Abstract
Very limited evidence has been reported on host T cell responses to the pandemic H1N1 swine-origin influenza A virus (S-OIV) infection in humans. Therefore, we investigated the proportions of peripheral T cell subsets and analyzed the relationship of T helper subset changes with T cell activation during this infection. We found that these S-OIV-infected patients exhibited rapid lymphopenia, T cell activation and preferential loss of Th17 subset at the early stage of acute infection. Statistical analysis indicated that CD4 depletion and loss of Th17 cells, rather than Th1 or Treg cells, were correlated with CD4 T cell activation. More importantly, up-regulated IFN-α likely contributed to the functional loss of Th17 cells. Thus, rapidly generalized lymphopenia, preferential loss of Th17 population and T cell activation presented as characteristics of the early immune response in S-OIV-infected patients. These findings, therefore, may be helpful for an earlier diagnosis and further studies of immune pathogenesis of S-OIV infection.
Abbreviations: APC, Allophycocyanin; PE, Phycoerythrin; FITC, Fluorescein isothiocyanate; PerCP, Peridin chlorophyll protein; PBMCs, Peripheral blood mononuclear cells; Treg, Regulatory T cell; Th17 cells, Interleukin-17-producing CD4 T cells; Th1 cells, IFN-γ-producing CD4 T cells; HC, Healthy control; S-OIV, Swine-origin influenza A virus.
Keywords: Pandemic H1N1 swine-origin influenza A virus, Activation, T cell depletion, Th17 cells
1. Introduction
Pandemic H1N1 swine-origin influenza A virus (S-OIV) is a pandemic acute respiratory illness caused by a novel influenza H1N1 strain that first emerged in humans in Mexico during March of 2009. Since then, S-OIV has spread rapidly across the globe, resulting in sustained community transmission in more than 207 countries on five continents with over 322,482 cumulative cases and at least 7826 deaths up to November 22, 2009 [1]. The incubation period of S-OIV infection is usually 1–7 days, and the clinical manifestations often include a fever, cough, stethocatharsis, pharyngodynia, headache, myalgias, diarrhea and vomiting [2]. Severe cases will rapidly progress to respiratory failure or multiple organ failure [3]. Therefore, identification of early immunological parameters will be critical for the study of the pathogenesis of this disease.
Increasing evidence suggest that the host immune response plays a critical role in determining various outcomes of influenza virus infection. Moderate increases of pro-inflammatory activation in virus-infected patients may favor virus clearance. However, hyper-activated inflammatory responses may lead to detrimental effects for the host [4]. For example, it is known that the high mortality rate from the pathogenic avian influenza virus infections is a consequence of an overactive inflammatory response, and the severity of the disease is closely related with the virus-induced cytokine storm [5]. Severe acute respiratory syndrome further reinforces the concept in that an overactive inflammatory response is associated with progression of pulmonary infiltrates, necrosis and tissue destruction [6]. This abundance of virus-induced inflammatory cytokines after innate immunity activation can lead to subsequent antigen-nonspecific T cell activation in mice and human with viral infection [7], [8]. Furthermore, the pathogenesis induced by activated nonspecific T cells in liver or relative tissues has been demonstrated in human with chronic HIV, HBV or HCV infection [9], [10], [11], [12], in which type 1 IFN plays a crucial role in inducing systemic inflammation and loss of lymphocyte numbers in the early stages of viral infection [7], [9], [13], [28], [29], [30], [31], [32]. These studies provide evidence linking systemic inflammatory activation and activated nonspecific T cell induced tissue damage.
Helper CD4+ T cells, in general, can orchestrate host immune responses through their release of distinct cytokine profiles [14], and Th1 subsets that secrete endogenous IFN-γ have conventionally been thought to exacerbate tissue damage and control viral infection. Regulatory T cells (Tregs) are immunosuppressive and play an important role in the regulation of immune responses [15]. IL-17-expressing CD4+ T (Th17) cells, producing the pro-inflammatory cytokine IL-17, have been causally related both to chronic inflammation [16] and to host defenses against pulmonary microbial agents [17]. A recent report demonstrated that Th17 cells play an important role in protecting naïve mice against lethal doses of influenza challenge [18], [19]. Furthermore, Th1 and Th17 hypercytokinemia as early host response signature has been observed in severe pandemic influenza [20]. However, whether the change of T helper subsets could bridge the inflammatory activation and T cell activation during host-pathogen interactions at an early stage of A/H1N1 infection has not been well-defined.
To address the issues mentioned above, we comprehensively analyzed peripheral T cell subsets in acute S-OIV-infected patients and found that there were rapidly generalized lymphopenia and preferential loss of the Th17 population in patients at the early stage of acute S-OIV infection, which was subsequently associated with CD4 T cell activation. Our findings may be helpful for further study of pathogenesis of this disease.
