Highlights
-
•
Sero-type 3 reovirus strain MPC/04 transiently activated the PI3K/Akt pathway.
-
•
Blockage of PI3K/Akt activation increased viral RNA synthesis and yield.
-
•
The downstream effectors MDM2/p53 of PI3K/Akt were activated.
-
•
Disturbing MDM2 and p53 cross-talk enhanced reovirus replication.
-
•
Overexpression or knockdown of p53 promoted or inhibited reovirus replication.
Abbreviations: CHT, α-Chymotrypsin; LY, LY294002; Wort, Wortmannin; p-Akt, phosphorylated Akt; p-MDM2, phosphorylated MDM2
Keywords: Reovirus, PI3K/Akt, p53, MDM2, Replication
Abstract
Viral infections activate many host signaling pathways, including the phosphatidylinositol 3-kinase (PI3K)/Akt pathway, which has recently attracted considerable interest due to its central role in modulating virus replication. This study demonstrated that the sero-type 3 reovirus strain Masked Palm Civet/China/2004 (MPC/04) could transiently activate the PI3K/Akt pathway in A549 cells at earlier time points of infection. The blockage of PI3K/Akt activation increased viral RNA synthesis and yield. The role of the downstream effectors MDM2/p53 of PI3K/Akt in regulating reovirus replication was further analyzed. We found that during reovirus infection, the level of phosphorylated MDM2 (p-MDM2) was increased and the expression of p53 was reduced. In addition, the blockage of PI3K/Akt by Ly294002 or knockdown of Akt by siRNA reduced the level of p-MDM2 and increased the level of p53. Both indicated that the downstream effectors MDM2/p53 of PI3K/Akt were activated. Pre-treatment with Nutlin, which can destroy MDM2 and p53 cross-talk and increase the expression of p53 RNA and protein, dose-dependently enhanced reovirus replication. Additionally, the overexpression of p53 alone also supported reovirus replication, and knockdown of p53 significantly inhibited viral replication. This study demonstrates that PI3K/Akt/p53 activated by mammalian reovirus can serve as a pathway for inhibiting virus replication/infection, yet the precise mechanism of this process remains under further investigation.
1. Introduction
Reoviruses are non-enveloped, double-stranded RNA viruses in the family Reoviridae. Members of the genus Orthoreovirus have been isolated from a broad range of mammalian, avian, and reptilian hosts (Fields et al., 2001, Jackson and Muldoon, 1973, Rosen et al., 1960). The mammalian orthoreoviruses (MRV) were first isolated from humans in 1951. These viruses commonly infected humans but are rarely pathogenic in adults (Tyler et al., 2004). However, recent studies have demonstrated that reoviruses that spilled over from wild animals can cause acute and severe clinical disease in humans (Cheng et al., 2009, Chua et al., 2007, Chua et al., 2008, Chua et al., 2011). These recent discoveries have raised concerns about future zoonotic reovirus infections in humans and also demonstrated the need to better understand virus-host interactions.
Mammalian reoviruses are highly tractable models for understanding virus-host interactions (Danthi et al., 2008). Studies using these viruses have improved our understanding of the pathogenesis of viral encephalitis. Viruses have evolved to exploit the host cellular machinery to ensure efficient replication. However, limited information exists about the role of many host-signaling pathways during reovirus replication.
The PI3K/Akt pathway has recently been shown to be required not only for viral cell entry but also for subsequent intracellular trafficking and viral replication (Cooray, 2004). Feng et al. reported that the induction of the PI3K/Akt signaling pathway by exogenous ALV infection regulated viral entry (Feng et al., 2011). PI3K activation was required for the human immunodeficiency virus type 1 (HIV-1) infection of CD4+ T-cells, and the suppression of PI3K signaling reduced HIV infection post-viral entry and post-reverse transcription, but prior to HIV gene expression (Francois and Klotman, 2003). The activation of the PI3K/Akt pathway is a strategy for Zaire Ebola virus to regulate vesicular trafficking and cellular entry (Saeed et al., 2008). Influenza A virus NS1 protein activated the PI3K/Akt pathway to augment its efficient replication (Ehrhardt et al., 2007, Shin et al., 2007). The direct relevance of the PI3K/Akt signaling pathway to reovirus infection is currently unknown.
The p53 tumor suppressor protein is an important component of the PI3K/Akt signaling pathway that responds to various cellular stresses, such as DNA damage and deregulated oncogene expression (Gottlieb et al., 1996, Khanna and Jackson, 2001). Studies have demonstrated that type I interferons (IFN-α/β) enhance p53 transcription, and p53 also contributes to the immune responses that lead to the eradication of pathogens, such as viruses (Takaoka et al., 2003). p53 is involved in influenza virus-induced cell death, and inhibiting p53 could increase viral replication (Turpin et al., 2005). Another study demonstrated that p53 plays a crucial role in the cellular innate defence against the hepatitis C virus (HCV) (Dharel et al., 2008). However, the encephalomyocarditis virus (EMCV) and human parainfluenza virus type 3 (HPIV3) could down-regulate p53 in infected cells (Marques et al., 2005). Moreover, the absence of p53 inhibits the replication of both EMCV and HPIV3 (Marques et al., 2005). The role of p53 in reovirus infection is of concern.
To examine the ability of PI3K/Akt to regulate reovirus replication, we analyzed the role of this signaling pathway in sero-type 3 reovirus infection. Our study found that Akt can be phosphorylated in a PI3K-dependent manner early during infection, and the blockage of Akt activation could promote viral replication. Following the induction of PI3K/Akt, the downstream effectors MDM2/p53 of PI3K/Akt were activated during reovirus infection. The up-regulation of p53 using an inhibitor or the overexpression of p53 in cells increased reovirus production, and knockdown of p53 by siRNA reduced reovirus replication. This study demonstrates that PI3K/Akt/p53 is involved in the regulation of mammalian reovirus replication.
