This cohort study evaluates the association of persistent Staphylococcus aureus colonization and household environmental contamination with recurrent skin and soft tissue infection in households with children with community-associated methicillin-resistant S aureus (MRSA) skin and soft tissue infection.
Key Points
Question
Is there an association between environmental contamination, persistent personal colonization, and recurrent skin and soft tissue infection among households with children with community-associated methicillin-resistant Staphylococcus aureus (MRSA) skin and soft tissue infection?
Findings
In this cohort study of 692 household members from 150 households with children with community-associated MRSA skin and soft tissue infection, 39% of participants were persistently colonized with S aureus. A total of 53% of index patients and 19% of household contacts reported interval skin and soft tissue infections, more often when persistently colonized with MRSA.
Meaning
The findings suggest that MRSA colonization of household members and contamination of environmental surfaces in the household may be associated with MRSA skin and soft tissue infections in children.
Abstract
Importance
The longitudinal association among persistent Staphylococcus aureus colonization, household environmental contamination, and recurrent skin and soft tissue infection (SSTI) is largely unexplored to date.
Objectives
To identify factors associated with persistent S aureus colonization and recurrent SSTI in households with children with community-associated methicillin-resistant S aureus (MRSA) SSTI.
Design, Setting, and Participants
This 12-month prospective cohort study included 150 children with community-associated MRSA SSTI, 542 household contacts, and 154 pets enrolled from January 3, 2012, through October 20, 2015. A total of 5 quarterly home visits were made to 150 households in the St Louis, Missouri, region. Statistical analysis was performed from September 18, 2018, to January 7, 2020.
Exposures
Covariates used in S aureus strain persistence and interval SSTI models included S aureus colonization and contamination measures, personal hygiene and sharing habits, health history, activities external to the home, and household characteristics (eg, cleanliness, crowding, home ownership, and pets). Serial samples to detect S aureus were collected from household members at 3 anatomic sites, from pets at 2 anatomic sites, and from environmental surfaces at 21 sites.
Main Outcomes and Measures
Molecular epidemiologic findings of S aureus isolates were assessed via repetitive-sequence polymerase chain reaction. Individual persistent colonization was defined as colonization by an identical strain for 2 consecutive samplings. Longitudinal, multivariable generalized mixed-effects logistic regression models were used to assess factors associated with persistent S aureus personal colonization, environmental contamination, and interval SSTI.
Results
Among 692 household members in 150 households, 326 (47%) were male and 366 (53%) were female, with a median age of 14.82 years (range, 0.05-82.25 years). Of 540 participants completing all 5 samplings, 213 (39%) were persistently colonized with S aureus, most often in the nares and with the strain infecting the index patient at enrollment. Nine pets (8%) were persistently colonized with S aureus. Participants reporting interval intranasal mupirocin application were less likely to experience persistent colonization (odds ratio [OR], 0.44; 95% credible interval [CrI], 0.30-0.66), whereas increasing strain-specific environmental contamination pressure was associated with increased individual persistent colonization (OR, 1.17; 95% CrI, 1.06-1.30). Strains with higher colonization pressure (OR, 1.47; 95% CrI, 1.25-1.71) and MRSA strains (OR, 1.57; 95% CrI, 1.16-2.19) were more likely to persist. Seventy-six index patients (53%) and 101 household contacts (19%) reported interval SSTIs. Individuals persistently colonized with MRSA (OR, 1.56; 95% CrI, 1.17-2.11), those with a history of SSTI (OR, 2.55; 95% CrI, 1.88-3.47), and index patients (OR, 1.54; 95% CrI, 1.07-2.23) were more likely to report an interval SSTI.
Conclusions and Relevance
The study findings suggest that recurrent SSTI is associated with persistent MRSA colonization of household members and contamination of environmental surfaces. Future studies may elucidate the effectiveness of specific combinations of personal decolonization and environmental decontamination efforts in eradicating persistent strains and mitigating recurrent SSTIs.
Introduction
Staphylococcus aureus is the most common cause of skin and soft tissue infection (SSTI) in the United States.1,2 The emergence of community-associated methicillin-resistant S aureus (MRSA) in recent decades has posed unique infection prevention challenges because community-associated MRSA establishes community reservoirs and spreads via asymptomatic colonization.3,4
States of S aureus colonization include noncarriage, intermittent colonization, and persistent colonization.5,6 Because colonization is associated with increased risk for infection,7 understanding these states is central to addressing the epidemic of community-associated MRSA infection. Although colonization can resolve spontaneously or with topical antimicrobial use,5,6 the reported median duration of MRSA colonization is approximately 4 months despite systemic antibiotic and topical antimicrobial use.8 Persistently colonized individuals can be sources of transmission to others and their environment and thus warrant specific attention.
Studies9,10,11 have shown that community-associated MRSA infection is frequently spread among households, with personal S aureus colonization, environmental contamination, and pet carriage as reservoirs.12,13,14,15,16 Recurrent SSTIs in index patients and colonization and subsequent infection in household contacts are common and concerning.9,10,14,17,18 Recent studies9,13,17 showed an SSTI recurrence rate of 43% to 51% in index patients within 6 months and subsequent SSTI in 13% of household contacts. Baseline MRSA environmental contamination was a risk factor for recurrent SSTIs in index patients13,17 and likewise was associated with recurrent environmental contamination19 and human reacquisition20 in the 3 months after baseline infection.
Although previous studies9,13,17,19,20 provided insights into persistent colonization and recurrent infection, to date, little is known about their association over time. We investigated factors associated with persistent S aureus colonization and recurrent SSTI for 1 year among households with children with community-associated MRSA SSTI to ascertain the association among environmental contamination, persistent personal colonization, and recurrent SSTI and to identify targets for intervention to disrupt the household reservoir that perpetuates colonization and recurrent SSTIs. We thus used household-level, high-resolution molecular typing with comprehensive, longitudinal sampling of household members, environmental surfaces, and pets.
Methods
Participants and Data Collection
Patients with culture-confirmed, community-associated MRSA SSTI were recruited for the Household Observation of MRSA in the Environment (HOME) study12,15,16,21 from hospitals and community practices in metropolitan St Louis, Missouri, from January 3, 2012, through October 20, 2015. We conducted a baseline visit at the index patient’s primary home at a median of 20 days (interquartile range [IQR], 13-29 days) after infection. Household contacts who slept in the home at least 4 nights per week and dogs and cats who lived indoors were enrolled. Written informed consent and assent were obtained for household members and pets; study procedures were approved by the Washington University institutional review board and Institutional Animal Care and Use Committee, St Louis, Missouri. Data were analyzed from September 18, 2018, to January 7, 2020.
