Abstract
Three subtypes of influenza A virus cause human disease: H1N1, H2N2, and H3N2. Although all result in respiratory illness, little is known about how these subtypes infect differentiated airway epithelia. Therefore, we assayed A/PR/8/34 (H1N1), A/Japan/305/57 (H2N2), and X31 (H3N2) influenza virus strains for binding and infection on fully differentiated primary cultures of airway epithelia isolated from human bronchus, grown on semiporous filters at an air–liquid interface. In this model system, viral infectivity was highest when virus was applied to the apical versus the basolateral surface; Japan was most infectious, followed by PR8. The X31 strain showed very low levels of infectivity. Confocal microscopy and fluorescence-resonance energy transfer studies indicated that Japan virus could enter and fuse with cellular membranes, while infection with X31 virions was greatly inhibited. Japan virus could also productively infect human trachea explant tissues. These data show that influenza viruses with SAα2,3Gal binding specificity, like Japan, productively infect differentiated human airway epithelia from the apical surface. These data are important to consider in the development of pseudotyped recombinant viral vectors for gene transfer to human airway epithelia for gene therapy.
Keywords: HA, gene therapy, pseudotype
Introduction
Influenza viruses comprise some of the most common pathogens of the human upper and lower respiratory tract (1). The virus lipid envelope surrounds a segmented RNA genome, with the envelope protein, hemagglutinin (HA), responsible for binding and fusion to host cells (1). Another viral surface glycoprotein, neuraminidase, is important in both budding and entry (1). Humans are infected by influenza A virus subtypes with the classification of H1N1, H2N2, and H3N2.
The HA proteins bind to sialic acid-bearing receptors on host cells. Specificity for sialic acid residues terminating in α2,6-galactose β1,4-N-acetyl glucosamine (SAα2,6Gal) or SAα2,3Gal is determined by HA protein sequence (2, 3, 4, 5, 6). Earlier studies showed that many human isolates preferentially bind and agglutinate red blood cells bearing oligosaccharides terminating in SAα2,6Gal while the avian strains often exhibit specificity for erythrocytes bearing SAα2,3Gal receptors (3, 4, 7). In subtypes cultured in the laboratory, the host cell used to culture the virus can direct HA-coding sequence alteration (5, 8, 9, 10) and alter sialic acid-binding specificity.
Cells in the human trachea express both types of oligosaccharides (11). Using specific lectins it was shown that ciliated cells express SAα2,6Gal moieties on their surface, while mucus-secreting goblet cells stain positive for SAα2,3Gal. The ability of influenza to infect differentiated airway cells expressing one, both, or neither oligosaccharide is not currently known. However, an earlier report showed that an H3 human virus strain with SAα2,6Gal specificity could bind to formaldehyde-fixed human respiratory epithelium (12).
Experiments that examined influenza virus–host cell interactions, using dog renal epithelia (13, 14, 15), showed that virus binding and budding occur preferentially at the apical surface of polarized cells. Binding to cultured airway cells or tissues has also been examined. Prior reports demonstrated that an H1N1 strain could bind and propagate in dissociated airway epithelia cultured from fetal lung tissue explants (16). The strain PR8 was also shown to productively infect airway epithelial cells grown as dissociated cells on plastic dishes (17). Thus these experiments together with earlier studies (11) do not directly address the polarity of infection or budding of influenza strains on differentiated respiratory epithelia.
A culture system which closely resembles the airway epithelium both morphologically and physiologically has been described (18, 19, 20, 21). In this model, airway epithelia cells are isolated from human trachea or bronchus and the cells dissociated. The cells are grown on filter inserts and cultured at the air–liquid interface. In approximately 2 weeks, the culture is multilayered and polarized, with basal cells, intermediate cells, and columnar cells present. The most apical layer contains both ciliated and nonciliated cells. This model has been used to demonstrate the polarity of infection of recombinant retroviruses (21), recombinant adenoviruses (22, 23), and recombinant adeno-associated viruses (24).
We examined the polarity of influenza virus binding, fusion, and budding in human airway epithelia cultures. The H3 strain, X31, with SAα2,6Gal specificity; A/Japan/305/57, an H2N2 influenza strain with SAα2,3Gal specificity; and a strain with mixed specificity (A/PR8, H1N1) were tested. Our results demonstrate that influenza A strains bind to the apical surface of the epithelia. For H2N2 and H1N1 this results in a productive infection. We also demonstrate entry and fusion of the H2N2 virus with cellular membranes and show that X31 virions can bind but do not productively infect differentiated airway epithelia.
Materials and Methods
Primary culture of human airway epithelia
Primary cultures of human airway epithelia were prepared from bronchi by enzymatic dispersion according to established methods (18, 22). Briefly, epithelial cells were disassociated and seeded onto collagen-coated, semipermeable membranes with a 0.4-μm pore size (Millicell-HA; surface area, 0.6 cm2; Millipore Corp., Bedford, MA). Millicell inserts were placed into 24-well plastic cell culture cluster (Costar, Cambridge, MA). Twenty-four hours after seeding, the mucosal medium was removed and the cells were allowed to grow at the air–liquid interface as reported previously (21). The culture medium, which consisted of a 1:1 mixture of Dulbecco's modified Eagle medium and Ham's F-12 with 2% Ultroser G (Sepracor, Inc., Marlborough, MA), 100 U of penicillin/ml, and 100 μg of streptomycin/ml, was added to the wells to feed cells from the basolateral surface. Only well-differentiated cultures >2 weeks old were used in these studies, with approximately 4 × 105 cells/Millicell. The presence of tight junctions was confirmed by transepithelial resistance measurements (resistance >500 Ω · cm2).
