Abstract
Objective
HSV-2 infection has increased significantly in recent years, which is closely associated with cervical cancer and HIV infection. The lack of success in vaccine development and the emergence of drug resistance to commonly used drugs emphasize the urgent need for alternative antivirals against HSV-2 infection. Arbidol (ARB) has been demonstrated to be a broad spectrum antiviral drug that exhibits immunomodulatory properties that affect the HSV-2 life cycle. This study investigated the efficacy and mechanism of ARB against HSV-2 in vivo and in vitro to further explore the clinical application of ARB.
Methods
The efficacy of ARB on HSV-2 infection in vitro was examined by CPE and MTT assays. A vaginitis model was established to monitor changes in histopathology and inflammatory cytokine (IL-2, IL-4, TNF-α and TGF-β) expression by H&E staining and ELISA, respectively, and the efficacy of ARB was evaluated accordingly. Furthermore, flow cytometry was used to determine the ratio of CD4+/CD8+ T cells in the peripheral blood of the vaginitis animals. Considering the balance of efficacy and pharmacokinetics, ARB ointment was strictly prepared to observe formulation efficacy differences compared to the oral dosing form.
Results
The results showed that, in vitro, the TC50 and IC50 of ARB were 32.32 μg/mL and 4.77 μg/mL (SI = 6.82), respectively, indicating that ARB presents effective activity against HSV-2 in a dose-dependent manner. The results of the time-course assay suggested that 25 μg/mL ARB affected the late stage of HSV-2 replication. However, ARB did not inhibit viral attachment or cell penetration. The in vivo results showed that ARB ointment can improve the survival rate, prolong the survival time and reduce the reproductive tract injury in mice infected with HSV-2, regulate cytokine expression; and balance the CD4+ and CD8+ T lymphocyte ratio in the peripheral blood to participate in the regulation of immune response.
Conclusion
ARB showed anti-HSV-2 activity in vitro in a dose-dependent manner and played a role in inhibiting the late replication cycle of the virus. The vaginitis model was successfully established, according to immunomodulation outcomes, responded better to ARB in ointment form than in oral form.
Keywords: Arbidol hydrochloride, HSV-2, Mechanism of action, In vitro, In vivo
Highlights
-
•
ARB showed anti-HSV-2 activity in vitro in a dose-dependent manner.
-
•
ARB inhibited the late replication cycle of HSV-2.
-
•
ARB ointment participated in the regulation of immune response to reduce the reproductive tract injury.
-
•
ARB in ointment form responded to vaginitis better than in oral form.
1. Introduction
Sexually transmitted diseases (STDs), with >1 million acquired every day worldwide, are now the most common group of notifiable infectious diseases in most countries, particularly in the age group of 15 to 50 years and infants [1]. Over eight pathogens are linked to the STDs with the greatest incidence, among which four are currently incurable viral infections: hepatitis B, herpes simplex virus (HSV), human immunodeficiency virus (HIV) and human papillomavirus (HPV) [2].
Herpesvirus is a family of medium-sized enveloped DNA virus, divided into α, β, γ subfamilies, and HSV belongs to the human Herpesviridae α subfamily. HSV is one of the most common pathogens in humans and is frequently reported to be responsible for herpetic encephalitis, herpes keratitis, genital herpes, neonatal infections, myocarditis and other diseases that are widely spread by humans to humans [3]. There are two distinct types in HSV infections of the central nervous system, HSV-1 (which produces most cold sores) and HSV-2 (which produces most genital herpes) [4]. It is estimated that >500 million people aged 15–49, approximately 16% of the population globally in this age range, are carriers of HSV-2, a virus with a risk of mortality up to 70% when left untreated [5]. HSV-2 infection usually causes characteristic lesions on the mucous membranes and skin surrounding the genitals [5]. After the initial or primary infection, some infected people experience sporadic episodes of viral reactivation or outbreaks. In addition, the seroprevalence of HSV-2 in women is higher than that in men and transmission of HSV-2 during childbirth can cause complications in neonates resulting in brain damage or death. Simultaneously, HSV-2 infection increases the risk of HIV acquisition by approximately three-fold [6], and the increase in risk is even greater in those with a newly-acquired HSV-2 infection.
The nucleoside analog acyclovir (ACV) discovered in 1974 is currently the only Food and Drug Administration (FDA)-approved treatment for HSV-2 infection [7]. In clinical trials, it can modulate the course of the disease and decrease the transmission risk but cannot cure the disease. Furthermore, ACV has a single mechanism of action against HSV-2 that requires active site coordination, which can easily lead to drug resistance and cross-resistance. This resistance is a major threat that could reduce the impact of antiviral drugs against STIs worldwide, especially among HIV-infected patients in regions and immunocompromised patients where only limited treatment options are available [8]. Therefore, there is an urgent need to develop a novel drug with different mechanism of action against HSV-2 infection due to the disease severity.
ARB, a small molecule indole derivative developed by the Russian Center for Medicinal Chemistry in 1993 and already licensed in Russia and China, is reported as an anti-influenza agent with immunomodulatory properties [9], but its clinical application has been limited regionally. It can not only interfere with virus-induced membrane fusion, but also degrade viral RNA (mRNA) to inhibit protein synthesis and thereby block the early replication of the virus [10]. In addition, ARB can also induce interferon (IFN) release in the host peripheral blood [11]. Recent studies extended its inhibitory activity to other human viruses, including respiratory syncytial virus (RSV), rhinovirus (RHV), hepatitis C virus (HCV), coxsackievirus (CVB), severe acute respiratory syndrome (SARS), herpes simplex virus type I (HSV-1) and the influenza mutant strains resistant to amantadine and oseltamivir [12]. YS Boriskin et al. demonstrated that ARB has a prophylactic effect on HCV infection and inhibits HCV replication [9]. ARB exerted even significantly antiviral activity against Hantaan virus (HTNV) that reduced pathological changes, decreased viral loads and regulated serum TNF-α levels in infected tissues [13]. ARB inhibits CVB-5 virus replication in vitro and in vivo, the mechanism by which is to block viral adsorption and the late stage(s) of viral replication, thus exerting a long-lasting antiviral effect [14]. In addition, recent data showed that ARB exerts a direct antiviral effect on the early viral replication of SARS virus in GMK-AH-1 cells [15], but its specific mechanism and clinical application still need further study. Perfetto et al. revealed that ARB exhibited the ability to influence HSV-1 replication through reduction of viral expression and modulation of the cytokine pathway [16]. ARB showed a strong therapeutic effect, even better than ACV on HSV-2 infection in the rabbit model of viral keratitis [17]. In two trials, the superior efficacy of ARB compared with ribavirin was demonstrated in patients with influenza virus and the prophylactic use of ARB reduced acute respiratory virus infection and the incidence of secondary viral- bacterial pneumonia [18,19]. Based on ARB's chemical structure, 17 metabolites, of which some may play antiviral and anti-inflammatory roles and could be useful as drug prototypes, could be identified in human urine after administration of a single 300 mg-dose of ARB to healthy people [20]. On the other hand, the clinical value of ARB is underestimated due to its regional application in China and Russia. ARB, with its unique pharmacological action, low toxicity and good tolerability, is considered a promising broad-spectrum antiviral drug.
In previous work, our results clarified the activity of ARB in both suppressing influenza virus propagation and modulating the expression of inflammatory cytokines in vitro and in vivo [21]. To explore the pharmacological activity of different ARB administration methods against other human infectious diseases and to better understand its antiviral mechanism of action to improve its clinical promotion, we first evaluated the in vitro efficacy of ARB against HSV-2 in nontoxic concentrations and determined its potential antiviral mechanisms. We then accessed the effect of ARB on pathological morphology, inflammatory cytokine levels, and changes in CD4+ and CD8+ peripheral blood T lymphocyte subsets in a mouse vaginitis model with HSV-2.
