Abstract
This study determined the effects of chicken egg yolk antibodies (IgY) on immune responses in the intestinal mucosal of mice infected with Salmonella typhimurium. Sixty, 28-day-old mice were divided into 4 groups and treated with streptomycin or sterile water for 2 days followed by 1 day without treatment. The control group was unchallenged whereas the mice in the other three groups were treated twice with 109 CFU mL− 1S. typhimurium. For the next 3 days, control mice continued to receive no treatment whereas the mice in the remaining three groups were orally administered with 20 mg mL− 1 of specific IgY, 20 mg mL− 1 of nonspecific IgY or PBS. S. typhimurium activated gut-associated lymphoid tissue, increasing the release of IFN-γ and TNF-α in the mucosa and increased the number of activated T-lymphocytes and cytotoxic T-γδ. Specific IgY attenuated the increase in IFN-γ and TNF-α and the decrease in IL-10. S. typhimurium induced mobilization of CD8+ and CD8+ TCRγδ T cells in the epithelium and CD4+ and CD8+ T cells in the lamina propria reflecting an inflammatory process that was attenuated by IgY. These results suggest that specific IgY modulates intestinal mucosal immune responses during a S. typhimurium infection.
Abbreviations: FBS, fetal bovine serum; FITC, fluorescein isothiocyanate; GALT, gut-associated lymphoid tissue; HE, hematoxylin and eosin; IgY, immunoglobulin Y; IFN-γ, interferon-γ; IL, interleukin; PBS, phosphate buffered saline; PE, phycoerythrin; TNF-α, tumor necrosis factor-α
Keywords: Egg yolk antibodies, IgY, Salmonella typhimurium, Mucosal immune response, Intestinal inflammation, Gut-associated lymphoid tissue
Highlights
-
•
Specific IgY could effectively alleviate S. typhimurium-inflicted damage to the jejunum.
-
•
Specific IgY attenuated an increase in the cytokines IFN-γ and TNF-α in the mucosa.
-
•
IgY attenuated changes in lymphocyte numbers in Peyer's patches, epithelium and lamina propria.
-
•
Specific IgY has an important immune-modulatory role on the intestinal mucosal immune response.
-
•
Specific IgY limits the consequences of intestinal inflammation induced by S. typhimurium.
1. Introduction
Chicken egg yolk antibody, commonly referred to as immunoglobulin Y (IgY), has attracted considerable interest as an alternative to antibiotics for the control of infectious diseases in animals and humans [1], [2], [3]. IgY possesses several advantages over mammalian IgG such as cost-effectiveness, convenience and high yield [4].
Numerous studies have demonstrated that specific IgY antibodies are highly effective against a variety of important intestinal pathogens such as Escherichia coli, Salmonella, rotaviruses, coronavirus, transmissible gastroenteritis virus and epidemic diarrhea virus [1], [2], [3], [4], [5]. The exact mechanism through which IgY counteracts pathogen activity has not been determined. However, several mechanisms have been proposed to show how specific IgY may counteract pathogen activity, including inhibition of adhesion [6], agglutination [6], [7], opsonization followed by phagocytosis [8] and toxin neutralization [9].
The gut is the main site of IgY activity against intestinal pathogens [10]. The mucosal immune system of the gastrointestinal tract, generally referred to as gut-associated lymphoid tissue (GALT) plays an important role as the first line of defense in protecting against ingested pathogens. GALT accounts for up to 80% of the mucosal immune system and is distributed throughout the intestine either as organized GALT (i.e., Peyer's patches, isolated follicles and mesenteric lymph nodes), or as diffuse GALT consisting of lymphocytes scattered throughout the epithelium and the lamina propria [11].
Diet modulates the immunological functions of GALT in multiple ways and affects host resistance to infections. For example, it has been demonstrated that spray dried animal plasma and the immunoglobulins present in it can modulate the mucosal and systemic immune response of weaned rats challenged with Staphylococcal aureus enterotoxin B [12]. However, few studies have examined the immuno-modulatory effects of IgY on the host's immune response in conditions associated with gut-barrier dysfunction and inflammatory response.
In the present study, we examined the effects of IgY on the intestinal mucosal immune system using a mouse model of intestinal infection induced by Salmonella typhimurium. The objectives were to examine the effects of IgY on gut histology, to determine the effects of IgY on the cytokines that are involved in inflammatory responses and finally to determine the effects of IgY on the lymphocyte populations of GALT.
2. Materials and methods
2.1. Bacteria and culture conditions
A standard strain of S. typhimurium (CVCC 50115) was obtained from the National Institute for Food and Drug Control (Beijing, China). S. typhimurium was grown in Tryptone Soya Broth (Oxoid, Hampshire, UK) at 37 °C for 7 h. The cells were harvested by centrifugation at 5000 × g for 10 min at 4 °C, washed twice with 1 mM PBS solution (pH 7.2), and then re-suspended in PBS. The suspension was adjusted to a cell density of 109 CFU mL− 1 and used for immunization of the hens and challenge infection of the mice.
2.2. Production of IgY against S. typhimurium
Specific IgY against S. typhimurium was prepared in a manner similar to our previously reported method [10]. Briefly, IgY was produced using 10 White Leghorn laying hens immunized with formaldehyde-killed S. typhimurium at a concentration of 109 CFU mL− 1 aided by Freund's adjuvant (Sigma-Aldrich, St Louis, MO). IgY antibodies were subsequently isolated from the egg yolks by several purification steps including salt precipitation (precipitation with 50% saturated ammonium sulfate followed by precipitation with 14% (w/v) sodium sulfate) and ultrafiltration using a Vivaflow 50 Tangential Flow Ultrafilter (Vivascience, Hannover, Germany) with a 100 kDa cut-off membrane. IgY powder was obtained by freeze-drying the ultra-filtration fractions. The titer of IgY was determined by Enzyme-Linked Immunosorbent Assay as described by Zhen et al. [13]. The powdered IgY with a titer ≥ 5000 was combined and used for the IgY protection test. Non-specific IgY was obtained from 5 additional non-immunized laying hens using similar methods.
2.3. Experimental animals
Sixty, 28-day-old, weaned Kunming mice (equal male to female ratio, weighing 18 to 20 g) were purchased from the Dalian Medical University Laboratory Animal Centre (Dalian, China) using the Permission number of SCXK 2008-0002. All animals were allowed to acclimate to our laboratory facilities for 7 days before their inclusion in the experiment. They were housed under standard laboratory conditions (22 ± 3 °C; relative humidity 50–55%; 12 h light/dark cycle) and given food and water ad libitum. This experiment was performed according to the Experimental Animal Management Law of China and approved by the Animal Ethics Committee of Dalian Medical University (Dalian, China).