2. Materials and methods
2.1. Cells and viruses
The human lung-epithelial cell line A549 was cultured in F-12K medium. Mouse L929 cells were maintained in 1640 medium. Fetal bovine serum (10%) and 1% antibiotics were added to the medium, and all cells were incubated at 37 °C in 5% CO2. Masked Palm Civet/China/Liu/2004 (MPC/04) was isolated from the masked palm civet in Yunnan, China in 2003 and propagated in L929 cells. The virus stock was purified using CsCl gradients (Hand and Tamm, 1971, Smith et al., 1969) and stored at −80 °C for the following studies.
The S1 gene from the Masked Palm Civet/China/Liu/2004 (MPC/04) shared 85.6% nucleotide identity with the S1 gene of reovirus serotype 3 Dear (T3D). A phylogenetic analysis revealed that the S1 gene of MPC/04 belonged to serotype 3. Infection studies via the intranasal inoculation of BALB/c mice indicated that the virus can cause clinical diseases and death in 80% of infected animals.
2.2. Preparation of UV-inactivated virus
The virus was diluted to 107 PFU/ml in DMEM without serum and irradiated in microtiter plates at a distance of 6 cm with shortwave (254 nm) UV light for 20 min (Helentjaris and Ehrenfeld, 1977). UV-irradiated virus infectivity was tested with a plaque assay. Secondly, UV-inactivated virus was inoculated into A549 cells for 2 h, and they were fixed with cold absolute methanol and kept at −20 °C for 15 min. Viral antigen was detected by indirect immunofluorescence with mouse polyclonal antibody anti-reovirus and followed by goat anti-mouse IgG-FITC secondary antibody (Sigma, St. Louis, MO, USA). The treated viruses were unable to form plaques and viral antigens could be detected by indirect immunofluorescence, which was considered to be UV-inactivated (Soares et al., 2009).
2.3. Titration
The virus titers of the samples were determined with plaque assays, as previously described (Middleton et al., 2007). Briefly, the samples were diluted and used to infect 6-well plates of L929 cells by incubation for 1 h. The monolayers were covered with 2 ml of 1% Bacto Agar (DIFCO) and serum-free medium 199 (Irvine Scientific) containing 10 μg/ml TLCK-treated α-Chymotrypsin (CHT) (Sigma–Aldrich). The plaques were counted 2 to 4 days later. CHT plaque assays were first fixed by incubation with 1 ml of 10% paraformaldehyde in PBS for 45 min at room temperature. The agar overlays were then peeled off, and the cells were stained by covering them with 0.05% crystal violet (Sigma–Aldrich) in 10% paraformaldehyde for 5 min at room temperature, followed by two washes with water.
2.4. Virus infection
Serum-starved A549 cells were washed with phosphate-buffered saline (PBS) and incubated with virus at the indicated multiplicities of infection (MOI) diluted in F-12K containing 0.2% bovine serum albumin (BSA), 100 U/ml penicillin, and 0.1 mg/ml streptomycin at 37 °C for the indicated times.
2.5. Analysis of viral replication after inhibitors treatment
Serum-starved A549 cells were treated with LY294002 (LY) (10–50 μM), Wortmannin (Wort) (0.1–1 μM), Nutlin (5–20 μM) or solvent DMSO (0.4%, v/v) before virus infection for 1 h and then infected at a MOI of 5. The inoculum was aspirated, and cells were incubated with F-12K in the presence of drug. Twenty-four hours post-infection (p.i.), the virus titer in cell supernatant was determined with a plaque assay, and the virus RNA was analyzed by real-time PCR. To study the time-course of infection, either Ly294002 or Nutlin was added at the indicated time points. The infection was terminated 24 h p.i., the virus titer in cell supernatant was determined with a plaque assay and the viral RNA was analyzed by real-time PCR.
2.6. Western blot analysis
The cell monolayers were washed with PBS and lysed at the indicated times. The lysates were collected and incubated on ice for 10 min. The lysates were cleared by centrifugation at 10,000g for 5 min at 4 °C. The supernatants were analyzed for total protein content with a BCA protein-assay kit (Beyotime). The total protein (30 μg) was resolved by 10% SDS–PAGE and transferred onto nitrocellulose membranes (Millipore). The membranes were blocked with 5% skim milk for 1 h at 37 °C and then incubated overnight at 4 °C with specific antisera: rabbit anti-phospho-Akt antibody (Ser473) (ab81283, Abcam), rabbit anti-Akt antibody (ab32505, Abcam), rabbit anti-phospho-MDM2 antibody (Ser166) (ab170880, Abcam), rabbit anti-MDM2 antibody (ab38618, Abcam), rabbit PI3K p85 antibody (ab40755, Abcam), rabbit anti-glyceraldehyde 3-phosphate dehydrogenase (GADPH) antibody (ab22555, Abcam) and rabbit anti-p53 antibody (9282, CST). After three rinses in TBST buffer, the membranes were incubated at 37 °C for 1 h with IRDye 800DX-conjugated anti-rabbit IgG (1:8000; Rockland Immunochemicals) diluted in TBST as a secondary antibody. The membranes were washed three times in PBST, then visualized and analyzed with an Odyssey infrared imaging system (LI-COR Biosciences).
2.7. Knockdown of PI3K p85α and Akt
p85α siRNA (6231, CST), Akt siRNA (6510, CST) and control siRNA (6201, CST) were obtained from Cell Signaling Technology. A549 cells grown in 24-well plates were transfected with 50 nM of siRNA using Lipofectamine™ 2000 (Invitrogen). Twenty-four hours after transfection, the expressions of p85 and Akt were determined with western blotting at 24 h post-transfection.
