Summary
First identified in humans in Hong Kong, influenza A/H5N1, known commonly as avian influenza, has caused human disease in 15 countries around the world. Although the current number of confirmed patients is tiny compared to seasonal and the recently emerged H1N1 ‘swine’ influenza, H5N1 remains a candidate for the next highly pathogenic influenza pandemic. Currently, H5N1 has very limited ability to spread from person-to-person but this may change because of mutation or reassortment with other influenza viruses leading to an influenza pandemic with high mortality. If this occurs travellers are likely to be affected and travel medicine doctors will need to consider avian influenza in returning febrile travellers. The early clinical features may be dismissed easily as ‘the flu’ resulting in delayed treatment. Treatment options are limited. Oral oseltamivir alone has been the most commonly used drug but mortality remains substantial, up to 80% in Indonesia. Intravenous peramivir has been filed for registration and IV zanamivir is being developed. This review will focus on the epidemiological and clinical features of influenza A/H5N1 avian influenza and will highlight aspects relevant to travel medicine doctors.
Keywords: Avian influenza, Travellers, H5N1, ARDS
Introduction
Avian influenza due to the influenza A/H5N1 virus has emerged as a potential global threat.1 The H5N1 virus joins a list of other respiratory pathogens that have emerged in the past 40 years and which often cause severe clinical disease like Legionella pneumophila, SARS coronavirus and Hanta virus pulmonary syndrome.2, 3, 4
When a new pathogen emerges and causes a high mortality, much angst is generated globally. The main question is whether there is potential for efficient spread from person-to-person and, therefore, the risk of an epidemic or pandemic. To date, person-to-person transmission of H5N1 has been very rare and associated with close human contact.5 Never the less, the threat remains that H5N1 may cause the next pandemic with substantial mortality.
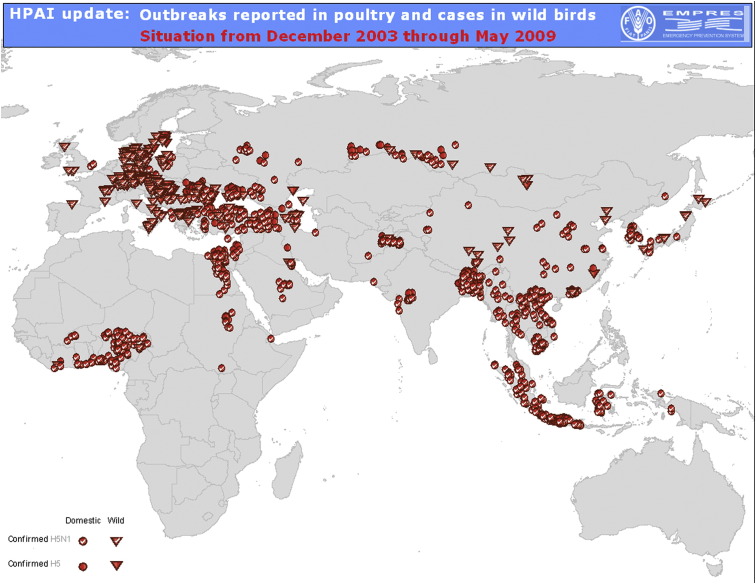
The global spread of avian influenza has been through the poultry trade and infected birds and many countries have experienced poultry outbreaks (Fig. 1 ). By contrast, the SARS epidemic and the current (2009) pandemic of H1N1 swine influenza are good examples of how global travel played an important role in the spread of these two viruses.3, 6 If H5N1 acquired the ability to spread easily, then air travel would be a very efficient way to transport infected passengers and cause outbreaks far from their original source.
Figure 1.
Map of the global distribution of poultry outbreaks since the first detection of H5N1 in 2003 to June 2009.
H5N1 infection of humans was first reported in Hong Kong in 1997, causing 6 deaths in 18 confirmed patients. Infected poultry in Hong Kong's wet markets were the likely source.7, 8 Mass culling of chickens was necessary to curtail this small epidemic. As of September 2009, H5N1 has been documented and reported to the WHO in 442 patients in 15 countries of which 6 are in SE Asia (www.who.int/csr/disease/avianinfluenza/country/casestable200809_10/en/index.htm, accessed November, 2009). H5N1 infections in wild birds, chickens and other poultry have been reported in 61 countries in Africa, Asia and Europe (Fig. 1).
The history of influenza pandemics explains the anxiety regarding H5N1. There have been three pandemics of influenza in the past 100 years and the one upper most in our minds is the 1918 pandemic because of its size and high, total mortality of some 40 million people.9 Patients of all ages died, including young adults aged 20–40 years. This was a distinct difference from the usual pattern of mortality of seasonal influenza epidemics in which patients at the extremes of age suffer the greatest mortality. A lack of influenza immunity was an important reason for its easy spread and high mortality which has also been attributed to viral genes that facilitate high viral loads, excessive innate inflammatory response and fatal primary viral pneumonia.10, 11, 12, 13 A recent re-examination of microbiological data and lung tissue from 1918 has suggested strongly that secondary bacterial pneumonia also played a key role in the high mortality.14 In 2009, the world is experiencing a fourth pandemic of a novel influenza A/H1N1 virus. Our knowledge of this infection is increasing and important lessons learnt may impact our thinking regarding H5N1.15, 16
One theme is common to the three major influenza pandemics and the ‘swine’ flu pandemic; the viruses responsible had an avian link. The H1N1 1918 pandemic is thought to have arisen through the adaptation of an avian like influenza virus that allowed for easy human to human transmission whereas the H2N2 (1957 pandemic) and H3N2 (1968 pandemic) influenza viruses acquired new genes from Eurasian avian viruses by genetic reassortment with the circulating human H1N1 virus.9 The 2009 ‘swine’ H1N1 influenza virus is an assortment of avian, human and swine influenza viruses.17 Currently, highly pathogenic H5N1 avian influenza virus continues to cause outbreaks in poultry and has gained the ability to jump species and infect humans and other mammals.18
The travel medicine doctor may be the first person a sick patient sees when returning from a trip overseas. Although still rare, avian influenza now needs to be on the differential diagnosis in a returning, febrile traveller. Travel medicine doctors often see rare diseases that capture the imagination. However romantic this notion, we should not forget that they are an important early defence in the potential spread of imported infectious diseases.
This review will focus on those aspects of H5N1 pertinent to the travel medicine doctors and will include the epidemiology, clinical features, diagnostics and management of a patient with avian influenza.
Clinical features
Human influenza is well known as a cause of, usually, a self-limiting infection of the upper respiratory tract. Influenza related mortality is confined generally to the very young, the elderly, the immunocompromised, patients with underlying cardiorespiratory illness and influenza complications, notably, secondary bacterial pneumonia, encephalitis, rhabdomyolysis and myocarditis.19, 20, 21
Avian influenza has important contrasting features. It affects individuals of all ages, causes frequently a severe and rapidly progressive pneumonitis and a high mortality even in fit and healthy patients.7, 22 The mean incubation period is 2–3 days with a reported range of 2–9 days. Most cases are clinically manifest within 7 days of exposure to e.g. sick poultry.23, 24, 25 Therefore, a traveller can easily become symptomatic once back in his/her home country or visiting another country where H5N1 may not have occurred.
Most of the clinical experience and published literature are based on hospitalised patients, so the clinical picture is biased towards those with greater illness severity. In general, individual clinical series have been small (<30 patients) but two larger studies have been published recently from Indonesia (n = 127) and Vietnam (n = 67).26, 27 Clearly, combining all these disparate databases would be helpful to define better the clinical features of the disease, assess robustly poor prognostic factors and enhance our ability to conduct clinical research.
