Abstract
Proper adherence to infection control precautions, including appropriate selection and use of personal protective equipment (PPE), is of significant importance to the health and well-being of perioperative personnel. Surgical masks are intended for use as a barrier to protect the wearer’s face from large droplets and splashes of blood and other body fluids; however, surgical and high-filtration surgical laser masks do not provide enough protection to be considered respiratory PPE. Potential exposure to airborne contaminants and infectious agents, including those present in surgical smoke, necessitates the use of respiratory PPE, such as a surgical N95 particulate filtering facepiece respirator. Filtering facepiece respirators greatly reduce a wide size range of particles from entering the wearer’s breathing zone and are designed to protect the user from both droplet and airborne particles. Every health care worker who must use a respirator to control hazardous exposures in the workplace must be trained to properly use the respirator and pass a fit test before using it in the workplace.
Key Words: surgical mask, filtering facepiece respirator, surgical N95 respirator, surgical smoke, smoke inhalation, health care worker safety
Continuing Education: Proper Use of Surgical N95 Respirators and Surgical Masks in the OR.
Continuing Education Contact Hours
indicates that continuing education contact hours are available for this activity. Earn the contact hours by reading this article, reviewing the purpose/goal and objectives, and completing the online Examination and Learner Evaluation at http://www.aorn.org/CE. A score of 70% correct on the examination is required for credit. Participants receive feedback on incorrect answers. Each applicant who successfully completes this program can immediately print a certificate of completion.
Event: #13510
Session: #0001
Fee: Members $12.60, Nonmembers $25.20
The contact hours for this article expire April 30, 2016.
Purpose/Goal
To enable the learner to correctly use surgical masks and surgical N95 respirators.
Objectives
-
1.
Discuss risks associated with surgical smoke.
-
2.
Explain the differences between surgical masks and surgical N95 respirators.
-
3.
Describe tests used to certify a surgical N95 respirator.
-
4.
Identify when use of a surgical N95 respirator is advised.
Accreditation
AORN is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation.
Approvals
This program meets criteria for CNOR and CRNFA recertification, as well as other continuing education requirements.
AORN is provider-approved by the California Board of Registered Nursing, Provider Number CEP13019. Check with your state board of nursing for acceptance of this activity for relicensure.
Conflict of Interest Disclosures
Ms Benson, Dr Novak, and Ms Ogg have no declared affiliations that could be perceived as posing potential conflicts of interest in the publication of this article.
The behavioral objectives for this program were created by Rebecca Holm, MSN, RN, CNOR, clinical editor, with consultation from Susan Bakewell, MS, RN-BC, director, Perioperative Education. Ms Holm and Ms Bakewell have no declared affiliations that could be perceived as posing potential conflicts of interest in the publication of this article.
Sponsorship or Commercial Support
No sponsorship or commercial support was received for this article.
Disclaimer
AORN recognizes these activities as continuing education for registered nurses. This recognition does not imply that AORN or the American Nurses Credentialing Center approves or endorses products mentioned in the activity.
Surgical personnel are vulnerable to a variety of hazardous substances, including potentially infectious agents present in surgical smoke given their prolonged exposures to surgical smoke during the course of their career.1, 2, 3 A growing body of scientific evidence indicates that 95% of surgical smoke consists of water, but the remaining 5% may contain potentially hazardous particles, including dead and live cellular material; blood fragments; bacteria; viruses; toxic gases and vapors (eg, benzene, hydrogen cyanide, formaldehyde); and lung-damaging dust.1, 4 Typically, within five minutes of the beginning of an electrosurgical procedure, particulate matter in the immediate area increases from a baseline measurement of approximately 60,000 particles per cubic foot to more than one million particles per cubic foot.5 Burning 1 g of tissue releases the same level of mutagenic toxins as smoking three to six cigarettes.6 In addition, it takes the typical OR airflow recirculating system approximately 20 minutes to return particle concentrations to normal after a surgical procedure is complete.5
Several reports within the past decade have indicated that health care workers are inconsistent with and have suboptimal adherence (ie, less than 60%) to recommended infection control precautions.7, 8, 9 For example, the lack of adherence to proper infection control precautions, including use of respiratory personal protective equipment (PPE), was documented during an H1N1 influenza outbreak.10, 11, 12 Both the Occupational Safety and Health Administration (OSHA) and the Centers for Disease Control and Prevention Healthcare Infection Control Practices Advisory Committee (CDC/HICPAC) have stated that, based on the evidence, there appears to be marginal compliance with proper use of respiratory protection guidelines by health care workers. In addition, OSHA warns that, given the emergence of “new infectious diseases that affect both patients and [health care workers], compliance with recommended infection control practices is an increasingly important issue.”13 Therefore, reinforcing and monitoring day-to-day compliance are necessary steps that must be undertaken by both the individual employee and employer.13
Do-Not-Use Abbreviation.
Surgical N95 respirators prevent the passage of a wide size range of hazardous airborne particulate matter from entering the wearer’s breathing space. Airborne particulate matter is measured in micrometers, formerly called microns. The Greek letter μ is often used to show the prefix micro, and μm is used for the unit of measure micrometer. This Greek letter, however, can be mistaken to mean unit, 0, cc, or mg; therefore, the Institute for Safe Medication Practices has placed μ on its list of abbreviations, symbols, and designations that should never be used when communicating medical information. As a result, practitioners should spell out the word micrometer or use the abbreviation mcm.1
-
1.
