Highlights
-
•
A novel RT-LAMP method was developed to detect canine distemper virus (CDV).
-
•
A set of four primers were designed to target the H gene for the specific detection of wild-type CDV variants.
-
•
The assay was 100-fold more sensitive than conventional RT-PCR.
-
•
The system showed a preference for wild-type CDV, and exhibited less sensitivity to CPV.
Keywords: Canine distemper virus (CDV), Loop-mediated isothermal amplification (LAMP), Attenuated vaccine, Differential diagnosis
Abstract
Although widespread vaccination against canine distemper virus (CDV) has been conducted for many decades, several canine distemper outbreaks in vaccinated animals have been reported frequently. In order to detect and differentiate the wild-type and vaccine strains of the CDV from the vaccinated animals, a novel reverse transcription loop-mediated isothermal amplification (RT-LAMP) method was developed. A set of four primers—two internal and two external—were designed to target the H gene for the specific detection of wild-type CDV variants. The CDV-H RT-LAMP assay rapidly amplified the target gene, within 60 min, using a water bath held at a constant temperature of 65 °C. The assay was 100-fold more sensitive than conventional RT-PCR, with a detection limit of 10−1 TCID50 ml−1. The system showed a preference for wild-type CDV, and exhibited less sensitivity to canine parvovirus, canine adenovirus type 1 and type 2, canine coronavirus, and canine parainfluenza virus. The assay was validated using 102 clinical samples obtained from vaccinated dog farms, and the results were comparable to a multiplex nested RT-PCR assay. The specific CDV-H RT-LAMP assay provides a simple, rapid, and sensitive tool for the detection of canines infected with wild-type CDV from canines vaccinated with attenuated vaccine.
1. Introduction
Canine distemper (CD) is a highly contagious and fatal disease of dogs and other terrestrial carnivores. CD is caused by the canine distemper virus (CDV), a single-stranded negative RNA virus of the genus Morbillivirus, family Paramyxoviridae, and is closely related to the viruses responsible for measles, rinderpest, peste des petits ruminants, phocine distemper, dolphin distemper, porpoise distemper, and equine morbillivirus (Fenner, 1976, Haas and Barrett, 1996, McCarthy et al., 2011, Soltan and Abd-Eldaim, 2014, Bellière et al., 2011).
It has been widely documented that CDV causes severe immunosuppression and persistent infection in the central nervous system in dogs (Müller et al., 1995, Wu et al., 2000, Meertens et al., 2003, Vandevelde and Zurbriggen, 2005). Moreover, the host spectrum of CDV is gradually increasing (Qiu et al., 2011). Currently, vaccination is the most effective means of controlling CD and one of the most frequently used vaccines is a modified live attenuated vaccine. Although widespread vaccination against CD has been conducted for many decades, this infection still represents an important disease for dogs (Elia et al., 2006). There is, therefore, a need for the development of assays that can distinguish dogs vaccinated with modified live attenuated vaccine from those infected with wild-type virus in herds.
Rapid and sensitive detection methods, including the reverse transcription polymerase chain reaction (RT-PCR) and multiplex PCR, are currently available for the diagnosis of CDV. However, the occurrence of false-positive PCR products for each of these methods hinders the identification of wild-type CDV strains (Ralston et al., 2007). Real-time quantitative PCR methods have also been reported to detect CDV. The advantages of real-time PCR, compared with conventional RT-PCR, include higher speed, greater sensitivity, and less handling of PCR products. However, real-time PCR instruments are expensive and may not be readily available in many laboratories (Coleman et al., 2014). Other methods such as serological assays (Indirect immunofluorescence assay (IFA), and Enzyme linked immunosorbent assay), virus isolation and in situ hybridization have also been developed, but are not applicable for the fast and sensitive diagnosis of CDV (Gray et al., 2012, Gaedke et al., 1997, Rzezutka and Mizak, 2002, Frisk et al., 1999, von Messling et al., 1999).
Loop-mediated isothermal amplification (LAMP) is a novel method for the rapid, specific, and sensitive amplification of nucleic acids (Notomi et al., 2000). With its advantages, it has been applied widely in the detection of pathogenic microorganisms (Dinh et al., 2011, Li and Cai, 2011, Njiru et al., 2012) and genetically modified foods (Fukuta et al., 2004), analysis of shelf-life in melon (Fukuta et al., 2006) and chromosomal chimerism of bovine (Hirayama et al., 2007).
Rapid and cost-effective RT-LAMP assays for the pre-clinical detection of CDV have been described elsewhere (Cho and Park, 2005, Liu et al., 2010, Wen et al., 2012, Hu et al., 2012). As these assays target both wild-type CDV variants and the live attenuated vaccine strain, the interpretation of the results may be complicated in terms of eradication and control where a vaccination policy is practiced.
