. Number with other complications inherent to the procedure.
Search methods for identification of studies
We did not impose any language or other restrictions on the searches and we identified trials from the sources listed below.
Electronic searches
We identified relevant trials from the Specialised Register of Trials of the Cochrane Incontinence Group (date of last search 31 March 2014). The Register contains trials identified from the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, MEDLINE in process, ClinicalTrials.gov, and handsearching of journals and conference proceedings. The methods used to derive this, including the search strategy, are described under the Group's module in The Cochrane Library. The search terms used to search the Cochrane Incontinence Group Specialised Register are given in Appendix 1. We also performed a broader search of computerised bibliographic databases: MEDLINE via PubMed (1980 to April 2014), EMBASE via Ovid (1980 to April 2014), and the Literature Latino‐Americana e do Caribe em Ciências da Saúde ‐ LILACS via Bireme (1982 to April 2014). For the search terms used, see Appendix 2 (PubMed), Appendix 3 (EMBASE), and Appendix 4 (LILACS). We performed these searches in April 2014. We searched the UK Clinical Research Network Study Portfolio in May 2014 using the terms given in Appendix 5.
Searching other resources
We scrutinised the reference lists of the identified relevant studies for additional citations. We contacted specialists in the field for any possible unpublished data.
Data collection and analysis
Selection of studies
Two review authors (LAS and EMKS) independently screened the trials identified by the literature search for eligibility. We consulted a third review author (RBA) when disagreement occurred. We included data from the trials in question until we reached a consensus. We obtained the full papers for the studies considered eligible. If there was any uncertainty on the eligibility of the studies based on title and abstract, the same two review authors obtained and reviewed the full paper. We listed studies formally considered for the review but excluded, with reasons given for their exclusion (Characteristics of excluded studies).
Data extraction and management
We passes studies that met the methodological criteria (randomised or quasi‐randomised clinical trial) and subject relevance to data abstraction stage. Two review authors (LAS and EMKS) independently abstracted data relevant to the pre‐stated outcome measures, characteristics of the study, interventions, and participants. Where data may have been collected, but not reported, we sought further clarification from the trialists. We processed included trial data as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Analyses were based on available data from the included trial relevant to the comparisons and outcomes of interest. We presented and considered data according to the comparisons and grouped them by outcomes. We resolved any differences of opinion related to the data extracted by discussion.
Assessment of risk of bias in included studies
Two review authors (LAS and EMKS) evaluated all relevant clinical studies independently for methodological quality. We resolved any disagreements by discussion and consultation with a third author (RBA). Each review author undertook assessment of methodological quality using The Cochrane Collaboration's 'Risk of bias' tool, which included quality of random allocation concealment, description of dropout and withdrawal, intention‐to‐treat analysis, and blinding procedures for treatment and outcome assessments (Higgins 2011). We synthesised qualitative information relative to methods, risk of bias, description of participants, and outcomes measures and inserted them in the Characteristics of included studies table and Figure 1.
1.
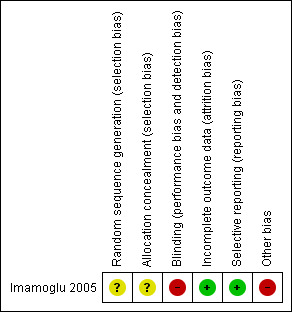
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) and 95% confidence intervals (CIs). For continuous outcomes, we calculated the mean difference (MD) and 95% CIs. Where sufficient data were not reported to allow summary results to be calculated, we reported the available data in the 'Additional table' section.
Unit of analysis issues
The unit of analysis was based on the individual participant (unit to be randomised for interventions to be compared).
Dealing with missing data
Irrespective of the type of data, we reported drop‐out rates in the Characteristics of included studies table. We used the reported available data since there were no losses reported in the study included.
Assessment of heterogeneity
Only one study met the criteria for this review and we did not need to do evaluation of heterogeneity. In future updates, if necessary, we will qualify inconsistency among the pooled estimates using the I2 test, where Q is the Chi2 statistic and df its degrees of freedom. This illustrates the percentage of the variability in effect estimates resulting from heterogeneity rather than sampling error (Deeks 2011; Higgins 2011).
Assessment of reporting biases
In future updates of this review, we will assess publication bias by drawing a funnel plot (trial effect versus trial size), if sufficient studies are included in the review.
Data synthesis
For dichotomous variables, we calculated the RR and 95% CI. For continuous variables, we calculated the MD and 95% CI.
In subsequent updates of this review, if continuous data are relative to the same aspect, but are measured with different instruments (different and not interchangeable units of measure), we will pool these data using the standardised mean difference (SMD). For all statistical methods when pooling data, we will report the 95% CI. If we identify no significant heterogeneity, we will compute pooled estimates of the treatment effect for each outcome using a fixed‐effect model. Otherwise, if we identify significant heterogeneity, we will use a random‐effects analysis.
Subgroup analysis and investigation of heterogeneity
We carried out subgroup analysis according to type of clinical population (type of prostate disease, either BPE or prostate cancer) and according to severity of incontinence (minimal or severe).
In subsequent updates of this review, if we find significant heterogeneity we will investigate the possible causes of this further by exploring the impact of methodological quality and condition of the individuals (i.e. severity of disease, duration of treatment). If we find sources of heterogeneity and if there are sufficient data, we will conduct meta‐analysis by subgroups (by types of dosage and duration of intervention). If we identify no significant heterogeneity, we will compute pooled estimates of the treatment effect for each outcome using a fixed‐effect model.
Sensitivity analysis
As we included only one study in this review, we did not performed a sensitivity analysis, which will be performed if there are an adequate number of studies in future updates. In this case, we will perform a sensitivity analysis to explore causes of heterogeneity and the robustness of the results.
We will include the following factors in the sensitivity analysis, separating studies according to:
allocation concealment quality (adequate or unclear or inadequate);
blinding of participants, carers, and outcome assessment (adequate or unclear or inadequate or not performed);
rates of withdrawal for each outcome.
Results
Description of studies
Results of the search
The search strategy retrieved 1356 records: Cochrane Group Specialised Register (all surgery for UI, both genders) 760 references; MEDLINE (PubMed), 391 references; EMBASE, 162 references; and LILACS, 43 references.
We also scrutinised the bibliographical references of these papers for further potentially eligible studies and found no additional references. After examination of the titles and abstracts of these references, we subsequently excluded all except four studies from further analysis (Altinova 2009; Imamoglu 2005; Lima 2013; Noguchi 2008) (see PRISMA flow chart in Figure 2). We obtained full‐text copies of these remaining studies and then subjected them to further assessment. Following examination of the full‐text articles, we concluded that only one study fulfilled the inclusion criteria of this review (Imamoglu 2005). In addition, we found three ongoing studies (Abrams 2014; Haab 2014; Ockrim 2013).
2.

PRISMA study flow diagram.
Included studies
Trial design
The only study included in this review was a prospective randomised controlled trial comparing AUS implantation and endourethral Macroplastique injection for the treatment of post‐prostatectomy incontinence (Imamoglu 2005).
Participants
The trial included 45 men who had urinary incontinence lasting one year (radical retropubic prostatectomy (RRP)) or six months (TURP/transvesical prostatectomy (TVP)) despite conservative treatment following RRP (12 men), TVP (16 men), TURP (16 men), or TURP plus TVP (one man). Men were divided into two groups as minimal incontinence (group I) and total incontinence (group II) depending on the symptom scores, and weight and mean number of pads used per day. Minimal incontinence was defined as total number of pads of two or fewer, total weight of pads of 100 g or less, and QoL score of 30 or less. The values above these numbers were accepted as total incontinence. The mean age of the men who underwent AUS implantation was 64 years (range 52 to 76) and mean age of the men who underwent Macroplastique injection was 62 years (range 55 to 75). There was no statistically significant difference at baseline. Further details are given in the Characteristics of included studies table.