Influenza viruses
Influenza virus preparations were purchased from Spafas Laboratory (Storrs, CT). Preparations were provided as concentrated and partially sucrose-gradient-purified preparations. Three strains of influenza A virus were used in this study: A/PR/8/34 (H1N1) (PR8), A/Japan/305/57 (H2N2) (Japan), and X31 (H3N2). The latter strain is a reassortment with surface glycoprotein genes from A/Aichi/2/68 (H3N2) and internal genes from A/PR/8/34 (25). The total protein concentration of each virus preparation was 2 mg/ml. Viruses were titrated by serial dilutions on MDCK cells in the presence of 2 μg/ml TPCK-treated trypsin (Sigma, St. Louis, MO) (26). Infected wells were determined by the presence of hemagglutination activity in the culture medium. The tissue culture infectious dose 50% (TCID50) was calculated using previously described methods (27).
Infection of airway epithelial cells
For measurement of the kinetics of influenza virus production from infected airway cells in one infectious cycle, 100 μl of the virus preparation was diluted in Dulbecco's PBS (Life Technologies, Inc., Grand Island, NY) and applied to the apical surface of the epithelia (m.o.i. of 0.1). After incubation for 30 min at 37°C the cells were washed three times with PBS and incubation at 37°C was continued for various time periods with 100 μl of PBS left on the surface of epithelia. Release of the progeny virus into PBS or basolateral cell culture medium was assayed by hemagglutination with human donor erythrocytes (7). There was no hemagglutination activity in the third PBS wash.
To infect airway epithelia with different influenza virus strains from the basolateral side, the Millicell culture insert was turned over and virus preparations diluted in PBS were applied in a 100-μl volume to the bottom of the membrane (m.o.i. = 0.1). After incubation for 30 min at 37°C, the suspension was removed and the membranes were washed three times with PBS. The Millicell insert was returned to its regular upright position with 100 μl of PBS added to the apical side. After incubation for 16 h at 37°C, virus hemagglutination titer was assayed in both the apical PBS and the basolateral cell culture medium.
For testing viral infectivity during a multiple cycle infection, 100 μl of the virus preparations was added to the apical side of the airway epithelia at an m.o.i. of 0.1 and incubated for 30 min at 37°C. The viral suspension was then removed and the cells were washed three times with PBS. Epithelia were incubated at 37°C for 18 h to allow for initial virus production. To assay for virus release, 100 μl of PBS was added to the mucosal surface and cells were incubated at 37°C for 1 h. To test the effects of protease on influenza virus HA, TPCK-treated trypsin was added to the PBS (final concentration of 2 μg/ml) in some experiments. After incubation, surface liquid was collected and assayed by hemagglutination for the presence of the virus. The cells were incubated further at 37°C without medium on the apical side. These steps were repeated every 24 h for 4 days. After 96 h of incubation the cells were lysed in Laemmli sample buffer (28), and viral proteins were analyzed by immunoblot assay.
Metabolic labeling of virus proteins
Airway epithelia were infected with different influenza virus strains from the apical side, at an m.o.i. of 0.1, for 2 h at 37°C. After the initial infection epithelia were incubated in methionine- and cysteine-free cell culture medium (Life Technologies, Inc.) for 8 h at 37°C to accommodate the initial steps of the virus replication cycle. [35S]Methionine (NEN Life Science Products, Boston, MA) was then added to the basolateral culture medium (50 μCi/ml final) and incubation continued for 10 h at 37°C. Cell-free virus was collected from the apical side and cells were lysed in Laemmli sample buffer. Analysis of radioactively labeled proteins was done by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) with subsequent autoradiography.
Antiviral polyclonal antibodies
Polyclonal antibodies against Japan and X31 strains were generated by inoculation of sheep with the corresponding partially purified formalin-inactivated virus by Elmira Biologicals (Iowa City, IA). The antibodies were purified from serum using a combination of ammonium sulfate precipitation and DEAE-cellulose chromatography (29). For immunostaining, purified antibodies were labeled with either Texas red or fluorescein isothiocyanate (FITC) using FluoReporter Protein Labeling kits (Molecular Probes, Inc., Eugene, OR). Reactions were performed according to the manufacturer's recommendations.
Immunoblot assay
Following PAGE, proteins were transferred to nitrocellulose membranes (Immobilon-NC; Millipore Corp.) using a semidry transfer cell (Trans-Blot SD; Bio-Rad Laboratories, Hercules, CA), 0.5 h at 50 mA. Membranes were blocked with 5% fat-free dry milk, 2 h at room temperature, followed by overnight incubation at 4°C with anti-Japan sheep serum diluted 1:5000 in 0.1% Tween 20/PBS solution. Specific virus proteins were stained with peroxidase-conjugated AffiniPure donkey anti-sheep IgG (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), used at 1:20,000 dilution for 2 h at room temperature. The bands were visualized on X-ray film after development with the ECL kit (Amersham Pharmacia Biotech UK Ltd., Little Chalfont, England). Staining of membranes with preimmune serum was negative.
Influenza virus interactions with airway epithelia
Partially purified preparations of influenza virus were labeled with N-(lissamine rhodamine B sulfonyl) diacyl phosphatidylethanolamine (Rh-PE) (Avanti Polar Lipids, Inc., Alabaster, AL) by passive insertion into the virus envelope according to earlier described methods (30). Cellular membranes were labeled with N-octadecyl-N’-(5-(fluoresceinyl))thiourea (F18; Molecular Probes) by addition of the dye into the basolateral culture medium at a final concentration of 10 μM and incubation for 2 h at 37°C. F18-labeled airway epithelia were infected with Rh-PE-labeled influenza virus by adding 100 μl of virus suspension to the apical surface (m.o.i. = 5). Virus was allowed to internalize for 30 min at 37°C. Cells were then washed three times with PBS and fixed with 2% paraformaldehyde for 30 min at room temperature. Cells were then washed two times with PBS and the Millicell inserts removed and mounted onto slides for microscopy. Cells were scanned using a MRC confocal microscope (Bio-Rad). Images were acquired using two photomultipliers, set for a double-labeling protocol (fluorescein and PE-rhodamine), and analyzed using Cut Plane Program software, allowing for xy and xz axis stacking of sections. For fluorescence-resonance energy transfer (FRET) assays cells were scanned using customized settings with the excitation wavelength set for fluorescein only. Images were acquired using the same double-labeling protocol as above. At this excitation wavelength, detection of red indicates FRET.