Therefore, this study will provide more support that ARB could be used clinically to provide some protection against other STDs, such as herpes, and take to prophylactically treat patients to decrease the risk of HIV acquisition.
2. Materials and methods
2.1. Cell culture and virus preparation
An African green monkey kidney cell line (Vero cells) was purchased from the American Tissue Culture Collection (ATCC) and was cultured using Dulbecco's modified Eagle's medium (DMEM; Gibco) supplemented with 10% fetal bovine serum (FBS) and 1% glutamine at 37 °C in a humidified atmosphere of 5% CO2. Cells were grown as a monolayer with a complete medium change every 2–3 days, and then trypsinized and seeded in a variety of microplates in serum-free medium for each experiment.
The HSV-2 virus (333 strains) gifted from the Institute of Tropical Medicine in Guangzhou University of Chinese Medicine was passaged and titred in Vero cells in our laboratory and stored at −80 °C for further use.
2.2. Preparation of ARB stock
ARB (Lot No. 20140204) was supplied by Shijiazhuang NO.4 Pharmaceutical Co. Ltd., Hebei, China. Acyclovir (ACV, Lot No: LRAA 9058) was purchased from Sigma Company, USA. ARB powders and ACV were dissolved at 10 mg/mL in dimethyl sulfoxide (DMSO), filtered through a 0.22 μm membrane and stored at 4 °C. If necessary, DMEM was used to dilute the substances to a suitable concentration.
Ointment was prepared as previously described with modifications [22,23]. 80 mg ARB powder was extracted three times with 10 mL 100% ethanol. Then, the all ethanolic extracts were combined and concentrated at 70 °C. Simultaneously, 40 mg of Vaseline, followed by 40 mg of olive oil, was melted in another beaker as the oily phase of the mixture. The extract was heated to approximately 70 °C, and the hot solution was slowly added to the oily phase with continuous stirring until the mixture cooled to form a 50% ARB ointment.
The average body weight of the mice was calculated to be 16 g according to the experimental rules, and approximately 0.8 mg of ARB extract (namely, 1.6 mg ARB ointment) was administered vaginally daily, so the dosage of ARB ointment was assumed to be 100 mg/kg/d.
2.3. Toxicity test (MTT)
Vero cells were seeded into 96-well plates at 2 × 104 cells per well [24]. Once the cells were grown into confluent monolayers, the culture was aspirated, washed with PBS twice, and the drugs were added at 100 μL/well (2-fold dilution). Then, the cells were incubated at 37 °C and 5% CO2 for 3 days, MTT solution (5 mg/mL) was then added, and the cells were incubated at 37 °C and 5% CO2 for an additional 4 h. The supernatant was discarded, and 100 μL of dimethylsulfoxide (DMSO) was added to each well. The absorbance was measured at 490 nm using a microplate reader. The 50% toxicity concentration (TC50) was calculated using the Reed-Muench method [25].
2.4. Antiviral effect assay
The antiviral activity of ARB was detected with the cytopathogenic efficiency (CPE) method and the MTT assay. Drugs were dissolved in DMEM medium and serially diluted to seven concentrations. Vero cells were inoculated into 96-well plates, grown to confluency (approximately 24 h) and then washed twice with PBS.
For the treatment assay, 100 μL of diluted HSV-2 (100 TCID50) was added to each well. After incubation at 37 °C and 5% CO2 for 2 h, the supernatant was discarded, and the drug was added to the wells for incubation at 37 °C and 5% CO2 for another 72 h. The 50% inhibitory concentration (IC50) was calculated with the Reed-Muench method. The pretreatment assay [26] was performed with cells treated with ARB at the indicated concentration for 2 h at 37 °C before infection. For the direct action assay [27,28], the virus was preincubated with ARB at the indicated concentrations for 2 h before being added to the cell culture. Both infections were incubated at 37 °C in 5% CO2 for 2 h, and the medium was then aspirated. Cells were rinsed with PBS and incubated with medium for another 72 h. Finally, the supernatant was collected for the TCID50 assay.
The attachment assay described by De Logu et al. [29] was performed in this study with a minor modification. The cell monolayer was infected with virus in the presence of serial dilutions of ARB at the indicated concentrations and incubated at 4 °C for 2 h. After that, the medium was aspirated to remove free virus. The infected cells were then washed with PBS three times and added to medium for further 72 h incubation. The supernatant was collected for the TCID50 assay.
According to published papers [24,26], the penetration assay of HSV-2 into Vero cells was performed with minor modifications. The cell monolayer was infected with virus and incubated at 4 °C for 2 h to allow the attachment of HSV-2. Then, ARB was added at the indicated concentrations, and the culture was incubated at 37 °C for 3 h to allow the entry of the virus. The infected cell monolayer was treated with PBS (pH 3) for 1 min to inactivate nonpenetrated virus. Subsequently, the PBS was removed, and fresh medium was added to the infected cells. After a further 72 h of incubation, the supernatant was collected to determine the virus titers by the TCID50 assay.
2.5. Time-course assay
To determine the possible stage(s) of the viral life cycle targeted by ARB, a time-course assay was performed as previously described [26] with minor modifications. Vero cells were seeded in 24-well plates at a density of 1.0 × l05 cells/mL. The cells were cultured at 37 °C and 5% CO2 until the cells grew into monolayers and then were washed twice with PBS. Vero cell monolayers were infected with HSV-2 (100 TCID50) and then treated with ARB at the indicated time points. After incubation at 37 °C and 5% CO2 for 24 h, the virus supernatant and cells were harvested, and virus yields were determined by the TCID50 method.
2.6. In vivo antiviral activity of ARB
To evaluate the anti-HSV-2 activity of ARB in vivo, in this study, we established a mouse vaginitis model by vaginal infection with the HSV-2. All animal experiments were performed in the Animal Research Center at the Guangzhou Institute of Respiratory Health. Female SPF Kunming mice (20–22 g) were randomly divided into 6 groups with 10 mice in each group as follows: normal group, virus group, ACV-treated (100 mg/kg/d) group, ARB-treated (100 mg/kg/d) group, ARB ointment-treated (100 mg/kg/d) group and ointment vehicle group. Progesterone (0.1 mL) was injected subcutaneously 5 days prior to infection, making animals enter the estrous cycle, which increases susceptibility to HSV-2 [30]. The mice in the ARB treatment groups were treated with ARB (100 mg/kg/d) orally or intravaginally, twice per day for 10 days; the ARB ointment was applied with a micropipette and spread over the infected site. ACV (100 mg/kg/d) was given orally according to the manufacturer's instructions. The mice in the normal and negative groups were administered equivalent amounts of saline. The ointment vehicle group was treated with an equivalent amount of control ointment after infection. 50 μL HSV-2 solutions (106 PFU/mL) were used to infect in the vaginal surface area, while saline was administered to the normal control. The mice in each group were observed daily for 15 days to calculate the survival rate, death protection, survival time and body weight change.
For the infection assay experiment, vaginal lavages were obtained 3 days after infection and evaluated for viral shedding. As reported in the literature, input virus could not be detected within 6 h of inoculation, and viral shedding by infected animals reached a maximum at 3 days. 40 μL precooled DMEM was delivered to the vagina and pipetted in and out 12 times to maximize viral recovery. The vagina was rinsed twice, and the collected vaginal lavages were mixed. To remove any drug that might remain in the lavage fluid, the mixed lavage fluid was ultrafiltered with an Amicon Ultra ultrafiltration tube. All the liquid in the centrifuge tube was recovered. The total volume was adjusted to 150 μL with DMEM in a 1.5 mL tube [31]. The supernatant was then placed on Vero cells to determine the viral titer by TCID50 according to the Reed-Muench method. Cytopathic effect (CPE) was scored 48 h later, and mice whose lavage cultures displayed CPE were considered infected. This assay is more rapid and sensitive than the observation of visible lesions or death, both of which are more dependent on the hormonal and immune status of the mice [32].