ISMP’s List of error-prone abbreviations, symbols, and dose designations. Institute for Safe Medication Practices. http://www.ismp.org/Tools/errorproneabbreviations.pdf. Accessed January 11, 2013.
Surgical masks are the most commonly used protective facemask in perioperative and other hospital settings.14 These masks are intended for use as a barrier to protect the wearer’s face from large droplets and splashes of blood and other body fluids. In certain clinical situations, however, potential exposure to airborne contaminants and infectious agents also necessitates the use of respiratory PPE, such as an N95 particulate filtering facepiece respirator (ie, surgical N95 respirator).15, 16 The term N95 refers to a National Institute for Occupational Safety and Health (NIOSH)-approved and US Food and Drug Administration (FDA)-cleared particulate filtering facepiece respirator that can filter at least 95% of airborne particles. The term surgical mask refers to an FDA-cleared laser, isolation, or medical procedure mask, with or without a face shield, worn “to protect both the surgical patient and perioperative team members from transfer of microorganisms and body fluids. Surgical masks are also used to protect health care providers from contact with large infectious droplets (> 5 micrometer [mcm] in size).”17 (p357) The term protective facemask refers to a surgical N95 respirator or surgical mask that covers at least the nose and mouth to reduce the wearer’s risk of inhaling hazardous airborne particles (including dust particles and infectious agents).
The primary objective of this article is to differentiate between surgical N95 respirators and surgical masks, a most significant and perhaps easily misunderstood issue. The secondary objective is to reinforce awareness of common workplace hazards in which voluntary and precautionary use of surgical N95 respirators may be advantageous to the health and safety of surgical health care workers.
Protective Differences
Varying degrees of protection are associated with different types of respiratory PPE.18 Although several types of respirators are available, those most commonly used by health care workers usually fall into the category of air-purifying filtering facepiece respirators. These fit-tested devices are personal protective devices that are worn on the face, cover at least the nose and mouth, and are composed of a filter that prevents the passage of a wide size range of hazardous airborne particulate matter, including dust particles and infectious agents, from entering the wearer’s breathing space.
All filtering facepiece respirators must pass certification tests conducted by NIOSH.18 To help ensure adequate performance, the NIOSH certification test challenges filtering facepiece respirators at a high flow rate using charged-neutralized aerosols in the most penetrating size range for most filters.19 Those filtering facepiece respirators that prevent at least 95% of particles from passing through the filter during a worst-case test are given a 95 designation. Similarly, those that prevent 99% and 99.97% of the particles from passing through the filter are given the designation of 99 and 100, respectively.19
As part of the OSHA-required respiratory protection program (29 CFR 1910.134), every health care worker who must use a respirator to control hazardous exposures in the workplace must be trained to properly use the respirator and pass a fit test before using it in the workplace.20 Fit tests can be performed in a qualitative or quantitative manner. During a qualitative fit test, the taste or smell of the test aerosol is a sign of an improperly fitting respirator. It is a subjective test that is dependent on the sensitivity of the health care worker. Quantitative fit tests require the use of an instrument that permits a technician to monitor particles in the room air as well as particles inside the respirator while it is being worn by the health care worker. The ratio between the room count and the count inside the mask determines the health care worker’s fit factor for that surgical N95 respirator. For a passing test, the fit factor must be greater than or equal to 100.20
The fit test ensures that the health care worker is wearing a surgical N95 respirator that can provide a good facial seal and, therefore, expected levels of protection. One key aspect of determining the effectiveness of a surgical N95 respirator is performing a user seal check. Every time a person dons a N95 respirator, he or she should perform the manufacturer's recommended user seal check method to ensure that an adequate seal has been obtained. The surgical N95 respirator only provides protection when the respirator is adequately sealed to the wearer's face, and a user seal check aids the health care worker in determining whether the respirator is fitting properly.
If the health care worker feels air coming in or going out around his or her eyes or chin or feels his or her glasses fogging during a user seal check, the surgical N95 respirator should be adjusted or replaced. User seal checks are not substitutes for qualitative or quantitative fit tests.
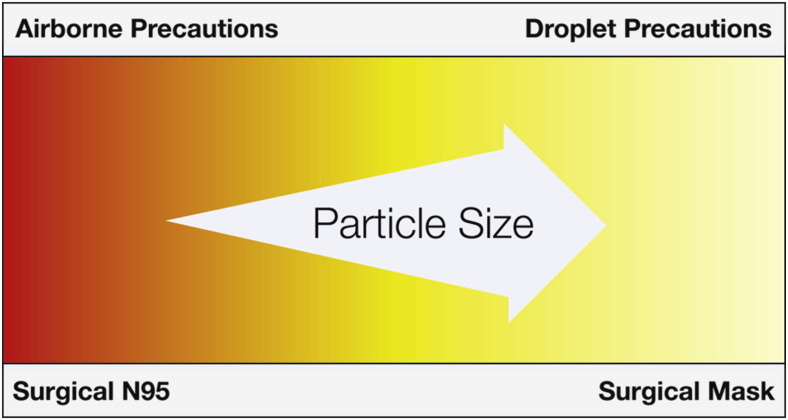
The CDC/HICPAC infection control transmission precautions instruct health care workers to use droplet precautions for certain pathogens and airborne precautions for others. This guidance is based on a pathogen’s known epidemiology (ie, patterns of transmissibility and infectivity).15 Particle size does not affect the infectivity of the pathogen; however, the principle that smaller airborne particles can travel farther and remain suspended in room air longer than larger particles (ie, droplets) is also an important factor to consider in selecting the best protective facemask (Figure 1 ). Filtering facepiece respirators, such as N95 respirators, are designed to protect the user from both droplet and airborne particles, and they come in many sizes and are designed to seal to the face. Both surgical N95 respirators and surgical masks are disposable, single-use devices that should be worn during a single patient encounter by a single person and should not be shared between wearers.18
Figure 1.
