Summary
Despite the high risk of acquiring respiratory infections, healthcare workers who treat pilgrims at Hajj have not been studied in previous research on respiratory diseases during Hajj. The objective of this study was to determine the prevalence of different respiratory viruses among healthcare workers who treated pilgrims during Hajj 2009, the year of the influenza A H1N1 pandemic. A cross-sectional study was performed just before and after Hajj (25–29 November, 2009). Nasal and throat swabs were tested for 18 respiratory virus types and subtypes. A total of 184 healthcare workers were examined. Most were men (85%) with an average age of 41 years. Before the Hajj, rates of seasonal influenza vaccination were higher (51%) than rates of pandemic influenza A H1N1 vaccination (22%). After the Hajj, participants reported high rates of maintaining hand hygiene (98%), cough etiquette (89%), and wearing a face mask (90%). Among all the viruses tested, only two were detected: rhinovirus was detected in 12.6% and Coronavirus 229E in 0.6%. Rhinovirus was detected in 21% of those who had respiratory symptoms during Hajj. Influenza A (including H1N1), influenza B. respiratory syncytial virus, other coronaviruses, parainfluenza viruses, human metapneumovirus, adenovirus, and human bocavirus were not detected. The finding of high rates of rhinovirus infection corresponds to their frequent occurrence in adults. None of the participants had influenza A H1N1 2009, possibly because it was also infrequent among the 2009 pilgrims.
Keywords: Hajj, Viral, Respiratory, Healthcare workers, H1N1
Background
Healthcare workers are exposed to many respiratory infections when they see patients, and they may transmit these infections to their patients or colleagues. For example, during the outbreak of severe acute respiratory syndrome (SARS) in 2003, attack rates were more than 50% in healthcare workers.1 Healthcare workers who see pilgrims during Hajj (the annual Muslim pilgrimage to Makkah) may be at higher risk of acquiring respiratory and other infections.2, 3 Because of these risks, special immunization requirements have been proposed to protect healthcare workers,4 although healthcare workers have not been included in previous studies of respiratory diseases during Hajj.2, 5
Hajj is the largest annual mass gathering in the world; it brings more than two million people from different countries together in a small, confined area. The extreme overcrowding of pilgrims during Hajj reaches about 7 persons per meter; combined with fatigue and extremely hot weather during much of the year, this crowding may increase the risk of transmitting air- and droplet-borne infectious diseases, particularly respiratory viruses.3, 5, 6, 7 An estimated one in three pilgrims experience respiratory symptoms.6 Several transmissible bacterial and viral respiratory pathogens have been reported among pilgrims, notably meningococci of all serotypes, Streptococcus pneumoniae, gram-negative organisms, atypical organisms, Mycobacterium tuberculosis, influenza A and B viruses, rhinoviruses, respiratory syncytial virus (RSV), parainfluenza viruses, enteroviruses, and adenoviruses.5, 6, 8, 9, 10
In April of 2009, a novel influenza A strain (H1N1 2009 strain) in Mexico spread globally.11, 12 The toll was particularly heavy in Saudi Arabia, which ranked fourth of 22 countries in the Eastern Mediterranean Region in deaths and probable H1N1 cases.13 The 2009 Hajj took place in November, six months later, and presented a public health challenge for infection control authorities in Saudi Arabia.14 Several practices to minimize disease transmission among pilgrims and healthcare workers were instituted even before the beginning of Hajj season, since pre-Hajj data showed low acceptance rates of H1N1 vaccine among healthcare workers.15, 16 This study evaluated the prevalence of viral respiratory pathogens among healthcare workers during the 2009 Hajj which coincided with the influenza A H1N1 2009 pandemic.
Methods
Population
Healthcare workers, including physicians, nurses, health inspectors, and others, who served pilgrims during the 2009 Hajj season were included. Three-fourths of the healthcare workers in the study were from the Saudi Ministry of Health (MOH), and the rest were from medical missions other than MOH. Most of the healthcare workers had treated pilgrims previously and had been practicing medicine for more than 10 years.
2009 Hajj season
The main religious activities of the 2009 Hajj season started on 25th November 2009 with a visit to the Holy Kaaba and continued for 5 or 6 days at different holy sites in Mina, Arafat, and Muzdalifa.
