This randomized clinical trial assesses the effectiveness of 2 interventions to reduce radiation doses in patients undergoing computed tomography (CT).
Key Points
Question
Is there an effective intervention to reduce radiation doses in patients undergoing computed tomography (CT) to enhance patient safety?
Findings
In this randomized clinical trial of 864 080 adults undergoing CT at 100 facilities, audit feedback with tailored suggestions, educational seminars, and sharing of best practices was more effective for reducing the organ doses associated with CT compared with audit feedback alone, and radiologists’ satisfaction with CT image quality was unchanged.
Meaning
Medical imaging facilities can use detailed feedback on CT doses combined with specific suggestions and an educational quality improvement program to reduce radiation doses without compromising the quality of images.
Abstract
Importance
Computed tomography (CT) radiation doses vary across institutions and are often higher than needed.
Objective
To assess the effectiveness of 2 interventions to reduce radiation doses in patients undergoing CT.
Design, Setting, and Participants
This randomized clinical trial included 864 080 adults older than 18 years who underwent CT of the abdomen, chest, combined abdomen and chest, or head at 100 facilities in 6 countries from November 1, 2015, to September 21, 2017. Data analysis was performed from October 4, 2017, to December 14, 2018.
Interventions
Imaging facilities received audit feedback alone comparing radiation-dose metrics with those of other facilities followed by the multicomponent intervention, including audit feedback with targeted suggestions, a 7-week quality improvement collaborative, and best-practice sharing. Facilities were randomly allocated to the time crossing from usual care to the intervention.
Main Outcomes and Measures
Primary outcomes were the proportion of high-dose CT scans and mean effective dose at the facility level. Secondary outcomes were organ doses. Outcomes after interventions were compared with those before interventions using hierarchical generalized linear models adjusting for temporal trends and patient characteristics.
Results
Across 100 facilities, 864 080 adults underwent 1 156 657 CT scans. The multicomponent intervention significantly reduced proportions of high-dose CT scans, measured using effective dose. Absolute changes in proportions of high-dose scans were 1.1% to 7.9%, with percentage reductions in the proportion of high-dose scans of 4% to 30% (abdomen: odds ratio [OR], 0.82; 95% CI, 0.77-0.88; P < .001; chest: OR, 0.92; 95% CI, 0.86-0.99; P = .03; combined abdomen and chest: OR, 0.49; 95% CI, 0.41-0.59; P < .001; and head: OR, 0.71; 95% CI, 0.66-0.76; P < .001). Reductions in the proportions of high-dose scans were greater when measured using organ doses. The absolute reduction in the proportion of high-dose scans was 6.0% to 17.2%, reflecting 23% to 58% reductions in the proportions of high-dose scans across anatomical areas. Mean effective doses were significantly reduced after multicomponent intervention for abdomen (6% reduction, P < .001), chest (4%, P < .001), and chest and abdomen (14%, P < .001) CT scans. Larger reductions in mean organ doses were 8% to 43% across anatomical areas. Audit feedback alone reduced the proportions of high-dose scans and mean dose, but reductions in observed dose were smaller. Radiologist’s satisfaction with CT image quality was unchanged and high during all periods.
Conclusions and Relevance
For imaging facilities, detailed feedback on CT radiation dose combined with actionable suggestions and quality improvement education significantly reduced doses, particularly organ doses. Effects of audit feedback alone were modest.
Trial Registration
ClinicalTrials.gov Identifier: NCT03000751
Introduction
Exposures to ionizing radiation approximately doubled during 3 decades, mainly because of increased use of computed tomography (CT).1,2 Ionizing radiation is a known carcinogen3,4,5,6,7,8,9; an estimated 2% to 5% of cancers in the United States result from medical imaging radiation exposure.10,11 Children exposed to CT have increased leukemia and brain cancer risks,12 and adults have elevated risks of all cancers.12,13 Computed tomography radiation doses are orders of magnitude higher than those of conventional radiography and frequently higher than needed for diagnosis based on dose variations across patients and institutions.14,15,16 Generally, radiation dose and image quality are inversely related, and efforts to reduce doses must consider quality to avoid compromising diagnoses. However, interpopulation dose variations and observational quality improvement (QI) projects14,17,18,19,20 suggest that 50% or greater dose reductions based on recent observed doses do not affect diagnostic accuracy. Optimizing CT doses is a goal of US and European professional societies, oversight organizations, and government programs.21,22
Few CT dose-reducing approaches have been tested across multiple institutions in randomized clinical trials. Most dose-lowering QI studies are small, single center, and observational.17,18,19,20,23,24 Since 2010,25 all CT scanners have been required to report radiation doses using standardized nomenclature, allowing reliable dose assessments and cross-institutional benchmarks.14 We hypothesized that using standardized data to provide institutions with comparative feedback on their CT doses would reduce high-dose examinations, particularly when combined with QI guidance and a forum for best-practice sharing.
This randomized clinical trial compared 2 interventions for optimizing CT doses: single-component audit feedback and multicomponent audit feedback with targeted suggestions for lowering doses plus 7-week QI collaborative with best-practice sharing. The trial sought to assess whether these interventions would be effective for reducing radiation exposure in patients undergoing CT.
Methods
Trial Design
In this randomized clinical trial using a pre-post experiment design (trial protocol in Supplement 1), outcomes were observed at sites first after usual care, second after audit feedback, and third after the multicomponent intervention of audit feedback, QI collaborative, and best-practice sharing (eFigure in Supplement 2). The institutional review boards of participating organizations and the University of California, San Francisco (UCSF) approved the study, including a waiver of informed consent because of the nature of the education and minimal associated risk. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Methods to minimize confounding, particularly from secular changes, were as follows: (1) randomly allocating the timing for sites to cross from usual care to the interventions into 1 of 3 groups; (2) adjusting for a common linear trend in the date of each scan, validated by checking for nonlinearity; (3) adjusting for patient characteristics to control potential confounding due to changes in case mix over time; and (4) including a fixed effect for site in models so that each served as its own control.
