Summary
Severe acute respiratory syndrome (SARS) was a new human disease in the autumn of 2002. It first occurred in Southern China in November 2002 and was transported to Hong Kong on February 21, 2003 by an infected and ill patient. Ten secondary cases spread the infection to two hospitals in Hong Kong and to Singapore, Toronto and Hanoi. In March 2003 a novel coronavirus (SARS-CoV) was found to be the causative agent. Within 11 weeks from the first SARS case in Hong Kong it had spread to an additional 27 countries or special administrative regions. The mini pandemic peaked during the last week of May 2003 and the last new probable case was on July 13, 2003. There were a total of 8096 probable cases and 774 deaths. Sixty-six per cent of the cases occurred in China, 22% in Hong Kong, 4% in Taiwan and 3% in both Singapore and Canada. Twenty-one per cent of all cases occurred in healthcare workers.
Keywords: SARS, pneumonia, respiratory distress, SARS chronology
INTRODUCTION
It is an honour to have been asked to contribute the lead paper in this symposium on paediatric SARS. Of all the participants in this symposium I have the distinction of having had no first-hand experience with SARS. Nevertheless, I do have some credentials. In March of 2003 I travelled to and through Hong Kong, Shanghai, Taipei, Seoul and Narita and realised on my trip back to Los Angeles on March 17th that I needed a chapter on SARS for the 5th edition of our book ‘Textbook of Pediatric Infectious Diseases’ which was soon to be published. So, with the help of four Hong Kong colleagues this chapter was written in under 2 weeks and is the most complete reference to date relating to paediatrics.1
SARS was apparently a new human disease which first occurred in Southern China in the autumn of 2002 and spread to 29 countries or regions with a total of 8096 cases and 774 deaths.[1], [2], [3] The aetiologic agent of SARS (a previously unrecognised coronavirus; SARS-CoV) was isolated and its genome sequenced in record time.[3], [4], [5], [6], [7], [8] Following an incredible global public health effort, the mini pandemic was brought under control within 7 months of its initial occurrence.9 The overall effort demonstrated unprecedented international cooperation and this was facilitated by electronic transmission of information. In general, worldwide media coverage was unusually accurate and provided pictures to augment scientific data.
As of June 1st, 2004 there were approximately 2000 citations related to SARS in the medical literature. However, of these publications only about 0.1% are related to children.
CASE DEFINITIONS
During the third week of March 2003 both the WHO and the CDC published preliminary case definitions for severe acute respiratory syndrome (SARS). Table 1 contains the WHO initial definitions of suspect and probably cases and Table 2 the CDC original definition of a suspected case.[10], [11] Following the rapidly expanding knowledge about SARS the WHO case definitions were revised on May 1, 2003 (Table 3 ).12 The CDC case definition was updated on four occasions throughout the epidemic as understanding of the clinical, laboratory, and transmission characteristics of SARS associated coronavirus increased.13 The most recent version from the CDC, which is exceedingly complex, is presented in Table 4 .
Table 1.
WHO SARS case definitions as of 16 March 2003*.
| Suspect case |
| A person presenting after 1 February, 2003 with history of high fever (> 38 °C) |
| and one or more respiratory symptoms, including cough, shortness of breath, difficulty breathing and one or more of the following: |
| • Close contact,† within 10 days of onset of symptoms, with a person who has been diagnosed with SARS |
| • History of travel, within 10 days of onset of symptoms, to an area in which there are reported foci of transmission of SARS |
| Probable case |
| A suspect case with chest X-ray findings of pneumonia or respiratory distress syndrome. or a suspect case with an unexplained respiratory illness resulting in death, with an autopsy examination demonstrating the pathology of respiratory distress syndrome without an identifiable cause |
From: the World Health Organization, Weekly Epidemiological Record No. 12, 21 March, 2003.
Defined as having cared for, having lived with, or having had direct contact with respiratory secretions and/or body fluids of a person suspected of having SARS.
Table 2.