Type of isolation precaution associated with proper selection of a protective facemask.
High-filtration surgical laser masks were designed as a form of barrier or source control. When placed on a contagious patient, surgical masks limit the spread of large-sized droplet respiratory secretions to others. When worn by a health care worker, the patient’s incisions are protected from infectious agents present in the worker’s mucus and saliva, while at the same time, the worker is protected from any sprays of blood or body fluid coming from the patient. Only protective facemasks cleared by the FDA as medical devices are recommended for use in health care workplaces. The FDA does not independently test the devices but rather reviews the manufacturer’s testing data for
In addition, with regard to the filter efficiency requirements for FDA clearance, a surgical mask must perform as well as one other cleared surgical mask currently on the market.19
Two key features associated with the level of protection provided by high-filtration surgical laser masks are filtration efficiency and fit. Studies have shown that the filtration efficiency for surgical masks is highly variable depending on the type of mask and the manufacturer.22, 23 The filtration efficiency can be as high as that of a surgical N95 respirator, but even when surgical masks with the most efficient filter media (ie, high-filtration surgical laser masks) are properly worn, the fit factor obtained is quite low. Oberg and Brosseau22 conducted filtration performance and fit tests with nine types of surgical masks and found that none of the models tested exhibited adequate filter performance and facial fit characteristics to be considered equivalent to a surgical N95 respirator. Surgical masks are not designed to seal to the face. Without an adequate seal to the face, inhaled breath is not forced through the filter and instead flows through the gaps around the seal area, providing minimal protection by allowing potentially hazardous contaminants to enter the workers’ breathing zone through gaps between the wearer’s face and the mask. Therefore, surgical and high-filtration surgical laser masks do not provide the degree of protection to be considered respiratory PPE.
Airborne Contaminants and Respiratory Hazards
Several characteristics of airborne contaminants should be taken into consideration for choosing the proper type of protective facemask:
-
■
What is the hazard?
-
■
How infectious is the hazard?
-
■
What is the dominant mode(s) of transmission (ie, contact, droplet, aerosol)?
-
■
What is the particle size of the airborne hazard?
If any of these factors are unknown, being cautious and using at least a surgical N95 respirator may be beneficial. Because of its greater filtration capacity, a surgical N95 respirator will greatly reduce a wide size range of particles from entering the wearer’s breathing zone. Therefore, a surgical N95 respirator provides more protection from a wider range of pathogen-carrying particles than a surgical mask.
When people speak, sneeze, or cough, they release particles that may contain pathogens. The size of the airborne pathogen is important because the smaller the particle, the longer it can remain aerosolized (ie, suspended in the air). In addition to suspension time, particle-size range affects how far an inhaled particle can travel; smaller particles can travel deeper into the airway, which increases the risk of potentially adverse health effects. Bac-teria can be as large as 30 mcm or smaller than 0.3 mcm.24 Viruses are smaller and can range in size from 0.01 mcm to 0.3 mcm.14 Primary infection for Mycobacterium tuberculosis occurs deep with-in the lung, whereas smallpox and pneumonic plague can occur in both the upper and lower respiratory tract.25
Every day, health care workers encounter hazardous particulates while treating patients, and these encounters necessitate different forms of protective precautions. Droplet precautions are used to prevent large droplets generated from coughs and sneezes from reaching the mucous membranes of the nose and mouth of the health care worker. This level of protection can be achieved with a surgical mask or a surgical N95 respirator. Diseases requiring droplet precautions include meningitis, pneumonia, mumps, and rubella. As an example, occupational transmission of Neisseria meningitides was reported in 2009 as a result of exposure of health care workers who were not wearing any type of protective facemask.26 Another case reported health care worker transmission of bacterial meningitis to five patients after intrapartum spinal anesthesia where established infection-control recommendations, including the use of a mask by health care workers, were not followed.27
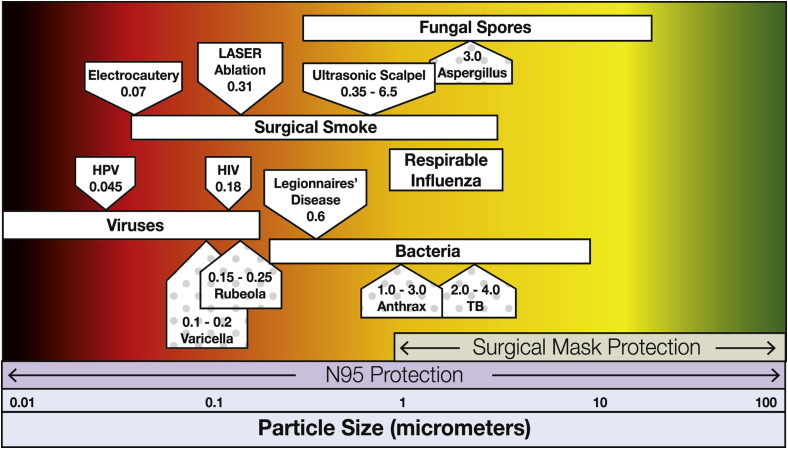
Regardless of their size, certain diseases can be transmitted through airborne routes and require more stringent precautions: negative pressure rooms, isolation rooms, and respiratory protection at least as protective as a surgical N95 respirator. Various pathogens, their size ranges, and the corresponding comparison of surgical mask and surgical N95 respirator ranges of particulate protection are shown in Figure 2 with examples of airborne-transmissible diseases (ie, tuberculosis, chickenpox, rubeola) depicted by the dotted boxes.28
Figure 2.
