Abstract
Japanese encephalitis (JE) is a serious leading health complication emerging expansively that has severely affected the survival rate of human beings. This fatal disease is caused by JE Virus (JEV). The current study was carried out for designing a multi-epitope loaded peptide vaccine to prevent JEV. Based on reverse vaccinology and in silico approaches, octapeptide B-cell and hexapeptide T-cell epitopes belonging to five proteins, viz. E, prM, NS1, NS3 and NS5 of JEV were determined. Hydrophilicity, antigenicity, immunogenicity and aliphatic amino acids of the epitopes were estimated. Further, the epitopes were analyzed for different physicochemical parameters, e.g. total net charges, amino acid composition and Boman index. Out of all the epitopes, a total of four T-cell epitopes namely KRADSS, KRSRRS, SKRSRR and KECPDE and one B-cell epitope i.e. PKPCSKGD were found to have potential for raising immunity in human against the pathogen. Taking into account the outcome of this study, the pharmaceutical industries could initiate efforts to combine the identified epitopes together with adjuvant or carrier protein to develop a multi-epitope-loaded peptide vaccine against JEV. The peptide vaccine, being cost effective, could be administered as a prophylactic measure and in JEV infected individuals to combat the spread of this virus in human population. However, prior to administration into human beings, the vaccine must pass through several clinical trials.
Keywords: JEV, T-cell epitope, B-cell epitope, Peptide vaccine
Highlights
-
•
Potential T and B-cell epitopes of 5 proteins in Japanese encephalitis virus (JEV) were determined using bioinformatics.
-
•
All epitopes were analyzed for hydrophilicity, immunogenicity, antigenicity and other physicochemical parameters.
-
•
Identified epitopes could be used for developing a multi-epitope-loaded peptide vaccine to combat JEV.
1. Introduction
Japanese encephalitis (JE) is a major emerging, dreadful infection worldwide as this fatal disease has affected the lives of many individuals resulting into15,000 deaths and 50,000 cases of infection per year (Solomon, 1997; Tsai, 1997). The causal agent of the disease is JE virus (JEV). JEV is transmitted by mosquitoes and is categorized into genus Flavivirus and family Flaviviridae (Westaway et al., 1985). JEV severely affects the central nervous system of human and results into infectious disease. The transmission cycle of JEV occurs between mosquitoes and birds or swine. However, the transmission of the virus to humans usually takes place through infected mosquitoes of the species, Culex tritaeniorrhynchus (Porterfield, 1995). JEV is found to prevail in many Asian nations namely India, Nepal, Sri Lanka, China, Japan, Korea, Vietnam, Thailand, Myanmar, Taiwan, Siberia, Cambodia, Bhutan, Bangladesh, Malaysia and Indonesia. The JE epidemic has spread from Eastern Asia to Southeast and Southern Asia (Burke and Leake, 1988b; Oya, 1988; Vaughn and Hoke Jr, 1992; Endy and Nisalak, 2002; Mackenzie et al., 2006). Apart from Asia, JE has affected many geographic regions of other continents as well, namely Northern Australia and Western Pacific (Paul et al., 1993; Hanna et al., 1996; Hanna et al., 1999). The outbreak of JE was first observed in Japan during 1870s and the first isolate of JEV was obtained by culturing the brain cells of an infected individual in 1935 (Solomon et al., 2000).
Although children are the primary targets of JE infection, it also causes dreadful infection in adolescents and adults. In temperate regions of Asia JE outbreaks mainly occur in summer; while outbreaks in torrid zone and subtropics of Asia prevail throughout the year and the occurrence of JE infections rapidly increases during rainy days (Burke and Leake, 1988b; Halstead and Jacobson, 2008; Fischer et al., 2010). Symptoms of JEV infection include fever, meningoencephalopmyelitis, aseptic meningitis, seizures or poliomyelitis-like paralysis (Solomon et al., 1998; Solomon et al., 2002; Solomon and Vaughn, 2002). Death occurs in about 20–30% of JE infected cases and about 30–50% of surviving people usually encounter constant abnormalities associated with nervous system, e.g. mental disorientation, mental retardation and hemiparesis (Solomon et al., 2000; Fischer et al., 2008; Ooi et al., 2008).
There are five different genotypes of JEV (Uchil and Satchidanandam, 2001; Solomon et al., 2003) and all the strains belong to only one serotype (Tsarev et al., 2000; Erra and Kantele, 2015). JEV genome consists of a positive-sense single stranded RNA molecule of size 11 kb (Westaway et al., 1985). The viral RNA synthesizes one polyprotein, which undergoes proteolytic cleavages within the JEV-infected cells and produces 10 proteins, viz. envelope (E), capsid (C), membrane (M or precursor membrane i.e. prM), NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5 proteins (NS-non-structural) (Chambers et al., 1990; Marin et al., 1995; Rice, 1996; Zanotto et al., 1996). The envelope of JEV, made up of glycoprotein, has a size of 50 nm. This envelope encircles the nucleocapsid formed by the joining of capsid and RNA. The E protein helps the virus adhere and penetrate into the host cell; in addition to helping at the time of membrane fusion (Allison et al., 2001; Kuhn et al., 2002). The prM protein is secreted only during immature stage of the virion. At the later stage of viral infection, prM protein is broken down into M protein with the help of proteases. As a result, the virion develops into a mature virion. Sometimes, prM protein fails to break down into M protein (Bray and Lai, 1991). In JEV-affected host cells, the virus produces NS1 protein externally and it acquires importance in virion maturation (Fan and Mason, 1990; Rice, 1996). The two other proteins namely NS3 and NS5 help JEV undergo replication process (Rice et al., 1985; Bartholomeusz and Wright, 1993).
Various vaccines are formulated time to time for preventing the spread of JE. The vaccines include inactivated cells acquired from mouse brain or vero cell culture, live-attenuated vaccines developed from JEV or other viruses resulting into the formation of chimera (Halstead and Thomas, 2010; Baig et al., 2013; Hegde and Gore, 2017). All these vaccines have shown effective results in infected individuals. The WHO suggests to utilize these vaccines in those regions of the world where JEV has caused acute infection (Organization, 2015). Novel vaccines based on replicon (Kofler et al., 2004), subviral particles (Konishi et al., 1992; Konishi et al., 2001), and JEV-pulsed dendritic cells (Li et al., 2009) have been under development and tested only in animal models.
Reverse vaccinology approach is based on the genomics of a pathogen and elucidates the antigenic determinants in the proteins of the pathogen (Rinaudo et al., 2009; Tantray et al., 2014). It, in fact, follows an in silico approach to screen the entire genome of the infectious pathogens to determine the epitopes of proteins that could be used as potential molecules for peptide vaccine formulation and development. In comparison to the traditional technique of vaccine design that requires laborious as well as expensive wet lab experiments, the reverse vaccinology approach relies mostly on genomic information and involves much lesser cost and time. The technique of reverse vaccinology provides enormous advantage in designing peptide vaccines against highly infectious pathogens, including those which cannot be cultured in lab due to high risk imposed on researchers and health professionals. Antigenic determinants or epitopes are the regions of the proteins that interact with the receptors on the T-cells or with antibodies produced by the B-cells and generate significant immune response in host. For this purpose, various bioinformatic tools have been developed to identify the potential epitopes for peptide vaccine formulation (Weiss and Littman, 1994). Peptide vaccines are not only safer, less expensive and easily manufacturable but also require less time for manufacture as compared to the traditional vaccines (Von Hoff, 2005; Tang et al., 2012). Unlike traditional vaccines, peptide vaccines do not cause autoimmune disorders or skin irritations (Skwarczynski and Toth, 2016). Moreover, the newly discovered epitopes need to be well characterized for biological functions.
