Abstract
Despite the considerable advances in medical and pharmaceutical research during the past years, diseases caused by viruses have remained a major burden to public health. Virtual in silico screening has repeatedly proven to be useful to meet the special challenges of antiviral drug discovery. Large virtual compound libraries are filtered by different computational screening methods such as docking, ligand-based similarity searches or pharmacophore-based screening, reducing the number of candidate molecules to a smaller set of promising candidates that are then tested biologically. This rational approach makes the drug discovery process more goal-oriented and saves resources in terms of time and money. In this review we discuss how different virtual screening techniques can be applied to antiviral drug discovery, present recent success stories in this field and finally address the main differences between the methods.
Introduction
Viral infections can result not only in acute potentially lethal clinical conditions (e.g. Influenza, Dengue), but also in chronic illnesses (e.g. HIV, Hepatitis C) that have to be treated for long periods of time and therefore lead to high expenses for the health care system. Especially the potential of some of these viral infections to reach pandemic dimensions is a major concern these days [1]. A primary challenge in the development of effective antiviral therapies is the high variability of viral genomes, which makes it easy for them to adapt to new conditions rapidly or to develop resistance against used drugs. This fact demands for a fast and effective drug discovery process. In the past decades, computational techniques like virtual screening have proven to be of great use to make the drug development process faster and less expensive [2]. The applications of virtual screening (VS) in the drug development process against viral targets vary. The first steps of target and lead structure identification as well as further steps like compound development and optimization can be rationalized and improved by VS.
This review aims to provide an explanation of the methods most commonly employed for VS against viral targets, with an overview of the latest findings achieved in this research field. Here for we focus on the development since 2009. For a review on VS against viral targets before this date see Kirchmair et al. [3].
Methods for virtual screening
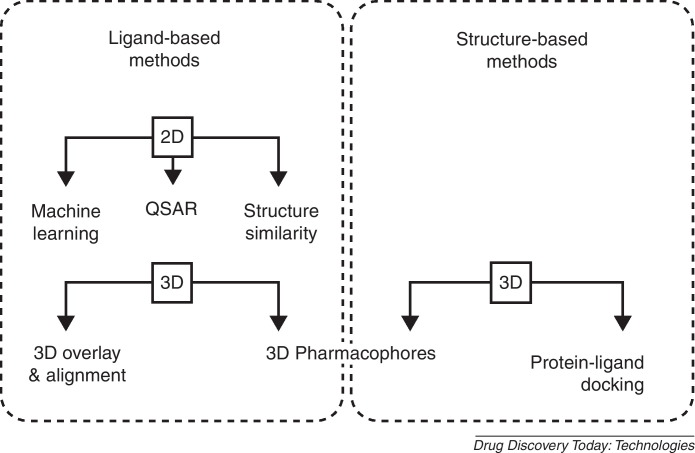
In silico VS uses computational models to describe macromolecule–ligand interactions. A multitude of methods exist for this purpose, which can mainly be categorized into 2D and 3D approaches. Descriptor-based methods (2D methods) rely on the calculation and comparison of scalar molecular properties and aim at identifying molecules that are similar with respect to their calculated molecular attributes. The selection and weighing of descriptors relevant for a certain target binding can be performed using machine-learning techniques, such as neural networks or by linear correlation of measured biological activity (quantitative structure–activity relationships, QSAR) with selected calculated descriptors. Recently, most computational approaches for the discovery of anti-viral compounds rely on 3D methods, which aim to describe the steric and chemical complementarity of the 3D conformations of a macromolecular target and the binding ligand (Fig. 1 ). There are two approaches for developing 3D models: Either from (i) the experimentally determined 3D structure of a disease-relevant macromolecular biological target (structure-based design) or (ii) from a set of ligands, which are supposed to bind to the same target at the same location (ligand-based design). For structure-based design, X-ray crystallography is the main source of information, and the Protein Data Bank (PDB) [4] is an excellent data source for such approaches. Ideally, the binding pocket location is already known, which is the case when a ligand is co-crystallized with the target structure. Ligand-based design, by contrast, is employed for those cases, where no structural information on the target is available. It requires a set of already known biologically active molecules, whose common binding location should be verified experimentally, for example, by mutational studies. This allows the search for novel potential ligands that show a similar 3D overlay either in terms of molecule volume (molecular shape) or in terms of chemical functions (such as hydrogen bond donors, acceptors, lipophilic areas or charges).
Figure 1.
Virtual screening approaches employed in anti-viral research.