Centers
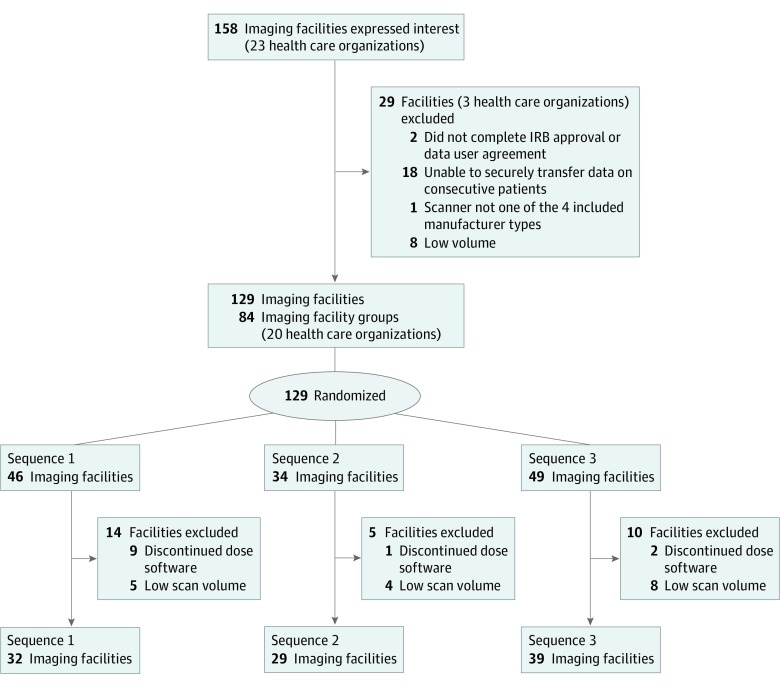
All organizations used the Radimetrics dose-management program (licensed by Bayer AG) throughout the study period, allowing standardized dose data collection. Bayer invited all users by email to contact UCSF staff if interested in participating in the trial (Figure 1), and 158 imaging facilities affiliated with 23 health care organizations in the United States, England, Germany, the Netherlands, Switzerland, and Japan responded. Facilities were eligible if they could complete institutional review board approval and data use agreement (2 facilities excluded), could establish data connections and electronically transfer data on consecutive patients to UCSF (18 excluded), had CT machines from 1 of the 4 largest manufacturers (1 excluded), and contributed a minimum 120 CT scans of included anatomical areas in a 3-month assessment period (8 excluded). A total of 129 imaging facilities were randomized, and 29 were excluded for discontinuing use of the Radimetrics dose-management program or having extremely low scan volumes (suggesting incomplete data submission to UCSF).
Figure 1. CONSORT Flow Diagram.
Imaging facility groups were formed by grouping imaging facilities that shared practices and/or personnel to reduce contamination when randomizing. Members of these facility groups were randomized together. Analysis was at the level of the facility. IRB indicates institutional review board.
Randomization and Masking
The nature of the interventions prevented blinding. We cluster randomized facilities because interventions targeted facilities to ensure all practitioners at a facility simultaneously received interventions and because our intervention shared facility-level results. Facilities for which directors consented to participate were randomly assigned to 1 of 3 sequences for crossing from control to interventions by a biostatistician unaffiliated with the project using matching and rerandomization to balance potential confounders.26,27 We constrained randomization to group facilities by time zone so that webinars were conducted during work hours. Some facilities were highly cointegrated in a health care organization, including sharing staff and management. To reduce between-facility contamination, we assessed facility independence before randomization using a survey about staffing and decision-making. Highly interdependent facilities were randomized together, resulting in 84 randomized imaging-facility groups. Randomization constraints led to unequally sized groups. Because health care organizations were not randomized, the imaging facilities for these organizations followed different sequences.
Patients
Data were from consecutive adults older than 18 years who underwent CT of the abdomen (abdomen and/or pelvis), chest, combined abdomen and chest (abdomen-chest), or head from November 1, 2015, to September 21, 2017, reflecting 87% of the diagnostic CTs during the study period. CT to guide radiation therapy, diagnostic procedures, or combined with positron emission tomography or single-photon emission tomography was excluded. CTs at external facilities but imported to participating facilities were excluded. Data analysis was performed from October 4, 2017, to December 14, 2018.
Data Collection
Radiation dose data on consecutive CT scans during each period were assessed. Data included radiation dose metrics, some information on why the CT scan was obtained, and patient age and sex. Examinations were pooled directly from CT scanners or picture archiving and communication systems of facilities and stored on local servers. Data were stripped of identifiers and submitted in real time to the UCSF International CT Radiation Dose Registry. Preintervention and postintervention surveys asked radiologists and medical technologists the strength of their agreement that their facility’s CT images had good diagnostic quality for the abdomen, chest, and head.
Interventions
Audit feedback was provided to facilities based on doses for consecutive patients receiving CT during a pretrial baseline period (eFigure in Supplement 2). Audits included descriptive tables and figures summarizing doses by anatomical area and scanning reason compared with all other study facilities and facilities with the same scanner make and model. Feedback highlighted modifiable factors (eg, scan length and multiphase studies).
The multicomponent intervention provided audit feedback with actionable dose-lowering suggestions (eAppendix in Supplement 2). For example, reports identified high-dose protocols and provided guidance on modifying technical parameters to match best-performing facilities with the same scanner make and model. Facilities also participated in a 7-week QI collaborative and learning community. Eight webinars covered topics, such as chest CT, with a standard format: 30-minute interactive lecture by a content expert, 30-minute case review, and 30-minute discussion on QI approaches and institution-specific QI projects. Sessions included education, QI approaches, and sharing experiences.28,29,30 Facilities were encouraged to always have the same participants (typically a radiologist and a technologist). Reports were sent to facility lead champions (radiologist, medical physicist, or technologist), who were encouraged to share reports with other facility contributors.
