Summary
The Middle East respiratory syndrome coronavirus (MERS-CoV) that causes a severe lower respiratory tract infection in humans is now considered a pandemic threat to the Gulf region. Since its discovery in 2012, MERS-CoV has reached 23 countries affecting about 1100 people, including a dozen children, and claiming over 400 lives. Compared to SARS (severe acute respiratory syndrome), MERS-CoV appears to kill more people (40% versus 10%), more quickly, and is especially more severe in those with pre-existing medical conditions. Most MERS-CoV cases (>85%) reported thus far have a history of residence in, or travel to the Middle East. The current epidemiology is characterised by slow and sustained transmission with occasional sparks. The dromedary camel is the intermediate host of MERS-CoV, but the transmission cycle is not fully understood. In this current review, we have briefly summarised the latest information on the epidemiology, clinical features, diagnosis, treatment and prevention of MERS-CoV especially highlighting the knowledge gaps in its transmission dynamics, diagnosis and preventive strategy.
Keywords: Dromedary, MERS-CoV, Middle East, Respiratory tract infection, SARS, Transmission chain
Introduction
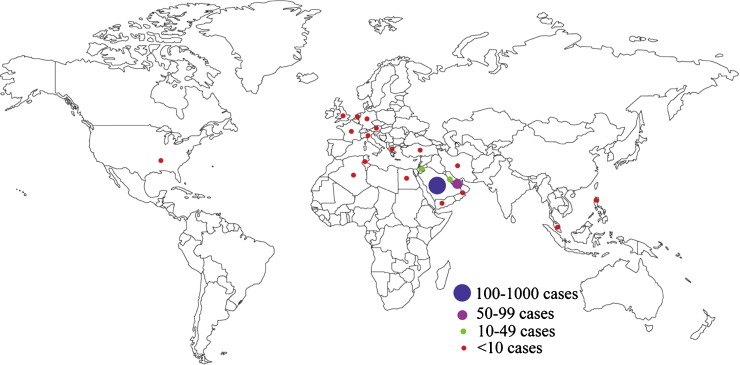
The Middle East respiratory syndrome coronavirus (MERS-CoV) was first isolated by Zaki and co-workers in June 2012 from a Saudi male aged 60 years who died of severe pneumonia and renal failure [1]. Currently, the virus has affected about 1100 individuals in 23 countries across the globe, claiming 439 (40%) lives, and thus poses a public health challenge to the Arabian Peninsula and elsewhere (Figure 1 ) [2], [3]. MERS-CoV outbreak began a decade after the epidemic of the severe acute respiratory syndrome (SARS) that caused a global scare in 2002-2003 affecting over 8000 people across the world killing one tenth of them [4], [5], [6]. However, unlike SARS, which disappeared within a year, the MERS-CoV epidemic continues (Table 1 ).
Figure 1.
Geographical distribution of confirmed MERS-CoV cases
Table 1.
Contrast between SARS and MERS-CoV in respect to their virology, epidemiology and clinical outcomes
| MERS-CoV | SARS | |
|---|---|---|
| Virology | Betacoronavirus lineage 2C | Betacoronavirus lineage 2B |
| Receptor | hDPP4 | ACE2 |
| Genome size | 29.9 kb | 29.3kb |
| Source | Not yet confirmed, camel is the likely host | Civet cat |
| Epidemiology | Limited human to human transmission, the disease is mostly localised in the Middle East | Human to human transmission is well-recognised, affected many countries but spared the Middle East |
| Cases (as of 24th April, 2015) | ∼1100 (deaths 439) | ∼8100 (deaths 774) |
| R0 | 2-3 (for Jeddah 3.5-6.7, for Riyadh 2-2.8) | Variable, ranges from 2-6 |
| Superspreading events | Not known | Reported |
| M:F | 1.74:1 | 0.75:1 |
| Median age (range) in years | 48 (1-99) | 40 (1-91) |
| Mean incubation period in days (range) | 5 (2-15) | 4 (2-14) |
| Comorbidities | Three quarter of the patients had comorbidities | Less than a third had Comorbidities |
| Clinical presentation | Unpredictable and erratic clinical course ranging from asymptomatic illness to severe pneumonia | A typical biphasic clinical course |
| Haemoptysis | More common | Less common |
| Respiratory failure | Presents relatively early | Presents relatively late |
| Travel association | Limited travel-associated exposure | Recognised travel-associated exposure |
| Time from symptom onset to hospitalisation | 0-16 days | 2-8 days |
| Median time from symptom onset to death | 12 days | 21 days |
Since its discovery, a number of excellent reviews have been published describing the important aspects of MERS-CoV, but few have summed up the current research gaps in light of the spectrum of burning questions ranging from its clinical presentation to molecular virology [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. In this review, we have summarised the latest findings in the field of MERS-CoV, especially its molecular virology, epidemiology, clinical features, diagnosis and infection control management plans, and highlighted the knowledge gaps in transmission modes, and attempted to explain unanswered questions raised by other investigators.
Literature searches were performed in PubMed, Promed Mail, Google and Google Scholar on articles published between September 2012 and March 2015. The following vocabulary terms were used: ‘coronavirus’, ‘mers-cov’, ‘coronavirus mers-cov’, ‘mers-cov review’, ‘mers-cov infection’, ‘mers-cov outbreak’, ‘mers-cov case studies/reports’, ‘mers-cov in camel’, ‘mers-cov in bat’, ‘mers-cov transmission’, ‘mers-cov pathogenesis’, ‘mers-cov antibodies’, ‘mers-cov receptor’, ‘mers-cov in animal’, ‘mers-cov diagnosis’, ‘mers-cov treatment’, ‘mers-cov vaccine’, ‘mers-cov in saudi’ and ‘mers-cov in health care worker’.