Development of immunity in a host against a pathogen or antigen stems from the presence of T- and B- lymphocytes. T-lymphocytes recognize the antigen only when it is displayed by a class of proteins, called major histocompatibility complex (MHC) molecules, as exterior part to the antigen presenting cells (APCs). The T-cell receptor binds with the antigen and causes its destruction (Lafuente and Reche, 2009). On the other hand, the destruction of antigen by B-cells takes place via the secretion of antibodies that interact with the antigen. B-cells are also capable of providing immunity during future exposure to the antigen as some B-cells differentiate into memory cells in the host.
The current study was performed for predicting the potential T-cell and B-cell epitopes of five proteins in JEV namely E, prM, NS1, NS3 and NS5 of JEV for designing a multi-epitope loaded peptide vaccine. This study allows the identification of unique peptides as vaccine candidates that might be much cheaper than the existing vaccine formulation against JEV. Our analysis suggested that E, prM and NS1 proteins possess capability for generating immunological response in the host body (Monath et al., 1996). Another study reported that NS3 and NS5 proteins can induce immune reaction in the host body (Turtle et al., 2016). The predicted epitopes identified in five proteins selected in this study could be promising for formulating a peptide vaccine against JEV and hence, could prevent the spread of JEV in affected individuals.
2. Materials and methods
2.1. Extraction of protein sequences
Complete sequences of the proteins, viz. E, prM, NS1, NS3 and NS5 of JEV were collected from the Protein database of National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov). All the proteins were identified to possess their respective accession numbers: NP_775666.1, NP_775664.1, NP_775667.1, NP_775670.1 and NP_775674.1.
2.2. Determination of T-cell epitopes
Using the algorithm based on wet lab experiments and established by Hopp and Woods, the linear hexamer T-cell epitopes in five selected proteins of JEV were identified (Hopp and Woods, 1981). Further, the set of linear octamer B-cell epitopes in five selected JEV proteins was determined by using the algorithm outlined by Kolaskar and Tongaonkar (Kolaskar and Tongaonkar, 1990).
2.3. Determination of hydrophilicity score
Hydrophilicity score is a measure that describes the hydrophilicity of an epitope or a protein. It is the mean of the hydrophilicity values of all the amino acids contained in an epitope or a protein. It is useful in determining the structure of the protein (Hopp and Woods, 1981).
2.4. Hydropathy index
Hydropathy index, a measure of hydrophobicity of a given epitope or a protein, was determined as the average of hydrophobicity values from all amino acids present in an epitope or a protein (Kyte and Doolittle, 1982). Estimation of Hydropathy index values of all epitopes was achieved by applying the AbDesigner algorithm (Pisitkun et al., 2011).
2.5. Chou-Fasman conformation
Beta-turn regions within a protein have significant role in generating immunogenicity. Chou- Fasman algorithm was used to determine the properties of secondary structure of peptide (Chou and Fasman, 1978).
2.6. Immunogenicity score
Immunogenicity score reports the immunogenic nature of a given epitope or protein. The immunogenicity scores of all the epitopes were estimated on the basis of hydropathy index and Chou- Fasman conformation parameters of each epitope. Further, hydrophilicity, antigenicity and immunogenicity scores as well as aliphatic amino acids in each epitope were estimated using a computer program written in PERL language by SC (corresponding author).
2.7. Determination of physicochemical parameters of epitopes
The physico-chemical parameters of epitopes such as net charge and amino acid composition were determined in ProtParam tool of ExPASy (https://web.expasy.org/protparam/). Further, the cleavage sites in epitopes were identified using the online tool Peptide cutter (https://web.expasy.org/peptide_cutter/) (shown in S1 and S2). Boman index i.e. the protein binding potential of each epitope was determined by using the online tool APD3 i.e. (http://aps.unmc.edu/AP/prediction/prediction_main.php). The epitopes that acquire Boman index values higher than 2.48 kcal/mol are considered being capable of attaching to the MHC molecules or antibodies.
3. Results
3.1. Hydrophilicity scores of epitopes
With the help of bioinformatic tools, five hexapeptide T-cells epitopes and five octapeptide B-cell epitopes were identified in five proteins namely E, prM, NS1, NS3 and NS5 of JEV.
In order to combat the spread of JEV, hexapeptide T-cell epitopes would possess the ability to raise high immune response in the host (Hopp and Woods, 1981). The epitope positions, hydrophilicity scores and total net charges of all T-cell epitopes were determined (Table 1a ). Out of five epitopes in E protein, the epitope EKRADS (epitope position 83–88) was recorded with the greatest hydrophilicity score (11.8) but without any net charge. Two epitopes namely KRSRRS (epitope position 88–93) and SKRSRR (epitope position 87–92) located within prM protein exhibited the greatest hydrophilicity value (12.6) with positive net charge (+4). Similarly, within NS1 protein, two epitopes with maximum hydrophilicity score of 11.9 were identified as EESTDE (173–178) and REESTD (172–177) carrying net charges of −4 and − 2, respectively. The epitope RGEEKK (527–532) of NS3 protein was estimated with the highest hydrophilicity value (15) along with a positive net charge (+1). In NS5 protein, the epitope DEEREN (438–443) was recorded with high hydrophilicity value (15.2) associated with a negative net charge (−3). High hydrophilicity scores of T-cell epitopes along with their positions usually represent the hydrophilic sections in proteins and these regions tend to remain exposed on the surface of the proteins. These regions can bind easily with the MHC molecules and the complexes so formed would be exhibited by the APCs on their surfaces. The T-cells can easily recognize these cells and destroy them.
Table 1a.