2D and 3D methods differ in terms of computational performance and applicability: 2D methods show substantially lower calculation times and therefore are mostly used as first classification filters to reduce the number of molecules to screen in later stages. Structure-based 3D approaches position the putatively active ligand in the relevant binding site and are therefore frequently able to provide ideas for further chemical optimization of the compound (lead optimization). Ligand-based 3D approaches show the same advantage to some extent, because an overlay to known active compounds is provided, which can help in analyzing aspects important for lead optimization.
Protein-ligand docking is the most important structure-based 3D approach, which aims at predicting the binding mode of a ligand for a protein with known 3D structure, assuming the protein binding site to be mostly rigid and the ligand to be flexible. There are several docking tools available, which have been reviewed recently [5]. They differ in terms of algorithms used for ligand placement and scoring. These include ligand fragmentation and incremental reconstruction approaches (e.g. FlexX [6]), algorithms based on molecular shape (e.g. DOCK [7], FRED [8]), genetic algorithms (e.g. GOLD [9] and AutoDock [10]), systematic search (e.g. Glide [11]), Monte Carlo optimization (e.g. LigandFit [12]) and surface-based molecular similarity (e.g. Surflex [13]). While ligand placement works reliably for sampling possible ligand binding geometries, scoring functions are not working dependably for all targets [14], mainly due to the impossibility to estimate entropic effects correctly. Nevertheless, manually developed empirical scoring functions tailored for a defined application scope are still useful, and due to the visualization of a binding hypothesis docking remains one of the methods that provides many ideas for rational lead optimization.
3D pharmacophore modeling is based on the concept that a three-dimensional arrangement of chemical features (such as hydrogen bonds, charges, lipophilic areas) forms a pattern that describes the binding of a ligand to a macromolecule. The advantages of pharmacophore models are their easy interpretability and way of modification, which allows researchers to incorporate their knowledge about a specific binding mode into a transparent, but still highly descriptive model. The careful arrangement of chemical feature markers in 3D space to search molecules that fulfill the required interactions is one of the most successful approaches of VS and has led to many success stories for retrospective explanation of ligand affinity and subsequently to prospective ligand design [15, 16, 17, 18, 19, 20]. While ligand-based pharmacophore creation has been introduced in the early days of molecular modeling, structure-based 3D pharmacophore design has been introduced more recently: The program LigandScout [21, 22, 23] derives pharmacophores from protein–ligand complexes, interprets ligand geometries, assigns correct hybridization states and applies a set of rules that classifies plausible protein-ligand interactions. For subsequent VS, a computationally efficient pattern-matching algorithm was implemented that allows for geometrically highly accurate VS. Other programs such as catalyst [24], MOE [25] and phase [26] use cascading n-point pharmacophore fingerprints for pharmacophore-molecule superpositioning. The simplicity of 3D pharmacophore representations also means that the complete biophysical nature of drug interactions is not addressed. Understanding the limitations of this approach is essential for its successful application.
Shape-based similarity screening is a purely ligand-based method, which allows searching for 3D analogs of a single compound. Despite its simplicity, it is a very powerful and frequently used filtering method due to its straightforward way of use. One of the most popular programs for shape-based search is ROCS [27], which implements an algorithm based on Gaussian spheres to compare molecular volume. ROCS additionally uses a basic pharmacophore-like similarity scheme to include chemical information in their overlay results.
All these methods have successfully been used for recent discovery of anti-viral compounds by screening different compound databases for compounds with previously unknown antiviral activity. In the following we will give an overview about success stories for the prospective discovery of antiviral compounds using rational drug design techniques and VS. More extensive information on the mentioned publications can be found in the summary table provided in the supplementary materials.
Success stories in virtual screening for anti-viral agents
Influenza
Influenza is a seasonal epidemic acute respiratory disease persistently threatening public health caused by ribonucleic acid (RNA) influenza viruses of the orthomyxoviridae family [28]. Drugs designed to fight Influenza viruses mainly target neuraminidase (NA), an enzyme enabling the transport of the virus through the host mucus [29]. With this regard, VS has been successfully used to identify novel inhibitors of this target by Kirchmair et al. They reported a study in which a shape and chemical feature-based search for analogs of a validated NA inhibitor, katsumadain A, was performed against the National Cancer Institute (NCI) compound database (http://cactus.nci.nih.gov/download/nci/). Five of the compounds evaluated for their NA-inhibiting potential (including four flavonoids) demonstrated strong activities against three oseltamivir-susceptible H1N1 strains in a micromolar range [30].
