Abstract
We have developed a new photocatalyst thin film that has strong antibacterial action in visible light. In this study the radiofrequency (RF) sputter technique was used to deposit a defective titanium dioxide (TiOx, x < 2) photocatalyst thin film (120 nm thickness) on glass and steel substrates. In the ultraviolet (UV)-visible spectrum analysis, the defective TiOx thin film was found to generate the red shift effect. To determine whether the defective TiOx thin film has antibacterial ability under visible light, we designed a series of experiments according to the Japanese Industrial Standards (JIS) Committee standard for testing antibacterial performance and for exploring the impact of surface roughness of substrate. Our results show that the antibacterial performance rate against Escherichia coli could reach 99.99% in visible light. We also proved that the coating technology can be applied effectively to surfaces with different degrees of roughness. It is suitable for protecting both human health and the natural environment.
Key words: Photocatalyst, RF sputter technique, Defective TiOx thin films, Escherichia coli
Introduction
Many antiviral products have recently been developed to prevent infectious diseases such as severe acute respiratory syndrome (SARS). Several studies have concluded that titanium dioxide (TiO2) photocatalyst is capable of deactivating the SARS virus with high efficiency. For this reason, much effort has been devoted to understanding the mechanism and fabrication methods of photocatalysts. In 1972 Fujishima and Honda successfully fabricated TiO2 ceramic nanoparticles that can be used in photocatalytic reactors for degrading contaminants in water and air [1]. To effectively detoxify noxious organic pollutants the semiconductor photocatalyst generally requires ultraviolet (UV) light as the excitant source; the reason is that UV energy is greater than the band gap of the semiconductor, and it will deduce the electron hole pairs generated when the semiconductor is illuminated by UV. The electrons subsequently react with O2 in the sample to form superoxide radicals, while the holes react with the surface OH groups to form OH radicals. These radicals attack the organic molecule, which is eventually oxidized to become CO2, H2O, and HCl, thus achieving an antibacterial effect.
It is widely known that TiO2 photocatalysts have minimal antibacterial efficacy in visible light. Therefore, it became essential to develop a new photocatalyst that could be excited by visible light. Briefly, there are three ways to modify photocatalysts while working within the spectrum of visible light. One is by dropping the metal elements into the ceramal material and then exciting this photocatalyst in visible light. For example, Zou's team discovered that TiO4 doped with nickel would be excited by visible light; alternatively, they doped with platinum to enhance water splitting [2], [3], [4]. The second approach is by doping the transition elements into the UV photocatalyst to create the red shift effect [5]. For example, TiO2 ceramal-doped transition elements were used to prepare a visible light photocatalyst with decreasing band gap energy [6], [7]. That is to say, when the transition elements are doped into TiO2 the TiOx structure (x < 2) would then be formed, and the red shift could be achieved by reducing the highest occupied molecular orbital gap energy. Unfortunately, thermal instability and short half-life are its disadvantages. The third way is to change the structure of the photocatalyst by the defection effect, thus achieving surface adsorption in visible light [8]. This approach has many advantages, such as thermal stability, ease of fabrication, and large red shift [9].
Of concern is that photocatalyst nanoparticles could have an adverse effect on human health. The photocatalyst has a high surface-to-volume ratio (S/V) to promote its antibacterial action. That is, the volume of photocatalyst must be small. For example, the diameter of nanoparticles is smaller than the human sweat duct (<10 nm) and may have untoward effects on the human body when the photocatalyst penetrates into skin via breathing or touching. For this reason, Rochester's researcher indicated that the metabolic mechanism of nanoparticles in the human body must be addressed. Our defective TiOx (x < 2) photocatalyst thin film has an excellent mechanical function and is effective against both viruses and bacteria under the visible light spectrum. Further details will be reported in this study.
Theory
The semiconductor photocatalyst is excited by the appropriate electromagnetic wave to produce electron and hole pairs. The electron combines with O2 to produce superoxide free radicals, and the hole captures the electron from H2O in air to generate hydroxyl free radicals. Both free radicals have powerful bonding ability with bacteria and fungi. Therefore, bacteria and fungi will be suppressed with DNA damaged by superoxide and hydroxyl free radicals [10]. The defective lattice TiOx structure was usually fabricated by doping nitrogen into pure TiO2 during the sputter process [11]. Because the strength of O-H bonding on metal oxides depends on the electronegative property of the metal ions, it is presumed that the N-H bonding is weaker than the O-H bonding (electronegative property of nitrogen is 3.0, oxygen is 3.5). Therefore, the N-H sites of the nitrogen-doped TiO2 surface act as acidic sites, and the defective lattice structure TiO2 - xNx is obtained. The structure shows adsorption in the spectrum of visible light [12]. In this work we used argon to replace nitrogen in the RF sputter process. Because the ion of argon has no electronegativity, it can barely bond with ionic oxygen, even though the plasma has high energy. That is, the large argon ion occupied the position of oxygen during the composition process of TiO2. Consequently, the thin film was altered to be TiOx (x < 2) photocatalyst, and its absorption spectrum has a larger red shift than pure TiO2. In this work the method of defection effect is used to produce a defective photocatalyst thin film that shows the characteristic of visible adsorption.
