Key Points
Question
Does treatment with remote ischemic perconditioning reduce brain infarction volume growth in patients with acute ischemic stroke?
Findings
In this randomized clinical trial of 188 patients with acute ischemic stroke, brain infarction volume was used as an outcome measure. Treatment with remote ischemic perconditioning within 6 hours of symptom onset was found not to reduce brain infarction volume growth.
Meaning
Contrary to the observations reported in preclinical studies, treatment with in-hospital remote ischemic perconditioning (in addition to reperfusion therapies) did not reduce brain infarction volume growth.
Abstract
Importance
Treatment with remote ischemic perconditioning has been reported to reduce brain infarction volume in animal models of stroke. Whether this neuroprotective effect was observed in patients with acute ischemic stroke remains unknown.
Objective
To determine whether treatment with remote ischemic perconditioning administered to the leg of patients with acute ischemic stroke can reduce brain infarction volume growth.
Design, Setting, and Participants
This proof-of-concept multicenter prospective randomized open-label with blinded end point clinical trial was performed from January 12, 2015, to May 2, 2018. Patients were recruited from 11 stroke centers in France. Of the 188 patients who received magnetic resonance imaging within 6 hours of symptom onset and were confirmed to have carotid ischemic stroke, 93 were randomized to receive treatment with lower-limb remote ischemic perconditioning in addition to standard care (the intervention group), and 95 were randomized to receive standard care alone (the control group).
Interventions
Randomization on a 1:1 ratio to receive treatment with remote ischemic perconditioning (4 cycles of 5-minute inflations and 5-minute deflations to the thigh to 110 mm Hg above systolic blood pressure) in addition to standard care or standard care alone.
Main Outcomes and Measures
The change in brain infarction volume growth between baseline and 24 hours, measured by a diffusion-weighted sequence of magnetic resonance imaging scans of the brain.
Results
A total of 188 patients (mean [SD] age, 67.2 [15.7] years; 98 men [52.1%]) were included in this intention-to-treat analysis. At hospital admission, the median National Institutes of Health Stroke Scale score was 10 (interquartile range [IQR], 6-16) and the median brain infarction volume was 11.4 cm3 (IQR, 3.6-35.8 cm3); 164 patients (87.2%) received intravenous thrombolysis, and 64 patients (34.0%) underwent mechanical thrombectomy. The median increase in brain infarction growth was 0.30 cm3 (IQR, 0.11-0.48 cm3) in the intervention group and 0.37 cm3 (IQR, 0.19-0.55 cm3) in the control group (mean between-group difference on loge-transformed change, –0.07; 95% CI, –0.33 to 0.18; P = .57). An excellent outcome (defined as a score of 0-1 on the 90-day modified Rankin Scale or a score equal to the prestroke modified Rankin Scale score) was observed in 46 of 90 patients (51.1%) in the intervention group and 37 of 91 patients (40.7%) in the control group (P = .12). No significant differences in 90-day mortality were observed between the intervention and control groups (14 of 90 patients; Kaplan-Meier estimate, 15.8% vs 10 of 91 patients; Kaplan-Meier estimate, 10.4%, respectively; P = .45) or with symptomatic intracerebral hemorrhage (4 of 88 patients [4.5%] in both groups; P = .97).
Conclusions and Relevance
In this study, treatment with remote ischemic perconditioning, during or after reperfusion therapies, had no significant effect on brain infarction volume growth at 24 hours after symptom onset.
Trial Registration
ClinicalTrials.gov Identifier: NCT02189928
This randomized clinical trial examines whether in-hospital treatment with remote ischemic perconditioning that is administered within 6 hours of symptom onset, with or without reperfusion therapy, can reduce brain infarction volume growth in patients with acute ischemic stroke.
Introduction
Despite substantial progress in reperfusion therapies, such as intravenous (IV) thrombolysis and mechanical thrombectomy, more than 50% of patients with stroke continue to have a disability at 3 months after treatment.1,2 Therefore, a need exists for neuroprotective interventions that can reduce infarction growth, and consequently disability, in patients who receive reperfusion therapy as well as patients with persistent arterial occlusion.3,4 One promising neuroprotective intervention is ischemic conditioning.5 This therapy consists of inducing transient ischemia without necrosis and can be applied directly to the organ experiencing spontaneous ischemia (conventional conditioning) or indirectly (ie, at a distance from the affected organ) through the application of an inflatable cuff that is placed around a patient’s arm or leg (remote conditioning).5 Remote ischemic preconditioning, perconditioning, and postconditioning are defined based on the timing of the intervention, which can occur before, during, or after the ischemic event (and after the reopening of the artery suspected to be responsible for the ischemic event). In high-quality preclinical studies that followed Stroke Therapy Academic Industry Roundtable (STAIR) recommendations,3 treatment with remote ischemic perconditioning was reported to be effective in animals6,7,8 and had an additive effect on final brain infarction volume when used in combination with alteplase thrombolytic medication.9 Although the exact mechanisms of remote ischemic perconditioning are not known to date, several potential mechanisms have been postulated. The cytoprotective effect in the target organ involves activation of the reperfusion injury salvage kinases and the protection of mitochondria by preventing the opening of the mitochondrial permeability transition pore.5
Treatment with remote ischemic perconditioning has been reported to reduce the final size of myocardial infarction in patients,10 but a large randomized clinical trial of more than 5000 patients with myocardial infarction reported no effect on clinical outcomes.11 The advantages of treatment with remote ischemic perconditioning include simplicity and low cost, and no safety issues have been raised among patients with ischemic stroke.12 We designed a proof-of-concept randomized clinical trial of in-hospital remote ischemic perconditioning that was performed between 2 magnetic resonance imaging (MRI) scans of the brain. Our aim was to evaluate whether treatment with remote ischemic perconditioning, with or without reperfusion therapies (including IV thrombolysis and mechanical thrombectomy), which was applied to the leg of a patient with acute ischemic stroke within 6 hours of symptom onset, could reduce brain infarction volume growth.
Methods
Clinical Trial Design and Participants
The Remote Ischemic Conditioning in Acute Brain Infarction (RESCUE BRAIN) study is a multicenter prospective randomized open-label with blinded end point (PROBE) efficacy clinical trial13 that was conducted in 11 stroke centers in France, 8 of which were comprehensive stroke centers. The trial protocol is available in Supplement 1. Participating centers included Versailles Mignot Hospital (Versailles), Hôpital Foch (Suresnes), Centre Hospitalier Universitaire de Lille (Lille), Bichat University Hospital (Paris), Hôpital Pitié-Salpêtrière (Paris), Centre Hospitalier Sud Francilien (Corbeil-Essonnes), Fondation Ophtalmologique Adolphe de Rothschild (Paris), Henri Mondor Hospital (Creteil), University Hospital of Nantes (Nantes), Strasbourg University Hospital (Strasbourg), and Rennes University Hospital (Rennes) (eMethods 1 in Supplement 2). Details of the study design have previously been published.14 The clinical trial was conducted according to the standard operating procedures of the Centre Hospitalier de Versailles and in accordance with Good Clinical Practice guidelines15 and the Declaration of Helsinki.16 The clinical trial protocol was approved by the institutional review board of the Comité de Protection des Personnes Ile de France XI. All patients (or their legal representatives) provided written informed consent. According to French legislation, consent was not immediately required if the patient had a severe language disturbance or a loss of consciousness and if no legal proxy or family member was present at hospital admission. In this case, in which emergency consent was necessary, the patient or the patient’s legal representative was asked to provide written informed consent during follow-up.