Virology of MERS-CoV
MERS-CoV is a positive-sense, enveloped, single-stranded RNA virus belonging to the genus of Betacoronavirus within the subfamily Coronavirinae [21]. Known in the begining variously as novel corona virus (nCoV), London1_novel CoV 2012, MERS-CoV has become the first lineage of Betacoronavirus known to infect humans [7], [9]. Having 90% sequence homology, MERS-CoV is more closely related to bat coronaviruses HKU4 and HKU5 (lineage 2C) than it is to SARS (lineage 2B) [4], [21], and is genetically related to other Betacoronaviruses isolated from bats in Europe, Mexico, Africa, Hong Kong and China [22], [23], [24].
Following its first isolation, the virus was propagated in African green monkey and rhesus macaque kidney cells (Vero and LLC-MK2 cell lines, respectively) [1], [25], and subsequently, in other cell lines including human derived cells like Calu-3, HFL, Caco-2, Huh-7, HEK, and His-1 [25]. MERS-CoV propagates well in human bronchial and lung tissues [26]. Cell lines derived from camels and goats were more functional for the replication of MERS-CoV than those from other species including dogs, cats, mice, hamsters, ferrets and equids [13], [27], [28], [29], indicating that the binding site of MERS-CoV could vary by species [27], [30].
Hemida et al. (2014) described the full genome of MERS-CoV from dromedaries demonstrating that it had 99.9% homology with human clade B MERS-CoV [31], which was confirmed by a subsequent study [13]. MERS-CoV has also high sequence homology (94%) with coronavirus carried by Pipistrellus bats [32].
Electron microscopy reveals that the virion contains club-like projections, representing viral spike peplomers, emanating from the viral membrane. MERS-CoV gains entry into target cells by binding to the receptor binding domain (RBD) on its spike glycoprotein to human receptor dipeptidyl peptidase 4 (hDPP4), whereas SARS enters the target cells via angiotensin converting enzyme 2 (ACE2) [25], [33], [34]. Both ACE2 and hDPP4 are commonly expressed in some human organs such as kidneys [33]. MERS-CoV utilises host proteases to gain entry into lung cells [35]. The spike protein on the viral envelope is activated by a protease, called furin, and mediates membrane fusion which eventually supports the virus entry into a host cell [33], [36], [37]. Consequently, protease inhibitors (e.g., camostat) are found to block MERS-CoV entry into cells [35].
MERS-CoV inoculated into rhesus macaques caused a transient lower respiratory tract infection, a multifocal, mild to marked interstitial pneumonia, with virus replication occurring mainly in alveolar pneumocytes [38], and caused extensive lethal pneumonia in the common marmoset [39]. In vitro experiments suggest that unlike SARS, MERS-CoV has cytopathic effects on kidney cells, and compared to SARS, MERS patients are more likely to develop acute renal failure [33].
Detailed pathogenesis of MERS-CoV either in humans or in other animals is not available [40], but, both the dromedary and human strains of MERS-CoV have comparable replication (in Vero-E6 cells) and respiratory tropism, and both disrupt the interferon responses [26], [41]. Both strains cause alveolar epithelial damage and apoptosis in various parts of the lungs [42], [43]. In transgenic mouse model, MERS viral particles were observed in lung, brain, spleen, intestine and heart tissues [44]. Further analysis is necessary to understand the virus-host interaction and the detailed pathogenesis of MERS-CoV infection in in vivo.
Epidemiology of MERS-CoV
Since its first discovery, MERS-CoV has reached more than twenty countries. As of March 2015, most of the MERS-CoV cases had been reported in Saudi Arabia (n = 938), followed by the United Arab Emirates, Jordan and Qatar. A handful of cases have been reported from Oman, Egypt, France, Germany, Tunisia, Italy, Algeria, Iran, the Netherlands, Greece, Kuwait, Lebanon, Malaysia, Philippines, Yemen, Austria, Turkey, United Kingdom and United States (Figure 1) [45], [46], [47], [48], [49].
Although the progression of the disease in the initial phase was much slower, with the basic reproduction number (R0) of 0·69 (95% CI 0·50-0·92); the latest estimate suggests that the disease actually has a higher but variable R0 (ranging from 2 to 6.7) depending on the geographic region (Table 1) [50], [51].
Available data suggest that healthcare exposure is the most important risk factor for MERS-CoV infection [52], [53], [54]. Mathematical modelling suggests that nosocomial transmission is over four times higher than community transmission [55]. Other risk factors like low humidity and high temperature are also important [56], and limited studies suggested a seasonal pattern with spikes in March -April [57].
Clinical features
Men aged over 45 years, people with pre-existing medical conditions and health care workers are the high risk groups for MERS-CoV infection [54]. The median incubation period for human-to-human transmission is approximately 5 days (range 2–15 days) [9].
MERS-CoV causes symptoms similar to that of SARS but with a distinct clinical course and a high case fatality rate of 35% to 50% [58], [59]. Most cases present with symptoms of influenza-like illness (ILI) such as fever, cough (predominantly dry), malaise, myalgia, sore throat, headache, rhinorrhoea, nausea, vomiting, abdominal pain, diarrhoea, and even renal failure occur occasionally [53], [57], [60]. Dyspnoea is a frequent complaint, and the majority of the patients develop pneumonia (70%) and ultimately require admission into an intensive care unit (ICU). Concomitant infections and hypoalbuminemia were identified as the predictors of severe infection in individuals aged >65 years [53]. Other extra-pulmonary organ dysfunction such as circulatory collapse, abnormal liver functions and hematological derangements are common in critically ill patients [61]. A second-trimester stillbirth has been reported in a pregnant woman with MERS-CoV [62].