Baseline surveys were administered to each household member to assess S aureus infection history, hygiene practices, activities outside the home, pet and household characteristics, and household cleaning habits. A household cleanliness score, adapted from The Environmental Cleanliness and Clutter Scale,22 (score: 1 [above average], 2 [average], 3 [below average], and 4 [very dirty]), was assigned by the research team to control potential reporting bias about cleaning practices. At 4 quarterly follow-up visits, participants were asked about interval SSTIs, emergency department and hospital visits, and systemic and topical antimicrobial use. Medical records were requested for treated SSTIs.
Samples were obtained from the anterior nares, axillae, and inguinal folds of household members (Eswab; Becton Dickinson), nares (minitip Eswab; Becton Dickinson) and dorsal fur (Eswab) of indoor pets as well as up to 21 environmental surfaces to test for S aureus at each visit. Sampled environmental surfaces (Eswabs and Baird Parker Agar contact plates [Hardy Diagnostics]) included shared electronics (n = 4) and kitchen (n = 5), bathroom (n = 11), and bedroom (n = 1) surfaces (eTable 1 in the Supplement).23
Microbiological Methods
Eswabs were processed using broth-enrichment (Becton Dickinson) culture methods; from contact plates, colonies were selected based on morphologic features. S aureus was isolated and antibiotic susceptibility testing was performed using established techniques (eMethods in the Supplement).24 When available, SSTI isolates from the index patients’ enrollment infections (infecting strains; n = 91) and interval SSTI isolates from household members (n = 19) were obtained from clinical microbiologic laboratories; alternatively, antibiotic susceptibility profiles were obtained. To discern distinct S aureus strains, molecular typing was performed via repetitive-sequence polymerase chain reaction using a 95% similarity cutoff.25,26
Definitions
Strains were defined based on the composite of repetitive-sequence polymerase chain reaction designation and methicillin-resistance profile for each S aureus isolate recovered within the context of a single household. An individual persistent colonization event was colonization of an individual by an identical strain for 2 consecutive samples from 1 or more anatomic sites. Colonization with an acquired novel strain did not constitute persistent colonization (ie, persistence). Persistence degree was considered to be the number of consecutive persistence events observed (ie, number of consecutive colonization events minus 1). Colonization at 2, 3, 4, or 5 consecutive samplings represented a persistence degree of 1, 2, 3, or 4, respectively.
Normalized persistence events were the number of persistence events divided by the number of potential persistence events (maximum of 4 events) × 100. Events were not necessarily consecutive in contrast to persistence degree.
In personal persistence, a strain was observed colonizing 1 or more individuals at 2 or more consecutive samplings. In environmental persistence, a strain was found contaminating any set of household environmental sites at consecutive samplings. In household persistence, a strain persisted across any personal, pet, and/or environmental sites at consecutive samplings.
Personal colonization pressure was the number of anatomic sites (3 per person) colonized with S aureus, methicillin-susceptible S aureus (MSSA), MRSA, or a given strain divided by the total number of anatomic sites sampled per household. Environmental contamination pressure was the number of contaminated surfaces divided by the total number of surfaces sampled per household.
Statistical Analysis
Univariate analyses were conducted in Python 2.7.15 (Python Software Foundation) using the scipy package 1.1.027 or in R (R Foundation for Statistical Computing).28 Categorical data were analyzed with Fisher exact test. For continuous data, Kruskal-Wallis nonparametric 1-way analysis of variance was used for pairwise comparisons and Spearman’s ρ was calculated to determine correlations. All statistical tests were 2-sided.
The individual persistence, interval SSTI, household persistence, and infecting-strain persistence models were bayesian, longitudinal, multivariable generalized mixed-effects logistic regression models fitted using R library MCMCglmm.29 Random intercepts were included to control for repeated longitudinal sampling at the individual, household, and strain-type levels. The threshold family link function was used with a χ2 prior for random intercepts and a normal prior for fixed effects. A heuristic, 2-stage covariate selection scheme driven by domain expertise controlled for the large number of covariates measured. In the first stage, 11 covariates were selected based on a priori hypotheses and statistically significant variables in univariate analysis for each outcome. All possible combinations were fitted and the optimal model selected based on the lowest deviance information criterion. In the second stage, each model was reestimated with the individual addition of other covariates of interest, which were maintained in the final, presented models when model deviance information criterion was reduced and the covariate exhibited a Markov chain Monte Carlo P < .05. eTables 2-5 in the Supplement list primary and secondary model covariates; the eMethods in the Supplement gives further details.
A first-order Markov model of household strain persistence was fitted using R library msm30 (eMethods in the Supplement). This model included 5 states to describe colonization by a given strain within a household: at least 1 child, at least 1 adult, or both a child and adult colonized, exclusively environmental contamination, and no colonization or contamination. Transition probabilities and 95% CI estimates are presented for comparison.
Results
Study Population and Longitudinal Colonization Status
From January 3, 2012, through October 20, 2015, 150 households were enrolled in a 12-month longitudinal study, including 150 index patients (70 [47%] male; median age, 2.99 years [range, 0.08-18.57 years]) presenting with community-associated MRSA infection, their 542 household contacts (256 [47%] male; median age, 25.80 years [range, 0.05-82.25 years]), and their 154 indoor pets (116 dogs, 38 cats) (eTable 6 in the Supplement). Median household size was 4 persons (range, 2-13 persons). Of 150 households enrolled, 135 (90%) completed the 12-month study visit; eTable 6 in the Supplement provides further details of longitudinal sampling completion for household members, environmental surfaces, and pets. A total of 513 of 692 participants (74%) were colonized with S aureus at least once, 319 (46%) were colonized with MRSA. Of 150 household environments, 136 (91%) were contaminated with S aureus at least once, and 104 (69%) were contaminated with MRSA. Of 154 pets, 68 (44%) carried S aureus at least once, and 44 (29%) carried MRSA. Over the study period, 290 of 671 participants (43%) received decolonization treatment at least once: 148 (22%) applied mupirocin to their anterior nares and 243 (36%) performed antiseptic body washes.