Interventions
The 45 men were classified in two subgroups as minimal incontinence (21 men) and total incontinence (24 men). Each group was randomised to either AUS (AMS 800®) implantation (11 men in group I, 11 men in group II) or injectable treatment with Macroplastique (10 men in group I, 13 men in group II). Macroplastique injection was repeated one month after the first injection if there was no improvement of symptoms: after that, persisting incontinence was considered a failure and men were offered AUS.
Outcomes
Outcomes were measured at one, six, and 12 months while the men were followed up for 48 or 60 months in the two surgical groups. Success was measured based on pad use: men who did not need to use pads were regarded as ''dry'', men using fewer than one pad per day were accepted as ''socially continent'', and men using more than one pad were classified as ''incontinent''. It was unclear when the reported outcome was assessed.
Excluded studies
We excluded three studies (Altinova 2009; Lima 2013; Noguchi 2008). We excluded one study because it compared two different types of sling (Lima 2013). We excluded two trials of 'preventive surgery' as they included all men having prostate surgery rather than only those men with persistent UI after surgery (Altinova 2009; Noguchi 2008 (see Characteristics of excluded studies table). In addition, it was not clear whether one of these was actually a randomised controlled trial (Altinova 2009).
The updated search conducted in March 2014 identified three ongoing studies (Abrams 2014; Haab 2014; Ockrim 2013) (see Characteristics of ongoing studies). These trials are comparing:
male synthetic sling versus Artificial urinary Sphincter Trial (MASTER, Abrams 2014);
periurethral Pro‐ACT™ Balloons and AdvanceXP™ retrourethral male sling (Haab 2014);
Advance™ male sling and AMS 800 AUS (Ockrim 2013).
Risk of bias in included studies
Allocation
Generation of randomisation sequence was unclear in the included trial (Imamoglu 2005), and, therefore, we assessed risk of bias as unclear. Attempts to contact the authors were unsuccessful. Allocation concealment was not discussed in the trial, resulting in risk of bias also being classed as unclear.
Blinding
The allocated treatment could not be blinded from the men or the surgeons. There was no reference to who conducted the primary outcome assessment (use of pads and weight of pads) but the information reported by the men could not be blinded. Therefore, we judged that the study may have some risk of bias.
Incomplete outcome data
The study authors do not mention whether any men were lost to follow‐up.
Selective reporting
There may be selective reporting, due to important clinical outcomes not being evaluated.
Other potential sources of bias
The study authors reported a large range of follow‐up time (mean 48 months (range six to 84) for the injection group and 60 months (range eight to 120) for the AUS implantation group). It was not clear when the outcomes were measured in both groups.
Furthermore, besides the limited sample size, two men were allocated to the AUS group without randomisation, according to the study authors because of previous treatment for urethral strictures, but it is unclear whether these men were analysed as if they were allocated to the randomised AUS group.
Effects of interventions
See: Table 1
Cure of urinary incontinence
For evaluating the success of the treatment, men who did not need to use pads were regarded as "dry". Men who used fewer than one pad were accepted as "socially continent", and men who were using more than one pad were classified as "incontinent". In the trial as a whole, the men treated with AUS (18/20, 82%) were more likely to be dry than men who had the injectable treatment (11/23, 46%) (odds ratio (OR) 5.67, 95% CI 1.28 to 25.10: Analysis 1.1; Figure 3). However, this effect was only statistically significant for the men with more severe ('total') incontinence (OR 8.89, 95% CI 1.40 to 56.57: Analysis 1.1) and the CIs were wide. When the data were analysed according to clinical type of prostate surgery, the same trend was apparent but the numbers were too few to be reliable (Analysis 1.2; Analysis 1.3; Figure 4; Figure 5).
1.1. Analysis.

Comparison 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, Outcome 1 Success rate ‐ dry.
3.

Forest plot of comparison: 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, outcome: 1.1 Success rate ‐ dry.
1.2. Analysis.

Comparison 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, Outcome 2 Group I ‐ success rate ‐ dry, according to aetiology.
1.3. Analysis.
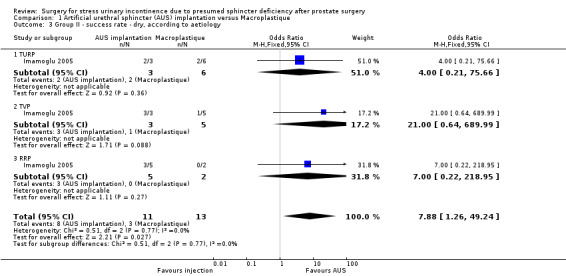
Comparison 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, Outcome 3 Group II ‐ success rate ‐ dry, according to aetiology.
4.

Forest plot of comparison: 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, outcome: 1.2 Group I ‐ success rate ‐ dry, according to aetiology.
5.
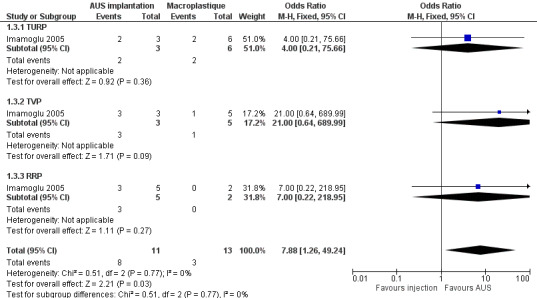
Forest plot of comparison: 1 Artificial urethral sphincter (AUS) implantation versus Macroplastique, outcome: 1.3 Group II ‐ success rate ‐ dry, according to aetiology.
We assessed the quality of this evidence as very low (Table 1).
Quantification of symptoms
While there was a significant difference in all groups of men from preoperatively to postoperatively in terms of number of pads used, mean pad weights, and QoL (Table 2), data on dispersion (e.g. standard deviations) were not presented and so it was not possible to compare the postoperative data from the groups statistically. While the men receiving AUS used fewer pads, had lower pad weights, and better QoL scores compared with men receiving injectables (Table 2), the small numbers of men make it difficult to assess the importance of these differences.
1. Comparison of treatment modalities according to success criteria.
| Macroplastique injection | AUS implantation | |||||
| Preoperative | Postoperative | P value | Preoperative | Postoperative | P value | |
|
Minimal incontinence (injection vs. AUS P value < 0.2) | ||||||
| Mean number of pads | 1.52 | 0.34 | < 0.001 | 1.33 | 0.09 | < 0.001 |
| Mean pad weight (g) | 84.0 | 20.2 | < 0.001 | 76.3 | 4.1 | < 0.001 |
| Quality‐of‐life scale | 29.9 | 8.9 | < 0.001 | 26.75 | 6.8 | < 0.001 |
|
Total incontinence (injection vs. AUS P value < 0.01) | ||||||
| Mean number of pads | 2.46 | 1.41 | < 0.001 | 2.27 | 0.36 | < 0.001 |
| Mean pad weight (g) | 174.2 | 98.6 | < 0.001 | 153.1 | 25.9 | < 0.001 |
| Quality‐of‐life scale | 33.7 | 20.1 | < 0.001 | 33.3 | 9.2 | < 0.001 |
AUS: artificial urinary sphincter.
Other relevant outcomes such as frequency of incontinence episodes from self report or diary or frequency of daytime or night‐time micturition were not reported in the included trial.
Clinician‐reported measures
The trial did not present any clinician‐reported outcomes such as the cough stress test or urodynamic measurements/studies.
Quality of life
The trialists did not report any general health status measures (e.g. Short Form 36 (Ware 1993).