Learning Periods
After each intervention, a 4-week transition allowed facilities to change imaging processes.17 Facility leaders developed individual improvement plans (eg, modifying protocols, using a high-dose protocol less frequently).
Outcomes
Facility outcomes assessed before and after interventions were proportion of high-dose CT scans and mean dose, each stratified by anatomical area. The proportion of high-dose examinations is most relevant for assessing change because high-dose CT scans should be avoided when possible at any facility. Their reduction is therefore a meaningful sign of QI. In contrast, mean doses at a facility may already be optimized; therefore, reduction may not consistently reflect high-quality imaging. Outcomes were measured using effective dose (primary outcome), organ doses, and volume CT dose index (CTDIvol) (secondary outcomes).
Effective dose in millisieverts (mSv) accounts for scanner radiation output, body areas imaged, and sensitivity of irradiated areas to developing cancer from the exposure. Effective dose is a weighted sum of exposures to different body parts that reflects a mean whole-body exposure. Organ dose in milligrays (mGy) is radiation absorbed by an organ and more closely reflects imaged areas (eg, for head CT, brain dose is high but colon dose is negligible). We report lung dose for chest CT, colon for abdomen CT, colon and lung for combined abdomen and chest CT, and brain for head CT. CTDIvol in milligrays measures mean radiation from the scanner for a single section, reflecting parameters such as x-ray tube current chosen by the technologist. The mean CTDIvol does not correlate well with total radiation; it does not increase with craniocaudal length scanned or number of acquisitions, such as scans with and without contrast enhancement in a complete CT study.
For patients with more than 1 acquisition, by convention, effective and organ doses were summed and CTDIvol averaged. Effective dose was calculated from machine-reported dose-length product multiplied by a standard weighting coefficient for scan type.31 Organ doses were estimated using Radimetrics software with Monte Carlo modeling. The Digital Imaging and Communication in Medicine standard as reported for each scan was used for CTDIvol. For each outcome and anatomical area, mean doses and proportion of doses above the 75th percentile of observed doses in the UCSF International CT Radiation Dose Registry during the preintervention period were calculated. For example, for effective doses, doses above the 75th percentile for each anatomical area were considered high (abdomen, 16 mSv; chest, 10 mSv; chest-abdomen, 25 mSv; and head, 2.7 mSv). Data were purposely collected for this study. Radiologists and technologists from imaging facilities were surveyed before and after interventions about image quality satisfaction for abdomen, chest, and head CT.
Timing
Outcomes were calculated for the preintervention period, transition after audit intervention (first learning period), postaudit intervention, transition after multicomponent intervention (second learning period), and postmulticomponent intervention. Doses during postintervention periods were compared with doses before intervention excluding transition data. The 2 interventions were not directly compared. Preintervention and postintervention periods varied with intervention and sequence (eFigure in Supplement 2).
Sample Size
Sample size calculations used R statistical software (R Foundation for Statistical Computing), with a 2-sided α = .05, within-cluster coefficient of variation ranging from 0.60 to 0.85, and intracluster correlation ranging from 0.16 to 0.32, depending on the anatomical area. Values roughly reflected the first and third quartiles of coefficient of variations from preliminary subpopulation studies of facility groups. We were powered to detect a 5–percentage point decrease in the proportion of patients undergoing high-dose CT examinations. We estimated that with 84 facility clusters (facilities or groups of facilities) randomized into 3 (approximately equal) sequences, a sample of 30 examinations per cluster per measurement period and anatomical area would achieve at least 80% statistical power per intervention.
Statistical Analysis
All analyses were at the level of complete CT study. Descriptive statistics were calculated during each period. For each anatomical area and dose metric, we modeled the proportion of high-dose examinations using hierarchical logistic regression and change in mean dose using hierarchical linear regression. Each intervention and learning period were fixed effects with the preintervention period as the reference. Examination date, coded as days since study start, was included to account for time trends. Random effects for CT scanner, facility, and facility cluster accounted for correlation among scans performed within clusters. Models adjusted for facility-level characteristics used in randomization and patient-level covariates that might influence dose (patient age, sex, and scanned region diameter measured on CT) as fixed effects. Medians and 75th percentiles in dose over time compared with before intervention were graphed by week before or after interventions. Fisher exact tests were used to detect changes in practitioner (radiologist or technologist) satisfaction with image quality using 2-sided significance testing with P < .05 considered to be statistically significant.
Results
We randomized 129 imaging facilities to 1 of 3 sequences (Figure 1). After excluding 29 facilities for low scan volume or discontinued use of dose-monitoring software, data were available from 100 facilities. Across facilities, 864 080 adults underwent 1 156 657 CT examinations. Exclusion of scans obtained during transition periods yielded 978 884 scans from 745 802 patients for inclusion in analyses, with no meaningful differences in sex, age, patient size, or CT scan type during preintervention and postintervention periods (Table 1).