Individual S aureus Strain Persistence
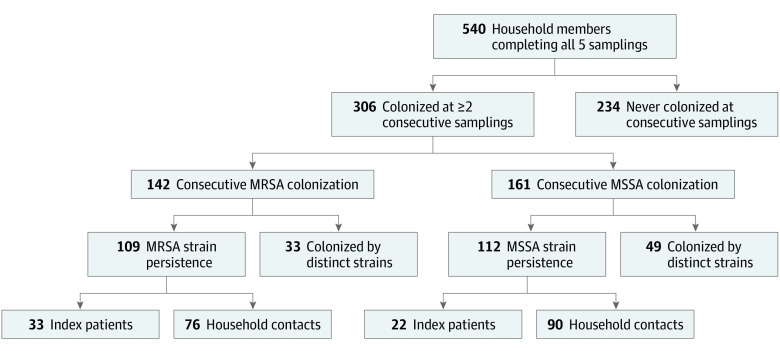
Of participants completing all 5 samplings (n = 540), 306 (57%) were colonized at 2 or more consecutive samples with any strain of S aureus; of these, 213 (70%; 213 of 540 [39%] overall) exhibited strain persistence (Table 1). Of 142 individuals (26%) colonized consecutively with any strain of MRSA, 109 (77%) exhibited strain persistence (Figure 1). Of 1171 S aureus colonization events with a subsequent sampling, persistent colonization was observed in 471 events (40%). Individuals colonized in the nares were most likely to exhibit persistent colonization (at any site) (eTable 7 in the Supplement). Persistence degree (number of consecutive persistence events) was similar for MRSA (median, 2 events [IQR, 2-4 events]) and MSSA (3 events [IQR, 2-4 events]; P = .80) (eFigure 1 in the Supplement). Environmental surfaces infrequently exhibited persistence compared with people (1.9% vs 11.7% normalized persistence events; P < .001) (eFigure 2 and eTable 1 in the Supplement). Among 91 households in which the infecting MRSA strain was available, index patients were more likely to be persistently colonized with the infecting strain than were household contacts (7.6% vs 3.8% normalized persistence events; P = .02) (eFigure 2 in the Supplement). Exemplar longitudinal strain persistence is illustrated in Figure 2 and eFigure 3 in the Supplement.
Table 1. Colonization Events and Association With Interval SSTIs.
| Colonization prevalence and frequencya | All individuals (n = 540) | Index patients (n = 128) | Household contacts (n = 412) | ||||
|---|---|---|---|---|---|---|---|
| No. (%)b | Positive samplings, median (IQR) | No. (%)b | Positive samplings, median (IQR) | No. (%)b | Positive samplings, median (IQR) | ||
| Any S aureus | 420 (78) | 2 (1-4) | 100 (78) | 2 (1-4) | 320 (78) | 2 (1-4) | |
| MRSAc | 265 (49) | 0 (0-2) | 76 (59) | 1 (0-2) | 189 (46) | 0 (0-1) | |
| MSSA | 286 (53) | 1 (0-2) | 65 (51) | 1 (0-2) | 221 (54) | 1 (0-2) | |
| Infecting straind | 123 (23) | 0 (0-1) | 40 (31) | 1 (0-1) | 83 (20) | 0 (0-1) | |
| Strain persistence and degree | No. (%)e | Persistence degree, median (IQR)f | No. (%)e | Persistence degree, median (IQR)f | No. (%)e | Persistence degree, median (IQR)f | |
| Any S aureus | 213 (39) | 2 (1-3) | 52 (41) | 1.5 (1-2) | 161 (39) | 2 (1-3) | |
| MRSAc | 109 (20) | 1 (1-3) | 33 (26) | 1 (1-3) | 76 (18) | 1 (1-3) | |
| MSSA | 112 (21) | 2 (1-2) | 22 (17) | 1 (1-2) | 90 (22) | 2 (1-2) | |
| Infecting straind | 48 (9) | 2 (1-3) | 19 (15) | 1 (1-3) | 29 (7) | 2 (1-3) | |
| Association with No. of interval SSTIsg | ρ | P value | ρ | P value | ρ | P value | |
| Colonization frequency | |||||||
| Any S aureus | 0.11 | .03 | 0.18 | .07 | 0.09 | .10 | |
| MRSAc | 0.29 | <.001 | 0.42 | <.001 | 0.21 | <.001 | |
| MSSA | −0.14 | .005 | −0.19 | .06 | −0.11 | .06 | |
| Infecting strainh | 0.36 | <.001 | 0.42 | <.001 | 0.29 | <.001 | |
| Strain persistence degree | |||||||
| Any S aureus | 0.09 | .07 | 0.18 | .08 | 0.07 | .18 | |
| MRSAc | 0.27 | <.001 | 0.35 | <.001 | 0.24 | <.001 | |
| MSSA | −0.14 | .003 | −0.18 | .07 | −0.12 | .03 | |
| Infecting strainh | 0.34 | <.001 | 0.40 | .001 | 0.28 | <.001 | |
Abbreviations: IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus; SSTI, skin and soft tissue infection.
Eligible individuals for this analysis were those who underwent all 5 samplings (n = 540).
The number of individuals colonized at least once.
All MRSA, including infecting-strain MRSA.
The isolate recovered from the index patient’s enrollment infection (infecting strain) was available in 91 households; thus, the number for the all individuals (n = 312), index patients (n = 77), and household contacts (n = 235) columns was reduced in rows associated with the infecting strain.
The number of individuals colonized with the same strain at consecutive samplings at least once (persistence degree ≥1).
The median (IQR) is for individuals with persistence degree of 1 or greater.
Eligible individuals for this analysis were those who underwent all 5 samplings and were colonized at least once (420 overall, 100 index patients, and 320 household contacts). Spearman correlation test was used.
The infecting strain was available in 91 households; thus, the number for all individuals (n = 238), index patients (n = 63), and household contacts (n = 175) was reduced in rows associated with the infecting strain.
Figure 1. Flow Diagram of Individual Staphylococcus aureus Strain Persistence.
Flow diagram of 540 household members completing all 5 samplings; 213 (39%) were persistently colonized with S aureus (ie, colonized by an identical S aureus strain for ≥2 consecutive samplings). Consecutive colonization with methicillin-resistant S aureus (MRSA) and then methicillin-susceptible S aureus (MSSA) or MSSA and then MRSA occurred; these people were colonized at 2 or more consecutive samplings (n = 306) but did not represent consecutive MRSA (n = 142) or MSSA (n = 161) colonization. Of note, 8 participants were persistently colonized with both MRSA and MSSA strains throughout the study, and are included in both sides of the flow diagram. Index patients (n = 128) were not more likely than household contacts (n = 412) to be persistently colonized with MRSA or MSSA.
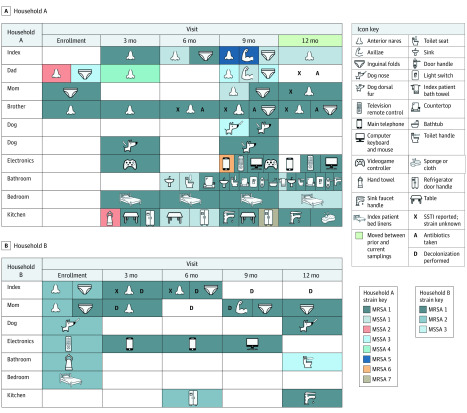
Figure 2. Longitudinal Strain Persistence and Interval Skin and Soft Tissue Infection (SSTI) in Exemplar Households.
