Abstract
This study describes the nationwide shift from brand-name adalimumab (originator) to adalimumab biosimilars in Denmark and analyzes what a similar shift in the US would look like.
Brand-name adalimumab (Humira, hereinafter originator) is by far the most cost-intensive pharmaceutical product in the US with global sales of $19.9 billion in 2018.1 Five adalimumab biosimilars have been approved by the US Food and Drug Administration, but none are marketed owing to patent disputes. The first to become marketed is expected in January 2023.2 A 2019 Viewpoint argues that cost savings in the US due to adalimumab biosimilars might vanish as patients might switch to newer and better drugs before the biosimilars become available.3 The lack of adalimumab biosimilars results in substantial premiums being paid by patients and payers when much cheaper biosimilar drugs are available outside the US.
Denmark, a member of the European Union with 5.8 million inhabitants, has benefited from substantial discounts owing to a well-planned implementation of adalimumab biosimilars. In Denmark, adalimumab is provided to patients in public outpatient clinics and bought through national tenders. There is no automated substitution to biosimilars, but the treatment recommendations from the Danish Medicines Council was changed to adalimumab biosimilars for all indications following the patent expiration of the adalimumab originator. This included switching patients to a biosimilar who were already well treated with the originator (nonmedical shift). We present the nationwide shift to adalimumab biosimilars across all indications in Denmark and calculate the actual associated cost reductions.
Methods
Monthly data (January 2017 to October 2019) on drug sales to all Danish Hospital departments from Amgros, the purchaser of all hospital drugs, were used to assess the biosimilar uptake proportion and subsequent changes in cost. Institutional review board approval was waived because the data used did not have information on individual drug use. Owing to low stocks and a high turnover in all departments, sales data were mentioned as consumption. Quantity was normalized to 40-mg adalimumab pens, and cost was converted from Danish kroner to US dollars using the official exchange rate on October 16, 2018. The statistical software R, version 3.6.0 (R Foundation for Statistical Computing) was used. Analysis began November 2019.
Results
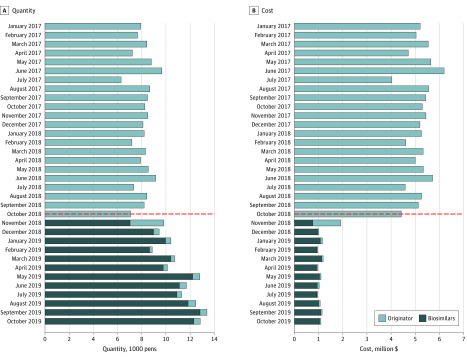
A complete shift to adalimumab biosimilars was seen immediately after the patent expiration of the originator on October 16, 2018 (Figure). A tendering process was conducted and 3 biosimilars won the tender, which resulted in 1 biosimilar for children and 2 biosimilars for adult patients, depending on the region. The proportion of adalimumab biosimilars increased from 71.6% (7040 of 9829 pens) in November 2018 to 95.1% (8974 of 9438 pens) in December 2018. Costs of adalimumab decreased by 82.8% from September 2018 to December 2018 (September: 8197 pens at $5.13 million; December: 9438 pens at $1.01 million). The proportion of biosimilars in December 2018 was 77.9% (438 of 562 pens) for pediatrics, 94.7% (4348 of 4590 pens) for adults in Western Denmark, and 97.7% (4188 of 4286 pens) for adults in Eastern Denmark. There were no differences in biosimilar proportions between dermatology, gastroenterology, and rheumatology.
Figure. Monthly Adalimumab Consumption by Quantity and Cost in Denmark.
The dashed horizontal line represents the Danish patent expiration of the brand-name adalimumab on October 16, 2018. The patent expiration was immediately followed by a shift to biosimilars with a substantial cost reduction.
Discussion
The price cuts of adalimumab to less than one-fifth was crucial to maximize health care services given the health budget. Previously, Denmark had succeeded with fast and near complete shifts of infliximab and etanercept to biosimilars, resulting in substantial price cuts.4 Like generic drugs, there is no reason to switch if the originator matched the price of biosimilars, but access to generic and biosimilar drugs is generally needed to ensure market competition and lower drug prices. Biosimilars in the US have low uptake owing to several reasons including no automated substitution, not included in formularies, and rebate traps, where manufacturers can withdraw substantial bio-originator drug discounts (up to 50%) if the payer uses biosimilars.5 Despite the rebates, originator adalimumab profited from US sales with a net revenue of $3.9 billion in the third quarter of 2019.6 Thorough preparation and multidisciplinary collaboration between all stakeholders were key points in the Danish implementation, and they are crucial for successful implementation in the US. Given the previous US experiences, a successful US implementation with substantial cost savings seems unlikely. While other countries, including Denmark, have benefitted from substantial price reductions after patent expiration of the cost-intensive adalimumab, US health care payers, and ultimately the patients, continue to pay a tremendous premium owing to the lack of adalimumab biosimilars in the US.
References
- 1.Urquhart L. Top drugs and companies by sales in 2018. Nat Rev Drug Discov. 2019;18:245-245. doi: 10.1038/d41573-019-00049-0 [DOI] [PubMed] [Google Scholar]
- 2.Zhai MZ, Sarpatwari A, Kesselheim AS. Why are biosimilars not living up to their promise in the US? AMA J Ethics. 2019;21(8):E668-E678. doi: 10.1001/amajethics.2019.668 [DOI] [PubMed] [Google Scholar]
- 3.Gellad WF, Good CB. Adalimumab and the challenges for biosimilars. JAMA. Published online October 23, 2019. doi: 10.1001/jama.2019.16275 [DOI] [PubMed] [Google Scholar]
- 4.Jensen TB, Bartels D, Sædder EA, et al. . The Danish model for the quick and safe implementation of infliximab and etanercept biosimilars. Eur J Clin Pharmacol. 2020;76(1):35-40. doi: 10.1007/s00228-019-02765-3 [DOI] [PubMed] [Google Scholar]
- 5.Hakim A, Ross JS. Obstacles to the adoption of biosimilars for chronic diseases. JAMA. 2017;317(21):2163-2164. doi: 10.1001/jama.2017.5202 [DOI] [PubMed] [Google Scholar]
- 6.AbbVie Investors. AbbVie reports third-quarter 2019 financial results. Accessed November 19, 2019. https://investors.abbvie.com/news-releases/news-release-details/abbvie-reports-third-quarter-2019-financial-results