CDC preliminary case definition for SARS as of March 19, 2003*.
| Suspect case |
| Respiratory illness of unknown aetiology with onset since February 1, 2003 and the following criteria: |
| • Documented temperature > 100.4 °F (38 °C) |
| • One or more symptoms of respiratory illness (e.g. cough, shortness of breath, difficulty breathing or radiographic findings of pneumonia or acute respiratory distress syndrome) |
| • Close contact† within 10 days of onset of symptoms with a person under investigation for or suspected of having SARS or travel within 10 days of onset of symptoms to an area with documented transmission of SARS as defined by the WHO. |
From: Centers for Disease Control and Prevention. Outbreak of severe acute respiratory syndrome-Worldwide, 2003. MMWR 2003, 52:226–228.
Defined as having cared for, having lived with, or having had direct contact with respiratory secretions and/or body fluids of a person suspected of having SARS.
Table 3.
WHO SARS case definitions* (Revised 1 May, 2003).
| Suspect case |
| 1. A person presenting after 1 November 2002a with history of: |
| - high fever (>38 °C) |
| and |
| - cough or breathing difficulty |
| and one or more of the following exposures during the 10 days prior to onset of symptoms: |
| - close contactb with a person who is a suspect or probable case of SARS |
| - history of travel to an area with recent local transmission of SARS |
| - residing in an area with recent local transmission of SARS |
| 2. A person with an unexplained acute respiratory illness resulting in death after 1 November, 2002,a but on whom no autopsy has been performed and one or more of the following exposures during to 10 days prior to onset of symptoms: |
| - close contact,b with a person who is a suspect or probable case of SARS |
| - history of travel to an area with recent local transmission of SARS |
| - residing in an area with recent local transmission of SARS |
| Probable case |
| 1. A suspect case with radiographic evidence of infiltrates consistent with pneumonia or respiratory distress syndrome (RDS) on chest X-ray (CXR) |
| 2. A suspect case of SARS that is positive for SARS coronavirus by one or more assays (See: Use of laboratory methods for SARS diagnosis.) |
| 3. A suspect case with autopsy findings consistent with the pathology of RDS without an identifiable cause |
| Exclusion criteria |
| A case should be excluded if an alternative diagnosis can fully explain their illness. |
| Reclassification of cases |
| As SARS is currently a diagnosis of exclusion, the status of a reported case may change over time. A patient should always be managed as clinically appropriate, regardless of their case status |
| - A case initially classified as suspect or probable, for whom an alternative diagnosis can fully explain the illness, should be discarded after carefully considering the possibility of co-infection |
| - A suspect case who, after investigation, fulfils the probable case definition should be reclassified as ‘probable’ |
| - A suspect case with a normal CXR should be treated as deemed appropriate and monitored for 7 days. Those cases in which recovery is inadequate should be re-evaluated by CXR |
| - Those suspect cases in whom recovery is adequate but whose illness cannot be fully explained by an alternative diagnosis should remain as ‘suspect’ |
| - A suspect case who dies, on whom no autopsy is conducted, should remain classified as ‘suspect’. However, if this case is identified as being part of a chain transmission of SARS, the case should be reclassified as ‘probable’ |
| - If an autopsy is conducted and no pathological evidence of RDS is found, the case should be ‘discarded’ |
The surveillance period begins on 1 November 2002 to capture cases of atypical pneumonia in China now recognized as SARS. International transmission of SARS was first reported in March 2003 for cases with onset in February 2003.
Close contact: having cared for, lived with, or had direct contact with respiratory secretions or body fluids of a suspect or probable case of SARS.
From: the World Health Organization Case Definitions for Surveillance of severe acute respiratory syndrome (SARS). http://www.who.int/csr/sars/casedefinition/en/.
Table 4.