Particle sizes and associated type of protection against pathogens and atmospheric hazards commonly found in hospital environments. The boxes with the dotted background are airborne-transmissible diseases.
Certain treatments may require health care workers to convert from using droplet precautions to using higher order control measures provided with airborne precautions. Aerosol-generating procedures create smaller droplets than those generated by sneezing or coughing, thereby increasing the suspension time of the particles.29 Aerosol-generating procedures include bronchoscopy, endotracheal intubation, suctioning, and nebulized medication administration. Assisting in such procedures requires health care workers to be within 3 ft of the patient, which leaves them vulnerable to droplet sprays as well. Health care worker involvement in these high-risk procedures has been linked to increased transmission of severe acute respiratory syndrome (SARS).29, 30 A systematic review of studies revealed that health care workers who performed endotracheal intubation on patients diagnosed with SARS were 13 times more likely to contract the illness than were health care workers who cared for patients with SARS but did not assist in the intubation.31
Various surgical techniques also generate aerosols known as surgical smoke or surgical plume. Health care workers assigned to surgical suites must be aware that this environment has two types of particulate hazards: the particles themselves and the viable biological pathogens that account for some particles. As stated earlier, the size of the particle affects how long it remains suspended in the air and how far it can travel into the respiratory tract. Electrosurgical procedures generate particles about 0.07 mcm in size, laser procedures create particles about 0.31 mcm in size,2, 32 and ultrasonic scalpels produce particles that range in size from 0.35 mcm to 6.5 mcm.2 Particles smaller than 5 mcm are categorized as lung-damaging dust, and working in surgical smoke can result in acute and chronic respiratory changes including emphysema, asthma, and chronic bronchitis.2 It has been reported that small particles less than 1.1 mcm in diameter constitute 77% of the particulate matter found in surgical smoke.33, 34 Surgical laser masks do not seal to the face and offer no protection from particles smaller than 1 mcm in size.32 In fact, “if the wearer wants to reduce inhalation of smaller, inhalable particles (those less than 100 [mcm]), they need to obtain and properly use a NIOSH-certified respirator.”16
Cells, including aerosolized blood,35 bacteria, and viruses, make up the biological components of surgical smoke.34 The viability of the pathogens released during surgery depends on
-
■
the length of the surgery,
-
■
the type of instrumentation used,
-
■
the presence of bacteria or virus in the patient, and
-
■
the type of procedure.2
Bacteria and virus cells have been detected in surgical smoke and can remain viable for up to 72 hours.34 Surgical smoke generated during laparoscopic procedures has been found to contain whole mesothelial and blood cells.36 One study found that nine out of 10 surgeries using high-speed surgical instruments and 16 of 17 coagulation devices produced aerosolized blood mists in the OR.37 Biologically active bacteria (ie, Staphylococcus, Corynebacterium, Neisseria) have been detected in laser resurfacing plume.38 Human papillomavirus (HPV) DNA has been detected in carbon dioxide laser smoke during laser vaporization of warts.39, 40 There are two documented cases of health care workers contracting HPV as a result of occupational exposure. In 1990, a 44-year-old surgeon was treated for removal of laryngeal papillomas. The DNA tests of the lesions indicated HPV types 6 and 11, and the only source of contamination was traced back to the surgeon’s patients who had undergone laser surgery.41 A second case involved a 28-year-old gynecological OR nurse who repeatedly assisted in laser surgical and electrosurgical excisions of anogenital condylomas. This case was defined as an occupational disease because of the high correlation between the nurse’s repeated exposures and her contraction of HPV.42
Recommended Practices
Given the known hazards, several professional and national organizations have established recommended practices to use during smoke-generating procedures in the OR. AORN,15 the Laser Institute of America,43 the American National Standards Institute,43 and The Joint Commission44 each recommend that surgical smoke be filtered and evacuated through the use of room ventilation and local exhaust ventilation methods; NIOSH recommends a combination of OR air exchanges and local exhaust ventilations as the first line of protection for controlling surgical smoke.4, 18, 19
Internet Resources.
-
■
Making things clear about surgical smoke. LINA-Medical. http://www.linamedical.co.uk/assets/files/penetration_brochure.pdf.
-
■
Respirator trusted source information. Section 3: Ancillary respirator information. The National Personal Protective Technology Laboratory (NPPTL). http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/RespSource3healthcare.html#m.
-
■
OSHA Fact Sheet. Respiratory infection control: respirators versus surgical masks. Occupational Safety and Health Administration. http://www.osha.gov/Publications/respirators-vs-surgicalmasks-factsheet.html.
-
■
Surgical smoke tool kit. AORN, Inc. http://www.aorn.org/Clinical_Practice/ToolKits/Tool_Kits.aspx.
-
■
Smoke plume. Occupational Safety and Health Administration. http://www.osha.gov/SLTC/etools/hospital/surgical/surgical.html#LaserPlume.
-
■
Respirator safety. US Department of Labor. http://www.youtube.com/watch?v=Tzpz5fko-fg.