Patients were eligible for study inclusion if they were age 18 years or older, had a score between 5 and 25 on the National Institutes of Health Stroke Scale (NIHSS) (score range, 0-42, with 0 indicating no stroke symptoms and 42 indicating severe stroke), had a carotid-territory brain infarction identified through diffusion-weighted MRI scan, and were able to receive treatment with remote ischemic perconditioning within 6 hours of stroke symptom onset. The main exclusion criteria included a history of peripheral arterial disease or leg phlebitis, the presence of a leg ulcer or a severe skin condition in the lower limb, or a diagnosis of sickle cell disease.
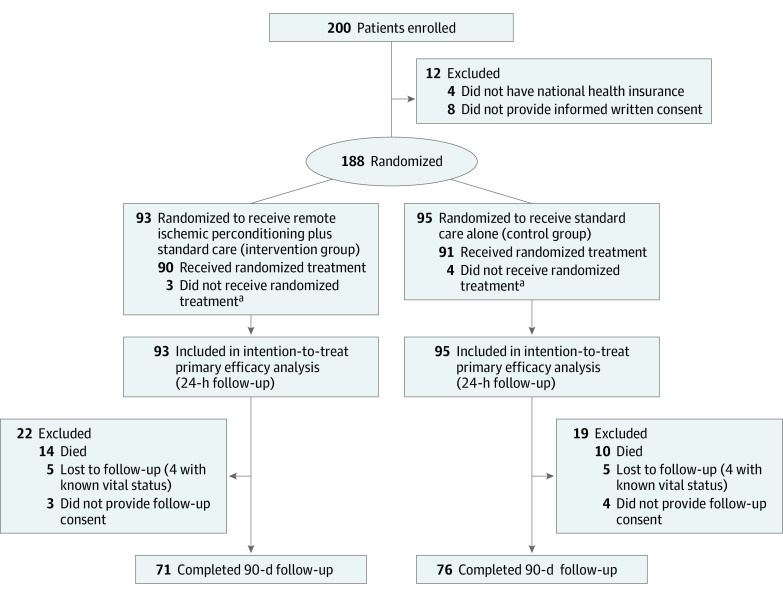
Between January 12, 2015, and May 2, 2018, the study enrolled 200 patients who received an MRI scan within 6 hours of symptom onset and were confirmed to have carotid ischemic stroke based on MRI results and scores on the NIHSS. A total of 12 patients were excluded (Figure). The remaining 188 patients were randomized, with 93 patients randomized to receive treatment with lower-limb remote ischemic perconditioning in addition to standard care (the intervention group) and 95 patients randomized to receive standard care alone (the control group). At an interim analysis in March 2017, with 102 patients included, the Data Safety Monitoring Board (eMethods 1 in Supplement 2) observed that the rate of symptomatic hemorrhagic transformation was similar in the intervention and control groups and recommended that the clinical trial be continued.
Figure. CONSORT Diagram.
aA total of 7 patients (3 in the intervention group and 4 in the control group) did not receive the randomized treatment: 1 patient had sickle cell disease, 5 patients had a history of peripheral arterial disease or leg phlebitis, and 1 patient had a prestroke modified Rankin score greater than 2.
Randomization and Masking
Randomization was performed on a 1:1 ratio using a predefined computer-generated table that was centrally issued by an interactive web server coupled with the CleanWEB electronic case report form (Telemedicine Technologies). The CleanWEB electronic case report server was audited and complied with all required Good Clinical Practice guidelines.
Randomization was stratified by stroke center and receipt of IV thrombolysis, with blocks of randomly varying size. At the time the clinical trial protocol was written (2013), treatment with mechanical thrombectomy had not been validated for use in patients in the acute phase of ischemic stroke; therefore, we allowed the investigators to include mechanical thrombectomy therapy but did not consider it a stratification parameter.
Intervention Procedures
In all patients, the cuff of a pneumatic electronic tourniquet with 1 regulated pressure circuit (Dessillons & Dutrillaux) was placed around the thigh of the unaffected side within 6 hours of symptom onset. Patients in the intervention group underwent 4 cycles of cuff inflation (to 110 mm Hg above their systolic blood pressure for 5 minutes) and deflation (for 5 minutes), for a total procedure time of 40 minutes.17,18 After the blood pressure inflation target was set in the device by a trained nurse, the electronic tourniquet automatically delivered the cycles. A printed report was provided at the end of the process, which summarized the number of inflation cycles delivered. Patients in the control group had the cuff placed around the thigh of the unaffected side for 40 minutes, without any inflations.
Blood pressure was recorded before cuff inflation and at the end of 4 cycles of cuff inflation. Discomfort and pain were assessed using a visual analog scale (scale range, 1-10, with 0 indicating no pain and 10 indicating maximum pain). In each stroke center, the nurses and physicians involved in the clinical trial were trained by the principal investigator (F.P.) and the project lead (A.C.) to place the cuff at the midthigh of the unaffected leg and to enter the target blood pressure into the electronic tourniquet.
Patients in both groups received standard care, at the discretion of the local investigator at each participating stroke center and according to international guidelines.19,20 Patients deemed eligible to receive IV thrombolysis were administered 0.9 mg/kg (10% bolus; 90% by infusion pump during 1 hour) of recombinant tissue plasminogen activator (Actilyse; Boehringer Ingelheim).
The same MRI protocol was performed in each stroke center (eMethods 2 in Supplement 2). The MRI scans were performed at hospital admission (within 6 hours of symptom onset and before any intervention) and at 24 hours after symptom onset (a maximum of 36 hours).
Outcomes
The primary outcome was the absolute change in brain infarction volume (ie, infarction growth) from baseline to 24 hours after the first MRI scan. The baseline infarction volume was defined as the hyperintense area measured on the initial diffusion-weighted MRI scan (b value = 1000 seconds/mm2), which was delineated by interactive manual outlining using Mango software (Research Imaging Institute, University of Texas). The follow-up diffusion-weighted MRI infarction volume was determined using a similar methodology. Infarction volume was computed using a previously validated method21 by an experienced neurologist (C.H.) who was previously trained in this method22 and who was blinded to the clinical data and patient randomization group.