Condition‐specific health measures (specific instruments designed to assess incontinence)
Condition‐specific QoL was measured using the Stress‐related leak, Emptying ability, Anatomy, Protection, Inhibition, Quality of life, Mobility and Mental status Incontinence Classification System (SEAPI‐QMM) Incontinence Classification System (Raz 1992), but the data could not be compared statistically between the groups because measures of dispersion were not provided (Table 2). However, the scores were lower (better) for all men in each group after surgery compared with preoperatively (Table 2).
Socioeconomic measures
Health economic measures were not reported in the included trial. Per capita costs per man were USD 6142 for men receiving an AUS, compared with USD 2264 for men randomised to Macroplastique, including the costs for five men who received two injections and two men who needed a second AUS implantation. However, it was not clear whether these costs include those of the five men who subsequently underwent a new AUS treatment after failed injections. The costs were stated to include the cost of treatment (implant or injectable), treatment of complications, and hospitalisation charge.
Surgical outcome measures
None of the pre‐specified surgical outcome measures (length of operating time, length of inpatient hospital stay, time to return to normal activity level, or time to catheter removal) were reported. However, 5/23 men who remained incontinent after injectable treatment opted to have an AUS subsequently: it is unclear what treatment was given to the four men who were incontinent after AUS opted for injectable treatment later. However, 2/22 men in the AUS group needed a second AUS implantation due to complications after the first procedure.
Adverse events
The study report that the complication rates were significantly higher in the AUS group, but they could not make a statistical comparison between the two groups, as the types of complications were different for each group. AUS implantation was complicated in 5/22 (23%) men: the implant had to be removed from one man because of infection and in one man due to the erosion of the cuff, in one man the pump was changed due to mechanical failure, in one man there was migration to the intraperitoneal region, and one man experienced scrotal erosion. In the injectable group, 3/23 (13%) men had a complication: one man treated with Macroplastique injection had to be catheterised because of urinary retention and two men developed urinary tract infections.
Discussion
Summary of main results
This review assessed surgery for urinary incontinence post‐prostatectomy for benign or malignant disease including a wide range of procedures. However, we identified only one randomised controlled trial that met the inclusion criteria (Imamoglu 2005). The other published studies identified by the search were observational or did not address urinary incontinence after prostate surgery.
More men were 'dry' after AUS implantation than after Macroplastique injection (OR 5.67, 95% CI 1.28 to 25.10; Analysis 1.1; Figure 3). In a subgroup analysis, this effect was only statistically significant in the group of men who had more severe ('total') incontinence (OR 8.89, 95% CI 1.40 to 56.57) and the CIs were wide. The numbers were too small to identify differences between different types of prostate disease reliably.
The trial report that the complication rates were significantly more frequent (23% with AUS versus 13% with Macroplastique) and of a more severe nature in the AUS group, but they could not make a statistical comparison between the two groups, as the types of complications were different for each group.
Overall completeness and applicability of evidence
Although there are several options for surgical treatment of urinary incontinence post‐prostatectomy, the search found only one randomised controlled trial comparing AUS implantation and injectable treatment with Macroplastique. For other surgical procedures, such as male sling, Pro‐ACT™ system, other bulking agents and stem‐cell therapy, the search identified only non‐randomised studies. The three ongoing clinical trials identified in the review update are still in the process of recruiting participants. One study aims to compare Pro‐ACT™ with transobturator sling (Haab 2014), and two studies aim to compare the AUS with sling (Abrams 2014; Ockrim 2013). In future updates of this review, we expect to include the results of these studies.
Therefore, of all the options available for surgical treatment of SUI post‐prostatectomy there was only limited evidence from one small study of the possible superiority of AUS implantation compared with injection of Macroplastique, especially in men with severe UI, but at a higher cost and risk of complications.
Quality of the evidence
There was considerable risk of bias in this trial, due to the small sample size, the lack of blinding, and uncertainty about the method of randomisation and allocation concealment. Furthermore, the interpretation of the data is difficult because the length of follow‐up was variable, it was uncertain when the reported outcomes were measured, and whether they were measured before or after extra treatments such as repeat injectables or AUS, or a new AUS.
Potential biases in the review process
Our attempts to contact the authors of the included study were unsuccessful and, perhaps, they could provide additional data affecting the results and conclusions of this review.
No other sources of potential bias are known to have affected the review process. We applied no language or other restrictions in the search for trials.
Agreements and disagreements with other studies or reviews
We identified only one clinical trial and no systematic reviews to assess the effectiveness of the surgeries for urinary incontinence after prostate surgery; however, there are two reviews assessing the current standards of diagnosis and management of post‐prostatectomy incontinence (Bauer 2009; Herschorn 2010), and several cohort studies and case series. The AUS is generally considered the gold standard for severe incontinence.
Authors' conclusions
Implications for practice.
There was very low quality evidence of possible superiority of artificial urethral sphincter (AUS) implantation compared with injectable treatment with Macroplastique in men with severe urinary incontinence post‐prostatectomy, but with a higher risk of complications and cost. No trials have tested the effect of the others surgical options (e.g. Pro‐ACT™ system, male sling, and stem cell therapy).
Implications for research.
The search results are replete with cohort studies, case series, and case reports, but only one clinical trial. There were no standardised protocols and outcome measures and complete reporting of adverse events and long‐term results. The mechanism of post‐prostatectomy incontinence is not fully understood.
There is an urgent need for well‐designed clinical trials to clarify the role of the therapies, using standardised protocols and outcome measures including condition‐specific quality of life and complete reporting of complications and costs. Studies must report long‐term monitoring and standardised reporting of durability. As AUS is considered the gold standard operation, any new operations should be compared with AUS.
What's new
| Date | Event | Description |
|---|---|---|
| 2 September 2014 | New citation required but conclusions have not changed | Search updated. No additional trial included in this update. Identified three ongoing trials (Abrams 2014; Haab 2014; Ockrim 2013). Incorporated Table 1. |
| 2 September 2014 | New search has been performed | Search updated. No additional trial included in this update. Identified three ongoing trials (Abrams 2014; Haab 2014; Ockrim 2013). Incorporated Table 1. |
Acknowledgements
We would like to thank June Cody, Sheila Wallace, and Bronwyn Davidson for their extensive collaboration in the first edition of this review and, now, Sheila Wallace and Muhammad Imran Omar from the Incontinence Review Group for their collaboration in this update.
Appendices
Appendix 1. Search terms ‐ Cochrane Incontinence Group Specialised Register
(({DESIGN.CCT*} OR {DESIGN.RCT*}) AND {INTVENT.SURG*} AND {TOPIC.URINE.INCON*})
(All searches were of the keyword field of Reference Manager 2012). Date of last search: 31 March 2014.
Appendix 2. Search strategy ‐ MEDLINE via PubMed
Last searched in April 2014.