-
■
The difference between respirators and surgical masks. US Department of Labor. http://www.youtube.com/watch?v=ovSLAuY8ib8.
Web site access verified December 18, 2012.
Despite these recommendations, a 2007 survey of 623 AORN members indicated weak compliance with recommended control measures.45 In 2010, a follow-up survey of 1,356 AORN members indicated that the use of wall suction, a less-effective local exhaust ventilation method, had increased significantly for almost all smoke-generating procedures, as did the use of high-filtration surgical laser masks.46 However, the use of smoke evacuators and the use of NIOSH-approved surgical N95 respirators had not changed significantly.46
In addition, a recently published comprehensive analysis of all literature pertaining to laser-generated air contaminants suggests that the known exposure risks coupled with the inconsistent use and marginal adoption of recommended local exhaust ventilation and respiratory control measures warrant a campaign for targeted health and safety training.47 Based on the compendium of evidence, the authors of the literature review recommend the use of a surgical N95 respirator in the presence of laser-generated air contaminants as a prudent course of action.47 High-filtration surgical laser masks are not designed to provide protection from the atmospheric contaminants present in surgical smoke,43 and high-filtration surgical laser masks provide less protection to the wearer from particulate hazards than a fit-tested surgical N95 respirator.47 According to the AORN “Recommended practices for laser safety in perioperative practice settings,” during high-risk or aerosol-generating procedures in patients with or suspected of having transmissible infections (eg, HPV, tuberculosis, rubeola, varicella), respiratory protection that is at least as protective as a fit-tested surgical N95 respirator should be used in conjunction with local exhaust ventilation.15 For instance, according to AORN's “Recommended practices for laser safety in perioperative practice settings,” a surgical N95 respirator should be used in conjunction with a smoke evacuator during laser evaporation of a condyloma.15
Conclusion
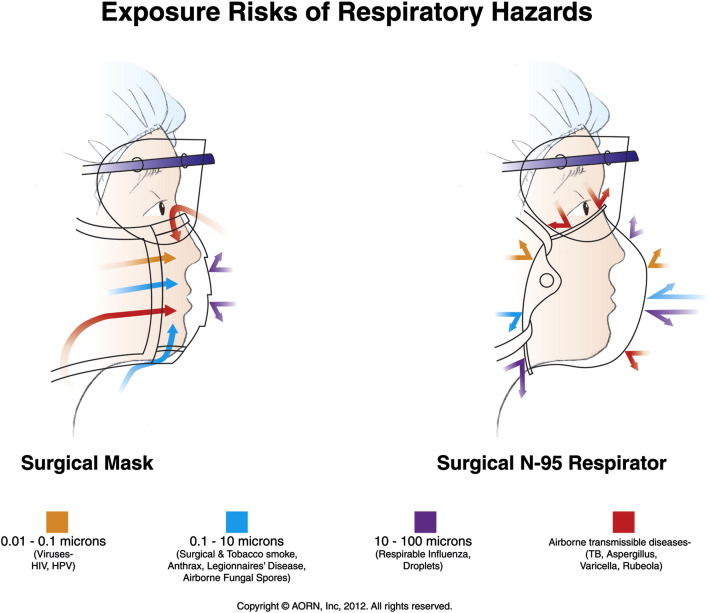
The CDC/HICPAC-recommended precautions for transmissible diseases provide a generally reliable method for selection and use of PPE.48 Consistent compliance with recommended precautions and practices is an important indicator of a health care organization’s commitment to a culture of safety. This includes the appropriate selection of protective facemasks. The differences in the protective features of high-filtration surgical laser masks and surgical N95 respirators for commonly encountered respiratory hazards are illustrated in Figure 3 , which can be used as a selection guide.
Figure 3.
Exposure risks of respiratory hazards. Proper selection of protection depends on particle size and mode of infectious agent transmission (eg, droplet, airborne).
Health care administrators should encourage individual health care workers, in conjunction with infection preventionists and occupational health or industrial hygienists, to examine the appropriate selection and use of protective facemasks. Health care workers should personally consider the patient’s diagnosis, how closely they will be working with the patient, and the treatments being performed.
When working in the OR during smoke-generating procedures, especially laser procedures, if a health care worker can smell the plume, potentially dangerous and infectious debris and contaminants are being released into the atmosphere that could cause adverse health effects.47, 49 All health care workers should be vigilant because bloodborne pathogens are known to be released during procedures on patients infected with HPV, HIV, and hepatitis.50 In such situations, at a minimum, health care workers should use a fit-tested surgical N95 respirator to ensure appropriate protection. Respiratory PPE is the health care worker’s last line of defense, but the best respirator will do little to protect an individual who does not know when to use it or how to use it properly.
Biographies
Stacey M. Benson, MS, is a doctoral student in the Epidemiology Program at the University of Pittsburgh Graduate School of Public Health, PA. Ms Benson has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Debra A. Novak, DSN, RN, is a senior service fellow at the Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, National Personal Protective Technology Laboratory, Atlanta, GA. Dr Novak has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Mary J. Ogg, MSN, RN, CNOR, is a perioperative nursing specialist in the Center for Nursing Practice at AORN, Inc, Denver, CO. Ms Ogg has no declared affiliation that could be perceived as posing a potential conflict of interest in the publication of this article.
Examination. Continuing Education Program
Proper Use of Surgical N95 Respirators and Surgical Masks in the OR
Purpose/Goal
To enable the learner to correctly use surgical masks and surgical N95 respirators.