Influence of influenza virus infection on fluid-phase endocytosis in airway cells
Airway epithelia were cooled on ice and influenza virus preparations diluted 10-fold in PBS were added to the apical side (m.o.i. of 1). The virus was allowed to adsorb for 30 min at 4°C. Virus was then removed, cells were washed three times with cold PBS, and 37°C-warm FITC–dextran solution (3000 MW, anionic, lysine fixable; Molecular Probes) 0.5 mg/ml in PBS was added. Cells were incubated at 37°C for 5 min, washed three times with PBS, and fixed with 2% paraformaldehyde for 30 min at room temperature. Cells were counterstained with Texas red-labeled lectin from Maackia amurensis (Sigma), specific for sialic acid.
Infection of tracheal explants
Blocks of human trachea, approximately 0.1 cm2 in size, were placed in airway culture medium in a 24-well plastic cluster dish. Explants were infected with the influenza virus preparations diluted to the same concentration as for evaluation of fluid-phase endocytosis (above). Virus was incubated for 2 h at 37°C. Excess virus was then removed by four washings with PBS, with the last wash tested for virus by hemagglutination assay. Infection of airway epithelia was allowed to proceed overnight at 37°C in the airway culture medium, and the release of the progeny virus was assayed by hemagglutination. Twenty-four hours after infection explants were washed two times with PBS and fixed with 2% paraformaldehyde. Fixed tissues were cryoprotected and embedded and 15-μm sections obtained for staining. The sections were stained with FITC-or Texas red-labeled sheep IgG against Japan or X31 strains. Photomicrographs were acquired using a Leica DM RBE fluorescence microscope equipped with a Sony digital camera and captured with Adobe PhotoShop software.
Results
Influenza Virus Infection of Airway Epithelia
We first compared the abilities of X31 (H3N2), Japan (H2N2), and PR8 (H1N1) strains to infect cultures of primary human airway epithelia. This model culture system, in which human airway epithelia are cultured at the air–liquid interface (18, 22), has been invaluable in testing how other viruses can or cannot access intracellular compartments when applied to the mucosal surface (21, 22, 31). The titers of the strains tested were determined in MDCK cells, and SA-Gal specificities were confirmed on erythrocytes. Titers and SA-Gal specificities are listed in Table 1 .
TABLE 1.
Characteristics of Influenza Virus Strains Used in the Study
| Strain name | Subtype | Receptor specificitya | HA titerb | Titer in HAI with NHSc | TCID50 in MDCK cellsd |
|---|---|---|---|---|---|
| A/Japan/305/57 | H2N2 | SAα2,3Gal | 16,000 | 4 | 6.3 × 107 ± 7.8 |
| X31 | H3N2 | SAα2,6Gal | 16,000 | 20,480 | 1.1 × 107 ± 4.2 |
| A/PR/8/34 | H1N1 | Both | 16,000 | 64 | 2.5 × 107 ± 5.1 |
As determined previously by hemagglutination assay with derivatized erythrocytes (7, 29).
Hemagglutination titers are expressed as the maximum dilution giving complete agglutination of human donor erythrocytes.
Hemagglutination inhibition titers are expressed as the reciprocal of the highest dilution of heat-inactivated normal horse serum (NHS) causing inhibition of agglutination by 4 hemagglutination units of virus.
Average TCID50 titers and standard deviations from six independent titrations are shown.
The viruses were applied to the apical side of the airway epithelia at m.o.i. equal to 0.1. The amount of the virus released to the apical surface fluid was measured at different time points by hemagglutination. The results of a representative experiment are shown in Fig. 1 . In all cases, virus production in airway epithelia was highest for the Japan strain. The number of virus progeny produced by PR8 was considerably lower than that of Japan, but higher than that of X31. For Japan, virus release started 10 h after virus inoculation and continued to rise throughout the 18-h time course. The virus production was somewhat delayed compared to the MDCK cell model, in which maximal virus production was reached in approximately 10 h (32). However, the kinetics of virus production observed in our experiments were very similar to previous reports for human airway cell lines infected with the H1N1 influenza A strain (16). There was no drop in transepithelial resistance measurements throughout the study (data not shown), indicating that the epithelia remained intact.
FIG. 1.

Kinetics of influenza virus production in human airway epithelia cultures. At time 0 cells were infected from the apical side with Japan, PR8, or X31 strains. Virus was recovered at the indicated times and assayed by hemagglutination. The data shown are representative of three independent experiments done with cells derived from different donor lungs.
Our first experiment tested the ability of influenza A viruses to productively infect airway epithelia when applied to the mucosal surface. We next compared the amount of virus produced after basolateral or apical application. The virus released into the basolateral or apical compartments was measured by hemagglutination. Apical infection of the airway epithelia produced 8- to 16-fold more virus than basolateral infections (Fig. 2 ). When Japan strain was allowed to infect from the top of the cultures, approximately 2-fold more virions were generated than when PR8 was applied and approximately 100-fold more viruses than following X31 infection. In similar studies, we found no cross-release of the virus (data not shown).
FIG. 2.