Table 1. Distribution of Patient Variables and CT Scans During Trial Periodsa.
| Variable | CT Scans | |||
|---|---|---|---|---|
| Total No. (%) | Intervention Periods | |||
| Baseline | After Intervention | |||
| Simple Audit | Multicomponent Intervention | |||
| Total | 978 884 | 526 439 (54) | 251 409 (26) | 207 576 (21) |
| Sex | ||||
| Female | 521 701 (53) | 278 841 (53) | 132 772 (53) | 110 088 (53) |
| Male | 463 723 (47) | 247 598 (47) | 118 637 (47) | 97 488 (47) |
| Age distribution, y | ||||
| 18-29 | 73 881 (7) | 40 022 (8) | 18 468 (7) | 15 391 (7) |
| 30-39 | 84 693 (9) | 45 880 (9) | 21 198 (8) | 17 615 (9) |
| 40-49 | 118 142 (12) | 64 299 (12) | 29 492 (12) | 24 351 (12) |
| 50-59 | 182 997 (19) | 98 457 (19) | 46 589 (19) | 37 951 (18) |
| 60-69 | 212 418 (22) | 114 161 (22) | 54 155 (22) | 44 102 (21) |
| 70-79 | 177 416 (18) | 92 374 (18) | 45 969 (18) | 39 073 (19) |
| ≥80 | 135 877 (14) | 71 246 (14) | 35 538 (14) | 29 093 (14) |
| Type of scan | ||||
| Abdomen | 345 273 (35) | 185 671 (35) | 87 518 (35) | 72 084 (35) |
| Chest | 269 208 (27) | 143 120 (27) | 69 678 (28) | 56 410 (27) |
| Combined abdomen and chest | 59 889 (6) | 30 689 (6) | 14 784 (6) | 14 416 (7) |
| Head | 311 054 (32) | 166 959 (32) | 79 429 (32) | 64 666 (31) |
| Age, mean (SD), y | ||||
| Abdomen | NA | 56.0 (18.2) | 56.3 (18.3) | 56.4 (18.3) |
| Chest | NA | 61.6 (15.3) | 62.1 (15.2) | 62.0 (15.2) |
| Combined abdomen and chest | NA | 62.5 (15.3) | 62.8 (15.5) | 63.4 (15.4) |
| Brain | NA | 60.1 (19.9) | 60.7 (19.9) | 60.6 (19.9) |
| Diameter, mean (SD), cm | ||||
| Abdomen | NA | 30.7 (4.6) | 30.8 (4.7) | 30.9 (4.7) |
| Chest | NA | 28.9 (4.9) | 29.0 (4.9) | 28.9 (5.1) |
| Combined abdomen and chest | NA | 29.7 (4.1) | 29.5 (4.2) | 29.7 (4.2) |
| Brain | NA | 17.1 (1.5) | 17.1 (1.5) | 17.2 (1.5) |
Abbreviations: CT, computed tomography; NA, not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated.
Change in Proportion of High Radiation Doses After Multicomponent Intervention
Proportions of high-dose examinations measured using effective dose were significantly reduced across anatomical areas after the multicomponent intervention (Table 2). Absolute change in the proportion of high-dose examinations was 1.1% to 7.9%, with a corresponding percentage reduction in the proportion of high-dose examinations of 4% to 30% (Figure 2). For example, abdomen CT scans demonstrated a reduction of 2.4% (10% reduction in proportion of high-dose examinations; odds ratio [OR], 0.82; 95% CI, 0.77-0.88; P < .001). Combined abdomen and chest CT scans had the largest change with a reduction of 7.9% (30% high-dose reduction; OR, 0.49; 95% CI, 0.41-0.59; P < .001). Although all changes were statistically significant, reductions in the proportion of high-dose examinations measured by effective dose exceeded the 5% used to assess statistical power only for combined abdomen and chest CT scans.
Table 2. Changes in the Proportion of High-Dose Studies (Proportion Over Benchmark) After Interventions by Anatomical Area for Effective Dose, Organ Dose, and CTDIvola.
| Variable | Proportion Over Benchmark Before Intervention | After Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Audit Alone | Multicomponent Intervention | ||||||||
| Proportion Over Benchmark | Change in Proportion Over Benchmark Absolute (%) | OR (95% CI) | P Value | Proportion Over Benchmark | Change in Proportion Over Benchmark Absolute (%) | OR (95% CI) | P Value | ||
| Effective dose | |||||||||
| Abdomen | 24.7 | 24.7 | 0.0 (0) | 1.00 (0.96-1.05) | .86 | 22.2 | −2.4 (−10) | 0.82 (0.77-0.88) | <.001 |
| Chest | 26 | 25.5 | −0.5 (−2) | 0.96 (0.90-0.98) | .10 | 24.9 | −1.1 (−4) | 0.92 (0.86-0.99) | .03 |
| Combined abdomen and chest | 26.7 | 23 | −3.7 (−14) | 0.73 (0.66-0.81) | <.001 | 18.8 | −7.9 (−30) | 0.49 (0.41-0.59) | <.001 |
| Head | 26.2 | 26 | −0.2 (−1) | 0.99 (0.90-0.97) | .50 | 21.8 | −4.4 (−17) | 0.71 (0.66-0.76) | <.001 |
| Organ doses | |||||||||
| Abdomenb | 24.9 | 22.6 | −2.3 (−9) | 0.84 (0.81-0.88) | <.001 | 18.0 | −6.9 (−28) | 0.59 (0.55-0.63) | <.001 |
| Chestc | 25.6 | 22.5 | −3.1 (−12) | 0.80 (0.77-0.84) | <.001 | 19.7 | −6.0 (−23) | 0.65 (0.60-0.69) | <.001 |
| Combined abdomen and chestc | 30.2 | 20.8 | −9.4 (−31) | 0.48 (0.43-0.53) | <.001 | 13.3 | −17.2 (−57) | 0.23 (0.19-0.27) | <.001 |
| Combined abdomen and chestb | 28.3 | 13.6 | −14.7 (−52) | 0.29 (0.26-0.33) | <.001 | 12.0 | −16.3 (−58) | 0.25 (0.21-0.30) | <.001 |
| Headd | 27.5 | 25.6 | −1.9 (−7) | 0.88 (0.85-0.92) | <.001 | 21.0 | −6.5 (−24) | 0.62 (0.59-0.67) | <.001 |
| CTDIvol | |||||||||
| Abdomen | 24.5 | 24.9 | 0.4 (2) | 1.04 (0.99-1.09) | .08 | 24.1 | −0.4 (−2) | 0.96 (0.89-1.03) | .25 |
| Chest | 26 | 25.8 | −0.2 (−1) | 0.98 (0.94-1.03) | .44 | 24.1 | −2.0 (−8) | 0.85 (0.79-0.92) | <.001 |
| Combined abdomen and chest | 23.6 | 22.7 | −0.9 (−4) | 0.90 (0.79-1.03) | .12 | 19.9 | −3.7 (−16) | 0.64 (0.51-0.80) | <.001 |
| Head | 24.4 | 25.0 | 0.6 (2) | 1.07 (1.02-1.13) | .007 | 17.9 | −6.5 (−27) | 0.44 (0.40-0.48) | <.001 |
Abbreviations: CTDIvol, volume computed tomographic dose index; OR, odds ratio.