While MERS-CoV is common in adults; a dozen paediatric cases, including a fatality, have been reported to date [63]. Most of the paediatric cases were asymptomatic and found during the screening process among close family contacts of MERS-CoV patients in the community or in hospital. Children with underlying medical conditions are at higher risk of MERS-CoV infection [63]. Mortality from MERS-CoV is higher in men and in those with pre-existing medical conditions [7]. The median time from symptom onset to hospitalisation is approximately 4 days, and time to ICU admission is approximately 5 days, and ventilatory care is required for a median of 16 days. The duration of an ICU stay is about 30 days however fatality occurs at a median of 12 days following symptom onset. The 90 day mortality for MERS patients in ICU is 58% [9].
The chest radiograph of a MERS-CoV patient typically shows bilateral enhanced hilar vascular shadows (more prominent on the left side), accentuated bronchovascular markings with multiple patchy opacities in the middle and lower lung fields. Consolidation of the right upper lobe can occur as early as one day after the onset of illness and ground glass opacities and consolidation of the left lower lobe can occur within 4 days of symptom onset, bilateral ground-glass opacities and consolidation and can occur respectively 7 and 9 days after the onset of illness [4], [64].
Diagnosis of MERS-CoV
Since most MERS-CoV cases present with symptoms similar to other respiratory viral infections, case detection purely based on syndromic diagnosis is challenging. Since the first discovery of the virus, comprehensive laboratory testings have been developed [56], [65]. Serological assays have been widely used to detect MERS-CoV antibody in dromedary camels [56], [66], [67]. Conventional and rapid biologically safe immunofluorescence assays to detect MERS-CoV antibodies have been published [68]. IgG and IgM antibodies in serum samples could be determined using an anti-MERS-CoV indirect immunofluorescence assay [56], [67], [69].
In addition, the enzyme-linked immunosorbent assays (ELISA), protein microarray technology and micro-neutralisation (MN) assays have also been developed, and have high sensitivity and specificity to detect MERS-CoV antibodies [56], [67], [70]. For seroepidemiological studies, assays like pseudoparticle virus neutralisation test (ppNT) and a conventional MN assay could be used to detect antibodies to MERS-CoV [49], [71]. Western blotting is also useful in serological diagnosis of MERS-CoV [71], [72]. However, serological tests may lack validity and cross react with other coronaviruses [56].
According to the World Health Organization (WHO) and experts in the field, the screening RT-PCR targeting Up E gene should be conducted on samples from suspected MERS patients. All positive samples should undergo confirmatory testing by tagerting ORF 1a, ORF 1b or N gene [35], [56]. Endeavours should be made to obtain lower respiratory tract samples such as bronchoalveolar lavage and tracheal aspirate, since the viral loads and genome fractions are higher in lower respiratory tract samples [73]. Sequencing data can be used to construct a phylogenetic tree in order to measure the genetic distance between the viruses of different intermediate hosts [66], [74].
Management of MERS-CoV
To date, no approved antiviral therapy or vaccination is available for MERS-CoV [58], [75]. Based on in vitro experiments or experience from SARS patients various treatment options have been attempted or suggested [39], [76]. Omrani et al. (2014) showed that in patients with severe MERS-CoV infection, ribavirin and interferon alfa-2a therapy are significantly associated with improved survival at 14 days, but not at 28 days [58]. In vitro studies also suggest that the ribavirin and interferon alpha-2b combination therapy has significant antiviral effects [77].
Using distinct clones of anti-CD26 monoclonal antibodies, the domains of CD26 involved in the binding of MERS-CoV have been identified [78]. It has been suggested that 2F9, a clone of CD26, and YS110, a humanised monoclonal antibody against CD26, could be a potential therapeutic agent for MERS-CoV [78]. One of the antibodies, m336, neutralises the virus with exceptional potency, and therefore, has potential as a candidate drug and could be even useful in vaccine design [79]. Additionally, human MicroRNAs might be useful as antiviral therapy against MERS-CoV infection [80]. In case of hospitalised patients, the untested convalescent-phase plasma has been suggested as a supportive therapy to minimise the severity of infection [81].
A few candidate vaccines have been tested in mice with some promising results [82]. Subunit vaccines based on MERS-CoV spike protein and its RBD could be useful in the development of MERS-CoV vaccine [83], [84]. Inhibiting papain-like or 3C-like proteases of MERS-CoV which regulate the polyproteins in MERS-CoV genomic RNA could be a useful concept in vaccine design [85].
The control of MERS-CoV primarily relies on case-based surveillance; early diagnosis is warranted when infection is suspected [86]. The role of respiratory protective equipment such as surgical mask and N95 respirators have been discussed but not yet proven [87]. A large study which is examining the role of facemasks against MERS-CoV among Hajj pilgrims is currently underway [88].
Although person to person transmission is limited, travellers to the Middle East could be at risk of exposure to MERS-CoV [89], [90], [91], [92]. Raising awareness of MERS-CoV transmission is important in view of the fact that many travellers are not aware of the MERS-CoV outbreak [93].
It is essential to intensify infection control measures in health care settings, particularly through health education and awareness [94]. Health-care workers also need to follow stringent precautions while handling suspected MERS-CoV patients including using eye protectors and other personal protective equipments [55]. Immunocompromised individuals and those with pre-existing medical conditions should avoid close contact with dromedary camels particularly if the virus is known to be circulating in an area. Similarly raw camel milk, meat and urine should be avoided.