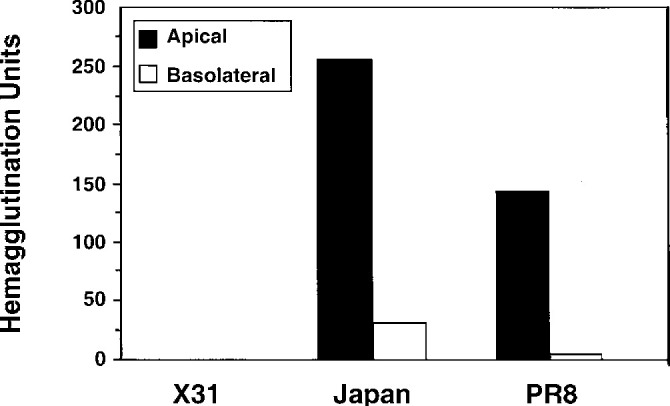
Polarized production of influenza virus following application of Japan, PR8, or X31 to the apical or basolateral side of human airway epithelia cultures. Hemagglutination titers were assayed 18 h later, with the results of a representative experiment shown. There was no drop in the transepithelial resistances throughout the culture period.
Cleavage of hemagglutinin is an important, and often a host-limiting, step of influenza virus infection. In most cell culture systems used for replication of influenza virus the addition of exogenous trypsin is required for multiple cell infection. We asked whether a multiple-cycle infection could proceed in airway cells without the addition of proteases. To perform this experiment, airway epithelia were infected with X31, PR8, or Japan strains and then washed with PBS with or without addition of trypsin. Virus production was monitored by hemagglutination. Protease treatments and assays were repeated on days 2, 3, and 4 after infection. It was found that the difference in TCID50 between Japan virus-infected filters treated and untreated with trypsin did not exceed 1 log (Fig. 3A ). Infectivity of X31 strain under either condition was very low (data not shown), precluding our ability to test for cleaved HA.
FIG. 3.

Replication of Japan influenza strain in human airway epithelia in the presence and absence of trypsin. (A) Airway epithelia were infected with Japan virus, and on subsequent days virus was collected in apical washings in the absence or presence of trypsin (2 μg/ml). (B) The status of HA in apical washes as determined by immunoblot assay. Apical washes were collected from cultures infected 4 days earlier with Japan strain (Jp sups) and subjected to SDS–PAGE and immunoblot using anti-Japan antibody. The +T and –T lanes refer to washes containing or devoid of exogenously added trypsin, respectively. Recombinant HA (rHA) expressed from an SV40 vector in CV-1 cells was used as a positive control for HA0. Partially purified Japan virus preparation (Jp) was used as a positive control for HA1. The positions of HA0, NP, and HA1 are indicated (arrowheads).
Immunoblot assays were done to confirm that Japan virus hemagglutinin was cleaved in the absence of exogenously added proteases (Fig. 3B). Japan strain hemagglutinin precursor (HA0) in the absence of trypsin can be seen in Fig. 3B (lane –T). The addition of trypsin facilitated more complete digestion of the hemagglutinin protein (Fig. 3B, lane +T), but as noted this additional cleavage did not dramatically increase virus production.
Infection of Human Airway Epithelia with X31 Strain Is Blocked at the Stage of Endocytosis
The X31 strain is a reassortment virus generated for vaccination purposes (33). Prior work showed that H3N2 strains similar to X31 could productively infect nasal polyps grown as organ cultures (34). Also, other work showed effective binding of X31 to formaldehyde-fixed tracheal tissues. To determine if the block in X31 infection was after entry or after binding, both metabolic labeling and binding assays were performed.
Cultures of airway epithelia were infected with X31 or Japan strains and metabolically labeled with [35S]methionine 8 h after infection. Virions released to the apical side were analyzed by SDS–PAGE. The data in Fig. 4A show accumulation of influenza nucleoprotein (NP) in apical washings from Japan-infected cells (Fig. 4A, lane JS) but not X31-infected cells (Fig. 4A, lane XS). The authenticity of viral NP protein was confirmed using an immunoblot with anti-Japan antiserum (Fig. 4B). This result indicates that the hindrance to X31 infection occurs at early stages of the infectious cycle.
FIG. 4.
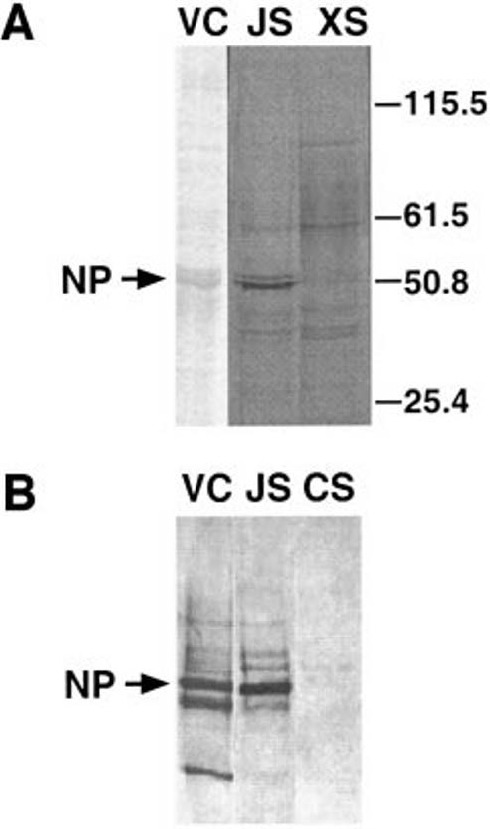
Synthesis of viral proteins in human airway epithelia. (A) Airway cells were infected with Japan or X31 viruses and viral protein synthesis was detected using [35S]methionine-metabolic labeling followed by SDS–PAGE and autoradiography. VC, Coomassie-stained lane of Japan virus control (vector control); JS and XS, apical washings after Japan or X31 infection, respectively. (B) Confirmation of Japan NP protein synthesis by immunoblot assay with anti-Japan antibodies. JS, Japan supernatant. CS, control supernatant from a mock-infected culture.