In households A and B, the infecting strain in the index patient at enrollment was MRSA 1 [methicillin-resistant S aureus]. In household B, the index patient had an SSTI with MRSA 1 nineteen months before their enrollment infection; the infection reported at their 6-month follow-up visit was also MRSA 1. Decolonization measures included nasal mupirocin, bleach baths, or chlorhexidine body washes. MSSA indicates methicillin-susceptible S aureus.
In univariate analysis, eczema diagnosis (odds ratio [OR], 1.77; 95% CI, 1.07-2.94), colonization with the infecting strain at previous sampling (OR, 1.46; 95% CI, 1.04-2.05), and sharing a bath towel (OR, 1.81; 95% CI, 1.21-2.71) were associated with persistence (eTable 8 in the Supplement). Although interval application of intranasal mupirocin was associated with reduced persistence (OR, 0.39; 95% CI, 0.24-0.65), bleach baths or chlorhexidine body washes were not (OR, 0.96; 95% CI, 0.68-1.36). Fewer persistence events were observed in households that were owned (median, 0.5 [IQR, 0.2-1.0]) vs rented (median, 1.0 [IQR, 0.5-1.7]; P < .001) or were rated clean per the interviewers’ household cleanliness score (0.6 [IQR, 0.2-1.2] vs 0.9 [IQR, 0.5-1.5]; P = .03). The number of persistence events correlated with the number of individuals per square foot in a household (ρ, 0.24; P = .004), warmer weather (ρ, 0.11; P = .02), and personal S aureus colonization pressure (ρ, 0.43; P < .001) and environmental S aureus contamination pressure (ρ, 0.27; P < .001) at previous sampling.
In the longitudinal, multivariable generalized mixed-effects logistic regression model, individuals reporting interval application of intranasal mupirocin were less likely to experience persistence (OR, 0.44; 95% credible interval [CrI], 0.30-0.66); those performing frequent handwashing were more likely to exhibit persistence (OR, 1.39; 95% CrI, 1.11-1.74) (Table 2). Increasing environmental strain contamination pressure was associated with increased individual persistence (OR, 1.17; 95% CrI, 1.06-1.30), whereas home ownership (OR, 0.75; 95% CrI, 0.59-0.97) and increasing personal colonization pressure of other strains colonizing household contacts (OR, 0.86; 95% CrI, 0.77-0.96) were associated with decreased likelihood of persistence (Figure 2A).
Table 2. Factors Retained in Persistence and Interval SSTI Multivariable Modelsa.
| Model, covariate | Odds ratio (95% credible interval) | Markov chain Monte Carlo P value |
|---|---|---|
| Individual persistenceb | ||
| Interval application of intranasal mupirocin vs no mupirocin application | 0.44 (0.30-0.66) | <.001 |
| Environmental strain contamination pressure (increase of 1 SD, 0.16, from the mean 0.11) | 1.17 (1.06-1.30) | <.001 |
| Frequent handwashing score vs infrequentc | 1.39 (1.11-1.74) | .003 |
| Personal colonization pressure of other household strains (increase of 1 SD, 0.15, from the mean 0.12) | 0.86 (0.77-0.96) | .002 |
| Home ownership vs renting | 0.75 (0.59-0.97) | .03 |
| Towel washed after each use vs less frequently | 0.81 (0.65-1.01) | .06 |
| Shares brush or comb vs does not share | 0.85 (0.68-1.06) | .17 |
| Infecting strain persistenced | ||
| Interval application of intranasal mupirocin, proportion of household members (increase of 1 SD, 0.28, from the mean 0.09) | 0.46 (0.27-0.78) | .002 |
| Interval bleach baths, proportion of household members (increase of 1 SD, 0.29, from the mean 0.14) | 2.09 (1.15-3.80) | .005 |
| Interval SSTI, proportion of household members (increase of 1 SD, 0.21, from the mean 0.15) | 1.60 (1.10-2.33) | .01 |
| People per bedroom (increase of 1 SD, 0.57, from the mean 1.46) | 0.62 (0.34-1.11) | .08 |
| Home ownership vs renting | 0.62 (0.18-1.80) | .40 |
| Interval chlorhexidine body washes, proportion of household members (increase of 1 SD, 0.19, from the mean 0.05) | 0.89 (0.55-1.38) | .60 |
| Household persistencee | ||
| Personal strain colonization pressure (increase of 1 SD, 0.11, from the mean 0.11) | 1.47 (1.25-1.71) | <.001 |
| Strain types, No. (increase of 1 SD, 1.33, from the mean 2.27) | 1.88 (1.59-2.26) | <.001 |
| Personal colonization pressure of other household strains (increase of 1 SD, 0.15, from the mean 0.15) | 0.80 (0.69-0.93) | .003 |
| MRSA strain type vs MSSA | 1.57 (1.16-2.19) | .005 |
| Interval systemic antibiotic use, proportion of household members (increase of 1 SD, 0.13, from the mean 0.06) | 0.91 (0.78-1.04) | .17 |
| People per 1000 ft2 (increase of 1 SD, 1.89, from the mean 3.67) | 0.96 (0.81-1.16) | .63 |
| Home ownership vs renting | 0.94 (0.64-1.38) | .76 |
| Interval SSTIf | ||
| SSTI in past year vs no SSTI in past year | 2.55 (1.88-3.47) | <.001 |
| MRSA persistence vs no MRSA persistence | 1.56 (1.17-2.11) | .004 |
| MSSA persistence vs no MSSA persistence | 0.53 (0.32-0.85) | .007 |
| Index patient vs household contact | 1.54 (1.07-2.23) | .02 |
| Environmental MRSA contamination pressure (increase of 1 SD, 0.14, from the mean 0.08) | 1.09 (0.99-1.21) | .07 |
| Shares bedroom with MRSA-colonized individual vs does not share | 1.23 (0.94-1.60) | .12 |
| Frequent handwashing score vs infrequentc | 1.07 (0.83-1.42) | .60 |
| Child vs adult | 0.92 (0.67-1.25) | .60 |
Abbreviations: MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible S aureus; SSTI, skin and soft tissue infection.
For all observations across models, all values are complete observations with no missing values. Odds ratios for continuous covariates are associated with an increase of 1 SD from the mean (zero-centered).
Eligible individuals were those colonized with S aureus at 1 sampling and sampled subsequently, totaling 907 observations of 443 individuals in 133 households across samplings.
Aggregate variable defined as washing hands always after using bathroom, always before preparing food, at least frequently before eating, and at least frequently after changing a diaper (when applicable).
Eligible households were those with an available infecting strain (n = 91) present within the household in at least 1 subsequent sampling, totaling 169 observations in 71 households across samplings.
Eligible households included household members and environmental surfaces sampled consecutively at least once with at least 1 S. aureus strain type present at the first sampling, totaling 780 observations in 119 households across samplings.