To further determine whether ARB could affect the immune status of the mice, the spleen was scored by the spleen index. After ARB treatment, mouse body weight was recorded, and the mice were sacrificed to dissect their spleens. After rinsing with 0.9% saline and drying with absorbent paper, the spleen was weighed. The spleen index and spleen index inhibition rate were calculated according to the following formula [33].
Groups of four other mice were set up for histopathological experiments. The animals in each group were sacrificed on day 5 post viral exposure before dissecting out the vagina. The vagina were harvested and fixed in 4% paraformaldehyde solution. Subsequently, the tissues were processed for paraffin embedding and cut into 4-μm-thick sections for standard hematoxylin and eosin (H&E) staining.
2.7. Detection of cytokines by ELISA assay
After finishing treatment (5 days after infection), the blood was immediately collected from three randomly selected mice from each group, and the serum obtained by centrifugation at 4000 r/min, 4 °C for 10 min was used for the detection of serum cytokines. The levels of pro-inflammatory cytokines (IL-2, IL-4, TNF-α and TGF-β) were evaluated using enzyme-linked immunosorbent assays (ELISA) (USA R&D Systems) used according to the manufacturer's instructions. The OD at 450 nm was read immediately using a microtiter plate reader. The serum was aliquoted into an EP tube and stored at −20 °C. Samples were not repeatedly frozen and thawed.
2.8. Effect on T lymphocyte subsets in the peripheral blood of mice
The animal model was established as described in Section 2.6. On the 5th day after infection, the peripheral blood was taken from the mouse eyeball by retro-orbital puncture. 100 μL of blood was incubated with FITC-labeled anti-mouse CD4 monoclonal antibody (eBioscience, 11-0042-82), PE-labeled CD8 anti-mouse monoclonal antibody (eBioscience, 17-0081-82) and PE-Cyanine7- labeled anti-mouse CD3e monoclonal antibody (eBioscience, 25-0031-82) for 30 min in the dark. After washed 3 times with PBS, the stained blood was incubated with red blood cell lysis buffer for 15 min; then, the blood was analyzed for the percentage of CD4+ and CD8+ cells by flow cytometry. The experimental results were analyzed using FlowJo software.
2.9. Statistical analysis
The data were processed using GraphPad Prism 5 and expressed as the means ± S.D. Statistical differences between 2 groups were determined using Student's t-test. For multiple groups, one-way ANOVA analysis was used to compare the means. p < 0.05 was considered as a significant difference between groups.
3. Results
3.1. Cytotoxicity of ARB on Vero cells
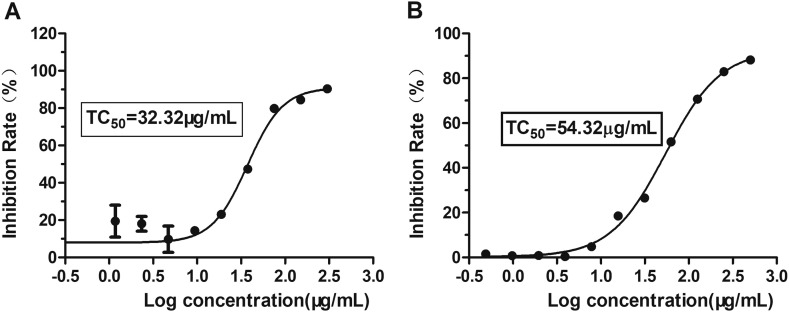
While growing in monolayers, Vero cells were treated with ARB at concentrations of 300, 150, 75, 37.5, 18.750… 0.293 μg/mL to investigate ARB cytotoxicity. After incubation for 72 h, cell viability was evaluated by CPE and MTT assays. ARB showed toxicity, and the 50% toxic concentration (TC50) of ARB on Vero cells was 32.32 μg/mL (Fig. 1A), which was calculated by regression analysis of the dose-viability curve. Similarly, the TC50 of ARB in the cell pretreatment assay and the virus pretreatment assay was 54.32 μg/mL when the drug was incubated for 2 h (Fig. 1B). Therefore, 25 μg/mL was adopted as the experimental maximum concentration, and had a >70% cell survival rate.
Fig. 1.
The toxicity of ARB on Vero cells. MDCK cells were treated with ARB at the indicated concentrations for 72 h (A) and 2 h (B, in the cell pretreatment assay and the virus pretreatment assay). The TC50 of ARB was determined by using MTT assay. Each spot is representative of three independent experiments. Data are expressed as the mean ± S.D.
3.2. Antiviral activity of ARB on HSV-2 in vitro
The antiviral efficacy of ARB against HSV-2 in vitro was first evaluated by MTT assay. With continuous ARB presence at 2 h post infection, a dose-dependent inhibition of infection was observed with a 50% inhibitory concentration (IC50) value of 4.77 μg/mL (Fig. 2 ) (SI = 6.82). Additionally, the effect of the positive control drug ACV against HSV-2 was investigated through comparison with the virus control group, and showed a good protective effect with an IC50 value of 33.135 μg/mL (SI = 5.91) (Table 1 ).
Fig. 2.
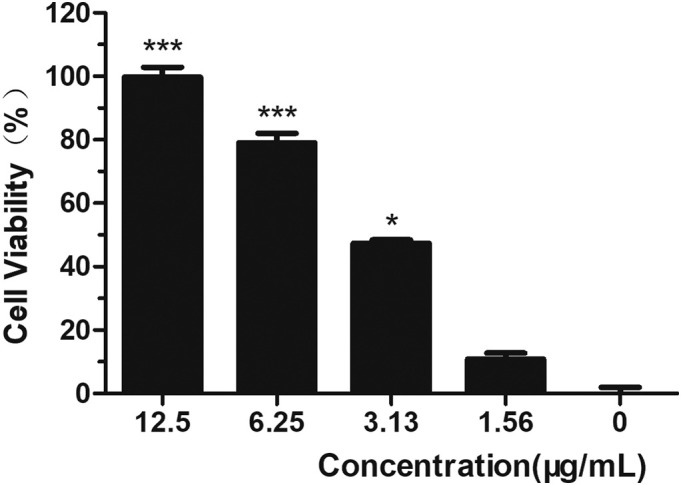
Cell viability of ARB on HSV-2 in vitro in dose-dependent manner.
Table 1.
IC50 values of ARB on Vero cells as determined by the CPE method and MTT assay.
| Drugs | TC50 (μg/m) | HSV-2 (CPE assay) |
HSV-2 (MTT assay) |
||
|---|---|---|---|---|---|
| IC50 (μg/mL) | SIa | IC50 (μg/mL) | SIa | ||
| ARB | 32.605 ± 0.02 | 5.045 ± 0.33 | 6.46 | 4.77 ± 0.29 | 6.82 |
| ACV | 195.64 ± 11.37 | 38.30 ± 6.67 | 5.11 | 33.135 ± 7.01 | 5.91 |
IC50 (μg/mL) values and TC50 (μg/mL) values were predicted according to the best fitting nonlinear regression formula calculated for each set of data.
“SI” is the abbreviation of “the selection index”. SI > 2 means low toxicity; 2 > SI > 1 means high toxic and low effect; SI ≤ 1 means no effect.
Some drugs can exert their antiviral activities by modifying membrane components of target cells [34] or directly targeting the virus [24]. To elucidate whether these are possible mechanisms for the anti-HSV-2 activity of ARB, cells and viruses were pretreated with ARB before the incubation for the virus yield assay. The results indicated that pretreatment of Vero cells with ARB at a concentration of 25 μg/mL did not significantly inhibit HSV-2 replication (IC50 > 32 μg/mL, SI < 1, data not shown). In addition, pretreatment of HSV-2 with 25 μg/mL ARB did not profoundly depress progeny viral yield (IC50 > 50.85 μg/mL, SI < 1, data not shown). These results suggested that the antiviral activity of ARB did not exist in cells or viruses before viral entry.