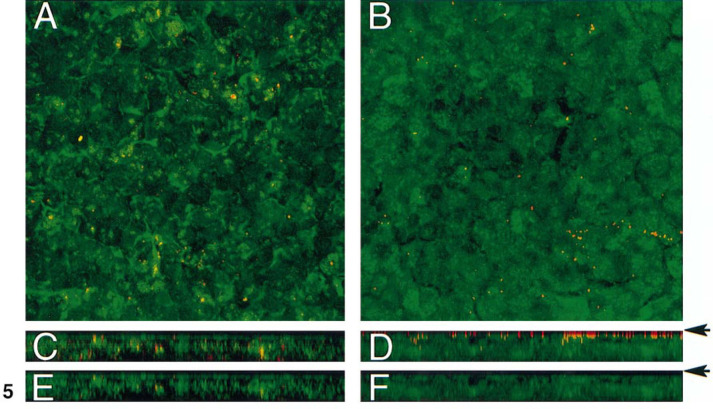
Japan and X31 strain infection was monitored by confocal microscopy after application of fluorescently labeled virions to the cultures (m.o.i. = 5) to determine if the X31 strain was capable of entry into human airway epithelia. To distinguish between viruses and cells, the rhodamine-labeled lipid probe Rh-PE was introduced into the viral envelope, and cellular membranes were labeled with F18 (fluorescein lipid probe). Thirty minutes after application of virus, cells were washed, fixed, and observed by confocal microscopy. The photomicrographs in Fig. 5 show an en face view (Figs. 5A and 5B) or x-z stacked series (Figs. 5C and 5D) from the acquired images of influenza virus-infected airway epithelia. Japan virions were noted both apically and internally (Fig. 5C). In contrast, the X31 strain is localized to the apical surface only (Fig. 5D). We next tested for evidence of fusion using the FRET assay. The assay was designed to test for FRET between the fluorescein label in cellular membranes and the rhodamine fluorophore in the viral envelopes. This process can take place only if the distance between the two dyes is in the range of 10 to 100 Å (35), as can be accomplished during membrane fusion processes. Figures 5E and 5F are representative of the level of FRET occurring in the presence of excitation of fluorescein only. FRET, detected as excitation of the Ph-RE label, was observed throughout the thickness of the culture after application of labeled Japan strain (Fig. 5E). For cultures to which Rh-PE-labeled X31 was applied, FRET was rarely detected (Fig. 5F).
FIG. 5.
Analysis of Japan and X31 virus entry into human airway epithelia. Influenza viruses were labeled with Rh-PE and cell membranes were stained with F18 as described under Materials and Methods. (A, B) Photomicrographs depicting an en face view of airway epithelia cultures 30 min after application of fluorescently labeled Japan (A) or X31 (B) virions. (C, D) Stacked x-z series from A and B, respectively. (E, F) FRET evaluation of the series shown in C and D, respectively. Arrows denote top of epithelium.
Fluid-phase endocytosis is minimal in fully differentiated airway epithelia (36). To investigate if application of influenza virus would increase endocytosis, FITC-labeled dextran, as a fluid-phase marker, was applied to the cells after their infection with Japan or X31 strains. After incubation with virus for 5 min at 37°C (m.o.i. = 1), cells were washed and intracellular fluorescence was observed using confocal microscopy. The results of this experiment show that endocytosis in the cells infected with Japan strain was significantly enhanced, probably as a result of virus uptake (Fig. 6A ). In contrast, FITC–dextran uptake in the cells infected with X31 was very low (Fig. 6B) and was indistinguishable from that of control, uninfected cells (not shown).
FIG. 6.
Fluid-phase endocytosis in human airway epithelia infected with Japan or X31 strain of influenza virus. Influenza virus preparations were added to airway epithelia in the cold, followed by addition of a 37°C solution of FITC–dextran to detect fluid-phase endocytosis. After a 5-min incubation cells were fixed, stained with Texas red-labeled lectin to label the apical surface, and observed by confocal microscopy. The photomicrographs are a stacked x-z series. Background levels of endocytosis (not shown) in the absence of added virus were uniformly low as previously published (36).
Infection of Human Trachea Explants with Influenza Virus
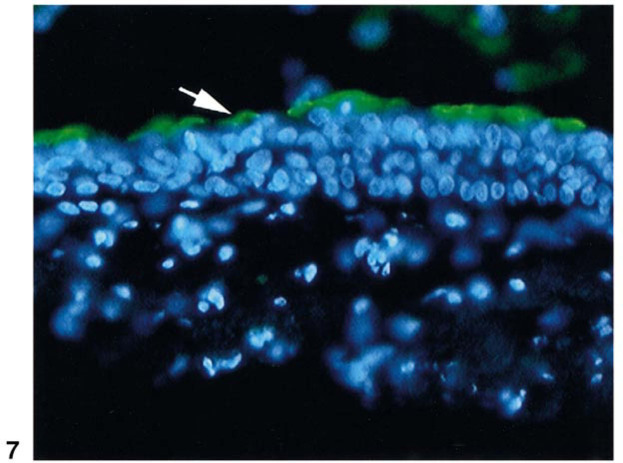
To confirm the ability of Japan to productively infect human airway cells, experiments were done on freshly explanted human trachea. After overnight infection with Japan strain, culture fluid was collected and assayed by hemagglutination for the presence of the virus. The Japan strain produced a HA titer of 1:8. In similar studies with PR8 and X31, the titers were 1:8 and undetectable, respectively. Thus qualitatively, in both the cell culture and the explant models, Japan and PR8 influenza strains were capable of infection, while X31 was not. The infection of the tracheal epithelium by Japan virus was also confirmed by immunostaining (Fig. 7 ). Notably, only the apical surface was positive for influenza proteins. The detection of virus proteins was replication-dependent, as staining of control tracheal explants, fixed immediately after adsorption of the viruses, was negative (data not shown).