#1. surgery
#2. operative therapy
#3. operative procedures
#4. invasive procedures
#5. operations
#6. peroperative procedures
#7. perioperative procedures
#8. preoperative procedures
#9. intraoperative procedures
#10. Operative Surgical Procedure
#11. Operative Surgical Procedures
#12. Operative Procedures
#13. Operative Procedure
#14. Suburethral Slings
#15. Suburethral Sling
#16. Transobturator Tape
#17. Transobturator Tapes
#18. Transobturator Suburethral Tape
#19. Trans‐Obturator Tape
#20. Artificial Urinary Sphincter
#21. Artificial Urinary Sphincters
#22. Artificial Genitourinary Sphincter
#23. Artificial Genitourinary Sphincters
#24. bulking agents
#25. augmentation agents
#26. Prostheses
#27. Implants
#28. GAX‐collagen
#29. Glutaral
#30. Glutaraldehyde
#31. Glutardialdehyde
#32. Gludesin
#33. Sonacide
#34. Sporicidin
#35. Cidex
#36. Korsolex
#37. Bode Brand of Glutaral
#38. Novaruca
#39. Bioglan Brand of Glutaral
#40. Sekumatic
#41. Ecolab Brand of Glutaral
#42. Diswart
#43. Dermatech Brand of Glutaral
#44. Glutarol
#45. Dermal Brand of Glutaral
#46. Cahill May Roberts Brand of Glutaral
#47. ethylene‐vinyl alcohol copolymer
#48. Enteryx
#49. polyethylene vinyl alcohol
#50. Tegress
#51. Uryx
#52. EVOH
#53. EVA
#54. Permacol
#55. operative therapy
#56. operative procedures
#57. invasive procedures
#58. operations
#59. peroperative procedures
#60. perioperative procedures
#61. preoperative procedures
#62. intraoperative procedures
#63. porcine collagen
#64. pig collagen
#65. swine collagen
#66. dermal collagen
#67. skin collagen
#68. Zuidex
#69. NASHA
#70. Dextranomer
#71. hyaluronic acid
#72. Hyaluronan
#73. Hyaluronate
#74. HA
#75. dextran polymer
#76. bulkamid
#77. aquamid
#78. Polyacrylamide
#79. Hydrogel
#80. PAHG
#81. Polyacrylamide grafted substrates
#82. Jung embedding medium
#83. polyacrylamide hydrogels
#84. ceramic microsphere
#85. coaptite
#86. hydroxylapatite
#87. hydroxyapatite
#88. CaHA
#89. trisacryl gelatin microspheres
#90. tris‐acryl gelatin microspheres
#91. Embosphere Microspheres
#92. Embospheres
#93. durasphere
#94. ACYST
#95. CCZB
#96. carbon coat
#97. carbon bead
#98. pyrolytic carbon
#99. low temperature isotropic carbon
#100. LTIC
#101. zirconium oxide
#102. zirconium dioxide
#103. Zirconia
#104. DC‐zirkon
#105. zirconium oxide hydrate
#106. gatekeeper
#107. hydrogel prostheses
#108. Teflon
#109. Polytetrafluoroethylene
#110. Polytef
#111. Tetrafluoroethylene
#112. PTP
#113. Bioplastique
#114. PTQ
#115. Macroplastique
#116. Proctoplastique
#117. Silicon
#118. Polydimethylsiloxane
#119. PDMS
#120. Dimethicone
#121. Dimethysilicone
#122. Poly‐dimethyl‐siloxane
#123. Elastomer
#124. Embolotherapy
#125. Embolotherapies
#126. Therapeutic Embolization
#127. Therapeutic Embolizations
#128. autologous fat
#129. urovive
#130. implant microballoon
#131. implant micro‐balloon
#132. colposuspension
#133. Open retropubic colposuspension
#134. Colporrhaphia
#135. Bladder neck needle suspension
#136. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119 OR #120 OR #121 OR #122 OR #123 OR #124 OR #125 OR #126 OR #127 OR #128 OR #129 OR #130 OR #131 OR #132 OR #133 OR #134 OR #135 OR #136
#137 Urinary Incontinence
#138. Urinary Urge Incontinence
#139. Urge Incontinence
#140. Urinary Reflex Incontinence
#141. Urinary Stress Incontinence
#142. #137 OR #138 OR #139 #140 OR #141 OR #142
#143. post prostatectomy
#144. post‐prostatectomy
#145. Prostatectomy
#146. Prostatectomies
#147. Suprapubic Prostatectomies
#148. Suprapubic Prostatectomy
#149. Retropubic Prostatectomies
#150. Retropubic Prostatectomy
#151. #143 OR #144 OR #145 OR #146 OR #147 OR #148 OR #149 OR #150
#152. #142 AND #151
#153. #136 AND #152
#154. randomised controlled trial [pt]
#155. controlled clinical trial [pt]
#156. randomized [tiab]
#157. placebo [tiab]
#158. drug therapy [sh]
#159. randomly [tiab]
#169. trial [tiab]
#170. groups [tiab]
#172. #154 OR #155 OR #157 OR #158 OR #159 OR #160 OR #170
#173. animals [mh] NOT humans [mh]
#174. #172 NOT #173
#175 #136 AND #153 AND #174
Appendix 3. Search strategy ‐ EMBASE via Ovid
Last searched in April 2014.
#1. surgery
#2. operative therapy
#3. operative procedures
#4. invasive procedures
#5. operations
#6. peroperative procedures
#7. perioperative procedures
#8. preoperative procedures
#9. intraoperative procedures
#10. Operative Surgical Procedure
#11. Operative Surgical Procedures
#12. Operative Procedures
#13. Operative Procedure
#14. Suburethral Slings
#15. Suburethral Sling
#16. Transobturator Tape
#17. Transobturator Tapes
#18. Transobturator Suburethral Tape
#19. Trans‐Obturator Tape
#20. Artificial Urinary Sphincter
#21. Artificial Urinary Sphincters
#22. Artificial Genitourinary Sphincter
#23. Artificial Genitourinary Sphincters
#24. bulking agents
#25. augmentation agents
#26. Prostheses
#27. Implants
#28. GAX‐collagen
#29. Glutaral
#30. Glutaraldehyde
#31. Glutardialdehyde
#32. Gludesin
#33. Sonacide
#34. Sporicidin
#35. Cidex
#36. Korsolex
#37. Bode Brand of Glutaral
#38. Novaruca
#39. Bioglan Brand of Glutaral
#40. Sekumatic
#41. Ecolab Brand of Glutaral
#42. Diswart
#43. Dermatech Brand of Glutaral
#44. Glutarol
#45. Dermal Brand of Glutaral
#46. Cahill May Roberts Brand of Glutaral
#47. ethylene‐vinyl alcohol copolymer
#48. Enteryx
#49. polyethylene vinyl alcohol
#50. Tegress
#51. Uryx
#52. EVOH
#53. EVA
#54. Permacol
#55. operative therapy
#56. operative procedures
#57. invasive procedures
#58. operations
#59. peroperative procedures
#60. perioperative procedures
#61. preoperative procedures
#62. intraoperative procedures
#63. porcine collagen
#64. pig collagen
#65. swine collagen
#66. dermal collagen
#67. skin collagen
#68. Zuidex
#69. NASHA
#70. Dextranomer
#71. hyaluronic acid
#72. Hyaluronan
#73. Hyaluronate
#74. HA
#75. dextran polymer
#76. bulkamid
#77. aquamid
#78. Polyacrylamide
#79. Hydrogel
#80. PAHG
#81. Polyacrylamide grafted substrates
#82. Jung embedding medium
#83. polyacrylamide hydrogels
#84. ceramic microsphere
#85. coaptite
#86. hydroxylapatite
#87. hydroxyapatite
#88. CaHA
#89. trisacryl gelatin microspheres
#90. tris‐acryl gelatin microspheres
#91. Embosphere Microspheres
#92. Embospheres
#93. durasphere
#94. ACYST
#95. CCZB
#96. carbon coat
#97. carbon bead
#98. pyrolytic carbon
#99. low temperature isotropic carbon
#100. LTIC
#101. zirconium oxide
#102. zirconium dioxide
#103. Zirconia
#104. DC‐zirkon
#105. zirconium oxide hydrate
#106. gatekeeper
#107. hydrogel prostheses
#108. Teflon
#109. Polytetrafluoroethylene
#110. Polytef
#111. Tetrafluoroethylene
#112. PTP
#113. Bioplastique
#114. PTQ
#115. Macroplastique
#116. Proctoplastique
#117. Silicon
#118. Polydimethylsiloxane
#119. PDMS
#120. Dimethicone
#121. Dimethysilicone
#122. Poly‐dimethyl‐siloxane
#123. Elastomer
#124. Embolotherapy
#125. Embolotherapies
#126. Therapeutic Embolization
#127. Therapeutic Embolizations
#128. autologous fat
#129. urovive
#130. implant microballoon
#131. implant micro‐balloon
#132. colposuspension
#133. Open retropubic colposuspension
#134. Colporrhaphia
#135. Bladder neck needle suspension
#136. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119 OR #120 OR #121 OR #122 OR #123 OR #124 OR #125 OR #126 OR #127 OR #128 OR #129 OR #130 OR #131 OR #132 OR #133 OR #134 OR #135 OR #136
#137 Urinary Incontinence
#138. Urinary Urge Incontinence
#139. Urge Incontinence
#140. Urinary Reflex Incontinence
#141. Urinary Stress Incontinence
#142. #137 OR #138 OR #139 #140 OR #141 OR #142
#143. post prostatectomy
#144. post‐prostatectomy
#145. Prostatectomy
#146. Prostatectomies
#147. Suprapubic Prostatectomies
#148. Suprapubic Prostatectomy
#149. Retropubic Prostatectomies
#150. Retropubic Prostatectomy
#151. #143 OR #144 OR #145 OR #146 OR #147 OR #148 OR #149 OR #150
#152. #142 AND #151
#153. #136 AND #152
#154. random$
#155. factorial$
#156. crossover$
#157. cross over$
#158. cross‐over$
#159. placebo$
#160. double$ adj blind$
#161. singl$ adj blind$
#162. assign$
#163. allocate$
#164. volunteer$
#165. cross‐over procedure
#166. double‐blind procedure
#167. randomized controlled trial
#168. single‐blind procedure
#169. #154 OR #155 OR #156 OR #157 OR #158 OR #159 OR #160 OR #161 OR #162 OR #163 OR #164 OR #165 OR #166 OR #167 OR #168
#170. #136 AND #142 AND #153 AND #169
Appendix 4. Search strategy ‐ LILACS via Bireme
Last searched in April 2014.