This study aimed to establish a RT-LAMP method which was specific for wild-type CDV, could differentiate from canines vaccinated with attenuated CDV vaccine, and which could be applied with relative ease in minimally-equipped laboratories and under field conditions.
2. Materials and methods
2.1. Viruses and field samples
CDV strains: wild-type CDV YB strain (Asia-1 subgroup), LDH (06) strain (Asia-1 subgroup), and ZH (05) strain (Asia-2 subgroup) were isolated by using Madin–Darby Canine Kidney expressed SLAM of dog (MDCK-SLAM) cells, and were identified and maintained at the Harbin Veterinary Research Institute (Harbin, China). Three locally supplied CDV vaccine products Vacc-A (Intervet, Netherlands: Onderstepoort strain), Vacc-B (Pfizer, USA: Snyder Hill strain) and Vacc-C (Merial, France: RECOMBITEK C4) were tested in the current study. All other non-CDV canine viruses: canine parvovirus (CPV), canine adenovirus type 1 and type 2 (CAV-1 and CAV-2), canine coronavirus (CCoV), and canine parainfluenza virus (CPIV) were provided by the Harbin Veterinary Research Institute (Harbin, China). Field samples (n = 102; including 37 rectal swabs, 20 conjunctival swabs, 15 urine samples, and 30 whole blood samples) (Table 1 ) were collected from different farms across several provinces of China where vaccination with live attenuated vaccine was in operation.
Table 1.
The information of the clinical samples collected from domestic dogs in 2012.
| Location (Province) | Specimens | Numbers | Clinical signs |
|---|---|---|---|
| Heilongjiang | Rectal swabs | 15 | Fever, sneeze, depression, cough, diarrhea or bloody stool |
| Conjunctival swabs | 13 | ||
| Urine | 5 | ||
| Whole blood | 13 | ||
| Jilin | Rectal swabs | 10 | Spasm, fever, depression, sneeze and cough |
| Urine | 4 | ||
| Whole blood | 10 | ||
| Liaoning | Rectal swabs | 12 | Depression and diarrhea |
| Whole blood | 7 | ||
| Shandong | Conjunctival swabs | 7 | Sneeze, cough, spasm and fever |
| Urine samples | 6 | ||
2.2. Ethical considerations
This study was approved by the Harbin Veterinary Research Institute Experimental Animal Welfare Ethics Committee (HVRI-EAWEC), and conducted under the guidance of the HVRI-EAWEC. The animals from which specimens were collected were handled in accordance with animal protection law of the People’ Republic of China. Our sampling processes were assisted by local authorities and veterinarians. All the owners of the dogs gave permission for their animals’ samples to be used in this study.
2.3. Primer design
Genomic sequences of N and H gene were aligned using the MegAlign program of DNAStar software (DNAStar, Madison, WI, USA). With online LAMP designing software PrimerExplorer V4 (http://primerexplorer.jp/e/), two sets primers (F3-N and B3-N, FIP-N and BIP-N) and (F3-H and B3-H, FIP-H and BIP-H) were designed based on the wild-type CDV YB strain (Table 2 ). The former was common for all types of CDV strains; the latter was conserved among wild-type CDV strains but not for the live attenuated CDV vaccine strains.
Table 2.
Details of RT-LAMP and multiplex nested RT-PCR primers for CDV.
| Primersa | Type | Sequences |
|---|---|---|
| F3-N | Forward outer primer | 5′-GCAAAGTAAGCTCGGCACTT-3′ |
| B3-N | Reverse outer primer | 5′-TGGTTTTCGGACCTCTTGTT-3′ |
| FIP-N | Forward inner primer | 5′-CGGTCCTCCGTTGTCTTGGATGGCTTGGCATCACCAAGGAA-3′ |
| BIP-N | Reverse inner primer | 5′-CGCTGCTGGTCCCAAGCAATGGGGTTGTTGATTAGCGACT-3′ |
| F3-H | Forward outer primer | 5′-GCCTGTATTGGTCTCTGAG-3′ |
| B3-H | Reverse outer primer | 5′-CTCCG TTCAGTATAACCGG-3′ |
| FIP-H | Forward inner primer | 5′-TGCACATAGGGTAGGATTTTCTGAACAAGAGGAGCAAAAAAACTG-3′ |
| BIP-H | Reverse inner primer | 5′-AGTTGCCTTCTTATGGGCGGATGTTAAGTTGAAGGTCAATGC-3′ |
| CDV-P1 | F (For CDV species) | 5′-AAATCCTGTGTTACCCGCTC-3′ |
| CDV-P2 | F (For wild-type strain) | 5′-TGGTGGCTCTGCAATATGAA-3′ |
| CDV-P3 | F (For vaccine strain) | 5′-AATGAATGGATGCCTGGGGTTT-3′ |
| CDV-P4 | R(For CDV species) | 5′-ACGTCCTGGACCCTAAGTTTTG-3′ |
The primers of F3-N, B3-N, FIP-N, BIP-N, F3-H, B3-H, FIP-H, BIP-H were for RT-LAMP Primers. CDV-P1, CDV-P2, CDV-P3 and CDV-P4 were applied in multiplex nested RT-PCR.