HIV
Human immunodeficiency virus (HIV) is a member of the retrovirus family and the cause of acquired immunodeficiency syndrome (AIDS), one of the major worldwide health problems of our times. Different general approaches are pursued to fight this retrovirus: (i) preventing its genetic material to be replicated or inserted within the host cell, (ii) blocking the entry of the virus inside the cell or (iii) inhibiting its assembly and maturation.
The reverse transcriptase (RT) of HIV-1 is one of the major targets of the antiretroviral drug therapies used for the treatment of AIDS. RT is responsible for the retro transcription of RNA to DNA in the first phase of the intracellular viral replication. In an attempt to target this protein, Bustanji et al. selected 2800 fragment-like compounds from the NCI database, performed a high throughput docking and selected the six best hits for testing based on a consensus docking score of which four were found to inhibit RT in biological testing [31]. Similarly, Nichols et al. developed a docking-based VS strategy. They started with the ZINC database [32] and screened the 2 million molecules with the wild-type viral proteins and two clinically relevant RT-mutants. In this study, activities against RT were measured for nine hit compounds and three showed low-micro molar antiviral activity toward either one or both the wild-type and the Y181C mutant HIV-1 strains [33]. Another VS screening was performed to identify novel compounds targeting RT as well as other viral functions associated with RNA transcription. A shape-based screening was applied on the NCI database in a first VS run using dihydroxy benzoyl naphthyl hydrazone, a known RT inhibitor, as query compound. The most active hits identified through this process were employed for a second VS now using a combined ligand-based strategy comprising 3D-, 2D-similarity searches and ligand-based pharmacophore screening. When tested on the RT functions, several of the selected compounds characterized by new scaffolds were shown to inhibit both RT-associated ribonuclease H and RT activities in a low micromolar range [34].
Another therapeutic strategy against HIV is to inhibit the integration of the viral DNA into the host cell genome by targeting the HIV-1 Integrase (IN). Recently, a target-based pharmacophore search was performed against a commercial database leading to 10 hit compounds selected for biological investigation. One compound showed significant inhibitory potency against IN and proved to moderately inhibit HIV-1 replication [35].
The process of HIV entry can also be inhibited at the level of viral envelope glycoprotein gp120 binding to both the CD4 receptor and one of CXCR4 or CCR5 chemokine co-receptors. Lalonde et al. described a docking plus a shape-based similarity search to identify amine-building blocks that, when conjugated to their core scaffold, yielded novel analogs that maintain similar affinity for gp120 [36].
Finally, the Capsid protein plays an important role in HIV-1 assembly and maturation, and has been recognized as a potential target for the development of a new generation of drugs for anti-AIDS therapy. Curelli et al. undertook a docking-based VS and subsequent analog search. Small molecule inhibitors targeting the C-terminal domain of the HIV-1 capsid were identified. The most active compounds showed drastically reduced infectivity in HIV-1 infected cells [37].
Flaviviridae family
The Flaviviridae family contains many relevant pathogenic viruses, such as the hepatitis C virus (HCV), the dengue virus (DENV), the yellow fever virus (YFV), the West Nile virus (WNV) and the tick-borne encephalitis virus (TBEV) [38]. The viral genome of viruses belonging to this family encodes three structural proteins (core, E1 and E2) forming the viral particle and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5). These participate in genome replication and virion assembly [39]. So far, only the enzymatic activities of NS3 and NS5 have been elucidated. The E protein is crucial for fusion activity, making this protein and NS3 and NS5 suitable antiviral targets.
For the E-protein, several successful docking studies have been reported. Kampmann et al. performed a docking-based VS with the ectodomain of the DENV E-protein structure [40]. Antiviral activity in a low micromolar range could be measured for two hit compounds against DENV; interestingly one compound also showed antiviral activity against WNV and YFV. In another study published by Wang et al. high-throughput docking led to the identification of an antiviral thiophen-pyrimidine with an EC50 of 119 nM against DENV-serotype2 in a human cell line [41]. Poh et al. discovered a compound that inhibits dengue fusion in low micromolar range through docking of over 1 million in-house compounds [42]. Yennamalli and coworkers combined three different docking algorithms to identify inhibitors of DENV E-protein out of a collection of thirteen available databases [38]. One of seven tested molecules showed an IC50 value in the micromolar range against DENV-serotype2. Umamaheswari et al. identified ten new lead structures as fusion inhibitors for YFV which can be used as a starting point for designing antiviral drugs against yellow fever [43].