Current research gaps
An important current research gap is that the mechanism by which most people acquired MERS-CoV is unclear; the mechanism of exposure (whether direct or indirect) is difficult to explain and the experiments are fraught with challenges [47], [95]. Serological and molecular evidence of the presence of the virus have been established in domestic dromedaries [96], [97], [98] but not in other livestock [99], [100]. The virus has also been recovered from respiratory, gastrointestinal and other bodily secretions or samples of dromedary camels and fruit bats [66], [96], [101], [102], but serological evidence of MERS-CoV in animal workers is rare [103]. MERS-CoV has been detected from air samples collected from a barn that sheltered an infected camel owned by an infected patient indicating possible airborne transmission of the virus [104].
Secondly, the direction of transmission, whether from humans to camels or vice versa is unknown. MERS-CoV was identified in dromedary camels in a Qatari barn, which was linked to two confirmed human cases who have since recovered [66], and it was also possible to inoculate MERS-CoV into healthy camels ultimately producing upper respiratory tract symptoms [105], but it could not be established whether the people on the farm were infected by the camels or vice versa, or if a third source was responsible [60].
Thirdly, it remains unclear if a third (intermediate) host is playing a key role in the transmission chain. Phylogenetic studies have revealed a close relationship between MERS-CoV in humans and coronaviruses in bats but the exact virus has not been confirmed in bats [21], [106]. It is noteworthy that most other human coronaviruses have emerged upon transmission from bats to other animal species [13], [104]. Although others reported that a short genomic sequence isolated from an Egyptian tomb bat (Taphozous perforates) was identical to that of EMC/2012 MERS-CoV Essen isolate (KC875821); there is a dearth of information establishing association with the bat virus [60].
Fourthly, a few reports suggested that MERS-CoV can transmit from human-to- human [107], [108], [109], but, the available data show uncertainty or only limited human-to-human transmission of the virus [30], [55], [108], [110], [111], [112], [113], [114]. Low levels of virus shedding might be an explanation why human-to-human transmission is limited or unlikely [66], [115].
Finally, the evolutionary background of MERS-CoV is still unclear [116]. MERS-CoV might have been circulating in camels in Saudi Arabia since at least 1992 [100]. MERS-CoV antibody was found in African camels during 1992-2013 suggesting that the virus has existed in camels for long time [117], [118]. However, MERS-CoV in camels may have undergone a mutation several years ago, allowing the virus to infect humans.
Future research directions
-
•
There is a paucity of data describing the transmission cycle of MERS-CoV in various hosts. Well-designed large scale case-control studies are needed to define the transmission chain of MERS-CoV.
-
•
Research into safe and effective antiviral treatments need to be prioritised for a condition with a high mortality rate that threatens as a pandemic.
Educational aims
The reader will be able to:
-
•
Get the latest update on MERS-CoV, including its clinical features, diagnosis and management.
-
•
Learn about the adult and paediatric aspects of MERS-CoV.
-
•
Understand the gaps in current knowledge and future research in the field of MERS-CoV.
Acknowledgements
We thank Dr Mohamed Tashani for his help with Figure 1. G. Khandaker is supported by NHMRC Health Early Career Fellowship (1054414). Conflicts of interest
No conflicts of interest are declared.
References
- 1.Zaki A., van Boheemen S., Bestebroer T. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 2.Berry M., Gamieldien J., Fielding B.C. Identification of new respiratory viruses in the new Millennium. Viruses. 2015;7:996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Centre for Disease prevention and control (ECDC) updated rapid risk assessment on MERS-CoV. 8th March, 2015; http://www.ecdc.europa.eu/en/healthtopics/coronavirus infections/Pages/publications.aspx. (accessed 24 April 2015).
- 4.Álvarez E., Donado-Campos J., Morilla F. New coronavirus outbreak. Lessons learned from the severe acute respiratory syndrome epidemic. Epidemiol Infect. 2015:1–12. doi: 10.1017/S095026881400377X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joob B., Wiwanitkit V. MERS-CoV. Oman Med J. 2014;29:381. doi: 10.5001/omj.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharif-Yakan A., Kanj S.S. Emergence of MERS-CoV in the Middle East: origins, transmission, treatment, and perspectives. PLoS Pathog. 2014:10e1004457. doi: 10.1371/journal.ppat.1004457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jadav H.A. Middle East respiratory syndrome- corona virus (MERS-CoV): A deadly Killer. IOSR JPBS. 2013;8:74–81. [Google Scholar]
- 8.Al-Tawfiq J.A., Memish Z.A. Middle East respiratory syndrome coronavirus: transmission and phylogenetic evolution. Trends Microbiol. 2014;22:573–579. doi: 10.1016/j.tim.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha C.B., Opal S.M. Middle East respiratory syndrome (MERS): A new zoonotic viral pneumonia. Virulence. 2014;5:650–654. doi: 10.4161/viru.32077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdel-Moneim A. Middle East respiratory syndrome coronavirus (MERS-CoV): evidence and speculations. Arch Virol. 2014;159:1575–1584. doi: 10.1007/s00705-014-1995-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou N., Zhang Y., Zhang J.-C. The receptor binding domain of MERS-CoV: The dawn of vaccine and treatment development. J Formos Med Assoc. 2014;113:143–147. doi: 10.1016/j.jfma.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geng H., Tan W. A novel human coronavirus: Middle East respiratory syndrome human coronavirus. Sci China Life Sci. 2013;56:683–687. doi: 10.1007/s11427-013-4519-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raj V.S., Osterhaus A.D.M.E., Fouchier R.A.M. MERS: emergence of a novel human coronavirus. Curr Opin virol. 2014;5:58–62. doi: 10.1016/j.coviro.2014.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milne-Price S., Miazgowicz K.L., Munster V.J. The emergence of the Middle East respiratory syndrome coronavirus. Pathog Dis. 2014;71:121–136. doi: 10.1111/2049-632X.12166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Payne B., Bellamy R. Novel respiratory viruses: what should the clinician be alert for? Clin Med. 2014;14:12–16. doi: 10.7861/clinmedicine.14-6-s12. [DOI] [PubMed] [Google Scholar]
- 16.Hayward A., Fragaszy E. Emerging respiratory infections: influenza, MERS-CoV, and extensively drug-resistant tuberculosis. Lancet Respir Med. 2014;2:970–972. doi: 10.1016/S2213-2600(14)70250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Al-Tawfiq J.A. Middle East respiratory syndrome-coronavirus infection: An overview. J Infect Public Health. 2013;6:319–322. doi: 10.1016/j.jiph.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cabral L. Update on Middle East respiratory syndrome coronavirus (MERS-CoV) Aust Vet J. 2014;92:N16. [PubMed] [Google Scholar]