T-cell epitopes from five proteins of JEV representing their hydrophilicity scores and total net charges (Hopp & Woods approach, 1981).
| Name of virus | Name of proteins | Accession number | Hexapeptide epitope | Epitope position | Hydrophilicity score | Total net charge |
|---|---|---|---|---|---|---|
| Japanese encephalitis | E protein | NP_775666.1 | EKRADS | 83–88 | 11.8 | 0 |
| NEKRAD | 82–87 | 11.7 | 0 | |||
| GRGDKQ | 386–391 | 9.2 | 1 | |||
| KRADSS | 84–89 | 9.1 | 1 | |||
| NARDRS | 474–479 | 9 | 1 | |||
| prM protein | NP_775664.1 | KRSRRS | 88–93 | 12.6 | 4 | |
| SKRSRR | 87–92 | 12.6 | 4 | |||
| DPEDVD | 61–66 | 10.5 | −-4 | |||
| SKGENR | 28–33 | 9.5 | 1 | |||
| GNDPED | 59–64 | 9.2 | −-3 | |||
| NS1 protein | NP_775667.1 | EESTDE | 173–178 | 11.9 | −-4 | |
| REESTD | 172–177 | 11.9 | −-2 | |||
| KHNRRE | 253–258 | 11.7 | 2 | |||
| EDCGKR | 289–294 | 11 | 0 | |||
| KECPDE | 141–146 | 11 | −-2 | |||
| NS3 protein | NP_775670.1 | RGEEKK | 527–532 | 15 | 1 | |
| GEEKKN | 528–533 | 12.2 | 0 | |||
| DRQEEP | 169–174 | 12.2 | −-2 | |||
| GDRQEE | 168–173 | 12.2 | −-2 | |||
| GPEREK | 511–516 | 12 | 0 | |||
| NS5 protein | NP_775674.1 | DEEREN | 438–443 | 15.2 | −-3 | |
| KREKKP | 459–464 | 15 | 3 | |||
| GKREKK | 458–463 | 15 | 3 | |||
| REKRPR | 389–394 | 15 | 3 | |||
| RRARRE | 41–-46 | 14.5 | 3 |
The epitope positions, hydrophilicity scores and total net charge of each octapeptide B-cell epitope were evaluated (Table 1b ). In E protein, the epitope SGSDGPCK (329–336) occupied maximal hydrophilicity value (5.6) but without any net charge. In the case of prM protein, two epitopes namely SKRSRRSV (87–94) and KRSRRSVS (88–95) were identified with the highest hydrophilicity score of 11.4 with net charge of +4 each. In NS1 protein, the epitope TKECPDEH (140–147) was recorded with the highest hydrophilicity value (10.4) and a negative net charge (−2). In NS3 protein, the epitope PKPCSKGD (10–17) was found to possess high hydrophilicity value (8.3) with a positive net charge (+1). In NS5 protein, the epitope PYVGKRED (842–849) was found to possess the highest hydrophilicity score of 8.2 without any net charge. High hydrophilicity scores of B-cell epitopes imply that these epitopes lie in the hydrophilic regions of the respective proteins and can interact with the antibodies produced by B-cells. This interaction might lead to the generation of immunological response in host against JEV.
Table 1b.
B-cell epitopes from five proteins of JEV representing their hydrophilicity scores and total net charges (Kolaskar & Tongaonkar approach, 1990).
| Name of virus | Name of proteins | Accession number | Octapeptide epitope | Epitope position | Hydrophilicity score | Total Net charge |
|---|---|---|---|---|---|---|
| Japanese encephalitis | E protein | NP_775666.1 | SGSDGPCK | 329–336 | 5.6 | 0 |
| YSGSDGPC | 328–335 | 0.3 | − | |||
| EPPFGDSY | 375–382 | 1.5 | − | |||
| LSYSGSDG | 326–333 | − | − | |||
| ELSYSGSD | 325–332 | 2.8 | − | |||
| prM protein | NP_775664.1 | NDPEDVDC | 60–67 | 9.7 | − | |
| DCWCDNQE | 66–73 | 4 | − | |||
| SKRSRRSV | 87–94 | 11.4 | 4 | |||
| KRSRRSVS | 88–95 | 11.4 | 4 | |||
| RCTRTRHS | 80–87 | 7 | 3 | |||
| NS1 protein | NP_775667.1 | TKECPDEH | 140–147 | 10.1 | − | |
| DRYKYLPE | 30–37 | 5.6 | 0 | |||
| KPVGRYRS | 92–99 | 5.5 | 3 | |||
| ECPDEHRA | 142–149 | 10 | − | |||
| KYLPETPR | 33–40 | 4.5 | 1 | |||
| NS3 protein | NP_775670.1 | YPKCKNGD | 395–402 | 5.9 | 1 | |
| PKPCSKGD | 10–17 | 8.3 | 1 | |||
| PSPKPCSK | 8–15 | 5.6 | 2 | |||
| EYPKCKNG | 394–401 | 5.9 | 1 | |||
| DTPSPKPC | 6–13 | 4.9 | 0 | |||
| NS5 protein | NP_775674.1 | HKDPEHPY | 295–302 | 5.7 | − | |
| GKENYVDY | 880–887 | 3.1 | − | |||
| PYVGKRED | 842–849 | 8.2 | 0 | |||
| FYKPSEPS | 133–140 | 1.8 | 0 | |||
| CGRGGWSY | 82–89 | − | 1 |
3.2. Antigenicity scores of epitopes
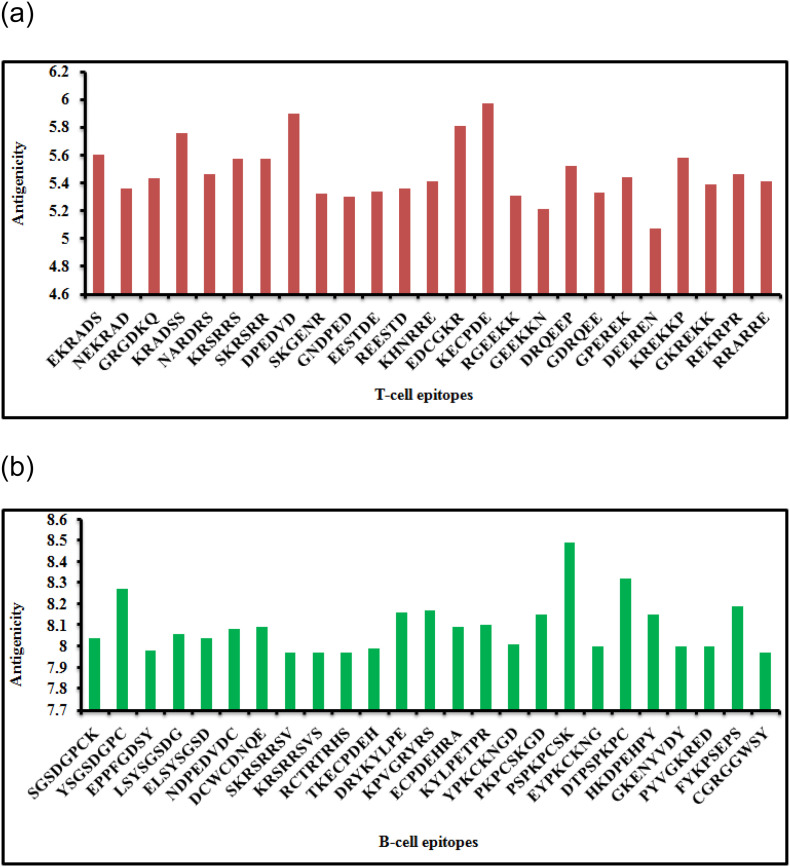
All T-cell epitopes obtained from five proteins were examined and evaluated for antigenicity (Fig. 1 a). Antigenicity values of the 25 T-cell epitopes were found to range from 5.07 (DEEREN) to 5.97 (KECPDE). Likewise, total 25 B-cell epitopes were also analyzed for antigenicity score (Fig. 1b) and the antigenicity scores of B-cell epitopes varied from 7.97 (SKRSRRSV, KRSRRSVS, RCTRTRHS and CGRGGWSY) to 8.49 (PSPKPCSK). The epitopes possessing high antigenicity values normally represent highly antigenic nature of the epitopes. Notably, high antigenicity score of an epitope is treated as a salient feature for the formulation of a peptide vaccine.