#1. surgery
#2. operative therapy
#3. operative procedures
#4. invasive procedures
#5. operations
#6. peroperative procedures
#7. perioperative procedures
#8. preoperative procedures
#9. intraoperative procedures
#10. Operative Surgical Procedure
#11. Operative Surgical Procedures
#12. Operative Procedures
#13. Operative Procedure
#14. Suburethral Slings
#15. Suburethral Sling
#16. Transobturator Tape
#17. Transobturator Tapes
#18. Transobturator Suburethral Tape
#19. Trans‐Obturator Tape
#20. Artificial Urinary Sphincter
#21. Artificial Urinary Sphincters
#22. Artificial Genitourinary Sphincter
#23. Artificial Genitourinary Sphincters
#24. bulking agents
#25. augmentation agents
#26. Prostheses
#27. Implants
#28. GAX‐collagen
#29. Glutaral
#30. Glutaraldehyde
#31. Glutardialdehyde
#32. Gludesin
#33. Sonacide
#34. Sporicidin
#35. Cidex
#36. Korsolex
#37. Bode Brand of Glutaral
#38. Novaruca
#39. Bioglan Brand of Glutaral
#40. Sekumatic
#41. Ecolab Brand of Glutaral
#42. Diswart
#43. Dermatech Brand of Glutaral
#44. Glutarol
#45. Dermal Brand of Glutaral
#46. Cahill May Roberts Brand of Glutaral
#47. ethylene‐vinyl alcohol copolymer
#48. Enteryx
#49. polyethylene vinyl alcohol
#50. Tegress
#51. Uryx
#52. EVOH
#53. EVA
#54. Permacol
#55. operative therapy
#56. operative procedures
#57. invasive procedures
#58. operations
#59. peroperative procedures
#60. perioperative procedures
#61. preoperative procedures
#62. intraoperative procedures
#63. porcine collagen
#64. pig collagen
#65. swine collagen
#66. dermal collagen
#67. skin collagen
#68. Zuidex
#69. NASHA
#70. Dextranomer
#71. hyaluronic acid
#72. Hyaluronan
#73. Hyaluronate
#74. HA
#75. dextran polymer
#76. bulkamid
#77. aquamid
#78. Polyacrylamide
#79. Hydrogel
#80. PAHG
#81. Polyacrylamide grafted substrates
#82. Jung embedding medium
#83. polyacrylamide hydrogels
#84. ceramic microsphere
#85. coaptite
#86. hydroxylapatite
#87. hydroxyapatite
#88. CaHA
#89. trisacryl gelatin microspheres
#90. tris‐acryl gelatin microspheres
#91. Embosphere Microspheres
#92. Embospheres
#93. durasphere
#94. ACYST
#95. CCZB
#96. carbon coat
#97. carbon bead
#98. pyrolytic carbon
#99. low temperature isotropic carbon
#100. LTIC
#101. zirconium oxide
#102. zirconium dioxide
#103. Zirconia
#104. DC‐zirkon
#105. zirconium oxide hydrate
#106. gatekeeper
#107. hydrogel prostheses
#108. Teflon
#109. Polytetrafluoroethylene
#110. Polytef
#111. Tetrafluoroethylene
#112. PTP
#113. Bioplastique
#114. PTQ
#115. Macroplastique
#116. Proctoplastique
#117. Silicon
#118. Polydimethylsiloxane
#119. PDMS
#120. Dimethicone
#121. Dimethysilicone
#122. Poly‐dimethyl‐siloxane
#123. Elastomer
#124. Embolotherapy
#125. Embolotherapies
#126. Therapeutic Embolization
#127. Therapeutic Embolizations
#128. autologous fat
#129. urovive
#130. implant microballoon
#131. implant micro‐balloon
#132. colposuspension
#133. Open retropubic colposuspension
#134. Colporrhaphia
#135. Bladder neck needle suspension
#136. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 OR #55 OR #56 OR #57 OR #58 OR #59 OR #60 OR #61 OR #62 OR #63 OR #64 OR #65 OR #66 OR #67 OR #68 OR #69 OR #70 OR #71 OR #72 OR #73 OR #74 OR #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 OR #84 OR #85 OR #86 OR #87 OR #88 OR #89 OR #90 OR #91 OR #92 OR #93 OR #94 OR #95 OR #96 OR #97 OR #98 OR #99 OR #100 OR #101 OR #102 OR #103 OR #104 OR #105 OR #106 OR #107 OR #108 OR #109 OR #110 OR #111 OR #112 OR #113 OR #114 OR #115 OR #116 OR #117 OR #118 OR #119 OR #120 OR #121 OR #122 OR #123 OR #124 OR #125 OR #126 OR #127 OR #128 OR #129 OR #130 OR #131 OR #132 OR #133 OR #134 OR #135 OR #136
#137 Urinary Incontinence
#138. Urinary Urge Incontinence
#139. Urge Incontinence
#140. Urinary Reflex Incontinence
#141. Urinary Stress Incontinence
#142. #137 OR #138 OR #139 #140 OR #141 OR #142
#143. post prostatectomy
#144. post‐prostatectomy
#145. Prostatectomy
#146. Prostatectomies
#147. Suprapubic Prostatectomies
#148. Suprapubic Prostatectomy
#149. Retropubic Prostatectomies
#150. Retropubic Prostatectomy
#151. #143 OR #144 OR #145 OR #146 OR #147 OR #148 OR #149 OR #150
#152. #142 AND #151
#153. #136 AND #152
#154. ((Pt ENSAIO CONTROLADO ALEATORIO OR Pt ENSAIO CLINICO CONTROLADO OR Mh ENSAIOS CONTROLADOS ALEATORIOS OR Mh DISTRIBUICAO ALEATORIA OR Mh MÉTODO DUPLO‐CEGO OR Mh MÉTODO SIMPLES‐CEGO or PT ESTUDO MULTICENTRICO) or ((tw ensaio or tw ensayo or tw trial) and (tw azar or tw acaso or tw placebo or tw control$ or tw aleat$ or tw random$ or (tw duplo and tw cego) or (tw doble and tw ciego) or (tw double and tw blind)) and tw clinic$)) AND NOT (Ct ANIMAIS OR ct coelhos or ct camundongos or MH ANIMAIS OR MH RATOS OR MH PRIMATAS OR MH CAES OR MH COELHOS OR MH SUINOS) [Palavras]
#155. #136 AND #153 AND #154
Appendix 5. UK Clinical Research Network (UKCRN)
UKCRN Study Portfolio (public) available at: public.ukcrn.org.uk/search/
Each term shown below was searched separately. (Note: cannot truncate. Only looked at 'Interventional'.)