Revised Council of State and Territorial Epidemiologists surveillance case definition for SARS, December 2003*.
| Clinical criteria |
| Early illness |
| • Presence of two or more of the following features: fever (might be subjective), chills, rigors, myalgia, headache, diarrhea, sore throat, or rhinorrhea |
| Mild-to-moderate respiratory illness |
| • Temperature of >100.4 °F (> 38 °C)*and |
| • One or more clinical findings of lower respiratory illness (e.g. cough, shortness of breath or difficulty breathing) |
| Severe respiratory illness |
| • Meets clinical criteria of mild-to-moderate respiratory illness and |
| • One or more of the following findings: |
| — Radiographic evidence of pneumonia or |
| — Acute respiratory distress syndrome or |
| — Autopsy findings consistent with pneumonia or acute respiratory distress syndrome without an identifiable cause |
| Epidemiologic criteria |
| Possible exposure to SARS-associated coronavirus (SARS-CoV) |
| One or more of the following exposures in the 10 days before onset of symptoms: |
| • Travel to a foreign or domestic location with documented or suspected recent transmission of SARS-CoVaor |
| • Close contactb with a person with mild-to-moderate or severe respiratory illness and history of travel in the 10 days before onset of symptoms to a foreign or domestic location with documented or suspected recent transmission of SARS-CoVa |
| Likely exposure to SARS-CoV |
| One or more of the following exposures in the 10 days before onset of symptoms: |
| • Close contactb with a person with confirmed SARS-CoV disease or |
| • Close contactb with a person with mild-to-moderate or severe respiratory illness for whom a chain of transmission can be linked to a confirmed case of SARS-CoV disease in the 10 days before onset of symptoms |
| Laboratory criteria |
| Tests to detect SARS-CoV are being refined and their performance characteristics assessedc; therefore, criteria for laboratory diagnosis of SARS-CoV are changing. The following are general criteria for laboratory confirmation of SARS-CoV: |
| • Detection of serum antibody to SARS-CoV by a test validated by CDC (e.g. enzyme immunoassay) or |
| • Isolation in cell culture of SARS-CoV from a clinical specimen or |
| • Detection of SARS-CoV RNA by a reverse transcription polymerase chain reaction test validated by CDC and with subsequent confirmation in a reference laboratory (e.g. CDC) |
| Information about the current criteria for laboratory diagnosis of SARS-CoV is available at http://www.cdc.gov/ncidod/sars/labdiagnosis.htm |
| Exclusion criteria |
| A case may be excluded as a SARS report under investigation (SARS RUI), including as a CDC-defined probable SARS-CoV case, if any of the following apply: |
| • An alternative diagnosis can explain the illness fullydor |
| • Antibody to SARS-CoV is undetectable in a serum specimen obtained > 28 days after onset of illnesseor |
| • The case was reported on the basis of contact with a person who was subsequently excluded as a case of SARS-CoV disease; then the reported case is also excluded, provided other epidemiologic or laboratory criteria are not present |
| Case classification |
| SARS RUI |
| Reports in persons from areas where SARS is not known to be active |
| • SARS RUI-1: cases compatible with SARS in groups likely to be first affected by SARS-CoVf if SARS-CoV is introduced from a person without clear epidemiologic links to known cases of SARS-CoV disease or places with known ongoing transmission of SARS-CoV |
| Reports in persons from areas where SARS activity is occurring |
| • SARS RUI-2: cases meeting the clinical criteria for mild-to-moderate illness and the epidemiologic criteria for possible exposure (spring 2003 CDC definition for suspect casesg) |
| • SARS RUI-3: cases meeting the clinical criteria for severe illness and the epidemiologic criteria for possible exposure (spring 2003 CDC definition for probable casesg) |
| • SARS RUI-4: cases meeting the clinical criteria for early or mild-to-moderate illness and the epidemiologic criteria for likely exposure to SARS-CoV |
| SARS-CoV disease |
| • Probable case of SARS-CoV disease: meets the clinical criteria for severe respiratory illness and the epidemiologic criteria for likely exposure to SARS-CoV |
| • Confirmed case of SARS-CoV disease: clinically compatible illness (i.e. early, mild-to-moderate, or severe) that is laboratory confirmed |
*From: Centers for Disease Control and Prevention. Revised U.S. Surveillance Case Definition for severe acute respiratory syndrome (SARS) and Update of SARS Cases – United States and Worldwide, December 2003. MMWR 2003, 52:1202–1206.