2.4. Challenge infection and IgY protection tests
The mice were randomly divided into 4 groups, with 15 animals per group. Following an acclimation period of 1 week, each group was supplied with 200 mL of a sterile solution containing 5 g/L streptomycin or 200 mL of sterile water (control group) for 2 days followed by 1 day without streptomycin. They were then fasted for 12 h. Control mice were unchallenged whereas the mice in the remaining three groups were orally challenged with 0.4 mL of S. typhimurium at a concentration of 109 CFU mL− 1 at 0 and 24 h of the experiment. After the 2nd S. typhimurium challenge, the mice in the control group continued to receive no treatment, whereas the mice in the remaining 3 groups were orally administered with 0.4 mL of a solution containing 20 mg mL− 1 specific IgY, 20 mg mL− 1 of nonspecific IgY or PBS once a day for 7 consecutive days. The clinical response of mice was monitored throughout the experiment in terms of lethargy, in appetence, weight gain and survival rate.
2.5. Histopathology
Random segments of the jejunum were excised and fixed with formalin, embedded in paraffin, sectioned into 6-μm slices and stained with hematoxylin and eosin (HE) according to standard procedures. Stained sections were examined microscopically by 2 pathologists unaware of the experimental treatments to which the mice had been subjected using a scoring system described by [14] (Table 2).
Table 2.
Histological scoring criteria.
| Pathological state | Score | Definition |
|---|---|---|
| Submucosal edemaa | 0 | No pathological changes |
| 1 | Mild edema (the submucosa is < 0.20 mm wide and accounts for 50% of the diameter of the entire intestinal wall [tunica muscularis to epithelium]) | |
| 2 | Moderate edema (the submucosa is 0.21 to 0.45 mm wide and accounts for 50 to 80% of the diameter of the entire intestinal wall) | |
| 3 | Profound edema (the submucosa is > 0.46 mm wide and accounts for 80% of the diameter of the entire intestinal wall) | |
| Leucocyte infiltration | 0 | Absent |
| 1 | 2–10/high-power field | |
| 2 | 11–20/high-power field | |
| 3 | 21–30/high-power field | |
| 4 | > 31/high-power field | |
| Epithelial damage | 0 | No pathological changes detectable in 10 high-power field (× 400 magnification) |
| 1 | Epithelial desquamation | |
| 2 | Erosion of the epithelial surface (gaps of 1 to 10 epithelial cells/lesion) | |
| 3 | Epithelial ulceration |
The submucosa widths were determined by quantitative microscopy and represent the averages of 30 evenly spaced radial measurements of the distance between the tunica muscularis and the lamina mucosalis mucosae.
2.6. RNA extraction and analysis for cytokine gene expression
Excised Peyer's patches from the small intestine and 0.5 cm samples of jejunum were washed with 0.1% diethypyrocarbonate-treated water, frozen in liquid nitrogen and then homogenized. The homogenate was subjected to total RNA extraction using a Simple Total RNA Kit (Tiangen Biotech Company, Beijing, China) ccording to the manufacturer's instructions. The purity and integrity of the RNA were confirmed by Agarose Gel Electrophoresis and the A260/A280 ratio. UV absorbance was measured with a UV Spectrophotometer (Beijing Purkinje General Instrument Company, Beijing, China). The RNA concentration was determined by measuring absorbance at 260 nm. Subsequently, 500 ng of the RNA was reverse transcribed into cDNA using PrimeScript® RT Reagent Kit (Perfect Real Time, TaKaRa, Dalian, China) according to the manufacturer's instruction.
The mRNA expression of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and IL-10 were measured in the intestinal mucosa by Real-Time Quantitative PCR. The reaction mixture (25 μL) contained 2 μL of diluted cDNA as the template, 0.5 μL of each primer (10 μM), 12.5 μL of 2 × SYBR® Premic Ex Taq™ (TaKaRa, Dalian, China) and 9.5 μL of sterile distilled water. The primers specific for each cytokine used in this work were designed using Gene Tool 1.0 Software (Bio Tools Incorporated, Edmonton, Canada) and their sequences are shown in Table 1 . To ensure the sensitivity and accuracy of the results obtained by Real-Time Quantitative PCR, internal normalization of the gene expression analysis was carried out using β-actin as the internal control gene (reference gene). Real-time Quantitative PCR was performed in a 96-well plate format using a Thermal Cycler Dice® Real Time System (TaKaRa, Dalian, China) with the following parameters: initial denaturation at 95 °C for 10 s to activate the DNA polymerase, followed by 40 cycles of 95 °C for 5 s and 60 °C for 30 s. Each sample was analyzed in triplicate. The data were analyzed with the Thermal Cycler Dice Real Time System Analysis Software (TaKaRa, Dalian, China).
Table 1.
List of genes and primer sequences used for RTQ-PCR analysis of cytokines.
| Gene name | Forward primer (5′–3′) | Reverse primer (3′–5′) | Accession number |
|---|---|---|---|
| β-Actin | tgagagggaaatcgtgcgtgac | aagaaggaaggctggaaaagag | NM_007393.3 |
| IFN-γ | tttgcccagactcgagctcctg | gggtgcaggttcgggattcaac | NR_038116.1 |
| TNF-α | tcacccacaccgtcagccgattt | gtctgggccatagaactgatg | NM_013693.2 |
| IL-6 | aaagagttgtgcaatggcaatt | cagtttggtagcatccatcat | NM_031168.1 |
| IL-10 | aacatactgctaaccgactcct | ctgccttgctcttattttcaca | NM_010548.2 |
2.7. Immunohistochemistry of lymphocyte populations
Jejunal fragments (1 cm) were fixed in 10% formalin, dehydrated in a graded series of ethanol, and embedded in paraffin wax before sectioning. Sections were dewaxed and rehydrated. After washing in PBS, sections were immersed in a 3% H2O2 solution at room temperature for 10 min. The sections were then pre-incubated with non-immune serum for 15 min and subsequently replaced with the corresponding primary mouse monoclonal antibody in a humidified chamber and incubated overnight at 4 °C. The primary antibodies used were anti-CD3 (17A2), anti-CD4 (GK1.5), anti-CD8a (53-6.7), and anti-T cell receptor γδ (GL3) (all from BioLegend, San Diego, CA). The slices were washed with PBS and incubated with horseradish peroxidase conjugated goat anti-mouse IgG antibody (BioLegend) at 37 °C for 30 min. The localization of the antigen was indicated by a brown color obtained by staining with 3,3′-diaminobenzidine in a humidified chamber for 30 min at room temperature. Afterwards, the samples were washed with PBS and counterstained with hematoxylin for 5 min at room temperature. They were then washed again with PBS and sealed with mounting medium. Negative controls were performed without the primary antibodies. The samples were stored until observation by microscopy.
2.8. Isolation of lymphocytes
Lymphocytes from the intra-epithelium, lamina propria, and Peyer's patches of the small intestine were obtained as previously described [15]. Briefly, for isolation of lymphocytes in the intestinal intra-epithelial, the small intestine was removed and cleaned with PBS. Mesentery nodes and Peyer's patches were carefully excised. The intestine was then opened longitudinally and cut into 0.5 cm pieces. These tissues were incubated in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) (GE Healthcare Life Sciences, Marlborough, MA), 1 mM of dithiothreitol (Sigma-Aldrich, St Louis, MO) and 1 mM of ethylenediaminetetraacetic acid for 30 min at 37 °C. Cell suspensions were filtered through a 70-μm nylon mesh and then centrifuged (600 × g, 10 min). The pellet was purified using a continuous 40 to 70% Percoll gradient (GE Healthcare Life Sciences, Marlborough, MA). Briefly, the pellet was re-suspended in 40% (v/v) Percoll and placed over 70% (v/v) Percoll. After centrifugation (600 × g for 30 min), the interface between the 40 and 70% layers was removed, and the cells were washed in RPMI-1640 and harvested by centrifugation (600 × g for 10 min).