2.4. RNA extraction and cDNA synthesis
Viral RNA was extracted using the QIAamp Viral RNA Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. Reverse transcription was performed in a final reaction volume of 20 μl containing: 10 μl RNA, 4 μl 5× RT Buffer, 1 μl dNTP (10 mM each), 50 pmol 9-Random Hexamers, 10 U M-MLV reverse transcriptase (Takara, Dalian, China), and 20 U RNase inhibitor (Takara, Dalian, China). The reaction was incubated at42 °C for 1 h followed by incubation at 70 °C for 10 min.
2.5. The universal CDV-N RT-LAMP assay for detection of CDV
The CDV-N RT-LAMP assay was performed in a total volume of 25 μl consisting of: 0.3 μM F3-N and B3-N primers, 2.4 μM FIP-N and BIP-N primers, 1.4 mM dNTPs, 2.5 μl 1× Thermo Pol buffer, 8 UBst DNA polymerase (New England BioLabs, Herts, UK), and 2 μl cDNA. The reaction mixture was incubatedin a water bath for 45–50 min at 65 °C prior to incubation at 80 °C for 2 min. 6 μl of the LAMP products were subjected to electrophoresis on a 2%(w/v) agarose gel.
2.6. The specific CDV-H RT-LAMP assay for detection of wild-type CDV
The CDV-H RT-LAMP assay was performed in a total volume of 25 μl consisting of: 0.2 μM F3-H and B3-H primers, 1.2 μM FIP-H and BIP-H primers, 1.4 mM dNTPs, 2.5 μl 1× Thermo Pol buffer, 12 U Bst DNA polymerase (New England BioLabs, Herts, UK) and 2 μl cDNA. The reaction condition was same as above.
2.7. Evaluation of the specificity, sensitivity and reproducibility of CDV-H RT-LAMP assay
Specificity of the CDV-H RT-LAMP assay for wild-type CDV was assessed against CDV vaccine strains (Vacc-A, Vacc-B and Vacc-C), CDV wild-type isolates (ZH (05) and LDH (06)), and non-CDV canine viruses (CPV, CAV-1, CAV-2, CPIV, and CCoV). PCR products amplified from the CDV-YB strain with two external primers F3 and B3 were cloned into pMD18-T Vector (Takara, Dalian, China) and sequenced to validate the results of CDV-H RT-LAMP assay. To establish the sensitivity of the CDV-H RT-LAMP and RT-PCR assay with the primers of CDV-P1/CDV-P4, CDV-YB viral stocks obtained from infected cells were titrated, their titers determined, 10-fold serial dilutions prepared, and viral RNA extracted from 140 μl per dilution. Inter- and intra-assay reproducibility tests were performed in triplicate by testing four cell cultures infected with the CDV-YB strain against four mock-infected cells (data not shown).
2.8. Comparison of the CDV-H RT-LAMP and a multiplex nested RT-PCR assay for detection of CDV in field samples
Field samples (n = 102) from different dog farms in China were tested in parallel with both the CDV-H RT-LAMP assay and a multiplex nested RT-PCR assay (Si et al., 2010).
2.9. Virus isolation
Virus isolation of field samples was performed by using MDCK-SLAM cells (Seki et al., 2003). At least three passages in cell cultures were conducted, followed by IFA using the antiserums for CDV.