The protease/helicase NS3 is another potential drug-target in the Flaviviridae family. In a study targeting this protein, a new induced-fit docking program (GENIUS) developed by Takaya was used for the discovery of 13 new HCV NS3 protease inhibitors [44]. Shiryaev performed a docking study with the compounds of the NCI database into the NS2B cofactor binding site of the NS3 protease crystal structure of WNV, leading to one biologically validated inhibitor with nanomolar activity [45].
NS5 polymerase is a key target for the discovery of antiviral agents for the treatment of HCV and other Flaviviridae infections. A pharmacophore-based approach was followed by Kim et al. [46]. A 3D-pharmacophore model of the HCV NS5 (NNI site IV) was built and used to screen a commercial compound library. Out of 18 tested hits, the best compound showed EC50 value in a low micromolar range.
In a docking-based VS study, Lin and coworkers screened 57,177 chemicals and found an isoxazole with significant dose-dependent inhibition of HCV RdRp activity and replication [47]. Talele et al. identified two novel chemotypes as allosteric inhibitors of HCV NS5B through molecular docking of compounds from a commercially available database into the allosteric pocket-1 of NS5 [48]. Musmuca et al. used 3-D QSAR as scoring function for the docking-based VS of the NCI database, resulting in one compound with inhibitory activity [49]. Podvinec et al. also used molecular docking to screen a library, containing more than 5 million commercially available compounds, against the two binding sites of DENV NS5 [50].
Herpes viruses
Eight different types of viruses belonging to the herpes family are known to infect humans and cause a variety of diseases. The most commonly targeted protein in these viruses is the thymidine kinase [3]; other targets like viral helicases, proteases or the protein–protein interfaces of replication proteins are also being investigated. Especially available crystal structures for viral proteins are the basis for VS efforts regarding new therapeutical targets. For example, Li et al. recently published a study in which Epstein-Barr nuclear antigen 1 was proposed as a target against Epstein-Barr viruses [51]. A compound collection was screened through a workflow based on solubility prediction and docking with several scoring functions. Like this, four inhibitors out of 30 biologically tested compounds could be identified.
Severe acute respiratory syndrome-coronavirus (SARS-CoV)
In 2002, an outbreak of severe acute respiratory syndrome (SARS) in China with nearly pandemic status triggered a huge amount of efforts regarding the development of therapeutic drugs against the coronavirus (CoV) causing the disease. In the development of drugs against SARS-CoV mostly two proteins are targeted: the SARS-CoV Spike Protein (S Protein) and the SARS-CoV Main Proteinase (Mpro) also called SARS-Co 3CLpro [3]. VS has been successfully used to identify and optimize compounds that inhibit SARS-CoV 3CLpro.
In a study published in 2011, Ngoyen et al. describe a VS workflow by which two compounds with inhibitory activity against SARS-CoV 3CLpro at low micromolar concentrations could be identified [52]. To achieve this, a commercial compound database was screened in a procedure involving (i) filtering by predicted toxical properties, (ii) docking, (iii) filtering by free energy calculation and potential H-bond formation between the protein and the ligand, and (iv) a clustering and visual inspection step.
The way in which VS can successfully be used to optimize inhibitors for SARS-Co 3CLpro was demonstrated by Mukherjee and coworkers [53]. Based on two micromolar inhibitors previously identified by structure-based VS, a combined ligand and structure-based study was performed to improve the inhibitory potency and analyze the available chemical space. The ligand-based part of the screening consisted of a similarity search with further filtering by substructure substitution. This was followed by a docking study with rescoring performed to select hits for biological validation.
VS has also enabled the exploration of further processes in the viral lifecycle as potential drug targets. As for many other viruses RNA pseudoknot formation and the subsequent −1 ribosomal frameshift are necessary for the translation of viral proteins. It has been shown that diminished −1 ribosomal frameshift causes a decreased production of viral particles, making it a potential target to fight viral infections. In an example of successful VS, Park et al. could identify a compound that binds to the SARS pseudoknot and inhibits −1 RF in SARS viruses [54]. For this purpose the 3D structure of the pseudoknot was first computationally modeled. Through a docking-based procedure 58 compounds were selected for biological testing, leading to one potent −1 RF inhibitor.