For isolation of lymphocytes in the lamina propria, the remaining tissues were digested by collagenase II (Sigma-Aldrich, St Louis, MO) for 40 min at 37 °C. The cell suspensions were filtered through a 70-μm nylon mesh and then centrifuged (600 × g for 30 min). The pellet was purified by a continuous 40 to 70% Percoll gradient as described above.
For isolation of lymphocytes in the Peyer's patches, sections of Peyer's patches (~ 2 cm) were ground with a syringe plunger on a 70-μm nylon mesh in RPMI-1640 medium supplemented with 10% (v/v) FBS. Lymphocytes were then obtained by centrifugation (600 × g for 10 min) and re-suspended in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 mg/mL streptomycin. In all cases, lymphocyte samples were stored at 4 °C until processing for immuno-staining and flow cytometry.
2.9. Analysis of lymphoid tissue cell subsets
Lymphocyte subsets were determined after double staining with a panel of anti-mouse monoclonal antibodies and analyzed by flow cytometry. The phenotype of the lymphocyte subsets were identified by staining with anti-CD3 (17A2, phycoerythrin (PE)-conjugated, BioLegend), anti-CD4 (GK1.5, fluorescein isothiocyanate (FITC)-conjugated, BioLegend), anti-CD8a (53-6.7, FITC-conjugated, BioLegend), anti-CD25 (PC61, PE-conjugated, BioLegend), and anti-TCRγδ (GL3, PE-conjugated, BioLegend).
Cells (1 × 106) were incubated for 30 min with a mixture of FITC- and PE-conjugated mouse monoclonal antibodies diluted in PBS containing 10% (v/v) FBS at 4 °C in the dark, then centrifuged at 600 × g for 3 min. The cells were washed 3 times with PBS and fixed in PBS containing 0.5% paraformaldehyde and then stored at 4 °C in the dark until analysis by a Flow Cytometer (FACSCanto, BD Biosciences, San Jose, CA).
2.10. Statistical analysis
Statistical analysis of differences in survival rates between the treated and control groups was assessed by the Chi-square test using SPSS 11.5 for Windows (IBM, Armonk, NY). To study the effect of IgY on the intestinal inflammation model, the levels of significance for collected data were evaluated using one-way analysis of variance with the Student–Neuman–Keuls Post-Hoc Test. Differences were considered significant at P < 0.05.
3. Results
3.1. Oral administration of specific IgY confers protection against S. typhimurium infection
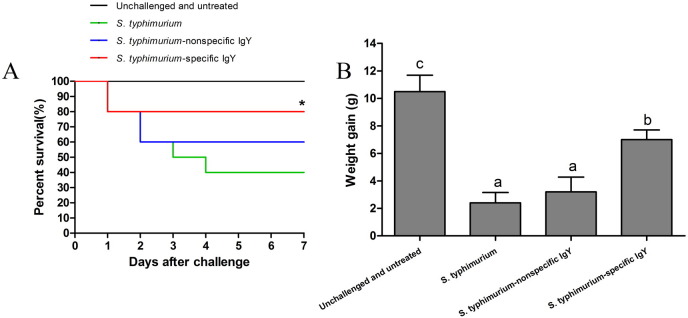
Mice that received the S. typhimurium challenge showed typical clinical signs of infection, including lethargy, inappetence, and piloerection within 5 h of challenge, whereas control mice did not exhibit any symptoms of S. typhimurium infection. The survival rates for control mice, challenged-untreated mice as well as challenged mice treated with nonspecific and specific IgY were 100, 40, 60, and 80% respectively 7 days post-challenge (Fig. 1A). The mice treated with specific IgY showed significant difference in survival rates compared with the positive control group (P < 0.05). With a 40% survival rate for the challenged-untreated mice, only 6 mice remained in this treatment at the end of the experiment. Therefore, to keep the sample sizes equal, 6 mice were randomly selected from each group for all subsequent analysis.
Fig. 1.
A. Survival rate of mice orally challenged with 0.4 mL of viable S. typhimurium organisms (109 CFU mL− 1 per mouse) at 0 and 24 h. Unchallenged mice received no treatment, whereas the challenged mice were treated once a day (after the 2nd bacterial challenge) for 7 consecutive days with 0.4 mL of 20 mg mL− 1 specific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS (n = 15 per group). Data were analyzed by the Chi-square test. *P < 0.05 compared with no treatment.
B. Weight gain of unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Data are expressed as means ± SD and analyzed by ANOVA followed by SNK. Means without the same letter are significantly different (P < 0.05).
Mice in the specific IgY-treated group and the control group had similar body weight gains that were significantly higher (P < 0.05) than those challenged and untreated, or treated with nonspecific IgY (Fig. 1B). These findings indicate that oral administration of specific IgY was able to provide an effective passive protection against S. typhimurium challenge in mice.