2.8. RNA analysis
The total RNA was extracted from cells using the RNeasy Mini Kit (QIAGEN, Dusseldorf, Germany) according to the manufacturer’s protocol. The RNA concentrations were measured with a spectrophotometer (260 nm/280 nm). The cDNA was generated with a reverse-transcription kit (Invitrogen). Real-time PCR was used to determine the expressions of GAPDH and the viral S4 gene or p53 with the SYBR Premix Ex Taq (Takara). After an initial denaturation at 95 °C for 30 s, amplification was performed over 40 cycles, each consisting of denaturation at 95 °C for 15 s, primer annealing at 55 °C for 30 s, and DNA extension at 72 °C for 20 s. The relative quantification of gene expression was analyzed using the 2−ΔΔCT method. The relative expression levels of the targeted gene were normalized to the expression level of the GAPDH gene. The following primers were used: reovirus S4 forward: 5′-TTGTCGCAATGGAGGTGTGC-3′ and reverse: 5′-TAGACATTGCATGCAGACGA-3′; p53 forward: 5′-CATAGTGTGGTGGTGCCCTAT-3′ and reverse: 5′-CAAAGCTGTTCCGTCCCAGTA-3′; GAPDH forward: 5′-TGACCACAGTCCATGCCATC-3′ and reverse: 5′-GCCAGTGAGCTTCCCGTTCA-3′.
2.9. Over-expression or knockdown of p53 and antiviral effect analysis
Plasmid coding for human p53 was constructed by inserting the corresponding cDNA into pcDNA3.1. p53 siRNA (6231, CST) and control siRNA (6201, CST) were obtained from Cell Signaling Technology. A549 cells grown in 24-well plates were transfected with 1 μg/well of plasmid or 50 nM of siRNA using Lipofectamine™ 2000 (Invitrogen). Twenty-four hours after transfection, the cells were infected at an MOI of 5. The viral RNA level and virus titers of cell supernatants were determined 24 h after infection.
2.10. Statistical analysis
The significance of differences between experimental groups was determined with a paired t-test and a one-way ANOVA using the Prism 5.0 software (GraphPad Software). A p-value <0.05 was selected to indicate significance.
3. Results
3.1. MPC/04 infection results in a transient activation of Akt in a PI3K-dependent manner
Given the biological importance of the PI3K/Akt pathway in viral infection, we initially investigated whether MPC/04 infection activates this pathway. In contrast to mock-treated cells (Fig. 1 C), MPC/04 infection rapidly increased Akt phosphorylation within 5–15 min post-infection (p.i.), which was followed by a decline in Akt phosphorylation 30 min p.i., with a return to background levels by 6 h p.i. (Fig. 1A). To demonstrate that Akt phosphorylation depended on viral infection, the UV-inactivated virus was prepared and verified with a plaque assay. The result showed that inactivated virus did not lose the ability to activate p-Akt (Fig. 1B). To determine whether Akt phosphorylation was PI3K-dependent, the role of PI3K in Akt phosphorylation following reovirus infection was investigated using the specific PI3K inhibitors LY and Wort or siRNA targeting the PI3K p85α subunit. As expected, pre-treatment with LY (50 μM) (Fig. 1D) or Wort (0.1 μM) (Fig. 1D) completely inhibited Akt phosphorylation. MPC/04 infection did not increase the levels of p-Akt in cells transfected with siRNA targeting the PI3K p85α subunit (Fig. 1F).
Fig. 1.
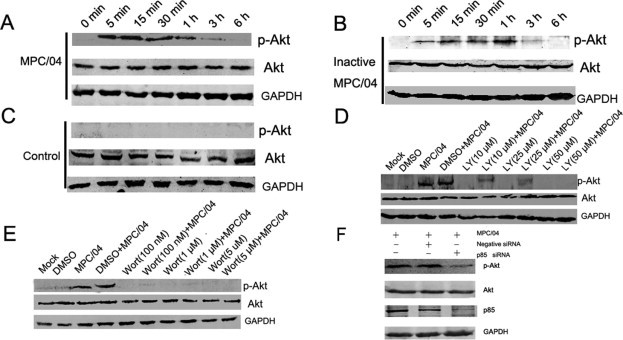
Determination of Akt phosphorylation in A549 cells. (A)–(C) Serum-starved A549 cells were infected with live MPC/04 (A) at an MOI of 5 (B) with UV-treated MPC/04 or (C) with mock virus. The phosphorylated Akt and total Akt levels were examined at the indicated times points by western blots. (D) and (E) Serum-starved A549 cells were pre-incubated with LY294002 (LY; 10–50 μM) (D), Wortmannin (Wort; 100 nM–1 μM) (E) or DMSO (0.4%, v/v) for 1 h and subsequently infected with MPC/04 at an MOI of 5. A mock-infected lane was included in each panel as a negative control. After 30 min, the phosphorylated Akt and total Akt levels were examined by western blots. (F) A549 cells (105) were transfected with 50 nM siRNA targeting p85 or negative siRNA, and then infected with MPC/04 at an MOI of 5 at 24 h after transfection. The expression of p-Akt, total Akt, p85 and GAPDH was determined 30 min post-infection. The data represent the results of three independent experiments.
3.2. PI3K/Akt pathway regulates viral replication
To determine whether activated PI3K/Akt is required for reovirus replication, the viral RNA transcription and progeny virus titers were measured after PI3K inhibition. The real-time PCR results showed that pre-treatment with 50 μM LY significantly increased MPC/04 RNA synthesis (p < 0.05; Fig. 2 A). Pre-treatment with Wort at all tested concentrations led to a 3-to-5-fold increase (p < 0.05) in MPC/04 RNA (Fig. 2B). The examination of virus titers agreed with the RNA results (Fig. 2C and D). The growth of MPC/04 increased 9.5 ± 2.2-fold in A549 cells pre-treated with LY (50 μM) (p < 0.05; Fig. 2C); Wort (1 μM) pre-treatment led to increases of 11.3 ± 2.4-fold (p < 0.05; Fig. 2D). To determine the time point at which PI3K/Akt played a role in reovirus replication, LY (50 μM) was added to cells −1, 0, 1, 3, and 5 h p.i. The real-time PCR and virus titer results showed that the addition of LY after infection could not affect virus replication (Fig. 2E and F). These results suggest that PI3K/Akt functioned at the early stage of reovirus infection.