Study design
The current study was a cross-sectional study performed in two phases. The first phase was conducted during the week before the start of Hajj on November 25th, and the second phase was conducted in the week following the end of Hajj on November 30th. Healthcare workers were asked to answer a questionnaire and provide nasal and throat swabs both before and after Hajj.
Data collection
The pre-Hajj questionnaire was about demographics (age, sex, occupation, and nationality), medical history (chronic disease and smoking), vaccination history (including H1N1 and seasonal influenza), and knowledge of H1N1 influenza (symptoms, transmission, and prevention). The post-Hajj questionnaire included questions about exposure to infections during Hajj and compliance with infection control practices (hand hygiene, cough etiquette, and wearing a mask).
Laboratory methods
Nasal and throat swabs were collected using the same method during both phases of the study. Nose and throat swabs were collected in viral transport media using Dacron swabs on stainless steel wire and plastic shafts, respectively (Remel, MicroTest M4RT, USA). Immediately after collection, samples were transported to the Jeddah Regional Laboratory where they were stored at −80 °C until tested. Nucleic acid was extracted using the X-Tractor Gene, Corbett from Qiagen using 25101 VX DNA/RNA purification protocol. The multiplex PCR using micro fluid arrays and Luminex x-Map system, with xTAG Respiratory Viral Panel FAST Assay (Manufactured By Luminex Molecular Diagnostics, Inc, Toronto, ON, Canada, distributed by Abbott Molecular, Wiesbaden-Delkenheim, Germany) was used for nucleic acid testing for 18 circulating respiratory virus types and subtypes: influenza A, influenza A H1, influenza A H3, influenza B, RSV, coronavirus 229E, coronavirus OC 43, coronavirus NL63, coronavirus HKU1, parainfluenza 1, parainfluenza 2, parainfluenza 3, parainfluenza 4, human metapneumovirus, rhinovirus, adenovirus, and human bocavirus.
The xTAG Data Analysis Software for RVP FAST(TDAS RVP FAST) analyzed the data and provided a report summarizing which viruses were present. The RVP FAST detects influenza B, influenza A H1 seasonal, and influenza A H3 only. If any other subtypes are present, it will indicate the presence of influenza A matrix protein only. Therefore the samples in which influenza A matrix protein was detected were run separately by a singleplex PCR to detect H1 2009 pandemic strain using Artus Inf/H1 LC/RG RT-PCR Kit (Qiagen) and for avian influenza A H5N1 (subtype Asia) by LightMix Kit (TIB, MOLBIOL,GmbH, Berlin, Germany), according to the manufacturer’s instructions. Any strain in which RNA was not detected for these four influenza A types (i.e., H1, H3 seasonal in RVP FAST and independent singleplex PCRs for H1 2009 pandemic strain, and H5N1 [subtype Asia]) was labeled as unsubtypeable influenza A virus.
Data analysis
Demographics, medical history, vaccination history, knowledge of H1N1 influenza, and compliance with infection control practices are presented as frequencies. The prevalence of respiratory viruses is presented as number of viruses per 1000 healthcare workers. Differences in the prevalence of respiratory viruses before and after the Hajj were examined using non-parametric paired statistics (McNemar test). Differences in the prevalence of respiratory viruses between potential confounding groups, such as wearing a mask or getting a vaccine, were examined using chi-square or Fisher exact test, as appropriate. All P-values were two-tailed. P-value <0.05 was considered significant. SPSS (release 17.0, SPSS Inc., Chicago, U.S.) software was used for all statistical analyses.
Results
A total of 184 healthcare workers who treated pilgrims during the 2009 Hajj season were included in the study. Of these, 161 answered the (main) pre-Hajj questionnaire and 104 answered the (short) post-Hajj questionnaire. A total of 120 combined nasal and throat swabs were obtained during the pre- and post-Hajj periods. Demographic and clinical characteristics of the sample are shown in Table 1 . The majority of the healthcare workers were males (85%) with an average age of 40.9 ± 9.2 years (range 23–59 years), Non-Saudi (71%), physicians (75%), with more than 10 years of medical experience (60%) as well as previous experience of serving in Hajj medical services (83%).
Table 1.