3.2. Effect of IgY on histopathological features in the jejunum of S. typhimurium-challenged mice
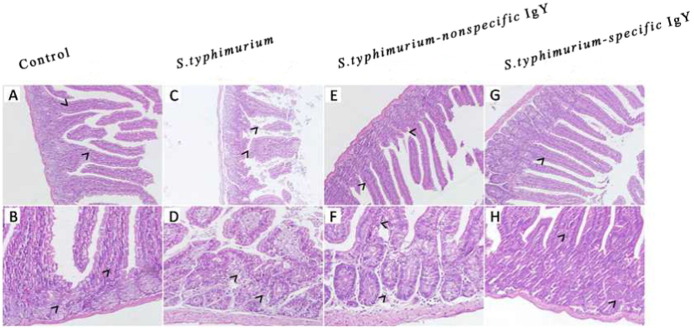
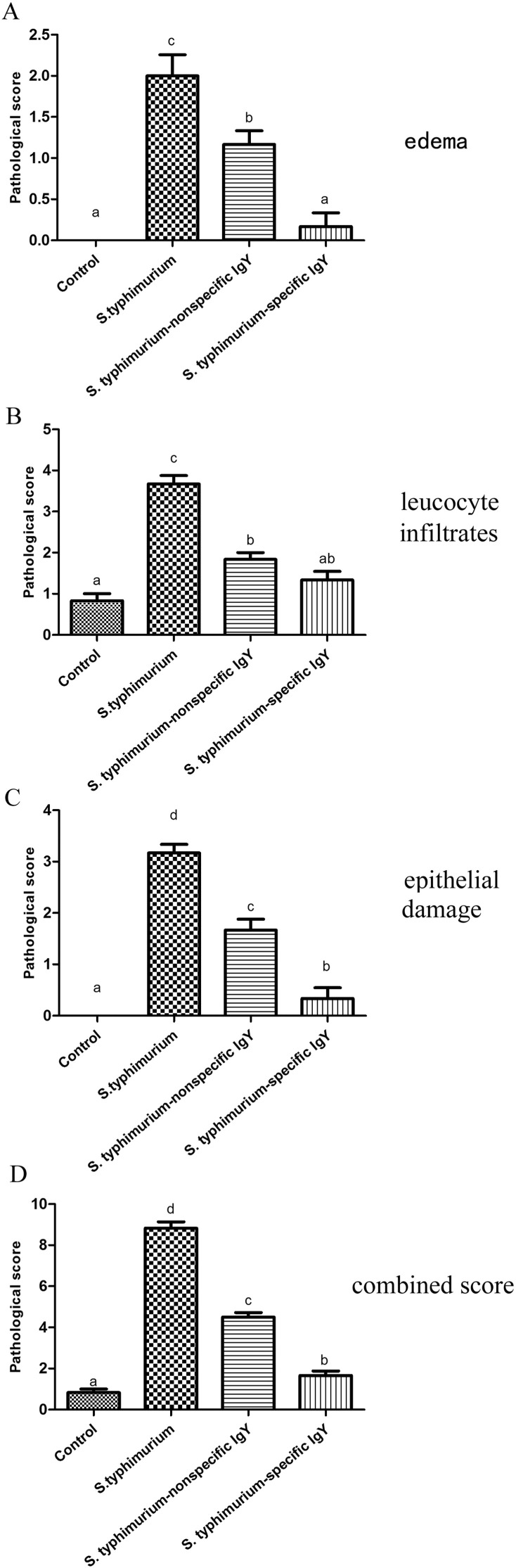
The histopathological features in the jejunum of the mice were examined and are shown in Fig. 2 . In the infected mice, mucosal damage and intestinal inflammation were observed. After challenge, the jejunum of challenged-untreated mice had severe submucosal edema with increased mononuclear inflammatory cells infiltrating the lamina propria and epithelial damage (Fig. 2C and D). Treatment with nonspecific IgY or specific IgY alleviated the damage inflicted by S. typhimurium challenge, and specific IgY treatment effectively reduced jejunum ulceration, transmural inflammation, and edema to a greater extent than treatment (Fig. 2E to H). The statistical analysis on the intestinal pathological injury by the scoring standard also showed the protective function of IgY (Fig. 3 ). The jejunum from specific IgY or nonspecific IgY group had a lower inflammatory score compared with challenged-untreated group, indicating attenuation of the inflammatory response.
Fig. 2.
Representative histopathology of the jejunum from unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge. (A, C, E, G) Magnification: 200 ×. Jejunum mucosa sections show a focal area of rupture and ulceration after challenge that was cured after administration of IgY (arrows). (B, D, F, H) Magnification: 400 ×. The mucosa, submucosa and remaining jejunum wall show evidence of inflammation after challenge which was minimized by administration of IgY (arrows).
Fig. 3.
Pathological score analysis for HE staining. Edema in the submucosa (A), leucocyte infiltrates (B) and epithelial damage (C) was scored separately. The combined score equals the sum of the separate scores (D). Statistical analyses are shown for the separate scores and for the combined score. Data are expressed as means ± SD and analyzed by ANOVA followed by SNK (n = 6 per group). Means without the same letter are significantly different (P < 0.05).
3.3. Effects of IgY on cytokine production in S. typhimurium-challenged mice
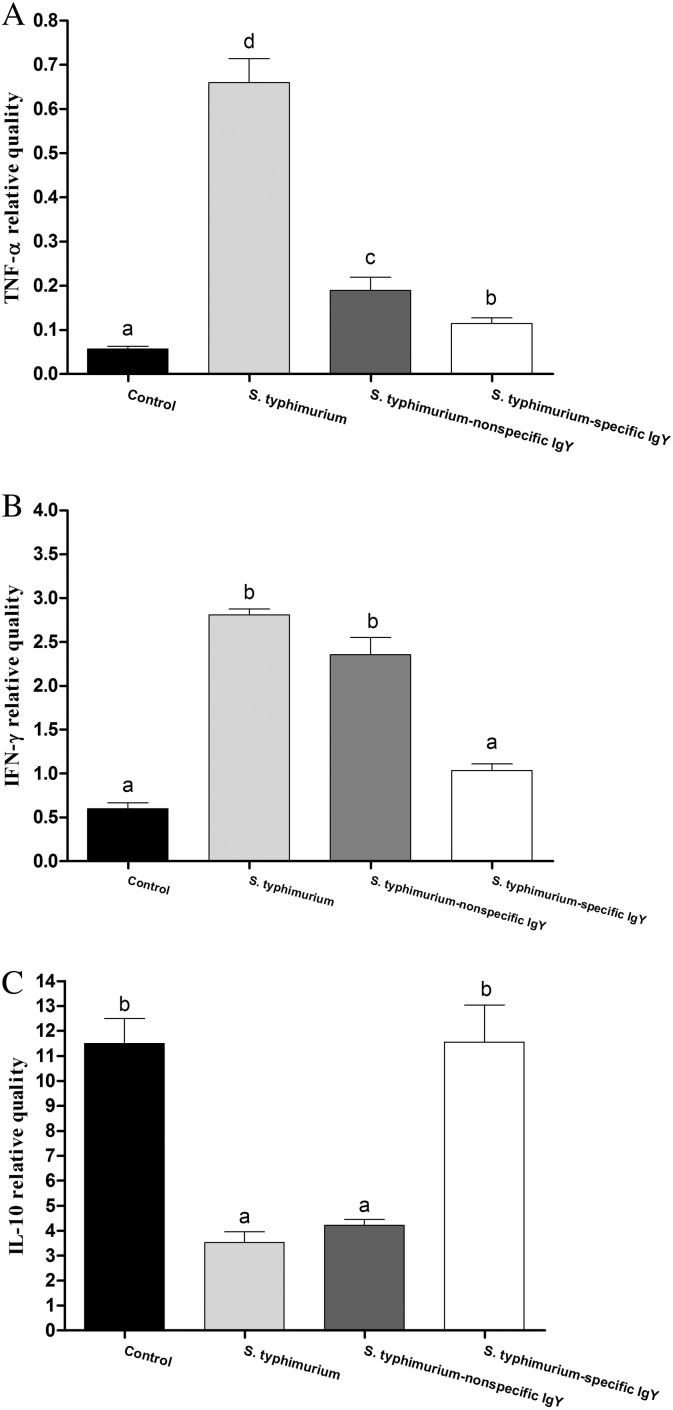
The expression level of the pro-inflammatory cytokines TNF-α and IFN-γ as well as the anti-inflammatory cytokine IL-10 were measured in the intestinal mucosa. S. typhimurium challenge increased the expression levels of TNF-α and IFN-γ (P < 0.05; Fig. 4A and B). Nonspecific IgY did not alter the effect of S. typhimurium on IFN-γ but reduced the expression of TNF-α (P < 0.05). Specific IgY prevented S. typhimurium-induced increases in TNF-α and IFN-γ (P < 0.05). In addition, the results indicate that the infection decreased the expression of the anti-inflammatory cytokine IL-10 (Fig. 4C). Nonspecific IgY did not affect the mucosal expression of IL-10 in challenged mice. However, specific IgY significantly increased the expression of IL-10 (P < 0.05).