Date of search: 8 May 2014.
Incontinence (in Research Summary)
Incontinent (in Research Summary)
Continent (in Research Summary)
Continence (in Research Summary)
Data and analyses
Comparison 1. Artificial urethral sphincter (AUS) implantation versus Macroplastique.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Success rate ‐ dry | 1 | 45 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.67 [1.28, 25.10] |
| 1.1 Group I ‐ minimal incontinence | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.19, 32.80] |
| 1.2 Group II ‐ total incontinence | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 8.89 [1.40, 56.57] |
| 2 Group I ‐ success rate ‐ dry, according to aetiology | 1 | 20 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.25, 10.09] |
| 2.1 Transurethral resection of prostate (TURP) | 1 | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.09, 102.05] |
| 2.2 Transvesical prostatectomy (TVP) | 1 | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.86 [0.12, 126.73] |
| 2.3 Radical retropubic prostatectomy (RRP) | 1 | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 12.82] |
| 3 Group II ‐ success rate ‐ dry, according to aetiology | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.88 [1.26, 49.24] |
| 3.1 TURP | 1 | 9 | Odds Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.21, 75.66] |
| 3.2 TVP | 1 | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | 21.0 [0.64, 689.99] |
| 3.3 RRP | 1 | 7 | Odds Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.22, 218.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Imamoglu 2005.
| Methods | Prospective randomised controlled trial Men having Macroplastique injection were followed up for 48 months (range 6 to 84) Men having artificial urinary sphincter implantation were followed up for 60 months (range 8 to120) |
|
| Participants | 45 men with urinary incontinence lasting 1 year (radical retropubic prostatectomy) or 6 months (transurethral prostatectomy/transvesical prostatectomy) despite conservative treatment following radical retropubic prostatectomy (12 men), transvesical prostatectomy (16 men), transurethral prostatectomy (16 men), or transurethral prostatectomy plus transvesical prostatectomy (1 man). The selected men needed more than 1 pad/day in the last month and had not received prior surgical treatment for post‐prostatectomy incontinence. None of the men were given radiotherapy for prostate carcinoma. The men were diagnosed with urodynamic studies, sphincteric electromyogram, and cytoscopic examinations. All men had a minimum bladder capacity of 150 mL and they did not have detrusor instability or hyper‐reflexia. They had urethral pressure profiles < 20 cmH2O and leak point pressures < 40 cmH2O. Most of these men had decreased sphincteric activity in electromyogram and some men did not have any activity. The cases were divided into 2 groups as minimal incontinence (group I) and total incontinence (group II) depending on the symptom scores, and weight and mean number of pads used per day. Minimal incontinence was defined as total number of pads ≤ 2, total weight of pads ≤ 100g, and score of quality of life scale ≤ 30. The values above these numbers were accepted as total incontinence Mean age of the men who underwent artificial urinary sphincter implantation was 64 years (52 to 76) and mean age of the men who underwent Macroplastique injection was 62 (55 to 75) years. There was no statistically significant difference between men with artificial urinary sphincter versus with Macroplastique injection considering urethral pressure profile implantation (5‐15 cmH2O) or leak point pressure (0‐20 cmH2O) |
|
| Interventions | Artificial urinary sphincter implantation (AMS 800) vs. endourethral Macroplastique Injection | |
| Outcomes | Mean number of pads used per day, weight of the pads, and score of quality of life survey scale. Mean cost of treatments For evaluating the success of the treatment, the mean number of pads used by the man per day and the weight of the pads were calculated for each group both in the preoperative and postoperative periods. Men who did not need to use pads were regarded as "dry". Men using 1 pad per day were accepted as "socially continent", and men using > 1 pad classified as "incontinent". The authors mention that there were control visits at 1, 6, and 12 months after treatment: however, they did not specify which visit was used to measure the outcome |
|
| Notes | Sources of funding not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | We found no information about generation of randomisation sequence. Randomisation was stratified according to severity of incontinence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | The authors do not mention any blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The author do not mention any loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | All relevant outcome reported |
| Other bias | High risk | 2 men were allocated to the artificial urinary sphincter group without randomisation, because of previous treatment for urethral strictures The follow‐up was variable between participants without the minimum of 12 months, and the author did not indicate when the outcomes were measured |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altinova 2009 | Rectus fascia sling inserted at the time of radical prostate surgery. Unclear whether truly a randomised controlled trial ("men randomly selected") |
| Lima 2013 | Compared 2 types of sling |
| Noguchi 2008 | Randomised controlled trial of a surgical suspension technique at the time of radical prostate surgery |
Characteristics of ongoing studies [ordered by study ID]
Abrams 2014.
| Trial name or title | Male Synthetic Sling versus Artificial Urinary Sphincter Trial for Men with Urodynamic Stress Incontinence after Prostate Surgery: Evaluation by Randomised Controlled Trial (MASTER) |
| Methods | Multicentre randomised controlled non‐inferiority trial |
| Participants | Adult men who have decided in discussion with their urologist to have surgery for urodynamic stress incontinence resulting from prostate surgery |
| Interventions | 2 surgical operations for male urinary incontinence, a synthetic male sling and an AUS implantation will be evaluated |
| Outcomes | Primary 1. Clinical effectiveness of implantation of the male sling compared with AUS in terms of self reported incontinence at 12 months 2. Cost effectiveness of a policy of primary implantation of the male sling compared with AUS, measured by incremental cost per quality‐adjusted life‐year at 24 months Secondary 1. Risks of each type of surgery 2. Costs of the benefits and risks of each treatment policy 3. Subsequent National Health Service use (including repeat surgery) needed for men with persistent or recurrent problems 4. Differential effects of the operations on other outcomes such as quality of life and general health 5. Satisfaction of the men with each procedure |
| Starting date | 1 July 2013 |
| Contact information | Prof. Paul Abrams. Bristol Urological Institute Southmead Hospital. Email: paul.abrams@bui.ac.uk |
| Notes |
Haab 2014.
| Trial name or title | Periurethral Pro‐ACT͐™ Balloons vs Retrourethral AdvanceXP™ Male Sling for Post‐prostatectomy Incontinence (BALLANCE) |
| Methods | Prospective, randomised, multicentric, comparative trial of the 2 devices (with a superiority hypothesis) |
| Participants | Men presenting stress urinary incontinence following radical prostatectomy for prostate cancer. Men must be aged > 18 years, without cancer recurrence, and must present pure stress urinary incontinence on urodynamics (without detrusor overactivity), and mild‐to‐moderate incontinence (24‐hour pad test < 300 g). All men showing urethral stricture at preoperative cystoscopy will be excluded from the study |
| Interventions | AdvanceXP™ retrourethral male sling vs. Periurethral Pro‐ACT™ balloons |
| Outcomes | Efficacy, side effects, quality of life, participant satisfaction, and cost‐effectiveness |
| Starting date | March 2013 |
| Contact information | François Haab, MD, PhD; telephone: +33 (0)1 56 01 64 95; email: francois.haab@tnn.aphp.fr |
| Notes |
Ockrim 2013.
| Trial name or title | Single‐site, Two‐arm Randomised Controlled Study: a Comparison of Effectiveness of the Advance Male Sling and AMS 800 Artificial Urinary Sphincter for Mild to Moderate Post Prostatectomy Incontinence |
| Methods | Single‐site, 2‐arm randomised controlled study |
| Participants | Post‐prostatectomy men at least 6 months after surgery Mild‐to‐moderate stress urinary incontinence (mild 50‐200 mL 1‐2 pads/day; moderate 200‐400 mL 3‐4 pads/day) Able and willing to participate in the study for its duration Upper age limit 80 years; lower age limit 40 years |
| Interventions | Advance™ male sling (polypropylene mesh, retro‐urethral transobturator position. Inserted using 2 needle passers and through a perineal incision) vs. AUS (mechanical device made of silicon, has 3 components: cuff, pump, and a balloon. Implanted through a perineal incision and inguinal incision) Follow‐up length: 12 months |
| Outcomes | Difference in 24‐hour pad weight; time points: 3, 6, 12 months after surgery |
| Starting date | 8 February 2013 |
| Contact information | Mr Antoine Kass‐Iliyya: email: antione.kass‐iliyya@uclh.nhs.uk |
| Notes |
AUS: artificial urinary sphincter.