Symptoms and signs
Our knowledge of the early stages of H5N1 is somewhat scant because most series are from hospitalised patients with advanced disease. The best data are from the series of Kandun et al. The majority of patients reported a mild prodromal phase characterised by fever, cough and runny nose. Only a minority (9%) complained of early dyspnoea; a clinical picture that may easily be dismissed as the common cold or another infectious disease prevalent in that setting.26, 28 As a result, patients may have visited several clinics or hospitals before the proverbial ‘penny drops’.27, 29, 30 With this time delay, the clinical picture is likely to have progressed significantly when cough (88%) and dyspnoea (84%) dominate the clinical picture.26 Missing the diagnosis of a patient with a non descript febrile illness is not new in travel medicine and has occurred in patients with early malaria, a disease that is often placed first in the differential diagnosis of a fever in a traveller.31, 32
The prodromal symptoms of H5N1 are followed by increasing shortness of breath that may become incapacitating within several days, necessitating hospital admission. However, a minority of patients do not develop severe disease and have an otherwise mild course that has responded well to oseltamivir.7, 22 Other reported symptoms have included myalgia (13%), headache (13%), vomiting (16%), diarrhoea (14%), nausea (7%), epigastric pain (4%), convulsion (2%) and constipation (2%).22, 26
Physical signs depend on the severity of illness at presentation and may include fever, tachypnoea, dyspnoea, tachycardia, a normal or low blood pressure, and inspiratory crepitations; wheezes may also be heard but less frequently than crepitations. Signs of a pleural effusion may also be present. In our experience, pneumothoraces have generally occurred during mechanical or noninvasive ventilation but have also occurred in non ventilated patients; cutaneous crepitus may be found. There are no human data on the possible occurrence of H5N1 induced myocarditis.
As patients deteriorate clinically, their degree of respiratory failure often worsens and they develop the acute respiratory distress syndrome (ARDS); this has occurred between 4 and 13 days (median 6 days) of illness.24, 33, 34 We have not seen acute renal failure requiring haemofiltration or haemodialysis nor the classic signs of acute hepatic failure. In <10% of patients, there is spontaneous bleeding from mucosal or venepuncture sites.24, 27, 35
Patients usually die of progressive respiratory failure, a number of whom are also on inotropic support for hypotension. Death has occurred between 6 and 16 days of illness.23, 26, 35 Some patients have responded favourably to oseltamivir and cleared their H5N1 virus only to die of a ventilator associated pneumonia due to e.g. by multi drug resistant Acinetobacter baumanii.
Although a predominantly respiratory illness, clinicians should be aware of atypical, nonrespiratory presentations. Case reports have documented a child who first presented with severe diarrhoea followed by convulsions and coma36 and an adult female who presented with ‘gastroenteritis’ followed by severe pneumonia.37 Both died.
Laboratory results
The full blood count in most patients shows either a normal haemoglobin or a mild anaemia. White cell count abnormalities are common. Leukopenia, defined as either a total white cell count <5000 or 4000/μL occurs in 27–84%, lymphopenia [<1000 (>7 years) to <1500/μL (2 to ≤6 years)] in 16–55%, any degree of neutropenia (<2000 or <2200/μL) in 30–50% of patients in Vietnam and Indonesia, severe neutropenia (<1000/μL) in <10%. Thrombocytopenia (platelet count <150,000/μL) is common (31–64%)22, 35 and severe thrombocytopenia (<50,000/μL) affected 10% of Indonesian patients.30
Using the International Society of Thrombosis and Homeostasis definition,38 disseminated intravascular coagulation is present in up to 20–25% of patients but spontaneous bleeding from mucosal or venepuncture sites is reported in <10%.24, 27, 35 A number of patients may have raised d-dimers but normal PTs and aPTTs with a low or normal fibrinogen concentrations, consistent with H5N1 fibrinolysis.24, 30
Liver enzymes are generally moderately elevated with higher AST (3–10 × ULN) values compared to ALT (up to 4 × ULN) values; AST and ALT values exceeding 1000 and 400, respectively, have been seen in some patients. Where measured the lactate dehydrogenase (LDH) has also been high (2–10 × ULN) and is most likely to be of lung origin.27, 33, 35
The serum creatinine may be mild or moderately raised and urine microscopy, where reported, has been unremarkable aside from proteinuria and microscopic haematuria. The creatinine phosphokinase (CPK) may be raised up to 10 fold above the upper limit of normal but acute rhabdomyolysis with myoglobinuria and renal impairment have not yet been reported. There are no published data on the CPKmb fraction or on cardiac troponins. Raised blood glucose concentrations in patients without known diabetes mellitus have been observed at presentation, consistent with a stress induced hyperglycaemia, and during hospital admission, usually in association with steroid use and have required intravenous insulin treatment in some patients.24, 28, 34, 35
Pleural fluid may be straw coloured or haemorrhagic with high total protein and LDH concentrations, meeting the Light criteria for an exudate, and may be H5N1 positive.30, 39
Radiology
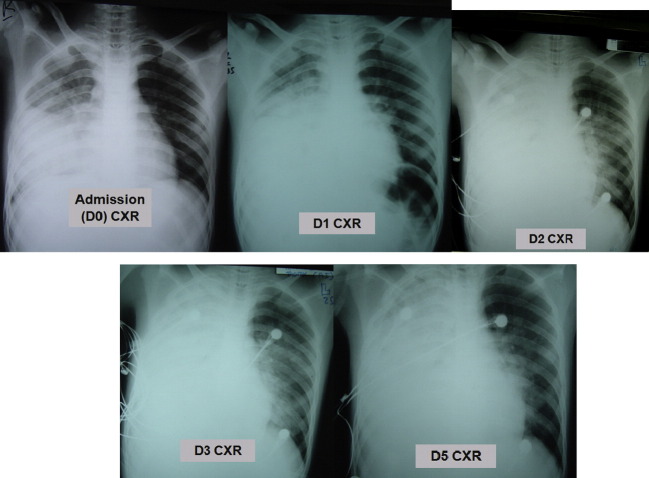
Radiographic pneumonia has occurred in almost all hospitalised patients but these changes are not H5N1 specific. Chest X-ray (CXR) shadowing has included diffuse, multi-focal or patchy infiltrates, interstitial infiltrates, lobular consolidation with air bronchograms, pneumothoraces and pneumomediastina.40, 41 Sequential CXR shadows may worsen in parallel with the clinical state of the patient (Fig. 3 ). Findings on chest CT scan have been consistent but have also shown destroyed lung architecture, persistent ground glass attenuation, segmental consolidation, pseudocavitation, pneumatoceles, lymphadenopathy, centrilobular nodules and post recovery fibrosis (Fig. 3).
Figure 3.
Chest X-ray series in an adult male patient with fatal H5N1. He was admitted after 8 days of illness. Admission (D0) CXR shows opacification in the right mid zone, outlining the horizontal fissure and obscuring the right heart border, in keeping with middle lobe consolidation. On Day 1 there has been development of additional right lower lobe consolidation, as demonstrated by obscuration of the right hemidiaphragm. By Day 2, the patient has been intubated; there is now complete opacification of the right hemithorax and left perihilar consolidation, likely representing additional pulmonary oedema. There has been little radiographic change at Day 3. No CXR was done on Day 4. Day 5: the parenchymal opacification is stable but there is now a moderate right pleural effusion. He died on Day 6.
Poor prognostic features
There are many reported poor prognostic factors. Delayed oseltamivir treatment was an important, independent factor in the 127 patient Indonesian series.26 Many other factors have not been robustly tested for independence in multivariate analyses but they are probably useful clinical pointers: (i) admission leukopenia, lymphopenia, neutropenia, thrombocytopenia, (ii) increased LDH on admission, (iii) no oseltamivir/other antiviral treatment, (iv) the use of corticosteroids, (v) bilateral CXR shadowing, (vi) the development of ARDS, (vii) blood positive for H5N1 (H5N1 viraemia) and (viii) high cytokine concentrations.7, 23, 26, 28, 33, 35, 42 Data from Indonesia suggest hyperglycaemia and raised d-dimers are also poor prognostic factors.30
H5N1 infections have caused high mortality across all age groups. In some clinical series, younger patients have experienced a higher mortality which may be due to cross protective immunity from previous human influenza in older individuals.33
Transmission
The main route of transmission of H5N1 from poultry to humans is probably via the respiratory tract but the eye, via the conjunctival mucosa, and gut are other possible routes. Infection through the gut could occur by licking contaminated fingers or swallowing water contaminated by poultry infected faeces. Poultry excrete large amounts of H5N1 virus in their faeces that can survive in soil and water.43, 44 Poultry faeces is used as a fertiliser and fish feed in some countries.1
Close contact with chickens or direct handling of infected, poultry faeces represents a risk factor for infection. In the H5N1 outbreak in Turkey, some children shared their living space with the infected chickens24 and in SE Asia, where home rearing of poultry is common, chickens and ducks may gather to deposit their faeces in ponds which are commonly used by children for bathing and playing.45 Infected poultry may appear well yet be excreting virus in the environment. So apparently healthy poultry pose a risk.