Fig. 4.
mRNA levels of TNF-α (A), IFN-γ (B) and IL-10 (C) in the jejunal mucosal from unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Data are expressed as means ± SD and analyzed by ANOVA followed by SNK. Means without the same letter are significantly different (P < 0.05).
3.4. Effect of IgY on lymphocyte populations in the GALT of S. typhimurium-challenged mice
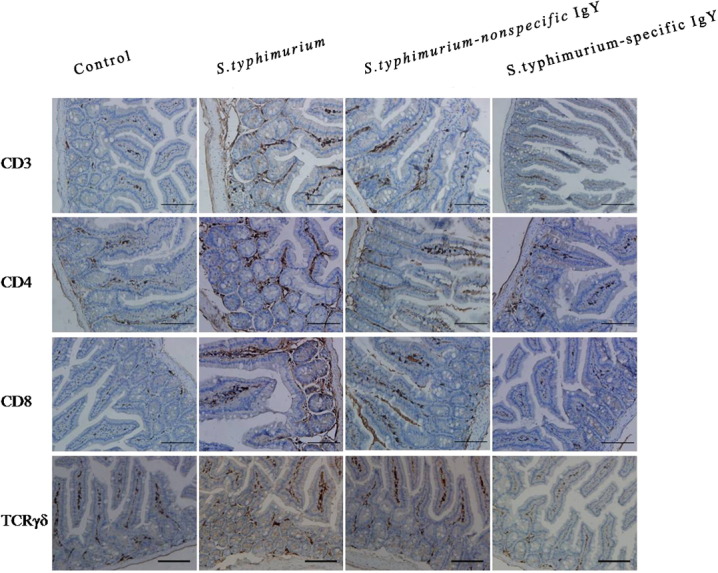
Lymphocyte populations in the jejunum were examined (Fig. 5 ). The brown area shows the different lymphocyte populations. Quantification of lymphocyte populations is shown in Fig. 6 .
Fig. 5.
Representative images of the immunohistochemical localization of lymphocyte populations in the jejunum of unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Jejunum sections immuno-stained with anti-CD3, anti-CD4, anti-CD8, anti-TCRγδ and counterstained with hematoxylin (nuclear marker) are shown at 40 × magnification. Staining for lymphocyte markers is shown in brown and nuclei in blue.
Fig. 6.
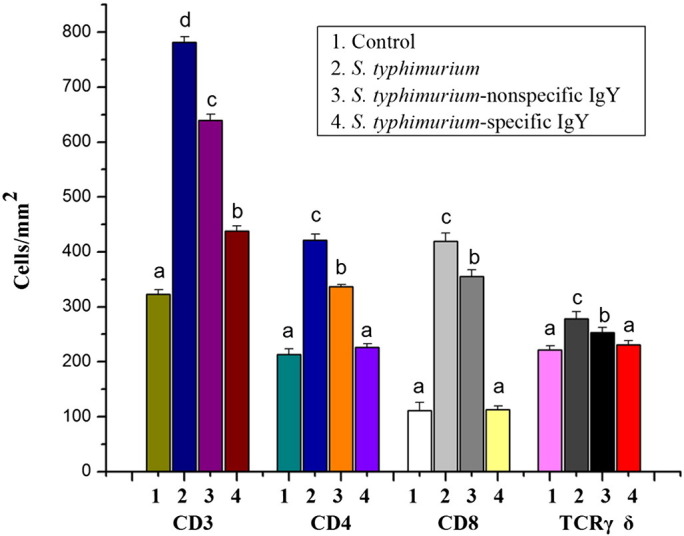
Quantification of lymphocyte populations in the jejunum of unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Values are means ± SD and analyzed by ANOVA followed by SNK. Means without the same letter are significantly different (P < 0.05).
3.4.1. T lymphocytes
S. typhimurium challenge significantly (P < 0.05) increased the number of T lymphocytes in the lamina propria compartment (Fig. 6). Both nonspecific IgY and specific IgY reduced the effects of S. typhimurium in this compartment (P < 0.05; Fig. 5).
3.4.2. T helper lymphocytes
T lymphocytes are composed of 2 main subpopulations namely CD4+ lymphocytes (also called T helper lymphocytes) and CD8+ lymphocytes (T suppressor/cytotoxic lymphocytes). The number of T helper lymphocytes in S. typhimurium-challenged mice was higher than in control mice (P < 0.05; Fig. 6). Both nonspecific IgY and specific IgY attenuated the increase in the number of T helper lymphocytes induced by S. typhimurium challenge (P < 0.05). In addition, more T suppressor/cytotoxic lymphocytes were observed in the untreated S. typhimurium-challenged group than the control group. The number of T suppressor/cytotoxic lymphocytes was decreased by both nonspecific IgY and specific IgY (P < 0.05).
3.4.3. Tγδ lymphocytes
The number of Tγδ lymphocytes in the untreated S. typhimurium-challenged group was increased by 25% compared with the control group (P < 0.05). Administration of nonspecific IgY or specific IgY attenuated the increase in the number of Tγδ lymphocytes which occurred as a result of the S. typhimurium challenge (P < 0.05).
3.5. Effects of IgY on lymphocyte subsets in S. typhimurium-challenged mice
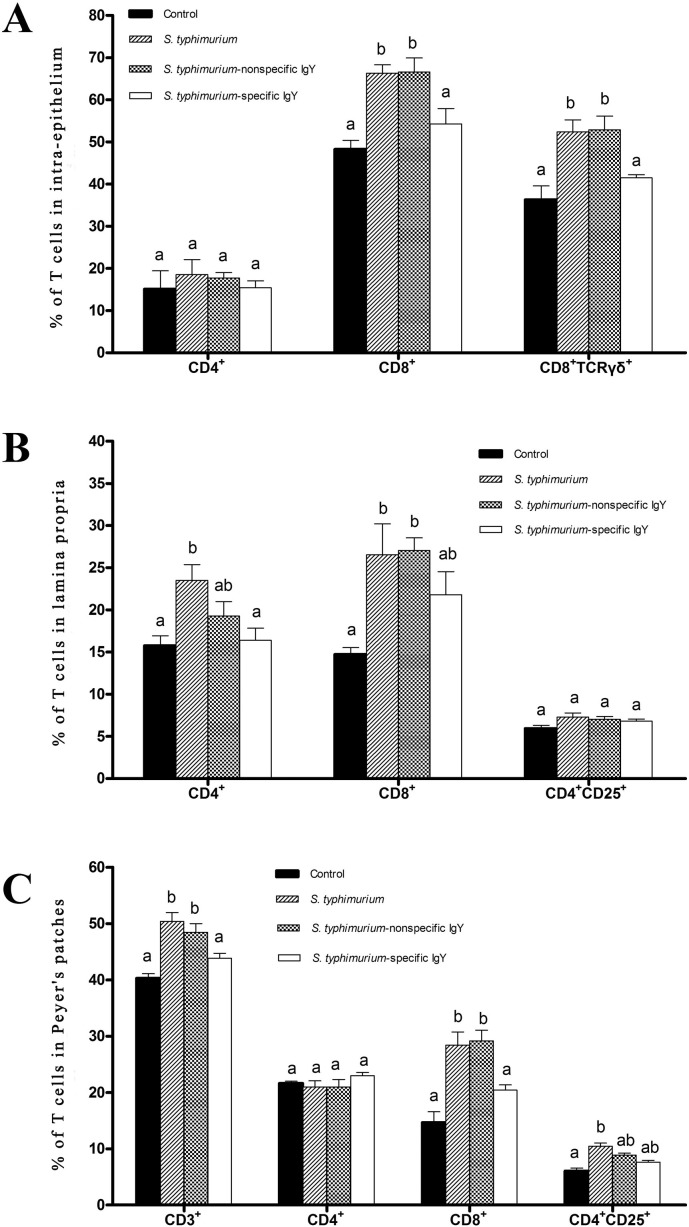
S. typhimurium challenge modified the percentage of T cells in the intestinal intra-epithelial lymphocyte population. In particular, the percentage of CD8+ TCRγδ intestinal intra-epithelial lymphocytes increased from 36.3% in the control mice to 52.4% in the untreated mice challenged with S. typhimurium (P < 0.05; Fig. 7, Fig. 8A ). S. typhimurium administration also increased the percentage of CD8+ T cells compared with control mice in the intestinal intra-epithelial lymphocytes (P < 0.05). The percentage of CD8+ and CD8+ TCRγδ T cells were significantly decreased in the mice treated with specific IgY (P < 0.05). Nonspecific IgY had no effect on lymphocyte percentages.