Demographic and clinical characteristics of HCWs serving pilgrims of 2009 Hajj season.
| Demographic characteristics | N (%)a |
|---|---|
| Age (mean ± SD, years) | 40.9 ± 9.2 |
| Age (range, years) | 23–59 |
| Age group (years) | |
| <35 | 44 (27.7%) |
| 35–44 | 50 (31.4%) |
| ≥45 | 65 (40.9%) |
| Total | 159 (100.0%) |
| Gender | |
| Male | 136 (85.0%) |
| Female | 24 (15.0%) |
| Total | 160 (100.0%) |
| Nationality | |
| Saudi | 42 (29.4%) |
| Non-Saudi | 101 (70.6%) |
| Total | 143 (100.0%) |
| Occupation | |
| Physician | 119 (75.3%) |
| Nurse | 31 (19.6%) |
| Other HCWs | 8 (5.1%) |
| Total | 158 (100.0%) |
| Years of medical experience | |
| <10 years | 59 (39.1%) |
| 10–20 years | 50 (33.1%) |
| >20 years | 42 (27.8%) |
| Total | 158 (100.0%) |
| Own description of general health | |
| Excellent | 58 (37.7%) |
| Very good | 86 (55.8%) |
| Good | 0 (0.0%) |
| Fair | 10 (6.5%) |
| Poor | 0 (0.0%) |
| Total | 154 (100.0%) |
| Chronic diseases | |
| Yes | 26 (14.7%) |
| No | 151 (85.3%) |
| Total | 177 (100.0%) |
| Smoking | |
| Never | 82.6% |
| Previous | 6.5% |
| Current | 11.0% |
| Total | 155 (100.0%) |
Unless mentioned otherwise.
Most of the healthcare workers (93%) described their own health as very good to excellent.
Chronic disease, namely hypertension, diabetes, and asthma were present in 15% and 11% were current smokers. Compliance of healthcare workers with pre-Hajj vaccination and infection control is shown in Table 2 . Eighty four percent of them got at least one vaccine before Hajj. The coverage of hepatitis B, meningococcal and seasonal influenza vaccines were relatively high (73%, 67% and 51%, respectively), while the coverage of H1N1 vaccine was considerably low (22%). The main reasons described for not getting the vaccine were worries about the side effects, (42%), non-availability (34%), and fear of developing H1N1 symptoms (22%). Approximately 50% of the healthcare workers did not get seasonal influenza vaccine in the past year due to the belief of being healthy (29%), lack of knowledge about the place to get the vaccine (22%), and the assumption that influenza is not a serious illness (18%). Compliance with hand hygiene was noted in 98%, cough etiquette in 89% and wearing face mask in 90% of the healthcare workers. The Exposure Risk as defined by being within 1 m from a person with ILI was reported in 61%, handling biological specimens in 34% and examining patients in 76%. About 20% of them got sick or injured during Hajj.
Table 2.
Compliance with vaccination and infection control practices among HCWs serving pilgrims of 2009 Hajj season.
| N (%) | |
|---|---|
| Receiving any vaccine before Hajj | 135 (83.9%) |
| Meningococcal | 108 (67.1%) |
| Seasonal influenza | 82 (50.9%) |
| H1N1 | 35 (21.7%) |
| Hepatitis B | 118 (73.3%) |
| Tetanus toxoid | 4 (2.2%) |
| MMR | 3 (1.9%) |
| Causes of not getting H1N1 influenza vaccine? | N = 126 |
| Worried about the vaccine side effects | 53 (42.4%) |
| Non-availability of the vaccine | 43 (34.1%) |
| Fear of developing H1N1 symptoms | 28 (22.2%) |
| Not sure about the vaccine protection | 9 (7.1%) |
| Worried about getting H1N1 from the vaccine | 6 (4.8%) |
| Receiving seasonal influenza vaccine in the past year | 85 (52.8%) |
| Causes of not receiving seasonal influenza vaccine in the past year? | N = 76 |
| Being healthy | 22 (28.9%) |
| Don’t know where to get the seasonal influenza vaccine | 17 (22.4%) |
| Influenza is not a serious illness | 14 (18.4%) |
| Don’t know if I had to get the seasonal influenza vaccine | 9 (11.8%) |
| Worried about seasonal influenza vaccine side effects | 8 (10.5%) |
| Not sure about the seasonal influenza vaccine protection | 7 (9.2%) |
| Non of the above | 12 (15.8%) |
| Compliance with infection control measures during Hajj | |
| Hand hygiene | 77 (97.5%) |
| Cough etiquette | 65 (89.0%) |
| Wearing a mask | |
| Always | 37 (48.7%) |
| Sometimes | 31 (40.8%) |
| Only when with patients | 6 (7.9%) |
| Seldom | 2 (2.6%) |
| Total | 76 (100.0%) |
| Exposure to infections during Hajj: | |
| Within 1 m distance from a person with influenza-like illness | 35 (61.4%) |
| Handling biological specimens | 21 (33.9%) |
| Examining patients | 50 (75.8%) |
| Sickness during Hajj: | |
| Getting any medical or dental care | 23 (22.1%) |
| Getting sick or injured | 21 (20.2%) |
| Respiratory symptoms | 11 (52.4%) |
Background knowledge of the healthcare workers about H1N1 2009 is shown in Table 3, Table 4 . Eighty five percent believed that H1N1 is a serious disease, 80% were worried about catching H1N1 influenza during Hajj and 75% were aware of the main symptoms of H1N1 influenza. The main source or vehicle of H1N1 transmission as recognized by them were contact with people infected with H1N1 (86%), contaminated fomites (72%) and air (65%). Appreciable level of knowledge about measures to avoid H1N1 infection were noted as described by maintenance of hand hygiene (91%), wearing a mask (76%), cough/sneeze etiquette (76%), staying away from sick people (65%), using hand sanitizer (63%), avoiding crowds/public gatherings (62%) and taking H1N1 vaccine (58%).