2. Materials and methods
2.1. Study subjects
Blood samples were collected from 53 confirmed S-OIV-infected patients and 21 healthy controls. The diagnoses complied with the diagnoses criteria of the WHO Global Influenza Program. Briefly, laboratory diagnosis of the S-OIV was made by RT–PCR on combined nasal and throat swabs (the primers were designed by the Chinese National Influenza Center). All individuals with concurrent hepatitis C virus, hepatitis B virus and HIV-1 infections and autoimmune diseases and who met clinical or biological criteria of bacterial or fungal infection were excluded.
The patients were assigned to three groups according to the day of the first clinical manifestation. The early stage group (n = 15) was defined as patients enrolled within 3 days after onset of clinical symptoms of S-OIV. The intermediate stage group (n = 18) was defined as patients enrolled between 4 and 7 days after onset of clinical symptoms of S-OIV. The late stage group (n = 20) was defined as patients enrolled after suffering from S-OIV infection for more than 8 days. All patients were hospitalized or followed-up in our unit at the Beijing 302 Hospital and treated with oseltamivir immediately according to the treatment recommendations [21]. None of the patients developed into more severe status throughout the treatment period according to the Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses. In each group the convalescent stage was defined as the period beginning after 10 days of therapy. Twenty-one age- and sex-matched healthy individuals were enrolled as controls. The study protocol was approved by the ethics committee of our unit, and written informed consent was obtained from each subject. Basic characteristics of these subjects are listed in Table 1 . Plasma was stored at − 80 °C prior to analysis.
Table 1.
Clinical characteristics of the populations enrolled in the study.
| Clinical symptoms (yes/no) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Cases | Fever | Cough | Sputum production | Running nose | Sore throat | Headache | Nausea | Vomiting | Diarrhea |
| Total | 53 | 48/5 | 24/29 | 5/48 | 4/49 | 33/20 | 21/32 | 5/48 | 4/49 | 9/44 |
| Early stage (1–3 days) | 15 | 13/2 | 12/3 | 4/11 | 2/13 | 14/1 | 11/4 | 4/11 | 3/12 | 6/9 |
| Intermediate stage (4–7 days) | 18 | 17/1 | 10/8 | 1/17 | 2/16 | 12/6 | 10/8 | 1/17 | 1/18 | 3/15 |
| Later stage (8–10 days) | 20 | 18/2 | 2/18 | 0/20 | 0/20 | 7/13 | 0/20 | 0/20 | 0/20 | 0/20 |
2.2. Flow cytometric analysis
All antibodies were purchased from BD Biosciences (San Jose, CA, USA), except for phycoerythrin (PE) conjugated anti-IL-17A mAb and fluorescein isothiocyanate (FITC) conjugated anti-FoxP3, which were from eBioscience (San Diego, CA).
For T cell counts, cocktails of mAbs (Beckman–Coulter) specific for CD3, CD4, and CD8 cells were used to directly measure peripheral blood CD3, CD4, and CD8 T cell counts.
To determine T cell surface activation marker, cocktails of mAbs including PE conjugated anti-CD4, allophycocyanin (APC) conjugated anti-CD8, peridin chlorophyll protein conjucted anti-HLA-DR and FITC-conjugated anti-CD38 antibodies were used. To determine the frequencies of Tregs in peripheral blood, PerCP conjugated anti-CD3, APC conjugated anti-CD4, PE conjugated anti-CD25 and FITC-conjugated anti-FoxP3 were used. Isotype control antibodies were used to separate positive and negative cells in the PerCP, FITC, PE, and APC fluorescence channels. Cells were incubated and stained at 4 °C in the dark for 30 min. The blood was then lysed with FACS™ lysing solution (BD PharMingen, San Jose, CA). For intracellular Foxp3 staining, cells were further permeabilized and stained with the FITC-conjugated anti-FoxP3 and fixed using eBioscience fix/perm according to the manufacturer's instructions.
For intracellular IL-17 and IFN-γ staining, fresh heparinized peripheral blood (200 μl) was incubated with PMA (300 ng/ml) and ionomycin (1 μg/ml) in 800 μl RPMI 1640 medium supplemented with 10% fetal calf serum (FCS) for 6 h. Monensin (0.4 μM) was added during the 1st hour of incubation. The blood was then lysed with FACS™ lysing solution and further permeabilized, stained with the corresponding intracellular antibody, fixed, and analyzed using FACSCalibur and FlowJo software (TreeStar, Ashland, OR, USA) as previously described [22].