FIG. 7.
Cryosection of trachea explant infected with Japan virus. Trachea explants were left uninfected or were infected with the Japan influenza strain as described under Materials and Methods. Cryosections (15 μm) were stained with FITC-labeled antibodies against Japan virus. The photomicrograph shown is representative of the level of anti-Japan immunoreactivity seen in three independent experiments using blocks of trachea from different donor lungs. Sections from control tissues, or mock-infected tissues, were negative (not shown). The arrow points to the apical side of tracheal epithelium. Nuclei were detected using DAPI staining.
Discussion
We compared the infectious activity of three influenza A strains in differentiated primary human airway epithelia grown at an air–liquid interface. Each of the viruses tested was representative of a different HA subtype. X31 (H3N2) has binding specificity for SAα2,6Gal, Japan (H2N2) binds preferentially to SAα2,3Gal, and PR8 (H1N1) is able to utilize both types of oligosaccharides (6). We confirmed the SA-Gal specificity (3, 7) of our viruses in hemagglutination inhibition tests with normal horse serum (Table 1). Based on studies of virus binding to fixed sections of human trachea (12), and human respiratory tissue (34), we initially hypothesized that application of viruses with SAα2,6Gal specificity would be most likely to result in a productive infection in human airway epithelia. However, our data show that influenza strains with preferential binding to SAα2,3Gal receptors productively infect human airway epithelia. This was recently confirmed by clinical observations; avian influenza viruses isolated from infected patients in Hong Kong were found to be SAα2,3Gal specific (37).
Prior studies using specific lectin staining showed that tracheal epithelia express sialyloligosaccharides with both SAα2,3Gal and SAα2,6Gal linkages (11, 12). In earlier work by Baum and Paulson (11), goblet cells and their corresponding mucus droplets stained with lectins specific for SAα2,3Gal, while ciliated cells stained predominantly with lectins specific for SAα2,6Gal. These data supported studies done by Liu (38), showing binding of dye-labeled human isolates with SAα2,6Gal specificity to ciliated epithelia of the ferret trachea. Similar studies, using paraformaldehyde-fixed preparations of human trachea, confirmed binding of viruses with SAα2,6Gal specificity to ciliated cells (11). Interestingly, the authors of this study also noted labeled virus with SAα2,6Gal specificity situated over goblet cells, but attributed this binding to tissue preparation artifact. In our experiments binding and infection were tested using viable cells, with clear evidence of SAα2,3Gal-specific strain binding and fusion with the apical membranes of polarized airway epithelia.
Like Japan, X31 could bind to the apical surface of airway epithelia. However, it could not enter the cells; there was minimal evidence of membrane fusion within the cells as was found with the Japan strain, and there was little to no virus produced after 18 h of infection. The block in infection was not due to defective virus, since the titer in MDCK cells was similar to those of the other viruses tested. Also, we did not find an overall block in infection by other H3N2 strains with SAα2,6Gal specificity, since other H3N2 human isolates could productively infect human airway epithelia from the apical surface (39). One possibility for the inability of the X31 strain to infect airway epithelia could be mutations within HA that do not alter binding specificity, but do inhibit interaction with a secondary receptor or entry of the virus into cells. Sequencing of the HA RNA encoding the HA1 subunit from X31 and A/Aichi/2/68 virions revealed only a conservative mutation: Ile182 in Aichi to Val in X31 (data not shown). However, we cannot discount that mutations in HA2 or other proteins were responsible for the low infectious activity of X31 in airway epithelia. A second possibility may relate to the susceptibility of Japan or X31 HA to inactivation (40). Work by Korte and colleagues demonstrated that X31 HA is more sensitive to inactivation after preincubation in low-pH buffer (pH 5.0–5.2) than Japan virus. Those studies showed that a 10-min preincubation of X31 at pH 5.0 reduced the fusogenic capacity of the virus by 70%. Japan was more stable under these conditions. When similar experiments were done using PR8, the rate and extent of inactivation were greater than for X31. However, we noted that PR8 was more infections than X31 in airway epithelia, suggesting that inactivation of X31 HA may not be the sole determinant for its inability to infect airway epithelia in this study. Finally, the observed differences between the parent strains (PR8 and A/Aichi) and their reassortant (X31) could be due to altered interactions between surface glycoproteins derived from the H3N2 strain and the membrane proteins (M1 and M2) originating from H1N1 virus. How the requirements for these interactions would differ between kidney cells and airway epithelia is unclear at this time.
Infection of epithelial cells by most viruses is polarized in terms of entry and release of virus particles (reviewed in (41)). We noted previously that entry of Moloney murine retrovirus vectors with amphotropic or xenotropic envelopes into human airway epithelia was restricted to the basolateral surface (21). In contrast, influenza virus infection resulted from binding to the apical plasma membrane ((32) and this study), similar to coronavirus (42, 43) and measles virus (44). Researchers investigating viral vectors for application to the treatment of cystic fibrosis have shown that one of the major limitations to infection of airway epithelia from the apical side is receptor inaccessibility (21, 22, 23, 24). Recently, methods have been developed to transiently disrupt the tight junctions to allow envelope viruses access to all cell layers of the epithelia (45). An alternative method to target retroviral vectors to airway epithelia from the mucosal side may be to pseudotype them with envelopes shown to allow for productive infection when applied to the apical surface. Our data suggest that Japan, but not X31, could be a candidate envelope protein for such an approach.