Fig 1.
(a): Antigenicity scores of hexapeptide T-cell epitopes obtained from five proteins of JEV. Fig 1(b): Antigenicity scores of octapeptide B-cell epitopes obtained from five proteins of JEV.
3.3. Immunogenicity scores of epitopes
Like antigenicity analysis, all the hexapeptide T-cell epitopes were evaluated for their immunogenicity scores (Table 2a ). In E protein, the epitope NARDRS was found to possess the highest immunogenicity score (8.19) whereas, in prM protein the epitope GNDPED recorded maximum immunogenicity score (9.89). In NS1 protein the epitope KHNRRE was identified with the highest immunogenicity score (8.6) but in NS3 protein the epitope DRQEEP was found to possess the maximum immunogenicity score of 8.4. In NS5 protein two epitopes, viz. DEEREN and REKRPR each possessed the highest immunogenicity score (8.42).
Table 2a.
Immunogenicity (Ig) and number of aliphatic amino acids in T-cell epitopes of JEV (Hopp & Woods approach, 1981).
| Name of virus | Name of proteins | Accession number | Hexapeptide epitope | Aliphatic amino acids | Ig analysis of hexapeptide epitopes |
||
|---|---|---|---|---|---|---|---|
| Hydropathy index | Chou-Fasman conformation | Ig score | |||||
| Japanese encephalitis | E protein | NP_775666.1 | EKRADS | 2 | 6.9 | 1.04 | 7.18 |
| NEKRAD | 2 | 7.35 | 1.06 | 7.79 | |||
| GRGDKQ | 3 | 7.2 | 1.25 | 9 | |||
| KRADSS | 2 | 6.45 | 1.16 | 7.48 | |||
| NARDRS | 1 | 7 | 1.17 | 8.19 | |||
| PreM protein | NP_775664.1 | KRSRRS | 1 | 7.67 | 1.12 | 8.59 | |
| SKRSRR | 1 | 7.67 | 1.12 | 8.59 | |||
| DPEDVD | 1 | 6.4 | 1.19 | 7.62 | |||
| SKGENR | 2 | 7.27 | 1.21 | 8.8 | |||
| GNDPED | 1 | 7.17 | 1.38 | 9.89 | |||
| NS1 protein | NP_775667.1 | EESTDE | 0 | 7.08 | 1.01 | 7.15 | |
| REESTD | 0 | 7.25 | 1.05 | 7.61 | |||
| KHNRRE | 1 | 8.35 | 1.03 | 8.6 | |||
| EDCGKR | 2 | 6.72 | 1.15 | 7.73 | |||
| KECPDE | 1 | 6.75 | 1.11 | 7.49 | |||
| NS3 protein | NP_775670.1 | RGEEKK | 3 | 7.78 | 1 | 7.78 | |
| GEEKKN | 3 | 7.62 | 1.1 | 8.38 | |||
| DRQEEP | 0 | 7.85 | 1.07 | 8.4 | |||
| GDRQEE | 1 | 7.65 | 1.07 | 8.19 | |||
| GPEREK | 2 | 7.4 | 1.09 | 8.07 | |||
| NS5 protein | NP_775674.1 | DEEREN | 0 | 8.17 | 1.03 | 8.42 | |
| KREKKP | 3 | 8.05 | 1.04 | 8.37 | |||
| GKREKK | 4 | 7.85 | 1.05 | 8.24 | |||
| REKRPR | 1 | 8.25 | 1.02 | 8.42 | |||
| RRARRE | 1 | 7.78 | 0.87 | 6.77 | |||
Aliphatic amino acid plays an important role in binding affinity. It is believed that the epitopes comprising of aliphatic amino acids viz. Ala, Gly, Ile, Lys, Leu, Met and Val can interact easily with either of the two lymphocytes. In the present study, the T-cell epitopes were examined to calculate their aliphatic amino acid number (Table 2a). One epitope, GKREKK of NS5 protein consisted of 4 aliphatic amino acids. Four epitopes, namely GRGDKQ of E protein, RGEEKK and GEEKKN of NS3 protein, and KREKKP of NS5 protein had 3 aliphatic amino acids each. Six epitopes were identified to consist of 2 aliphatic amino acids namely EKRADS, NEKRAD and KRADSS of E protein; SKGENR of prM protein; EDCGKR of NS1 protein; and GPEREK of NS3 protein. Ten epitopes, i.e. NARDRS of E protein; KRSRRS, SKRSRR, DPEDVD and GNDPED of prM protein; KHNRRE and KECPDE of NS1 protein; GDRQEE of NS3 protein; and REKRPR and RRARRE of NS5 protein contained a single aliphatic amino acid. However, four epitopes were identified with no aliphatic amino acid and these were EESTDE and REESTD of NS1 protein, DRQEEP of NS3 protein, and DEEREN of NS5 protein.
In the case of all octapeptide B-cell epitopes, the immunogenicity score as well as the number of aliphatic amino acids contained in each B-cell epitope were determined (Table 2b ). In E protein the epitope YSGSDGPC was identified to possess the highest immunogenicity score of 7.46 whereas, in prM protein the epitope NDPEDVDC was recorded with maximal immunogenicity score (7.5). In NS1 protein the B-cell epitope TKECPDEH was estimated with maximum immunogenicity score of 7.14. Further, in NS3 protein the epitope YPKCKNGD recorded the highest immunogenicity score (8.45) whereas, in NS5 protein the epitope HKDPEHPY recorded the maximum immunogenicity score of 8.39.
Table 2b.