A measured documented temperature of >100.4 °F (> 38 °C) is expected. However, clinical judgment may allow a small proportion of patients without a documented fever to meet this criterion. Factors that might be considered include: patients’ self-report of fever; use of antipyretics; presence of immunocompromising conditions or therapies; lack of access to healthcare; or inability to obtain a measured temperature. Initial case classification based on reported information might change, and reclassification might be required.
Types of locations specified will vary (e.g. country, airport, city, building, or floor of building). The last date a location may be a criterion for exposure is 10 days (one incubation period) after removal of that location from CDC travel alert status. The patient's travel should have occurred on or before the last date the travel alert was in place. Transit through a foreign airport meets the epidemiologic criteria for possible exposure in a location for which a CDC travel advisory is in effect. Information about CDC travel alerts and advisories and assistance in determining appropriate dates are available at http://www.cdc.gov/ncidod/sars/travel.htm.
Close contact is defined as having cared for or lived with a person with SARS or having a high likelihood of direct contact with respiratory secretions and/or body fluids of a person with SARS (during encounters with the patient or through contact with materials contaminated by the patient) either during the period the person was clinically ill or within 10 days of resolution of symptoms. Examples of close contact include kissing or embracing, sharing eating or drinking utensils, close (i.e. <3 feet) conversation, physical examination, and any other direct physical contact between persons. Close contact does not include activities such as walking by a person or sitting across a waiting room or office for a brief time.
The identification of the aetiologic agent of SARS (i.e. SARS-CoV) led to the rapid development of enzyme immunoassays and immunofluorescence assays for serologic diagnosis and reverse transcription polymerase chain reaction assays for detection of SARS-CoV RNA in clinical samples. These assays can be very sensitive and specific for detecting antibody and RNA, respectively, in the later stages of SARS-CoV disease. However, both are less sensitive for detecting infection early in illness. The majority of patients in the early stages of SARS-CoV disease have a low titre of virus in respiratory and other secretions and require time to mount an antibody response. SARS-CoV antibody tests might be positive as early as 8–10 days after onset of illness and often by 14 days after onset of illness, but sometimes not until 28 days after onset of illness. Information about the current criteria for laboratory diagnosis of SARS-CoV is available at http://www.cdc.gov/ncidod/sars/labdiagnosis.htm.
Factors that may be considered in assigning alternate diagnoses include the strength of the epidemiologic exposure criteria for SARS-CoV disease, the specificity of the alternate diagnostic test, and the compatibility of the clinical presentation and course of illness with the alternative diagnosis.
Current data indicate that > 95% of patients with SARS-CoV disease mount an antibody response to SARS-CoV. However, health officials may choose not to exclude a case on the basis of lack of a serologic response if reasonable concern exists that an antibody response could not be mounted.
Consensus guidance is in development between CDC and CSTE on which groups are most likely to be affected first by SARS-CoV if it re-emerges. SARS-CoV disease should be considered at a minimum in the differential diagnoses for persons requiring hospitalisation for pneumonia confirmed radiographically or acute respiratory distress syndrome without identifiable aetiology and who have one of the following risk factors in the 10 days before the onset of illness: (1) Travel to mainland China, Hong Kong, or Taiwan, or close contact with an ill person with a history of recent travel to one of these areas, or; (2) Employment in an occupation associated with a risk for SARS-CoV exposure (e.g. healthcare worker with direct patient contact or worker in a laboratory that contains live SARS-CoV) or; (3) Part of a cluster of cases of atypical pneumonia without an alternative diagnosis. Guidelines for the identification, evaluation, and management of these patients are available at http://www.cdc.gov/ncidod/sars/absenceofsars.htm.