Human targets for antiviral therapy
The advent of viral escape mutants is one of the principal adversities that has to be faced in antiviral therapy, as they can possibly turn drugs targeting viral proteins inactive. To avoid this, one strategy is targeting host proteins that are necessary for virus replication but nonessential for the host. Like this, to inhibit the process of HIV entry instead of the viral envelope glycoprotein, the human CXCR4 receptor can also be targeted. Perez-Nueno et al. developed and validated a combined structure- (docking) and a ligand-based (QSAR analyses, pharmacophore modeling and shape matching) VS workflow targeting the CXCR4 receptor. This approach was used to select five virtual hits for synthesis from a database of virtual molecules, which could be validated as inhibitors by biological testing [55].
Conclusion
VS has proven to be useful in the discovery of new antiviral drugs. The advantages of a rationalized and faster drug discovery process are obvious, especially in anti-viral research, where the sudden emergence of new diseases of drug resistances is common. During the past years, VS has been mainly used for lead-discovery and, to a smaller extent for compound development and optimization.
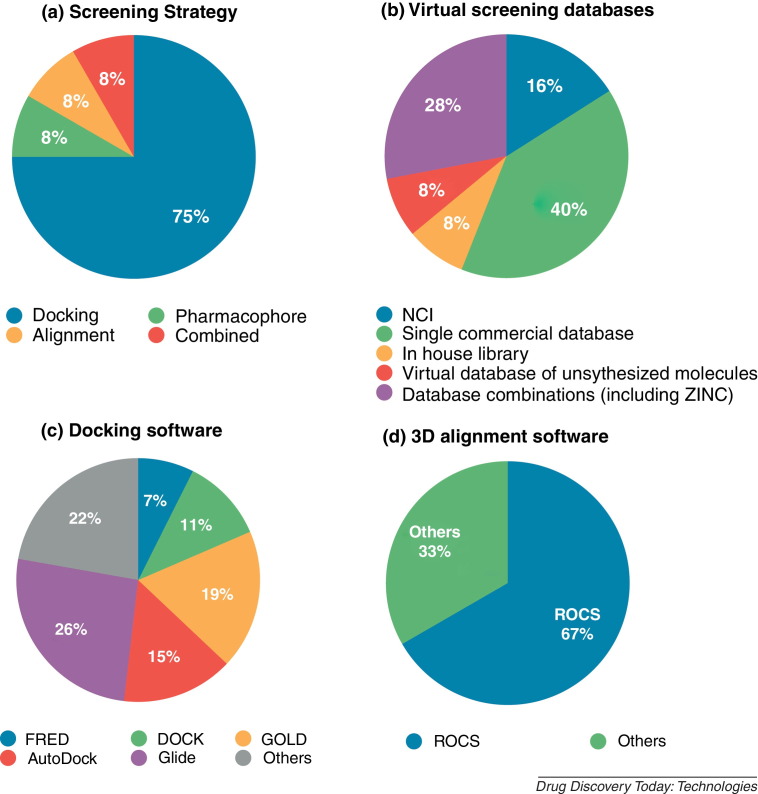
Most virtual screening efforts employ docking as their principal screening technique. Less common are 2D or 3D similarity searches with validated ligands or pharmacophore-based screening. An overview on the percentage of studies applying the respective techniques is shown in Fig. 2 . Their distinct requirements and features can explain the different frequencies with which the methods are employed. The major advantage of docking for VS is that it can be utilized to screen for compounds acting on a certain protein as soon as one crystal structure exists. This is especially useful in early lead discovery, as at this point only scarce information is accessible. To employ similarity search methods for screening, at least one known ligand must exist. To build a pharmacophore, several known ligands or a crystal structure with co-crystallized ligand are necessary, which makes these methods difficult to employ when little information is available. By contrast, in later drug development stages with more experimental data available, especially 3D pharmacophores can be efficiently used for VS leading to hit compounds with a high structural variability. One main advantage of similarity searches is their efficiency in terms of computational time, especially in comparison to docking which is less suitable for the large-scale virtual screening campaigns. Requirements, advantages and disadvantages of the reported techniques are summarized in Table 1 . It remains obvious that the drug development process still needs to be improved as it is far from being fast enough to face suddenly appearing viral diseases in an appropriate time scale. Besides, several severe diseases like HIV remain without cure despite high expenses in research. Further efforts have to be made to improve the accuracy of VS methods as well as to improve the quality and availability of experimental data. This can only be achieved by a closer collaboration between experimental and theoretical groups. As mentioned above, promising advances have been made in identifying and targeting host proteins, which could be an option to avoid problems related to viral variability.
Figure 2.
Overview on screening methods, databases and software employed in recent virtual screening studies. The following distributions are depicted: (a) employed virtual screening method, (b) type of database screened, (c) docking software utilized and (d) employed alignment software.
Table 1.