Fig. 2.
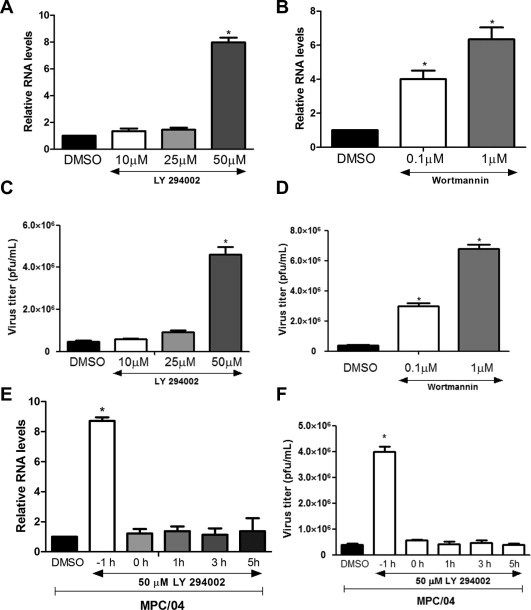
Blocking the PI3K/Akt pathway promotes reovirus replication. (A)–(D) Serum-starved A549 cells were pre-incubated with increasing concentrations of LY294002 (A and C), Wortmannin (B and D) or DMSO for 1 h and then infected with MPC/04 at an MOI of 5. Twenty-four hours p.i., the relative expression of the viral S4 gene was analyzed by real-time PCR (A and B), and the cell supernatants were harvested for an analysis of the viral titers (C and D). (E) and (F) Serum-starved A549 cells were inoculated with LY294002 (50 μM) or DMSO at the indicated time points during the infection of MPC/04 at an MOI of 5. Twenty-four hours after infection, the relative expression of the S4 gene was analyzed by real-time PCR (E), and the cell supernatants were harvested for an analysis of the virus titers (F). The data represent the results of three independent experiments. *p < 0.05, **p < 0.001. Error bars indicate SD.
3.3. Reovirus infection induces the phosphorylation of MDM2 and reduces the expression of p53
MPC/04 infection transiently activated Akt in a PI3K-dependent manner early during infection, which could suppress virus replication. To further analyze the downstream effectors of Akt responsible for the defence against viral infection, we examined the expression of phosphorylated MDM2 and p53 upon reovirus infection. After infection, the level of phosphorylated MDM2 significantly increased from 6 to 24 h p.i. (Fig. 3 A) and recovered to the normal level at 32 p.i. Under non-stressed conditions, the MDM2 protein tightly regulates p53 via an autoregulatory feedback loop (Vassilev et al., 2004). Phosphorylated MDM2 serves as an ubiquitin ligase that promotes p53 degradation (Vassilev et al., 2004). Because reovirus infection increased the level of phosphorylated MDM2, the expression of p53 was inhibited from 6 h to 28 h p.i. (Fig. 3B). To examine whether both p-MDM2 and p53 were regulated in a PI3K/Akt-dependent manner, cells pre-treated with the inhibitor LY or transfected with siRNA targeting Akt were infected with MPC/04, and the levels of both genes were analyzed at 12 h p.i. As expected, LY treatment (Fig. 3C) or the knockdown of Akt (Fig. 3D) decreased p-MDM2 expression and increased p53 expression compared with mock treatment. These results indicated that reovirus infection activated the PI3K/Akt/MDM2/p53 pathway.
Fig. 3.
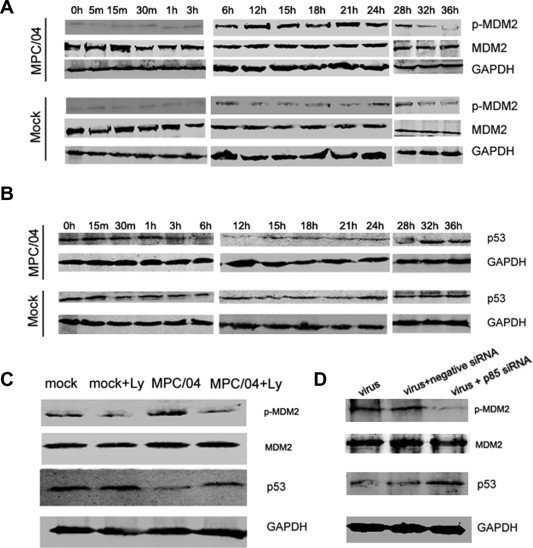
Assay of MDM2 and p53 during reovirus infection. (A) and (B) Serum-starved A549 cells were infected with MPC/04 at an MOI of 5 or mock virus. The lysates of reovirus-infected cells were harvested at the indicated times points for an analysis of phosphorylated MDM2, total MDM2, p53 and GAPDH. (C) Serum-starved A549 cells were pre-incubated with LY294002 (50 μM) for 1 h and subsequently infected with MPC/04 at an MOI of 5 or mock virus. The lysates from reovirus-infected cells were prepared 24 h p.i. for an analysis of p-MDM2, MDM2, p53 and GAPDH. (D) A549 cells (105) were transfected with 50 nM siRNA targeting Akt or negative siRNA, and then infected with MPC/04 at an MOI of 5 after 24 h transfection. The expression levels of p-MDM2, MDM2, p53, Akt and GAPDH were determined 12 h post-infection. The data represent the results of three independent experiments.