Eligible individuals were those sampled consecutively at least once, along with sampling of environmental surfaces, totaling 1952 observations of 640 individuals in 142 households across samplings.
Pet Strain Persistence
Of 106 pets (83 dogs, 23 cats) from 57 households with at least 2 consecutive samplings, 9 dogs were persistently colonized with S aureus, totaling 13 persistence events. In 7 (54%) of these events, the primary caretaker or household member who shared a bed with the pet was colonized with the persistent strain at the previous sampling (eFigure 3C in the Supplement). Pet age, health status, history of SSTI, and overall environmental and personal S aureus colonization pressure were not significantly associated with persistence in pets (eTable 9 in the Supplement).
Household Strain Persistence
Across 98 households in which household members and environmental surfaces were sampled at all 5 visits, 69 (70%) had at least 1 S aureus strain with a persistence degree of at least 2 (eTable 10 in the Supplement). Forty-five households (46%) exhibited MRSA persistence degree of at least 2; of these households, the persistent MRSA strain colonized at least 3 individuals at 1 or more sampling in 26 (58%) and contaminated the environment in 44 (98%). The MRSA strain infecting the index patient persisted longer than MSSA or noninfecting MRSA strains (persistence degree: median, 2 [IQR, 0-4] vs 1 [IQR, 0-2]; P = .01) (Figure 2 and eTable 10 and eFigure 3 in the Supplement). Moving to a new dwelling was associated with reduced persistence across household members, pets, and the environment (OR, 0.51; 95% CI, 0.25-0.99) (eFigure 3 in the Supplement). Neither the frequency of participant-reported environmental surface cleaning nor the assigned household cleanliness score was significantly associated with persistence on specified surfaces. More household members per bathroom was associated with increasing number of persistence events on bathroom surfaces (ρ, 0.22; P = .02).
We then modeled the association between persistence of the infecting strain (when known; n = 91) and reported interval SSTIs, controlling for socioeconomic status, crowding, and decolonization measures. The proportion of household members reporting interval SSTIs was associated with increased persistence of the index patient’s enrollment infecting strain at the household level (OR, 1.60; 95% CrI, 1.10-2.33) (Table 2). The proportion of household members reporting interval application of intranasal mupirocin was associated with reduced persistence of the index patient’s enrollment infecting strain at the household level (OR, 0.46; 95% CrI, 0.27-0.78); the proportion of household members reporting interval bleach baths was associated with increased persistence of the index patient’s infecting strain at the household level (OR, 2.09; 95% CrI, 1.15-3.80).
In the multivariable household persistence model, MRSA (vs MSSA: OR, 1.57; 95% CrI, 1.16-2.19), strains with higher personal colonization pressure (OR, 1.47; 95% CrI, 1.25-1.71), and strains in households with a higher number of distinct strains (OR, 1.88; 95% CrI, 1.59-2.26) exhibited a higher likelihood of persistence (Table 2). Strains in households with higher personal colonization pressure of other strains (OR, 0.80; 95% CrI, 0.69-0.93) (Figure 2A) were less likely to persist.
Markov Model of Household Strain Persistence Dynamics
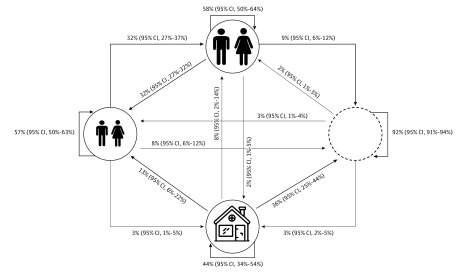
A first-order Markov model was constructed to capture the persistence dynamics of S aureus strains in households among 5 possible colonization states for adults, children, and the environment (Figure 3 and eMethods in the Supplement). Strains in both the adult and child states tended to persist, suggesting that strains most commonly remain within households by persistent colonization of 1 individual or by transitions between adults or between children. Strains in the environment-only state showed lower persistence.
Figure 3. Markov Model of Strain Persistence Among Children, Adults, and the Environment.
A 5-state Markov model was fit across all households and persistent strain types, with the resultant transition probabilities (and 95% CI estimates) re-scaled for the displayed 4-state model. Strains most commonly persist within households by persistent colonization of 1 individual or through transitions between adults or between children and less often through environment-only persistence. Smaller people indicate at least 1 child (aged <18 years) colonized; larger people, at least 1 adult (aged ≥18 years) colonized; house, at least 1 environmental site colonized (but no household members colonized); and empty circle, strain type not present in household.
Interval SSTI
Of 144 index patients with at least 1 follow-up visit, 76 (53%) reported 164 recurrent SSTIs, with 45 (31%) reporting 2 or more subsequent SSTIs (range, 1-9 SSTIs) (eTable 11 in the Supplement). Of 523 household contacts, 101 (19%) experienced 169 SSTIs (range, 1-13 SSTIs). Participants sought medical care for 178 of 333 reported SSTIs (53%), for which 80% were prescribed a systemic antibiotic; 19 (7%) interval infecting isolates were available for molecular characterization (eTable 12 in the Supplement). In 14 households with an available infecting strain at enrollment and interval SSTI strains, 11 isolate pairs (79%) were identical strains. Interval infecting strains were commonly found in the household at the previous sampling (14 of 19 [74%]), often colonizing the individual reporting the SSTI (8 of 19 [42%]) and/or contaminating the environment (9 of 17 [53%]) (Figure 2B and eFigure 3 in the Supplement). Additional exemplar interval SSTIs are illustrated in Figure 2 and eFigure 3 in the Supplement.
Individuals exhibiting a higher degree of MRSA strain persistence reported more interval SSTIs (ρ, 0.27; P < .001) (Table 1 and Figure 2A). Increasing persistence degree of the most persistently MRSA-colonized individual was associated with an increase in the total number of interval household SSTIs (ρ, 0.30; P < .001) (eFigure 4 in the Supplement). A higher degree of MSSA strain persistence was associated with fewer individual SSTIs (ρ, –0.14; P < .003) (Table 1 and eFigure 4 in the Supplement).
In univariate analysis, individuals reporting a history of SSTI in the year before enrollment (OR, 5.79; 95% CI, 3.93-8.53) and children (OR, 1.60; 95% CI, 1.12-2.27) were more likely to report at least 1 interval SSTI during the study (eTable 13 in the Supplement). Hygiene measures, such as showering (vs bathing: OR, 0.58; 95% CI, 0.40-0.85), frequent handwashing (OR, 0.69; 95% CI, 0.47-0.99), and frequent tooth brushing (OR, 0.59; 95% CI, 0.37-0.94), were inversely associated with interval SSTI. Individuals colonized with MRSA (OR, 2.91; 95% CI, 2.22-3.82) and/or colonized with the infecting strain (OR, 3.55; 95% CI, 2.42-5.20) at previous sampling or sharing a towel with a MRSA-colonized individual (OR, 1.48; 95% CI, 1.14-1.92) were at higher risk for interval SSTI, as were those colonized with MRSA at more anatomic sites during previous sampling (relative risk, 2.33; P < .001) (Figure 2 and eFigure 3 in the Supplement).