Infected individuals who visited wet markets (Fig. 2) where infected poultry were sold developed H5N1 about one week later.46, 47, 48 Determining how they became infected is speculative but could have been self-inoculation of the respiratory tract after touching H5N1-contaminated surfaces, or eggs or inhalation of aerosolized debris containing H5N1 virus.
Figure 2.

Ducks in a wet market in Hanoi, Vietnam.
Downloaded from the Food and Agriculture Organisation web site (http://www.fao.org).
Human to human transmission has been documented rarely and usually after close and prolonged contact in persons of the same familial bloodline (e.g. father to son).5, 26, 49 Nosocomial transmission, manifest as asymptomatic seroconversion, is also rare but this does not obviate the need for good infection control practices.50, 51, 52, 53 Importantly, in about a quarter of patients, there is no history of a high risk exposure; so, a lack of a history of a high risk exposure does not exclude the possibility of H5N1.
Pretravel advice
H5N1 is a cosmopolitan disease (Fig. 1). Three countries account for three quarters of the cases reported to the WHO: Indonesia, Vietnam and Egypt. Other avian viruses may also cause outbreaks, most notably, the 2003 H7N7 outbreak in Holland that affected 89 individuals and resulted in one death.54, 55 Given the increased awareness of H5N1, other highly pathogenic viruses like Corona SARS, and more recently the ‘swine’ flu pandemic, travellers are very likely to ask how to protect themselves.
The overall risk of travellers contracting H5N1 is very low. There are currently no travel restrictions to countries where bird or human cases have been or are being reported, and travellers are not recommended to carry standby oseltamivir. Nevertheless, it remains important to advise individual travellers so they are aware of the behaviours or exposures that have led to H5N1 disease in local populations. There is currently no readily available vaccine against H5N1 but a number have received regulatory approval.56 Vaccination against seasonal influenza should be considered for travellers at high risk of influenza complications, consistent with many national guidelines. Seasonal influenza vaccines do not offer protection against H5N1 so travellers should be advised of this. There are no data on the emerging 2009 ‘swine’ H1N1 vaccines.
General advice (Box 1 ) includes staying away from dead or live birds and poultry, like chickens, turkeys, geese, ducks and quail.57 This applies especially to children who may wish to play with their new found friends. For travellers who end up in wet markets, avoiding direct contact with chickens, ducks, eggs, dirty cages and slaughtering counters etc is wise. “Take your photographs from a distance, use the zoom function!” might be sensible advice. Well cooked poultry and eggs do not pose a threat to health but H5N1 virus can be detected in raw eggs. Good hand hygiene remains an important preventative measure and some travellers may wish to purchase commercially available hand sanitisers that are effective against H5N1 and other influenza viruses.
Box 1. An adaptation of advice offered to travellers by the UK Health Protection Agency and the US CDC (http://www.hpa.org.uk, http://wwwn.cdc.gov/travel/contentAvianFluAsia.aspx).
- Be careful with birds
- Avoid visiting live animal markets and poultry farms
- Avoid all direct contact with birds, including poultry (such as chickens and ducks) and wild birds.
- Avoid touching surfaces that have bird droppings (faeces) or other bird fluids on them.
- Avoid places such as poultry farms and bird markets where live birds are raised or kept.
- Do not pick up or touch dead or dying birds
- Do not eat or handle undercooked or raw poultry, egg, poultry blood or duck dishes
- Eat only bird meat or products that have been thoroughly cooked.
- Egg yolks should not be runny or liquid.
- Do not attempt to bring any live poultry products back to the UK
- Practice healthy habits to help stop the spread of germs
- Wash your hands often with soap and clean water.
- This removes germs from your skin and helps prevent diseases from spreading.
- Use waterless alcohol-based hand gels (containing at least 60% alcohol) when soap and clean water are not available and hands are not visibly dirty.
- Seek medical care if you feel sick
- If you become sick with a fever plus a cough and sore throat, or have trouble breathing, seek medical care right away. Tell the doctor if you have had contact with sick or dead birds.
If a traveller develops a febrile illness, he/she should seek medical advice early and report any potential exposure to chickens and other poultry, even if only fleetingly.
Several airborne transmitted diseases like tuberculosis and human influenza have caused small outbreaks within the confines of aircraft, buses and trains.58, 59, 60, 61 Given the very low transmission potential of H5N1 viruses, travellers can be assured that travel by air etc is safe.
Evaluation of a traveller with suspected avian influenza
Evaluation of the returned traveller with a fever is the bread and butter of all travel medicine doctors. General practitioners should also be aware of serious illnesses in recently returned travellers and should refer patients quickly for evaluation. There are several reviews on fever in the returned traveller.62, 63
Because the risk of H5N1 in travellers is extremely low and its early clinical manifestations can mimic other febrile illnesses like the ‘flu’, malaria, dengue and enteric fever; thinking of H5N1 requires a high index of suspicion. An awareness of the case definitions of avian influenza (Box 2 ) may be helpful to guide the taking of a good history, especially regarding potential exposure (http://www.who.int/csr/disease/avian_influenza/guidelines/casedefinition2006_08_29/en/,http://www.cdc.gov/flu/avian/professional/guidance-labtesting.htm).
Box 2. The WHO definition of a suspected and probable case of H5N1.
Suspected H5N1 case
A person presenting with unexplained acute lower respiratory illness with fever (>38 °C) and cough, shortness of breath or difficulty breathing AND One or more of the following exposures in the 7 days prior to symptom onset:
-
a.
Close contact (within 1 m) with a person (e.g. caring for, speaking with, or touching) who is a suspected, probable, or confirmed H5N1 case.
-
b.
Exposure (e.g. handling, slaughtering, defeathering, butchering, preparation for consumption) to poultry or wild birds or their remains or to environments contaminated by their faeces in an area where H5N1 infections in animals or humans have been suspected or confirmed in the last month.
-
c.
Consumption of raw or undercooked poultry products in an area where H5N1 infections in animals or humans have been suspected or confirmed in the last month.
-
d.
Close contact with a confirmed H5N1 infected animal other than poultry or wild birds (e.g. cat or pig).
-
e.
Handling samples (animal or human) suspected of containing H5N1 virus in a laboratory or other setting.
Probable H5N1 case (notify WHO)
-
Probable definition 1:
A person meeting the criteria for a suspected case AND one of the following additional criteria:-
a.infiltrates or evidence of an acute pneumonia on chest radiograph plus evidence of respiratory failure (hypoxemia, severe tachypnoea) OR
-
b.positive laboratory confirmation of an influenza A infection but insufficient laboratory evidence for H5N1 infection.
-
a.
-
Probable definition 2:
A person dying of an unexplained acute respiratory illness who is considered to be epidemiologically linked by time, place, and exposure to a probable or confirmed H5N1 case.
For patients who present with a predominantly respiratory illness, other respiratory pathogens may be thought of before H5N1 e.g. influenza A/H1N1 or H3N2, pneumococcal, legionella and mycoplasma pneumonia. Lung involvement also characterizes a number of systemic tropical infections, notably, falciparum and vivax malaria, severe typhoid fever, leptospirosis, typhus and melioidosis.64, 65, 66, 67, 68 It is also important to consider the existence of coinfections in travellers even if they may present with an apparently clear cut clinical illness.
Many other febrile illnesses will share the laboratory features of H5N1 e.g. low total white cell count, thrombocytopenia, mildly raised PT, aPTT, raised liver enzymes and mild renal impairment. Blood eosinophilia, however, would make H5N1 unlikely.