Comparison of virtual screening methods
| Method | Necessary data | Computational requirements | Hit structural diversity |
|---|---|---|---|
| Docking | Protein crystal structure | High | High |
| Pharmacophore | Several known ligands or protein crystal structure with bound ligand | Moderate | High |
| Ligand similarity search | Min. one known ligand | Low | Moderate |
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgement
We thank ‘Wallonie-Bruxelles International’ (WBI) for funding J.M.’s contribution to this work.
Section editors: Piet Herdewijn – Catholic University Leuven, Leuven, Belgium. Gilles Gosselin – Université Montpellier II, Montpellier, Cedex 5, France
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.ddtec.2012.07.009.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Neumann G. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McInnes C. Virtual screening strategies in drug discovery. Curr. Opin. Chem. Biol. 2007;11:494–502. doi: 10.1016/j.cbpa.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 3.Kirchmair J. Development of anti-viral agents using molecular modeling and virtual screening techniques. Infect. Disorders Drug Targets. 2011;11:64–93. doi: 10.2174/187152611794407782. [DOI] [PubMed] [Google Scholar]
- 4.Berman H.M. The Protein Data Bank. Acta Crystallogr. D. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- 5.Kalyaanamoorthy S., Chen Y-P.P. Structure-based drug design to augment hit discovery. Drug Discov. Today. 2011;16:831–839. doi: 10.1016/j.drudis.2011.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Rarey M. A fast flexible docking method using an incremental construction algorithm. J. Mol. Biol. 1996;261:470–489. doi: 10.1006/jmbi.1996.0477. [DOI] [PubMed] [Google Scholar]
- 7.Ewing T.J.A. DOCK 4.0: search strategies for automated molecular docking of flexible molecule databases. J. Comput. Aided Mol. Des. 2001;15:411–428. doi: 10.1023/a:1011115820450. [DOI] [PubMed] [Google Scholar]
- 8.McGann M.R. Gaussian docking functions. Biopolymers. 2003;68:76–90. doi: 10.1002/bip.10207. [DOI] [PubMed] [Google Scholar]
- 9.Jones G. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- 10.Morris G.M. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998;19:1639–1662. [Google Scholar]
- 11.Halgren T.A. Glide: a new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004;47:1750–1759. doi: 10.1021/jm030644s. [DOI] [PubMed] [Google Scholar]
- 12.Venkatachalam C.M. LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J. Mol. Graphics Model. 2003;21:289–307. doi: 10.1016/s1093-3263(02)00164-x. [DOI] [PubMed] [Google Scholar]
- 13.Jain A.N. Surflex: fully automatic flexible molecular docking using a molecular similarity-based search engine. J. Med. Chem. 2003;46:499–511. doi: 10.1021/jm020406h. [DOI] [PubMed] [Google Scholar]
- 14.Warren G.L. A critical assessment of docking programs and scoring functions. J. Med. Chem. 2006;49:5912–5931. doi: 10.1021/jm050362n. [DOI] [PubMed] [Google Scholar]
- 15.Noha S.M. Pharmacophore-based discovery of a novel cytosolic phospholipase A(2)alpha inhibitor. Bioorg. Med. Chem. Lett. 2012;22:1202–1207. doi: 10.1016/j.bmcl.2011.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Distinto S. Identification of HIV-1 reverse transcriptase dual inhibitors by a combined shape-, 2D-fingerprint- and pharmacophore-based virtual screening approach. Eur. J. Med. Chem. 2012;50:216–229. doi: 10.1016/j.ejmech.2012.01.056. [DOI] [PubMed] [Google Scholar]
- 17.Waltenberger B. Pharmacophore modeling and virtual screening for novel acidic inhibitors of microsomal prostaglandin E-2 synthase-1 (mPGES-1) J. Med. Chem. 2011;54:3163–3174. doi: 10.1021/jm101309g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schuster D. Pharmacophore-based discovery of FXR agonists. Part I. Model development and experimental validation. Bioorg. Med. Chem. 2011;19:7168–7180. doi: 10.1016/j.bmc.2011.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster D. Identification of chemically diverse, novel inhibitors of 17 beta-hydroxysteroid dehydrogenase type 3 and 5 by pharmacophore-based virtual screening. J. Steroid Biochem. Mol. Biol. 2011;125:148–161. doi: 10.1016/j.jsbmb.