- 19.Hui D., Memish Z.A., Zumla A. Severe acute respiratory syndrome vs. the Middle East respiratory syndrome. Curr Opin Pulm Med. 2014;20:233–241. doi: 10.1097/MCP.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 20.Alsolamy S. Middle East respiratory syndrome: Knowledge to date. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000000966. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 21.de Groot R.J., Baker S.C., Baric R.S. Middle East respiratory syndrome coronavirus (MERS-CoV): Announcement of the Coronavirus Study Group. J Virol. 2013;87:7790–7792. doi: 10.1128/JVI.01244-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang L., Wu Z., Ren X. MERS-related betacoronavirus in Vespertilio superans. bats, China. Emerg Infect Dis. 2014;20:1260–1262. doi: 10.3201/eid2007.140318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q., Qi J., Yuan Y. Bat origins of MERS-CoV supported by bat coronavirus HKU4 usage of human receptor CD26. Cell Host Microbe. 2014;16:328–337. doi: 10.1016/j.chom.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotten M., Lam T.T., Watson S.J. Full-genome deep sequencing and phylogenetic analysis of novel human betacoronavirus. Emerg Infect Dis. 2013;19:736. doi: 10.3201/eid1905.130057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shirato K., Kawase M., Matsuyama S. Middle East respiratory syndrome coronavirus infection mediated by the transmembrane serine protease TMPRSS2. J Virol. 2013;23:12552–12561. doi: 10.1128/JVI.01890-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan R.W.Y., Chan M.C.W., Agnihothram S. Tropism of and innate immune responses to the novel human betacoronavirus lineage C virus in human ex vivo respiratory organ cultures. J Virol. 2013;87:6604–6614. doi: 10.1128/JVI.00009-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raj V.S., Smits S.L., Provacia L.B. Adenosine deaminase acts as a natural antagonist for dipeptidyl peptidase 4-mediated entry of the Middle East respiratory syndrome coronavirus. J Virol. 2014;88:1834–1838. doi: 10.1128/JVI.02935-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyer B., García-Bocanegra I., Wernery U. Serologic assessment of possibility. for MERS-CoV infection in equids. Emerg Infect Dis. 2015;21:181–182. doi: 10.3201/eid2101.141342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckerle I., Corman V.M., Müller M.A. Replicative capacity of MERS coronavirus in livestock cell lines. Emerg Infect Dis. 2014;20:276–279. doi: 10.3201/eid2002.131182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van den Brand J.M.A., Smits S.L., Haagmans B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J Pathol. 2014;235:175–184. doi: 10.1002/path.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemida M., Chu D., Poon L. MERS coronavirus in dromedary camel herd, Saudi Arabia. Emerg Infect Dis. 2014;20:1231–1234. doi: 10.3201/eid2007.140571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Memish Z.A., Mishra N., Olival K.J. Middle East respiratory syndrome coronavirus in bats, Saudi Arabia. Emerg Infect Dis. 2013;19:1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eckerle I., Muller M., Kallies S. In-vitro renal epithelial cell infection reveals a viral kidney tropism as a potential mechanism for acute renal failure during Middle East respiratory syndrome (MERS) coronavirus infection. Virol J. 2013;10:359. doi: 10.1186/1743-422X-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song W., Wang Y., Wang N. Identification of residues on human receptor DPP4 critical for MERS-CoV binding and entry. Virology. 2014;471–473:49–53. doi: 10.1016/j.virol.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shirato K., Yano T., Senba S. Detection of Middle East respiratory syndrome coronavirus using reverse transcription loop-mediated isothermal amplification (RT-LAMP) Virol J. 2014;11:139. doi: 10.1186/1743-422X-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hofmann H., Pöhlmann S. Cellular entry of the SARS coronavirus. Trends Microbiol. 2004;12:466–472. doi: 10.1016/j.tim.2004.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimitrov D.S. Virus entry: molecular mechanisms and biomedical applications. Nat Rev Micro. 2004;2:109–122. doi: 10.1038/nrmicro817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Wit E., Rasmussen A.L., Falzarano D. Middle East respiratory syndrome coronavirus (MERS-CoV) causes transient lower respiratory tract infection in rhesus macaques. Proc Natl Acad Sci. 2013;110:16598–16603. doi: 10.1073/pnas.1310744110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falzarano D., de Wit E., Feldmann F. Infection with MERS-CoV causes lethal pneumonia in the common Marmoset. PLoS Pathog. 2014;10:e1004250. doi: 10.1371/journal.ppat.1004250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fehr A., Perlman S. Coronaviruses: an overview of their replication and pathogenesis. Methods Molecular Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scheuplein V.A., Seifried J., Malczyk A.H. High secretion of interferons by human plasmacytoid dendritic cells upon recognition of MERS-CoV. J Virol. 2015;89:3859–3869. doi: 10.1128/JVI.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hocke A.C., Becher A., Knepper J. Emerging human Middle East respiratory syndrome coronavirus causes widespread infection and alveolar damage in human Lungs. Am J Respir Crit Care Med. 2013;188:882–886. doi: 10.1164/rccm.201305-0954LE. [DOI] [PubMed] [Google Scholar]
- 43.de Wilde A.H., Raj V.S., Oudshoorn D. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J General Virol. 2013;94:1749–1760. doi: 10.1099/vir.0.052910-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agrawal A.S., Garron T., Tao X. Generation of transgenic mouse model of Middle East respiratory syndrome-coronavirus infection and disease. J Virol. 2015;89:3659–3670. doi: 10.1128/JVI.03427-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.The WHO MERS-CoV research group. State of knowledge and data gaps of Middle East respiratory syndrome coronavirus (MERS-CoV) in humans. PLoS Curr. 2013;5 doi: 10.1371/currents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. pii: ecurrents.outbreaks.0bf719e352e7478f8ad85fa30127ddb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yavarian J., Rezaei F., Shadab A. Cluster of Middle East respiratory syndrome coronavirus infections in Iran. Emerg Infect Dis. 2014;21:362–364. doi: 10.3201/eid2102.141405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gardner L.M., Rey D., Heywood A.E. A scenario-based evaluation of the Middle East respiratory syndrome coronavirus and the Hajj. Risk Anal. 2014;34:1391–1400. doi: 10.1111/risa.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization (Global alert and response). Middle East respiratory syndrome coronavirus (MERS-CoV) – update, 23rd July 2014; http://www.who.int/csr/don/2014_07_23_mers/en/. (accessed 24 April, 2015).