Experience to date with returned travellers is limited. In one report of 59 travellers with suspected avian influenza, all were H5N1 negative but 25 were positive for seasonal influenza. In an era of heightened awareness of H5N1, many returning travellers who are tested are likely to be H5N1 negative.69
Diagnostics
Faced with a number of possible diagnoses, choosing appropriate diagnostic tests is essential. Aside from the routine diagnostic tests ordered for febrile travellers, obtaining good quality, respiratory specimens is crucial. All specimens should be considered high risk, labelled ‘Biohazardous’ and transported to the laboratory, according to guidelines for infected specimens.
For patients who are well, a nasopharyngeal (NP) sample by either aspiration (NPA) or using an NP swab is the sample of choice and should be obtained observing all the necessary infection control measures. A fresh specimen is preferred but if laboratory processing is not possible immediately, the sample may be stored in a fridge at 4 °C for up to 24 h.
Throat and nose swabs should also be taken on admission and daily thereafter to document clearance of virus. For ventilated patients, an endotracheal aspirate should be obtained. Limited experience from SE Asia shows that an endotracheal aspirate has a higher yield than an NPA which in turn is better than a throat and nasal swab (H. Wertheim, unpublished observations).
Currently available, rapid commercial tests are designed primarily for human A and B influenza and have low sensitivity and specificity for H5N1 and should not be relied upon to diagnose H5N1. A number of user friendly, nucleic acid based, rapid tests are in development for the detection of H5N1 and should be available in the future.70 These will need independent evaluation by reference laboratories.71
At present, the best way to diagnose H5N1 is by reverse transcriptase polymerase chain reaction (RT PCR) on a respiratory specimen. RT PCR will tell the physician the type of influenza virus i.e. A, B or C and its H and N subtype e.g. H5N1, H3N2, H1N1, H7N7 etc. The result may take 8–12 h. Not all laboratories can do viral subtyping and will only report e.g. influenza A, subtype unknown. Full virus characterisation will then need to be done in a reference laboratory. A real time RT PCR method is also available to measure the viral load.
H5N1 is a systemic disease that has been detected in plasma, pleural fluid, rectal swabs and CSF.30, 36, 42, 72 Therefore, samples should not be restricted to the respiratory tract.
NPAs, swabs and plasma and, if applicable, post mortem biopsies, are valuable specimens and should be stored. Failure to detect H5N1 may mean another respiratory illness that may be diagnosed with the stored specimens.
Serology on paired serum samples can diagnose retrospectively H5N1. Detecting antibodies by haemagglutination inhibition (HI) and neutralisation are the two principal methods. The latter is more sensitive, detecting antibodies some 14 days post infection but is more labour intensive than HI and requires the use of a BSL-3 laboratory.1
Antiviral resistance and clades
The H5N1 family of viruses is composed of a number of clades (strains).35 Evidence to date suggests that different clades have similar clinical pictures.23, 73 Of therapeutic importance is their varying in vitro sensitivity to the currently available antiviral drugs (Table 1 ). Clade 1 H5N1 is intrinsically more sensitive than the clade 2 H5N1 to oseltamivir. The experience from seasonal influenza viruses is that sensitive viruses may mutate and develop reduced sensitivity to the neuraminidase inhibitors, oseltamivir, zanamivir and peramivir and the adamantanes, amantadine and rimantadine.74, 75, 76, 77, 78
Table 1.
Summary of the in vitro sensitivity of different H5N1 clades.a The IC50 is the concentration of drug that inhibits viral growth by 50%.
| Oseltamivir carboxylate | Zanamivir | Amantadine | |
|---|---|---|---|
| Clade 1: China, SE Asia | 0.5–2.9 | 1.2–1.9 | 95% |
| Clade 2.1: Indonesia | 11.5 | 1.4 | 80% |
| Clade 2.2: China, SE Asia,Middle East, Europe, Africa | 10.8 | 1.4 | No data |
| Clade 2.3.4: China, SE Asia | 2.5–3.8 | 1.2–1.3 | 0% |
A notable example is the recent and rapid emergence globally of seasonal, non ‘swine’, influenza A/H1N1 with reduced sensitivity to oseltamivir due to the H274Y mutation but in the absence of oseltamivir use in communities. The H274Y mutation confers about a marked increase in the IC50 against oseltamivir and peramivir for both seasonal and avian influenza. The reported increase varies widely from 200 to over 1000 fold.74, 75, 79, 80, 81 The majority of H5N1 viruses remain sensitive to oseltamivir but oseltamivir resistant H5N1 has emerged during oseltamivir treatment in H5N1 infected patients who were treated with 75 mg 12 hourly; the virus was found to have the H274Y mutation.72
Monitoring of in vitro sensitivity of influenza viruses by National Reference Laboratories within the Global Neuraminidase Inhibitor Susceptibility Network is an important surveillance activity and a source of up to date information.82, 83
Treatment
The number of registered antiviral drugs is limited to oral amantadine, rimantadine, oseltamivir and inhaled zanamivir. Intravenous zanamivir is being developed but is available for compassionate use on a named patient basis. IV peramivir has been filed for registration in Japan and has been authorised for use in the USA by the US Food and Drug Administration (FDA) for pandemic H1N1. The neuraminidase inhibitors inhibit viral neuraminidase from breaking the bond between newly formed virions and the sialic acid receptor on the epithelial cell surfaces, thus, preventing their release. The adamantanes inhibit the ion channel function of the M2 matrix protein which results in failure of the virus to uncoat after infecting a cell.
The current mainstay of treatment for avian influenza is oral oseltamivir alone. Recent retrospective data suggest oseltamivir may reduce the mortality of H5N1 by half compared to untreated patients (Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) 2009. Abstract V-533, 9/13/2009). There is no published experience on the use of antiviral combinations in humans but animal data suggest oseltamivir plus ribavirin and amantadine plus oseltamivir are synergistic but, for the latter, only with amantadine sensitive H5N1 virus. These animal studies may pave the way for trials in humans.84, 85
The optimal doses of antiviral drugs in H5N1 or other forms of severe influenza have not been evaluated rigorously in randomised trials. Given the rarity of H5N1, such trials may never be conducted. One ongoing study is evaluating double versus standard dose oseltamivir in the treatment of H5N1 and severe seasonal influenza in Vietnam, Indonesia and Thailand under the auspices of the SEA Infectious Diseases Clinical research Network (www.seaicrn.org).
Recommended doses of antivirals are based on mild human influenza but the WHO recommends that physicians consider a double dose of oseltamivir in severely ill patients.86 However, this is already common practice for H5N1 and for severe ‘swine’ H1N1 in countries like Vietnam and Indonesia. The somewhat cautious approach of the WHO seems unwarranted. Proper dose ranging studies are needed to determine the optimal dose of oseltamivir alone or in combination. Amantadine or rimantadine at the same doses used for human influenza can be added if the infecting clade is known to be sensitive e.g. the clade 2.3.4 in northern Vietnam and some 2.1 clades.73 The adamantanes have a narrower therapeutic index than oseltamivir (which is very well tolerated), thus limiting the possibility of increasing their doses. Using combinations may reduce the probability of the development of resistance on treatment.87
Oseltamivir
Oseltamivir phosphate, the parent drug, is well absorbed in patients with mild influenza. This short half life (∼2 h) prodrug lacks antiviral activity and is metabolised to the antivirally active oseltamivir carboxylate (OC). It is given 12 hourly. The uncertainty surrounding the absorption of oseltamivir in severely ill, influenza patients has been partly resolved. In a small clinical series, oseltamivir was well absorbed and achieved higher, steady state plasma concentrations compared to mild influenza patients because of renal impairment and a lower volume of distribution (W. Taylor, unpublished data).88
Based on very small numbers of patients to date, viral clearance in throat swabs has occurred within 5 days of oseltamivir treatment30, 72, 88; whether this is representative of the lungs is unknown. Continuing H5N1 viral replication despite oseltamivir has been documented and is associated with death.72 Given the high mortality of H5N1, even double dose oseltamivir alone fails clearly to reverse the pathology in many patients who may have also documented viral clearance. The optimal oseltamivir dose in severe influenza remains to be determined.