3.4. Blocking the MDM2-p53 interaction promotes virus replication
The specific MDM2 inhibitor Nutlin that can bind MDM2 in the p53-binding pocket and disrupt the MDM2-p53 interaction (Vassilev et al., 2004), was used to further examine the role of PI3K/Akt/MDM2/p53 in reovirus replication. Cells pre-treated with Nutlin were infected with MPC/04, and the viral RNA level and titers were determined. As shown in Fig. 4 A, Nutlin pre-treatment at a concentration of 5–20 μM significantly increased MPC/04 RNA synthesis (p < 0.05) in a dose-dependent manner. The virus titer results agreed with the RNA results (Fig. 4B), and Nutlin pre-treatment at all tested concentrations led to an 8- to 80-fold increase (p < 0.05). Moreover, Nutlin treatment significantly increased the RNA level of p53 (Fig. 4C). Nutlin treatment reversed the results MPC/04 infection-induced reduction in p53 expression. Infection significantly increased protein level of p53 in cells pre-treated with 20 μM Nutlin (Fig. 4D).
Fig. 4.
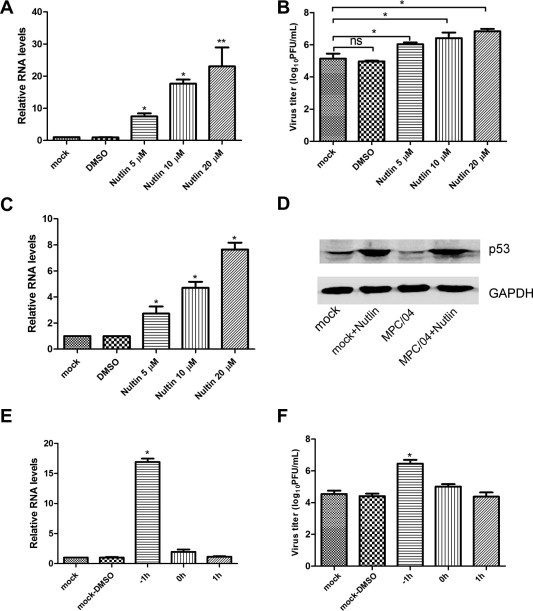
Nutlin treatment promotes reovirus replication. (A) and (B) Serum-starved A549 cells were pre-incubated with increasing concentrations of Nutlin or DMSO for 1 h and then infected with MPC/04 at an MOI of 5 in the presence of drug for 24 h. The relative expression of the viral S4 gene was analyzed by real-time PCR (A) and cell supernatants were harvested for an analysis of viral titer (B). (C) Serum-starved A549 cells were incubated with increasing concentrations of Nutlin or DMSO for 24 h. The relative expression of p53 gene was analyzed by real-time PCR. (D) Serum-starved A549 cells were incubated with Nutlin (20 μM) or DMSO for 1 h and then infected with MPC/04 at an MOI of 5 in the presence of each drug for 24 h. The lysates from reovirus-infected cells were prepared for an analysis of p53 and GAPDH (D). (E) and (F) Serum-starved A549 cells were inoculated with Nutlin (20 μM) or DMSO at the indicated time points and then infected with MPC/04 at an MOI of 5 in the presence of drug for 24 h. The relative expression of the viral S4 gene was analyzed by real-time PCR, (E) and the cell supernatants were harvested for an analysis of the virus titer (F). The data represent the results of three independent experiments. *p < 0.05, **p < 0.001. Error bars indicate SD.
To determine the time point at which PI3K/Akt/MDM2/p53 acted in the reovirus replication cycle, Nutlin (20 μM) was added to cells at −1, 0, 1 h p.i., respectively. The real-time RT-PCR results showed that the addition of Nutlin −1 h p.i. increased viral transcription approximately 15-fold compared with the vehicle-treated control (Fig. 4E). The viral titer was also significantly increased when the inhibitor was added 1 h before infection (Fig. 4F). Adding the inhibitor 0 and 1 h p.i did not produce significant differences in viral transcription and titers (Fig. 4F). These results suggested that blocking the MDM2-p53 interaction promoted virus replication.
3.5. p53 plays an important role in modulating reovirus replication
To further analyze the role of p53 in regulating viral replication, we first examined the effect of the over-expression of p53 on viral replication. p53 over-expression led to a 7.5-fold increase in viral RNA (Fig. 5 A) and 2 log PFU/mL-fold increase in the viral titer (Fig. 5B). Furthermore, the knockdown of p53 led to a 92% decrease in viral RNA (Fig. 5C) and 1.6 log PFU/mL-fold decrease in the viral titer (Fig. 5D). The inhibition of MDM2/p53 by Nutlin was expected to increase the expression of p53 and promoted viral replication. However, Nutlin treatment did not increase viral replication when p53 was knocked down (Fig. 5E and F). In contrast, viral RNA synthesis (Fig. 5E) and virus replication (Fig. 5F) was reduced, indicating that p53 was required to regulate reovirus replication via PI3K/Akt.
Fig. 5.