Immunogenicity (Ig) and number of aliphatic amino acids in B-cell epitopes of JEV (Kolaskar & Tongaonkar approach, 1990).
| Name of virus | Name of proteins | Accession number | Octapeptide epitope | Aliphatic amino acids | Ig analysis of octapeptide epitopes |
||
|---|---|---|---|---|---|---|---|
| Hydropathy index | Chou-Fasman conformation | Ig score | |||||
| Japanese encephalitis | E protein | NP_775666.1 | SGSDGPCK | 3 | 5.61 | 1.39 | 7.8 |
| YSGSDGPC | 2 | 5.29 | 1.41 | 7.46 | |||
| EPPFGDSY | 1 | 5.74 | 1.25 | 7.18 | |||
| LSYSGSDG | 3 | 5.03 | 1.33 | 6.69 | |||
| ELSYSGSD | 2 | 5.41 | 1.22 | 6.6 | |||
| PreM protein | NP_775664.1 | NDPEDVDC | 1 | 6.05 | 1.24 | 7.5 | |
| DCWCDNQE | 0 | 6.18 | 1.19 | 7.35 | |||
| SKRSRRSV | 2 | 6.45 | 1.08 | 6.97 | |||
| KRSRRSVS | 2 | 6.45 | 1.08 | 6.97 | |||
| RCTRTRHS | 0 | 6.55 | 1.04 | 6.81 | |||
| NS1 protein | NP_775667.1 | TKECPDEH | 1 | 6.67 | 1.07 | 7.14 | |
| DRYKYLPE | 2 | 6.48 | 1.07 | 6.93 | |||
| KPVGRYRS | 3 | 6.1 | 1.13 | 6.89 | |||
| ECPDEHRA | 1 | 6.44 | 1.03 | 6.63 | |||
| KYLPETPR | 2 | 6.16 | 1.05 | 6.47 | |||
| NS3 protein | NP_775670.1 | YPKCKNGD | 3 | 6.45 | 1.31 | 8.45 | |
| PKPCSKGD | 3 | 6.15 | 1.34 | 8.24 | |||
| PSPKPCSK | 2 | 5.96 | 1.33 | 7.93 | |||
| EYPKCKNG | 3 | 6.45 | 1.22 | 7.87 | |||
| DTPSPKPC | 1 | 5.9 | 1.33 | 7.85 | |||
| NS5 protein | NP_775674.1 | HKDPEHPY | 1 | 7.23 | 1.16 | 8.39 | |
| GKENYVDY | 3 | 6.15 | 1.14 | 7.01 | |||
| PYVGKRED | 3 | 6.31 | 1.11 | 7 | |||
| FYKPSEPS | 1 | 5.84 | 1.17 | 6.83 | |||
| CGRGGWSY | 3 | 5.27 | 1.29 | 6.8 | |||
Nine epitopes namely SGSDGPCK and LSYSGSDG of E protein; KPVGRYRS of NS1 protein; YPKCKNGD, PKPCSKGD and EYPKCKNG of NS3 protein; GKENYVDY, PYVGKRED and CGRGGWSY of NS5 protein were identified to contain 3 aliphatic amino acids. Seven epitopes were found to possess 2 aliphatic amino acids i.e. YSGSDGPC and ELSYSGSD of E protein, SKRSRRSV and KRSRRSVS of prM protein, DRYKYLPE and KYLPETPR of NS1 protein and PSPKPCSK of NS3 protein. Seven epitopes, i.e. EPPFGDSY of E protein, NDPEDVDC of prM protein, TKECPDEH and ECPDEHRA of NS1 protein, DTPSPKPC of NS3 protein, and HKDPEHPY and FYKPSEPS of NS5 protein contained a single aliphatic amino acid. Moreover, two epitopes namely DCWCDNQE and RCTRTRHS of prM protein contained no aliphatic amino acid. It is believed that the epitopes that possess high immunogenicity score are highly immunogenic and the immunogenicity score of a peptide epitope is a determining factor in choosing epitopes for peptide vaccine formulation.
3.4. Boman index of epitopes
Boman index values of all hexapeptide T-cell epitopes present in five JEV proteins were estimated (Fig. 2a). We used Boman value 2.48 kcal/mol as the minimum cut-off value. Notably, all the 25 T-cell epitopes in our study possessed Boman value >2.48 kcal/mol and were considered to have potential for binding successfully to MHC molecules. From these results we concluded that the complexes would be presented to T-cells by APCs and would elicit immune response in the host. Further, we noted that the Boman index of T-cell epitopes ranged from 4.43 (KECPDE) to 10.78 (RRARRE).
Fig 2.
(a): Hexapeptide T-cell epitopes from the proteins of JEV along with their Boman index values. Fig 2(b): Octapeptide B-cell epitopes from the proteins of JEV along with Boman index values.
Similarly, we estimated Boman index of all the 25 B-cell epitopes (Fig. 2b). Eight B-cell epitopes, viz. SGSDGPCK, YSGSDGPC, EPPFGDSY and LSYSGSDG of E protein; PSPKPCSK and DTPSPKPC of NS3 protein; and FYKPSEPS and CGRGGWSY of NS5 protein were found to have Boman index <2.48 kcal/mol which indicated they may not attach to immunoglobulins secreted by B-cells. The remaining epitopes possessed Boman index >2.48 kcal/mol and hence, these epitopes could bind to immunoglobulins effectively.
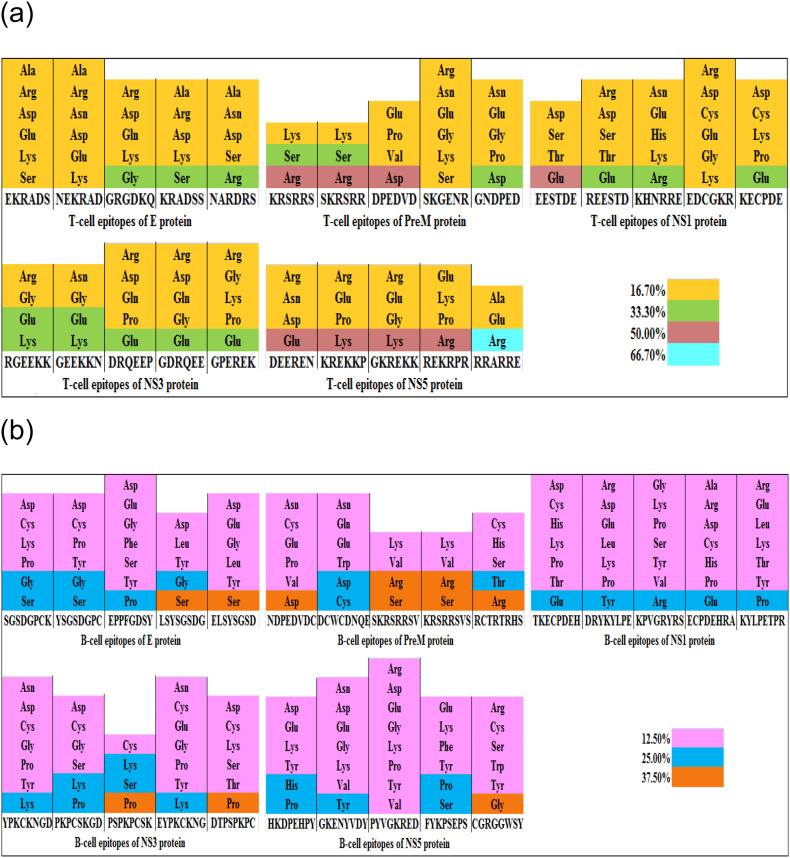
3.5. Amino acid composition of epitopes
The T-cell epitopes of JEV proteins were analyzed for their amino acid arrangement (Fig. 3a). Different amino acids were found in epitopes. Interestingly, some epitopes of E, prM, NS1 and NS3 proteins comprised of amino acids like Lys, Asp, Gly, Ser, Glu, Arg constituting nearly 33.30% of the total composition. On the other hand, some epitopes of prM, NS3 and NS5 proteins contained Arg, Asp, Glu and Lys to the extent of 50.00%. Only one epitope RRARRE of NS5 protein was found to contain Arg constituting 66.70% of the total epitope composition.