Because OC is renally excreted by glomerular filtration and secretion by the human anion 1 transporter in the proximal tubules, the manufacturer recommends that the dose of oseltamivir should be halved in renal disease (CrCl 10–<30 mL/min).89 OC loss via a haemofilter during haemofiltration is low and a top up dose is not required.88 The reverse is true for haemodialysis (HD) where about two thirds of the OC concentration is lost after a 4 h HD session, so top up doses should be given after dialysis (W. Taylor, unpublished data).90 The optimal oseltamivir dose in H5N1 patients with renal impairment is unknown.
Oseltamivir is usually well tolerated; dose related side effects are generally confined to headache, nausea and vomiting despite doses of up to 500 mg given 12 hourly. In post marketing surveillance, a number of severe adverse events have been observed like Stevens Johnson syndrome, cardiac arrhythmias but these reports are often difficult to ascribe to the drug. Given the severity and poor prognosis of H5N1, physicians should not be overly concerned with OC induced toxicity in patients, many of whom will be ventilated.
Amantadine and rimantadine
The roles of amantadine and rimantadine are limited because of the presence of the M2 gene mutation in many H5N1 isolates that confers adamantane resistance. In human influenza, the development of clinical resistance on treatment is well described and this is likely to occur in H5N1 if the adamantanes are used alone.91, 92, 93 Therefore, amantadine and rimantadine should always be used in combination with another antiviral drug. Rimantadine has fewer central nervous system toxicity (e.g. hyperexcitability, slurred speech, tremors, insomnia, dizziness, psychosis, convulsions) than amantadine and is preferred.94, 95 These side effects are related to the crossing of the blood brain barrier and are worse with higher plasma concentrations consequent to impaired renal function.96, 97 Therefore, dose reduction is required in renal disease.98 There is no PK interaction between oseltamivir and amantadine and tolerability was similar to the drugs when given individually in human normal volunteers.99
Amantadine crosses the placenta and is associated with toxicity in animal studies. It has also been associated rarely with teratogenic lesions in infants born to mothers with first trimester exposure.100, 101 There are no published data for rimantadine. Both should be avoided in pregnancy.
Adjunct treatment
The majority of H5N1 patients in hospital series have developed the acute respiratory distress syndrome, impairment of other organs and required intensive care and mechanical ventilation. The optimal ventilatory settings are unknown but the ARDS Network guidelines of lung protective ventilation is recommended pending more research data.102
Based on uncontrolled observational data, high dose, supplementary corticosteroids are not recommended by the WHO because their use in H5N1 patients may be associated with an increase in mortality.27, 35 However, steroids may have been given to patients with more severe disease.
Where evidence is lacking in severely ill H5N1 patients, and much of it is, clinicians can apply principles and recommendations formulated from treating patients with e.g. severe sepsis.103
There has been very limited but apparently favourable experience with intravenous immunoglobulin30 and the administration of serum to Chinese patients with H5N1 from patients who have recovered from H5N1,28 as was done also during the 1918 flu pandemic.104 Several groups are developing neutralising monoclonal antibodies for use in passive immunization.105 Immunomodulatory therapy should be explored as a therapeutic option in combination with antiviral drugs.
Other measures
Avian influenza is a dangerous disease and all suspected cases should be immediately reported to local public health authorities. Confirmed cases should be reported to WHO. Although the risk of person-to-person transmission has been low to date, it is not zero and the case fatality rate is extremely high.52, 106 Therefore, patients admitted with suspected avian influenza should be considered infectious and appropriate infection control measures instituted under the guidance of an infection control expert. These should include patient isolation, preferably in a negative pressure room, restriction of visitors, full respiratory and droplet protection for health staff caring for the patient i.e. gloves, goggles, respirators (e.g. N95), and gowns. Secondary cases have occurred in family members who have cared for confirmed cases. Therefore, health surveillance and post-exposure oseltamivir prophylaxis of close contacts of confirmed cases should be considered.
Concluding remarks
We are learning more about H5N1 infections with the passage of time and research. Early H5N1 infection may be deceptively mild and may not be thought of in the differential diagnosis. Mortality remains high despite the use of antiviral drugs. Pretravel consultations should include advice on H5N1 prevention. The travel doctor is likely to see patients with suspected H5N1 as part of his or her work. Therefore, H5N1 needs to be on the differential diagnosis along with a number of respiratory or systemic fevers. Early diagnosis is key and obtaining the necessary respiratory specimens for RT PCR is essential.
Conflict of interest statement
No conflict of interest for any author.
Acknowledgements
WRJT & HW are supported by the SEAICRN. PH is supported by the Wellcome Trust of Great Britain and AF is supported by the UK Medical Research Council.
References
- 1.Peiris J.S., de Jong M.D., Guan Y. Avian influenza virus (H5N1): a threat to human health. Clin Microbiol Rev. 2007;20(2):243–267. doi: 10.1128/CMR.00037-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legionnaire's disease. Lancet. 1977;1(8025):1295–1296. [PubMed] [Google Scholar]
- 3.Parashar U.D., Anderson L.J. Severe acute respiratory syndrome: review and lessons of the 2003 outbreak. Int J Epidemiol. 2004;33(4):628–634. doi: 10.1093/ije/dyh198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duchin J.S., Koster F.T., Peters C.J., Simpson G.L., Tempest B., Zaki S.R. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. The Hantavirus Study Group. N Engl J Med. 1994;330(14):949–955. doi: 10.1056/NEJM199404073301401. [DOI] [PubMed] [Google Scholar]
- 5.Ungchusak K., Auewarakul P., Dowell S.F., Kitphati R., Auwanit W., Puthavathana P. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352(4):333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 6.Khan K., Arino J., Hu W., Raposo P., Sears J., Calderon F. Spread of a novel influenza A (H1N1) virus via global airline transportation. N Engl J Med. 2009;361(2):212–214. doi: 10.1056/NEJMc0904559. [DOI] [PubMed] [Google Scholar]