Contributions of authors
Laercio Antonio da Silva (LAS): conception of the protocol.
LAS and Regis Bruni Andrioli (RBA): developing the search strategy and undertaking searches.
Edina Mariko Koga da Silva (EMKS) and LAS: screening search results, organising retrieval of papers, screening retrieved papers against the inclusion criteria, appraising quality of papers, extracting data; analysed and interpreted the data; wrote up results; and entered data into RevMan.
LAS: wrote to authors of papers for additional information and to locate potentially relevant unpublished or ongoing studies; provided a clinical perspective.
EMKS: responsible for data management for the review.
Alvaro Nagib Atallah (ANA): provided a methodological perspective.
Sources of support
Internal sources
No sources of support supplied
External sources
-
The National Institute for Health Research, UK.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Incontinence Group.
Declarations of interest
None of the review authors had a direct financial interest, paid consultancy or share‐holding in a company producing the intervention(s) under review or a rival treatment.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Imamoglu 2005 {published data only}
- Imamoglu MA, Tuygun C, Bakirtas H, Yigitbasi O, Kiper A. The comparison of artificial urinary sphyncter implantation and endourethral macroplastique injection for the treatment of postprostatectomy incontinence. European Urology 2005;47:209‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altinova 2009 {published data only}
- Altinova S, Demirci DA, Ozdemir AT, Akbulut Z, Atmaca AF, Caglayan A, et al. Incorporation of anterior rectus fascial sling into radical retropubic prostatectomy improves postoperative continence. Urologia Internationalis 2009;83(1):19‐21. [SRINCONT32094] [DOI] [PubMed] [Google Scholar]
Lima 2013 {published data only}
- Lima JPC, Pompeo ACL, Bezerra CA. Sub urethral sling for male urinary incontinence: randomized clinic trial of two slings. Neurourology and Urodynamics 2013;32(6):894‐5. [sr‐incont49193] [Google Scholar]
Noguchi 2008 {published data only}
- Noguchi M, Kakuma T, Suekane S, Nakashima O, Mohamed ER, Matsuoka K. A randomized clinical trial of suspension technique for improving early recovery of urinary continence after radical retropubic prostatectomy. BJU International 2008;102(8):958‐63. [SRINCONT27722] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Abrams 2014 {unpublished data only}
- Abrams P. Male synthetic sling versus Artificial urinary Sphincter Trial for men with urodynamic stress incontinence after prostate surgery: Evaluation by Randomised controlled trial (MASTER), 2014. www.controlled‐trials.com/ISRCTN49212975/MASTER (accessed 23 September 2014). [sr‐incont60070]
Haab 2014 {published and unpublished data}
- Haab F. Prospective, Randomized, Multicentric, Comparative Trial of Two Devices in Surgical Treatment of Post‐radical Prostatectomy Stress Urinary Incontinence: Periurethral Pro‐ACT(TM) Balloons and AdvanceXP(TM) Retrourethral Male Sling. clinicaltrials gov/show/NCT01500603 (accessed 23 September 2014). [srincont60065]
Ockrim 2013 {published and unpublished data}
- Ockrim J, Kass‐Iliyya A. Single‐site, two‐arm randomised controlled study: a comparison of effectiveness of the Advance male sling and AMS 800 artificial urinary sphincter for mild to moderate post prostatectomy incontinence. www controlled‐trials com/ISRCTN55599282 (accessed 23 September 2014). [srincont50321]
Additional references
Abrams 1991
- Abrams PH. Investigation of post prostatectomy problems. Urology 1991;15:209‐12. [DOI] [PubMed] [Google Scholar]
Abrams 2003
- Abrams P, Cardozo L, Fall M, Griffiths D, Rosier P. The standardization of terminology in lower urinary tract function: report from the standardization sub‐committee of the international continence society. Urology 2003;61:37‐41. [DOI] [PubMed] [Google Scholar]
Albertsen 1997
- Albertsen PC, Lu‐Yao GL, Warren J. Risk of complication and readmission associated with radical prostatectomy in community practice: a Medicare claims analysis. Journal of Urology 1997;157:93. [Google Scholar]
Armstrong 2009
- Armstrong N, Vale L, Deverill M, Nabi G, McClinton S, N'Dow J, et al. Surgical treatments for men with benign prostatic enlargement: cost effectiveness study. BMJ 2009;338:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Augustin 2002
- Augustin H, Pummer K, Daghofer F, Habermann H, Primus G, Hubmer G. Patient self‐reporting questionnaire on urological morbidity and bother after radical retropubic prostatectomy. European Urology 2002;42:112‐7. [DOI] [PubMed] [Google Scholar]
Bauer 2009
- Bauer RM, Bastian PJ, Gozzi C, Stief CG. Postprostatectomy Incontinence: all about diagnosis and management. European Urology 2009;55:322‐33. [DOI] [PubMed] [Google Scholar]
Black 2006
- Black L, Naslund MJ, Gilbert TD, Davis AE, Ollendorf DA. An examination of treatment patterns and costs of care among patients with benign prostatic hyperplasia. American Journal of Managed Care 2006;12(4):S99‐110. [PubMed] [Google Scholar]
Burkhard 2006
- Burkhard FC, Kessler TM, Fleischmann A, Thalmann GN, Schumacher M, Studer UE. Nerve‐sparing open radical retropubic prostatectomy? Does it have an impact on urinary continence?. Journal of Urology 2006;176:189‐95. [DOI] [PubMed] [Google Scholar]
Campbell 2012
- Campbell SE, Glazener CMA, Hunter KF, Cody JD, Moore KN. Conservative management for postprostatectomy urinary incontinence. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD001843.pub4] [DOI] [PubMed] [Google Scholar]
Catalona 1999
- Catalona WJ, Carvalhal GF, Mager DE, Smith DS. Potency, continence and complication rates in 1,870 consecutive radical retropubic prostatectomies. Journal of Urology 1999;162:433‐8. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. . . .