Table 3.
Knowledge of H1N1 among HCWs serving pilgrims of 2009 Hajj season.
| N (%) | |
|---|---|
| Do you think H1N1 Influenza is a serious disease? | |
| Serious | 120 (85.1%) |
| Not serious | 21 (14.9%) |
| How worried are you about catching H1N1 Influenza during Hajj? | |
| Worried | 122 (79.7%) |
| Not worried | 31 (20.3%) |
| What are the symptoms of H1N1 influenza | |
| Fever | 156 (96.9%) |
| Cough | 138 (85.7%) |
| Sore throat | 137 (85.1%) |
| Headache | 128 (79.5%) |
| Tierdness | 126 (78.3%) |
| Muscle or joint pain | 126 (78.3%) |
| Nauseas or vomiting | 120 (74.5%) |
| Trouble breathing | 119 (73.9%) |
| Diarrhea | 115 (71.4%) |
| Pneumonia | 95 (59.0%) |
| Chills | 94 (58.4%) |
| Congestion or stuffy nose | 90 (55.9%) |
| Vehicles/sources of H1N1 infection | |
| People with H1N1 | 138 (85.7%) |
| Contaminated patient objects | 116 (72.0%) |
| Air | 104 (64.6%) |
| Dirty or poor hygiene | 62 (38.5%) |
| Soil | 15 (9.3%) |
| Animals | 14 (8.7%) |
| Water | 13 (8.1%) |
| Food | 7 (4.3%) |
| Ways to avoid H1N1 infection | |
| Frequent washing of hands | 147 (91.3%) |
| Wearing a mask | 122 (75.8%) |
| Covering own cough or sneeze | 122 (75.8%) |
| Staying away from sick people | 105 (65.2%) |
| Using hand sanitizer | 101 (62.7%) |
| Avoiding crowds/public gatherings | 100 (62.1%) |
| Taking H1N1 Vaccine | 93 (57.8%) |
| Staying home from work/school | 35 (21.7%) |
| Checking with a doctor/nurse | 34 (21.1%) |
| Taking vitamins/herbs/natural remedies | 22 (13.7%) |
| Using antibiotics/medicine | 21 (13.0%) |
Table 4.
Prevalence (per 100 persons) of different types of respiratory viruses examined among HCWs serving pilgrims of 2009 Hajj season.
| Pre-Hajj (N = 120) | Post-Hajj (N = 120) | Total (N = 172) | p-value | |
|---|---|---|---|---|
| Influenza A | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| H1N1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Influenza B | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Respiratory Syncitial Virus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Corona Virus 229E | 0 (0.0%) | 1 (0.8%) | 1 (0.6%) | NSa |
| Corona Virus HKU1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Corona Virus NL63 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Corona Virus OC43 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Parainfluenza 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Parainfluenza 2 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Parainfluenza 3 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Parainfluenza 4 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Entero-Rhinovirus | 9 (7.5%) | 14 (11.7%) | 21 (12.6%) | NSa |
| Metapneumovirus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Adenovirus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| Human Bocavirus | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| All types of viruses | 9 (7.5%) | 15 (12.5%) | 22 (12.8%) | NSa |
Using exact p-value from McNemar Test.