Frequent cleaning of refrigerator handles was associated with fewer interval SSTIs (0.05 vs 0.09 SSTIs per person-sampling; P = .03) (eTable 13 in the Supplement). Households with at least 1 household member reporting an SSTI in the year before enrollment (not including the index patient’s SSTI at enrollment) had more interval SSTIs (0.10 vs 0 SSTIs per person-sampling; P < .001). Higher personal MRSA colonization pressure (ρ, 0.21; P < .001) and environmental MRSA contamination pressure (ρ, 0.13; P = .006) were associated with more interval SSTIs across household members.
In the multivariable interval SSTI model, individuals persistently colonized with MRSA (OR, 1.56; 95% CrI, 1.17-2.11), those with an SSTI in the year before enrollment (excluding index enrollment infection; OR, 2.55; 95% CrI, 1.88-3.47), and index patients (OR, 1.54; 95% CrI, 1.07-2.23) were more likely to report an interval SSTI (Table 2). Increasing environmental MRSA contamination pressure at the previous sampling (OR, 1.09; 95% CrI, 0.99-1.21) and sharing a bedroom with a MRSA-colonized individual (OR, 1.23; 95% CrI, 0.94-1.60) remained in the final model despite not reaching statistical significance (Figure 2A and eFigure 3 in the Supplement).
Discussion
The commensal state of S aureus challenges prevention efforts because colonization serves as a reservoir for endogenous infection, further perpetuating persistent colonization and transmission. This poses a significant burden, particularly in households.31 Although topical antimicrobials and antiseptics used by patients with SSTI have demonstrated eradication of S aureus carriage in decolonization trials, their effectiveness in preventing recurrent SSTI wanes over time, with more than 50% experiencing recurrence during 1 year.9,32,33,34 To develop more effective prevention strategies, it is essential to illuminate individual and household factors associated with persistent S aureus colonization and SSTI. The present work attempted to fill this knowledge gap by using a combination of longitudinal sampling of household members and their environments; high-resolution molecular typing of all recovered strains; detailed health, hygiene, and household behaviors; and tracking of incident SSTIs. We found that the index patient’s infecting MRSA strain was most likely to persist in the household; that environmental contamination, but not pets, was associated with personal strain persistence; and that personal MRSA strain persistence was associated with increased likelihood of development of subsequent SSTI.
A study in Pennsylvania8 found that index patients with community-associated MRSA who were colonized with MRSA at SSTI onset remained colonized for a median of 140 days; colonization never cleared in 40% during the 6-month study period. Persons in whom colonization cleared were more likely to experience reacquisition if there was a higher proportion of children in the home or if they lived with a MRSA-colonized household contact.35 Our study extended this knowledge because high environmental contamination burden (unmeasured in the previous study) was associated with individual persistent strain colonization. Moreover, although MRSA frequently colonized extranasal sites, nasal colonization was most likely to persist. Of interest, frequent handwashing alone was not associated with reduced persistence. Interval application of intranasal mupirocin was associated with protection against persistence, suggesting that measures beyond hand hygiene are necessary to interrupt persistent colonization. We found that the duration of persistent MRSA colonization was correlated with increased risk of SSTI in the modeled individual and in all household contacts.
Our study observed a difference in interval SSTI risk between individuals and households experiencing MRSA vs MSSA persistence. In households affected by community-associated MRSA SSTI, MRSA persistence was associated with increased likelihood of interval SSTI and MSSA persistence was inversely associated with SSTI incidence. Thus, strain competition, whether from noninfecting MRSA or MSSA strains, appears to oppose persistence of the MRSA infecting strain. Although contemporary community-associated SSTIs are most often attributable to MRSA, MSSA SSTIs are increasing.36 In households of patients with MSSA SSTI, persistence dynamics and risk factors for SSTI may be different.
The present study reinforces emerging observations of the household as the primary source of community-associated MRSA, as suggested by an agent-based simulation of community-associated MRSA colonization in Chicago, Illinois, by Macal et al.37 In our real-world study, we found no activities external to the household (secondary covariates in the multivariate models such as nail salons, sports, and occupation, as seen in eTables 2 and 3 in the Supplement) associated with persistent colonization or interval SSTI. We replicated the finding that individuals with an SSTI are at increased likelihood to experience subsequent SSTIs and cohabitate with others with a history of SSTI.9,17,38 The infecting strain at enrollment was the strain most likely to persist within a household, and its persistence was significantly associated with interval SSTI. In addition, strains causing interval SSTIs in household members were typically present within the household at prior sampling and were frequently concordant with the infecting strain at enrollment. Similarly, in a study conducted in Los Angeles, California, and Chicago, the infecting strain at enrollment was prevalent in approximately 50% of contaminated households for up to 3 months.19 These findings signify a cycle of MRSA colonization and infection in households: persistent personal MRSA colonization may lead to SSTI, and SSTI in turn may be associated with increased likelihood of MRSA acquisition and persistent colonization in household contacts.
We found that environmental MRSA contamination was associated with both strain-specific, personal-persistent colonization and interval SSTI. This finding is in contrast to the simulation by Macal et al37 that posited that community-associated MRSA was spread solely through interpersonal contact. In addition, although previous studies in New York, NY,13 and in Los Angeles and Chicago17 found household fomite S aureus contamination to be independently associated with interval SSTIs in the index patient, the longitudinal sampling and hierarchical modeling of our study assessed the associations among environmental contamination, persistent colonization, and interval SSTI. In our study, persistent colonization was reduced in households with higher cleanliness scores and frequent laundering of bathroom towels; cleaning of select surfaces was associated with reduced SSTI incidence. Taken together, our analyses suggest that long-term eradication and SSTI prevention measures should be targeted at both the personal and the household levels, pairing personal decolonization with environmental hygiene interventions associated with reduced contamination.
Strengths and Limitations
The strength of our study comes from its comprehensive modeling of clinical and molecular epidemiologic data in households (with 96% household member participation plus inclusion of pets) during a 12-month sampling time to provide a novel understanding of the association between persistent colonization and SSTI (with strain-type resolution), drawing from a diverse community sample. Previous studies lacked the temporal resolution, molecular resolution, environmental sampling, and/or sample size to provide a rigorous, longitudinal perspective of colonization persistence patterns and recurrent SSTI.8,13,17,19,35,39
Limitations include that interval SSTI isolates were usually unavailable for molecular typing. In addition, our findings may not be generalizable to geographic locales with less prevalent MRSA colonization and infection or to households with SSTI caused by MSSA strains.