3.3. ARB markedly inhibits the late stage of the HSV-2 replication cycle
To characterize the specific stage(s) of the HSV-2 replication cycle impeded by ARB, we performed a time-of-addition assay to characterize its inhibitory actions in vitro. Briefly, ARB was removed from Vero cells at the indicated time after virus addition: 3 h p.i., 6 h p.i. and 24 h p.i. Subsequently, the progeny virus yield was determined by the TCID50 method [35]. As shown in Fig. 3 , ARB inhibited progeny virus production by up to approximately 40% (p < 0.01) when treatment lasted 24 h, while no significant reduction of progeny virus was observed when ARB was removed after 3 h p.i. and 6 h p.i. (p > 0.05).
Fig. 3.
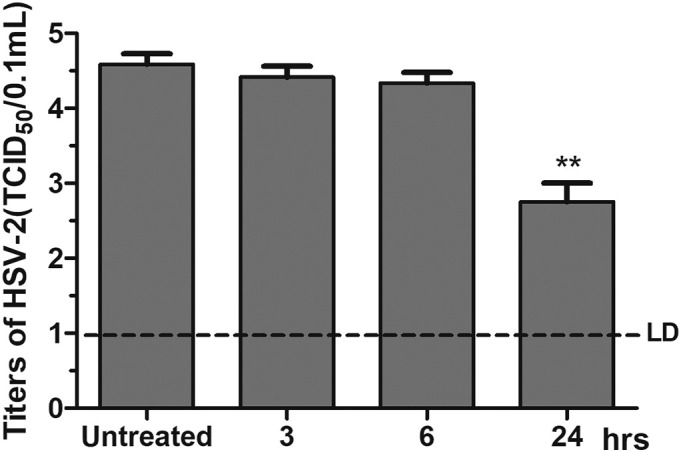
Time-course analysis of ARB inhibitory effects on HSV-2 virus replication. Vero cells infected with 100 TCID50 HSV-2 virus were treated with ARB (25 μg/mL). Then, ARB was removed at 3 h p.i., 6 h p.i. and 24 h p.i.. After freezing and thawing three times, the supernatants were collected and determined virus titers by TCID50 assay. Each value is represented as the mean ± standard deviation from three individual experiments (⁎⁎p < 0.01).
The results revealed that ARB probably inhibited viral assembly or release in the late step of the HSV-2 replication cycle unlike ACV, which inhibits the virus-encoded thymidine kinase and DNA polymerase activity at the middle step of the life cycle [36].
3.4. Effect of ARB on HSV-2 attachment and entry
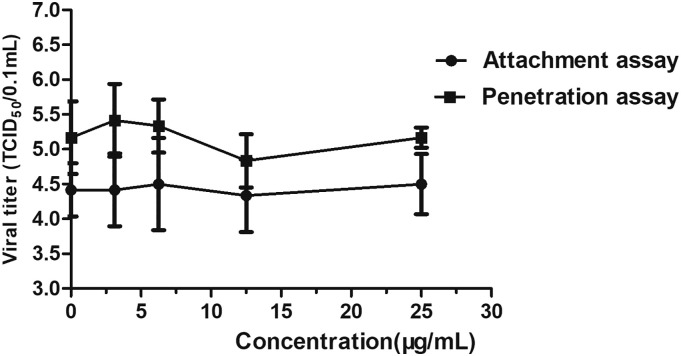
According to the results of the time-of-addition assay, ARB affected the late stage of HSV-2 infection. To further verify whether ARB blocks HSV-2 replication at the early stage, the attachment and entry assays for HSV-2 were individually carried out with ARB at 25 μg/mL. As shown in Fig. 4 , the virus yield from both HSV-2 attachment and penetration assays was not significantly decreased at an ARB concentration of 25 μg/mL. These results are consistent with the hypothesis that viral inhibition occurs in the late stage of the replication cycle.
Fig. 4.
Effect of ARB treatment on the attachment and penetration of HSV-2.
For the attachment assay, the cell monolayer was infected with 100 TCID50 HSV-2 in the absence or presence of ARB at the indicated concentrations and incubated at 4 °C for 2 h. For the penetration assay, the cell monolayer was infected with virus and incubated at 4 °C for 2 h. Then, ARB was added at the indicated concentrations for another 2 h of incubation. The infected cell monolayer was treated with PBS (pH 3) for 1 min. Fresh medium was then added to remove acidic PBS for 6 times. After that, fresh medium was added for further 72 h incubation. The supernatant was collected to determine the virus titers by the TCID50 assay. Each point represents the mean ± S.D. for three independent experiments.
3.5. In vivo efficacy of ARB against the HSV-2 infected mouse vaginal epithelium
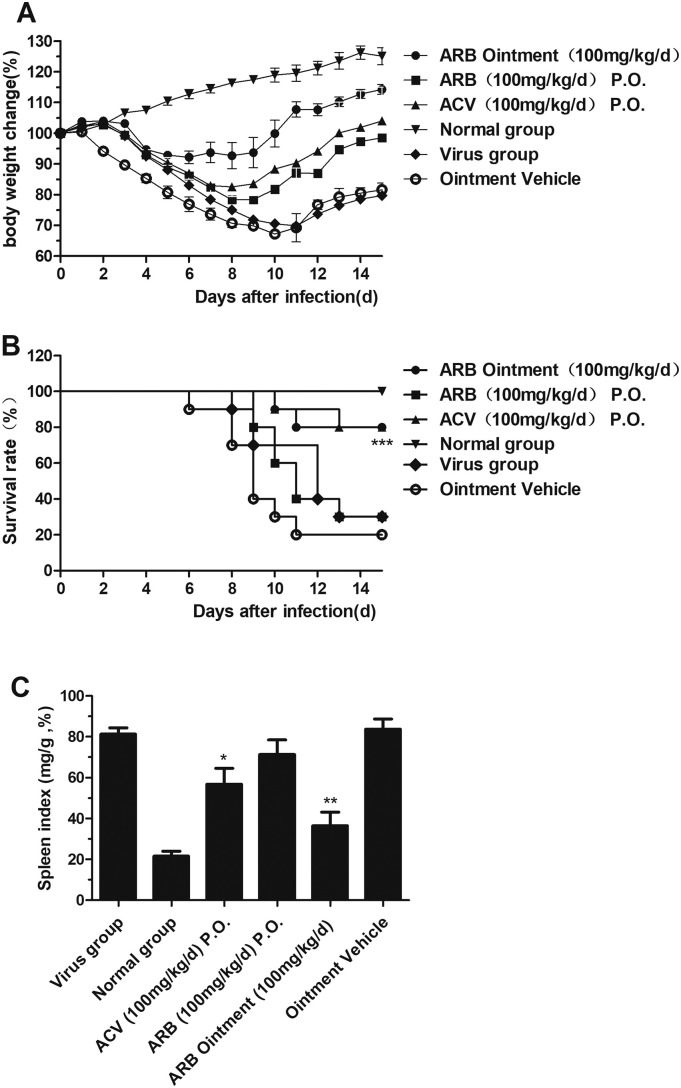
To evaluate the anti-HSV-2 activity of ARB in vivo, we tested the drug in a mouse model of vaginitis. The mice were observed daily, and symptom onset, body weight, survival time and the number of deaths were recorded for 15 days. The survival rate of mice administered ARB ointment intravaginally (100 mg/kg/d) was 80% when therapy was initiated 5 days before viral inoculation, which was unexpectedly the same as in the ACV-treated group (Fig. 5B and Table 2 ). ARB ointment (100 mg/kg/d) also inhibited the decrease in body weight (Fig. 5A) and suppressed the increase in mouse spleen index compared to the ointment vehicle group (Fig. 5C). Interestingly, the average survival time, average body weight change and spleen index showed no significant differences compared to the virus group (p > 0.05) when ARB (100 mg/kg/d) was orally administered.