Among the 18 circulating respiratory virus types and subtypes, only two were detected in the healthcare workers in the pre- and post-Hajj period: rhinovirus (N = 21, 12.6%) and coronavirus 229E (N = 1, 0.6%). Rhinovirus was detected more before the Hajj (N = 14, 11.7%) than after (N = 9, 7.5%), but the difference was not statistically significant. The only isolate of coronavirus was detected in the post-Hajj period. Two healthcare workers had rhinovirus detected both before and after the Hajj. Rhinovirus was detected in 21.1% of those who had respiratory symptoms and 30.0% of those who got sick during Hajj. No other respiratory viruses were detected in any of the samples.
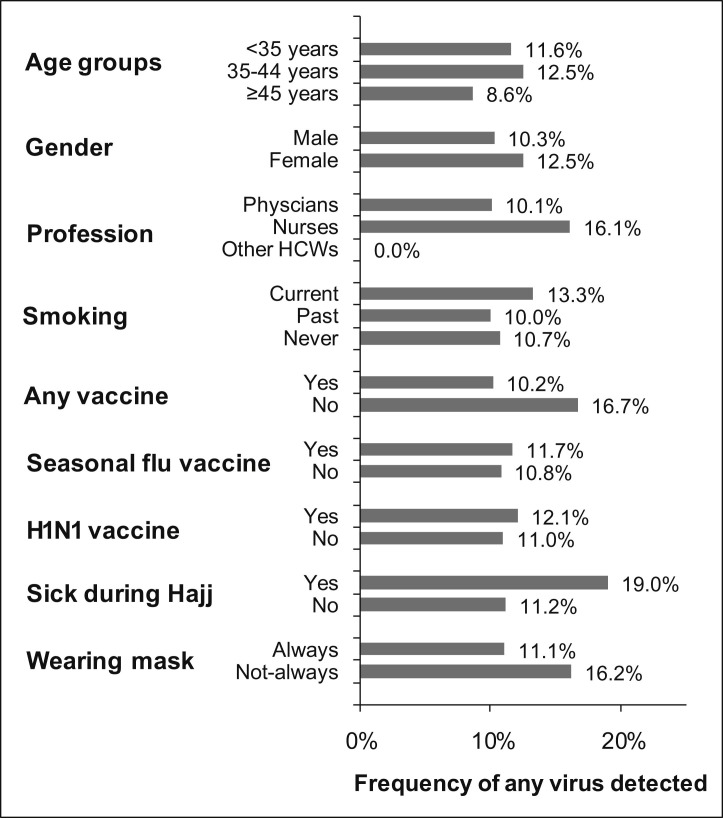
The prevalence (per 100 persons) of respiratory viruses according to age, sex, profession, smoking, vaccine, sickness, and wearing a mask is shown in Fig. 1 . The prevalence was slightly higher in healthcare workers who got sick during Hajj, in nurses, and in those who did not wear masks than in smokers; however, the difference was not statistically significant.
Figure 1.
Prevalence (per 100 persons) of all detected respiratory viruses* examined by demographic and clinical variables among HCWs serving at 2009 Hajj season (*Mainly Entero-Rhinovirus).
Discussion
Hajj, the annual pilgrimage of Muslims is a time of a unique mass gathering event in Makkah.
Around two million people are confined to small area and the chances of having infections acquired by respiratory tract are increased. Al-Tamami et al,10 during the 2001 Hajj, found 23 cases of meningitis of all types, mainly in Indians, whose ages ranged from 1 to 70 years, and in twice as many women as men. Balkhy et al,6 in 2004, studied 500 symptomatic pilgrims, 10.8% of whom had positive viral cultures. Of these, influenza B accounted for 50%, followed by herpes simplex virus (21.4%), RSV (12.9%), parainfluenza (7.4%) and influenza A (5.6%). A comparative study of respiratory tract infections in symptomatic UK and Saudi pilgrims by Rashid et al. in 20088 found infections in 25% of UK pilgrims but in only 13% of Saudi pilgrims. Half of the infections in UK pilgrims were due to rhinoviruses, followed by influenza virus, parainfluenza, and RSV. The Saudi pilgrims had higher infection rates with influenza virus (78.5%) than with rhinovirus (21.4%). In 2009, Alborzi et al.9 also reported that 32.5% of patients tested had viral pathogens: influenza in 25 (9.8%), parainfluenza in 19 (7.4%), rhinovirus in 15 (5.9%), adenovirus in 14 (5.4%), enterovirus in 5 (2%), and RSV in 4 (1.6%) and coinfection with two viruses in 1 patient (0.4%).