Experiments
Preparation of defective TiOx photocatalyst thin film
We used slide glass and steel as the substrate to be coated with the O2-defective TiOx (x < 2) photocatalyst thin film. Ar and O2 were introduced to the RF sputter chamber kept at 3.2 × 10−6 torr. Ar fluid was 16 sccm (standard cubic centimeter per minute) and O2 fluid was 33 sccm. The substrate of sputter was loaded with 100 V at 200 °C, and the target was Ti of 10 cm diameter, 6 mm thickness and 99.50% purity. After 120 seconds of deposition, the thickness of the TiOx thin film was about 120 nm, and the red shift was 0.65 to 1.12 eV in the adsorption spectrum. To compare the surface obtained with the defective TiOx to that obtained with pure TiO2, the samples were destroyed by ultrasonic waves and then imaged by scanning electron microscopy (SEM). The results are shown in Figure 1, A and B .
Fig 1.

SEM image of (A) TiO2 thin film surface of pure TiO2 and (B) surface of defective TiO2.
Preparation of bacteria
E. coli (ATCC No. 23505) was chosen as the standard bacterium in our antibacterial examinations because it is widely used as the biological index for testing the pollution of apparatus and water. We first prepared the Luria broth (LB) medium, which comprises 10 g tryptone, 10 g sodium chloride, and 5 g yeast extract in 1 L of water; we then added 20 g agar to the 1 L of LB medium, producing LB agar. After inoculating a single colony of E. coli into the LB medium we kept the culture at 200 rpm shaking for 12 hours at 37 °C.
Antibacterial experiment procedures
First, a bacterial solution containing 105 colonies/mL was dropped onto the samples of photocatalyst thin film, and the samples were covered with a coverslip (1 cm × 1 cm × 0.5 cm) to prevent medium evaporation. The control sample of photocatalyst thin film was placed in the dark inside a box covered with black polycarbonate. All samples were exposed to four 15-W fluorescent lamps for several hours. After exposure we flushed the bacteria with LB medium and inoculated this into LB agar for 12 to 16 hours at 37 °C. Figure 2, A is a sketch map of this experiment, and Figure 2, B is a photograph of the defective TiOx thin film on the steel surface (the TiOx thin film is purple).
Fig 2.

A, Diagram of platform for examination of antibacterial thin film. B, Photograph of the sample of defective TiOx thin film on the steel surface (the TiOx thin film is purple).
Method for analyzing antibacterial capacity
To allow an exact estimate of the antibacterial capacity of the sample, the E. coli must grow in an exponential curve in time to provide a standard growth curve for evaluating the concentration of bacteria solution. The selected single colony cultured in LB medium was kept at 37 °C and shaken at 200 rpm for 12 hours. It was then diluted to reduce the original concentration of bacteria in a stepwise manner. The diluted samples have different values of optical density at 600 nm (OD600) reflecting the different concentrations of bacteria in solution by UV-visible adsorption spectrum apparatus. Figure 3, A shows the plots of the OD600 values versus the corresponding logarithmic values of the multiple of the diluted bacteria concentration (M). This standard growth curve of E. coli could be used to evaluate the concentration of bacteria in solution and reduce the experimental error. Figure 3, B is a photomicrograph showing the E. coli on the sample surface; the bacteria were cultured in LB agar obtained by flushing with 100 mL LB medium after the experiment. The precise value of colony-forming units (CFU) of E. coli could be obtained from Figure 3, B, using the following three steps: (1) collecting all E. coli in LB medium after exposure, (2) inoculating E. coli onto LB agar for 12 to 16 hours at 37 °C, and (3) counting the yellow colonies of E. coli. The units for CFU are CFU/mL. According to a general rule, the survival rate of bacteria can be defined by a simple formula shown as follows:
| (1) |
where x and y are the CFU before and after exposure, respectively. Figure 4, A shows photographs of the sample exposed to fluorescent light for different lengths of time. The survival rate of the E. coli can be calculated from Figure 4, A and Eq. (1) using the method that has just been expressed. The result is shown in Figure 4, B.