2.3. Inhibitory assay of IFN-α on Th17 cells
To investigate whether IFN-α inhibit Th17 cell function in vitro, the isolated peripheral blood mononuclear cells (PBMCs, 5 × 105 cells/well) were incubated with IFN-α in a 96-well U-bottom plate at different concentrations (100 IU/ml, 500 IU/ml, 1000 IU/ml). These cells were then stimulated with PMA (300 ng/ml) and ionomycin (1 μg/ml) for 6 h to perform intracellular IL-17 and IFN-γ staining according to the aforementioned protocols.
2.4. ELISA
The plasma levels of IFN-α and IL-17 were measured by quantitative sandwich ELISA following the manufacturer's instructions (R&D Systems, Minneapolis, MN, USA). The minimal detectable concentrations were 12.5 pg/ml for IFN-α and 15 pg/ml for IL-17. Intra-assay and inter-assay coefficients of variation for all ELISAs were < 5% and < 10%, respectively. All samples were measured in duplicate.
2.5. Statistical analysis
All data were analyzed using SPSS software (SPSS Inc., Chicago, Illinois) and are summarized as means ± standard deviations. Comparisons between various individuals were performed using the Mann–Whitney U-test. Comparisons between the same individual were performed using the Wilcoxon matched pairs T test. Correlation analysis was evaluated by the Spearman rank correlation test. For all tests, 2-sided P < 0.05 was considered to be statistically significant.
3. Results
3.1. S-OIV infection results in generalized T cell depletion and T cell activation
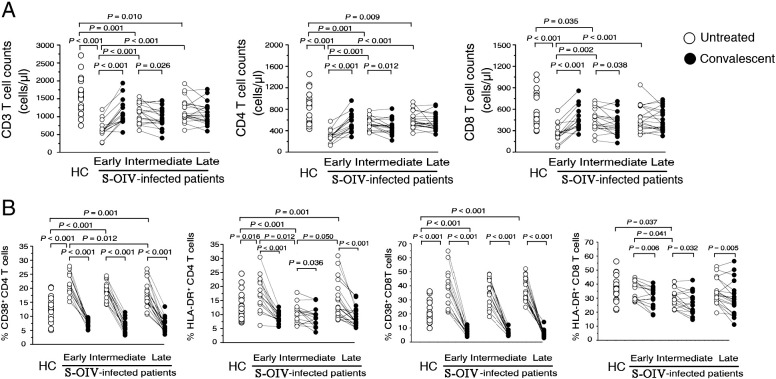
Using flow cytometric analysis, we first compared the absolute numbers of circulating CD3+, CD4+, and CD8+ T cells in 53 patients at the acute and convalescent stages of S-OIV infection with that of the healthy control individuals. As shown in Fig. 1A, patients infected with S-OIV clearly experienced T cell loss in peripheral blood during the acute stage of infection. The mean absolute CD3+, CD4+, and CD8+ T cell counts in healthy individuals were 1556, 8836, and 603 cells/μl, respectively; whereas those in patients with S-OIV infection were markedly lower, at 994, 492, and 410 cells/μl, respectively. Among the 53 patients, patients at early clinical onset showed lower T cell counts (649 cells/μl for CD3+, 304 cells/μl for CD4+, and 274 cells/μl for CD8+ T cells), and T cell counts were progressively increased in patients at the intermediate (1086 cells/μl for CD3+, 534 cells/μl for CD4+, and 444 cells/μl for CD8+ T cells) and late stages (1168 cells/μl for CD3+, 599 cells/μl for CD4+, and 476 cells/μl for CD8+ T cells). These data showed that S-OIV infection leads to a rapid depletion of CD3+, CD4+, and CD8+ T cell at the early stage (1–3 days), followed by a rapid and significant restoration of CD3+, CD4+, and CD8+ T cells at 4 days after the onset of illness. However, the disparate patterns of T cell restoration were observed in patients at the early and intermediate stages after oseltamivir therapy. As shown in Fig. 1, absolute cell counts almost doubled for CD3+, CD4+, and CD8+ T cell subsets when compared within patients between the early and convalescent stages (1136 cells/μl for CD3+, 551 cells/μl for CD4+, and 465 cells/μl for CD8+ T cells); however, absolute cell counts for CD3+, CD4+, and CD8+ T cell subsets showed a decreasing trend when compared within patients between the intermediate and convalescent stages (966 cells/μl for CD3+, 470 cells/μl for CD4+, and 393 cells/μl for CD8+ T cells), and we observed no significant differences in absolute cell counts for CD3+, CD4+, and CD8+ T cell subsets within patients from the late and convalescent stages.
Figure 1.
Peripheral T cell changes and T cell activation after S-OIV infection. (A) comparison of peripheral changes in CD3+, CD4+, and CD8+ T cell counts in S-OIV-infected patients at different clinical stages (early, intermediate, late and corresponding convalescent). (B) comparison of cells positive for HLA-DR and CD38 amongst total CD4+ and CD8+ T cells from peripheral blood at different clinical stages (early, intermediate, late and corresponding convalescent). The early stage: 1–3 days after the first clinical manifestation; the intermediate stage: 4–7 days; the late stage: 8–10 days. Each open dot represents one subject without oseltamivir treatment, and the solid dot represents the corresponding subject at the convalescent stage (day 10 after oseltamivir treatment).