The current study evaluated the prevalence of respiratory viruses in healthcare workers who saw pilgrims after the H1N1 2009 pandemic had been declared. We tested for 18 respiratory virus types and subtypes in the healthcare workers and found primarily rhinoviruses and a single coronavirus 229E. Rhinoviruses were more prevalent after the Hajj (11.7%) than before (7.5%). None of the healthcare workers tested positive for any influenza virus, including the H1N1 2009 pandemic strain. This is explained by the fact that among more than two million pilgrims in 2009, the Ministry of Health reported only 100 cases of H1N1 and 5 deaths.17 The high case-fatality ratio may be because pilgrims were committed to completing Hajj and delayed seeking medical care until their condition had worsened.17, 18 Since the overall number of cases among pilgrims was low, therefore, the chances of transmitting it to healthcare workers were very small.
Rhinoviruses are present in about two-thirds of persons with common colds and probably are responsible for more human infections than any other agents.19, 20 They are common in all age groups, occur throughout the year, and are present worldwide.21 Louis et al.22 found that rhinovirus was responsible for half of the respiratory infections in residents and staff in a long-term care facility for elderly persons, although in community-dwelling elderly, they cause 63% of respiratory infections.23 Renois et al24 found rhinoviruses to be most prevalent in cases of influenza-like illness in infections with one agent (25%) as well as in co-infections with influenza A H1N1 viruses (50%). Our finding that rhinoviruses are the most prevalent viruses in healthcare workers during the 2009 Hajj are consistent with the other studies of rhinoviruses in the general population, in patients with influenza-like illness, and also in pilgrims.
Arruda et al.25 studied the natural history of rhinovirus infections in adults during Autumn and found that among 346 persons with colds, 82% (283) had rhinovirus infections and 8% had coronavirus OC43 and 229E. These findings agree with our finding of rhinovirus as the predominantly isolated virus (12.6%), followed by coronavirus 229E (0.6%), in a group of subjectively healthy healthcare workers. Arruda et al. isolated a high percentage of viruses because the subjects were symptomatic, while in our study rhinovirus was detected in 21% of participants with respiratory symptoms and in 30% who got sick during Hajj.
Rhinoviruses spread efficiently in families, in school groups, among university students, and on military bases.26, 27 Linde et al.28 found an increase in the proportion and number of rhinovirus diagnoses that roughly parallels a decrease in influenza diagnoses, after the summer holidays and start of schools. They hypothesize that a rhinovirus epidemic could interfere with the spread of pandemic influenza in a warm and humid climate, which decreases the spread of influenza by aerosol. A similar phenomenon may be responsible for the frequent isolation of rhinoviruses in the present study. Rhinoviruses may protect the host from being infected by other viruses such as influenza A virus, parainfluenza virus, adenoviruses, coronaviruses, bocavirus, metapneumovirus, and RSV.29
Rhinovirus shedding is commonly limited to 10–14 days in immunocompetent subjects.24 However viral RNA may be present from days before symptoms occur to five or more weeks after they go away.30, 31
The influenza A H1N1 vaccination rate in healthcare workers has been reported to be lower than the seasonal influenza vaccination rate16, 32, 33 and was 22% versus 51% in the current study. These results are also comparable to the data obtained from the United States for the same period: where vaccination coverage for H1N1 in healthcare workers was 37% and for seasonal influenza it was 62%.32, 33
In conclusion, we found that rhinoviruses were the most frequently isolated viruses in a group of subjectively healthy middle-aged healthcare workers who treated Hajj pilgrims during the 2009 influenza A H1N1 pandemic. Respiratory symptoms were present in 21% of the healthcare workers in which the virus was detected. None of the participants had influenza A H1N1 2009, despite that only 22% of them were vaccinated against H1N1 vaccine, possibly because it was also infrequently found among pilgrims.
Conflict of interest
None declared.
Acknowledgments
Authors like to acknowledge the contribution of Dr. Shahul Ebrahim from the Centers for Disease Control & Prevention, USA, Dr Abduraman Abudawod and Dr. Nedal Almasri for their assistance in the data collection.