Fig. 7.
Representative plots of lymphocyte subsets in the small intestine of unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Lymphocytes from the intestinal intra-epithelium, lamina propria and Peyer's patches were isolated from the small intestine as described, pooled, and stained with different antibodies to identify various immune cells (CD3, CD4, CD8, CD25, and TCRγδ). Values are presented as the percentage of total lymphocytes. Figures shown are representative of three individual experiments.
Fig. 8.
Effects of IgY on lymphocyte populations in the intestinal intra-epithelium (A), lamina propria (B) and Peyer's patches (C) of unchallenged mice receiving no treatment, untreated mice challenged with S. typhimurium and challenged mice treated with 0.4 mL of 20 mg mL− 1 nonspecific IgY, 0.4 mL of 20 mg mL− 1 nonspecific IgY or 0.4 mL of PBS 7 days post-challenge (n = 6 per group). Values are means ± SD and analyzed by ANOVA followed by SNK. Means without the same letter are significantly different (P < 0.05).
In the lamina propria compartment, the percentages of CD4+ and CD8+ T cells were significantly increased by S. typhimurium challenge (P < 0.05; Fig. 7, Fig. 8B), but the percentage of activated T lymphocytes (mainly CD4+ CD25+) was not modified. Specific IgY attenuated the effects of S. typhimurium challenge on the percentage of CD4+ T cells in this compartment (P < 0.05). Neither specific IgY nor nonspecific IgY modified the S. typhimurium effect on numbers of CD8+ and CD4+ CD25+ cells.
In the Peyer's patches, S. typhimurium administration increased the percentage of total T lymphocytes (CD3+) and CD8+ T cells compared with control mice (P < 0.05; Fig. 7, Fig. 8C). In addition, S. typhimurium caused an increase in the number of Peyer's patches-activated lymphocytes (mainly CD4+ CD25+) compared with control mice (P < 0.05). Specific IgY diminished the effects of S. typhimurium on the numbers of total T lymphocytes and CD8+ T cells (P < 0.05). CD4+ CD25+ cells tended to decrease after specific IgY treatment. No effects of nonspecific IgY were observed on the various lymphocyte populations.
4. Discussion
Since 2003, when it was originally described, a streptomycin-pretreated mouse model has been increasingly used as the intestinal model of infection to investigate host-pathogen interactions underlying S. typhimurium induced gastro-enteritis (i.e. Typhlitis and Colitis). In this study, we established a streptomycin-pretreated mouse model of intestinal inflammation induced by S. typhimurium similar to that established by Barthel et al. [14].
In this study, mice were pretreated with streptomycin for 48 h before infection to modify the murine intestinal flora thus allowing S. typhimurium to evoke inflammation in the small intestine. We conducted our analysis in the jejunum to monitor the intestinal infection. In addition, we chose mice at weaning because the mucosal immune system is still immature at this time and is more susceptible to infection. In our model, streptomycin-pretreated mice exhibited intestinal inflammation as indicated by histopathological lesions and developed typical clinical signs, suggesting that this model can be a functional instrument to test the efficacy of IgY to treat enteric salmonellosis caused by S. typhimurium.
The protection conferred by oral administration of IgY was confirmed 7 days after challenge. The survival rates for control mice, challenged-untreated mice, challenged mice treated with nonspecific, and specific IgY were 100, 40, 60 and 80% respectively, 7 days post-challenge. This is accordance with the findings of Yokoyama et al. [16], who found that IgY antibodies specific for outer membrane protein, lipopolysaccharide, or flagella can control salmonellosis when orally administrated to mice following a challenge with S. typhimurium. Further confirming the above finding, histopathological analysis showed microvillus damage and evidence of inflammation in the cross section of the jejunum in untreated challenged mice while treatment of infected mice with specific IgY alleviated gut damage and inflammation. This observation clearly indicates that IgY can provide effective protection against S. typhimurium-induced intestinal infection.
We also investigated the effects of IgY on the immune system during S. typhimurium infection in order to better understand the mechanisms through which IgY confers protection. Previous studies have shown that IgY is associated with the immune system in the newborn bovine [17] and fish [18] but its immuno-modulatory functions during infection have not been fully characterized. The present study found an important immuno-modulatory role for IgY on cytokine gene expression and the lymphocyte populations of GALT in mice challenged with S. typhimurium.
A number of microorganisms have developed the capacity to invade the cells of the host and evoke an inflammatory response. Previous reports have shown that T cells play an important role during infection of intracellular pathogens such as S. typhimurium, by activating the anti-microbial activity of macrophages via IFN-γ secretion [19]. The up-regulation of pro-inflammatory cytokines is important to curb the extent of the infection. However, an excessive pro-inflammatory response is also known to be detrimental to the host. So these pro-inflammatory responses are being controlled in order to maintain a balanced immune system.
In the present study, IgY was observed to be regulating these responses during infection. The challenged mice treated with specific IgY significantly reduced their pro-inflammatory responses. The expression of genes for the pro-inflammatory cytokines, especially IFN-γ and TNF-α, were lower in the challenged mice treated with specific IgY compared with untreated infected mice. These observations suggest an important role for IgY in down-regulating cytokine expression. In addition, the stimulation of mucosal GALT induced by S. typhimurium can drive the mucosal immune response toward a Th1 type (cellular immunity) response, characterized by the production of large amounts of IFN-γ by Th1 cells [20], [21].
It has been clearly shown that T cells play important roles in the control of S. typhimurium infection and that CD4, CD8 and TCRγδ T cells in particular are involved [22]. In this study, S. typhimurium induced significant mobilization of lymphocytes including CD3, CD4, CD8 and TCRγδ T cells in the epithelium and lamina propria reflecting an inflammatory process that can be prevented by specific IgY. This effect was consistently observed in the GALT populations of the epithelium and lamina propria indicating that these two mucosal compartments may be the targets of the regulatory effects of IgY.