Objectives
-
1.
Discuss risks associated with surgical smoke.
-
2.
Explain the differences between surgical masks and surgical N95 respirators.
-
3.
Describe tests used to certify a surgical N95 respirator.
-
4.
Identify when use of a surgical N95 respirator is advised.
The Examination and Learner Evaluation are printed here for your convenience. To receive continuing education credit, you must complete the Examination and Learner Evaluation online at http://www.aorn.org/CE.
Questions
-
1.Surgical smoke may contain
-
1.bacteria and viruses.
-
2.benzene.
-
3.dead and live cellular material.
-
4.formaldehyde.
-
5.hydrogen cyanide.
-
6.lung-damaging dust.
-
a.1, 3, and 5
-
b.2, 4, and 6
-
c.2, 3, 5, and 6
-
d.1, 2, 3, 4, 5, and 6
-
a.
-
1.
-
2.The term N95 refers to a National Institute for Occupational Safety and Health-approved and US Food and Drug Administration-cleared particulate filtering facepiece respirator that can filter at least 95% of airborne particles.
-
a.true
-
b.false
-
a.
-
3.Air-purifying filtering facepiece respirators
-
1.are composed of a filter that prevents the passage of a percentage of hazardous airborne particulate matter.
-
2.cover at least the nose and mouth.
-
3.have a self-contained air supply.
-
4.are fit-tested personal protective devices.
-
5.prevent dust particles and infectious agents from entering the wearer’s breathing space.
-
a.4 and 5
-
b.1, 2, and 3
-
c.1, 2, 4, and 5
-
d.1, 2, 3, 4, and 5
-
a.
-
1.
-
4.During a ________, a technician uses an instrument to monitor particles in the room air as well as particles inside the respirator while it is being worn by the health care worker.
-
a.comfort fit test
-
b.qualitative fit test
-
c.quantitative fit test
-
d.user seal check
-
a.
-
5.High-filtration surgical laser masks provide adequate protection to be considered respiratory PPE.
-
a.true
-
b.false
-
a.
-
6.Considering the size of a pathogen is important because
-
a.the larger the particle, the longer it can remain aerosolized.
-
b.the larger the particle, the more infectious it is.
-
c.the smaller the particle, the deeper it can travel into the airway.
-
d.the smaller the particle, the less infectious it is.
-
a.
-
7.The level of protection necessitated by droplet precautions can only be achieved with a surgical N95 respirator.
-
a.true
-
b.false
-
a.
-
8.Certain diseases can be transmitted through airborne routes and require more stringent precautions such as:
-
1.negative pressure rooms.
-
2.isolation rooms.
-
3.respiratory protection at least as protective as a surgical N95 respirator.
-
a.1 and 2
-
b.1 and 3
-
c.2 and 3
-
d.1, 2 and 3
-
a.
-
1.
-
9.Bacteria and virus cells have been detected in surgical smoke and can remain viable for up to
-
a.72 hours.
-
b.96 hours.
-
c.108 hours.
-
d.120 hours.
-
a.
-
10.AORN recommends wearing a __________ in conjunction with using a smoke evacuator during a laser evaporation of a condyloma.
-
1.standard surgical mask
-
2.surgical N95 respirator
-
3.high-filtration surgical mask
-
a.2
-
b.3
-
c.1 or 2
-
d.1 or 3
-
a.
-
1.
Learner Evaluation. Continuing Education Program
Proper Use of Surgical N95 Respirators and Surgical Masks in the OR
This evaluation is used to determine the extent to which this continuing education program met your learning needs. Rate the items as described below.
Objectives
To what extent were the following objectives of this continuing education program achieved?
-
1.
Discuss risks associated with surgical smoke. Low 1. 2. 3. 4. 5. High
-
2.
Explain the differences between surgical masks and surgical N95 respirators. Low 1. 2. 3. 4. 5. High
-
3.
Describe tests used to certify a surgical N95 respirator. Low 1. 2. 3. 4. 5. High
-
4.
Identify when use of a surgical N95 respirator is advised. Low 1. 2. 3. 4. 5. High
Content
-
5.
To what extent did this article increase your knowledge of the subject matter? Low 1. 2. 3. 4. 5. High
-
6.
To what extent were your individual objectives met? Low 1. 2. 3. 4. 5. High
-
7.
Will you be able to use the information from this article in your work setting? 1. Yes 2. No
-
8.
Will you change your practice as a result of reading this article? (If yes, answer question #8A. If no, answer question #8B.)
-
8A.How will you change your practice? (Select all that apply)
-
1.I will provide education to my team regarding why change is needed.
-
2.I will work with management to change/implement a policy and procedure.
-
3.I will plan an informational meeting with physicians to seek their input and acceptance of the need for change.
-
4.I will implement change and evaluate the effect of the change at regular intervals until the change is incorporated as best practice.
-
5.Other: ________________________________
-
1.
-
8B.If you will not change your practice as a result of reading this article, why? (Select all that apply)
-
1.The content of the article is not relevant to my practice.
-
2.I do not have enough time to teach others about the purpose of the needed change.
-
3.I do not have management support to make a change.
-
4.Other: ________________________________
-
1.
-
9.