- 49.Hemida M., Perera R., Al Jassim R. Seroepidemiology of Middle East respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Euro Surveill. 2014;19:20828. doi: 10.2807/1560-7917.es2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Breban R., Riou J., Fontanet A. Interhuman transmissibility of Middle East respiratory syndrome coronavirus: estimation of pandemic risk. Lancet. 2013;382:694–699. doi: 10.1016/S0140-6736(13)61492-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Majumder M.S., Rivers C., Lofgren E. Estimation of MERS-coronavirus reproductive number and case fatality rate for the spring 2014 Saudi Arabia outbreak: insights from publicly available data. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. pii: ecurrents.outbreaks.98d2f8f3382d84f390736cd5f5fe133c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen E., Pollack M.M., Madoff L.C. Health-care associate transmission of Middle East respiratory syndrome Corona virus, MERS-CoV, in the Kingdom of Saudi Arabia. Int J Infect Dis. 2014;29:299–300. doi: 10.1016/j.ijid.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saad M., Omrani A.S., Baig K. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oboho I.K., Tomczyk S.M., Al-Asmari A.M. 2014 MERS-CoV outbreak in Jeddah-a link to health care facilities. N Engl J Med. 2015;372:846–854. doi: 10.1056/NEJMoa1408636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chowell G., Blumberg S., Simonsen L. Synthesizing data and models for the spread of MERS-CoV, 2013: Key role of index cases and hospital transmission. Epidemics. 2014;9:40–51. doi: 10.1016/j.epidem.2014.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balkhair A., Alawi F.B., Al Maamari K. MERS-CoV: Bridging the knowledge gaps. Oman Med J. 2014;29:169–171. doi: 10.5001/omj.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alghamdi I.G., Hussain I.I., Almalki S.S. The pattern of Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive epidemiological analysis of data from the Saudi Ministry of Health. Int J Gen Med. 2014;7:417–423. doi: 10.2147/IJGM.S67061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Omrani A.S., Saad M.M., Baig K. Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis. 2014;14:1090–1095. doi: 10.1016/S1473-3099(14)70920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sutton T.C., Subbarao K. Development of animal models against emerging coronaviruses: from SARS to MERS coronavirus. Virology. 2015 doi: 10.1016/j.virol.2015.02.030. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azhar E.I., El-Kafrawy S.A., Farraj S.A. Evidence for camel-to-human transmission of MERS Coronavirus. N Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 61.Das K.M., Lee E.Y., Enani M.A. CT correlation with outcomes in 15 Patients with acute Middle East respiratory syndrome coronavirus. AJR Am J Roentgenol. 2015;204:736–742. doi: 10.2214/AJR.14.13671. [DOI] [PubMed] [Google Scholar]
- 62.Payne D.C., Iblan I., Alqasrawi S. Stillbirth during infection with Middle East respiratory syndrome coronavirus. J Infect Dis. 2014;209:1870–1872. doi: 10.1093/infdis/jiu068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Memish Z.A., Al-Tawfiq J.A., Assiri A. Middle East respiratory syndrome coronavirus disease in children. Pediatr Infect Dis J. 2014;33:904–906. doi: 10.1097/INF.0000000000000325. [DOI] [PubMed] [Google Scholar]
- 64.Guery B., van der Werf S. Coronavirus: need for a therapeutic approach. Lancet Infect Dis. 2013;13:726–727. doi: 10.1016/S1473-3099(13)70153-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leclercq I., Batéjat C., Burguière A.M. Heat inactivation of the Middle East respiratory syndrome coronavirus. Influenza Other Respir Viruses. 2014;8:585–586. doi: 10.1111/irv.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Haagmans B.L., Al Dhahiry S.H., Reusken C.B. Middle East respiratory syndrome coronavirus in dromedary camels: an outbreak investigation. Lancet Infect Dis. 2014;14:140–145. doi: 10.1016/S1473-3099(13)70690-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Spanakis N., Tsiodras S., Haagmans B.L. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int Journal Antimicrob Agents. 2014;44:528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyer B., Drosten C., Müller M.A. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175–183. doi: 10.1016/j.virusres.2014.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reusken C.B.E.M., Haagmans B.L., Müller M.A. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet Infect Dis. 2013;13:859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gossner C., Danielson N., Gervelmeyer A. Human–dromedary camel interactions and the risk of acquiring zoonotic Middle East respiratory syndrome coronavirus infection. Zoonoses Public Health. 2014 doi: 10.1111/zph.12171. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Du L., Zhao G., Kou Z. Identification of a receptor-binding domain in the S protein of the novel human coronavirus Middle East respiratory syndrome coronavirus as an essential target for vaccine development. J Virol. 2013;87:9939–9942. doi: 10.1128/JVI.01048-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Raj V.S., Lamers M., Smits S. Identification of protein receptors for coronaviruses by mass spectrometry. Methods Mol Biol. 2015;1282:165–182. doi: 10.1007/978-1-4939-2438-7_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Memish Z.A., Al-Tawfiq J.A., Makhdoom H.Q. Respiratory tract samples, viral load, and genome fraction yield in patients with Middle East respiratory syndrome. J Infect Dis. 2014;210:1590–1594. doi: 10.1093/infdis/jiu292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stalin Raj V., Farag E.A.B.A., Reusken C.B.E.M. Isolation of MERS coronavirus from dromedary camel, Qatar, 2014. Emerg Infect Dis. 2014;8:1339–1342. doi: 10.3201/eid2008.140663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Memish Z.A., Al-Tawfiq J.A. Middle East respiratory syndrome coronavirus infection control: The missing piece? Am J Infect Control. 2014;42:1258–1260. doi: 10.1016/j.ajic.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al-Tawfiq J.A., Memish Z.A. What are our pharmacotherapeutic options for MERS-CoV? Expert Rev Clin Pharmacol. 2014;7:235–238. doi: 10.1586/17512433.2014.890515. [DOI] [PubMed] [Google Scholar]