Results are after audit alone and after complex intervention relative to before intervention adjusting for temporal trends and patient and facility characteristics by anatomical area.
Colon organ doses.
Lung organ doses.
Brain organ doses.
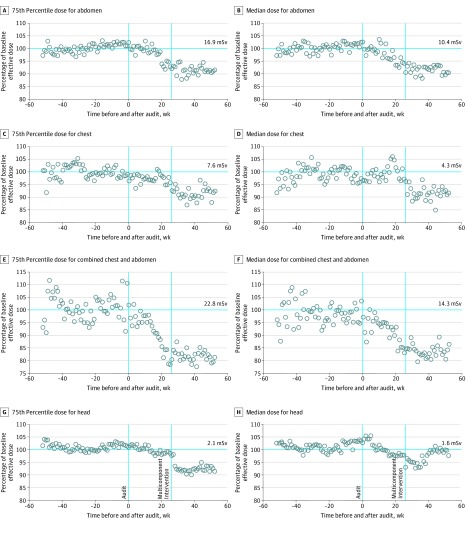
Figure 2. Effective Dose Compared With Preaudit Baseline.
Effective dose by week before or after the audit, shown as the percentage of the 75th percentile or median dose at pre-audit baseline dose. Numbers on the horizontal cyan lines in A, C, E, and G represent the 75th percentile in dose at baseline and in B, D, F, and H represent the 50th percentile in dose at baseline. Each circle reflects the 75th percentile dose (A, C, E, G) or the median dose (B, D, F, H) relative to the pre-audit baseline values across all imaging facilities. Doses equal to the baseline dose are 100%. Values less than 100% indicate the relative decrease in dose.
Reductions in the proportions of high-dose examinations after the multicomponent intervention were greater when measured using organ doses. Absolute reduction in the proportion of high-dose examinations was 6.0% to 17.2% (all above the 5% used to assess power), reflecting a 23% to 57% reduction in the proportion of high-dose examinations across anatomical areas. For example, at baseline, 24.9% of abdomen CT scans had high organ doses, whereas after the multicomponent intervention 18.0% did, reflecting a 28% reduction in the proportion of high colon doses.
Reductions in the proportions of high-dose examinations after the multicomponent intervention were smaller when measured using CTDIvol. High-dose examinations defined using CTDIvol decreased by 0.4% to 6.5% after multicomponent intervention, reflecting a 2% to 27% reduction across anatomical areas (Table 2). Reductions were statistically significant for chest (OR, 0.85; 95% CI, 0.79-0.92; P < .001), combined abdomen and chest (OR, 0.64; 95% CI, 0.51-0.80; P < .001), and head (OR, 0.44; 95% CI, 0.40-0.48; P < .001) CT scans.
Change in Proportion of High Radiation Doses After Audit Alone
Audit feedback alone reduced the proportions of high-dose examinations but with smaller magnitudes and significance for only some comparisons (Table 2). For effective dose, the absolute change in the proportion of high-dose CTs was 0.0 to 3.7 mSv, a 0% to 14% reduction in the proportion of high-dose examinations across anatomical areas. The change was statistically significant only for combined abdomen and chest CT scans (reduction of 3.7 mSv, 14%; OR, 0.73; 95% CI, 0.66-0.81; P < .001). For organ doses, absolute change in proportion of high-dose CT scans was 2.3% to 14.7% (9%-52% reduction in proportion of high-dose examinations) and was statistically significant for all anatomical areas (abdomen: OR, 0.84; 95% CI, 0.81-0.88; P < .001; chest: OR, 0.80; 95% CI, 0.77-0.84; P < .001; chest and abdomen [lung organ doses]: OR, 0.48; 95% CI, 0.43 to 0.53; P < .001; chest and abdomen [colon organ doses]: OR, 0.29; 95% CI, 0.26-0.33; P < .001; and head: OR, 0.88; 95% CI, 0.85-0.92; P < .001). There was little consistent change in high-dose examinations after audit, measured using CTDIvol.
Change in Mean Radiation Doses After Multicomponent Intervention
Mean effective doses were reduced across anatomical areas after the multicomponent intervention (Table 3). Reduction in mean effective dose was 0.05 to 2.0 mSv, corresponding to a 3% to 14% reduction. For example, abdomen CT scans demonstrated a mean change of −0.81 mSv (95% CI, −0.99 to −0.64 mSv), a 6% reduction. Combined chest and abdomen CT scans had a change of −2.04 mSv (95% CI, −2.55 to −1.53 mSv), a 14% reduction. The magnitude of changes to mean effective dose after multicomponent intervention were attenuated compared with changes in the proportion of high-dose examinations.