During the 2003 SARS epidemic, CDC case definitions were the following: Suspect case; (1) Meets the clinical criteria for mild-to-moderate respiratory illness and the epidemiologic criteria for possible exposure to SARS-CoV but does not meet any of the laboratory criteria and exclusion criteria or; (2) Unexplained acute respiratory illness that results in death of a person on whom an autopsy was not performed and that meets the epidemiologic criteria for possible exposure to SARS-CoV but does not meet any of the laboratory criteria and exclusion criteria; Probable case; (3) Meets the clinical criteria for severe respiratory illness and the epidemiologic criteria for possible exposure to SARS-CoV but does not meet any of the laboratory criteria and exclusion criteria.
THE EPIDEMIC ONSET
Transmission of SARS apparently first occurred in Fosham City, Guangdong Province, China.[1], [9] The primary case may have been a 46-year-old man who had a fever for 9 days and respiratory distress. He was hospitalised and treated in an intensive care unit. He recovered but his wife, aunt, and aunt's daughter all became ill.14 Subsequently, several independent clusters of cases were noted in seven municipalities in Southern China. From November 10, 2002 until February 9, 2003 there were 305 cases of SARS identified in Guangdong Province. Many of the afflicted patients were healthcare workers. The first identified spread of SARS to Hong Kong occurred on February 21, 2003. This involved a 64-year-old physician from Zhongshan University in Guangdong Province who travelled to Hong Kong to attend a wedding. Prior to his hospitalisation in Hong Kong he stayed in the Metropole Hotel in Hong Kong for 1 day. Subsequently, 10 secondary cases occurred in other hotel guests and these infected and ill persons led directly to tertiary cases in two Hong Kong hospitals and outbreaks in Singapore, Toronto and Hanoi.15
AETIOLOGY
Using classical virologic methods, the causative viral agent of SARS was identified in three laboratories in mid March 2003.[15], [16], [17], [18] Viral particles were seen on electron microscopic examination of swabs and sputum specimens from SARS patients. The SARS virus grew in Vero cell and fetal rhesus kidney cell tissue cultures. Cytopathic effect was noted in the Vero cell cultures in 5 to 6 days and in the fetal rhesus kidney cell cultures in 2 to 4 days.[16], [17] This virus, which was a novel coronavirus (SARS-CoV), was rapidly identified using both conventional virologic methods and cutting edge molecular biology. The genome was completely and rapidly sequenced in several laboratories.[4], [5], [6], [7], [8] This was a significant accomplishment when one realises that coronaviruses have the largest genomes (30 000 bases of positive-strand RNA) found in any RNA virus. Sequence data allowed the rapid development of highly specific diagnostic tests and also helped in the epidemiologic study of the mini pandemic.
The genome sequence data from human isolates assisted in the search for the origin of the disease when similar viruses were isolated from Himalayan palm civets and raccoon dogs in a live animal market in Shenzhen, China.19 The viral genomes recovered in nasal swabs from the palm civets were 99.8% homologous to the human SARS-CoV isolates. Early in the epidemic Orf8 reading frame sequences from human isolates were identical to those from the palm civets, which suggested animal to human transmission.
WORLDWIDE CHRONOLOGICAL EVENTS
As noted above, SARS originated in Southern China in November 2002. Presumably the first case or cases were the result of transmission from palm civets to humans. Subsequently, human to human transmission occurred. Apparently, human to human transmission did not occur outside of China until February 21, 2003 when the adult physician from Guangdong Province, whose illness commenced on February 15, stayed at the Metropole Hotel in Hong Kong.15 The chronology of events related to the Metropole Hotel is presented in Fig. 1 . Case A (the primary case) stayed on the ninth floor of the hotel on February 21, 2003 and was then admitted to hospital #2 the following day and died on February 23, 2003. Secondary cases related to Case A, in addition to cases B to K, were four healthcare workers at hospital #2 and two of the patient's family members.
Figure 1.