Conclusions
Recurrent SSTI is associated with strain-specific, persistent MRSA colonization of household members and contamination of environmental surfaces. Future randomized clinical trials are necessary to define the effectiveness of specific combinations of personal decolonization and environmental decontamination efforts in preventing recurrent SSTI in households. Our findings also suggest that the relative prevalence of other strains in the household may be associated with destabilization of a given strain’s capacity to persist longitudinally on an individual. These data highlight the need for future studies of S aureus ecologic features and how other components of the skin and nasal microbiota may prevent or facilitate the transition of MRSA from colonization to infection.
eMethods
eAcknowledgments
eReferences
eTable 1. Longitudinal S aureus Colonization Pressure and Normalized Persistence Events on Environmental Surfaces
eTable 2. Individual Persistence Model, Primary and Secondary Covariates
eTable 3. Interval SSTI Model, Primary and Secondary Covariates
eTable 4. Household Persistence Model, Primary and Secondary Covariates
eTable 5. Infecting-strain Persistence Model, Covariates
eTable 6. Study Population and Sampling Statistics: Participants, the Environment, and Their Pets
eTable 7. Individual Strain Persistence by Anatomic Site
eTable 8. Factors Associated With Persistence, Univariate Analysis
eTable 9. Factors Associated With Strain Persistence in Pets
eTable 10. Strain Persistence Measures of Strains Present at Enrollment or 3-Month Follow-up Visit
eTable 11. Interval Skin and Soft-Tissue Infections
eTable 12. Interval Skin and Soft-Tissue Infections, Isolate Available
eTable 13. Factors Associated with Interval Skin and Soft-Tissue Infection, Univariate Analysis
eFigure 1. Degree of Persistent Colonization Between Children and Adults
eFigure 2. S aureus Strain Persistence by Sex, Age, and Environmental Site
eFigure 3. Longitudinal Strain Persistence and Interval SSTI in Exemplar Households (C, D, E, and F)
eFigure 4. Degree of Persistent Colonization and Risk of Interval SSTI
References
- 1.Kaplan SL. Community-acquired methicillin-resistant Staphylococcus aureus infections in children. Semin Pediatr Infect Dis. 2006;17(3):113-119. doi: 10.1053/j.spid.2006.06.004 [DOI] [PubMed] [Google Scholar]
- 2.Miller LG, Diep BA. Clinical practice: colonization, fomites, and virulence: rethinking the pathogenesis of community-associated methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(5):752-760. doi: 10.1086/526773 [DOI] [PubMed] [Google Scholar]
- 3.Dukic VM, Lauderdale DS, Wilder J, Daum RS, David MZ. Epidemics of community-associated methicillin-resistant Staphylococcus aureus in the United States: a meta-analysis. PLoS One. 2013;8(1):e52722. doi: 10.1371/journal.pone.0052722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto M. Community-associated MRSA: a dangerous epidemic. Future Microbiol. 2007;2(5):457-459. doi: 10.2217/17460913.2.5.457 [DOI] [PubMed] [Google Scholar]
- 5.Nouwen JL, Ott A, Kluytmans-Vandenbergh MF, et al. Predicting the Staphylococcus aureus nasal carrier state: derivation and validation of a “culture rule”. Clin Infect Dis. 2004;39(6):806-811. doi: 10.1086/423376 [DOI] [PubMed] [Google Scholar]
- 6.Sollid JUE, Furberg AS, Hanssen AM, Johannessen M. Staphylococcus aureus: determinants of human carriage. Infect Genet Evol. 2014;21:531-541. doi: 10.1016/j.meegid.2013.03.020 [DOI] [PubMed] [Google Scholar]
- 7.Fritz SA, Epplin EK, Garbutt J, Storch GA. Skin infection in children colonized with community-associated methicillin-resistant Staphylococcus aureus. J Infect. 2009;59(6):394-401. doi: 10.1016/j.jinf.2009.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cluzet VC, Gerber JS, Nachamkin I, et al. Duration of colonization and determinants of earlier clearance of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2015;60(10):1489-1496. doi: 10.1093/cid/civ075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis. 2012;54(6):743-751. doi: 10.1093/cid/cir919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med. 2012;166(6):551-557. doi: 10.1001/archpediatrics.2011.900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez M, Hogan PG, Krauss M, Warren DK, Fritz SA. Measurement and impact of Staphylococcus aureus colonization pressure in households. J Pediatric Infect Dis Soc. 2013;2(2):147-154. doi: 10.1093/jpids/pit002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritz SA, Hogan PG, Singh LN, et al. Contamination of environmental surfaces with Staphylococcus aureus in households with children infected with methicillin-resistant S aureus. JAMA Pediatr. 2014;168(11):1030-1038. doi: 10.1001/jamapediatrics.2014.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knox J, Sullivan SB, Urena J, et al. Association of environmental contamination in the home with the risk for recurrent community-associated, methicillin-resistant Staphylococcus aureus infection. JAMA Intern Med. 2016;176(6):807-815. doi: 10.1001/jamainternmed.2016.1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ng W, Faheem A, McGeer A, et al. Community- and healthcare-associated methicillin-resistant Staphylococcus aureus strains: an investigation into household transmission, risk factors, and environmental contamination. Infect Control Hosp Epidemiol. 2017;38(1):61-67. doi: 10.1017/ice.2016.245 [DOI] [PubMed] [Google Scholar]
- 15.Hogan PG, Mork RL, Boyle MG, et al. Interplay of personal, pet, and environmental colonization in households affected by community-associated methicillin-resistant Staphylococcus aureus. J Infect. 2019;78(3):200-207. doi: 10.1016/j.jinf.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mork RL, Hogan PG, Muenks CE, et al. Comprehensive modeling reveals proximity, seasonality, and hygiene practices as key determinants of MRSA colonization in exposed households. Pediatr Res. 2018;84(5):668-676. doi: 10.1038/s41390-018-0113-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller LG, Eells SJ, David MZ, et al. Staphylococcus aureus skin infection recurrences among household members: an examination of host, behavioral, and pathogen-level predictors. Clin Infect Dis. 2015;60(5):753-763. doi: 10.1093/cid/ciu943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nerby JM, Gorwitz R, Lesher L, et al. Risk factors for household transmission of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2011;30(11):927-932. doi: 10.1097/INF.0b013e31822256c3 [DOI] [PubMed] [Google Scholar]
- 19.Eells SJ, David MZ, Taylor A, et al. Persistent environmental contamination with USA300 methicillin-resistant Staphylococcus aureus and other pathogenic strain types in households with S aureus skin infections. Infect Control Hosp Epidemiol. 2014;35(11):1373-1382. doi: 10.1086/678414 [DOI] [PubMed] [Google Scholar]