Fig. 5.
Antiviral activity of ARB against HSV-2 infection in a mouse vaginitis model. (A) Body weight change of mice after HSV-2 infection. (B) Survival analysis of HSV-2-infected and ARB-treated Kunming mice. (C) Spleen index was calculated as ratio of spleen weight (mg/mouse) to body weight (g/mouse) on day 5 d.p.i. Asterisks indicate statistical differences compared the virus group (⁎p < 0.05, ⁎⁎p < 0.01, ⁎⁎⁎p < 0.001).
Table 2.
Effect of ARB treatment on the protection of mice infected intravaginally with HSV-2.
| Group | Survivors/total | Protection index | Average survival time (d) |
|---|---|---|---|
| ARB ointment (100 mg/kg/d) | 8/10⁎⁎⁎ | 71.4% | 14.1 ± 1.91 |
| ARB (100 mg/kg/d) | 3/10 | 0% | 11.8 ± 2.49 |
| ACV (100 mg/kg/d) | 8/10⁎⁎⁎ | 71.4% | 14.3 ± 1.64 |
| Virus group | 3/10# | – | 12.0 ± 2.62 |
| Normal group | 10/10 | 100% | >15.0 |
Note: Drug group compared with virus group: ⁎⁎⁎p < 0.001. Virus group compared with normal group: #p < 0.05.
3.6. Effect of ARB on viral titers in vaginal lavage fluid in a mouse vaginitis model
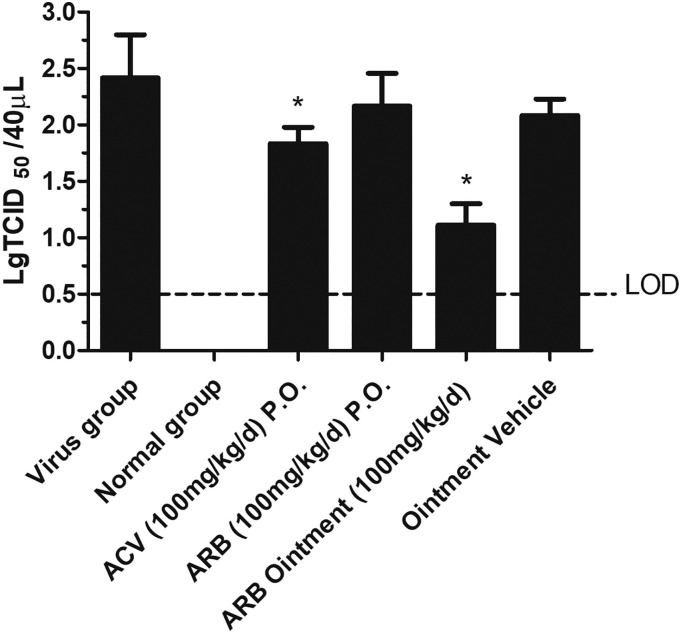
The progeny virus replication in the vagina was then assessed in female mice infected with HSV-2. Following intravaginal infection with 50 μL HSV-2, vaginal lavage fluid was collected by washing repeatedly with precooled DMEM at 3 d.p.i to quantify virus titers by the TCID50 assay (Fig. 6 ). Consistent with the ACV-treated group, ARB ointment (100 mg/kg/d) could significantly attenuate progeny virus replication in the infected vagina with statistical significance compared to the ointment vehicle group (p < 0.05), whereas HSV-2 was present at similarly high titers in the ARB oral treatment group and the virus group (p > 0.05) (Fig. 6).
Fig. 6.
Replication of HSV-2 after ARB ointment treatment is markedly attenuated in mice vaginitis model compared to that of mice in the virus group. The asterisks refer to the level of significance: ⁎p < 0.05.
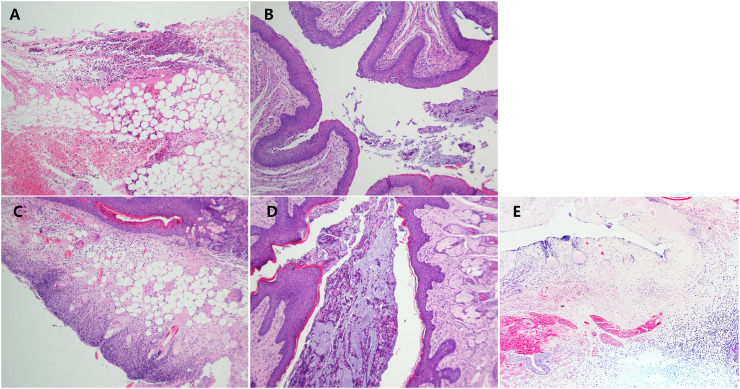
3.7. ARB ointment alleviated vaginal pathology induced by HSV-2 infection
On day 3 post infection, many neutrophils, necrotic areas and lymphocyte aggregates microscopically were observed in the vaginal submucosa and adipose tissue in the mice of the virus group and the ointment vehicle group (Fig. 7A and E). As expected, unapparent vaginal mucosal lesions were observed in the ARB ointment-treated group (Fig. 7D), with a few neutrophil infiltrations and hemorrhages in the mucosal layer, which was not significantly different from the ACV-treated group (Fig. 7B). It is also noticeable that in the vaginal tissue of female mice treated orally with 100 mg/kg/d of ARB, the lesions were as serious as the severe acute purulent inflammation with necrosis in the vaginal epithelium seen in untreated infected tissue (Fig. 7C). These results clearly demonstrated the potent activity of ARB ointment (100 mg/kg/d) against vaginitis caused by HSV-2 infection in vaginal tissue.
Fig. 7.
ARB ointment improved vaginal epithelial histopathological changes induced by HSV-2 infection. A: Virus group; B: ACV-treated group (100 mg/kg/d); C: ARB-treated group (100 mg/kg/d); D: ARB ointment-treated group (100 mg/kg/d). Sections are histopathologic findings by hematoxylin and eosin-stained (H&E stain) for vaginal tissues isolated from HSV-2-infected female mice at 3 d.p.i. Magnification 100×.
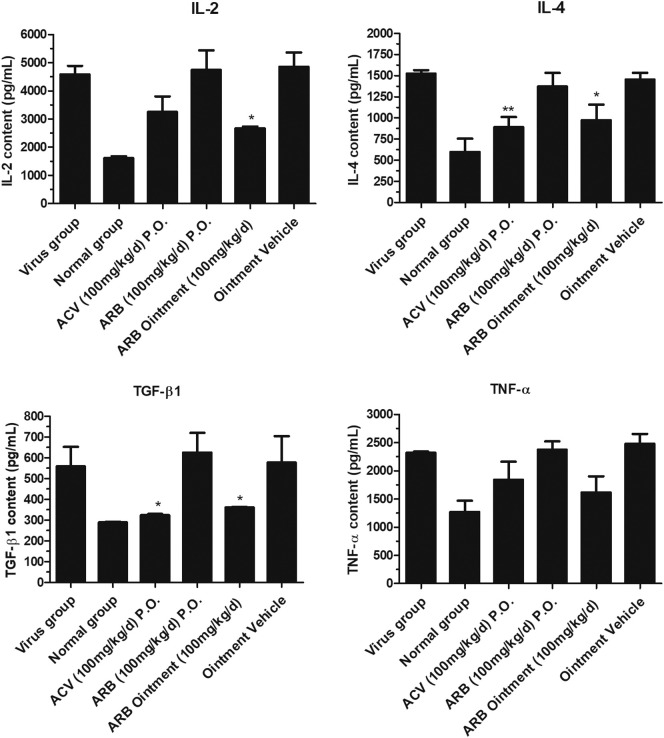
3.8. Effects of ARB on inflammatory cytokines in the peripheral serum of mice infected with HSV-2
To evaluate whether ARB affects virus-induced inflammatory cytokines, ELISA assays for IL-2, IL-4, TNF-α and TGF-β were conducted on day 5 post-infection. As shown in Fig. 8 , IL-2, IL-4 and TGF-β were significantly downregulated following infection with HSV-2 and posttreatment with ARB ointment (100 mg/kg/d) compared to the levels in the mice in the virus group and the ointment vehicle group (p > 0.05). However, the level of TNF-α was showed no significant difference (p < 0.05).