We further analyzed CD38 and HLA-DR antigen expression in these patients. As shown in Fig. 1B, we found that acute S-OIV-infected patients at the early stage had higher percentages of CD38 and HLA-DR in all CD4+ T cells (21.6% for CD38 and 17.5% for HLA-DR) than in healthy controls (13.0% for CD38 and 13.1% for HLA-DR); in contrast, we observed up-regulated CD38 expression and no significant differences in HLA-DR expression on CD8+ T cells in acute S-OIV-infected patients at the early stage (38.4% for CD38 and 35.1% for HLA-DR) when compared with healthy controls (20.5% for CD38 and 35.9% for HLA-DR). The expressions of CD38 and HLA-DR on CD4+ and CD8+ T cells gradually decreased in patients at the intermediate stage (19.1% for CD38 and 11.1% for HLA-DR in CD4+ cells; 35.1% for CD38 and 30.1% for HLA-DR in CD8+ T cells) and late stage (18.0% for CD38 and 14.7% for HLA-DR in CD4+ cells; 37.9% for CD38 and 34.5% for HLA-DR in CD8+ T cells) when compared with acute S-OIV-infected patients at the early stage. Notably, the CD38 and HLA-DR expressions on CD4+ and CD8+ T cells were significantly increased at various stages within patients compared with that at the convalescent stages. The decreased T cell activation closely approximated the kinetics of T cell restoration in acute S-OIV-infected patients at the early stage.
3.2. Preferential loss of IL-17-expressing Th17 cells after S-OIV infection
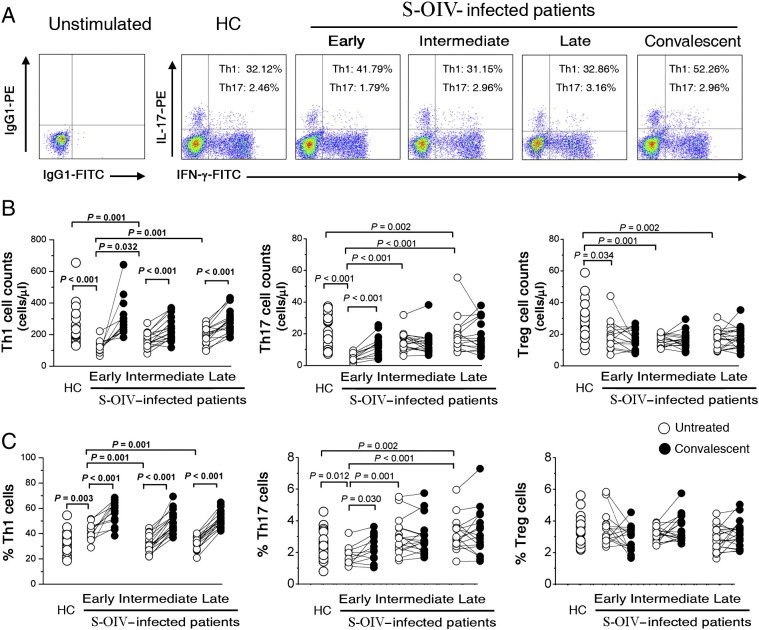
We then compared the frequencies of IFN-γ-producing CD4+ T cells (Th1), Th17 and Tregs in peripheral blood from healthy controls and acute S-OIV-infected patients. As shown in Figs 2A and B, the distribution of Th1 and Th17 subsets in S-OIV-infected subjects differed from that of HC subjects. We found that the absolute cell counts of Th1, Th17 and Tregs cells were significantly decreased in S-OIV-infected patients versus HC individuals. In particular, patients at the convalescent stage had full-scale restoration of CD4+ T cells. To understand whether the loss of these cell subsets in patients with S-OIV infection was selective or simply due to generalized CD4+ T cell depletion, we investigated more specifically the relative frequencies of these subset populations and found that the percentage of Th1 cells was significantly increased in S-OIV-infected patients as compared with HC individuals (P = 0.003, Fig. 2C). Furthermore, the average Th1 frequency in patients at the convalescent stage was even further increased over that in patients at the early stage (P < 0.001). These data agree with previous reports that Th1 cells play an important role in viral clearance. In contrast, the average Th17 frequency at early clinical onset was significantly decreased in S-OIV-infected patients compared with that of HC subjects, but showed a gradual increase from early to late phase (Figs. 2A and C). However, there was no difference in Treg frequency between S-OIV-infected patients and HCs. These data suggest that Th17 cells are more prone to be depleted at an early stage after S-OIV infection.