Table 3. Changes in Mean Dose After Interventions by Anatomical Area for Effective Dose, CTDIvol, and Organ Dosea.
| Variable | Mean Dose Before Intervention, mSv | After Intervention | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Audit Alone | Multicomponent Intervention | ||||||||
| Mean Dose After Audit | Change in Mean Dose (95% CI), mSv | Change, % | P Value | Mean Dose After Complex Intervention | Change in Mean Dose (95% CI), mSv or mGy | Change, % | P Value | ||
| Effective dose, mSv | |||||||||
| Abdomen | 14.7 | 14.6 | −0.13 (−0.24 to −0.02) | −1 | .02 | 13. 9 | −0.81 (−0.99 to −0.64) | −6 | <.001 |
| Chest | 7.1 | 7.0 | −0.14 (−0.23 to −0.05) | −2 | .002 | 6.8 | −0.26 (−0.40 to −0.11) | −4 | <.001 |
| Chest and abdomen | 14.9 | 14.0 | −0.88 (−1.18 to −0.57) | −6 | <.001 | 12.9 | −2.04 ( −2.55 to −1.53) | −14 | <.001 |
| Head | 1.7 | 1.7 | −0.01 (−0.06 to 0.04) | −1 | .68 | 1.7 | −0.05 (−0.13 to 0.02) | −3 | .18 |
| Organ doses, mGy | |||||||||
| Abdomenb | 62.6 | 56.6 | −6.0 (−6.9 to −5.0) | −10 | <.001 | 50.4 | −12.2 (−13.8 to −10.6) | −20 | <.001 |
| Chestc | 29.3 | 27.2 | −2.1 (−2.8 to −1.5) | −7 | <.001 | 25.6 | −3.7 (−4.7 to −2.7) | −13 | <.001 |
| Chest and abdomenc | 70.7 | 54.7 | −16.0 (−18.2 to −13.8) | −23 | <.001 | 40.6 | −30.1 (−33.8 to −26.4) | −43 | <.001 |
| Chest and abdomenb | 42.1 | 29.1 | −13.0 (−14.5 to −11.6) | −31 | <.001 | 24.5 | −17.6 (−20.0 to −15.1) | −42 | <.001 |
| Headd | 89.3 | 86.4 | −2.9 (−6.4 to 0.70) | −3 | .11 | 81.8 | −7.5 (−13.3 to −1.7) | −8 | .01 |
| CTDIvol, mGy | |||||||||
| Abdomen | 14.7 | 14.8 | 0.09 (0.01 to 0.16) | 0.6 | .03 | 14.7 | 0.03 (−0.10 to 0.15) | 0.2 | .68 |
| Chest | 11.6 | 11.6 | 0.04 (−0.07 to 0.14) | 0.3 | .50 | 11.5 | −0.11 (−0.29 to 0.06) | −1 | .20 |
| Chest and abdomen | 10.7 | 10.7 | −0.05 (−0.18 to 0.08) | −0.4 | .48 | 10.3 | −0.42 (−0.64 to −0.20) | −4 | <.001 |
| Head | 38.6 | 39.0 | 0.44 (0.27 to 0.62) | 1.1 | <.001 | 38.0 | −0.56 (−0.85 to −0.27) | −1 | <.001 |
Abbreviations: CTDIvol, volume computed tomographic dose index; mGy, milligrays; mSv, millisieverts.
Results are after audit alone and after complex multicomponent intervention relative to before intervention adjusting for temporal trends and patient and facility characteristics by anatomical area.
Colon organ doses.
Lung organ doses.
Brain organ doses.
Changes in mean radiation doses after the multicomponent intervention were greater when measured using organ doses (Table 3). Reductions in mean effective dose were 3.7 to 30.1 mGy per examination, reflecting a 13% to 43% statistically significant change in mean organ dose across anatomical areas (abdomen [colon organ doses]: change, −12.2 mGy; 95% CI, −13.8 to −10.6 mGy; −20% change; P < .001; chest [lung organ doses]: −3.7 mGy; 95% CI, −4.7 to −2.7 mGy; change, −13%; P < .001; chest and abdomen [lung organ doses]: −30.1 mGy; 95% CI, −33.8 to −26.4 mGy; change, −43%; P < .001; chest and abdomen [colon organ doses]: −17.6 mGy; 95% CI, −20.0 to −15.1 mGy; change, −42%; P < .001; and head [brain organ doses]: −7.5 mGy; 95% CI, −13.3 to −1.7 mGy; change, −8%; P = .01).
The largest reduction in mean effective dose was for combined abdomen and chest CT—a 30-mGy reduction in organ doses to lungs (43% reduction) and 17.6-mGy reduction to colon (42% reduction). The magnitude of change to mean organ dose was attenuated compared with changes in the proportion of high-dose examinations after the multicomponent intervention. Changes in mean CTDIvol after the multicomponent intervention were small when measured using organ doses.
Change in Mean Radiation Doses After Audit Alone
Audit feedback alone reduced mean doses but with smaller magnitudes than the multicomponent intervention and with statistical significance only for some comparisons. With effective dose to measure change, mean dose was reduced 0.01 to 0.88 mSv (1%-6% reduction). With organ doses, mean doses were reduced 2.1 to 16.0 mGy after audit, for a 7% to 23% change (abdomen [colon organ doses]: −6 mGy; 95% CI, −6.9 to −5.0 mGy; change, −10%; P < .001; chest [lung organ doses]: −2.1 mGy; 95% CI, −2.8 to −1.5 mGy; change, −7%; P < .001; chest-abdomen [lung organ doses]: −16.0 mGy; 95% CI, −18.2 to −13.8 mGy; change, −23%; P < .001; chest and abdomen [colon organ doses]: −13.0 mGy; 95% CI, −14.5 to −11.6 mGy; change, −31%; P < .001; and head [brain organ doses]: −2.9; 95% CI, −6.4 to 0.7; change, −3%; P = .11).
With CTDIvol, mean dose changes were 0.05 reduction to 0.09 increase for changes of 0.6% decrease to 1.1% increase. Median effective dose and 75th percentile for effective dose across the study were reduced after interventions (Figure 2).