- 77.Yao Y., Bao L., Deng W. An animal model of MERS produced by infection of rhesus macaques with MERS coronavirus. J Infect Dis. 2014;209:236–242. doi: 10.1093/infdis/jit590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohnuma K., Haagmans B.L., Hatano R. Inhibition of Middle East respiratory syndrome coronavirus infection by Anti-CD26 monoclonal antibody. J Virol. 2013;87:13892–13899. doi: 10.1128/JVI.02448-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ying T., Li H., Lu L. Development of human neutralizing monoclonal antibodies for prevention and therapy of MERS-CoV infections. Microbes Infect. 2014;17:142–148. doi: 10.1016/j.micinf.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hasan M.M., Akter R., Ullah M.S. A computational approach for predicting role of human microRNAs in MERS-CoV genome. Adv Bioinformatics. 2014;2014:967946. doi: 10.1155/2014/967946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dyall J., Coleman C.M., Hart B.J. Repurposing of clinically developed drugs for treatment of Middle East respiratory syndrome coronavirus infection. Antimicrob. Agents Chemother. 2014;58:4885–4893. doi: 10.1128/AAC.03036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao J., Li K., Wohlford-Lenane C. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang N., Jiang S., Du L. Current advancements and potential strategies in the development of MERS-CoV vaccines. Expert Rev Vaccines. 2014;13:761–774. doi: 10.1586/14760584.2014.912134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang N., Tang J., Lu L. Receptor-binding domain-based subunit vaccines against MERS-CoV. Virus Res. 2014 doi: 10.1016/j.virusres.2014.11.013. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang X., Chen X., Bian G. Proteolytic processing, deubiquitinase and interferon antagonist activities of Middle East respiratory syndrome coronavirus papain-like protease. J Gen Virol. 2014;95:614–626. doi: 10.1099/vir.0.059014-0. [DOI] [PubMed] [Google Scholar]
- 86.Kemp M., Høgh S.V., Skov M.N. Quick simultaneous analyses are important when MERS-coronavirus infection is suspected. Ugeskr Laeger. 2014:176. pii: V05140293. [PubMed] [Google Scholar]
- 87.Chung S.J., Ling M.L., Seto W.H. Debate on MERS-CoV respiratory precautions: surgical mask or N95 respirators? Singapore Med J. 2014;55:294–297. doi: 10.11622/smedj.2014076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang M., Barasheed O., Rashid H. A cluster-randomised controlled trial to test the efficacy of facemasks in preventing respiratory viral infection among Hajj pilgrims. J Epidemiol Glob Health. 2014 doi: 10.1016/j.jegh.2014.08.002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pavli A., Tsiodras S., Maltezou H.C. Middle East respiratory syndrome coronavirus. (MERS-CoV): Prevention in travelers. Travel Med Infectious Dis. 2014;12:602–608. doi: 10.1016/j.tmaid.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazarus R., Lim P.L. Avian influenza: recent epidemiology, travel-related risk, and management. Curr Infect Dis Rep. 2015;17:456. doi: 10.1007/s11908-014-0456-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soliman T., Cook A., Coker R. Pilgrims and MERS-CoV: what's the risk? Emerg Themes Epidemiol. 2015;12:1–3. doi: 10.1186/s12982-015-0025-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lessler J., Rodriguez-Barraquer I., Cummings D.A. The MERS-CoV scenario modeling working group -. estimating potential incidence of MERS-CoV associated with Hajj pilgrims to Saudi Arabia, 2014. PLoS Curr. 2014;6 doi: 10.1371/currents.outbreaks.c5c9c9abd636164a9b6fd4dbda974369. pii: ecurrents.outbreaks.c04478c7fbd9854ef6ba923cc81eb799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tashani M., Alfelali M., Barasheed O. Australian Hajj pilgrims’ knowledge about MERS-CoV and other respiratory infections. Virol Sin. 2014;29:1–3. doi: 10.1007/s12250-014-3506-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maltezou H.C., Tsiodras S. Middle East respiratory syndrome coronavirus: Implications for health care facilities. Am J Infect Control. 2014;42:1261–1265. doi: 10.1016/j.ajic.2014.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cotten M., Watson S.J., Kellam P. Transmission and evolution of the Middle East respiratory syndrome coronavirus in Saudi Arabia: a descriptive genomic study. Lancet. 2013;382:1993–2002. doi: 10.1016/S0140-6736(13)61887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gardner L., MacIntyre C. Unanswered questions about the Middle East respiratory syndrome coronavirus (MERS-CoV) BMC Research Notes. 2014;7:358. doi: 10.1186/1756-0500-7-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wang LF, Eaton BT. Bats, Civets and the Emergence of SARS. Wildlife and emerging zoonotic diseases: the biology, circumstances and consequences of cross-species transmission: Springer Berlin Heidelberg 2007;315:325–44.