Fig 3.
(a): Amino acid composition of each hexapeptide T-cell epitope of JEV. Fig 3(b): Amino acid composition of each octapeptide B-cell epitope of JEV.
Likewise, the B-cell epitopes of JEV proteins were analyzed for amino acid composition (Fig. 3b). All the epitopes contained varying amino acid composition. Some B-cell epitopes contained amino acids namely Tyr, His, Asp, Ser, Gly, Lys, Thr, Cys, Glu, Pro and Arg constituting nearly 25.00% of the total composition. Further, some other epitopes of E, prM, NS3 and NS5 proteins were found to possess Ser, Gly, Arg, Pro and Asp constituting 37.50% of the total epitope composition.
Further, the T-cell and B-cell epitopes against JEV are amenable to cleavage by various enzymes and chemicals and the results of cleavage are presented in supplementary tables (S1, S2).
4. Discussion
In this study, the potential epitopes for peptide vaccine formulation were identified in five proteins of JEV namely E, prM, NS1, NS3 and NS5. All the epitopes were analyzed for various parameters using different bioinformatic tools. Only a few epitopes were found to possess the properties essential for generating immune response in the host against JEV and the selected epitopes might be used in formulating an effective peptide vaccine to combat the menace of JEV.
Earlier several studies were carried out by other researchers for designing peptide vaccine based on genome derived or reverse vaccinology approach. In a study conducted by Wei et al. (2010), the epitopes existing in E protein of JEV were identified that possessed the ability for developing peptide vaccine. Two T-cell epitopes were determined positioning at amino acid sequences 436–445 and 60–68, while six B-cell epitopes positioning at amino acid sequences 356–362, 75–92, 258–285, 149–163, 397–403, and 373–399 were identified (Wei et al., 2010).
Chikungunya infection, a viral disease spread by Aedes mosquitoes, is caused by Chikungunya virus (CHIKV). Kori et al. (2015) identified the epitopes from the proteins of three CHIKV strains having potential for peptide vaccine formulation. The T-cell epitopes identified by them included DAEKEAEEEREAELT, AEEEREAEL and KKKPGRRERMCMKIE whereas, the B-cell epitopes were found to be QVLKAKNIGL and SSKYDLECAQ (Kori et al., 2015).
Zika virus (ZIKV) that causes deadly infectious disease results in malformed babies and the decline of survival rate in human. Yadav et al. (2017) identified epitopes in the envelope glycoprotein sequence of ZIKV that could be employed for creating a peptide vaccine to prevent ZIKV. The T-cell epitope YRIMLSVHG possessed the competence for developing immunity (Yadav et al., 2017).
Nipah virus (NiV) causes respiratory infection and encephalitis in human and the transmission usually occurs through pigs or bats. In order to design peptide vaccines for treatment of NiV diseases, two potential epitopes from NiV proteins i.e. Glycoprotein (G) and Fusion (F) protein were detected with the help of immunoinformatics approach. It was reported that either the epitope EWISIVPNFILVRNT from G-protein or the epitope GPKVSLIDTSSTITI from F-protein could be used for vaccine development (Sakib et al., 2014).
High-risk human papillomavirus (hrHPVs) is known to cause viral infections as well as cervical cancers in human. In a study, the probable epitopes were identified from E6 protein of hrHPVs and these epitopes were reported to possess competence in preparing successful peptide vaccine against hrHPVs. Based on in silico approach, it was suggested that the T-cell epitopes FAFRDLCIVYR and RREVYDFAF or their mixture could be considered for vaccine formulation and development. Moreover, another potential T-cell epitope positioning at 65–76 amino acids was also identified as a potential peptide vaccine candidate (Khan et al., 2018).
Human coronavirus (HCoV) has been reported to cause pneumonia as well as some diseases of the respiratory and the gastrointestinal tracts in human. For developing peptide vaccines against this virus, Oany et al. (2014) identified the epitopes in spike proteins that was suggested for boosting immunological response within human body against HCoV. From the study, it was evident that the B-cell epitope positioned at 88–94 amino acids and the T-cell epitope KSSTGFVYF had the ability for designing a peptide vaccine (Oany et al., 2014).
Ebola virus disease (EVD) is considered to be one of the deadly viral diseases affecting human beings. Epitope-based peptide vaccine formulation against EVD using reverse vaccinology approach was reported, wherein the potential T-cell epitopes viz., RRTRRE, RRKRRD, KTGKKG and DEDDED and the potential B-cell epitopes viz., HLGLDDQ, PDYDDCH, QPKCNPN, DQEKKIL, SHYEPPN, DYDDCHS, PTSPPQD and EYTYPDS were identified from the viral surface proteins (Chakraborty, 2014).
5. Conclusion
The present study suggested that a multi-epitope-based peptide vaccine against JEV could be developed by combining the promising B-cell and T-cell epitopes found in E, prM, NS1, NS3 and NS5 proteins. In order to design a peptide vaccine, the first criterion is to select the epitopes possessing high antigenicity scores. Out of 25 T-cell epitopes examined in five proteins of JEV, eight epitopes were identified to possess high antigenicity scores. These epitopes were EKRADS, KRSRRS, SKRSRR, DPEDVD, EDCGKR, KECPDE and KREKKP. The epitope EKRADS possessed low immunogenicity score and so, it was excluded from further analysis. The remaining seven epitopes possessed high immunogenicity and hydrophilicity values in addition to the desired Boman index value. On the basis of total net charges, three epitopes, i.e. DPEDVD, EDCGKR and KECPDE were excluded from the study as these might not bind with the virus. We concluded that four T-cell epitopes, viz. KRADSS, KRSRRS, SKRSRR and KREKKP possess the potential to be used in epitope-based peptide vaccine formulation against JEV.
Among 25 octapeptide B-cell epitopes, seven epitopes namely DRYKYLPE, KPVGRYRS, PKPCSKGD, PSPKPCSK, DTPSPKPC, HKDPEHPY and FYKPSEPS were estimated to possess high antigenicity scores. Only one epitope PKPCSKGD was identified with excessive immunogenicity, hydrophilicity as well as Boman index value. This B-cell epitope could be used in formulating the peptide vaccine.
Pharmaceutical companies could initiate efforts to synthesize a multi-epitope-loaded peptide vaccine by combining the promising four T-cell epitopes and one B-cell epitope in varying proportions with a strong adjuvant or carrier protein. The peptide vaccine could be administered in JEV affected persons following proper clinical trials to ensure its safety and efficacy and might prevent the spread of JEV by raising immunity in human.
In view of the endless struggle between human and JEV in evolutionary context, it is advisable to construct different sets of peptide vaccines comprising the epitopes in varied combinations. To build up a continuing resistance against JEV through vaccination, the temporal and spatial deployment strategy (over time and space) of vaccine administration could be followed using different peptide vaccine sets in the countries frequently affected by JEV.
The following are the supplementary data related to this article
List of predicted potential cleavage sites of proteases or chemicals in T-cell epitopes from five proteins of JEV.