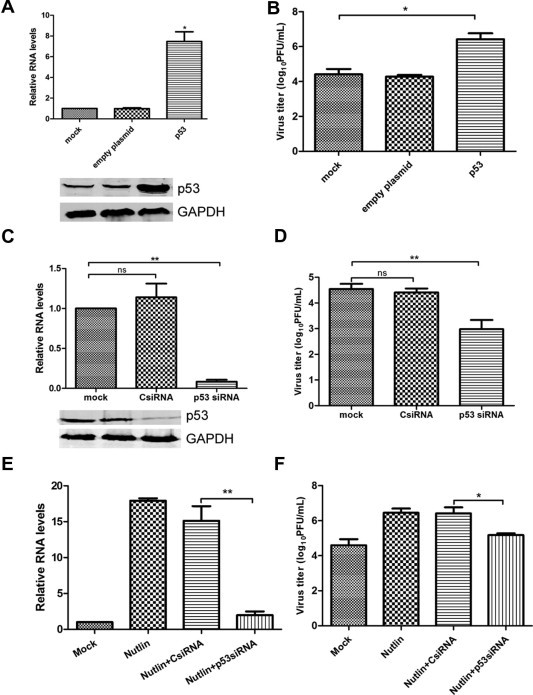
Role of p53 in reovirus replication. (A)–(D) A549 cells were transfected with a plasmid expressing p53 or siRNA targeting p53 and then infected with MPC/04 at an MOI of 5 after 24 h of transfection. Twenty-four hours after transfection, the relative expression of the viral S4 gene was analyzed by real-time PCR (A and C). The expression of p53 was examined by western blots (A and C). The virus titers were measured (B and D). (E) and (F) Twenty-four hours after transfection with siRNA targeting p53, A549 cells were inoculated with Nutlin (20 μM) or DMSO before infection and then infected with MPC/04 at an MOI of 5 in the presence of drug for 24 h. The relative expression of the viral S4 gene was analyzed by real-time PCR, (E) and the cell supernatants were harvested for an analysis of the virus titer (F). Data represent the results of three independent experiments. *p < 0.05, **p < 0.001. Error bars indicate SD.
4. Discussion
In this study, we analyzed the role of PI3K/Akt/p53 signaling pathway upon sero-type 3 reovirus-MPC/04 infection. We detected PI3K-dependent Akt phosphorylation at the early stage of infection, and the suppression of Akt activation significantly increased viral replication. Furthermore, the downstream effector MDM2/p53 of PI3K/Akt was activated during reovirus infection and involved in the regulation of viral replication. These findings demonstrate that the activation of the PI3K/Akt signaling pathway is important for inhibiting reovirus replication/infection.
Many wildlife species are reservoirs of pathogens (such as the Ebola virus and HIV) that threaten the health of domestic animals and humans. After the outbreaks of severe acute respiratory syndrome (SARS) in 2002–2003, epidemiological and pathogen investigations indicated that masked palm civets and bats are most likely amplifying reservoir hosts for the SARS coronavirus (Ge et al., 2013, Kan et al., 2005, Wang et al., 2005). Since then, other viruses have also been detected or isolated from these two animal groups, including reoviruses (Du et al., 2010, Lelli et al., 2013). The mammalian reovirus used in this study was isolated from civets in 2004 (MPC/04). It was isolated from apparently healthy animals in a surveillance study after the SARS outbreaks. Understanding the mechanisms of infection and pathogenesis of viruses of wildlife origin will provide insight into the prevention and control of infectious disease emergence.
The PI3K/Akt signaling pathway plays an important role in cell growth and survival. The PI3K/Akt pathway has been demonstrated to be involved in the replication of many viruses. The blockage of PI3K/Akt during infection with the ALV, vaccinia virus, and other viruses inhibits viral replication, but we observed that PI3K/Akt pathway activation antagonized rather than supported the virus. While the PI3K/Akt pathway is involved in the replication of many viruses, the precise molecular mechanism(s) by which PI3K/Akt regulates viral replication are not yet fully understood. In this study, we found that the downstream effector MDM2/p53 of PI3K/Akt was activated during reovirus infection, which inhibited p53 expression in cells. The up-regulation of p53 using inhibitors or the overexpression of p53 in cells promoted reovirus replication, and the knockdown of p53 by siRNA inhibited reovirus replication. Studies demonstrated that type I interferons (IFN-α/β) regulate p53 transcription, and p53 also promotes the immune responses that lead to the inhibition of viral replication. However, the replication of both EMCV and HPIV3 was inhibited after the down-regulation of p53 (Marques et al., 2005), which is consistent with our results. In our study, Nutlin treatment did not affect IFN-α/β expression (data not shown) suggesting that type I interferons did not play a role in regulating reovirus replication. The activation of PI3K, a heterodimer composed of a catalytic subunit p110 and an adaptor/regulatory subunit p85 (Ram and Ethier, 1996), leads to Akt activation. Phosphorylated Akt inactivates several pro-apoptotic proteins, including GSK-3β, to promote cell survival (Cross et al., 1995). Akt activation leads to MDM2 phosphorylation and then inhibits p53 transcription (Pan et al., 2011), which results in cell survival. p53 accumulation significantly enhanced reovirus-induced apoptosis, virus release and dissemination (Pan et al., 2011), which may be a key factor for increasing reovirus production. Another study from Zhe Liu’s research group showed that the PI3K–Akt pathway facilitates hepatitis C virus entry (Liu et al., 2012). A significant increase in p53 was observed 6 h p.i., indicating that p53 may play a role during viral replication. However, this does not necessarily rule out the possible role of the PI3K/Akt pathway and p53 in reovirus entry. Because the virus attachment and adsorption may be inhibited during the second wave of virus infection by activating the PI3K/Akt pathway and then reducing the p53 level. This could be a potential mechanism for p53 regulating reovirus infection.
In this study, all LY and Nutlin treatments as well as the overexpression of p53 could increase the viral titers to various extents. The latter two treatments were more effective than LY treatment. Other pathways that can affect p53 may be associated with this effect. Direct intervention to affect the downstream effectors may preclude other factors and benefit the study of targeted proteins.
Some studies have reported that p53 affects reoviral oncolysis. Kim et al. (2010) reported that p53 deficiency in certain cells renders them susceptible to reovirus infection and that the reovirus preferentially infects p53-deficient cancer cells compared with cells retaining the genes’ normal counterparts. Pan et al. (2011) subsequently demonstrated that the reovirus yield did not significantly differ between HCT116 p53+/+ and p53−/− cells, but treating p53 wild-type A549 cells with Nutlin-3a enhanced reovirus-induced cell death. Our study showed that p53 promoted reovirus replication in A549 cells. Thus, the function of p53 may depend on cell type. The precise molecular mechanism by which p53 regulates reovirus replication remains to be explored further.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Grant No. 31402201).