- 98.Reusken CB1, Ababneh M., Raj V.S. Middle East respiratory scoronavirus (MERS-CoV) serology in major livestock species in an affected region in Jordan, June to September 2013. Euro Surveill. 2013;18:20662. doi: 10.2807/1560-7917.es2013.18.50.20662. [DOI] [PubMed] [Google Scholar]
- 99.Hemida M., Perera R., Wang P. Middle East respiratory syndrome (MERS) coronavirus seroprevalence in domestic livestock in Saudi Arabia, 2010 to 2013. Euro Surveill. 2013;18:20659. doi: 10.2807/1560-7917.es2013.18.50.20659. [DOI] [PubMed] [Google Scholar]
- 100.Alagaili A.N., Briese T., Mishra N. Middle East respiratory syndrome coronavirus infection in dromedary camels in Saudi Arabia. mBio. 2014;5:e00884–e914. doi: 10.1128/mBio.00884-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lu G., Hu Y., Wang Q. Molecular basis of binding between novel human coronavirus MERS-CoV and its receptor CD26. Nature. 2013;8:227–231. doi: 10.1038/nature12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ferguson N.M., Van Kerkhove M.D. Identification of MERS-CoV in dromedary camels. Lancet Infect Dis. 2014;14:93–94. doi: 10.1016/S1473-3099(13)70691-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Memish Z.A., Alsahly A., Masri Ma Sparse evidence of MERS-CoV infection among animal workers living in Southern Saudi Arabia during 2012. Influenza Other Respir Viruses. 2015;9:64–67. doi: 10.1111/irv.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Azhar E.L., Hashem A.M., El-Kafrawy S.A. Detection of the Middle East respiratory syndrome coronavirus genome in an air sample originating from a camel barn owned by an infected patient. mBio. 2014;5:e01450–e1514. doi: 10.1128/mBio.01450-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adney D.R., van Doremalen N., Brown V.R. Replication and shedding of MERS-CoV in upper respiratory tract of inoculated dromedary camels. Emerg Infect Dis. 2014;20:1999–2005. doi: 10.3201/eid2012.141280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Woo P.C.Y., Lau S.K.P., Li K.S.M. Molecular diversity of coronaviruses in bats. Virology. 2006;351:180–187. doi: 10.1016/j.virol.2006.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Memish Z., Zumla A., Al-Hakeem R. Family cluster of Middle East respiratory syndrome coronavirus infections. N Engl J Med. 2013;368:2487–2494. doi: 10.1056/NEJMoa1303729. [DOI] [PubMed] [Google Scholar]
- 108.Assiri A., McGeer A., Allison P. Hospital outbreak of Middle East respiratory syndrome coronavirus. N Engl J Med. 2013;369:407–416. doi: 10.1056/NEJMoa1306742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Omrani A.S., Matin M.A., Haddad Q. A family cluster of Middle East respiratory syndrome coronavirus infections related to a likely unrecognized asymptomatic or mild case. Int J Infect Dis. 2013;17:668–672. doi: 10.1016/j.ijid.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Al-Abdallat M.M., Payne D.C., Alqasrawi S. Hospital-associated outbreak of Middle East respiratory syndrome Coronavirus: A serologic, epidemiologic, and clinical description. Clin Infect Dis. 2014;59:1225–1233. doi: 10.1093/cid/ciu359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Al-Tawfiq J.A., Hinedi K., Ghandour J. Middle East respiratory syndrome- coronavirus (MERS-CoV): a case-control study of hospitalized patients. Clin Infect Dis. 2014;59:160–165. doi: 10.1093/cid/ciu226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Memish Z.A., Al-Tawfiq J., Makhdoom H. Screening for Middle East respiratory syndrome coronavirus infection in hospital patients and their health care worker and family contacts: a prospective descriptive study. Clin Microbiol Infect. 2014;20:469–474. doi: 10.1111/1469-0691.12562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cotten M., Watson S., Zumla A. Spread, circulation, and evolution of the Middle East respiratory syndrome coronavirus. MBio. 2014;5:01062–1113. doi: 10.1128/mBio.01062-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Guery B., Poissy J., el Mansouf L. Clinical features and viral diagnosis of two cases of infection with Middle East respiratory syndrome coronavirus: a report of nosocomial transmission. Lancet. 2013;381:2265–2272. doi: 10.1016/S0140-6736(13)60982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Corman V.M., Ithete N.L., Richards L.R. Rooting the phylogenetic tree of MERS-coronavirus by characterization of a conspecific virus from an African Bat. J Virol. 2014;88:11297–11303. doi: 10.1128/JVI.01498-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Corman V.M., Jores J., Meyer B. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992-2013. Emerg Infect Dis. 2014;20:1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Müller M.A., Corman V.M., Jores J. MERS coronavirus neutralizing antibodies in camels, eastern Africa, 1983-1997. Emerg Infect Dis. 2014;20:2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]