Secondary efficacy outcomes were (1) relative change in brain infarction volume from baseline to 24 hours after the first MRI scan (expressed as a percentage increase); (2) absolute change in NIHSS score between baseline and 24 hours after symptom onset; (3) activities of daily living scores at 90 days (percentage of patients with a score ≥95 on the Barthel Index for Activities of Daily Living scale [score range, 0-99, with 0 indicating total dependency and 99 indicating slight dependency]); (4) excellent outcome (defined as a score of 0-1 on the 90-day modified Rankin Scale [mRS]) (score range, 0-6, with 0 indicating no symptoms of disability and 6 indicating death or a score equal to the prestroke mRS score); (5) degree of disability, which was assessed by the overall distribution of mRS scores at 90 days (scores of 5 [indicating severe disability] and 6 were combined for the shift analysis)23; and (6) successful recanalization (defined as a measurement of grade 2b or 3 on the modified Treatment in Cerebral Infarction scale [grade range, 0-3, with 0 indicating no perfusion and 3 indicating complete antegrade reperfusion] at the 24-hour MRI scan) in patients treated with IV thrombolysis.
Safety outcomes were (1) symptomatic hemorrhagic transformation (using European Cooperative Acute Stroke Study [ECASS] 3 classification24); (2) early neurological deterioration (a score increase of ≥4 on the NIHSS from baseline to 24 hours after symptom onset); (3) 90-day all-cause mortality; and (4) tolerance and adverse effects of remote ischemic perconditioning, particularly local complications (pain [evaluated by visual analog scale], erythema, hematoma, limb ischemia, skin ulcer, and phlebitis).
Statistical Analysis
The study was designed to have a statistical power of 80% with a 2-sided α level of .05 to allow assessment of the efficacy of treatment with remote ischemic perconditioning to decrease infarction growth within 24 hours of symptom onset compared with standard care. The sample size was calculated based on published25,26 and data from 2 of us (C.R. and Y.S.) from the INSULINFARCT clinical trial,25 which had a similar target population of patients with ischemic stroke and a similar MRI design. These data were used to estimate a minimal clinically relevant difference of 15 cm3 of infarction growth between the intervention and control groups. Another team also found this cutoff value effective in discriminating between better and worse clinical outcomes.27 With an SD of 36 cm3, and assuming an estimate of 10% unusable MRI results, a total of 200 patients (100 per group) was required.
The primary efficacy analysis was performed on all randomized patients in each group after analyzing missing values for baseline and 24-hour infarction volumes using a multiple imputation procedure. The primary outcome (absolute change in brain infarction volume from baseline to 24 hours after the first MRI scan) was estimated and compared between the intervention and control groups using the constrained longitudinal data analysis model,28,29 adjusting for randomization stratification variables by including IV thrombolysis as a fixed effect and stroke center as a random effect.
In the constrained longitudinal data analysis, both baseline and 24-hour brain infarction volumes were modeled as dependent variables using a linear mixed model (with an unstructured covariance pattern model), and the true baseline means were constrained to be the same for the intervention and control groups. We chose a constrained longitudinal data analysis model because, in comparison with the standard analysis of covariance model, it was able to better analyze differential baseline-dependent dropout data and missing data.29 Because we observed an asymmetric distribution of brain infarction volume, the constrained longitudinal data analysis model was performed on log-transformed brain infarction volumes.
Primary outcome values that were missing (for whatever reason) were analyzed through a multiple imputation procedure with a missing-at-random assumption by using a regression switching approach (ie, multivariable imputation by chained equations with m = 10 imputations). The imputation procedure was performed using the main baseline characteristics of participants, a predictive mean matching method for continuous variables for the intervention and control groups, and logistic regression models (binomial, ordinal, or multinomial) for categorical variables.
A comparison of the primary outcome was performed after adjustment for age, sex, NIHSS score at admission, glucose level at admission, receipt of endovascular treatment, and time from symptom onset to MRI scan. This comparison was conducted according to prespecified sensitivity analyses, which included a complete-case analysis and a per-protocol analysis.
Before analyzing missing values through the multiple imputation procedure, the effect of treatment modification on the primary outcome was explored in 2 prespecified subgroups: receipt of IV thrombolysis (yes or no) and arterial occlusion status (no initial occlusion, reperfusion, or persistent occlusion) at 24 hours after symptom onset. Unplanned subgroup analyses were also reported, including receipt of endovascular treatment (yes or no) and mismatch between NIHSS score and diffusion-weighted MRI result (yes or no), using the DAWN clinical trial criteria.30 Further details regarding the statistical analysis for secondary outcomes are available in the statistical analysis plan (eMethods 3 in Supplement 2). All statistical tests were 2-sided, and P < .05 was considered statistically significant. Because no adjustment for multiple testing was applied, all secondary end points were considered exploratory. All data were analyzed using SAS software, version 9.4 (SAS Institute Inc).
Results
Of 188 patients included in the intention-to-treat analysis, the mean (SD) age was 67.2 (15.7) years, and 98 patients (52.1%) were men. The intervention and control groups were well balanced in terms of baseline characteristics (Table 1).
Table 1. Baseline Patient Characteristics and Treatment.
| Characteristic | Intervention group, No. (%) (n = 93) | Control group, No. (%) (n = 95) |
|---|---|---|
| Age, mean (SD), y | 67.8 (15.1) | 66.7 (16.4) |
| Male | 45 (48.4) | 53 (55.8) |
| BMI, mean (SD)a | 26.2 (5.1) | 26.5 (5.8) |
| Medical history | ||
| Hypertension | 48 (51.6) | 50 (52.6) |
| Diabetes | 12 (12.9) | 18 (18.9) |
| Hypercholesterolemia | 32 (34.4) | 40 (42.1) |
| Current smoking, No./total No. (%) | 14/88 (15.9) | 16/91 (17.6) |
| Former smoking, No./total No. (%) | 20/88 (22.7) | 26/91 (28.6) |
| Coronary artery disease | 12 (12.9) | 12 (12.6) |
| Previous stroke or TIA | 11 (11.8) | 13 (13.7) |
| Previous atrial fibrillation | 18 (19.4) | 14 (14.7) |
| Previous cardiac insufficiency | 4 (4.3) | 8 (8.4) |
| Current ischemic event measurements at hospital admission | ||
| Systolic BP, mean (SD), mm Hg | 150 (24) | 147 (24) |
| Diastolic BP, mean (SD), mm Hg | 84 (15) | 82 (16) |
| Glucose level, median (IQR), mg/dLb | 121 (101-141) | 121 (101-150) |
| NIHSS score, median (IQR) | 9 (6-15) | 10 (7-17) |
| Prestroke mRS score | ||
| 0 | 73 (78.5) | 85 (89.5) |
| 1 | 10 (10,8) | 6 (6.3) |
| 2 | 9 (9.7) | 4 (4.2) |
| 3 | 1 (1.1) | 0 |
| Time from symptom onset to baseline MRI, median (IQR), min | 105 (81-147) | 103 (83-142) |
| Occlusion at baseline MRI | ||
| None | 30 (32.3) | 29 (30.5) |
| MCA | 45 (48.4) | 49 (51.6) |
| Cervical ICA | 6 (6.5) | 0 |
| Carotid T | 6 (6.5) | 5 (5.3) |
| Others | 6 (6.5) | 12 (12.6) |
| Acute stroke treatment | ||
| None | 7 (7.5) | 10 (10.5) |
| IVT | 56 (60.2) | 51 (53.7) |
| Endovascular | 4 (4.3) | 3 (3.2) |
| IVT and endovascular | 26 (28.0) | 31 (32.6) |
| Time from symptom onset to treatment, median (IQR), min | ||
| IVTc | 154 (111-186) | 145 (112-175) |
| Groin punctured | 187 (130-250) | 182 (150-245) |
| Remote ischemic perconditioning | 222 (176-275) | NA |
| Time from symptom onset to follow-up MRI, h | 27.9 (26.4-31.2) | 28.5 (26.8-31.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure; ICA, internal carotid artery; IQR, interquartile range; IVT, intravenous thrombolysis; MCA, middle cerebral artery; MRI, magnetic resonance imaging; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
SI conversion factor: To convert glucose to millimoles per liter, multiply by 0.0555.