- 7.Yuen K.Y., Chan P.K., Peiris M., Tsang D.N., Que T.L., Shortridge K.F. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351(9101):467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 8.Woo P.C., Lau S.K., Yuen K.Y. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr Opin Infect Dis. 2006;19(5):401–407. doi: 10.1097/01.qco.0000244043.08264.fc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taubenberger J.K., Morens D.M. 1918 Influenza: the mother of all pandemics. Emerg Infect Dis. 2006;12(1):15–22. doi: 10.3201/eid1201.050979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tumpey T.M., Garcia-Sastre A., Taubenberger J.K., Palese P., Swayne D.E., Basler C.F. Pathogenicity and immunogenicity of influenza viruses with genes from the 1918 pandemic virus. Proc Natl Acad Sci U S A. 2004;101(9):3166–3171. doi: 10.1073/pnas.0308391100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tumpey T.M., Garcia-Sastre A., Taubenberger J.K., Palese P., Swayne D.E., Pantin-Jackwood M.J. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79(23):14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kobasa D., Jones S.M., Shinya K., Kash J.C., Copps J., Ebihara H. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445(7125):319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe T., Watanabe S., Shinya K., Kim J.H., Hatta M., Kawaoka Y. Viral RNA polymerase complex promotes optimal growth of 1918 virus in the lower respiratory tract of ferrets. Proc Natl Acad Sci U S A. 2009;106(2):588–592. doi: 10.1073/pnas.0806959106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morens D.M., Taubenberger J.K., Fauci A.S. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198(7):962–970. doi: 10.1086/591708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson R.M. How well are we managing the influenza A/H1N1 pandemic in the UK? BMJ. 2009;339:b2897. doi: 10.1136/bmj.b2897. [DOI] [PubMed] [Google Scholar]
- 16.Perez-Padilla R., de la Rosa-Zamboni D., Ponce de Leon S., Hernandez M., Quinones-Falconi F., Bautista E. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361(7):680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 17.Garten R.J., Davis C.T., Russell C.A., Shu B., Lindstrom S., Balish A. Antigenic and genetic characteristics of swine-origin 2009 A(H1N1) influenza viruses circulating in humans. Science. 2009;325(5937):197–201. doi: 10.1126/science.1176225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thanawongnuwech R., Amonsin A., Tantilertcharoen R., Damrongwatanapokin S., Theamboonlers A., Payungporn S. Probable tiger-to-tiger transmission of avian influenza H5N1. Emerg Infect Dis. 2005;11(5):699–701. doi: 10.3201/eid1105.050007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jones S.R. Potential complications of influenza A infections. West J Med. 1976;125(5):341–346. [PMC free article] [PubMed] [Google Scholar]
- 20.Chow A., Ma S., Ling A.E., Chew S.K. Influenza-associated deaths in tropical Singapore. Emerg Infect Dis. 2006;12(1):114–121. doi: 10.3201/eid1201.050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson W.W., Shay D.K., Weintraub E., Brammer L., Cox N., Anderson L.J. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 22.Sedyaningsih E.R., Isfandari S., Setiawaty V., Rifati L., Harun S., Purba W. Epidemiology of cases of H5N1 virus infection in Indonesia, July 2005–June 2006. J Infect Dis. 2007;196(4):522–527. doi: 10.1086/519692. [DOI] [PubMed] [Google Scholar]
- 23.Beigel J.H., Farrar J., Han A.M., Hayden F.G., Hyer R., de Jong M.D. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353(13):1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- 24.Oner A.F., Bay A., Arslan S., Akdeniz H., Sahin H.A., Cesur Y. Avian influenza A (H5N1) infection in eastern Turkey in 2006. N Engl J Med. 2006;355(21):2179–2185. doi: 10.1056/NEJMoa060601. [DOI] [PubMed] [Google Scholar]
- 25.Dinh P.N., Long H.T., Tien N.T., Hien N.T., Mai le T.Q., Phong le H. Risk factors for human infection with avian influenza A H5N1, Vietnam, 2004. Emerg Infect Dis. 2006;12(12):1841–1847. doi: 10.3201/eid1212.060829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kandun I.N., Tresnaningsih E., Purba W.H., Lee V., Samaan G., Harun S. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372(9640):744–749. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 27.Liem N.T., Tung C.V., Hien N.D., Hien T.T., Chau N.Q., Long H.T. Clinical features of human influenza A (H5N1) infection in Vietnam: 2004–2006. Clin Infect Dis. 2009;48(12):1639–1646. doi: 10.1086/599031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu H., Gao Z., Feng Z., Shu Y., Xiang N., Zhou L. Clinical characteristics of 26 human cases of highly pathogenic avian influenza A (H5N1) virus infection in China. PLoS ONE. 2008;3(8):e2985. doi: 10.1371/journal.pone.0002985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giriputro S., Agus R., Sulastri S., Murniati D., Darwis F., Wiweka I.B. Clinical and epidemiological features of patients with confirmed avian influenza presenting to Sulianti Saroso Infectious Diseases Hospital, Indonesia, 2005–2007. Ann Acad Med Singapore. 2008;37(6):454–457. [PubMed] [Google Scholar]
- 30.Soepandi PZ, Burhan E, Mangunnegoro H, Nawas A, Aditama TY, Partakusuma L, et al., in press. [DOI] [PMC free article] [PubMed]
- 31.Ellis C.J., Eykyn S.J., Watkins P., Bell M., Geddes A.M. Malaria in Birmingham and a London teaching hospital. BMJ. 1979;1(6160):385–388. doi: 10.1136/bmj.1.6160.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viani R.M., Bromberg K. Pediatric imported malaria in New York: delayed diagnosis. Clin Pediatr (Phila) 1999;38(6):333–337. doi: 10.1177/000992289903800603. [DOI] [PubMed] [Google Scholar]
- 33.Chotpitayasunondh T., Ungchusak K., Hanshaoworakul W., Chunsuthiwat S., Sawanpanyalert P., Kijphati R. Human disease from influenza A (H5N1), Thailand, 2004. Emerg Infect Dis. 2005;11(2):201–209. doi: 10.3201/eid1102.041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tran T.H., Nguyen T.L., Nguyen T.D., Luong T.S., Pham P.M., Nguyen V.C. Avian influenza A (H5N1) in 10 patients in Vietnam. N Engl J Med. 2004;350(12):1179–1188. doi: 10.1056/NEJMoa040419. [DOI] [PubMed] [Google Scholar]
- 35.Abdel-Ghafar A.N., Chotpitayasunondh T., Gao Z., Hayden F.G., Nguyen D.H., de Jong M.D. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med. 2008;358(3):261–273. doi: 10.1056/NEJMra0707279. [DOI] [PubMed] [Google Scholar]
- 36.de Jong M.D., Bach V.C., Phan T.Q., Vo M.H., Tran T.T., Nguyen B.H. Fatal avian influenza A (H5N1) in a child presenting with diarrhea followed by coma. N Engl J Med. 2005;352(7):686–691. doi: 10.1056/NEJMoa044307. [DOI] [PubMed] [Google Scholar]
- 37.Apisarnthanarak A., Kitphati R., Thongphubeth K., Patoomanunt P., Anthanont P., Auwanit W. Atypical avian influenza (H5N1) Emerg Infect Dis. 2004;10(7):1321–1324. doi: 10.3201/eid1007.040415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taylor F.B., Jr., Toh C.H., Hoots W.K., Wada H., Levi M. Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb Haemost. 2001;86(5):1327–1330. [PubMed] [Google Scholar]
- 39.Porcel J.M., Light R.W. Diagnostic approach to pleural effusion in adults. Am Fam Phys. 2006;73(7):1211–1220. [PubMed] [Google Scholar]
- 40.Bay A., Etlik O., Oner A.F., Unal O., Arslan H., Bora A. Radiological and clinical course of pneumonia in patients with avian influenza H5N1. Eur J Radiol. 2007;61(2):245–250. doi: 10.1016/j.ejrad.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 41.Qureshi N.R., Hien T.T., Farrar J., Gleeson F.V. The radiologic manifestations of H5N1 avian influenza. J Thorac Imag. 2006;21(4):259–264. doi: 10.1097/01.rti.0000213573.94032.53. [DOI] [PubMed] [Google Scholar]
- 42.de Jong M.D., Simmons C.P., Thanh T.T., Hien V.M., Smith G.J., Chau T.N. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12(10):1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vong S., Ly S., Mardy S., Holl D., Buchy P. Environmental contamination during influenza A virus (H5N1) outbreaks, Cambodia, 2006. Emerg Infect Dis. 2008;14(8):1303–1305. doi: 10.3201/eid1408.070912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown J.D., Swayne D.E., Cooper R.J., Burns R.E., Stallknecht D.E. Persistence of H5 and H7 avian influenza viruses in water. Avian Dis. 2007;51(1 Suppl.):285–289. doi: 10.1637/7636-042806R.1. [DOI] [PubMed] [Google Scholar]