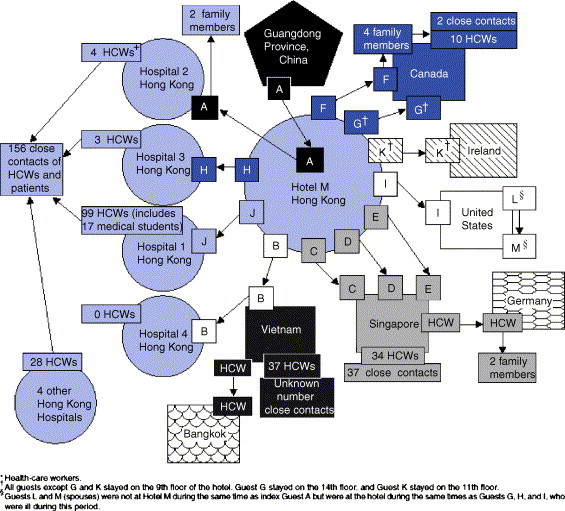
Chain of transmission among guests at Hotel M – Hong Kong, 2003. (From: Centers for Disease Control and Prevention. Update: Outbreak of severe acute respiratory syndrome – Worldwide, 2003. MMWR 2003; 52:241–248.).
Case B was an Asian-American businessman who stayed on the ninth floor of the Metropole Hotel at the same time as Case A. He travelled to Hanoi on February 23, 2003 and he also became ill on that day. He was hospitalised on February 26, 2003 at the Vietnam French Hospital in Hanoi and was placed on mechanical ventilation on March 2, 2003. On March 5, 2003 he was medically evacuated to hospital #4 in Hong Kong and died there on March 12, 2003. In the Hanoi hospital, 37 healthcare workers became secondary cases. A healthcare worker from Bangkok who was investigating the outbreak in Hanoi became ill and returned to Bangkok.
Patients C, D, and E were the primary cases in Singapore and their illnesses led to 70 cases in Singapore and three cases in Germany. Patients F and G were the initial cases in Canada and Case F was subsequently linked to a cluster of 16 cases in Toronto. Patients H and J were linked to outbreaks among healthcare workers in Hong Kong hospitals #1 and #3. Patients I, L, and M were suspect cases in the United States. Case K was a potential primary case in Ireland.
On February 28, 3003 the Hanoi office of the World Health Organization was contacted by officials at the Vietnam French Hospital.20 They were concerned about the unusual influenza-like illness that patient B had which they suspected could have been due to an avian influenza virus. Dr. Carlo Urbani from the WHO office answered the call and rapidly determined that the illness which had spread throughout the hospital was unusual. He carried out an extensive on-site investigation and worked with the hospital staff to set up quarantine procedures. His efforts led to the containment of SARS in Hanoi. Unfortunately, he became ill with SARS on March 11, 2003 and died in an isolation room in a Bangkok hospital on March 29, 2003. He did not live to see the worldwide successes of SARS control which resulted to a great extent from his early detection of SARS.
Following the recognition of SARS at the end of February in Hanoi, Vietnam the World Health Organization initiated the coordination of a global response. Beginning on March 17, 2003 the WHO published electronically a daily (exclusive of Sunday) summary of reported suspect and probable SARS cases.2 This continued until July 11, 2003. A final update of probable cases and the number of countries was posted on April 30, 2004 for the period November 1, 2002 to July 31, 2003. A weekly summary of these data are presented in Fig. 2 . The initial report on March 17, 2003 noted 167 cases in 7 countries or special administrative regions. Neither this report or the next Monday's report included China. (In Fig. 2 the initial 305 cases for Southern China as well as the entire country have been included for the March 17 and 24, 2003 time points.) This March 17th report included 5 countries, with cases linked to Hong Kong and the Metropole Hotel. It also includes two suspect or probable cases in Switzerland. Over the next several days the case counts in Switzerland varied between zero and seven but a subsequent study indicated only one case with no subsequent transmission.
Figure 2.