Image Quality
Surveys were from 88 facilities at baseline and 63 after multicomponent intervention. Before and after intervention, most respondents reported acceptable image quality for abdomen CT (94% before and 95% after intervention), chest CT (93% before and 95% after intervention), and head CT (92% before and 92% after intervention).
Discussion
Providing detailed feedback to imaging facilities on their CT doses combined with a forum for sharing best practices and a collaborative learning community significantly reduced proportions of high-dose examinations. Our findings suggest that CT radiation doses can be reduced through simple, inexpensive interventions without reducing radiologist satisfaction with image quality.
Reductions in high-dose CT examinations after the multicomponent intervention varied with dose measures. Effective dose, the primary outcome, reflects total radiation received by patients and future risk of all cancer types. Effective dose allows comparison across CT types, imaging modalities, and nonmedical radiation sources. However, because it averages exposures across the body, including nonimaged areas, it dilutes exposure to imaged areas. Organ doses more directly reflect total absorbed dose by scanned areas and future cancer risk to those organs and showed the greatest magnitude of change. The use of lower-dose CT techniques (protocols) will result in larger dose reductions to exposed organs but smaller reductions in effective dose. Effective dose is essentially a watered-down organ dose because its calculation includes nonimaged areas. We believe the larger effects seen with organ doses are clinically important.
Changes in effective dose were statistically significant, but are they meaningful? We believe that even a small absolute reduction in high-dose studies is clinically important because based on 85 million annual CT scans in the United States, if all imaging facilities performed 1% fewer high-dose CT examinations, 212 500 people would avoid receiving a high CT dose annually. High radiation exposures are usually unnecessary; thus, even small reductions in percentages of high-dose examinations are clinically meaningful. To put this in context, we estimated the number of cancers avoided if all imaging centers matched our results after the multicomponent intervention, even though cancer estimation models are inexact and sometimes considered conservative.11 On the basis of number of annual CT scans, distribution in scan types (Table 1), observed doses and dose reductions in this study, and risk of radiation-induced cancer associated with these examinations,11 the dose reductions that we observed could prevent approximately 6000 cancers annually in the United States.
Our interventions were simple audit feedback and a more intensive, multicomponent intervention that reflects an educational QI program. Audit reports prompted staff discussions about strategies and techniques to improve CT doses. The multicomponent intervention added specific recommendations for matching the performance of facilities with lower mean doses, education on optimal approaches, guidance on QI methods for organizational change, and a forum for sharing best practices and experiences implementing changes. Best performers described how they achieved goals, gave low performers concrete steps for effective action, and highlighted successful improvement. Our results support that information alone helps change behavior, but an organizational approach to quality improvement that affected systems, workflows, and processes has a greater impact.
A full discussion of intervention effects is beyond this article’s scope and strategies that lowered doses varied across sites. Nonetheless, we can make high-level points. For example, imaging facilities lowered doses for combined chest and abdomen scanning primarily by reducing numbers of acquisitions. In contrast, head CT typically uses a single acquisition, and reduced doses resulted from lowering acquisition settings. The primary conclusion—that interventions reduced CT radiation dose levels—is supported by all dose metrics and anatomical areas scanned.
The goal of dose optimization is appropriate radiation doses—not the lowest possible dose but the lowest dose needed to answer clinical questions. Proportions of high-dose examinations demonstrated greater reductions after interventions than mean doses. This finding was expected because many patients received appropriate doses, which should not be reduced. Instead, the interventions aimed to reduce doses that were unnecessarily high for clinical questions.
This trial to test interventions to lower CT radiation had geographic, socioeconomic, and racial/ethnic diversity, and facilities used diverse equipment from the 4 largest manufacturers. The sample of approximately 1 million CTs allowed detection of clinically meaningful changes. These features ensure generalizability to imaging facilities worldwide.
Radiologists who interpret images ensure their quality meets diagnostic needs. No accepted objective measure of image quality exists to date, but most imaging facilities completed surveys in which radiologists reported uniformly high satisfaction with image quality.
Limitations
Study limitations include that the multicomponent intervention occurred after simple audit; thus, some reductions could reflect its delayed effect. All participants volunteered, were using dose-management software, and were possibly committed to optimizing CT doses.
Conclusions
Exposure to unnecessary radiation from medical imaging is a quality and safety issue. Our results demonstrate that reviewing audit reports of doses and participating in a learning community can reduce radiation doses from CT to patients.
Trial Protocol
eFigure. Study Timeline
eAppendix. Sample Detailed Audit Report
Data Sharing Statement
References
- 1.National Council on Radiation Protection and Measurements Ionizing Radiation Exposure of the Population of the United States, 2009. Bethesda, MD: National Council on Radiation Protection and Measurements; 2009. NCRP report 160. [Google Scholar]
- 2.Medical Radiation Exposure of the European Population: Part 1/2. Luxenbourg, Sweden: Luxenbourg Publications Office of the European Union; 2014. European Commission Medical Protection No. 180.