Figure 2.
Preferential loss of IL-17 expressing Th17 cells after S-OIV infection. (A) representative dot plots of IL-17 and IFN-γ expression in peripheral CD4+ T cells of S-OIV-infected patients at different clinical stages (early, intermediate, late and corresponding convalescent). The values in the quadrants indicate the percentages of each CD4+ T cell subset. (B) comparison of peripheral changes in Th1, Th17, and Treg cell counts in S-OIV-infected patients at different clinical stages (early, intermediate, late and corresponding convalescent). The numbers of each subset were calculated as the CD4+ T lymphocyte count (cells/microliter) multiplied by the percentage of each subset in CD4+ T lymphocytes. (C) comparison of peripheral changes in Th1, Th17, and Treg cell frequencies in S-OIV-infected patients at different clinical stages (early, intermediate, late and corresponding convalescent). The early stage: 1–3 days after the first clinical manifestation; the intermediate stage: 4–7 days; the late stage: 8–10 days. Each open dot represents one subject without oseltamivir treatment, and the connected solid dot represents the same subject at the convalescent stage (day 10 after oseltamivir treatment). P-values were calculated using the nonparametric Mann–Whitney U-test.
3.3. Th17 cells and CD4 depletion at early clinical onset is associated with sustained CD4 T cell immune activation
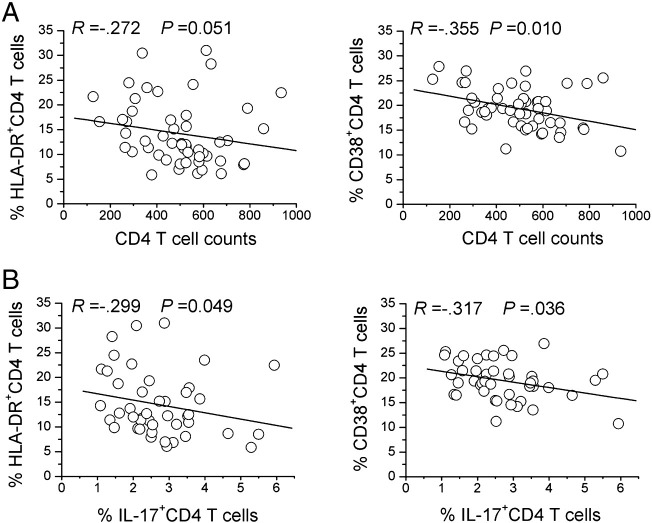
We investigated the impact of Th17 cells and CD4 depletion on T cell activation and found some significant negative correlations between CD4 T cell counts or Th17 frequency and CD38+ or HLA-DR+ T cells in these S-OIV-infected subjects at early clinical onset (Figs. 3A and B). Further analysis indicated that these negative associations occurred only with Th17 frequency but not with the Th1 or Treg subsets. Together, these results demonstrate that the CD4 depletion and selective loss of Th17 cells, not Th1 or Treg cells, were strongly associated with increased CD4+ T immune activation at the early phase of S-OIV infection.
Figure 3.
Th17 cells and CD4 depletion are associated with sustained CD4 T cell immune activation. A significant correlation existed between the levels of HLA-DR and CD38 expression on CD4+ T cells and (A) peripheral CD4 T cell counts or (B) peripheral Th17 frequencies in A/H1N1-infected subjects at early clinical stage as evaluated using the Spearman rank correlation test. Each symbol represents one individual.