3. Results
3.1. Performance of the CDV-N RT-LAMP assay
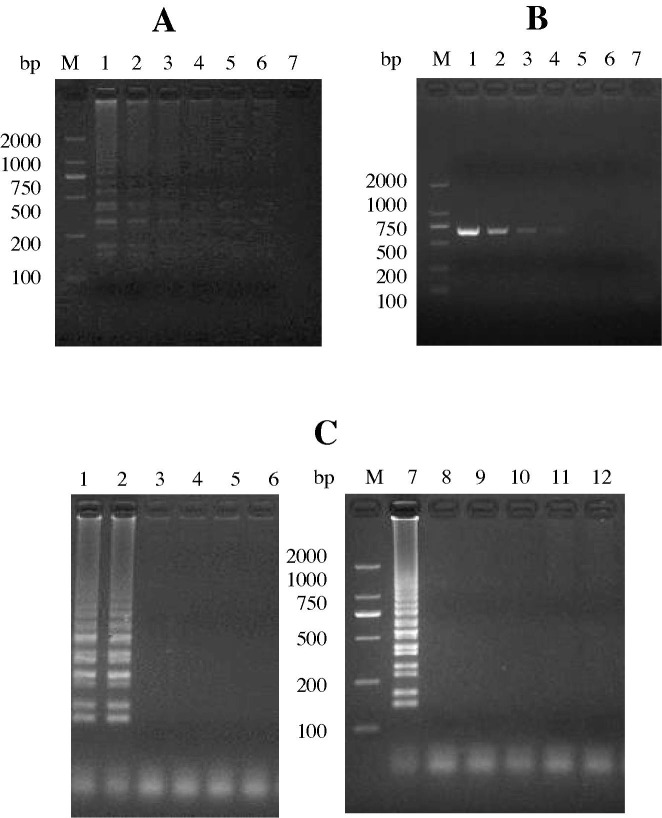
With regard to the dilution series for the CDV-YB strain, the CDV-N RT-LAMP assay detected a limit of 10−1 TCID50 ml−1 of CDV, as observed by gel electrophoresis (Fig. 1 A). The assay detected three strains of wild-type CDV and three vaccine strains, as demonstrated by the bands of differing sizes visualized by agarose electrophoresis, which is consistent with the presence of several inverted-repeat structures for RT-LAMP products (Fig. 1B). No RT-LAMP products were detected for CPV, CAV-1, CAV-2, CPIV and CCoV (Fig. 1C).
Fig. 1.
Sensitivity and specificity of the CDV-N RT-LAMP assay. (A) Sensitivity of the CDV-N RT-LAMP assay. M: DL2000 DNA marker; 1–7: cDNA from different titers of CDV-YB: 104 TCID50 ml−1 (1), 103 TCID50 ml−1 (2), 102 TCID50 ml−1 (3), 101 TCID50 ml−1 (4), 100 TCID50 ml−1 (5), 10−1 TCID50 ml−1 (6), and 10−2 TCID50 ml−1 (7). (B and C) Specificity of the CDV-N RT-LAMP assay. (B) M: DL2000 DNA marker; 1-6: ZH (05), YB, LDH (06), Vacc-A, Vacc-B and Vacc-C. (C) M: DL2000 DNA marker; 1-6: CDV-YB, CPV, CAV-1, CAV-2, CCoV and CPIV.
3.2. Performance of the CDV-H RT-LAMP assay
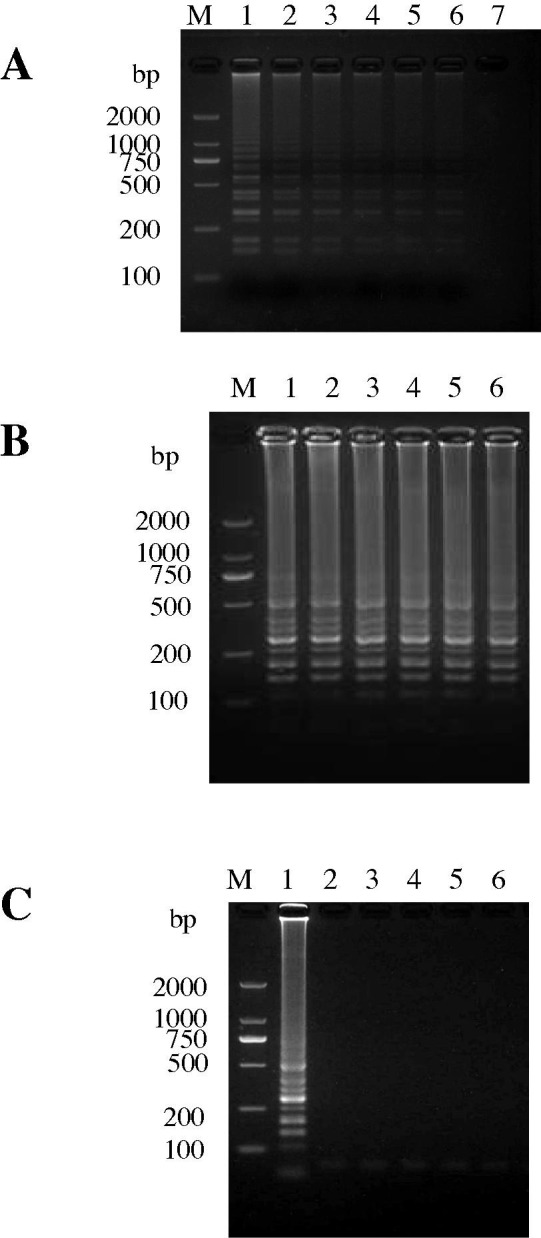
With regard to the dilution series for the CDV-YB strain, the CDV-H RT-LAMP assay detected a limit of 10−1 TCID50 ml−1of CDV, and was 100-fold more sensitive than conventional RT-PCR, as observed by gel electrophoresis (Fig. 2 A and B). The assay detected two strains of wild-type CDV, as demonstrated by the bands of differing sizes visualized by agarose electrophoresis, which is consistent with the presence of several inverted-repeat structures for RT-LAMP products (Fig. 2C). No RT-LAMP products were detected for CPV, CAV-1, CAV-2, CPIV and CCoV (Fig. 2C). Similar results were obtained from three independent tests of four positive and negative samples, performed in triplicate for the inter- and intra-assays, and which demonstrated the reproducibility of the assay (results not included).