A total of 8 patients were missing values (4 patients in the intervention group).
A total of 46 patients were missing values (19 patients in the intervention group).
Data reported for patients who received IVT treatment.
Data reported for patients who received endovascular treatment.
At hospital admission, the median NIHSS score was 10 (interquartile range [IQR], 6-16), and 129 of 188 patients (68.6%) had a documented vessel occlusion identified through a baseline MRI scan, which was performed at a median of 104 minutes (IQR, 82-145 minutes) after symptom onset (Table 1). The baseline median brain infarction volume was 11.4 cm3 (IQR, 3.6-35.8 cm3). Most patients (164 of 188 [87.2%]) received IV thrombolysis at a median of 149 minutes (IQR, 111-179 minutes) after symptom onset, and 64 of 188 patients (34.0%) underwent mechanical thrombectomy at a median of 187 minutes (IQR, 145-247 minutes) after symptom onset.
In the intervention group, 88 of 93 patients (94.6%) underwent the complete procedure of 4 cycles at a median of 222 minutes (IQR, 176-275 minutes) from symptom onset to the first cuff inflation. Of the remaining 5 patients, 1 received 7 cycles, 1 received 3 cycles, and 3 received no cycle. The cuff was placed on the left thigh of 57 of 93 patients (61.2%), and the mean (SD) blood pressure was 251 (31) mm Hg before the first cuff inflation. In the control group, 2 patients received 3 inflation cycles, and 2 received 4 inflation cycles. One patient received treatment with remote ischemic perconditioning before IV thrombolysis, and 6 patients received treatment with remote ischemic perconditioning before mechanical thrombectomy. The median time from symptom onset to cuff inflation was 3.7 hours (IQR, 2.9-4.6 hours).
Efficacy Outcomes
For the primary outcome, brain infarction volume growth was not significantly different between the intervention and control groups in the primary efficacy analysis, with a median absolute change (expressed as a mean loge increase from baseline to 24 hours after the first MRI scan) of 0.30 cm3 (IQR, 0.11-0.48 cm3) in the intervention group and 0.37 cm3 (IQR, 0.19-0.55 cm3) in the control group (mean loge between-group difference, –0.07; 95% CI, –0.33 to 0.18; Table 2). No significant difference was found after adjustment for age, NIHSS score at admission, glucose level at admission, receipt of endovascular treatment, and time from symptom onset to MRI scan (mean loge between-group difference, –0.05; 95% CI, –0.30 to 0.21). Similar results were obtained in the per-protocol analysis (mean loge between-group difference, –0.02; 95% CI, –0.28 to 0.24; Table 2) and the complete-case analysis (mean loge between-group difference, –0.07; 95% CI, –0.31 to 0.19; eTable in Supplement 2).
Table 2. Absolute Change in Brain Infarction Volume From Baseline to 24 Hours After Symptom Onset.
| Variable | Intervention group | Control group | Difference, mean loge (95% CI)a | P value | ||
|---|---|---|---|---|---|---|
| No. | Median (IQR), cm3 | No. | Median (IQR), cm3 | |||
| Primary efficacy analysisb | ||||||
| Baseline | 93 | 9.3 (3.4 to 38.3) | 95 | 12.2 (3.7 to 32.3) | NA | NA |
| 24 h | 93 | 13.0 (3.2 to 54.7) | 95 | 18.8 (4.9 to 66.7) | NA | NA |
| Change | 93 | 0.30 (0.11 to 0.48)a | 95 | 0.37 (0.19 to 0.55)a | –0.07 (–0.33 to 0.18) | .57 |
| Per-protocol analysis | ||||||
| Baseline | 86 | 9.1 (3.3 to 37.9) | 88 | 12.1 (3.8 to 32.0) | NA | NA |
| 24 h | 86 | 13.0 (3.6 to 45.4) | 88 | 18.5 (4.8 to 60.0) | NA | NA |
| Change | 86 | 0.30 (0.12 to 0.49)a | 88 | 0.33 (0.14 to 0.51)a | –0.02 (–0.28 to 0.24) | .86 |
Abbreviations: IQR, interquartile range; NA, not applicable.
Adjusted for baseline value using constrained longitudinal data analysis model on log transformed (+1) volume after adjustment for stroke center (included as a random effect) and receipt of IV thrombolysis.
Missing values for brain infarction volume at baseline: 1 patient in the intervention group. Missing values for brain infarction volume at 24 hours after symptom onset: 5 patients in the intervention group and 3 patients in the control group. Missing values were analyzed using a multiple imputation procedure (m = 10).
As shown in Table 3, none of the secondary efficacy outcomes differed significantly between the intervention and control groups. At 90 days, an excellent functional outcome was observed in 46 of 90 patients (51.1%) in the intervention group and 37 of 91 patients (40.7%) in the control group (odds ratio [OR], 1.60; 95% CI, 0.87-2.94; P = .12). A nonsignificant shift was noted in the mRS score distribution in the intervention group compared with the control group, with a common OR for 1-point improvement of 1.29 (95% CI, 0.75-2.22; P = .35).