There are studies which suggest that IL-10 is not involved in protection but rather reflects the severity of disease [23]. Low levels of IL-10 have been shown to increase the severity of Crohn's disease in patients compared with high levels of IL-10 [23]. Our results show that the anti-inflammatory cytokine IL-10 decreases during infection alone and showed an increase in the animals treated with specific IgY, suggesting that IL-10 levels increase during the phase of disease resolution in the presence of specific IgY. IL-10 expression was increased but regulatory T cells (mainly CD4+ CD25+ cells) which produce IL-10 were not changed in challenged mice treated with specific IgY. This might be explained by the fact that IL-10 can be produced by a variety of cells in addition to T cells [20].
Intestinal intra-epithelial lymphocytes play a critical role in the generation of a complex immuno-regulatory network during S. typhimurium infection and the ensuing host response [24]. The majority of intestinal intra-epithelial lymphocytes are CD8+ T lymphocytes [24]. Tγδ lymphocytes that lie in the mucosal and epithelial surfaces have been found to be associated with the repair of damage to epithelial cells during the protective immune response and form the first line of defense against pathogens [25], [26]. CD8+ T cells are effective in providing immunity against S. typhimurium [22].
In the present experiment, we found that S. typhimurium significantly increased the production of CD8+ T cells, particularly CD8+ TCRγδ T cells, which stimulated strong cellular immunity against S. typhimurium infection, reflecting an overstimulation of the immune system. This result is consistent with the observation that S. typhimurium promotes the expansion of intestinal intra-epithelial lymphocytes, particularly CD8+ TCRγδ T cells [24].
The increase in the number of CD8+ T cells and their activation were prevented by specific IgY, indicating that specific IgY could weaken the cellular immune activity possibly by recognizing and binding with S. typhimurium and alleviating an immune stress. In addition, the effects of S. typhimurium challenge on CD4+ T cells in the intestinal intra-epithelial lymphocytes were modest because the CD4+ T cells are a small subset in this mucosal compartment [27].
The lamina propria is one of the constituents of GALT involved in the effector phase of the intestinal immune response [28]. The present study observed that S. typhimurium infection stimulated the production of CD4+ and CD8+ T cells in this compartment, suggesting an overstimulation of the immune system. Specific IgY prevented the S. typhimurium-induced increase in this compartment and reduced the immune activation in the lamina propria.
Peyer's patches are the induction site of mucosal immune responses [28]. After oral infection and colonization of the small intestine, S. typhimurium penetrates the epithelial barrier by invasion of M cells in particular [29] or less efficiently, by transport via CD18/dendritic cells [30] and possibly by penetration of enterocytes [31]. After penetration of the epithelial barrier, S. typhimurium has been shown to colonize Peyer's patches. From there, bacteria move into the mesenteric lymph nodes and then spread via the efferent lymph to the circulatory system, leading to systemic infection.
Peyer's patches have often been used to evaluate the T lymphocyte associated responses as local lymphoid tissue in previous studies [12], [32]. In the present study, the S. typhimurium-induced increases in the percentage of CD8+ lymphocytes in Peyer's patches suggests that T cytotoxic lymphocytes are responsible for S. typhimurium-induced intestinal immune activation. Specific IgY attenuated the increase of CD8+ lymphocytes induced by S. typhimurium challenge.
The mechanism by which IgY inhibits lymphocyte proliferation is not known. For Salmonella, components expressed on the bacterial surface, such as lipopolysaccharide and outer membrane proteins are crucial virulent factors [22]. IgY was found to attenuate inflammatory responses via down regulation of T-lymphocyte function possibly by recognizing and binding with S. typhimurium. This binding may block surface structures on bacteria or neutralize toxic components such as lipopolysaccharide as well as preventing the adhesion of bacteria to the target surface (e.g. epithelial cells and M cells) of the host and penetration of bacteria into deeper tissue.
In addition, nonspecific IgY was effective to some extent at preventing the S. typhimurium-induced increase in proinflammatory cytokine TNF-α and T lymphocytes in the GALT. Several papers have reported that nonspecific IgY improved the phagocytosis of E. coli O111 [13] or Staphylococcus aureus [33]. So nonspecific IgY could weaken the cellular immune activity possibly by phagocytosis opsonization and alleviate an immune stress. However, this might be a good avenue for future research.
In this study, we focused on the effects of IgY on the intestinal mucosal immune responses during infection because the gut is the main site of IgY activity against intestinal pathogens [1] and the mucosal immune system [11]. The effects of IgY on the production of the cytokines and lymphocytes in the spleen (systemic tissue) and local lymphoid tissue (mesenteric lymph nodes) as well as the production of the cytokines in Peyer's patches have not been studied but would be a productive avenue of future research. The present results encourage us to continue evaluating the immuno-modulatory effects of IgY in detail.
In conclusion, we found that specific IgY effectively alleviates S. typhimurium-inflicted damage to the jejunum and plays an important role in limiting the consequences of intestinal inflammation induced by S. typhimurium. Our study highlights the interaction between IgY and the intestinal mucosal immune response during infection. Specific IgY positively modulates the intestinal mucosal immune response of organized and diffuse GALT, including reducing the lymphocyte populations in Peyer's patches, epithelium and lamina propria thus protecting these sites from possible excessive activation evoked by S. typhimurium. These effects were accompanied by an attenuation of the increase of the pro-inflammatory cytokines IFN-γ and TNF-α in the intestinal mucosa. Whether or not IgY functions in other tissues remains to be determined.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgments
Support for this work was provided by the National Natural Science Foundation of China (31001053) and the Special Fund of the Central Colleges Basic Scientific Research Operating Expenses (DUT13 JB04).