References
- 1.Smith W., Low J.G. Risk of respiratory infections in healthcare workers: lessons on infection control emerge from the SARS outbreak. Southeast Asian J Trop Med Public Health. 2005;36(2):481–488. [PubMed] [Google Scholar]
- 2.Al-Asmary S., Al-Shehri A.S., Abou-Zeid A., Abdel-Fattah M., Hifnawy T., El-Said T. Acute respiratory tract infections among Hajj medical mission personnel, Saudi Arabia. Int J Infect Dis. 2007;11:268–272. doi: 10.1016/j.ijid.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmed Q.A., Arabi Y.M., Memish Z.A. Health risks at the Hajj. Lancet. 2006;367:1008–1015. doi: 10.1016/S0140-6736(06)68429-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Association of National Health Occupational Physicians (ANHOPS) April 2006. Immunisation of healthcare workers.http://www.anhops.com/docs/29_9_immuninisation.pdf [accessed 07.02.10] [Google Scholar]
- 5.Alzeer A.H. Respiratory tract infection during Hajj. Ann Thorac Med. 2009;4:50–53. doi: 10.4103/1817-1737.49412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balkhy H.H., Memish Z.A., Bafaqeer S., Almuneef M.A. Influenza a common viral infection among Hajj pilgrims: time for routine surveillance and vaccination. J Travel Med. 2004;11:82–86. doi: 10.2310/7060.2004.17027. [DOI] [PubMed] [Google Scholar]
- 7.Memish Z.A., Ahmed Q.A.A. Mecca bound: the challenges ahead. J Travel Med. 2002;9:202–210. doi: 10.2310/7060.2002.24557. [DOI] [PubMed] [Google Scholar]
- 8.Rashid H., Shafi S., Haworth E., El Bashir H., Memish Z.A., Sudhanva M. Viral respiratory infections at the Hajj: comparison between UK and Saudi pilgrims. Clin Microbiol Infect. 2008;14:569–574. doi: 10.1111/j.1469-0691.2008.01987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alborzi A., Aelami M.H., Ziyaeyan M., Jamalidoust M., Moeini M., Pourabbas B. Viral etiology of acute respiratory infections among Iranian Hajj pilgrims. J Travel Med. 2006;2009(16):239–242. doi: 10.1111/j.1708-8305.2009.00301.x. [DOI] [PubMed] [Google Scholar]
- 10.Al-Tamami S., Bajeri K., Turkistani A., Al-Rabeah A., Mohammad A.G. Risk factors of bacterial meningitis in Makkah during Hajj 1421 H: a pilot study. Saudi Epidemiol Bull. 2001;8(4) [Oct-Dec] [Google Scholar]
- 11.CDC Outbreak of swine-origin influenza A (H1N1) virus infection – Mexico, March–April 2009. Morbidity Mortality Weekly Rep (MMWR) May 8, 2009;58(17):467–470. [PubMed] [Google Scholar]
- 12.CDC Update: swine influenza A (H1N1) infections – California and Texas, April 2009. Morbidity Mortality Weekly Rep (MMWR) May 1, 2009;58(16):435–437. [PubMed] [Google Scholar]
- 13.World Health Organization . 2009. The regional office of eastern mediterranean. Pandemic (H1N1)http://www.emro.who.int/csr/h1n1/h1n1_update.htm [accessed 05.04.11] [Google Scholar]
- 14.Khan K., Memish Z.A., Chabbra A., Liauw J., Hu W., Janes D.A. Global public health implications of a mass gathering in Mecca, Saudi Arabia during the midst of an influenza pandemic. J Travel Med. 2010;17:75–81. doi: 10.1111/j.1708-8305.2010.00397.x. [DOI] [PubMed] [Google Scholar]
- 15.Memish Z.A., McNabb S.J.N., Mahoney F., Alrabiah F., Marano N., Ahmed Q.A. Establishment of public health security in Saudi Arabia for the 2009 Hajj in response to pandemic influenza A H1N1. The Lancet. 2009;374:1786–1791. doi: 10.1016/S0140-6736(09)61927-9. [DOI] [PubMed] [Google Scholar]