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 20.Noha S.M. Discovery of a novel IKK-beta inhibitor by ligand-based virtual screening techniques. Bioorg. Med. Chem. Lett. 2011;21:577–583. doi: 10.1016/j.bmcl.2010.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidel T. Strategies for 3D pharmacophore-based virtual screening. Drug Discov. Today: Technol. 2010;7:e221–e228. doi: 10.1016/j.ddtec.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Wolber G. Efficient overlay of small organic molecules using 3D pharmacophores. J. Comput. Aided Mol. Des. 2006;20:773–788. doi: 10.1007/s10822-006-9078-7. [DOI] [PubMed] [Google Scholar]
- 23.Wolber G., Langer T. LigandScout: 3-d pharmacophores derived from protein-bound: ligands and their use as virtual screening filters. J. Chem. Inform. Model. 2005;45:160–169. doi: 10.1021/ci049885e. [DOI] [PubMed] [Google Scholar]
- 24.Guner O. Pharmacophore modeling and three dimensional database searching for drug design using catalyst: recent advances. Curr. Med. Chem. 2004;11:2991–3005. doi: 10.2174/0929867043364036. [DOI] [PubMed] [Google Scholar]
- 25.Cheong S.L. Pharmacophore elucidation for a new series of 2-aryl-pyrazolo-triazolo-pyrimidines as potent human A(3) adenosine receptor antagonists. Bioorg. Med. Chem. Lett. 2011;21:2898–2905. doi: 10.1016/j.bmcl.2011.03.073. [DOI] [PubMed] [Google Scholar]
- 26.Dixon S.L. PHASE: A novel approach to pharmacophore modeling and 3D database searching. Chem. Biol. Drug Design. 2006;67:370–372. doi: 10.1111/j.1747-0285.2006.00384.x. [DOI] [PubMed] [Google Scholar]
- 27.Grant J.A. A fast method of molecular shape comparison: A simple application of a Gaussian description of molecular shape. J. Comput. Chem. 1996;17:1653–1666. [Google Scholar]
- 28.Monto A.S. The risk of seasonal and pandemic influenza: prospects for control. Clin. Infect. Dis. 2009;48:S20–S25. doi: 10.1086/591853. [DOI] [PubMed] [Google Scholar]
- 29.Gong J. Structure and functions of influenza virus neuraminidase. Curr. Med. Chem. 2007;14:113–122. doi: 10.2174/092986707779313444. [DOI] [PubMed] [Google Scholar]
- 30.Kirchmair J. Novel neuraminidase inhibitors: identification, biological evaluation and investigations of the binding mode. Future Med. Chem. 2011;3:437–450. doi: 10.4155/fmc.10.292. [DOI] [PubMed] [Google Scholar]
- 31.Bustanji Y. In silico screening for non-nucleoside HIV-1 reverse transcriptase inhibitors using physicochemical filters and high-throughput docking followed by in vitro evaluation. Chem. Biol. Drug Design. 2009;74:258–265. doi: 10.1111/j.1747-0285.2009.00852.x. [DOI] [PubMed] [Google Scholar]
- 32.Irwin J.J., Shoichet B.K. ZINC – a free database of commercially available compounds for virtual screening. J. Chem. Inform. Model. 2005;45:177–182. doi: 10.1021/ci049714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nichols S.E. Discovery of wild-type and Y181C mutant non-nucleoside HIV-1 reverse transcriptase inhibitors using virtual screening with multiple protein structures. J. Chem. Inform. Model. 2009;49:1272–1279. doi: 10.1021/ci900068k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Distinto S. Synthesis and biological assessment of novel 2-thiazolylhydrazones and computational analysis of their recognition by monoamine oxidase B. Eur. J. Med. Chem. 2012;48:284–295. doi: 10.1016/j.ejmech.2011.12.027. [DOI] [PubMed] [Google Scholar]
- 35.Rajamaki S. Exploration of novel thiobarbituric acid-, rhodanine- and thiohydantoin-based HIV-1 integrase inhibitors. Bioorg. Med. Chem. Lett. 2009;19:3615–3618. doi: 10.1016/j.bmcl.2009.04.132. [DOI] [PubMed] [Google Scholar]
- 36.LaLonde J.M. Design, synthesis and biological evaluation of small molecule inhibitors of CD4-gp120 binding based on virtual screening. Bioorg. Med. Chem. 2011;19:91–101. doi: 10.1016/j.bmc.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curreli F. Virtual screening based identification of novel small-molecule inhibitors targeted to the HIV-1 capsid. Bioorg. Med. Chem. 2011;19:77–90. doi: 10.1016/j.bmc.2010.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yennamalli R. Identification of novel target sites and an inhibitor of the dengue virus E protein. J. Comput. Aided Mol. Des. 2009;23:333–341. doi: 10.1007/s10822-009-9263-6. [DOI] [PubMed] [Google Scholar]