Fig. 2.
Sensitivity and specificity of the CDV-H RT-LAMP assay. (A and B) Sensitivity of the CDV-H RT-LAMP assay and RT-PCR (CDV-P1/CDV-P4). M, DL2000 DNA marker; 1–7, cDNA from different titers of CDV-YB: 104 TCID50 ml−1 (1), 103 TCID50 ml−1 (2), 102 TCID50 ml−1 (3), 101 TCID50 ml−1 (4), 100 TCID50 ml−1 (5), 10−1 TCID50 ml−1 (6) and 10−2 TCID50 ml−1 (7). (C) Specificity of the CDV-H RT-LAMP assay. M: DL2000 DNA markers; 1: ZH (05); 2: LDH (06); 3–6: Vacc-A, Vacc-B, Vacc-C and Negative control; 7–12: CDV-YB, CPV, CAV-1, CAV-2, CCoV and CPIV.
3.3. Comparison of the CDV-H RT-LAMP assay with a multiplex nested RT-PCR assay and virus isolation for detection of CDV
Of the 102 field samples, 13 were positive for CDV using both assays. The remaining 89 samples were negative for CDV when tested with the RT-LAMP assay, while 87 samples were classified as negative for the multiplex nested RT-PCR assay. The result of CDV-H RT-LAMP assay was consistent with the virus isolation, which is the “gold standard” for the detection of CDV.
4. Discussion
Canine distemper virus is the etiological agent for a serious and often fatal disease in dogs and many other carnivores. CDV is responsible for several fatal outbreaks in wild carnivores, including the decimation of almost an entire population of African wild dogs (Lycaon pictus) in a Tanzanian reserve, the deaths of approximately 10,000 Caspian seals (Phoca caspica), and a loss of approximately 35% of Serengeti lions (Panthera leo) in an African national park (Van de Bildt et al., 2002, Kuiken et al., 2006, Roelke-Parker et al., 1996).
The control of canine distemper can realistically only be achieved through vaccination (Chappuis, 1995). One of the currently available vaccines (Onderstepoort strain) elicits robust immunity in dogs, but is insufficiently attenuated for other species; high levels of mortality can occur because of its remaining virulence (Carpenter et al., 1976, Barrett, 1999). In general, the vaccine is safe, and highly efficacious in preventing CD in dogs (Gassen et al., 2000). However, the unsuccessful immunisation of dogs against CD has been reported in several countries (Blixenkrone-Moller et al., 1993, Patronek et al., 1995, Gemma et al., 1996, Ek-Kommonen et al., 1997, Józwik and Frymus, 2002).
Many PCR assays can differentiate between dogs vaccinated with modified-live attenuated vaccine from those infected with wild-type virus in herds (Wang et al., 2011, Yi et al., 2012), but RT-LAMP is easier to perform and provides more rapid results.
The success of the RT-LAMP method relies on the specificities of the designed primers for the target RNA sequence. To develop a RT-LAMP method for the specific detection of wild-type CDV variants, we first designed specific primers by targeting the H gene. Under the reaction conditions, the amplification was completed in 45–50 min at a constant temperature of 65 °C. The results demonstrated that the RT-LAMP assay was able to differentiate wild-type CDV isolates from the vaccine strains, with a detection limit of 10−1 TCID50 ml−1 of CDV, and in the absence of cross-reactivity with CAV, CPV, CPIV and CCoV. Furthermore, 102 clinical samples obtained from vaccinated dogs were tested by both RT-LAMP and multiplex nested RT-PCR. Of the samples tested, 13 were positive for both assays. The remaining 89 samples were negative for the RT-LAMP assay, and 87 samples were classified as negative. These results indicate that the specific CDV-H RT-LAMP assay provides a simple, rapid, and sensitive tool for the detection of canines infected with wild-type CDV from canines vaccinated with attenuated vaccine, and has potential use for laboratory and clinical diagnosis practices.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31402201), the National Key Technology R&D Program (2013BAK11B01), the Open Fund of State Key Laboratory of Veterinary Biotechnology of Harbin veterinary research institute (SKLVBF201404), and the Basic Scientific Research Operation Cost of State-leveled Public Welfare Scientific Research Courtyard (0302014014), and the Heilongjiang Postdoctoral Financial Assistance (LBH-Z13043).