Our accrediting body requires that we verify the time you needed to complete the 2.1 continuing education contact hour (126-minute) program: _________________________________
References
- 1.Ulmer B.C. The hazards of surgical smoke. AORN J. 2008;87(4):721–734. doi: 10.1016/j.aorn.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 2.Alp E., Bijl D., Bleichrodt R.P., Hansson B., Voss A. Surgical smoke and infection control. J Hosp Infect. 2006;62(1):1–5. doi: 10.1016/j.jhin.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Ortolano G.A., Cervia J.S., Canonica F.P. Surgical smoke: a concern for infection control practitioners. Manag Infect Control. 2009;9(8):48–54. [Google Scholar]
- 4.Control of smoke from laser/electric surgical procedures. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/docs/hazardcontrol/hc11.html. Accessed December 3, 2012.
- 5.Brandon H.J., Young V.L. Characterization and removal of electrosurgical smoke. Surg Serv Manag. 1997;3(3):14–16. [Google Scholar]
- 6.Tomita Y., Mihashi S., Nagata K. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89(2):145–149. [PubMed] [Google Scholar]
- 7.Bryce E., Forrester L., Scharf S., Eshghpour M. What do healthcare workers think? A survey of facial protection equipment user preferences. J Hosp Infect. 2008;68(3):241–247. doi: 10.1016/j.jhin.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Moore D., Gamage B., Bryce E., Copes R., Yassi A., BC Interdisciplinary Respiratory Protection Study Group Protecting health care workers from SARS and other respiratory pathogens: organization and individual factors that affect adherence to infection control guidelines. Am J Infect Control. 2005;33(2):88–96. doi: 10.1016/j.ajic.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radonovich L.J., Jr., Hodgson M.J., Cohen H.J. Do respirators protect health-care workers from airborne infectious diseases? Respir Care. 2008;53(12):1660–1664. [PubMed] [Google Scholar]
- 10.Banach D.B., Bielang R., Calfee D.P. Factors associated with unprotected exposure to 2009 H1N1 influenza A among healthcare workers during the first wave of the pandemic. Infect Control Hosp Epidemiol. 2011;32(3):293–295. doi: 10.1086/658911. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Novel influenza A (H1N1) virus infections among health-care personnel—United States, April-May 2009. MMWR. 2009;58(23):641–645. [PubMed] [Google Scholar]
- 12.Wise M.E., DePerio M., Halpin J. Transmission of pandemic (H1N1) 2009 influenza to healthcare personnel in the United States. Clin Infect Dis. 2011;52(Suppl 1):S198–S204. doi: 10.1093/cid/ciq038. [DOI] [PubMed] [Google Scholar]
- 13.Infectious diseases; proposed rules. Fed Regist. 2010;75(87):24835–24844. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=FEDERAL_REGISTER&p_id=21497 Accessed December 3, 2012. [Google Scholar]
- 14.Making things clear about surgical smoke. LINA-Medical. http://www.lina-medical.com/files/34/penetration_brochure.pdf. Accessed December 3, 2012.
- 15.Perioperative Standards and Recommended Practices. AORN, Inc; Denver, CO: 2013. Recommended practices for laser safety in perioperative practice settings; pp. 143–156. [Google Scholar]
- 16.Respirators and surgical masks: a comparison. October 2009. 3M. http://multimedia.3m.com/mws/mediawebserver?LLLLLLuS1X&Leo4Lyo4LLlvv3wHkkkkJ-. Accessed December 3, 2012.
- 17.Perioperative Standards and Recommended Practices. AORN, Inc; Denver, CO: 2013. Recommended practices for prevention of transmissible infections in the perioperative setting; pp. 331–363. [Google Scholar]
- 18.The National Personal Protective Laboratory (NPPTL): respirator trusted-source information. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. http://www.cdc.gov/niosh/npptl/topics/respirators/disp_part/RespSource.html. Accessed December 3, 2012.
- 19.NIOSH Science Blog. N95 respirators and surgical masks. Centers for Disease Control and Prevention. National Institute for Occupational Safety and Health. http://blogs.cdc.gov/niosh-science-blog/2009/10/n95. Accessed December 3, 2012.
- 20.Occupational Safety and Health Standards. 29 CFR 1910.134. Personal protective equipment: respiratory protection. June 8, 2011. Occupational Safety & Health Administration. http://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=12716&p_table=standards. Accessed December 3, 2012.
- 21.Guidance for industry and FDA staff: surgical masks—premarket notification [510(k)] submissions; guidance for industry and FDA. US Food and Drug Administration. http://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072549.htm. Accessed December 3, 2012.
- 22.Oberg T., Brosseau L.M. Surgical mask filter and fit performance. Am J Infect Control. 2008;36(4):276–282. doi: 10.1016/j.ajic.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rengasamy S., Miller A., Eimer B.C., Shaffer R.E. Filtration performance of FDA-cleared high filtration surgical masks. JISRP. 2009;26(Spring-Summer):54–70. [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough N.V., Brosseau L.M. Selecting respirators for control of worker exposure to infectious aerosols. Infect Control Hosp Epidemiol. 1999;20(2):136–144. doi: 10.1086/501602. [DOI] [PubMed] [Google Scholar]
- 25.Nicas M., Nazaroff W.W., Hubbard A. Toward understanding the risk of secondary airborne infection: emission of respirable pathogens. J Occup Environ Hyg. 2005;2(3):143–154. doi: 10.1080/15459620590918466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Occupational transmission of Neisseria meningitides-California, 2009. MMWR. 2010;59(45):1480–1483. [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention Bacterial meningitis after intrapartum spinal anesthesia—New York and Ohio, 2008-2009. MMWR. 2010;59(3):65–69. [PubMed] [Google Scholar]
- 28.Infection Control Policies & Procedures (revised 2007). Yale University Office of Environmental Health. http://www.yale.edu/ehs/Documents/Bio/Yale%20Infection%20Control%20Manual.pdf. Accessed December 18, 2012.