Eastham 1996
- Eastham JA, Kattan MW, Rogers E, Goad JR, Ohori M, Boone TB, et al. Risk factors for urinary incontinence after radical prostatectomy. Journal of Urology 1996;156:1707‐13. [PubMed] [Google Scholar]
Fader 2008
- Fader M, Cottenden AM, Getliffe K. Absorbent products for moderate‐heavy urinary and/or faecal incontinence in women and men. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007408; CD007408] [DOI] [PMC free article] [PubMed] [Google Scholar]
Filocamo 2007
- Filocamo M, Li Marzi V, Popolo G, Cecconi F, Villari D, Marzocco M, et al. Pharmacologic treatment in postprostatectomy stress urinary incontinence. European Urology 2007;51:1559‐64. [DOI] [PubMed] [Google Scholar]
Foote 1991
- Foote J, Yun S, Leach GE. Post‐prostatectomy incontinence. Pathophysiology, evaluation, and management. Urology Clinics of North America 1991;18(2):229‐41. [PubMed] [Google Scholar]
García Montes 2007
- García Montes F, Knight SL, Greenwell T, Mundy AR, Craggs MD. "Flowsecure" artificial urinary sphincter: a new adjustable artificial urinary sphincter concept with conditional occlusion for stress urinary incontinence [Esfínter Urinario Artificial “FlowSecureTM”: Un nuevo concepto de esfínter artificial regulable y con oclusión condicional para la incontinencia urinaria de esfuerzo]. Actas Urol Esp 2007;31(7):752‐8. [DOI] [PubMed] [Google Scholar]
Haab 1996
- Haab F, Yamaguchi R, Leach GE. Postprostatectomy incontinence. Urology Clinics of North America 1996;23:447‐57. [DOI] [PubMed] [Google Scholar]
Hammerer 1997
- Hammerer P, Huland H. Urodynamic evaluation of changes in urinary control after radical retropubic prostatectomy. Journal of Urology 1997;157:233‐6. [PubMed] [Google Scholar]
Herr 1994
- Herr HW. Quality of life of incontinence men after radical prostatectomy. Journal of Urology 1994;151:652. [DOI] [PubMed] [Google Scholar]
Herschorn 2010
- Herschorn S, Bruschini H, Comiter C, Grise P, Hanus T, Kirschner‐Hermanns R, et al. Surgical treatment of stress incontinence in men. Neurology and Urodynamics 2010;29:179‐90. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICI 2008
- International Consultation on Incontinence. Fourth International Consultation on Incontinence (ICI) ‐ Surgery for Urinary Incontinence in Men Committee highlights. 2008 July 5‐8; Paris.
Jack 2005
- Jack GS, Almeida GF, Zhang R, Alfonso ZC, Zuk PA, Rodriguez LV. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. Journal of Urology 2005;174(5):2041‐5. [DOI] [PubMed] [Google Scholar]
Kundu 2004
- Kundu SD, Roehl KA, Eggener SE, Antenor JAV, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. Journal of Urology 2004;172:2227‐31. [DOI] [PubMed] [Google Scholar]
Ogah 2009
- Ogah J, Cody JD, Rogerson L. Minimally invasive synthetic suburethral sling operations for stress urinary incontinence in women. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD006375.pub2] [DOI] [PubMed] [Google Scholar]
Onur 2004
- Onur R, Rajpurkar A, Singla A. New perineal bone anchored male sling: lessons learned. Urology 2004;64:58‐61. [DOI] [PubMed] [Google Scholar]
Penson 2005
- Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FC, et al. Five‐year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. Journal of Urology 2005;173:1701‐5. [DOI] [PubMed] [Google Scholar]
Peyromaure 2002
- Peyromaure M, Ravery V, Boccon‐Gibod L. The management of stress urinary incontinence after radical prostatectomy. British Journal of Urology International 2002;90:155‐61. [DOI] [PubMed] [Google Scholar]
Raz 1992
- Raz S, Erickson DR. SEAPI QMM incontinence classification system. Neurourology and Urodynamics 1992;11(3):187‐99. [DOI] [PubMed] [Google Scholar]
Reference Manager 2012 [Computer program]
- Thomson Reuters. Reference Manager Professional Edition. Version 12. New York: Thomson Reuters, 2012.
Rehder 2007
- Rehder P, Gozzi C. Transobturator sling suspension for male urinary incontinence including post‐radical prostatectomy. European Urology 2007;52(3):860‐7. [DOI] [PubMed] [Google Scholar]
Sacco 2006
- Sacco E, Prayer‐Galetti T, Pinto F, Fracalanza S, Betto G, Pagano F, et al. Urinary incontinence after radical prostatectomy: incidence by definition, risk factors and temporal trend in a large series with a long‐term follow‐up. British Journal of Urology International 2006;97:1234‐41. [DOI] [PubMed] [Google Scholar]
Sanda 2008
- Sanda MG, Dunn RL, Michalski J, Sandler HM, Northouse L, Hembroff L, et al. Quality of life and satisfaction with outcome among prostate‐cancer survivors. New England Journal of Medicine 2008;358:1250‐61. [DOI] [PubMed] [Google Scholar]
Schlenker 2006
- Schlenker B, Gratzke C, Reich O, Schorsch I, Seitz M, Stief CG. Preliminary results on the off‐label use of duloxetine for the treatment of stress incontinence after radical prostatectomy or cystectomy. European Urology 2006;49:1075‐8. [DOI] [PubMed] [Google Scholar]
Secin 2005
- Secin FP, Martinez‐Salamanca JI, Eilber KS. Limited efficacy of permanent injectable agents in the treatment of stress urinary incontinence after radical prostatectomy. Archivos Espanoles de Urologia 2005;58(5):431‐6. [DOI] [PubMed] [Google Scholar]
Seckiner 2007
- Seckiner I, Yesilli C, Mungan NA, Aykanat A, Akduman B. Correlations between the ICIQ‐SF score and urodynamic findings. Neurourology & Urodynamics 2007;26:492‐4. [DOI] [PubMed] [Google Scholar]
Sierra 2006
- Sierra JM, Romano SV, Romo IG, Ortega JB, Casado JS, Moyano AS. New male sling "Argus" for the treatment of stress urinary incontinence. Archivos Espanoles de Urologia 2006;59:607‐13. [DOI] [PubMed] [Google Scholar]
Stamey 1981
- Stamey TA. Endoscopic suspension of the vesical neck for surgically curable incontinence in the female. Monograph in Urology 1981;2(3):387‐90. [Google Scholar]
Steiner 1991
- Steiner MS, Morton RA, Walsh PC. Impact of anatomical radical prostatectomy on urinary continence. Journal of Urology 1991;145:512‐5. [DOI] [PubMed] [Google Scholar]
Ullrich 2004
- Ullrich NFE, Comiter CV. The male sling for stress urinary incontinence: urodynamic and subjective assessment. Journal of Urology 2004;172:204‐6. [DOI] [PubMed] [Google Scholar]
van der Horst 2007
- Horst C, Naumann CM, Al‐Najaar A, Seif C, Stubinger SH, Junemann KP, et al. Etiology and pathophysiology of male stress incontinence. Urologe A 2007;46:229‐41. [DOI] [PubMed] [Google Scholar]
Veenema 1977
- Veenema RJ, Gursel EO, Lattimer JK. Radical retropubic prostatectomy for cancer: a 20‐year experience. Journal of Urology 1977;117:330‐1. [DOI] [PubMed] [Google Scholar]
Walsh 1980
- Walsh PC, Jewett HJ. Radical surgery for prostatic cancer. Cancer 1980;45(7 Suppl):1906‐11. [PubMed] [Google Scholar]
Walsh 1994
- Walsh PC, Partin AW, Epstein JI. Cancer control and QoL following anatomical radical retropubic prostatectomy: results at 10 years. Journal of Urology 1994;152:1831‐6. [DOI] [PubMed] [Google Scholar]
Walsh 2000
- Walsh PC, Marschke P, Ricker D, Burnett AL. Patient reported urinary continence and sexual function after anatomic radical prostatectomy. Urology 2000;55:58‐61. [DOI] [PubMed] [Google Scholar]
Wei 2000
- Wei JT, Montie JE. Comparison of patients and physicians rating of urinary incontinence following radical prostatectomy. Seminars in Urologic Oncology 2000;18:76‐80. [PubMed] [Google Scholar]
Zahariou 2006
- Zahariou A, Papaioannou P, Kalogirou G. Is HCl duloxetine effective in the management of urinary stress incontinence after radical prostatectomy?. Urology International 2006;77:9‐12. [DOI] [PubMed] [Google Scholar]