Fig. 8.
The expression levels of IL-2, IL-4, TGF-β and TNF-α in the peripheral blood in mice infected with HSV-2 after ARB treatment. Female mice were infected intravaginally with HSV-2 virus and serum was collected from peripheral blood in each experimental group on day 5 post infection. The supernatants were determined by ELISA assay (compared with virus group, ⁎p < 0.05, ⁎⁎p < 0.01).
3.9. ARB ointment significantly regulated CD4 and CD8 T lymphocyte subsets in the peripheral blood
To assess the influence of ARB ointment on the T cell subgroups in peripheral blood, we measured CD3+CD4+ and CD3+CD8+ T cell populations in the peripheral blood of mice treated with drugs or left untreated by flow cytometry. A significant higher ratio of CD4+/CD8+ T cells was found in the peripheral blood of the ARB ointment-treated group and ACV-treated group than in the virus group (Table 3 ).
Table 3.
The effect of ARB on T cell subgroup in blood mice ( ± S, n = 4).
| Group | Dose (mg/kg/d) | CD4+ T cell (%) | CD8+ T cell (%) | CD4+/CD8+ |
|---|---|---|---|---|
| Virus group | – | 55.750 ± 4.352 | 31.725 ± 5.899 | 1.786 ± 0.214 |
| Normal group | – | 62.550 ± 0.035 | 25.825 ± 2.567 | 2.438 ± 0.243 |
| ACV P.O. | 100 | 43.875 ± 5.775 | 19.75 ± 2.922 | 2.230 ± 0.183* |
| ARB P.O. | 100 | 50.150 ± 11.841 | 27.175 ± 8.178 | 1.873 ± 0.188 |
| ARB ointment | 100 | 64.325 ± 3.685 | 30.675 ± 2.822 | 2.104 ± 0.130* |
| Ointment vehicle | – | 44.925 ± 7.587 | 28.300 ± 4.799 | 1.591 ± 0.090 |
Note: Compared with virus group: ⁎p < 0.05.
4. Discussion
HSV is one of the pathogens that causes the most common sexually transmitted infections (STIs). Infection always leads to some severe diseases, such as genital herpes [37], vaginitis [37], and encephalitis [7], and increases the risk of HIV acquisition [38] so that HSV-2 infection has been recognized as an important marker for HIV-1 infection. HSV persists indefinitely, can be shed for years and can reactivate, which is probably one of the reasons that the prevalence of genital herpes in developing countries has increased in recent decades [39]. Therefore, the development of novel antiviral strategies is critically required for counteracting the emergence of HSV-2. Previous studies have shown that ARB has an inhibitory effect on enveloped and nonenveloped RNA and DNA viruses, including some variant strains of amantadine and oseltamivir [12,40,41], by targeting different the stages of virus replication cycle [42]. We have recently reported the activity of ARB in suppressing influenza A virus (H1N1) propagation in vitro [21]. Furthermore, in this paper, we found that a nontoxic concentration of ARB is effective for blocking the cytopathic effect induced by HSV-2 in a dose-dependent manner in the late stage of the virus replication cycle but has no direct inactivating effect on HSV-2 itself nor does it interfere with the adherence of the virus to the cell surface, which is consistent with the data documented for HSV-1 virus [43], HCV virus [9] and Hantaan virus [13]. Based on the results, we speculate that ARB is most likely to affect the assembly and release of progeny virus; however, further study is necessary to explain specific mechanism impacting the replication cycle.
Weight changes, survival rates and life protection rates are important indicators for evaluating the efficacy of antiviral drugs. In a recent study, ARB could ameliorate the development of skin lesions caused by HSV-1 in cutaneous infected guinea pig models [43]. Hai-ying Deng. et al. also demonstrated that oral administration of ARB increased both survival rate and mean time to death (MTD) in mice infected with HTNV virus [13]. In our study, however, oral administration of ARB only slightly improved the weight loss, survival rate and release of virus particles at the infected vaginal site caused by HSV-2 infection, and showed no significant difference compared to the virus group. This may be because the distribution of the drug in mice was rapid and extensive after intragastric administration of ARB, and the maximum concentrations of drug mainly appear in the digestive tract such as stomach, small intestine, and large intestine, followed by the target organs such as lung, spleen and liver, but the concentration in the genitalia is low due to the drug's pharmacokinetics [17]. It is also likely that ARB, rather than its metabolites, exerts antiviral and anti-inflammatory effects. Based on this, we specifically prepared ARB ointment for the treatment of vaginal infection sites. Interestingly, the results showed that ARB ointment could significantly alleviate vaginal pathology, increase the survival rate, prolong the survival time, and delay the weight loss induced by HSV-2 viral infection as well as inhibit the release of progeny virus.
Activating the immune response is an efficient way to interfere with viral infection, as this system is responsible for virus elimination. Cytokines play an important role in the activation of immune system, the regulation of the immune responses and the promotion of viral clearance [44]. Suppression of these cytokines can potentially control the severity of virus-induced inflammatory complications and essentially reduce mortality. Recent studies have found that most patients infected with HSV, especially those with recurrence, have an immunologic defect associated with lacking T cells [45]. Perfetto B. et al. further noted that ARB could regulate the HSV-induced overexpression of cytokines such as IL-6, TNF-α and TGF-β [16]. Previously, we reported that several proinflammatory cytokines induced by influenza (IL-10, TNF-α, IL-8, IP-10, MCP-1, RANTES and IL-6) were downregulated by posttreatment with ARB in mice and ferrets [21]. Therefore, we hypothesize that ARB may suppress HSV-2 infection by modulating the expression of inflammatory cytokines (IL-4, IL-2, TGF-β) because it could act as an immunomodulator to drive the host cells to exert an antiviral response against HSV-2.
It has been documented that the spleen is the largest peripheral immune organ and whose structure directly affects the host's immune function. To a certain extent, the spleen index is one of the important indicators of immune function, and its value change can reflect the state of the body's immune function after hypersensitivity occurs [46]. In this paper, the results showed that the spleen index values at 5 d.p.i. of the HSV-2-infected mice significantly increased compared with those of mice in the normal control group (p < 0.001), indicating that the immune function of HSV-2-infected mice was compromised; however, this is in contrary to previous reports [47]. This may be due to the difference in infection time. We calculated the spleen index on the 5th day after infection; however, the document indicated that at the early stages of viral infection, the immune function was disordered due to the infection, resulting in compensatory hyperplasia of the spleen [48], so that the spleen index values of the virus group and the ointment vehicle group were higher than that of the normal group. With the prolongation of the infection time, the condition of the infected mice gradually increased and the spleen atrophied. Another reason that might explain this difference is the different routes of infection. We directly inoculated the vagina with HSV-2, which activated the immune system earlier than oral administration. Based on our results, ARB ointment can delay the compensatory hyperplasia of the spleen and weight loss caused by HSV-2, indicating that ARB ointment can effectively modulate the body's immune function, balance the body's cytokine levels and suppress the disorders caused by HSV-2 infection.