For consideration of gene therapy for CF or other airway diseases, it would be advantageous to use an envelope not currently circulating in the human population (like Japan). This would preclude problems of existing neutralizing antibodies. Such envelopes, coupled with lentivirus vectors, which we previously showed to result in long-term expression (46), may be ideal.
In conclusion, we confirm the previously reported polarity of influenza virus binding and budding, but using a model of well-differentiated airway epithelia cultured at an air–liquid interface. Our data revealed interesting differences between X31 infectivity in MDCK vs airway epithelia and suggest that the block in X31 infection was due to inhibition of infection after binding to the cell surface. We demonstrate that Japan virus can bind and fuse with apical cellular membranes, increase fluid-phase endocytosis, and replicate in viable cultures of well-differentiated epithelia. Finally, we speculate that the HA from Japan, or from similar strains shown to productively infect airway epithelia models, may be useful for targeting other enveloped viruses, such as recombinant lentiviruses, to the apical surface of airway epithelia for gene therapy of lung diseases.
Acknowledgments
The authors acknowledge Phil Karp, Perry Weber, Royce Burns, and Camille Deering for technical assistance; Joseph Zabner for help with the tracheal experiments; and Michael J. Welsh for helpful discussions. We also thank Christine McLennan for expert secretarial assistance. This work was provided by support from the CF Foundation, the Carver Foundation (B.L.D.), and the NIH (DK54759).
References
- 1.1Kingsbury D.W. Orthomyxoviridae and their replication. In: Fields B.N., Knipe D.M., editors. Virology. Raven Press; New York: 1990. pp. 1075–1090. [Google Scholar]
- 2.Suzuki Y., Kato H., Naeve C.W., Webster R.G. Single-amino-acid substitution in an antigenic site of influenza virus hemagglutinin can alter the specificity of binding to cell membrane-associated gangliosides. J. Virol. 1989;63:4298–4302. doi: 10.1128/jvi.63.10.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Connor R.J., Kawaoka Y., Webster R.G., Paulson J.C. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- 4.Rogers G.N., D'Souza B.L. Receptor binding properties of human and animal H1 influenza virus isolates. Virology. 1989;173:317–322. doi: 10.1016/0042-6822(89)90249-3. [DOI] [PubMed] [Google Scholar]
- 5.Rogers G.N., Daniels R.S., Skehel J.J. Host-mediated selection of influenza virus receptor variants. J. Biol. Chem. 1985;260:7362–7367. [PubMed] [Google Scholar]
- 6.Rogers G.N., Paulson J.C. Receptor determinants of human and animal influenza virus isolates: Differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- 7.Rogers G.N., Pritchett T.J., Lane J.L., Paulson J.C. Differential sensitivity of human, avian, and equine influenza A viruses to a glycoprotein inhibitor of infection: Selection of receptor specific variants. Virology. 1983;131:394–408. doi: 10.1016/0042-6822(83)90507-x. [DOI] [PubMed] [Google Scholar]
- 8.Carroll S.M., Paulson J.C. Differential infection of receptor-modified host cells by receptor-specific influenza viruses. Virus Res. 1985;3:165–179. doi: 10.1016/0168-1702(85)90006-1. [DOI] [PubMed] [Google Scholar]
- 9.Ito T., Suzuki Y., Takada A. Differences in sialic acid-galactose linkages in the chicken egg amnion and allantois influence human influenza virus receptor specificity and variant selection. J. Virol. 1997;71:3357–3362. doi: 10.1128/jvi.71.4.3357-3362.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Katz J.M., Naeve C.W., Webster R.G. Host cell-mediated variation in H3N2 influenza viruses. Virology. 1987;156:386–395. doi: 10.1016/0042-6822(87)90418-1. [DOI] [PubMed] [Google Scholar]
- 11.Baum L.G., Paulson J.C. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem. Suppl. 1990;Band XL S:35–38. [PubMed] [Google Scholar]
- 12.Couceiro J.N.S.S., Paulson J.C., Baum L.G. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium: The role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- 13.Matlin K.S., Reggio H., Helenius A., Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J. Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nunes-Correia I., Ramalho-Santos J., Nir S., Pedroso de Lima M.C. Interactions of influenza virus with cultured cells: Detailed kinetic modeling of binding and endocytosis. Biochemistry. 1999;38:1095–1101. doi: 10.1021/bi9812524. [DOI] [PubMed] [Google Scholar]
- 15.Doms R.W., Gething M.-J., Henneberry J., White J., Helenius A. Variant influenza virus hemagglutinin that induces fusion at elevated pH. J. Virol. 1986;57:603–613. doi: 10.1128/jvi.57.2.603-613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiss T.F., Fruenert D.C., Nadal J.A., Jacoby D.B. Infection of cultured human airway epithelial cells by influenza A virus. Life Sci. 1991;49:1173–1181. doi: 10.1016/0024-3205(91)90565-s. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson P.J., Borland R. Persistent infection of human lung cells with influenza virus. Nature. 1972;238:153–155. doi: 10.1038/238153a0. [DOI] [PubMed] [Google Scholar]
- 18.Yamaya M., Finkbeiner W.E., Chun S.Y., Widdicombe J.H. Differentiated structure and function of cultures from human tracheal epithelium. Am. J. Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]