- 45.Ly S., Van Kerkhove M.D., Holl D., Froehlich Y., Vong S. Interaction between humans and poultry, rural Cambodia. Emerg Infect Dis. 2007;13(1):130–132. doi: 10.3201/eid1301.061014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu H., Feng Z., Zhang X., Xiang N., Huai Y., Zhou L. Human influenza A (H5N1) cases, urban areas of People's Republic of China, 2005–2006. Emerg Infect Dis. 2007;13(7):1061–1064. doi: 10.3201/eid1307.061557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mounts A.W., Kwong H., Izurieta H.S., Ho Y., Au T., Lee M. Case–control study of risk factors for avian influenza A (H5N1) disease, Hong Kong, 1997. J Infect Dis. 1999;180(2):505–508. doi: 10.1086/314903. [DOI] [PubMed] [Google Scholar]
- 48.Zhou L., Liao Q., Dong L., Huai Y., Bai T., Xiang N. Risk factors for human illness with avian influenza A (H5N1) virus infection in China. J Infect Dis. 2009;199(12):1726–1734. doi: 10.1086/599206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H., Feng Z., Shu Y., Yu H., Zhou L., Zu R. Probable limited person-to-person transmission of highly pathogenic avian influenza A (H5N1) virus in China. Lancet. 2008;371(9622):1427–1434. doi: 10.1016/S0140-6736(08)60493-6. [DOI] [PubMed] [Google Scholar]
- 50.Apisarnthanarak A., Erb S., Stephenson I., Katz J.M., Chittaganpitch M., Sangkitporn S. Seroprevalence of anti-H5 antibody among Thai health care workers after exposure to avian influenza (H5N1) in a tertiary care center. Clin Infect Dis. 2005;40(2):e16–e18. doi: 10.1086/427034. [DOI] [PubMed] [Google Scholar]
- 51.Buxton Bridges C., Katz J.M., Seto W.H., Chan P.K., Tsang D., Ho W. Risk of influenza A (H5N1) infection among health care workers exposed to patients with influenza A (H5N1), Hong Kong. J Infect Dis. 2000;181(1):344–348. doi: 10.1086/315213. [DOI] [PubMed] [Google Scholar]
- 52.Liem N.T., Lim W. Lack of H5N1 avian influenza transmission to hospital employees, Hanoi, 2004. Emerg Infect Dis. 2005;11(2):210–215. doi: 10.3201/eid1102.041075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tellier R. Review of aerosol transmission of influenza A virus. Emerg Infect Dis. 2006;12(11):1657–1662. doi: 10.3201/eid1211.060426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koopmans M., Wilbrink B., Conyn M., Natrop G., van der Nat H., Vennema H. Transmission of H7N7 avian influenza A virus to human beings during a large outbreak in commercial poultry farms in the Netherlands. Lancet. 2004;363(9409):587–593. doi: 10.1016/S0140-6736(04)15589-X. [DOI] [PubMed] [Google Scholar]
- 55.Fouchier R.A., Schneeberger P.M., Rozendaal F.W., Broekman J.M., Kemink S.A., Munster V. Avian influenza A virus (H7N7) associated with human conjunctivitis and a fatal case of acute respiratory distress syndrome. Proc Natl Acad Sci U S A. 2004;101(5):1356–1361. doi: 10.1073/pnas.0308352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Palache B., Krause R. Progress with human H5N1 vaccines: a perspective from industry. Expert Rev Vaccines. 2009;8(4):391–400. doi: 10.1586/erv.09.16. [DOI] [PubMed] [Google Scholar]
- 57.Sturm-Ramirez K.M., Hulse-Post D.J., Govorkova E.A., Humberd J., Seiler P., Puthavathana P. Are ducks contributing to the endemicity of highly pathogenic H5N1 influenza virus in Asia? J Virol. 2005;79(17):11269–11279. doi: 10.1128/JVI.79.17.11269-11279.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez L., Blanc L., Nunn P., Raviglione M. Tuberculosis and air travel: WHO guidance in the era of drug-resistant TB. Travel Med Infect Dis. 2008;6(4):177–181. doi: 10.1016/j.tmaid.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 59.Mangili A., Gendreau M.A. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365(9463):989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Neira-Munoz E., Smith J., Cockcroft P., Basher D., Abubakar I. Extensive transmission of Mycobacterium tuberculosis among children on a school bus. Pediatr Infect Dis J. 2008;27(9):835–837. doi: 10.1097/INF.0b013e31816ff7c5. [DOI] [PubMed] [Google Scholar]
- 61.Moore M., Valway S.E., Ihle W., Onorato I.M. A train passenger with pulmonary tuberculosis: evidence of limited transmission during travel. Clin Infect Dis. 1999;28(1):52–56. doi: 10.1086/515089. [DOI] [PubMed] [Google Scholar]
- 62.Ryan E.T., Wilson M.E., Kain K.C. Illness after international travel. N Engl J Med. 2002;347(7):505–516. doi: 10.1056/NEJMra020118. [DOI] [PubMed] [Google Scholar]
- 63.Doherty J.F., Grant A.D., Bryceson A.D. Fever as the presenting complaint of travellers returning from the tropics. QJM. 1995;88(4):277–281. [PubMed] [Google Scholar]
- 64.Taylor W.R., Canon V., White N.J. Pulmonary manifestations of malaria: recognition and management. Treat Respir Med. 2006;5(6):419–428. doi: 10.2165/00151829-200605060-00007. [DOI] [PubMed] [Google Scholar]
- 65.Buczko G.B., McLean J. Typhoid fever associated with adult respiratory distress syndrome. Chest. 1994;105(6):1873–1874. doi: 10.1378/chest.105.6.1873. [DOI] [PubMed] [Google Scholar]
- 66.Thammakumpee K., Silpapojakul K., Borrirak B. Leptospirosis and its pulmonary complications. Respirology. 2005;10(5):656–659. doi: 10.1111/j.1440-1843.2005.00764.x. [DOI] [PubMed] [Google Scholar]
- 67.Wang C.C., Liu S.F., Liu J.W., Chung Y.H., Su M.C., Lin M.C. Acute respiratory distress syndrome in scrub typhus. Am J Trop Med Hyg. 2007;76(6):1148–1152. [PubMed] [Google Scholar]
- 68.Currie B.J. Melioidosis: an important cause of pneumonia in residents of and travellers returned from endemic regions. Eur Respir J. 2003;22(3):542–550. doi: 10.1183/09031936.03.00006203. [DOI] [PubMed] [Google Scholar]
- 69.Ortiz J.R., Wallis T.R., Katz M.A., Berman L.S., Balish A., Lindstrom S.E. No evidence of avian influenza A (H5N1) among returning US travelers. Emerg Infect Dis. 2007;13(2):294–297. doi: 10.3201/eid1302.061052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Charlton B., Crossley B., Hietala S. Conventional and future diagnostics for avian influenza. Comp Immunol Microbiol Infect Dis. 2008 doi: 10.1016/j.cimid.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 71.Curran M.D., Ellis J.S., Wreghitt T.G., Zambon M.C. Establishment of a UK national influenza H5 laboratory network. J Med Microbiol. 2007;56(Pt 10):1263–1267. doi: 10.1099/jmm.0.47336-0. [DOI] [PubMed] [Google Scholar]
- 72.de Jong M.D., Tran T.T., Truong H.K., Vo M.H., Smith G.J., Nguyen V.C. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med. 2005;353(25):2667–2672. doi: 10.1056/NEJMoa054512. [DOI] [PubMed] [Google Scholar]
- 73.Le M.T., Wertheim H.F., Nguyen H.D., Taylor W., Hoang P.V., Vuong C.D. Influenza A H5N1 clade 2.3.4 virus with a different antiviral susceptibility profile replaced clade 1 virus in humans in northern Vietnam. PLoS ONE. 2008;3(10):e3339. doi: 10.1371/journal.pone.0003339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hurt A.C., Selleck P., Komadina N., Shaw R., Brown L., Barr I.G. Susceptibility of highly pathogenic A(H5N1) avian influenza viruses to the neuraminidase inhibitors and adamantanes. Antivir Res. 2007;73(3):228–231. doi: 10.1016/j.antiviral.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 75.McKimm-Breschkin J.L., Selleck P.W., Usman T.B., Johnson M.A. Reduced sensitivity of influenza A (H5N1) to oseltamivir. Emerg Infect Dis. 2007;13(9):1354–1357. doi: 10.3201/eid1309.07-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee J., Song Y.J., Park J.H., Lee J.H., Baek Y.H., Song M.S. Emergence of amantadine-resistant H3N2 avian influenza A virus in South Korea. J Clin Microbiol. 2008;46(11):3788–3790. doi: 10.1128/JCM.01427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheung C.L., Rayner J.M., Smith G.J., Wang P., Naipospos T.S., Zhang J. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J Infect Dis. 2006;193(12):1626–1629. doi: 10.1086/504723. [DOI] [PubMed] [Google Scholar]