Table 3. Secondary Efficacy Outcomesa.
| Outcome | Intervention group (n = 93) | Control group (n = 95) | Effect size | Value (95% CI) | P value |
|---|---|---|---|---|---|
| Percentage change in brain infarction volume at 24 h, median (IQR)b | 36.5 (–7.3 to 98.1) | 34.1 (–11.0 to 106.4) | NA | NA | .80c |
| NIHSS score, median (IQR) | |||||
| Baseline | 9 (6 to 15) | 10 (7 to 17) | NA | NA | NA |
| 24 h | 5 (2 to 9) | 7 (2 to 12) | NA | NA | NA |
| Change, mean (95% CI) | –0.59 (–0.75 to –0.43)d | –0.51 (–0.67 to –0.35)d | Mean loge differenced | –0.08 (–0.30 to 0.15)d | .48d |
| Successful reperfusion in patients treated with IV thrombolysis, No./total No. (%) | 62/82 (75.6) | 65/82 (79.3) | OR | 0.80 (0.29 to 2.13) | .65 |
| 90-d Barthel score ≥95, No./total No. (%)e | 61/90 (67.8) | 56/91 (61.5) | OR | 1.32 (0.68 to 2.57) | .41 |
| Excellent outcome, No./total No. (%)f | 46/90 (51.1) | 37/91 (40.7) | OR | 1.60 (0.87 to 2.94) | .12 |
| Favorable outcome, No./total No. (%)g | 62/90 (68.9) | 60/91 (65.9) | OR | 1.15 (0.59 to 2.22) | .68 |
| 90-d mRS score, median (IQR)h | 2 (1 to 3) | 2 (1 to 3) | Common ORi | 1.29 (0.75 to 2.22) | .35 |
Abbreviations: IQR, interquartile range; IV, intravenous; mRS, modified Rankin Scale; NA, not applicable; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio.
Missing values were analyzed using a multiple imputation procedure. Effect sizes were calculated after adjustment for randomization stratification variables (stroke center and receipt of IV thrombolysis).
Not calculable for 2 patients in the intervention group and 2 patients in the control group who had baseline volumes of zero.
Calculated using the mean value of normal approximation z statistics from Mann-Whitney U tests performed in each imputed data set.
Calculated using a constrained longitudinal data analysis model on log transformed (+1) values, adjusted for stroke center (included as a random effect) and receipt of IV thrombolysis.
Data among patients who provided follow-up consent.
Excellent outcome was defined as a 90-day mRS score of 0 to 1 or a score equal to the prestroke mRS score.
Favorable outcome was defined as a 90-day mRS score of 0 to 2 or a score equal to the prestroke mRS score.
Data among patients who provided follow-up consent (90 patients in the intervention group and 91 patients in the control group).
Common OR for 1-point improvement in mRS score (scores of 5 and 6 were combined) was computed from the ordinal mixed logistic model.
No significant differences were found in the primary or secondary efficacy outcomes, particularly regarding percentage changes in brain infarction volume between baseline and 24 hours after symptom onset, which were similar in the intervention group (36.5%; 95% CI, –7.3% to 98.1%) and control group (34.1%; 95% CI, –11.0% to 106.4%).
Safety Outcomes
No significant difference was noted in 90-day mortality between patients who received at least 1 cycle of cuff inflation and deflation (14 of 90 patients; Kaplan-Meier estimate, 15.8%) and patients who received no cycles of cuff inflation and deflation (10 of 91 patients; Kaplan-Meier estimate, 10.4%) nor in the rate of symptomatic intracerebral hemorrhage (4 of 88 patients [4.5%] in both groups; Table 4).
Table 4. Safety Outcomes.
| Outcome | Patient underwent ≥1 cycle of cuff inflation and deflation, No./total No. (%) | Effect sizec | Odds ratio (95% CI) | P value | |
|---|---|---|---|---|---|
| Yes (n = 94)a | No (n = 94)b | ||||
| Intracerebral hemorrhage at 24 h | 35/88 (39.8) | 37/88 (42.0) | OR | 0.91 (0.49 to 1.69) | .77 |
| Hemorrhagic infarction | 16/88 (18.2) | 26/88 (29.5) | NP | NA | NA |
| Type 1 | 3/88 (3.4) | 9/88 (10.2) | NP | NA | NA |
| Type 2 | 13/88 (14.8) | 17/88 (19.3) | NP | NA | NA |
| Parenchymal hematoma | 19/88 (21.6) | 11/88 (12.5) | NP | NA | NA |
| Type 1 | 8/88 (9.1) | 3/88 (3.4) | NP | NA | NA |
| Type 2 | 11/88 (12.5) | 8/88 (9.1) | NP | NA | NA |
| Symptomatic intracerebral hemorrhage | 4/88 (4.5) | 4/88 (4.5) | OR | 0.97 (0.23 to 4.09) | .97 |
| Early neurological improvement at 24 h | 7/94 (7.4) | 9/93 (9.7) | OR | 0.71 (0.25 to 2.03) | .52 |
| 90-d All-cause mortality, No. (estimated Kaplan-Meier %)d | 14/90 (15.6) | 10/91 (11.0) | HR | 1.37 (0.60 to 3.09) | .45 |
| Procedure-related events | 4/94 (4.3) | NA | NA | NA | NA |
| Hematoma in lower limb | 1/94 (1.1) | NA | NA | NA | NA |
| Cyanosis in lower limb | 2/94 (2.1) | NA | NA | NA | NA |
| Erythema in lower limb | 2/94 (2.1) | NA | NA | NA | NA |
| Pain during ischemic perconditioning proceduree | |||||
| VAS score >0 | 41/78 (52.6) | NA | NA | NA | NA |
| Median (IQR)f | 6 (3 to 7) | NA | NA | NA | NA |
| Mean (95% CI) change in hemodynamic measurements during ischemic perconditioning procedure | |||||
| Systolic BP, median (IQR), mm Hg | 1.5 (–2.2 to 5.3) | NA | NA | NA | NA |
| Diastolic BP, median (IQR), mm Hg | 1.6 (–1.5 to 4.7) | NA | NA | NA | NA |
| Other serious adverse events | |||||
| Seizure | 5/94 (5.3) | 5/94 (5.3) | NA | NA | NA |
| Pneumonia | 6/94 (6.4) | 8/94 (8.5) | NA | NA | NA |
| Deep vein thrombosis | 1/94 (1.1) | 1/94 (1.1) | NA | NA | NA |
| Pulmonary embolism | 0 | 2/94 (2.1) | NA | NA | NA |
| Myocardial infarction | 0 | 1/94 (1.1) | NA | NA | NA |
Abbreviations: BP, blood pressure; IQR, interquartile range; NA, not applicable; NP, not performed; VAS, visual analog scale.
Data reported for 90 patients randomized to the intervention group who received remote ischemic perconditioning and 4 patients randomized to the control group who also received remote ischemic perconditioning.
Data reported for 91 patients randomized to the control group and 3 patients randomized to the intervention group who did not receive remote ischemic perconditioning.