The elimination of viral antigens mainly relies on cellular immunity and humoral immunity, especially for the clearance of intracellular viruses. Among the immune participants, CD4+ T cells and CD8+ T cells play an important role. Studies have shown that in the absence of HSV-2 antibodies, initiation of replication-deficient virus-induced T cell responses may be effective against HSV-2 infection; CD4+ T cells are essential, and their secreted cytokines can promote CD8+ T cells enter into HSV-2-infected tissues [49]. Jia Zhu et al. found that when genital tract subclinical HSV-2 infections recur, virus-specific CD8+ T cells will accumulate around the sensory nerves of the genital tract [50], indicating that CD8+ T cells play an important role in clearing the activation virus. At the same time, the related cytokines secreted by CD4+ and CD8+ T cells may be involved in cellular immune responses during viral infection. Our results showed that the ratio of CD4+/CD8+ T cells in the peripheral blood of the ointment vehicle group and the virus group was lower than in the normal group. Interestingly, the ratio of CD4+/CD8+ is significantly higher after treatment, suggesting that ARB ointment exerts an immunomodulatory effect that enhances the immune function to eliminate HSV-2 in the host and prevents the recurrence of genital herpes.
Taken together, our results suggested that ARB potently possessed antiviral and immunomodulatory activities simultaneously, which blocked the proliferation of HSV-2 in a mouse vaginitis model. Moreover, we successfully prepared ARB ointment that could significantly reduce the expression of virally induced cytokine resulted in tissue damage. Consistently, immunohistochemistry analysis showed that ARB ointment dramatically attenuated the recruitment of inflammatory factors in vaginal epithelium. Despite this, the precise molecular mechanism through which ARB regulates host immunity remains unclear and the ointment preparation used in this study is not yet stable for clinical application. Therefore, more studies will be required to focus on the development of a topical formulation with, hopefully, more effectiveness and stability of ARB as a promising anti-HSV-2 drug. In addition, the degree to which these findings can be extended to other HSV-related disease but keratitis [17] and vaginitis requires further investigation.
Competing interests
The authors declare no conflict of interest.
Acknowledgments
Acknowledgements
This work was supported by the Ministry of Science and Technology of the People's Republic of China, China (Grant no. 2015DFM30010); the Science and Technology Development Fund in Macao Special Administrative Region, China (Grant no. 600 084/2015/A); the National Natural Science Foundation of China, China (Grant no. 81703788); the Engineering Technology Research Center (Development) 602 of Guangdong General Universities (GCZX-A1408). We thank Shijiazhuang No.4 Pharmaceutical Co., Ltd. and Jiangsu Wuzhong Pharmaceutical China Co., Ltd. for providing with ARB drug.
Author contributions
Zifeng Yang and Liehua Deng conceived and designed the study. Qiuling Du, Zhen Gu, Haiming Jiang and Runfeng Li performed the experiments. Qiuling Du, Zhen Gu and Irina Leneva analyzed data; Qiuling Du and Irina Leneva contributed to the drafting of the manuscript. All authors reviewed the manuscript.
Contributor Information
Liehua Deng, Email: liehuadeng@126.com.
Zifeng Yang, Email: jeffyah@163.com.
References
- 1.Schryver D., Meheus Epidemiology of sexually transmitted diseases: the global picture. Bull. World Health Organ. 1990;68:639–654. [PMC free article] [PubMed] [Google Scholar]
- 2.S.t.i. (STIs) 2017. http://www.who.int/en/
- 3.Kaufman H.E., Azcuy A.M., Varnell E.D., Sloop G.D., Thompson H.W., Hill J.M. HSV-1 DNA in tears and saliva of normal adults. Invest. Ophthalmol. Vis. Sci. 2005;46:241–247. doi: 10.1167/iovs.04-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith J.S., Robinson N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: a global review. J. Infect. Dis. 2002;186(Suppl. 1):S3–28. doi: 10.1086/343739. [DOI] [PubMed] [Google Scholar]
- 5.Looker K.J., Garnett G.P., Schmid G.P. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull. World Health Organ. 2008;86:805–812. doi: 10.2471/BLT.07.046128. (a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman E.E., Weiss H.A., Glynn J.R., Cross P.L., Whitworth J.A., Hayes R.J. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 7.Whitley R.J., Lakeman F. Herpes simplex virus infections of the central nervous system: therapeutic and diagnostic considerations. Clin. Infect. Dis. 1995;20:414–420. doi: 10.1093/clinids/20.2.414. [DOI] [PubMed] [Google Scholar]
- 8.Piret J., Boivin G. Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management. Curr. Opin. Infect. Dis. 2016;29:654–662. doi: 10.1097/QCO.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 9.Boriskin Y.S., Leneva I.A., Pecheur E.I., Polyak S.J. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- 10.Teissier E., Zandomeneghi G., Loquet A., Lavillette D., Lavergne J., Montserret R., Cosset F., Böckmann A., Meier B., Penin F., Pécheur E. Mechanism of inhibition of enveloped virus membrane fusion by the antiviral drug arbidol. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0015874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vigant F., Santos N.C., Lee B. Broad-spectrum antivirals against viral fusion. Nat. Rev. Microbiol. 2015;13:426–437. doi: 10.1038/nrmicro3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leneva I.A., Fediakina I.T., Eropkin M., Gudova N.V., Romanovskaia A.A., Danilenko D.M., Vinogradova S.M., Lepeshkin A., Shestopalov A.M. Study of the antiviral activity of Russian anti-influenza agents in cell culture and animal models. Vopr. Virusol. 2010;55:19–27. [PubMed] [Google Scholar]
- 13.Deng H.Y., Luo F., Shi L.Q., Zhong Q., Liu Y.J., Yang Z.Q. Efficacy of arbidol on lethal hantaan virus infections in suckling mice and in vitro. Acta Pharmacol. Sin. 2009;30:1015–1024. doi: 10.1038/aps.2009.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhong Q., Yang Z., Liu Y., Deng H., Xiao H., Shi L., He J. Antiviral activity of Arbidol against Coxsackie virus B5 in vitro and in vivo. Arch. Virol. 2009;154:601–607. doi: 10.1007/s00705-009-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khamitov R.A., Loginova S., Shchukina V.N., Borisevich S.V., Maksimov V.A., Shuster A.M. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr. Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 16.Perfetto B., Filosa R., De Gregorio V., Peduto A., La Gatta A., de Caprariis P., Tufano M.A., Donnarumma G. In vitro antiviral and immunomodulatory activity of arbidol and structurally related derivatives in herpes simplex virus type 1-infected human keratinocytes (HaCat) J. Med. Microbiol. 2014;63:1474–1483. doi: 10.1099/jmm.0.076612-0. [DOI] [PubMed] [Google Scholar]
- 17.Xiao L. Shenyang Pharmaceutical University; 2009. The Study on Pharmacokinetics of Arbidol Hydrochloride. [Google Scholar]
- 18.Wang M.Z., Cai B.Q., Li L.Y., Lin J.T., Su N., Yu H.X., Gao H., Zhao J.Z., Liu L. Efficacy and safety of arbidol in treatment of naturally acquired influenza. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2004;26:289–293. [PubMed] [Google Scholar]
- 19.Shumilov V.I., Shuster A.M., Lobastov S.P., Shevtsov V.A., Mednikov B.L., Piiavskii S.A., Litus V.I. Efficacy of arbidol in prophylaxis and treatment of acute respiratory viral infections in servicemen. Voen. Med. Zh. 2002;323:51–53. (96) [PubMed] [Google Scholar]