Contributor Information
Jin Tian, Email: 289533973@qq.com.
Yu-Ping Hua, Email: 112441457@qq.com.
Lian-Dong Qu, Email: qld@hvri.ac.cn.
References
- Barrett T. Morbillivirus infections, with special emphasis on morbilliviruses of carnivores. Vet. Microbiol. 1999;69:3–13. doi: 10.1016/s0378-1135(99)00080-2. [DOI] [PubMed] [Google Scholar]
- Bellière E.N., Esperón F., Sánchez-Vizcaíno J.M. Genetic comparison among dolphin morbillivirus in the 1990–1992 and 2006–2008 Mediterranean outbreaks. Infect. Genet. Evol. 2011;11:1913–1920. doi: 10.1016/j.meegid.2011.08.018. [DOI] [PubMed] [Google Scholar]
- Blixenkrone-Moller M., Svansson V., Have P., Orvell C., Appel M., Pedersen I.R., Dietz H.H., Henriken P. Studies on manifestations of canine distemper virus infection in an urban dog population. Vet. Microbiol. 1993;37:163–173. doi: 10.1016/0378-1135(93)90190-i. [DOI] [PubMed] [Google Scholar]
- Carpenter J.W., Appel M.J., Erickson R.C., Novilla M.N. Fatal vaccine induced canine distemper virus infection in black-footed ferrets. J. Am. Vet. Med. Assoc. 1976;169:961–964. [PubMed] [Google Scholar]
- Chappuis G. Control of canine distemper. Vet. Microbiol. 1995;44:351–358. doi: 10.1016/0378-1135(95)00028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.S., Park N.Y. Detection of canine distemper virus in blood samples by reverse transcription loop-mediated isothermal amplification. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2005;52:410–413. doi: 10.1111/j.1439-0450.2005.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J.W., Wright K.J., Wallace O.L., Sharma P., Arendt H., Martinez J., DeStefano J., Zamb T.P., Zhang X., Parks C.L. Development of a duplex real-time RT-qPCR assay to monitor genome replication, gene expression and gene insert stability during in vivo replication of a prototype live attenuated canine distemper virus vector encoding SIV gag. J. Virol. Methods. 2014;213C:26–37. doi: 10.1016/j.jviromet.2014.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh D.T., Le M.T., Vuong C.D., Hasebe F., Morita K. An updated loop-mediated isothermal amplification method for rapid diagnosis of H5N1 avian influenza viruses. Trop. Med. Health. 2011;39:3–7. doi: 10.2149/tmh.2010-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ek-Kommonen C., Sihvonen L., Pekkanen K., Rikula U., Nuotio L. Outbreak off canine distemper in vaccinated dogs in Finland. Vet. Rec. 1997;141:380–383. doi: 10.1136/vr.141.15.380. [DOI] [PubMed] [Google Scholar]
- Elia G., Decaro N., Martella V., Cirone F., Lucente M.S., Lorusso E., Di Trani L., Buonavoglia C. Detection of canine distemper virus in dogs by real-time RT-PCR. J. Virol. Methods. 2006;136:171–176. doi: 10.1016/j.jviromet.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Fenner F. Paramyxoviridae. Intervirology. 1976;7:59–60. [Google Scholar]
- Frisk A.L., König M., Moritz A., Baumgärtner W. Detection of canine distemper virus nucleoprotein RNA by reverse transcription-PCR using serum, whole blood, and cerebrospinal fluid from dogs with distemper. J. Clin. Microbiol. 1999;37:3634–3643. doi: 10.1128/jcm.37.11.3634-3643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuta S., Mizukami Y., Ishida A., Kanbe M. Development of loop-mediated isothermal amplification (LAMP)-based SNP markers for shelf-life in melon (Cucumis melo L.) J. Appl. Genet. 2006;47:303–308. doi: 10.1007/BF03194639. [DOI] [PubMed] [Google Scholar]
- Fukuta S., Mizukami Y., Ishida A., Ueda J., Hasegawa M., Hayashi I., Hashimoto M., Kanbe M. Real-time loop-mediated isothermal amplification for the CaMV-35S promoter as a screening method for genetically modified organisms. Eur. Food Res. Technol. 2004;218:496–500. [Google Scholar]