Effect size is reported for prespecified safety outcomes.
Data among patients who provided follow-up consent.
Pain measured by VAS (range, 0 indicating no pain to 10 indicating worst pain).
Data reported for patients with a VAS score greater than 0.
A total of 41 of 78 patients (52.6%) experienced pain during the perconditioning procedure, with a median visual analog scale rating of 6 (IQR, 3-7). One patient experienced a hematoma in the lower limb at the site at which the cuff was placed; no decrease in hemoglobin levels was noted. Two patients had transient cyanosis, and 2 patients had transient erythema after receiving remote ischemic perconditioning; no acute limb ischemia was noted. One patient in each group had deep vein thrombosis (Table 4).
Subgroup Analyses
In the prespecified subgroup analyses based on the receipt of IV thrombolysis and the presence of arterial occlusion at 24 hours after symptom onset, no significant heterogeneity was observed in the effect of treatment with remote ischemic perconditioning on the primary outcome (eFigure in Supplement 2).
Unplanned subgroup analyses based on the receipt of mechanical thrombectomy and the mismatch between MRI result and NIHSS score also indicated no treatment heterogeneity (eFigure in Supplement 2).
Discussion
To our knowledge, RESCUE BRAIN is the first randomized clinical trial of treatment with in-hospital remote ischemic perconditioning in patients in the acute phase of stroke using the same end point (brain infarction volume) as that of preclinical studies. We found treatment with remote ischemic perconditioning to be feasible and safe, although one-half of the patients experienced pain. The median time from symptom onset to cuff inflation was 3.7 hours, indicating that the target to deliver remote ischemic perconditioning treatment within the first 6 hours was achieved. However, contrary to the observations reported in preclinical studies, we did not find that treatment with remote ischemic perconditioning reduced infarction growth at 24 hours after symptom onset when treatment was performed primarily during or after the administration of reperfusion therapies. In addition, no significant effect on clinical end points was observed.
The primary outcome was the absolute difference in brain infarction volume between baseline and 24 hours after the first MRI scan in the intervention vs control groups. Infarction growth was chosen (rather than clinical scores at 90 days) because this biomarker was recommended by the STAIR3 and Stroke Imaging Research (STIR) groups.31,32 Further, this quantitative objective outcome has been associated with clinical outcome at 3 months.32,33 The STAIR group suggested that, before testing a neuroprotective agent or intervention in a large randomized clinical trial, the first step should be to verify, through a proof-of-concept randomized clinical trial, whether the biological effect of the neuroprotective agent or intervention (ie, brain infarction volume reduction) is conserved during bench-to-bedside translation and, if so, to evaluate the extent of conservation. Based on simulations performed using the Virtual International Stroke Trials Archive (VISTA) database, Whitehead et al34 found that the sample size used to evaluate lesion volumes (as was done in our study) should be approximately one-fourth of the sample size used to evaluate clinical outcomes, such as mRS scores.
In our study, remote ischemic perconditioning was primarily performed during or after the administration of reperfusion therapies. This approach of the coadministration of a neuroprotective intervention and reperfusion therapies has been advocated for use in neuroprotection clinical trials in the era of reperfusion therapies.35 This approach also makes sense, as treatment with remote ischemic perconditioning has been reported in preclinical studies to reduce neural damage after reperfusion therapy.36,37,38,39
Treatment with remote ischemic perconditioning did not modify absolute or relative infarction growth volume. We can confidently exclude a clinically meaningful effect of a single session of remote ischemic perconditioning treatment delivered at hospital admission for the following reasons: (1) all patients underwent an MRI scan of the brain before and after receiving remote ischemic perconditioning; (2) a sufficient number of patients (n = 188) was analyzed; and (3) no significant differences were found in primary or secondary efficacy outcomes, particularly regarding percentage changes in brain infarction volume between baseline and 24 hours after the first MRI scan, which were similar in the intervention (36.5%) and control (34.1%) groups. Therefore, we do not plan to perform a large randomized clinical trial with functional outcomes.
We propose 2 main hypotheses for our neutral results. We found that treatment with remote ischemic perconditioning in patients with acute ischemic stroke (as an add-on therapy to reperfusion treatment) had no beneficial effect. We note that, although efforts have been made to improve the quality of preclinical studies (such as the use of randomization, blinded end points, and rodents with comorbidities), the results of such studies do not necessarily indicate what will happen in randomized clinical trials. Moreover, a review of preclinical models indicated that no multicenter preclinical study has indicated an association between treatment with remote ischemic perconditioning and final brain infarction volume.40 The second explanation is that treatment with remote ischemic perconditioning was not beneficial because it was mainly performed during or after the receipt of reperfusion therapies, which have been reported to effectively reduce final brain infarction volume.41,42,43
Our clinical trial cannot rule out the possibility that treatment with remote ischemic perconditioning may be effective if administered differently than it was in the RESCUE BRAIN study; in particular, remote ischemic perconditioning treatment may be effective if it is provided in a prehospital setting before the delivery of reperfusion therapies with the aim of freezing the penumbra,2 if it is repeated twice daily for the first 5 or 7 days, or if it is administered to more selected patients than were included in our pragmatic clinical trial. As noted in a review, the underlying mechanisms of remote ischemic perconditioning are not fully known, and no available biomarker can objectively quantify the biological effect of treatment with remote ischemic perconditioning.44 Moreover, we used a protocol of remote ischemic perconditioning on the leg rather than the arm of patients because fluid infusion and blood pressure monitoring are necessary in patients in the acute phase of stroke. However, the optimal protocol for remote ischemic perconditioning treatment is still a matter of debate.44
Several ongoing clinical trials of remote ischemic perconditioning treatment are being conducted,44 some of which are administering remote ischemic perconditioning to patients with suspected ischemic stroke in a prehospital setting. These studies include the RESIST clinical trial in Denmark (NCT03481777) and the REMOTE-CAT clinical trial in Spain (NCT03375762). However, other researchers have chosen a better-characterized target population, in particular a population that specifically includes patients with acute ischemic stroke who are treated with mechanical thrombectomy, such as the REVISE 2 clinical trial in China (NCT03045055) and the RICE PAC clinical trial in England (NCT03152799).44
To date, one clinical trial of remote ischemic perconditioning treatment in patients with suspected stroke has been conducted in the prehospital setting,45 in which remote ischemic perconditioning was performed before the administration of the first MRI scan. In the single-center study, 18% of patients had a transportation time that was too brief to allow delivery of the full remote ischemic perconditioning protocol. Among 81 patients who had a brain infarction and were randomized to receive remote ischemic perconditioning treatment, 64 patients (79%) were included in the analysis of the primary outcome, and only 33 patients (41%) underwent the 4 planned cycles of cuff inflation. The median NIHSS score (4) was low in the intervention group, and the baseline median brain infarction volume (approximately 1 cm3) was also low. Treatment with remote ischemic perconditioning neither significantly reduced infarction size at 1 month nor improved clinical outcome at 3 months.