Contributor Information
Jin Tian, Email: tj6049345@126.com.
Liandong Qu, Email: qld@hvri.ac.cn.
References
- Cheng P., Lau C.S., Lai A., Ho E., Leung P., Chan F., Wong A., Lim W. A novel reovirus isolated from a patient with acute respiratory disease. J. Clin. Virol. 2009;45:79–80. doi: 10.1016/j.jcv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Chua K.B., Crameri G., Hyatt A., Yu M., Tompang M.R., Rosli J., McEachern J., Crameri S., Kumarasamy V., Eaton B.T., Wang L.F. A previously unknown reovirus of bat origin is associated with an acute respiratory disease in humans. Proc. Natl. Acad. Sci. U.S.A. 2007;104:11424–11429. doi: 10.1073/pnas.0701372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B., Voon K., Crameri G., Tan H.S., Rosli J., McEachern J.A., Suluraju S., Yu M., Wang L.F. Identification and characterization of a new orthoreovirus from patients with acute respiratory infections. PLoS ONE. 2008;3:e3803. doi: 10.1371/journal.pone.0003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B., Voon K., Yu M., Keniscope C., Abdul Rasid K., Wang L.F. Investigation of a potential zoonotic transmission of orthoreovirus associated with acute influenza-like illness in an adult patient. PLoS ONE. 2011;6:e25434. doi: 10.1371/journal.pone.0025434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooray S. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 2004;85:1065–1076. doi: 10.1099/vir.0.19771-0. [DOI] [PubMed] [Google Scholar]
- Cross D.A.E., Alessi D.R., Cohen P., Andjelkovich M., Hemmings B.A. Inhibition of glycogen-synthase kinase-3 by insulin-mediated by protein-kinase-B. Nature. 1995;378:785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- Danthi P., Coffey C.M., Parker J.S., Abel T.W., Dermody T.S. Independent regulation of reovirus membrane penetration and apoptosis by the mu1 phi domain. PLoS Pathog. 2008;4:e1000248. doi: 10.1371/journal.ppat.1000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharel N., Kato N., Muroyama R., Taniguchi H., Otsuka M., Wang Y., Jazag A., Shao R.X., Chang J.H., Adler M.K., Kawabe T., Omata M. Potential contribution of tumor suppressor p53 in the host defense against hepatitis C virus. Hepatology. 2008;47:1136–1149. doi: 10.1002/hep.22176. [DOI] [PubMed] [Google Scholar]
- Du L., Lu Z., Fan Y., Meng K., Jiang Y., Zhu Y., Wang S., Gu W., Zou X., Tu C. Xi River virus, a new bat reovirus isolated in southern China. Arch. Virol. 2010;155:1295–1299. doi: 10.1007/s00705-010-0690-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrhardt C., Wolff T., Pleschka S., Planz O., Beermann W., Bode J.G., Schmolke M., Ludwig S. Influenza A virus NS1 protein activates the PI3K/Akt pathway to mediate antiapoptotic signaling responses. J. Virol. 2007;81:3058–3067. doi: 10.1128/JVI.02082-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng S.Z., Cao W.S., Liao M. The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. J. Gen. Virol. 2011;92:1688–1697. doi: 10.1099/vir.0.030866-0. [DOI] [PubMed] [Google Scholar]
- Fields B.N., Knipe D.M., Howley P.M., Griffin D.E. 4th ed. Lippincott Williams & Wilkins; Philadelphia: 2001. Fields Virology. [Google Scholar]
- Francois F., Klotman M.E. Phosphatidylinositol 3-kinase regulates human immunodeficiency virus type 1 replication following viral entry in primary CD4+ T lymphocytes and macrophages. J. Virol. 2003;77:2539–2549. doi: 10.1128/JVI.77.4.2539-2549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., Zhang Y.J., Luo C.M., Tan B., Wang N., Zhu Y., Crameri G., Zhang S.Y., Wang L.F., Daszak P., Shi Z.L. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb E., Lindner S., Oren M. Relationship of sequence-specific transactivation and p53-regulated apoptosis in interleukin 3-dependent hematopoietic cells. Cell Growth Differ. 1996;7:301–310. [PubMed] [Google Scholar]
- Hand R., Tamm I. Reovirus: analysis of proteins from released and cell-associated virus. J. Gen. Virol. 1971;12:121–130. doi: 10.1099/0022-1317-12-2-121. [DOI] [PubMed] [Google Scholar]
- Helentjaris T., Ehrenfeld E. Inhibition of host cell protein synthesis by UV-inactivated poliovirus. J. Virol. 1977;21:259–267. doi: 10.1128/jvi.21.1.259-267.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G.G., Muldoon R.L. Viruses causing common respiratory infection in man. IV. Reoviruses and adenoviruses. J. Infect. Dis. 1973;128:811–866. doi: 10.1093/infdis/128.6.811. [DOI] [PubMed] [Google Scholar]
- Kan B., Wang M., Jing H., Xu H., Jiang X., Yan M., Liang W., Zheng H., Wan K., Liu Q., Cui B., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Qi X., Chen K., Du L., Gao K., Zhao Y.T., Zou X.Z., Feng Y.J., Gao Y.F., Hai R., Yu D., Guan Y., Xu J. Molecular evolution analysis and geographic investigation of severe acute respiratory syndrome coronavirus-like virus in palm civets at an animal market and on farms. J. Virol. 2005;79:11892–11900. doi: 10.1128/JVI.79.18.11892-11900.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna K.K., Jackson S.P. DNA double-strand breaks: signaling, repair and the cancer connection. Nat. Genet. 2001;27:247–254. doi: 10.1038/85798. [DOI] [PubMed] [Google Scholar]