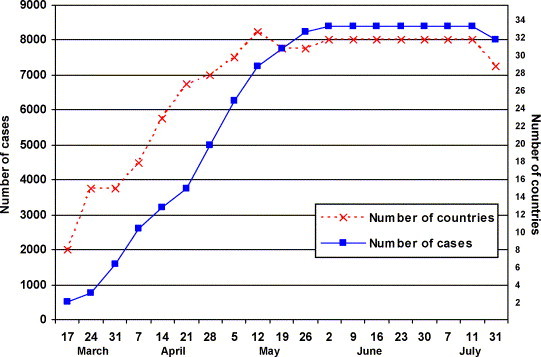
Cumulative number of reported suspect or probable SARS cases and countries or administrative regions in the world from March 17, 2003 to July 31, 2003. The number of countries decreased from 33 to 29 when cases in some countries were found not to be SARS. Similarly the final count of cases also decreased with the elimination of cases which were later found not to be SARS. The March 17 and 24, 2003 dates include the initial 305 cases from Southern China which were not included in the WHO reports.
By March 24, 2003 there were 761 cases in 15 countries or special administrative regions. New countries at this time were France, Italy, Ireland, Spain, Taiwan, the United Kingdom and the United States. In France the total cases reached seven, all of whom were imported with no local transmission within the country. In Italy the total number of cases reached four and all were imported. In Ireland there was one accepted case and, as noted above, this case had a link to the Metropole Hotel in Hong Kong. Throughout this reporting period there was one case in Spain, four cases in the United Kingdom and 27 cases in the United States, all cases in these three countries were imported. In Taiwan there were six cases noted on March 24, 2003 and there had been evidence of local transmission. The first case in Taiwan occurred on February 25 and the last probably case occurred on June 15, 2003. A total of 697 cases were reported in Taiwan at the peak of the epidemic but almost half were later excluded. In Taiwan the total number of imported cases was only 21 and the vast majority were the result of local transmission.
An addition to the effected countries on March 31, 2003 was Romania. Initially three cases were noted but the final count was one imported case with no further transmission. New countries on April 7, 2003 were Australia, Brazil and Malaysia. Of these countries Australia and Malaysia had six and five imported cases respectively, and Brazil was later removed from the list. New countries on April 14, 2003 included Indonesia, Kuwait, the Philippines, South Africa and Sweden. Of these countries, local secondary transmission occurred only in the Philippines. Seven of the 14 cases were importations. New countries as of April 21, 2003 were India, Japan and Mongolia. Of these countries, no secondary transmission occurred in India, Japan was later removed from the list and in Mongolia there were nine cases with eight of these being importations.
As of May 12, 2003 new countries or administration regions included Bulgaria, China, Macao SAR, Columbia, Finland, Poland and the Republic of Korea. Of these countries or regions only Macao SAR and the Republic of Korea had accepted cases and in both regions the cases were imported. In the June 2nd report one case in the Russian Federation was noted but this was subsequently removed from the data set.
Eighty-seven per cent of all probably SARS cases occurred in Hong Kong or China and information relating to these cases in presented elsewhere in this symposium. Only five other countries (Canada, Taiwan, Singapore, the United States and Vietnam) had more than 20 probable cases. In Canada there were 257 cases with the first case occurring on February 23, 2003 and the last case on June 12, 2003. The vast majority of cases in Canada (98%) were secondary to five primary importations and 43% of the cases occurred in healthcare workers. In Taiwan 6% of the cases were importations and 20% occurred in healthcare workers. In Singapore 3% of the cases were importations and 41% of the locally acquired cases were in healthcare workers. In Singapore the epidemic was controlled within 13 weeks of the initial importation. All 27 probable cases in the United States were importations. However, only eight of these were laboratory confirmed. In Vietnam, 62 of the 63 cases were the result of local transmission. The outbreak was controlled within 7 weeks of the first and only imported case.
In summary, the SARS mini pandemic of 2002–2003 probably does not deserve to be called a pandemic. However, the death rate in adults was sobering. In reality, significant epidemic disease occurred in only five countries and the special administrative region of Hong Kong.