Fig 3.

A, Plots of OD600 vs logarithm of the multiple of the diluted bacteria concentration in CFU/mL. B, The image of E. coli viewed by microscopy.
Fig 4.

A, Photographs of samples of E. coli colonies exposed and unexposed to fluorescent light in the antibacterial experiment. B, The survival rate of E. coli plotted against exposure time.
We can also use the JISL 1902-1998 rule to estimate the antibacterial effectiveness of the defective TiOx (x < 2) photocatalyst thin film. This rule has been used for estimating the toxicity of novel drugs. According to the rule, the antibacterial index (ABI) and kill-bacteria index (KBI) of the photocatalyst thin film can be calculated by the following formulas:
| (2) |
| (3) |
Here, A is the initial CFU value in which the bacteria was dropped onto the surface of a noncoated sample and was flushed immediately, B is the CFU value of a noncoated positive control sample exposed to fluorescent lamps or a coated sample in a dark field for 6 to 18 hours, and C is the CFU value of a coated photocatalyst thin film sample exposed to fluorescent lamps for 6 to 18 hours.
Results and discussion
Antibacterial capacity of anatase-TiO2 photocatalyst nanoparticles and defective TiOx photocatalyst thin film
To determine the antibacterial capacity of the defective TiOx photocatalyst thin film (glass substrate) we used anatase-TiO2 nanoparticles with 40 mm diameter as a reference. Both samples were illuminated by four 15-W UV lamps. The results are shown in Figure 5 . The survival rate of and the ABI of the samples can be obtained from (1), (2), respectively. The survival rate of E. coli on defective photocatalyst thin film was 49.3% (ABI of 1.3) after being exposed for 1 hour; that is, only 50.7% of E. coli would be restrained. The survival rate of E. coli on anatase-TiO2 nanoparticles was 57% (ABI of 1.7). That is to say, the antibacterial performance rate of defective TiOx thin film is about 1.17-fold that of the anatase-TiO2 nanoparticle after 1 hour. However, 3 hours later both samples reached the same survival rate of 3%. After 5 hours the value was below 0.01%; thus the antibacterial performance rate was above 99.99% for both samples. In contrast, the survival rate of bacteria in the dark field was 92%, for both samples at any time. Thus we proved that the defective photocatalyst thin film has the same antibacterial ability as the anatase-TiO2 nanoparticle.
Fig 5.
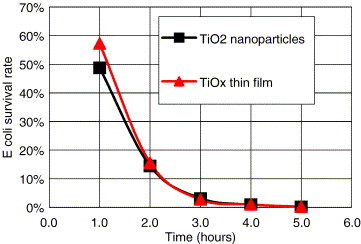
The survival rate of E. coli with defective TiOx photocatalyst thin film and TiO2 nanoparticles for samples exposed to UV light.
Influence of substrate roughness on coating by photocatalyst thin film
The surface of a steel substrate is generally always rougher than that of glass, and its real contact area with air is also larger than that of glass. Thus it was uncertain whether the defective TiOx photocatalyst thin film coated onto the steel surface would have greater antibacterial ability than when coated onto other surfaces of varying roughness. Thus we examined the ABI with substrates of varying surface roughness, including steel and glass. The excitant light source was replaced by four 15-W fluorescent lamps (in visible light). The results of the experiment are shown in Figure 6 . The survival rate of E. coli with a steel substrate was 69%, whereas that for glass was 49.3%, after the samples were exposed for 1 hour. After exposure for 5 hours, the survival rate with a steel substrate was less than 1%; thus the antibacterial performance rate was greater than 99%, and the corresponding ABI value is 1.69. The survival rate with the glass substrate is about 0.01%; thus the antibacterial performance rate can reach 99.99%, and the corresponding ABI value is 1.84. Although there is a small difference in the deviation of the ABI values corresponding to steel and glass respectively, the substrate roughness effect is still very slight. For comparison, the sample of anatase-TiO2 nanoparticle was also exposed under the same light source. The results are also shown in Figure 6. It can be seen here that the survival rate of E. coli is still greater than 50% after a 5-hour exposure; that is, the anatase-TiO2 nanoparticle has lower antibacterial efficiency in visible light than the defective TiOx photocatalyst thin film. In addition to the low antibacterial efficiency, the optimal value of ABI was only 1.3 after 5 hours. This is in contrast to the excellent performance of the defective TiOx photocatalyst thin film, regardless of the roughness of the coating. Moreover, to determine whether the defective TiOx thin film has the ability to kill bacteria we measured the KBI of the defective TiOx thin film (with glass substrate) and obtained the KBI from Eq. (3). The value was 0.22 in the UV experiment, whereas it was about 0.075 in the visible light experiment. These results led us to speculate that the chief agent responsible for killing bacteria is probably UV light and not the photocatalyst effect. Therefore, the defective TiOx photocatalyst thin film shows an excellent antibacterial ability, but it is an inefficient killer of bacteria. In addition to the issues already discussed, we are studying the influence of the thickness of the thin film on the antibacterial capacity. Furthermore, the friction effect is one of the most important variables and must be taken into account in industrial applications.