- 16.Ahmed G.Y., Balkhy H.H., Bafaqeer S., Al-Jasir B., Althaqafi A. Acceptance and adverse effects of H1N1 vaccinations among a Cohort of National Guard health care workers during the 2009 Hajj season. BMC Res Notes. 2011;4:61. doi: 10.1186/1756-0500-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haworth E., Rashid H., Booy R. Prevention of pandemic influenza after mass gatherings – learning from Hajj. J R Soc Med. 2010;103:79–80. doi: 10.1258/jrsm.2010.090463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Memish Z.A., Ebrahim S.H., Ahmed Q.A., Deming M., Assiri A. Pandemic H1N1 influenza at the 2009 Hajj: understanding the unexpectedly low H1N1 burden. J R Soc Med. 2010;103:386. doi: 10.1258/jrsm.2010.100263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mackay I.M. Human rhinoviruses: the cold wars resume. J Clin Virol. 2008;42:297–320. doi: 10.1016/j.jcv.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong C.Y., Lin R.T., Tan E.S., Chong P.N., Tan Y.S., Lew Y.J. Acute respiratory symptoms in adults in general practice. Fam Pract. 2004;21:317–323. doi: 10.1093/fampra/cmh319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monto A.S. The seasonality of rhinovirus infections and its implications for clinical recognition. Clin Ther. 2002;24(12):1987–1997. doi: 10.1016/S0149-2918(02)80093-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louie J.K., Yagi S., Nelson F.A., Kiang D., Glaser C.A., Rosenberg J. Rhinovirus outbreak in all long term care facility for elderly persons associated with unusually high mortality. Clin Infect Dis. 2005;41:262–265. doi: 10.1086/430915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson K.G., Kent J., Hammersley V., Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective, population based study of disease burden. Br Med J. 1977;315:1060–1064. doi: 10.1136/bmj.315.7115.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renois F., Talmud D., Huguenin A., Moutte L., Strady C., Cousson J. Rapid detection of respiratory tract viral infections and coinfections in patients with influenza-like illnesses by use of reverse transcription-PCR DNA microarray systems. J Clin Microbiol. 2010;48:3836–3842. doi: 10.1128/JCM.00733-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arruda E., Pitk˙aranta A., Witek T.J., Doyle C.A., Hayden F.G. Frequency and natural history of rhinovirus infections in adults during autumn. J Clin Microbiol. 1997;35:2864–2868. doi: 10.1128/jcm.35.11.2864-2868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fox J.P., Cooney M.K., Hall C.E. The Seattle virus watch. V. Epidemiologic observations of rhinovirus infections, 1965–1969, in families with young children. Am J Epidemiol. 1975;101:122–143. doi: 10.1093/oxfordjournals.aje.a112078. [DOI] [PubMed] [Google Scholar]
- 27.Fox J.P., Cooney M.K., Hall C.E., Foy H.M. Rhinoviruses in Seattle families, 1975–1979. Am J Epidemiol. 1985;122:830–846. doi: 10.1093/oxfordjournals.aje.a114166. [DOI] [PubMed] [Google Scholar]
- 28.Linde A., Rotzén-Östlund M., Zweygberg-Wirgart B., Rubinova S., Brytting M. Does viral interference affect spread of influenza? Euro Surveill. 2009;14(40) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19354 Available online: pii=19354. [PubMed] [Google Scholar]
- 29.Greer R.M., McErlean P., Arden K.E., Faux C.E., Nitsche A., Lambert S.B. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45:10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jartti T., Lehtinen P., Vuorinen P., Koskenvuo M., Ruuskanen O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J Med Virol. 2004;72:695–699. doi: 10.1002/jmv.20027. [DOI] [PubMed] [Google Scholar]
- 31.Winther B., Hayden F.G., Hendley J.O. Picornavirus infections in children diagnosed by RT-PCR during longitudinal surveillance with weekly sampling: association with symptomatic illness and effect of season. J Med Virol. 2006;78:644–665. doi: 10.1002/jmv.20588. [DOI] [PubMed] [Google Scholar]
- 32.Pearson M.L., Bridges C.B., Harper S.A. Influenza vaccination of health-care personnel: recommendations of the Healthcare Infection Control Practices Advisory Committee (HICPAC) and the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–16. [PubMed] [Google Scholar]
- 33.Jordan R., Hayward A. Should healthcare workers have the swine flu vaccine? Br Med J. 2009;339:b3398. doi: 10.1136/bmj.b3398. [DOI] [PubMed] [Google Scholar]