- 29.Muller M.P., McGeer A. Febrile respiratory illness in the intensive care unit setting: an infection control perspective. Curr Opin Crit Care. 2006;12(1):37–42. doi: 10.1097/01.ccx.0000198056.58083.a1. [DOI] [PubMed] [Google Scholar]
- 30.Peiris J.S., Phil D., Yuen K.Y., Osterhaus A.D.M.E., Stohr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431–2441. doi: 10.1056/NEJMra032498. [DOI] [PubMed] [Google Scholar]
- 31.Tran K., Cimon K., Severn M., Pessoa-Silva L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4):e35797. doi: 10.1371/journal.pone.0035797. http://www.plosone.org/article/info%3Adoi%2F10.1371%2Fjournal.pone.0035797 Accessed January 9, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewin J.M., Brauer J.A., Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65(3):636–641. doi: 10.1016/j.jaad.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Kunachak S., Sobhon P. The potential alveolar hazard of carbon dioxide laser-induced smoke. J Med Assoc Thai. 1998;81(4):278–282. [PubMed] [Google Scholar]
- 34.Brüske-Hohlfeld I, Preissler G, Jauch KW, et al. Surgical smoke and ultrafine particles. J Occup Med Toxicol. December 3, 2008;3:31. http://www.occup-med.com/content/3/1/31. Accessed January 23, 2013. [DOI] [PMC free article] [PubMed]
- 35.Andersen K. Safe use of lasers in the operating room—what perioperative nurses should know. AORN J. 2006;79(1):171–188. doi: 10.1016/s0001-2092(06)61151-4. [DOI] [PubMed] [Google Scholar]
- 36.Champault G., Taffinder N., Ziol M., Riskalla H., Catheline J.M. Cells are present in the smoke created during laparoscopic surgery. Br J Surg. 1997;84(7):993–995. doi: 10.1002/bjs.1800840724. [DOI] [PubMed] [Google Scholar]
- 37.Ishihama K., Sumioka S., Sakurada K., Kogo M. Floating aerial blood mists in the operating room. J Hazard Mater. 2010;181(1-3):1179–1181. doi: 10.1016/j.jhazmat.2010.05.110. [DOI] [PubMed] [Google Scholar]
- 38.Capizzi P.J., Clay R.P., Battey M.J. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23(3):172–174. doi: 10.1002/(sici)1096-9101(1998)23:3<172::aid-lsm7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Baggish M.S., Poiesz B.J., Joret D., Williamson P., Refai A. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11(3):197–203. doi: 10.1002/lsm.1900110302. [DOI] [PubMed] [Google Scholar]
- 40.Ferenczy A., Bergeron C., Richart R.M. Human papillomavirus DNA in CO2 laser-generated plume of smoke and its consequences to the surgeon. Obstet Gynecol. 1990;75(1):114–118. [PubMed] [Google Scholar]
- 41.Hallmo P., Naess O. Laryngeal papillomatosis with human papillomavirus DNA contracted by a laser surgeon. Eur Arch Otorhinolaryngol. 1991;248(7):425–427. doi: 10.1007/BF01463570. [DOI] [PubMed] [Google Scholar]
- 42.Calero L., Brusis T. Laryngeal papillomatosis—first recognition in Germany as an occupational disease in an operating room nurse. Laryngorhinootologie. 2003;82(11):790–793. doi: 10.1055/s-2003-44546. [in German] [DOI] [PubMed] [Google Scholar]
- 43.American National Standards Institute . Laser Institute of America; Orlando, FL: 2005. ANSI Z136.3—Safe Use of Lasers in Health Care Facilities. [Google Scholar]
- 44.The Joint Commission E-dition. The Joint Commission; Oakbrook Terrace, IL: 2013. Hospital environment of care EC.02.02.01. [Google Scholar]
- 45.Edwards B.E., Reiman R.E. Results of a survey on current surgical smoke control practices. AORN J. 2008;87(4):739–749. doi: 10.1016/j.aorn.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Edwards B.E., Reiman R.E. Comparison of current and past surgical smoke control practice. AORN J. 2012;95(3):337–350. doi: 10.1016/j.aorn.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 47.Pierce J.S., Lacey S.E., Lippert J.F., Lopez R., Franke J.E. Laser-generated air contaminants from medical laser applications: a state-of the-science review of exposure characterization, health effects and control. J Occup Environ Hyg. 2011;8(7):447–466. doi: 10.1080/15459624.2011.585888. [DOI] [PubMed] [Google Scholar]
- 48.Seigel J.D., Rhinehart E., Jackson M., Chiarello L., the Healthcare Infection Control Practices Advisory Committee . Centers for Disease Control and Prevention; Atlanta, GA: 2007. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare Settings.http://www.cdc.gov/hicpac/pdf/isolation/Isolation2007.pdf Accessed December 18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollock L. Hazards of electrosurgical smoke. Perioper Nurs Clin. 2007;2(2):127–138. [Google Scholar]
- 50.Fowler R.A., Guest C.B., Lapinsky S.E. Transmission of severe acute respiratory syndrome during intubation and mechanical ventilation. Am J Respir Crit Care Med. 2004;169(11):1198–1202. doi: 10.1164/rccm.200305-715OC. [DOI] [PubMed] [Google Scholar]