- 39.Fusco D.N., Chung R.T. Novel therapies for hepatitis C: insights from the structure of the virus. Annu. Rev. Med. 2012;63:373–387. doi: 10.1146/annurev-med-042010-085715. [DOI] [PubMed] [Google Scholar]
- 40.Kampmann T. In silico screening of small molecule libraries using the dengue virus envelope E protein has identified compounds with antiviral activity against multiple flaviviruses. Antiviral Res. 2009;84:234–241. doi: 10.1016/j.antiviral.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q-Y. A small-molecule dengue virus entry inhibitor. Antimicrob. Agents Chemother. 2009;53:1823–1831. doi: 10.1128/AAC.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poh M.K. A small molecule fusion inhibitor of dengue virus. Antiviral Res. 2009;84:260–266. doi: 10.1016/j.antiviral.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 43.Umamaheswari A. Docking studies towards exploring antiviral compounds against envelope protein of yellow fever virus. Interdiscip. Sci. Comput. Life Sci. 2011;3:64–77. doi: 10.1007/s12539-011-0064-y. [DOI] [PubMed] [Google Scholar]
- 44.Takaya D. A new method for induced fit docking (GENIUS) and its application to virtual screening of novel HCV NS3-4A protease inhibitors. Bioorg. Med. Chem. 2011;19:6892–6905. doi: 10.1016/j.bmc.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 45.Shiryaev S.A. Virtual ligand screening of the National Cancer Institute (NCI) compound library leads to the allosteric inhibitory scaffolds of the west Nile virus NS3 proteinase. Assay Drug Dev. Technol. 2011;9:69–78. doi: 10.1089/adt.2010.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim N.D. Discovery of novel HCV polymerase inhibitors using pharmacophore-based virtual screening. Bioorg. Med. Chem. Lett. 2011;21:3329–3334. doi: 10.1016/j.bmcl.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 47.Lin Y-T. Efficient in silico assay of inhibitors of hepatitis C virus RNA-dependent RNA polymerase by structure-based virtual screening and in vitro evaluation. Assay Drug Dev. Technol. 2011;9:290–298. doi: 10.1089/adt.2010.0341. [DOI] [PubMed] [Google Scholar]
- 48.Talele T.T. Structure-based virtual screening, synthesis and SAR of novel inhibitors of hepatitis C virus NS5B polymerase. Bioorg. Med. Chem. 2010;18:4630–4638. doi: 10.1016/j.bmc.2010.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Musmuca I. Combining 3-D quantitative structure-activity relationship with ligand based and structure based alignment procedures for in silico screening of new hepatitis C virus NS5B polymerase inhibitors. J. Chem. Inform. Model. 2010;50:662–676. doi: 10.1021/ci9004749. [DOI] [PubMed] [Google Scholar]
- 50.Podvinec M. Novel inhibitors of dengue virus methyltransferase: discovery by in vitro-driven virtual screening on a desktop computer grid. J. Med. Chem. 2010;53:1483–1495. doi: 10.1021/jm900776m. [DOI] [PubMed] [Google Scholar]
- 51.Li N. Discovery of selective inhibitors against EBNA1 via high throughput in silico virtual screening. PLoS One. 2010;5 doi: 10.1371/journal.pone.0010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nguyen T.T.H. Virtual screening identification of novel severe acute respiratory syndrome 3C-like protease inhibitors and in vitro confirmation. Bioorg. Med. Chem. Lett. 2011;21:3088–3091. doi: 10.1016/j.bmcl.2011.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mukherjee P. Inhibitors of SARS-3CL(pro): virtual screening, biological evaluation, and molecular dynamics simulation studies. J. Chem. Inform. Model. 2011;51:1376–1392. doi: 10.1021/ci1004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park S-J. Identification of RNA pseudoknot-binding ligand that inhibits the −1 ribosomal frameshifting of SARS-coronavirus by structure-based virtual screening (vol. 133, p. 10094, 2011) J. Am. Chem. Soc. 2011;133:14150. doi: 10.1021/ja206172p. [DOI] [PubMed] [Google Scholar]
- 55.Perez-Nueno V.I. Discovery of novel HIV entry inhibitors for the CXCR4 receptor by prospective virtual screening. J. Chem. Inform. Model. 2009;49:810–823. doi: 10.1021/ci800468q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.