3.4. S-OIV infection-induced IFN-α constricts Th17 responses
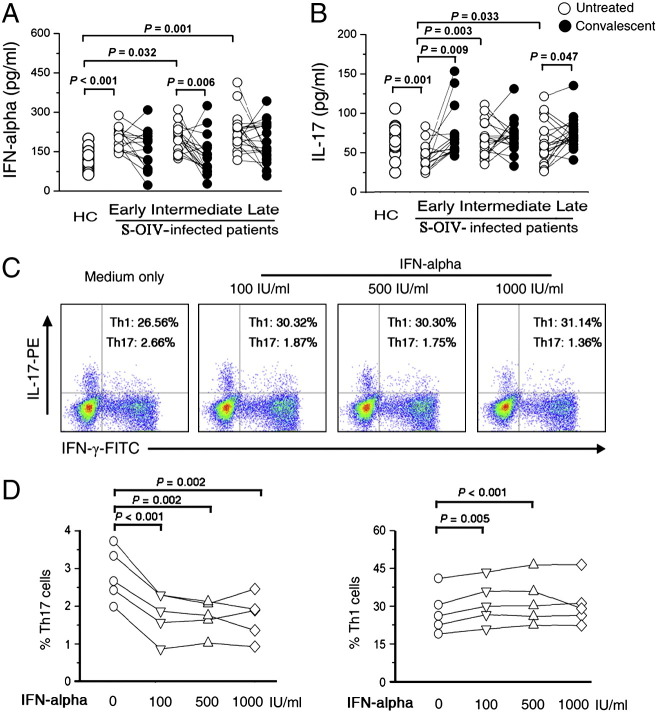
An early virus-induced peak in T cell attrition was shown to correlate with the peak in virus-induced IFN-α/β in mice [7], [28], [29], [30], [31], [32]. Here, we examined the serum concentration of IFN-α to address whether it is associated with the decrease of Th17 cells in S-OIV-infected patients. As expected, the serum IFN-α was highly up-regulated in S-OIV-infected patients when compared with healthy controls (Fig. 4A). Further analysis indicated that patients at the early stage had the lowest of serum concentrations of IL-17 (Fig. 4B). We then determined whether IFN-α influence the IL-17-production of Th17 cells in vitro. As shown in Fig. 4C, we observed that the IL-17-production from CD4+ T cells in the presence of IFN-α was significantly reduced. Surprisingly, we found that the IFN-γ secretions from CD4+ T cells were slightly increased in the presence of IFN-α. Pooled data confirmed that the results were consistent for five healthy controls from three independent detections (Fig. 4D). Taken together, these data suggest that S-OIV infection-induced IFN-α may partly constrict the function of Th17 cells.
Figure 4.
IFN-α controls the function of Th17 cells. Median serum levels of (A) IFN-α and (B) IL-17A concentrations in S-OIV-infected patients at different clinical stages (early, intermediate, late and corresponding convalescent). The early stage: 1–3 days after the first clinical manifestation; the intermediate stage: 4–7 days; the late stage: 8–10 days. Each open dot represents one subject without oseltamivir treatment, and the connected solid dot represents the same subject at the convalescent stage (day 10 after oseltamivir treatment). (C) representative dot plots of IL-17 and IFN-γ expression in peripheral CD4+ T cells from HC under IFN-α treatment in vitro. (D) pooled data indicating the IL-17 and IFN-γ expressions in peripheral CD4+ T cells in response to IFN-α at different concentrations (100 IU/ml, 500 IU/ml, 1000 IU/ml) in vitro. P-values were calculated using the nonparametric Mann–Whitney U-test.
4. Discussion
S-OIV causes the outbreaks of febrile respiratory infection ranging from self-limiting to severe illness. However, little is known about how the immune response develops in S-OIV-infected patients. Our clinical findings demonstrated that S-OIV infection leads to rapid and generalized lymphopenia and preferential loss of Th17 population in patients at the early stage of infection, which is subsequently associated with T cell activation. These properties of Th17 cells may represent an unknown mechanism leading to the pathogenesis of S-OIV infection.
Previous studies have hypothesized that virus-induced lymphopenia at early infection is beneficial for pathogen control [23], [24], [25], [26]. We then performed a comprehensive investigation of the peripheral lymphocyte subsets during different stages of infection and compared them within the same S-OIV-infected patients at the convalescent stage. Our results demonstrated that there was a rapid and generalized lymphopenia in patients with S-OIV at the early stage of infection. In patients who recovered from the initial A/H1N1 illness, an equally rapid and dramatic restoration of CD3+, CD4+, and CD8+ T cell counts was seen in the peripheral blood. These data support the notion that early loss of T cells may allow for the antigen-specific expansion of T cells [7]. We also found that the numbers of the Th17 subset were relatively preferentially decreased in S-OIV-infected patients compared with other CD4+ T cell subsets (including IFN-γ-producing Th1 cells and FoxP3-positive Treg cells). Th17 cells play an important role in host defenses against pulmonary microbial agents, and a recent study further indicated that Th17 protects naïve mice against lethal doses of influenza challenge [18], [19]. All these data suggest that Th17 cells might actively participate in the immune regulation of S-OIV-infected patients.
Although the precise mechanism of Th17 cell induced CD4+ T cell activation in S-OIV-infected patients remains unknown, the present study indicates that the loss of the Th17 subset may link the inflammatory response with T cell activation during early host-pathogen interactions. There are three pieces of evidence here to support this notion. First, similar to a recent report of macaques infected with SIV, the animal model of immunodeficiency virus infection similar to acute human HIV-1 infection [27], we found that there was a significantly decreased frequency of Th17 cells, and increased levels of IFN-α in S-OIV-infected patients. In addition, the present study also found that it was the frequency of Th17 cells, not Treg cells, which was negatively correlated with CD4+ T cell activation. Third, IFN-α, which is highly induced in acute infections in direct response to viral replication, exhibited a considerable inhibitory effect on IL-17-production of Th17 cells reminiscent of those found in S-OIV-infected patients at early phase. These results suggest that Th17 cells are closely associated with inflammatory activation and T cell activation in acute S-OIV infection. However, we observed there was no association of Th17 loss with the severity of S-OIV infection. Th17 cells produce a cocktail of cytokines such as IL-17A, IL-17F, IL-21, IL-22, IL-6 and TNF-α [16], [17] and future studies should investigate whether these Th17 cells in S-OIV-infected patients are functionally skewed to enhance inflammatory cytokine or to decrease anti-inflammatory cytokines.