Strengths and Limitations
Our study has both strengths and limitations. The strengths are that it was conducted in a multicenter setting, used a blinded end point, ensured that all patients had confirmed (rather than suspected) stroke, and resulted in 94.6% of patients in the intervention group receiving all 4 cycles of cuff inflation. Moreover, the remote ischemic perconditioning treatment was performed between the administration of the 2 MRI scans, and this design allowed us to evaluate the effect of the intervention on brain infarction volume growth.
Our study had several limitations. We only performed 1 follow-up MRI scan of the brain, which was performed soon after the first MRI scan. Therefore, we cannot exclude the possibility that treatment with remote ischemic perconditioning reduced brain infarction volume at later time points (eg, at 1 month after symptom onset). However, previous studies have observed an association between brain infarction volume at 1 day and 1 month.33,46 We chose the primary end point to be the change in infarction volume from baseline to 24 hours after symptom onset for 2 reasons. First, we aimed to minimize the amount of missing data owing to mortality or loss to follow-up. Second, brain imaging after the administration of IV thrombolysis is required; therefore, this research protocol was similar to daily practice, although computed tomographic scanning of the brain is performed more often than MRI scanning.
Conclusions
In our study, treatment with remote ischemic perconditioning, primarily during or after reperfusion therapy, did not reduce brain infarction growth at 24 hours after symptom onset. This result does not support the implementation of a large randomized clinical trial with a functional primary outcome for 1 cycle of remote ischemic perconditioning treatment at hospital admission for patients in the acute phase of brain infarction.
Clinical Trial Protocol
eMethods 1. RESCUE BRAIN Investigators and Contributors
eMethods 2. MRI Protocol
eMethods 3. Statistical Analysis Plan
eTable. Complete-Case Analysis of Primary and Secondary Efficacy Outcomes
eFigure. Subgroups Analyses for the Primary Outcome: Absolute Change in Brain Infarction Volume From Baseline to 24 Hours
Data Sharing Statement
References
- 1.Saver JL, Goyal M, van der Lugt A, et al. ; HERMES Collaborators . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA. 2016;316(12):1279-1288. doi: 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 2.Baron J-C. Protecting the ischaemic penumbra as an adjunct to thrombectomy for acute stroke. Nat Rev Neurol. 2018;14(6):325-337. doi: 10.1038/s41582-018-0002-2 [DOI] [PubMed] [Google Scholar]
- 3.Fisher M, Feuerstein G, Howells DW, et al. ; STAIR Group . Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40(6):2244-2250. doi: 10.1161/STROKEAHA.108.541128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Savitz SI, Baron J-C, Yenari MA, Sanossian N, Fisher M. Reconsidering neuroprotection in the reperfusion era. Stroke. 2017;48(12):3413-3419. doi: 10.1161/STROKEAHA.117.017283 [DOI] [PubMed] [Google Scholar]
- 5.Hess DC, Blauenfeldt RA, Andersen G, et al. Remote ischaemic conditioning—a new paradigm of self-protection in the brain. Nat Rev Neurol. 2015;11(12):698-710. doi: 10.1038/nrneurol.2015.223 [DOI] [PubMed] [Google Scholar]
- 6.Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88-94. doi: 10.1016/j.brainres.2009.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hahn CD, Manlhiot C, Schmidt MR, Nielsen TT, Redington AN. Remote ischemic per-conditioning: a novel therapy for acute stroke? Stroke. 2011;42(10):2960-2962. doi: 10.1161/STROKEAHA.111.622340 [DOI] [PubMed] [Google Scholar]
- 8.Hoda MN, Bhatia K, Hafez SS, et al. Remote ischemic perconditioning is effective after embolic stroke in ovariectomized female mice. Transl Stroke Res. 2014;5(4):484-490. doi: 10.1007/s12975-013-0318-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoda MN, Siddiqui S, Herberg S, et al. Remote ischemic perconditioning is effective alone and in combination with intravenous tissue-type plasminogen activator in murine model of embolic stroke. Stroke. 2012;43(10):2794-2799. doi: 10.1161/STROKEAHA.112.660373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Botker HE, Kharbanda R, Schmidt MR, et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375(9716):727-734. doi: 10.1016/S0140-6736(09)62001-8 [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy DJ, Kharbanda RK, Moller UK, et al. ; CONDI-2/ERIC-PPCI Investigators . Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet. 2019;394(10207):1415-1424. doi: 10.1016/S0140-6736(19)32039-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England TJ, Hedstrom A, O’Sullivan S, et al. RECAST (Remote Ischemic Conditioning After Stroke Trial): a pilot randomized placebo controlled phase II trial in acute ischemic stroke. Stroke. 2017;48(5):1412-1415. doi: 10.1161/STROKEAHA.116.016429 [DOI] [PubMed] [Google Scholar]
- 13.Hansson L, Hedner T, Dahlof B. Prospective randomized open blinded end-point (PROBE) study. a novel design for intervention trials. prospective randomized open blinded end-point. Blood Press. 1992;1(2):113-119. doi: 10.3109/08037059209077502 [DOI] [PubMed] [Google Scholar]
- 14.Pico F, Rosso C, Meseguer E, et al. A multicenter, randomized trial on neuroprotection with remote ischemic per-conditioning during acute ischemic stroke: the REmote iSchemic Conditioning in acUtE BRAin INfarction study protocol. Int J Stroke. 2016;11(8):938-943. doi: 10.1177/1747493016660098 [DOI] [PubMed] [Google Scholar]
- 15.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline. Guideline for Good Clinical Practice E6 (R1). https://apps.who.int/medicinedocs/documents/s22154en/s22154en.pdf
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb perconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42(5):1387-1391. doi: 10.1161/STROKEAHA.110.605840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hess DC, Hoda MN, Bhatia K. Remote limb perconditioning [corrected] and postconditioning: will it translate into a promising treatment for acute stroke? Stroke. 2013;44(4):1191-1197. doi: 10.1161/STROKEAHA.112.678482 [DOI] [PubMed] [Google Scholar]
- 19.Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi: 10.1161/STR.0b013e318284056a [DOI] [PubMed] [Google Scholar]
- 20.Powers WJ, Derdeyn CP, Biller J, et al. ; American Heart Association Stroke Council . 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(10):3020-3035. doi: 10.1161/STR.0000000000000074 [DOI] [PubMed] [Google Scholar]