References
- 1.Xu Y.P., Li X.Y., Jin L.J., Zhen Y.H., Lu Y.N., Li S.Y. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol. Adv. 2011;29:860–868. doi: 10.1016/j.biotechadv.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgY-technology): a review of progress in production and use in research and human and veterinary medicine. Altern. Lab. Anim. 2005;33:129–154. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- 3.Mine Y., Kovacs-Nolan J. Chicken egg yolk antibodies as therapeutics in enteric infectious disease: a review. J. Med. Food. 2002;5:159–169. doi: 10.1089/10966200260398198. [DOI] [PubMed] [Google Scholar]
- 4.Carlander D., Kollberg H., Wejaker P.E., Larsson A. Peroral immunotherapy with yolk antibodies for the prevention and treatment of enteric infections. Immunol. Res. 2000;21:1–6. doi: 10.1385/IR:21:1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Diraviyam T., Zhao B., Wang Y., Schade R., Michael A., Zhang X.Y. Effect of chicken egg yolk antibodies (IgY) against diarrhea in domesticated animals: a systematic review and meta-analysis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0097716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee E.N., Sunwoo H.H., Menninen K., Sim J.S. In vitro studies of chicken egg yolk antibody (IgY) against Salmonella enteritidis and Salmonella typhimurium. Poult. Sci. 2002;81:632–641. doi: 10.1093/ps/81.5.632. [DOI] [PubMed] [Google Scholar]
- 7.Tsubokura K., Berndtson E., Bogstedt A., Kaijser B., Kim M., Ozeki M. Oral administration of antibodies as prophylaxis and therapy in Campylobacter jejuni-infected chickens. Clin. Exp. Immunol. 1997;108:451–455. doi: 10.1046/j.1365-2249.1997.3901288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhen Y.H., Jin L.J., Guo J., Li X.Y., Li Z., Fang R. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Staphylococcus aureus. J. Appl. Microbiol. 2008;105:1529–1535. doi: 10.1111/j.1365-2672.2008.03920.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang L.H., Li X.Y., Jin L.J., You J.S., Zhou Y., Li S.Y. Characterization of chicken egg yolk immunoglobulins (IgYs) specific for the most prevalent capsular serotypes of mastitis-causing Staphylococcus aureus. Vet. Microbiol. 2011;149:415–421. doi: 10.1016/j.vetmic.2010.11.029. [DOI] [PubMed] [Google Scholar]
- 10.Li X.Y., Jin L.J., McAllister T.A., Stanford K., Xu J.Y., Lu Y.N. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY) J. Agric. Food Chem. 2007;55:2911–2917. doi: 10.1021/jf062900q. [DOI] [PubMed] [Google Scholar]
- 11.Granger N., Kevil C.G., Grisham M.B. Recruitment of inflammatory and immune cells in the gut: physiology and pathophysiology. In: Johnson L.R., Barrett K.E., Merchant J.L., Ghishan F.K., Said H.M., Wood J.D., editors. Physiology of the Gastrointestinal Tract. Elsevier; Amsterdam: 2006. pp. 1137–1162. [Google Scholar]
- 12.Perez-Bosque A., Pelegri C., Vicario M., Castell M., Russell L., Campbell J.M. Dietary plasma protein affects the immune response of weaned rats challenged with S. aureus super antigen. Br. J. Nutr. 2004;134:2667–2672. doi: 10.1093/jn/134.10.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhen Y.H., Jin L.J., Guo J., Li X.Y., Lu Y.N., Chen J. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Escherichia coli. Vet. Microbiol. 2008;130:126–133. doi: 10.1016/j.vetmic.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Barthel M., Hapfelmeier S., Quintanilla-Martinez L., Kremer M., Rohde M., Hogardt M. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 2003;71:2839–2858. doi: 10.1128/IAI.71.5.2839-2858.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davies M.D.J., Parrott D.M.V. Preparation and purification of lymphocytes from the epithelium and lamina propria of murine small intestine. Gut. 1981;22:481–488. doi: 10.1136/gut.22.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yokoyama H., Umeda K., Peralta R.C., Hashi T., Icatlo F.C., Jr., Kuroki M. Oral passive immunization against experimental salmonellosis in mice using chicken egg yolk antibodies specific for Salmonella enteritidis and S. typhimurium. Vaccine. 1998;16:388–393. doi: 10.1016/s0264-410x(97)80916-4. [DOI] [PubMed] [Google Scholar]
- 17.Vega C., Bok M., Chacana P., Saif L., Fernandez F., Parreno V. Egg yolk IgY: protection against rotavirus induced diarrhea and modulatory effect on the systemic and mucosal antibody responses in newborn calves. Vet. Immunol. Immunopathol. 2011;142:156–169. doi: 10.1016/j.vetimm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C.H., Lu X.J., Li D.F., Chen J. Passive protective effect of chicken egg yolk immunoglobulins against experimental Vibrio anguillarum infection in ayu (Plecoglossus altivelis) Fish Shellfish Immunol. 2014;37:108–114. doi: 10.1016/j.fsi.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 19.Bergman M.A., Cummings L.A., Alaniz R.C., Mayeda L., Fellnerova I., Cookson B.T. CD4+-T-cell responses generated during murine Salmonella enterica serovar Typhimurium infection are directed towards multiple epitopes within the natural antigen FliC. Infect. Immun. 2005;73:7226–7235. doi: 10.1128/IAI.73.11.7226-7235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pie S., Truffa-Bachi P., Pla M., Nauciel C. Th1 response in Salmonella typhimurium-infected mice with a high or low rate of bacterial clearance. Infect. Immun. 1997;65:4509–4514. doi: 10.1128/iai.65.11.4509-4514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramarathinam L., Shaban R.A., Niesel D.W., Klimpel G.R. Interferon-gamma (IFN-gamma) production by gut-associated lymphoid-tissue and spleen following oral Salmonella typhimurium challenge. Microb. Pathog. 1991;11:347–356. doi: 10.1016/0882-4010(91)90020-b. [DOI] [PubMed] [Google Scholar]
- 22.Mittrucker H.W., Kaufmann S.H.E. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 2000;67:457–463. doi: 10.1002/jlb.67.4.457. [DOI] [PubMed] [Google Scholar]
- 23.Marlow G., van Gent D., Ferguson L. Why interleukin-10 supplementation does not work in Crohn's disease patients. World J. Gastroenterol. 2013;19:3931–3941. doi: 10.3748/wjg.v19.i25.3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z.Y., Zhang C., Zhou Z.X., Zhang J.H., Zhang J., Tian Z.G. Small intestinal intraepithelial lymphocytes expressing CD8 and T cell receptor gamma delta are involved in bacterial clearance during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 2012;80:565–574. doi: 10.1128/IAI.05078-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carding S.R., Egan P.J. Gamma delta T cells: functional plasticity and heterogeneity. Nat. Rev. Immunol. 2002;2:336–345. doi: 10.1038/nri797. [DOI] [PubMed] [Google Scholar]
- 26.Hayday A., Tigelaar R. Immunoregulation in the tissues by gamma delta T cells. Nat. Rev. Immunol. 2003;3:233–242. doi: 10.1038/nri1030. [DOI] [PubMed] [Google Scholar]
- 27.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu. Rev. Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 28.Wershil B.K., Furuta G.T. 4. Gastrointestinal mucosal immunity. J. Allergy Clin. Immunol. 2008;121:S380–S383. doi: 10.1016/j.jaci.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 29.Neutra M.R., Frey A., Kraehenbuhl J.P. Epithelial M cells: gateways for mucosal infection and immunization. Cell. 1996;86:345–348. doi: 10.1016/s0092-8674(00)80106-3. [DOI] [PubMed] [Google Scholar]
- 30.Rescigno M., Urbano M., Valzasina B., Francolini M., Rotta G., Bonasio R. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat. Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 31.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- 32.Sofi M.H., Bhatnagar A., Sapra S., Mahmood A., Majumdar S. Immunoregulatory role of intestinal surfactant-like particles during Salmonella typhimurium infection. Int. J. Biol. Sci. 2007;3:446–454. doi: 10.7150/ijbs.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhen Y.H., Jin L.J., Guo J., Li X.Y., Li Z., Fang R., Xu Y.P. Characterization of specific egg yolk immunoglobulin (IgY) against mastitis-causing Staphylococcus aureus. J. Appl. Microbiol. 2008;105:1529–1535. doi: 10.1111/j.1365-2672.2008.03920.x. [DOI] [PubMed] [Google Scholar]