Notably, there is a difference in the time at which the responses were observed in the S-OIV-infected patients of this study and recent report regarding severe S-OIV infection and in the study performed on mice models. The IFN-induced T cell loses observed in mice models immediately after infection [7], [28], [29], [30], [31], [32]. By contrast, the S-OIV-infected patients are usually identified 7 days after S-OIV infection, when they become clinically symptomatic. Thus, although we found that the serum IFN-α level was significantly higher at clinical onset of infection than that in healthy subjects, even loss of Th17 cells response in the presence of IFN-α in healthy subject are reminiscent of those found in S-OIV-infected patients at early phase. The time difference suggested that the preferential decreased Th17 population in early S-OIV-infected patients may not simply be a direct result of IFN-induced attrition, an alternative explanation for the data is that the committed Th17 cells were still in peripheral blood with down-regulated synthesis of IL-17 in presence of IFN-α or these Th17 cells migrated into the lung tissue or lymph nodes. In contrast to recent data published by Bermejo-Martin JF [20], we observed similar levels of IL-17 in plasma of S-OIV-infected patients. However, we observed no association between levels of IL-17 and the severity of S-OIV infection.
In summary, this study provides the first indication that S-OIV infection leads to rapid and generalized lymphopenia and preferential loss of Th17 population in patients at the early stage of infection, and it is subsequently associated with T cell activation. This clinical characteristic may facilitate an earlier diagnosis and further the study of pathogenesis of S-OIV infection.
Acknowledgments
This study was supported by grants from the No.11 Five-year plan of Army Medical Research of China (09MA030), the National Grand Program on Key Infectious Disease (No. 2009ZX10004-103 and No. 2009ZX10004-309), and the National Natural Science Foundation of China (No. 30801039). We thank the volunteers who generously participated in this study.
Contributor Information
Fu-Sheng Wang, Email: fswang@public.bta.net.cn.
Min Zhao, Email: drzhaomin@sina.com.
References
- 1.Pandemic (H1N1) − update 76, World Health Organization, 2009. (Accessed at http://www.who.int/csr/don/2009_11_27a/en/index.html).
- 2.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 3.Neumann G., Noda T., Kawaoka Y. Emergence and pandemic potential of swine-origin H1N1 influenza virus. Nature. 2009;459:931–939. doi: 10.1038/nature08157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kimura K., Adlakha A., Simon P.M. Fatal case of swine influenza virus in an immunocompetent host. Mayo Clin. Proc. 1998;73:243–245. doi: 10.4065/73.3.243. [DOI] [PubMed] [Google Scholar]
- 5.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N., Hoang D.M., Chau N.V., Khanh T.H., Dong V.C., Qui P.T., Cam B.V., Ha do Q., Guan Y., Peiris J.S., Chinh N.T., Hien T.T., Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat. Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li T., Qiu Z., Zhang L., Han Y., He W., Liu Z., Ma X., Fan H., Lu W., Xie J., Wang H., Deng G., Wang A. Significant changes of peripheral T lymphocyte subsets in patients with severe acute respiratory syndrome. J. Infect. Dis. 2004;189:648–651. doi: 10.1086/381535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selin L.K., Brehm M.A., Naumov Y.A., Cornberg M., Kim S., Clute S.C., Welsh R.M. Memory of mice and men: CD8+ T cell cross-reactivity and heterologous immunity. Immunol. Rev. 2006;211:164–181. doi: 10.1111/j.0105-2896.2006.00394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mandl J.N., Barry A.P., Vanderford T.H., Kozyr N., Chavan R., Klucking S., Barrat F.J., Coffman R.J., Staprans SI S.I., Feinberg M.B. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat. Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 10.Appay V., Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J. Pathol. 2008;214:231–241. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 11.Wang F.S. Clinical immune characterization of hepatitis B virus infection and implications for immune intervention: progress and challenges. Hepatol. Res. 2007;37:S339–S346. doi: 10.1111/j.1872-034X.2007.00222.x. [DOI] [PubMed] [Google Scholar]