- 21.Luby M, Bykowski JL, Schellinger PD, Merino JG, Warach S. Intra- and interrater reliability of ischemic lesion volume measurements on diffusion-weighted, mean transit time and fluid-attenuated inversion recovery MRI. Stroke. 2006;37(12):2951-2956. doi: 10.1161/01.STR.0000249416.77132.1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hirel C, Nighoghossian N, Leveque Y, et al. Verbal and musical short-term memory: variety of auditory disorders after stroke. Brain Cogn. 2017;113:10-22. doi: 10.1016/j.bandc.2017.01.003 [DOI] [PubMed] [Google Scholar]
- 23.Savitz SI, Lew R, Bluhmki E, Hacke W, Fisher M. Shift analysis versus dichotomization of the modified Rankin scale outcome scores in the NINDS and ECASS-II trials. Stroke. 2007;38(12):3205-3212. doi: 10.1161/STROKEAHA.107.489351 [DOI] [PubMed] [Google Scholar]
- 24.Hacke W, Kaste M, Bluhmki E, et al. ; ECASS Investigators . Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008;359(13):1317-1329. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 25.Rosso C, Corvol J-C, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43(9):2343-2349. doi: 10.1161/STROKEAHA.112.657122 [DOI] [PubMed] [Google Scholar]
- 26.Rosso C, Attal Y, Deltour S, et al. Hyperglycemia and the fate of apparent diffusion coefficient-defined ischemic penumbra. AJNR Am J Neuroradiol. 2011;32(5):852-856. doi: 10.3174/ajnr.A2407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho K-H, Kwon SU, Lee DH, et al. Early infarct growth predicts long-term clinical outcome after thrombolysis. J Neurol Sci. 2012;316(1-2):99-103. doi: 10.1016/j.jns.2012.01.015 [DOI] [PubMed] [Google Scholar]
- 28.Liang K-Y, Zeger SL. Longitudinal data analysis of continuous and discrete responses for pre-post designs. Sankhya. 2000;62(1):134-148. [Google Scholar]
- 29.Liu GF, Lu K, Mogg R, Mallick M, Mehrotra DV. Should baseline be a covariate or dependent variable in analyses of change from baseline in clinical trials? Stat Med. 2009;28(20):2509-2530. doi: 10.1002/sim.3639 [DOI] [PubMed] [Google Scholar]
- 30.Nogueira RG, Jadhav AP, Haussen DC, et al. ; DAWN Trial Investigators . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11-21. doi: 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 31.Wintermark M, Albers GW, Broderick JP, et al. ; Stroke Imaging Research (STIR) and Virtual International Stroke Trials Archive (VISTA)–Imaging Investigators . Acute stroke imaging research roadmap II. Stroke. 2013;44(9):2628-2639. doi: 10.1161/STROKEAHA.113.002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warach SJ, Luby M, Albers GW, et al. ; Stroke Imaging Research (STIR) and VISTA-Imaging Investigators . Acute stroke imaging research roadmap III imaging selection and outcomes in acute stroke reperfusion clinical trials: consensus recommendations and further research priorities. Stroke. 2016;47(5):1389-1398. doi: 10.1161/STROKEAHA.115.012364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim SM, Kwon SU, Kim JS, Kang D-W. Early infarct growth predicts long-term clinical outcome in ischemic stroke. J Neurol Sci. 2014;347(1-2):205-209. doi: 10.1016/j.jns.2014.09.048 [DOI] [PubMed] [Google Scholar]
- 34.Whitehead J, Bolland K, Valdes-Marquez E, Lihic A, Ali M, Lees K; Virtual International Stroke Trials Archive Collaborators . Using historical lesion volume data in the design of a new phase II clinical trial in acute stroke. Stroke. 2009;40(4):1347-1352. doi: 10.1161/STROKEAHA.108.531442 [DOI] [PubMed] [Google Scholar]
- 35.Chamorro A. Neuroprotectants in the era of reperfusion therapy. J Stroke. 2018;20(2):197-207. doi: 10.5853/jos.2017.02901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Reis C, Applegate R II, Stier G, Martin R, Zhang JH. Ischemic conditioning–induced endogenous brain protection: applications pre-, per- or post-stroke. Exp Neurol. 2015;272:26-40. doi: 10.1016/j.expneurol.2015.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen G, Ye X, Zhang J, et al. Limb remote ischemic postconditioning reduces ischemia-reperfusion injury by inhibiting NADPH oxidase activation and MyD88-TRAF6-P38MAP–kinase pathway of neutrophils. Int J Mol Sci. 2016;17(12):E1971. doi: 10.3390/ijms17121971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zong Y, Jiang L, Zhang M, et al. Limb remote ischemic postconditioning protects cerebral ischemia from injury associated with expression of HIF-1α in rats. BMC Neurosci. 2015;16:97. doi: 10.1186/s12868-015-0235-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ren C, Wang P, Wang B, et al. Limb remote ischemic per-conditioning in combination with post-conditioning reduces brain damage and promotes neuroglobin expression in the rat brain after ischemic stroke. Restor Neurol Neurosci. 2015;33(3):369-379. doi: 10.3233/RNN-140413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neuhaus AA, Couch Y, Hadley G, Buchan AM. Neuroprotection in stroke: the importance of collaboration and reproducibility. Brain. 2017;140(8):2079-2092. doi: 10.1093/brain/awx126 [DOI] [PubMed] [Google Scholar]
- 41.Berkhemer OA, Fransen PSS, Beumer D, et al. ; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11-20. doi: 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 42.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. ; EXTEND-IA Investigators . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372(11):1009-1018. doi: 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 43.Jovin TG, Chamorro A, Cobo E, et al. ; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372(24):2296-2306. doi: 10.1056/NEJMoa1503780 [DOI] [PubMed] [Google Scholar]
- 44.Zhao W, Li S, Ren C, Meng R, Jin K, Ji X. Remote ischemic conditioning for stroke: clinical data, challenges, and future directions. Ann Clin Transl Neurol. 2018;6(1):186-196. doi: 10.1002/acn3.691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hougaard KD, Hjort N, Zeidler D, et al. Remote ischemic perconditioning as an adjunct therapy to thrombolysis in patients with acute ischemic stroke: a randomized trial. Stroke. 2014;45(1):159-167. doi: 10.1161/STROKEAHA.113.001346 [DOI] [PubMed] [Google Scholar]
- 46.Zaidi SF, Aghaebrahim A, Urra X, et al. Final infarct volume is a stronger predictor of outcome than recanalization in patients with proximal middle cerebral artery occlusion treated with endovascular therapy. Stroke. 2012;43(12):3238-3244. doi: 10.1161/STROKEAHA.112.671594 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical Trial Protocol
eMethods 1. RESCUE BRAIN Investigators and Contributors
eMethods 2. MRI Protocol
eMethods 3. Statistical Analysis Plan
eTable. Complete-Case Analysis of Primary and Secondary Efficacy Outcomes
eFigure. Subgroups Analyses for the Primary Outcome: Absolute Change in Brain Infarction Volume From Baseline to 24 Hours
Data Sharing Statement