- 78.He G., Qiao J., Dong C., He C., Zhao L., Tian Y. Amantadine-resistance among H5N1 avian influenza viruses isolated in Northern China. Antivir Res. 2008;77(1):72–76. doi: 10.1016/j.antiviral.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Hurt A.C., Ernest J., Deng Y.M., Iannello P., Besselaar T.G., Birch C. Emergence and spread of oseltamivir-resistant A(H1N1) influenza viruses in Oceania, South East Asia and South Africa. Antivir Res. 2009;83(1):90–93. doi: 10.1016/j.antiviral.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 80.Hurt A.C., Holien J.K., Barr I.G. In vitro generation of neuraminidase inhibitor resistance in A(H5N1) influenza viruses. Antimicrob Agents Chemother. 2009;53(10):4433–4440. doi: 10.1128/AAC.00334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abed Y., Nehme B., Baz M., Boivin G. Activity of the neuraminidase inhibitor A-315675 against oseltamivir-resistant influenza neuraminidases of N1 and N2 subtypes. Antivir Res. 2008;77(2):163–166. doi: 10.1016/j.antiviral.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Zambon M., Hayden F.G. Position statement: global neuraminidase inhibitor susceptibility network. Antivir Res. 2001;49(3):147–156. doi: 10.1016/s0166-3542(01)00124-3. [DOI] [PubMed] [Google Scholar]
- 83.Hayden F., Klimov A., Tashiro M., Hay A., Monto A., McKimm-Breschkin J. Neuraminidase inhibitor susceptibility network position statement: antiviral resistance in influenza A/H5N1 viruses. Antivir Ther. 2005;10(8):873–877. [PubMed] [Google Scholar]
- 84.Smee D.F., Hurst B.L., Wong M.H., Bailey K.W., Morrey J.D. Effects of double combinations of amantadine, oseltamivir, and ribavirin on influenza A (H5N1) virus infections in cell culture and in mice. Antimicrob Agents Chemother. 2009;53(5):2120–2128. doi: 10.1128/AAC.01012-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ilyushina N.A., Hoffmann E., Salomon R., Webster R.G., Govorkova E.A. Amantadine–oseltamivir combination therapy for H5N1 influenza virus infection in mice. Antivir Ther. 2007;12(3):363–370. [PubMed] [Google Scholar]
- 86.Schunemann H.J., Hill S.R., Kakad M., Bellamy R., Uyeki T.M., Hayden F.G. WHO rapid advice guidelines for pharmacological management of sporadic human infection with avian influenza A (H5N1) virus. Lancet Infect Dis. 2007;7(1):21–31. doi: 10.1016/S1473-3099(06)70684-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ilyushina N.A., Bovin N.V., Webster R.G., Govorkova E.A. Combination chemotherapy, a potential strategy for reducing the emergence of drug-resistant influenza A variants. Antivir Res. 2006;70(3):121–131. doi: 10.1016/j.antiviral.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 88.Taylor W.R., Thinh B.N., Anh G.T., Horby P., Wertheim H., Lindegardh N. Oseltamivir is adequately absorbed following nasogastric administration to adult patients with severe H5N1 influenza. PLoS ONE. 2008;3(10):e3410. doi: 10.1371/journal.pone.0003410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.He G., Massarella J., Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999;37(6):471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 90.Robson R., Buttimore A., Lynn K., Brewster M., Ward P. The pharmacokinetics and tolerability of oseltamivir suspension in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transpl. 2006;21(9):2556–2562. doi: 10.1093/ndt/gfl267. [DOI] [PubMed] [Google Scholar]
- 91.Drinka P.J., Haupt T. Emergence of rimantadine-resistant virus within 6 days of starting rimantadine prophylaxis with oseltamivir treatment of symptomatic cases. J Am Geriatr Soc. 2007;55(6):923–926. doi: 10.1111/j.1532-5415.2007.01172.x. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki H., Saito R., Masuda H., Oshitani H., Sato M., Sato I. Emergence of amantadine-resistant influenza A viruses: epidemiological study. J Infect Chemother. 2003;9(3):195–200. doi: 10.1007/s10156-003-0262-6. [DOI] [PubMed] [Google Scholar]
- 93.Shiraishi K., Mitamura K., Sakai-Tagawa Y., Goto H., Sugaya N., Kawaoka Y. High frequency of resistant viruses harboring different mutations in amantadine-treated children with influenza. J Infect Dis. 2003;188(1):57–61. doi: 10.1086/375799. [DOI] [PubMed] [Google Scholar]
- 94.Hayden F.G., Hoffman H.E., Spyker D.A. Differences in side effects of amantadine hydrochloride and rimantadine hydrochloride relate to differences in pharmacokinetics. Antimicrob Agents Chemother. 1983;23(3):458–464. doi: 10.1128/aac.23.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guay D.R. Amantadine and rimantadine prophylaxis of influenza A in nursing homes. A tolerability perspective. Drugs Aging. 1994;5(1):8–19. doi: 10.2165/00002512-199405010-00002. [DOI] [PubMed] [Google Scholar]
- 96.Geskey J.M., Thomas N.J. Amantadine penetration into cerebrospinal fluid of a child with influenza A encephalitis. Pediatr Infect Dis J. 2004;23(3):270–272. doi: 10.1097/01.inf.0000115647.66193.45. [DOI] [PubMed] [Google Scholar]
- 97.Spector R. Transport of amantadine and rimantadine through the blood-brain barrier. J Pharmacol Exp Ther. 1988;244(2):516–519. [PubMed] [Google Scholar]
- 98.Horadam V.W., Sharp J.G., Smilack J.D., McAnalley B.H., Garriott J.C., Stephens M.K. Pharmacokinetics of amantadine hydrochloride in subjects with normal and impaired renal function. Ann Intern Med. 1981;94(4 Pt 1):454–458. doi: 10.7326/0003-4819-94-4-454. [DOI] [PubMed] [Google Scholar]
- 99.Morrison D., Roy S., Rayner C., Amer A., Howard D., Smith J.R. A randomized, crossover study to evaluate the pharmacokinetics of amantadine and oseltamivir administered alone and in combination. PLoS ONE. 2007;2(12):e1305. doi: 10.1371/journal.pone.0001305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nora J.J., Nora A.H., Way G.L. Letter: cardiovascular maldevelopment associated with maternal exposure to amantadine. Lancet. 1975;2(7935):607. doi: 10.1016/s0140-6736(75)90198-1. [DOI] [PubMed] [Google Scholar]
- 101.Pandit P.B., Chitayat D., Jefferies A.L., Landes A., Qamar I.U., Koren G. Tibial hemimelia and tetralogy of Fallot associated with first trimester exposure to amantadine. Reprod Toxicol. 1994;8(1):89–92. doi: 10.1016/0890-6238(94)90072-8. [DOI] [PubMed] [Google Scholar]
- 102.Bernard G.R., Artigas A., Brigham K.L., Carlet J., Falke K., Hudson L. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. Intens Care Med. 1994;20(3):225–232. doi: 10.1007/BF01704707. The Consensus Committee. [DOI] [PubMed] [Google Scholar]
- 103.Dellinger R.P., Levy M.M., Carlet J.M., Bion J., Parker M.M., Jaeschke R. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 104.Luke T.C., Kilbane E.M., Jackson J.L., Hoffman S.L. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. 2006;145(8):599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- 105.Simmons C.P., Bernasconi N.L., Suguitan A.L., Mills K., Ward J.M., Chau N.V. Prophylactic and therapeutic efficacy of human monoclonal antibodies against H5N1 influenza. PLoS Med. 2007;4(5):e178. doi: 10.1371/journal.pmed.0040178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schultsz C., Dong V.C., Chau N.V., Le N.T., Lim W., Thanh T.T. Avian influenza H5N1 and healthcare workers. Emerg Infect Dis. 2005;11(7):1158–1159. doi: 10.3201/eid1107.050070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Govorkova E.A., Ilyushina N.A., McClaren J.L., Naipospos T.S., Douangngeun B., Webster R.G. Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model. Antimicrob Agents Chemother. 2009;53(7):3088–3096. doi: 10.1128/AAC.01667-08. [DOI] [PMC free article] [PubMed] [Google Scholar]