- Gaedke K., Zurbriggen A., Baumgärtner W. In vivo and in vitro detection of canine distemper virus nucleoprotein gene with digoxigenin-labelled RNA, double-stranded DNA probes and oligonucleotides by in situ hybridization. Zentralbl. Veterinarmed B. 1997;44:29–40. doi: 10.1111/j.1439-0450.1997.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Gassen U., Collins F.M., Duprex W.P., Rima B.K. Establishment of a rescue system for canine distemper virus. J. Virol. 2000;74:10737–10744. doi: 10.1128/jvi.74.22.10737-10744.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma T., Watari T., Akiyama K., Miyashita N., Shin Y.S., Iwatsuki K., Kai C., Mikami T. Epidemiological observations on recent outbreaks of canine distemper in Tokyo. J. Vet. Med. Sci. 1996;58:547–550. doi: 10.1292/jvms.58.547. [DOI] [PubMed] [Google Scholar]
- Gray L.K., Crawford P.C., Levy J.K., Dubovi E.J. Comparison of two assays for detection of antibodies against canine parvovirus and canine distemper virus in dogs admitted to a Florida animal shelter. J. Am. Vet. Med. Assoc. 2012;240:1084–1087. doi: 10.2460/javma.240.9.1084. [DOI] [PubMed] [Google Scholar]
- Haas L., Barrett T. Rinderpest and other animal morbillivirus infections: comparative aspects and recent developments. J. Vet. Med. 1996;43:411–420. doi: 10.1111/j.1439-0450.1996.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Hirayama H., Katagiri S., Kageyama S., Minamihashi A., Moriyasu S., Sawai K., Onoe S., Takahashi Y. Rapid sex chromosomal chimerism analysis in heterosexual twin female calves by loop-mediated isothermal amplification. Anim. Reprod. Sci. 2007;101:38–44. doi: 10.1016/j.anireprosci.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Hu J.X., Wen Y.J., Huo Z.Y., Shi X.C., Yang B.C., Wu H., Gao W.F. Establishment and preliminary application development of a loop-mediated isothermal amplification assay for detection of canine distemper virus. Chin. Anim. Husbandry Vet. Med. 2012;39:45–49. [Google Scholar]
- Józwik A., Frymus T. Natural distemper in vaccinated and unvaccinated dogs in Warsaw. J. Vet. Med. B Infect. Dis. Vet. Public Health. 2002;49:413–414. doi: 10.1046/j.1439-0450.2002.00549.x. [DOI] [PubMed] [Google Scholar]
- Kuiken T., Kennedy S., Barrett T., Van de Bildt M.W., Borgsteede F.H., Brew S.D., Codd G.A., Duck C., Deaville R., Eybatov T., Forsyth M.A., Foster G., Jepson P.D., Kydyrmanov A., Mitrofanov I., Ward C.J., Wilson S., Osterhaus A.D. The 2000 canine distemper epidemic in Caspian seals (Phoca caspica): pathology and analysis of contributory factors. Vet. Pathol. 2006;43:321–338. doi: 10.1354/vp.43-3-321. [DOI] [PubMed] [Google Scholar]
- Li Y., Cai S.H. Sensitive and rapid detection of the insect pathogenic fungus Metarhizium anisopliae var. anisopliae by loop-mediated isothermal amplification. Curr. Microbiol. 2011;62:1400–1404. doi: 10.1007/s00284-011-9872-x. [DOI] [PubMed] [Google Scholar]
- Liu C.Y., Wang J.G., Yuan D.L., Wang S., Zhu X.C., Liu W.Q. Development of a reverse transcriptase loop-mediated isothermal amplification (RT-LAMP) system for diagnosis of canine distemper virus. Chin. Vet. Sci. 2010;40:368–372. [Google Scholar]
- McCarthy A.J., Shaw M.A., Jepson P.D., Brasseur S.M., Reijnders P.J., Goodman S.J. Variation in European harbour seal immune response genes and susceptibility to phocine distemper virus (PDV) Infect. Genet. Evol. 2011;11:1616–1623. doi: 10.1016/j.meegid.2011.06.002. [DOI] [PubMed] [Google Scholar]
- Meertens N., Stoffel M.H., Cherpillod P., Wittek R., Vandevelde M., Zurbriggen A. Mechanism of reduction of virus release and cell–cell fusion in persistent canine distemper virus infection. Acta Neuropathol. 2003;106:303–310. doi: 10.1007/s00401-003-0731-0. [DOI] [PubMed] [Google Scholar]
- Müller C.F., Fatzer R.S., Beck K. Studies on canine distemper virus persistence in the nervous system. Acta Neuropathol. 1995;89:438–445. doi: 10.1007/BF00307649. [DOI] [PubMed] [Google Scholar]
- Njiru Z.K., Yeboah-Manu D., Stinear T.P., Fyfe J.A. Rapid and sensitive detection of Mycobacterium ulcerans using a loop-mediated isothermal amplification test. J. Clin. Microbiol. 2012;10:1737–1741. doi: 10.1128/JCM.06460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T., Okayama H., Masubuchi H., Yonekawa T., Watanabe K., Amino N., Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patronek G.J., Glickman L.T., Johnson R., Emerick T.J. Canine distemper infection in pet dogs: a case control study of risk factors during a suspected outbreak in Indiana. J. Am. Anim. Hosp. Assoc. 1995;31:230–235. doi: 10.5326/15473317-31-3-230. [DOI] [PubMed] [Google Scholar]