- 3.Board of Radiation Effects Research Division on Earth and Life Sciences National Research Council of the National Academies Health Risks From Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. Washington, DC: National Academies Press; 2006. [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer A Review of Human Carcinogenesis. Lyon, France: International Agency for Research on Cancer; 2012:100D:7-303. IARC monographs on the evaluation of carcinogenic risks to humans. [PMC free article] [PubMed] [Google Scholar]
- 5.Preston DL, Ron E, Tokuoka S, et al. Solid cancer incidence in atomic bomb survivors: 1958-1998. Radiat Res. 2007;168(1):1-64. doi: 10.1667/RR0763.1 [DOI] [PubMed] [Google Scholar]
- 6.Berrington de Gonzalez A, Salotti JA, McHugh K, et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br J Cancer. 2016;114(4):388-394. doi: 10.1038/bjc.2015.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson DB, Cardis E, Daniels RD, et al. Risk of cancer from occupational exposure to ionising radiation: retrospective cohort study of workers in France, the United Kingdom, and the United States (INWORKS). BMJ. 2015;351:h5359. doi: 10.1136/bmj.h5359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kamiya K, Ozasa K, Akiba S, et al. Long-term effects of radiation exposure on health. Lancet. 2015;386(9992):469-478. doi: 10.1016/S0140-6736(15)61167-9 [DOI] [PubMed] [Google Scholar]
- 9.Leuraud K, Richardson DB, Cardis E, et al. Ionising radiation and risk of death from leukaemia and lymphoma in radiation-monitored workers (INWORKS): an international cohort study. Lancet Haematol. 2015;2(7):e276-e281. doi: 10.1016/S2352-3026(15)00094-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brenner DJ, Hall EJ. Computed tomography: an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149 [DOI] [PubMed] [Google Scholar]
- 11.Berrington de González A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Intern Med. 2009;169(22):2071-2077. doi: 10.1001/archinternmed.2009.440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499-505. doi: 10.1016/S0140-6736(12)60815-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shuryak I, Sachs RK, Brenner DJ. Cancer risks after radiation exposure in middle age. J Natl Cancer Inst. 2010;102(21):1628-1636. doi: 10.1093/jnci/djq346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith-Bindman R, Wang Y, Chu P, et al. International variation in radiation dose for computed tomography examinations: prospective cohort study. BMJ. 2019;364:k4931. doi: 10.1136/bmj.k4931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med. 2009;169(22):2078-2086. doi: 10.1001/archinternmed.2009.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Commission Diagnostic Reference Levels in Thirty-six European Countries Part 2/2. Brussels, Belgium: Publications Office of the European Union; 2014. [Google Scholar]
- 17.Demb J, Chu P, Nelson T, et al. Optimizing radiation doses for computed tomography across institutions: dose auditing and best practices. JAMA Intern Med. 2017;177(6):810-817. doi: 10.1001/jamainternmed.2017.0445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tack D, Jahnen A, Kohler S, et al. Multidetector CT radiation dose optimisation in adults: short- and long-term effects of a clinical audit. Eur Radiol. 2014;24(1):169-175. doi: 10.1007/s00330-013-2994-8 [DOI] [PubMed] [Google Scholar]
- 19.Goenka AH, Dong F, Wildman B, Hulme K, Johnson P, Herts BR. CT Radiation dose optimization and tracking program at a large quaternary-care health care system. J Am Coll Radiol. 2015;12(7):703-710. doi: 10.1016/j.jacr.2015.03.037 [DOI] [PubMed] [Google Scholar]
- 20.Seuri R, Rehani MM, Kortesniemi M. How tracking radiologic procedures and dose helps: experience from Finland. AJR Am J Roentgenol. 2013;200(4):771-775. doi: 10.2214/AJR.12.10112 [DOI] [PubMed] [Google Scholar]
- 21.Rehani MM. ICRP and IAEA actions on radiation protection in computed tomography. Ann ICRP. 2012;41(3-4):154-160. doi: 10.1016/j.icrp.2012.06.029 [DOI] [PubMed] [Google Scholar]
- 22.Hricak H, Brenner DJ, Adelstein SJ, et al. Managing radiation use in medical imaging: a multifaceted challenge. Radiology. 2011:258(3):889-905. doi: 10.1148/radiol.10101157 [DOI] [PubMed] [Google Scholar]
- 23.Miglioretti DL, Zhang Y, Johnson E, et al. Personalized technologist dose audit feedback for reducing patient radiation exposure from CT. J Am Coll Radiol. 2014;11(3):300-308. doi: 10.1016/j.jacr.2013.10.017 [DOI] [PubMed] [Google Scholar]
- 24.Raff GL, Chinnaiyan KM, Share DA, et al. ; Advanced Cardiovascular Imaging Consortium Co-Investigators . Radiation dose from cardiac computed tomography before and after implementation of radiation dose-reduction techniques. JAMA. 2009;301(22):2340-2348. doi: 10.1001/jama.2009.814 [DOI] [PubMed] [Google Scholar]
- 25.Medical Imaging and Technology Alliance Nation’s CT Manufacturers Unveil New Industry-Wide Medical Radiation Patient Safety Features. Arlington, VA: Medical Imaging and Technology Alliance; 2010. [Google Scholar]
- 26.Greevy RA Jr, Grijalva CG, Roumie CL, et al. Reweighted Mahalanobis distance matching for cluster-randomized trials with missing data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 2):148-154. doi: 10.1002/pds.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greevy R, Lu B, Silber JH, Rosenbaum P. Optimal multivariate matching before randomization. Biostatistics. 2004;5(2):263-275. doi: 10.1093/biostatistics/5.2.263 [DOI] [PubMed] [Google Scholar]
- 28.Wells S, Tamir O, Gray J, Naidoo D, Bekhit M, Goldmann D. Are quality improvement collaboratives effective? a systematic review. BMJ Qual Saf. 2018;27(3):226-240. doi: 10.1136/bmjqs-2017-006926 [DOI] [PubMed] [Google Scholar]
- 29.Hulscher ME, Schouten LM, Grol RP, Buchan H. Determinants of success of quality improvement collaboratives: what does the literature show? BMJ Qual Saf. 2013;22(1):19-31. doi: 10.1136/bmjqs-2011-000651 [DOI] [PubMed] [Google Scholar]
- 30.Siegelman JR, Gress DA. Radiology stewardship and quality improvement: the process and costs of implementing a CT radiation dose optimization committee in a medium-sized community hospital system. J Am Coll Radiol. 2013;10(6):416-422. doi: 10.1016/j.jacr.2012.12.008 [DOI] [PubMed] [Google Scholar]
- 31.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194(4):881-889. doi: 10.2214/AJR.09.3462 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eFigure. Study Timeline
eAppendix. Sample Detailed Audit Report
Data Sharing Statement