- 12.Szabo G., Mandrekar P., Dolganiuc A. Innate immune response and hepatic inflammation. Semin. Liver Dis. 2007;27:339–350. doi: 10.1055/s-2007-991511. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J.Y., Zhang Z., Jin B., Zhang S.Y., Zhou C.B., Fu J.L., Wang F.S. Cutting edge: programmed death-1 up-regulation is involved in the attrition of cytomegalovirus-specific CD8+ T cells in acute self-limited hepatitis B virus infection. J. Immunol. 2008;181:3741–3744. doi: 10.4049/jimmunol.181.6.3741. [DOI] [PubMed] [Google Scholar]
- 14.Mosmann T.R., Coffman R.L. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 15.Beacher-Allan C., Brown J.A., Freeman G.J., Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 17.O'Quinn D.B., Palmer M.T., Lee Y.M., Weaver C.T. Emergence of the Th17 pathway and its role in host defense. Adv. Immunol. 2008;99:115–163. doi: 10.1016/S0065-2776(08)00605-6. [DOI] [PubMed] [Google Scholar]
- 18.McKinstry K.K., Strutt T.M., Buck A., Curtis J.D., Dibble J.P., Huston G., Tighe M., Hamada H., Sell S., Dutton R.M., Swain S.L. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J. Immunol. 2009;182:7353–7363. doi: 10.4049/jimmunol.0900657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hamada H., Garcia-Hernandez Mde L., Reome J.B., Misra S.K., Strutt T.M., McKinstry K.K., Cooper A.M., Swain S.L., Dutton R.W. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 2009;182:3469–3481. doi: 10.4049/jimmunol.0801814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bermejo-Martin J.F., Ortiz de Lejarazu R., Pumarola T., Rello J., Almansa R., Ramírez P., Martin-Loeches I. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit. Care. 2009;13:R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.WHO Guidelines for Pharmacological Management of Pandemic (H1N1) 2009 Influenza and other Influenza Viruses (http://www.cdc.gov/flu/).
- 22.Zhang J.Y., Zhang Z., Lin F., Zou Z.S., Xu R.N., Jin L., Fu J.L., Shi F., Shi M., Wang H.F., Wang F.S. Interleukin-17-producing CD4+ T cells increase with severity of liver damage in chronic hepatitis B patients. Hepatology. 2010;51:81–91. doi: 10.1002/hep.23273. [DOI] [PubMed] [Google Scholar]
- 23.Okada H., Kobune F., Sato T.A., Kohama T., Takeuchi Y., Abe T., Takayama N., Tsuchiya T., Tashiro M. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch. Virol. 2000;145:905–920. doi: 10.1007/s007050050683. [DOI] [PubMed] [Google Scholar]
- 24.Zitzow L.A., Rowe T., Morken T., Shieh W.J., Zaki S., Katz J.M. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bautista E.M., Ferman G.S., Golde W.T. Induction of lymphopenia and inhibition of T cell function during acute infection of swine with foot and mouth disease virus (FMDV) Vet. Immunol. Immunopathol. 2003;92:61–73. doi: 10.1016/s0165-2427(03)00004-7. [DOI] [PubMed] [Google Scholar]
- 26.O'Donnell D.R., Carrington D. Peripheral blood lymphopenia and neutrophilia in children with severe respiratory syncytial virus disease. Pediatr. Pulmonol. 2002;34:128–130. doi: 10.1002/ppul.10140. [DOI] [PubMed] [Google Scholar]
- 27.Favre D., Lederer S., Kanwar B., Ma Z.M., Proll S., Kasakow Z., Mold J., Swainson L., Barbour J.D., Baskin C.R., Palermo R., Pandrea I., Miller C.J., Katze M.G., McCune J.M. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5:e1000295. doi: 10.1371/journal.ppat.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selin L.K., Lin M.Y., Kraemer K.A., Pardoll D.M., Schneck J.P., Varga S.M., Santolucito P.A., Pinto A.K., Welsh R.M. Attrition of T cell memory: selective loss of LCMV epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–742. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 29.McNally J.M., Zarozinski C.C., Lin M.Y., Brehm M.A., Chen H.D., Welsh R.M. Attrition of bystander CD8 T cells during virus-induced T cell and interferon responses. J. Virol. 2001;75:5965–5976. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.K., Welsh R.M. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J. Immunol. 2004;172:3139–3150. doi: 10.4049/jimmunol.172.5.3139. [DOI] [PubMed] [Google Scholar]
- 31.Smith D.K., Dudani R., Pedras-Vasconcelos J.A., Chapdelaine Y., van Faassen H., Sad S. Cross-reactive antigen is required to prevent erosion of established T cell memory and tumor immunity: a heterologous bacterial model of attrition. J. Immunol. 2002;169:1197–1206. doi: 10.4049/jimmunol.169.3.1197. [DOI] [PubMed] [Google Scholar]
- 32.Bahl K., Kim S.K., Calcagno C., Ghersi D., Puzone R., Celada F., Selin L.K., Welsh R.M. Interferon-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J. Immunol. 2006;176:4284–4295. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]