- 19.Zabner J., Zeiher B.G., Friedman E., Welsh M.J. Adenovirus-mediated gene transfer to ciliated airway epithelia requires prolonged incubation time. J. Virol. 1996;70:6994–7003. doi: 10.1128/jvi.70.10.6994-7003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith J.J., Travis S.M., Greenberg E.P., Welsh M.J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 21.Wang G., Davidson B.L., Melchert P. Influence of cell polarity on retrovirus-mediated gene transfer to differentiated human airway epithelia. J. Virol. 1998;72:9818–9826. doi: 10.1128/jvi.72.12.9818-9826.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zabner J., Puga A., Freimuth P., Welsh M.J. Lack of high affinity fiber receptor activity explains the resistance of ciliated airway epithelia to adenovirus infection. J. Clin. Invest. 1997;100:1144–1149. doi: 10.1172/JCI119625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickles R.J., McCarty D., Matsui H., Hart P.J., Randell S.H., Boucher R.C. Limited entry of adenovirus vectors into well-differentiated airway epithelium is responsible for inefficient gene transfer. J. Virol. 1998;72:6014–6023. doi: 10.1128/jvi.72.7.6014-6023.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duan D., Yue Y., Yan Z., McCray P.B., Jr., Engelhardt J.F. Polarity influences the efficiency of recombinant adenoassociated virus infection in differentiated airway epithelia. Hum. Gene Ther. 1998;9:2761–2776. doi: 10.1089/hum.1998.9.18-2761. [DOI] [PubMed] [Google Scholar]
- 25.Kilbourne E.D. Future influenza vaccines and the use of genetic recombinants. Bull. World Health Organ. 1969;41:643–645. [PMC free article] [PubMed] [Google Scholar]
- 26.Levi R., Beeor-Tzahar T., Arnon R. Microculture virus titration—A simple colourimetric assay for influenza virus titration. J. Virol. Methods. 1995;52:55–64. doi: 10.1016/0166-0934(94)00137-6. [DOI] [PubMed] [Google Scholar]
- 27.Coico R. Detection and analysis of HIV. In: Coligan J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W., editors. Current Protocols in Immunology. Wiley; New York: 1999. pp. 12.2.5–12.2.6. [Google Scholar]
- 28.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Harlow E., Lane D. Antibodies: A Laboratory Manual. Cold Spring Harbor Laboratory Press; New York: 1988. Storing and purifying antibodies. p. 298–303. [Google Scholar]
- 30.Arbuzova A., Korte T., Muller P., Herrmann A. On the validity of lipid dequenching assays for estimating virus fusion kinetics. Biochim. Biophys. Acta. 1994;1190:360–366. doi: 10.1016/0005-2736(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.McCray P.B., Jr., Wang G., Kline J.N. Alveolar macrophages inhibit retrovirus-mediated gene transfer to airway epithelia. Hum. Gene Ther. 1997;8:1087–1093. doi: 10.1089/hum.1997.8.9-1087. [DOI] [PubMed] [Google Scholar]
- 32.Boulan E.R., Sabatini D.D. Asymmetric budding of viruses in epithelial monolayers: A model system for study of epithelial polarity. Proc. Natl. Acad. Sci. USA. 1978;75:5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higa H.H., Rogers G.N., Paulson J.C. Influenza virus hemagglutinins differentiate between receptor determinants bearing N-acetyl-, N-glycollyl-, and N,O-diacetylneuraminic acids. Virology. 1985;144:279–282. doi: 10.1016/0042-6822(85)90325-3. [DOI] [PubMed] [Google Scholar]
- 34.Ginzburg V.P., Rozina E.E., Sharova O.K., Ghendon Y.Z. Reproduction of human and animal influenza viruses in human nasal polyp organ cultures. Acta Virol. 1985;29:424–427. [PubMed] [Google Scholar]
- 35.Hoekstra D., Duzgunes N. Lipid mixing assays to determine fusion in liposome systems. Methods Enzymol. 1993;220:15–32. doi: 10.1016/0076-6879(93)20070-j. [DOI] [PubMed] [Google Scholar]
- 36.Drapkin P.T., O'Riordan C.R., Yi S.M. Targeting the urokinase plasminogen activator receptor enhances gene transfer to human airway epithelia. J. Clin. Invest. 2000;105:589–596. doi: 10.1172/JCI8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matrosovich M., Zhou N., Kawaoka Y., Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C. Studies on influenza infection in ferrets by means of fluorescein-labelled antibodies. J. Exp. Med. 1954;101:665–676. doi: 10.1084/jem.101.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slepushkin V., Staber P.D., Davidson B.L. Scientific Program and Abstracts of the 18th Annual Meeting, Amherst, MA. 1999. Influenza virus infection of cultured primary human airway cells. p. 79. [Google Scholar]
- 40.Korte T., Ludwig K., Booy F.P., Blumenthal R., Herrmann A. Conformational intermediates and fusion activity of influenza virus hemagglutinin. J. Virol. 1999;73:4567–4574. doi: 10.1128/jvi.73.6.4567-4574.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tucker S.P., Compans R.W. Advances in Virus Research. Academic Press; New York: 1993. Virus infection of polarized epithelial cells. p. 187–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rossen J.W.A., Voorhout W.F., Horzinek M.C., van der Ende A., Strous G.J.A.M., Rottier P.J.M. MHV-A59 enters polarized murine epithelial cells through the apical surface but is released basolaterally. Virology. 1995;210:54–66. doi: 10.1006/viro.1995.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang G., Deering C., Macke M. Human coronavirus 229E infects polarized airway epithelia from the apical surface. J. Virol. 2000;74:9234–9239. doi: 10.1128/jvi.74.19.9234-9239.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Blau D.M., Compans R.W. Entry and release of measles virus are polarized in epithelial cells. Virology. 1995;210:91–99. doi: 10.1006/viro.1995.1320. [DOI] [PubMed] [Google Scholar]
- 45.Wang G., Zabner J., Deering C. Increasing epithelial junction permeability enhances gene transfer to airway epithelia in vivo. Am. J. Respir. Cell Mol. Biol. 2000;22:129–138. doi: 10.1165/ajrcmb.22.2.3938. [DOI] [PubMed] [Google Scholar]
- 46.Wang G., Slepushkin V., Zabner J. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Invest. 1999;104:R55–R62. doi: 10.1172/JCI8390. [DOI] [PMC free article] [PubMed] [Google Scholar]