Nevertheless, SARS was an eye opener in regard to the number of healthcare workers who became infected. Hopefully the clinical lessons relating to isolation practices, personnel protection and the effective use of quarantine will be retained. Personally I will be less cavalier in my approach to patients with fever and respiratory illness.
References
- 1.Hon E., Li A., Nelson E.A.S., Leung C.W., Cherry J.D. Severe acute respiratory syndrome (SARS) In: Feigin R.D., Cherry J.D., Demmler G.J., Kaplan S., editors. Textbook of Pediatric Infectious Diseases. 5th Edition. WB Saunders Co; Philadelphia, PA: 2003. pp. 2389–2393. [Google Scholar]
- 2.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at http://www.who.int/scr/sars/country/table2004_04_21/en/print.html.
- 3.World Health Organization Consensus document on the epidemiology of severe acute respiratory syndrome (SARS) Global Health Security – Epidemic Alert & Response. 2003;11:1–44. [Google Scholar]
- 4.Marra M.A., Jones S.J.M., Astell C.R. The genome sequence of the SARS-associated coronavirus. Science. 2003;300:1399–1404. doi: 10.1126/science.1085953. [DOI] [PubMed] [Google Scholar]
- 5.Drosten C., Günther S., Preiser W. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N Engl J Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 6.Rota P.A., Oberste M.S., Monroe S.S. Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science. 2003;300:1394–1399. doi: 10.1126/science.1085952. [DOI] [PubMed] [Google Scholar]
- 7.Ksiazek T.G., Erdman D., Goldsmith C.S. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 8.Homes K.V., Enjuanes L. The SARS coronavirus: a postgenomic era. Science. 2003;300:1377–1378. doi: 10.1126/science.1086418. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Severe acute respiratory syndrome (SARS). Report by the Secretariat EB113/33 2003.
- 10.World Health Organization Outbreak news: severe acute respiratory syndrome (SARS) Weekly epidemiological record. 2003;12:81–88. [Google Scholar]
- 11.Centers for Disease Control and Prevention Trends in tuberculosis morbidity – United States, 1992–2002. MMWR. 2003;52:217–239. [PubMed] [Google Scholar]
- 12.World Health Organization. Case definitions for surveillance of severe acute respiratory syndrome (SARS). 2003. Available at http://www.who.int/csr/sars/casedefinition/en/print.html
- 13.Centers for Disease Control and Prevention Update: influenza activity – United States, 2003–04 season. MMWR. 2003;52:1197–1220. [Google Scholar]
- 14.Ni C.-C. Tracing the path of SARS: a tale of deadly infection. LA Times, April 22, 2003: A1&A5.
- 15.Centers for Disease Control and Prevention Update: outbreak of severe acute respiratory syndrome – worldwide. 2003;52:241–265. doi: 10.1001/jama.289.16.2059. [DOI] [PubMed] [Google Scholar]
- 16.Peiris J.S.M., Lai S.T., Poon L.L.M. Coronavirus as a possible cause of severe acute respiratory syndrome. The Lancet. 2003;631:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poutanen S.M., Low D.E., Henry B. Identification of severe acute respiratory syndrome in Canada. N Engl J Med. 2003;348:1995–2005. doi: 10.1056/NEJMoa030634. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization. Summary on major findings in relation to coronavirus by members of the WHO multi-centre collaborative network on SARS aetiology and diagnosis. 2003. Available at http://www.who.int/csr/sars/findings/en/print.html
- 19.Guan Y., Zheng B.J., He Y.Q. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern china. Science. 2003;302:276–278. doi: 10.1126/science.1087139. [DOI] [PubMed] [Google Scholar]
- 20.Reilley B., Van Herp M., Sermand D., Dentico N. SARS and Carlo Urbani. N Engl J Med. 2003;348:1951. doi: 10.1056/NEJMp030080. [DOI] [PubMed] [Google Scholar]