- Kim M., Williamson C.T., Prudhomme J., Bebb D.G., Riabowol K., Lee P.W.K., Lees-Miller S.P., Mori Y., Rahman M.M., McFadden G., Johnston R.N. The viral tropism of two distinct oncolytic viruses, reovirus and myxoma virus, is modulated by cellular tumor suppressor gene status. Oncogene. 2010;29:3990–3996. doi: 10.1038/onc.2010.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli D., Moreno A., Lavazza A., Bresaola M., Canelli E., Boniotti M.B., Cordioli P. Identification of Mammalian orthoreovirus type 3 in Italian bats. Zoonoses Public Health. 2013;60:84–92. doi: 10.1111/zph.12001. [DOI] [PubMed] [Google Scholar]
- Liu Z., Tian Y.J., Machida K., Lai M.M.C., Luo G.X., Foung S.K.H., Ou J.H.J. Transient activation of the PI3K–AKT pathway by Hepatitis C virus to enhance viral entry. J. Biol. Chem. 2012;287:41922–41930. doi: 10.1074/jbc.M112.414789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marques J.T., Rebouillat D., Ramana C.V., Murakami J., Hill J.E., Gudkov A., Silverman R.H., Stark G.R., Williams B.R. Down-regulation of p53 by double-stranded RNA modulates the antiviral response. J. Virol. 2005;79:11105–11114. doi: 10.1128/JVI.79.17.11105-11114.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton J.K., Agosto M.A., Severson T.F., Yin J., Nibert M.L. Thermostabilizing mutations in reovirus outer-capsid protein mu1 selected by heat inactivation of infectious subvirion particles. Virology. 2007;361:412–425. doi: 10.1016/j.virol.2006.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Pan L.Z., Hill R., Marcato P., Shmulevitz M., Vassilev L.T., Lee P.W.K. Stabilisation of p53 enhances reovirus-induced apoptosis and virus spread through p53-dependent NF-kappa B activation. Br. J. Cancer. 2011;105:1012–1022. doi: 10.1038/bjc.2011.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ram T.G., Ethier S.P. Phosphatidylinositol 3-kinase recruitment by p185(erbB-2) and erbB-3 is potently induced by neu differentiation factor/heregulin during mitogenesis and is constitutively elevated in growth factor-independent breast carcinoma cells with c-erbB-2 gene amplification. Cell Growth Differ. 1996;7:551–561. [PubMed] [Google Scholar]
- Rosen L., Hovis J.F., Mastrota F.M., Bell J.A., Huebner R.J. Observations on a newly recognized virus (Abney) of the reovirus family. Am. J. Hyg. 1960;71:258–265. doi: 10.1093/oxfordjournals.aje.a120109. [DOI] [PubMed] [Google Scholar]
- Saeed M.F., Kolokoltsov A.A., Freiberg A.N., Holbrook M.R., Davey R.A. Phosphoinositide-3 kinase-Akt pathway controls cellular entry of Ebola virus. PLoS Pathog. 2008;4:e1000141. doi: 10.1371/journal.ppat.1000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin Y.K., Liu Q., Tikoo S.K., Babiuk L.A., Zhou Y. Influenza A virus NS1 protein activates the phosphatidylinositol 3-kinase (PI3K)/Akt pathway by direct interaction with the p85 subunit of PI3K. J. Gen. Virol. 2007;88:13–18. doi: 10.1099/vir.0.82419-0. [DOI] [PubMed] [Google Scholar]
- Smith R.E., Zweerink H.J., Joklik W.K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology. 1969;39:791–810. doi: 10.1016/0042-6822(69)90017-8. [DOI] [PubMed] [Google Scholar]
- Soares J.A., Leite F.G., Andrade L.G., Torres A.A., De Sousa L.P., Barcelos L.S., Teixeira M.M., Ferreira P.C., Kroon E.G., Souto-Padron T., Bonjardim C.A. Activation of the PI3K/Akt pathway early during vaccinia and cowpox virus infections is required for both host survival and viral replication. J. Virol. 2009;83:6883–6899. doi: 10.1128/JVI.00245-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoka A., Hayakawa S., Yanai H., Stoiber D., Negishi H., Kikuchi H., Sasaki S., Imai K., Shibue T., Honda K., Taniguchi T. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424:516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- Turpin E., Luke K., Jones J., Tumpey T., Konan K., Schultz-Cherry S. Influenza virus infection increases p53 activity: role of p53 in cell death and viral replication. J. Virol. 2005;79:8802–8811. doi: 10.1128/JVI.79.14.8802-8811.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler K.L., Barton E.S., Ibach M.L., Robinson C., Campbell J.A., O’Donnell S.M., Valyi-Nagy T., Clarke P., Wetzel J.D., Dermody T.S. Isolation and molecular characterization of a novel type 3 reovirus from a child with meningitis. J. Infect. Dis. 2004;189:1664–1675. doi: 10.1086/383129. [DOI] [PubMed] [Google Scholar]
- Vassilev L.T., Vu B.T., Graves B., Carvajal D., Podlaski F., Filipovic Z., Kong N., Kammlott U., Lukacs C., Klein C., Fotouhi N., Liu E.A. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Wang M., Yan M., Xu H., Liang W., Kan B., Zheng B., Chen H., Zheng H., Xu Y., Zhang E., Wang H., Ye J., Li G., Li M., Cui Z., Liu Y.F., Guo R.T., Liu X.N., Zhan L.H., Zhou D.H., Zhao A., Hai R., Yu D., Guan Y., Xu J. SARS-CoV infection in a restaurant from palm civet. Emerg. Infect. Dis. 2005;11:1860–1865. doi: 10.3201/eid1112.041293. [DOI] [PMC free article] [PubMed] [Google Scholar]