List of predicted potential cleavage sites of proteases or chemicals in B-cell epitopes from five proteins of JEV.
Declaration of Competing Interest
Authors declare no conflict of interest in the manuscript.
Acknowledgments
Acknowledgement
The authors are grateful to Assam University, Silchar, Assam, India for providing necessary facilities to carry out this research work. Further, the research work is dedicated to those great souls who died of JEV due to lack of proper medication and affordable medical facility.
References
- Allison S.L., Schalich J. Mutational evidence for an internal fusion peptide in flavivirus envelope protein E. J. Virol. 2001;75(9):4268–4275. doi: 10.1128/JVI.75.9.4268-4275.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig S., Fox K.K. Japanese encephalitis surveillance and immunization—Asia and the Western Pacific, 2012. MMWR Morb. Mortal. Wkly Rep. 2013;62(33):658. [PMC free article] [PubMed] [Google Scholar]
- Bartholomeusz A., Wright P. Synthesis of dengue virus RNA in vitro: initiation and the involvement of proteins NS3 and NS5. Arch. Virol. 1993;128(1–2):111–121. doi: 10.1007/BF01309792. [DOI] [PubMed] [Google Scholar]
- Bray M., Lai C.-J. Dengue virus premembrane and membrane proteins elicit a protective immune response. Virology. 1991;185(1):505–508. doi: 10.1016/0042-6822(91)90809-p. [DOI] [PubMed] [Google Scholar]
- Burke D.S., Leake C.J. Japanese encephalitis. The Arboviruses. 1988;3:63–92. [Google Scholar]
- Chakraborty S. Ebola vaccine: multiple peptide-epitope loaded vaccine formulation from proteome using reverse vaccinology approach. Int J Pharm Pharm Sci. 2014;6:407–412. [Google Scholar]
- Chambers T.J., Hahn C.S. Flavivirus genome organization, expression, and replication. Annu. Rev. Microbiol. 1990;44(1):649–688. doi: 10.1146/annurev.mi.44.100190.003245. [DOI] [PubMed] [Google Scholar]
- Chou P.Y., Fasman G.D. Empirical predictions of protein conformation. Annu. Rev. Biochem. 1978;47(1):251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Endy T., Nisalak A. Japanese Encephalitis and West Nile Viruses. Springer; 2002. Japanese encephalitis virus: ecology and epidemiology; pp. 11–48. [DOI] [PubMed] [Google Scholar]
- Erra E.O., Kantele A. The Vero cell-derived, inactivated, SA14-14-2 strain-based vaccine (Ixiaro) for prevention of Japanese encephalitis. Expert Rev. Vacc. 2015;14(9):1167–1179. doi: 10.1586/14760584.2015.1061939. [DOI] [PubMed] [Google Scholar]
- Fan W., Mason P.W. Membrane association and secretion of the Japanese encephalitis virus NS1 protein from cells expressing NS1 cDNA. Virology. 1990;177(2):470–476. doi: 10.1016/0042-6822(90)90511-o. [DOI] [PubMed] [Google Scholar]
- Fischer M., Hills S. Emerging Infections 8. American Society of Microbiology; 2008. Japanese encephalitis prevention and control: advances, challenges, and new initiatives; pp. 93–124. [Google Scholar]
- Fischer M., Hills S. 2010. Japanese Encephalitis Vaccines; Recommendations of the Advisory Committee on Immunization Practices (ACIP) [PubMed] [Google Scholar]
- Halstead S., Jacobson J. Japanese encephalitis vaccines. In: Plotkin S.A., Orenstein W.A., Offit P.A., editors. Vaccines. Elsevier; Philadelphia: 2008. [Google Scholar]
- Halstead S.B., Thomas S.J. Japanese encephalitis: new options for active immunization. Clin. Infect. Dis. 2010;50(8):1155–1164. doi: 10.1086/651271. [DOI] [PubMed] [Google Scholar]
- Hanna J.N., Ritchie S.A. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med. J. Aust. 1996;165(5):256–260. doi: 10.5694/j.1326-5377.1996.tb124960.x. [DOI] [PubMed] [Google Scholar]
- Hanna J.N., Ritchie S.A. Japanese encephalitis in north Queensland, Australia, 1998. Med. J. Aust. 1999;170(11):533–536. doi: 10.5694/j.1326-5377.1999.tb127878.x. [DOI] [PubMed] [Google Scholar]
- Hegde N.R., Gore M.M. Japanese encephalitis vaccines: immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Human Vacc. Immunother. 2017;13(6):1320–1337. doi: 10.1080/21645515.2017.1285472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp T.P., Woods K.R. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. 1981;78(6):3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Junaid M. Computational identification, characterization and validation of potential antigenic peptide vaccines from hrHPVs E6 proteins using immunoinformatics and computational systems biology approaches. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler R.M., Aberle J.H. Mimicking live flavivirus immunization with a noninfectious RNA vaccine. Proc. Natl. Acad. Sci. 2004;101(7):1951–1956. doi: 10.1073/pnas.0307145101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolaskar A., Tongaonkar P.C. A semi-empirical method for prediction of antigenic determinants on protein antigens. FEBS Lett. 1990;276(1–2):172–174. doi: 10.1016/0014-5793(90)80535-q. [DOI] [PubMed] [Google Scholar]
- Konishi E., Pincus S. Mice immunized with a subviral particle containing the Japanese encephalitis virus prM/M and E proteins are protected from lethal JEV infection. Virology. 1992;188(2):714–720. doi: 10.1016/0042-6822(92)90526-u. [DOI] [PubMed] [Google Scholar]
- Konishi E., Fujii A. Generation and characterization of a mammalian cell line continuously expressing Japanese encephalitis virus subviral particles. J. Virol. 2001;75(5):2204–2212. doi: 10.1128/JVI.75.5.2204-2212.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori P., Sajjan S.S. In silico prediction of epitopes for Chikungunya viral strains. J. Pharmaceut. Invest. 2015;45(6):579–591. [Google Scholar]
- Kuhn R.J., Zhang W. Structure of dengue virus: implications for flavivirus organization, maturation and fusion. Cell. 2002;108(5):717–725. doi: 10.1016/s0092-8674(02)00660-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lafuente E.M., Reche P.A. Prediction of MHC-peptide binding: a systematic and comprehensive overview. Curr. Pharm. Des. 2009;15(28):3209–3220. doi: 10.2174/138161209789105162. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu M. Evaluation of murine bone marrow-derived dendritic cells loaded with inactivated virus as a vaccine against Japanese encephalitis virus. Vaccine. 2009;27(43):6004–6010. doi: 10.1016/j.vaccine.2009.07.078. [DOI] [PubMed] [Google Scholar]
- Mackenzie J.S., Williams D.T. Japanese encephalitis virus: the geographic distribution, incidence, and spread of a virus with a propensity to emerge in new areas. Perspect. Med. Virol. 2006;16:201–268. [Google Scholar]