- 20.Wang Y., Chen X., Li Q., Zhong D. Metabolite identification of arbidol in human urine by the study of CID fragmentation pathways using HPLC coupled with ion trap mass spectrometry. J. Mass Spectrom. 2008;43:1099–1109. doi: 10.1002/jms.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Y., Ding Y., Yang C., Li R., Du Q., Hao Y., Li Z., Jiang H., Zhao J., Chen Q., Yang Z., He Z. Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret) Biomed Pharmacother. 2017;91:393–401. doi: 10.1016/j.biopha.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 22.Z. Z.-p., Z. F.-l., Y. Xiao-xue. Evaluation of the effect of the ethanol extracts of styela plicata on vaginal infection of mice by HSV-2. Chin. J. Dermatovenereol. 2016;30:242–245. [Google Scholar]
- 23.Maduri B. Sairam, Atla Venkateshwar Reddy. Formulation of colchicine ointment for the treatment of acute gout. Singap. Med. J. 2012;53:750–754. [PubMed] [Google Scholar]
- 24.Su C., Hsu J., Hsieh H., Lin P., Chen T., Kao C., Lee C., Chang S. Anti-HSV activity of digitoxin and its possible mechanisms. Antivir. Res. 2008;79:62–70. doi: 10.1016/j.antiviral.2008.01.156. [DOI] [PubMed] [Google Scholar]
- 25.Brown W.F. Variance estimation in the Reed-Muench fifty percent end-point determination. Am. J. Hyg. 1964;79:37–46. doi: 10.1093/oxfordjournals.aje.a120362. [DOI] [PubMed] [Google Scholar]
- 26.Cheng H., Lin T., Yang C., Wang K., Lin L., Lin C. Putranjivain A from Euphorbia jolkini inhibits both virus entry and late stage replication of herpes simplex virus type 2 in vitro. J. Antimicrob. Chemother. 2004;53:577–583. doi: 10.1093/jac/dkh136. [DOI] [PubMed] [Google Scholar]
- 27.Gong Y., Wen A., Cheung D., Wong M., Sacks S. Preclinical evaluation of docusate as protective agent from herpes simplex viruses. Antivir. Res. 2001;52:25–32. doi: 10.1016/s0166-3542(01)00156-5. [DOI] [PubMed] [Google Scholar]
- 28.J. H.-Z., Deng Hong-Yi, Xu Qian. Experimental study on Fenmo II against Herpes Simplex Virus 2 in vitro. Chin. J. Integr. Tradit. West. Med. 2003;23:177–179. [Google Scholar]
- 29.De Logu A., Loy G., Pellerano M., Bonsignore L., Schivo M. Inactivation of HSV-1 and HSV-2 and prevention of cell-to-cell virus spread by Santolina insularis essential oil. Antivir. Res. 2000;48:177–185. doi: 10.1016/s0166-3542(00)00127-3. [DOI] [PubMed] [Google Scholar]
- 30.Milligan G., Bernstein D. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology. 1995;206:234–241. doi: 10.1016/s0042-6822(95)80038-7. [DOI] [PubMed] [Google Scholar]
- 31.Kratholm S., Iversen M., Reinert L., Jensen S., Hokland M., Andersen T., Rankin A., Young D., Frische S., Paludan S., Holm C. Interleukin-21 receptor signalling is important for innate immune protection against HSV-2 infections. PLoS ONE. 2013;8 doi: 10.1371/journal.pone.0081790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaushic C., Ashkar A.A., Reid L.A., Rosenthal K.L. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 2003;77:4558–4565. doi: 10.1128/JVI.77.8.4558-4565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peihua L.H.C. Progress of genital herpes research and treatment. J. Pract. Dermatol. 2010;3:21–23+27. [Google Scholar]
- 34.Kawamoto S., Oritani K., Asada H., Takahashi I., Ishikawa J., Yoshida H., Yamada M., Ishida N., Ujiie H., Masaie H., Tomiyama Y., Matsuzawa Y. Antiviral activity of limitin against encephalomyocarditis virus, herpes simplex virus, and mouse hepatitis virus: diverse requirements by limitin and alpha interferon for interferon regulatory factor 1. J. Virol. 2003;77:9622–9631. doi: 10.1128/JVI.77.17.9622-9631.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zamoiskii E.A. Evaluation of Reed-Muench method in determination of activity of biological preparations. Zh. Mikrobiol. Epidemiol. Immunobiol. 1956;27:77–83. [PubMed] [Google Scholar]
- 36.Jiang Y., Feng H., Lin Y., Guo X. New strategies against drug resistance to herpes simplex virus. Int. J. Oral Sci. 2016;8:1–6. doi: 10.1038/ijos.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta R., Warren T., Wald A. Genital herpes. Lancet. 2007;370:2127–2137. doi: 10.1016/S0140-6736(07)61908-4. [DOI] [PubMed] [Google Scholar]
- 38.Mehta S.D., Moses S., Parker C.B., Agot K., Maclean I., Bailey R.C. Circumcision status and incident herpes simplex virus type 2 infection, genital ulcer disease, and HIV infection. AIDS. 2012;26:1141–1149. doi: 10.1097/QAD.0b013e328352d116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Looker K.J., Magaret A.S., May M.T., Turner K.M.E., Vickerman P., Newman L.M., Gottlieb S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Health. 2017;5:e300–e309. doi: 10.1016/S2214-109X(16)30362-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burtseva E.I., Shevchenko E.S., Leneva I.A., Merkulova L.N., Oskerko T.A., Shliapnikova O.V., Zaplatnikov A.L., Shuster A.M., Slepushkin A.N. Rimantadine and arbidol sensitivity of influenza viruses that caused epidemic morbidity rise in Russia in the 2004–2005 season. Vopr. Virusol. 2007;52:24–29. [PubMed] [Google Scholar]
- 41.Shi L., Xiong H., He J., Deng H., Li Q., Zhong Q., Hou W., Cheng L., Xiao H., Yang Z. Antiviral activity of arbidol against influenza A virus, respiratory syncytial virus, rhinovirus, coxsackie virus and adenovirus in vitro and in vivo. Arch. Virol. 2007;152:1447–1455. doi: 10.1007/s00705-007-0974-5. [DOI] [PubMed] [Google Scholar]
- 42.Blaising J., Polyak S.J., Pecheur E.I. Arbidol as a broad-spectrum antiviral: an update. Antivir. Res. 2014;107:84–94. doi: 10.1016/j.antiviral.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li M.K., Liu Y.Y., Wei F., Shen M.X., Zhong Y., Li S., Chen L.J., Ma N., Liu B.Y., Mao Y.D., Li N., Hou W., Xiong H.R., Yang Z.Q. Antiviral activity of arbidol hydrochloride against herpes simplex virus I in vitro and in vivo. Int. J. Antimicrob. Agents. 2018;51:98–106. doi: 10.1016/j.ijantimicag.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Sladkova T., Kostolansky F. The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 2006;50:151–162. [PubMed] [Google Scholar]
- 45.Jiyun C.J.Z.W.W.R.T. Serum levels of IFN-γ, IL-2, IL-4 and IL-10 in patients with genital herpes and their significance. China J. Lepr. Skin Dis. 2007;23:968–969. [Google Scholar]
- 46.G. J.-h., Jia H.E., Liang Dong, Shi Su-yu. Influence of spleen-strengthening and lung-tonifying liquid on spleen index and thymus index of COPD in rats with Lung and spleen qi deficiency syndrome. Hainan Med. J. 2014;25:1412–1414. [Google Scholar]
- 47.Yang S., Niu S., Guo Z., Yuan Y., Xue K., Liu S., Jin H. Cross-protective immunity against influenza A/H1N1 virus challenge in mice immunized with recombinant vaccine expressing HA gene of influenza A/H5N1 virus. Virol. J. 2013;10:291. doi: 10.1186/1743-422X-10-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Qian L.X., Song Wu. Vol. 23. GanSu College of TCM; 2006. Experimental Study of the Change on Thymus Index and Spleen Index in Rats With Insufficiency of the Lung-qi of Pulmonary Emphysema; pp. 20–22. [Google Scholar]
- 49.Morrison L. Replication-defective virus vaccine-induced protection of mice from genital herpes simplex virus 2 requires CD4 T cells. Virology. 2008;376:205–210. doi: 10.1016/j.virol.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu J., Koelle D., Cao J., Vazquez J., Huang M., Hladik F., Wald A., Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J. Exp. Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]