- 20.Davis M, Morris D, Cluzet V, et al. Home environmental contamination is associated with community-associated methicillin-resistant Staphylococcus aureus re-colonization in treated patients. Open Forum Infect Dis. 2017;4(suppl 1):S7. doi: 10.1093/ofid/ofx162.016 [DOI] [Google Scholar]
- 21.Mork RL, Hogan PG, Muenks CE, et al. Longitudinal, strain-specific Staphylococcus aureus introduction and transmission events in households of children with community-associated meticillin-resistant S aureus skin and soft tissue infection: a prospective cohort study. Lancet Infect Dis. 2020;20(2):188-198. doi: 10.1016/S1473-3099(19)30570-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halliday G, Snowdon J. The Environmental Cleanliness and Clutter Scale (ECCS). Int Psychogeriatr. 2009;21(6):1041-1050. doi: 10.1017/S1041610209990135 [DOI] [PubMed] [Google Scholar]
- 23.Hogan PG, Burnham CA, Singh LN, et al. Evaluation of environmental sampling methods for detection of Staphylococcus aureus on fomites. Ann Public Health Res. 2015;2(1):1013. [PMC free article] [PubMed] [Google Scholar]
- 24.Cockerill F. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-third Informational Supplement. Clinical and Laboratory Standards Institute; 2013:M100-S23. [Google Scholar]
- 25.Rodriguez M, Hogan PG, Satola SW, et al. Discriminatory indices of typing methods for epidemiologic analysis of contemporary Staphylococcus aureus strains. Medicine (Baltimore). 2015;94(37):e1534. doi: 10.1097/MD.0000000000001534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Del Vecchio VG, Petroziello JM, Gress MJ, et al. Molecular genotyping of methicillin-resistant Staphylococcus aureus via fluorophore-enhanced repetitive-sequence PCR. J Clin Microbiol. 1995;33(8):2141-2144. doi: 10.1128/JCM.33.8.2141-2144.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Virtanen P, Gommers R, Oliphant TE, et al. ; SciPy 1.0 Contributors . SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat Methods. Published online February 3, 2020. doi: 10.1038/s41592-019-0686-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Foundation for Statistical Computing R: a language and environment statistical computing. Published 2016. Accessed September 18, 2018. https://www.R-project.org/
- 29.Hadfield J. MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw. 2010;33(2):1-22. doi: 10.18637/jss.v033.i02 20808728 [DOI] [Google Scholar]
- 30.Jackson C. Multi-state models for panel data: the msm package for R. J Stat Softw. 2011;38(8):1-28. doi: 10.18637/jss.v038.i08 [DOI] [Google Scholar]
- 31.Chisholm RH, Campbell PT, Wu Y, Tong SYC, McVernon J, Geard N. Implications of asymptomatic carriers for infectious disease transmission and control. R Soc Open Sci. 2018;5(2):172341. doi: 10.1098/rsos.172341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fritz SA, Camins BC, Eisenstein KA, et al. Effectiveness of measures to eradicate Staphylococcus aureus carriage in patients with community-associated skin and soft-tissue infections: a randomized trial. Infect Control Hosp Epidemiol. 2011;32(9):872-880. doi: 10.1086/661285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellis MW, Griffith ME, Dooley DP, et al. Targeted intranasal mupirocin to prevent colonization and infection by community-associated methicillin-resistant Staphylococcus aureus strains in soldiers: a cluster randomized controlled trial. Antimicrob Agents Chemother. 2007;51(10):3591-3598. doi: 10.1128/AAC.01086-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan SL, Forbes A, Hammerman WA, et al. Randomized trial of “bleach baths” plus routine hygienic measures vs. routine hygienic measures alone for prevention of recurrent infections. Clin Infect Dis. 2014;58(5):679-682. doi: 10.1093/cid/cit764 [DOI] [PubMed] [Google Scholar]
- 35.Cluzet VC, Gerber JS, Nachamkin I, et al. Risk factors for recurrent colonization with methicillin-resistant Staphylococcus aureus in community-dwelling adults and children. Infect Control Hosp Epidemiol. 2015;36(7):786-793. doi: 10.1017/ice.2015.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutter DE, Milburn E, Chukwuma U, Dzialowy N, Maranich AM, Hospenthal DR. Changing susceptibility of Staphylococcus aureus in a US pediatric population. Pediatrics. 2016;137(4):e20153099. doi: 10.1542/peds.2015-3099 [DOI] [PubMed] [Google Scholar]
- 37.Macal CM, North MJ, Collier N, et al. Modeling the transmission of community-associated methicillin-resistant Staphylococcus aureus: a dynamic agent-based simulation. J Transl Med. 2014;12:124. doi: 10.1186/1479-5876-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller LG, Eells SJ, Taylor AR, et al. Staphylococcus aureus colonization among household contacts of patients with skin infections: risk factors, strain discordance, and complex ecology. Clin Infect Dis. 2012;54(11):1523-1535. doi: 10.1093/cid/cis213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodriguez M, Hogan PG, Burnham C-A, Fritz SA. Molecular epidemiology of Staphylococcus aureus in households of children with community-associated S aureus skin and soft tissue infections. J Pediatr. 2014;164(1):105-111. doi: 10.1016/j.jpeds.2013.08.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eAcknowledgments
eReferences
eTable 1. Longitudinal S aureus Colonization Pressure and Normalized Persistence Events on Environmental Surfaces
eTable 2. Individual Persistence Model, Primary and Secondary Covariates
eTable 3. Interval SSTI Model, Primary and Secondary Covariates
eTable 4. Household Persistence Model, Primary and Secondary Covariates
eTable 5. Infecting-strain Persistence Model, Covariates
eTable 6. Study Population and Sampling Statistics: Participants, the Environment, and Their Pets
eTable 7. Individual Strain Persistence by Anatomic Site
eTable 8. Factors Associated With Persistence, Univariate Analysis
eTable 9. Factors Associated With Strain Persistence in Pets
eTable 10. Strain Persistence Measures of Strains Present at Enrollment or 3-Month Follow-up Visit
eTable 11. Interval Skin and Soft-Tissue Infections
eTable 12. Interval Skin and Soft-Tissue Infections, Isolate Available
eTable 13. Factors Associated with Interval Skin and Soft-Tissue Infection, Univariate Analysis
eFigure 1. Degree of Persistent Colonization Between Children and Adults
eFigure 2. S aureus Strain Persistence by Sex, Age, and Environmental Site
eFigure 3. Longitudinal Strain Persistence and Interval SSTI in Exemplar Households (C, D, E, and F)
eFigure 4. Degree of Persistent Colonization and Risk of Interval SSTI