Fig 6.

The survival rate of E. coli in samples exposed under four 15-W fluorescent lamps.
Conclusions
We used the defection effect in sputter technology and developed successfully the defective TiOx photocatalyst thin film. It is a simple and effective technique for coating photocatalyst thin film onto substrate. According to the JIS standard, the thin films have excellent antibacterial ability under visible light. The defective photocatalyst thin film overcame the drawback of a lower S/V ratio and could replace nanoparticles as a tool in the antibacteria field, especially, in an environment of weak sunlight. The results of our study of surface roughness of substrate show that the coating technology could be applied to surfaces of varying surface roughness. Moreover, the TiOx thin film possesses an excellent antibacterial ability but is inefficient at killing bacteria.
Footnotes
This work was supported by the National Science Council of the Republic of China under Contract No. NSC 93-2112-M-232-001.
No financial conflict of interest was reported by the authors of this paper.
References
- 1.Fujishima A., Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 2.Kasahara A., Nukumizu K., Takata T., Kondo J.N., Hara M., Kobayashi H. LaTiO2N as a visible-light (λ ≤ 600 nm) driven photocatalyst (2) J Phys Chem, B. 2003;107:791–797. [Google Scholar]
- 3.Linsebigler A.L., Lu G., Yates J.T. Photocatalysis on TiO2 surfaces: principles, mechanism, and selected results. Chem Rev. 1995;95:735–758. [Google Scholar]
- 4.Maldotti A., Molinari A., Amadelli R. Photocatalysis with organized systems for the oxofunctionalization of hydrocarbons by O2. Chem Rev. 2002;102:3811–3836. doi: 10.1021/cr010364p. [DOI] [PubMed] [Google Scholar]
- 5.Kato H., Kudo A. Visible-light-response and photocatalytic activities of TiO2 and SrTiO3 photocatalysts codoped with antimony and chromium. J Phys Chem, B. 2002;106:5029–5034. [Google Scholar]
- 6.Kawahara T., Konishi Y., Tada H., Tohge N., Nishii J., Ito S. Sichtbarmachen der reduktionsstellen durch abscheiden von Ag-nanopartikeln-untersucht (siehe Bild) Angew Chem Int Ed. 2002;41:2811–2819. doi: 10.1002/1521-3773(20020802)41:15<2811::AID-ANIE2811>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Ye J., Zou Z., Oshikiri M., Matsushita A., Shimoda M., Imai M. A novel hydrogen-evolving photocatalyst in VO4 active under visible light irradiation. Chem Phys Lett. 2002;356:221–226. [Google Scholar]
- 8.Taylor C.E., Noceti R.P. Other catalytic properties of V2O5-WO3/TiO2 EUROCAT catalysts. Catal Today. 2000;55:259–267. [Google Scholar]
- 9.Klabunde K.J., Stark J., Koper O., Mohs C., Park D.G. Nanocrystals as stoichiometric reagents with unique surface chemistry. J Phys Chem. 1996;100:12142–12148. [Google Scholar]
- 10.Zang L., Macyk W., Lange C., Maier W.F., Antonius C., Meissner D. Visible light detoxification and charge generation by transition metal chloride modified titania. Chem Eur J. 2000;6:379–384. doi: 10.1002/(SICI)1521-3765(20000117)6:2<379::AID-CHEM379>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Asahi R., Morikawa T., Ohwaki T., Aoki K., Taga Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science. 2001;293:269–271. doi: 10.1126/science.1061051. [DOI] [PubMed] [Google Scholar]
- 12.Zou Z., Ye J., Sayama K., Arakawa H. Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst. Nature. 2001;414:625–627. doi: 10.1038/414625a. [DOI] [PubMed] [Google Scholar]