- Marin M., Zanotto P. Phylogeny of TYU, SRE, and CFA virus: different evolutionary rates in the genus Flavivirus. Virology. 1995;206(2):1133–1139. doi: 10.1006/viro.1995.1038. [DOI] [PubMed] [Google Scholar]
- Monath T., Heinz F. Fields Virology. Vol. 1. 1996. Flaviviruses; pp. 961–1034. [Google Scholar]
- Oany A.R., Emran A.-A. Design of an epitope-based peptide vaccine against spike protein of human coronavirus: an in silico approach. Drug Des. Develop. Ther. 2014;8:1139. doi: 10.2147/DDDT.S67861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi M.H., Lewthwaite P. The epidemiology, clinical features, and long-term prognosis of Japanese encephalitis in central sarawak, malaysia, 1997–2005. Clin. Infect. Dis. 2008;47(4):458–468. doi: 10.1086/590008. [DOI] [PubMed] [Google Scholar]
- Organization, W. H . Weekly Epidemiological Record= Relevé Épidémiologique Hebdomadaire. 90(09) 2015. Japanese encephalitis vaccines: WHO position paper—February 2015; pp. 69–88. [PubMed] [Google Scholar]
- Oya A. Japanese encephalitis vaccine. Pediatr. Int. 1988;30(2):175–184. doi: 10.1111/j.1442-200x.1988.tb02516.x. [DOI] [PubMed] [Google Scholar]
- Paul W., Moore P. Outbreak of Japanese encephalitis on the island of Saipan, 1990. J. Infect. Dis. 1993;167(5):1053–1058. doi: 10.1093/infdis/167.5.1053. [DOI] [PubMed] [Google Scholar]
- Pisitkun T., Hoffert J.D. NHLBI-AbDesigner: an online tool for design of peptide-directed antibodies. Am. J. Phys. Cell Phys. 2011;302(1):C154–C164. doi: 10.1152/ajpcell.00325.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porterfield J.S. Chapman & Hall Medical Oxford; 1995. Exotic Viral Infections. [Google Scholar]
- Rice C. Flaviviridae: the viruses and their replication. In: Fields B.N., Knipe D.M., Howley P.M., editors. Fields Virology. Vol. 1. Lippincott-Raven Publishers; Philadelphia: 1996. [Google Scholar]
- Rice C.M., Lenches E.M. Nucleotide sequence of yellow fever virus: implications for flavivirus gene expression and evolution. Science. 1985;229(4715):726–733. doi: 10.1126/science.4023707. [DOI] [PubMed] [Google Scholar]
- Rinaudo C.D., Telford J.L. Vaccinology in the genome era. J. Clin. Invest. 2009;119(9):2515–2525. doi: 10.1172/JCI38330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakib M.S., Islam M. Prediction of epitope-based peptides for the utility of vaccine development from fusion and glycoprotein of nipah virus using in silico approach. Adv. Bioinforma. 2014;2014 doi: 10.1155/2014/402492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skwarczynski M., Toth I. Peptide-based synthetic vaccines. Chem. Sci. 2016;7(2):842–854. doi: 10.1039/c5sc03892h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T. Viral encephalitis in southeast Asia. Neurol. Infect. Epidemiol. 1997;2:191–199. [Google Scholar]
- Solomon T., Vaughn D. Japanese Encephalitis and West Nile Viruses. Springer; 2002. Pathogenesis and clinical features of Japanese encephalitis and West Nile virus infections; pp. 171–194. [DOI] [PubMed] [Google Scholar]
- Solomon T., Kneen R. Poliomyelitis-like illness due to Japanese encephalitis virus. Lancet. 1998;351(9109):1094–1097. doi: 10.1016/S0140-6736(97)07509-0. [DOI] [PubMed] [Google Scholar]
- Solomon T., Dung N.M. Japanese encephalitis. J. Neurol. Neurosurg. Psychiatry. 2000;68(4):405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon T., Dung N.M. Seizures and raised intracranial pressure in Vietnamese patients with Japanese encephalitis. Brain. 2002;125(5):1084–1093. doi: 10.1093/brain/awf116. [DOI] [PubMed] [Google Scholar]
- Solomon T., Ni H. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J. Virol. 2003;77(5):3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Liu X.S. The epitopes of foot and mouth disease. Asian J. Anim. Vet. Adv. 2012;7:1261–1265. [Google Scholar]
- Tantray V.G., Giri V. A new vaccine candidate using reverse vaccinology for vibrio Cholera0395 in cholera disease. Int. J. Innovat. Res. Stud. 2014;3(5) [Google Scholar]
- Tsai T. Factors in the changing epidemiology of Japanese encephalitis and West Nile fever. Fac. Emerg. Arbovirus Dis. 1997:179–189. [Google Scholar]
- Tsarev S., Sanders M. Phylogenetic analysis suggests only one serotype of Japanese encephalitis virus. Vaccine. 2000;18:36–43. doi: 10.1016/s0264-410x(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Turtle L., Bali T. Human T cell responses to Japanese encephalitis virus in health and disease. J. Exp. Med. 2016;213(7):1331–1352. doi: 10.1084/jem.20151517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchil P.D., Satchidanandam V. Phylogenetic analysis of Japanese encephalitis virus: envelope gene based analysis reveals a fifth genotype, geographic clustering, and multiple introductions of the virus into the Indian subcontinent. Am. J. Trop. Med. Hyg. 2001;65(3):242–251. doi: 10.4269/ajtmh.2001.65.242. [DOI] [PubMed] [Google Scholar]
- Vaughn D.W., Hoke C.H., Jr. The epidemiology of Japanese encephalitis: prospects for prevention. Epidemiol. Rev. 1992;14(1):197–221. doi: 10.1093/oxfordjournals.epirev.a036087. [DOI] [PubMed] [Google Scholar]
- Von Hoff D. Pancreatic cancer; Jones & Bartlett Learning: 2005. D. [Google Scholar]
- Wei J., Huang Y. Design and evaluation of a multi-epitope peptide against Japanese encephalitis virus infection in BALB/c mice. Biochem. Biophys. Res. Commun. 2010;396(4):787–792. doi: 10.1016/j.bbrc.2010.04.133. [DOI] [PubMed] [Google Scholar]
- Weiss A., Littman D.R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Westaway E., Brinton M. In: Flaviviridae Intervirology. Lvov D.K., Porterfield J.S., Russell P.K., Trent D.W., editors. Vol. 24. 1985. pp. 183–192. [DOI] [PubMed] [Google Scholar]
- Yadav G., Rao R. Computational modeling and analysis of prominent T-cell epitopes for assisting in designing vaccine of ZIKA virus. J. App. Pharmaceut. Sci. 2017;7(08):116–122. [Google Scholar]
- Zanotto P., Gould E.A. Population dynamics of flaviviruses revealed by molecular phylogenies. Proc. Natl. Acad. Sci. 1996;93(2):548–553. doi: 10.1073/pnas.93.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of predicted potential cleavage sites of proteases or chemicals in T-cell epitopes from five proteins of JEV.
List of predicted potential cleavage sites of proteases or chemicals in B-cell epitopes from five proteins of JEV.