- Qiu W., Zheng Y., Zhang S.F., Fan Q.S., Liu H., Zhang F.Q., Wang W., Liao G.Y., Hu R.L. Canine distemper outbreak in rhesus monkeys, China. Emerg. Infect. Dis. 2011;17:1541–1543. doi: 10.3201/eid1708.101153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston S.H., Afzal M.A., Helfrich M.H., Fraser W.D., Gallagher J.A., Mee A., Rima B. Multicenter blinded analysis of RT-PCR detection methods for paramyxoviruses in relation to Paget’s disease of bone. J. Bone Miner Res. 2007;22:569–577. doi: 10.1359/jbmr.070103. [DOI] [PubMed] [Google Scholar]
- Roelke-Parker M.E., Munson L., Packer C., Kock R., Cleaveland S., Carpenter M., O’Brien S.J., Pospischil A., Hofmann-Lehmann R., Lutz H., Mwamengele G.L., Mgasa M.N., Machange G.A., Summers B.A., Appel M.J. A canine distemper virus epidemic in Serengeti lions (Panthera leo) Nature. 1996;379:441–445. doi: 10.1038/379441a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rzezutka A., Mizak B. Application of N-PCR for diagnosis of distemper in dogs and fur animals. Vet. Microbiol. 2002;88:95–103. doi: 10.1016/s0378-1135(02)00097-4. [DOI] [PubMed] [Google Scholar]
- Seki F., Ono N., Yamaguchi R., Yanagi Y. Efficient isolation of wild strains of canine distemper virus in Vero cells expressing canine SLAM (CD150) and their adaptability to marmoset B95a cells. J. Virol. 2003;77:9943–9950. doi: 10.1128/JVI.77.18.9943-9950.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Si W., Zhou S., Wang Z., Cui S.J. A multiplex reverse transcription-nested polymerase chain reaction for detection and differentiation of wild-type and vaccine strains of canine distemper virus. Virol. J. 2010;7:86. doi: 10.1186/1743-422X-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltan M.A., Abd-Eldaim M.M. Emergence of peste des petits ruminants virus lineage IV in Ismailia Province, Egypt. Infect. Genet. Evol. 2014;28:44–47. doi: 10.1016/j.meegid.2014.08.016. [DOI] [PubMed] [Google Scholar]
- Van de Bildt M.W., Kuiken T., Visee A.M., Lema S., Fitzjohn T.R., Osterhaus A.D. Distemper outbreak and its effect on African wild dog conservation. Emerg. Infect. Dis. 2002;8:211–213. doi: 10.3201/eid0802.010314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevelde M., Zurbriggen A. Demyelination in canine distemper virus infection: a review. Acta Neuropathol. 2005;109:56–68. doi: 10.1007/s00401-004-0958-4. [DOI] [PubMed] [Google Scholar]
- von Messling V., Harder T.C., Moennig V., Rautenberg P., Nolte I., Haas L. Rapid and sensitive detection of immunoglobulin M (IgM) and IgG antibodies against canine distemper virus by a new recombinant nucleocapsid protein-based enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1999;37:1049–1056. doi: 10.1128/jcm.37.4.1049-1056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Yan X., Chai X., Zhang H., Zhao J., Wen Y., Wu W. Differentiation of canine distemper virus isolates in fur animals from various vaccine strains by reverse transcription-polymerase chain reaction-restriction fragment length polymorphism according to phylogenetic relations in china. Virol. J. 2011;27:85. doi: 10.1186/1743-422X-8-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Qin H.B., He X.L., Zhu Q., Gao Y.L., Zhang H.D. Detection of canine distemper virus by reverse transcriptase loop-mediated isothermal amplification method. Chin. J. Comp. Med. 2012;22:51–55. [Google Scholar]
- Wu S.C., Chueh L.L., Pang V.F., Jeng C.R., Lee C.C., Liu C.H. Pathology and RT-PCR employed in the identification of canine distemper virus infection in Taiwan. J. Chin. Soc. Vet. Sci. 2000;26:328–338. [Google Scholar]
- Yi L., Cheng S., Xu H., Wang J., Cheng Y., Yang S., Luo B. Development of a combined canine distemper virus specific RT-PCR protocol for the differentiation of infected and vaccinated animals (DIVA) and genetic characterization of the hemagglutinin gene of seven Chinese strains demonstrated in dogs. J. Virol. Methods. 2012;179:281–287. doi: 10.1016/j.jviromet.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]