Abstract
Background
Sorafenib, a multi-kinase inhibitor approved for treatment of advanced renal cell carcinoma and other malignancies, has been shown as a modulator for dendritic cells. This study was designed to examine the effects of sorafenib on macrophages, the major ontogeny of innate immunity.
Materials and methods
Macrophages were derived from sorted CD14+ monocytes of human peripheral blood mononuclear cells. Cell viability and surface antigens were examined by trypan blue analysis. Autophagy was characterized by light microscopy and transmission electron microscopy for morphology, Western blotting for microtubule associated light chain protein 3B (LC-3B) I lipidation, and acridine orange staining for acidic component vacuoles. Soluble factors contained in culture medium and serum were measured by ELISA.
Results
We found that sorafenib inhibited the viability of macrophages accompanied by morphological changes characteristic of autophagy. This autophagy-inducing effect was validated by LC3B-I lipidation and autophagosome accumulation. The surface antigen expression and the function of activated macrophages were inhibited by sorafenib, including the expression of co-stimulatory molecule CD80, phagocytosis, and the production of reactive oxygen species. The secretion of IL-10, but not IL-6, TNF-α nor TGF-β, was reduced by sorafenib.
Conclusion
Sorafenib, in addition to being a cancer targeted therapeutic agent, can induce autophagy and modulate the function of human macrophages.
Keywords: Sorafenib, Macrophage, Autophagy
Highlights
► Sorafenib is a multi-kinase inhibitor approved for cancer treatment. ► We examine the immunomodulatory effect of sorafenib on macrophages. ► Sorafenib induces autophagy and alters functions of human macrophages.
1. Introduction
After being recruited from bone marrow to peripheral blood, monocytes enter tissues and differentiate into macrophages, the major cell lineage of the innate immune system [1]. By phagocytosis and the removal of infected microorganisms and cellular debris, macrophages can process and present antigens to initiate a specific immune response.
Sorafenib is a multi-kinase inhibitor capable of blocking the RAF/MEK/ERK pathway, the vascular endothelial growth factor receptors (VEGFR)-2 and VEGFR-3, platelet-derived growth factor receptor (PDGFR)-β, c-Kit, and FMS-like tyrosine kinase (Flt)-3 [2]. This drug has been approved for the treatment of advanced renal cell carcinoma [3], hepatocellular carcinoma [4], [5] and others [6]. Other than the anti-cancer activity, sorafenib has been reported capable of modulating the immunobiological activity of dendritic cells and a murine regulatory macrophage population secreting relatively high levels of anti-inflammatory interleukin (IL)-10 [7], [8].
Autophagy, known as type II programmed cell death, is a distinct cellular event [9]. Autophagy is characterized by the formation of autophagosomes and the degradation of intracellular organelles and materials within autophagosomes fused with lysosomes. The process occurs at a basal level and in response to stress, such as serum starvation, radiation, or pharmacological agents [10]. The pathophysiological significance of autophagy developed in macrophages remains controversial [11]. The role of autophagy in cell survival remains inconclusive. For example, cells undergoing starvation stress may process autophagy as a pro-survival event. Cancer treatments such as chemotherapeutics or radiation therapy may cause an anti-survival manner of autophagy.
In the present study, we investigated the effects of sorafenib on primary cultured human macrophages and revealed an autophagy-inducing activity of sorafenib on macrophages. The accompanying alteration of macrophage function was also examined.
2. Materials and methods
2.1. Generation of human macrophages
Macrophages were generated from human peripheral blood monocytes. Briefly, peripheral blood mononuclear cells were obtained from healthy donors by Histopaque density gradient centrifugation (Amersham, Buckinghamshire, UK). This study was approved by our institutional ethics committee. Erythrocytes were lysed by treatment with 0.9% ammonium chloride. Subsequently, CD14+ cells were purified by high-gradient magnetic sorting using the miniMACS system with anti-CD14 microbeads. The purity of isolated CD14+ monocytes was more than 90% according to flow cytometric analysis. Macrophages were generated from CD14+ monocytes by culture in an RPMI 1640 medium supplemented with 10% fetal-calf serum every 3 days for 6 days in a humidified 5% CO2 incubator. Various concentrations of sorafenib (Bayer, Leverkusen, Germany), which were dissolved in dimethyl sulfoxide (DMSO), were added at the beginning of the CD14+ cell cultures to evaluate the effects on macrophages. In some experiments, macrophages were activated by adding LPS (100 μg/mL) for a further 24 h. In each experiment, an equal amount of DMSO was added as a vehicle control.
2.2. Cell viability
Trypan blue could not be uptaken into viable macrophages that had intact cell membranes. To assess cell membrane integrity, cells were counted on day 7 after treatment with various concentrations (0, 2.5, 5, 7.5, and 10 μM) of sorafenib using the trypan blue (Gibco) dye exclusion method. In another set of experiments, cells were counted on days 1, 3, and 5 after treatment with 7.5 μM sorafenib. Cells were collected using 0.25% trypsin (Sigma) solution and were washed with PBS. Equal volumes of cell suspension and 0.4% trypan blue solution were mixed to count the number of viable cells.
2.3. Flow cytometric analysis for CD80, acridine orange staining, and reactive oxygen species production
Dual-color immunolabeling was performed using fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-conjugated monoclonal antibodies (mAbs). The mouse anti-human mAbs IgG1:FITC and appropriate isotype controls were purchased from Serotec (Oxford, UK) and used for anti-CD80 (for IgG-FITC). Macrophages were incubated with saturating concentrations of primary CD80 mAbs followed by IgG-FITC at 4 °C for 30 min. After washing twice with PBS, 106 cells were applied to a FACS caliber flow cytometer (BD Biosciences, San Jose, CA). Data were collected and analyzed using CellQuest Software (BD Biosciences). For acridine orange staining, macrophages were stained with acridine orange (10 ng/mL) for 15 min and subjected to flow cytometry. For testing reactive oxygen species production, cells collected from day 6 cultures were treated with lipopolysaccharide (LPS) (1 μM for 24 h) and then were incubated for 30 min at 37 °C in PBS containing 25 μM DCFH-DA. Thereafter, cells were washed with PBS two times and subjected to flow cytometry.
2.4. Morphological observation by Liu's stain and light microscope
Cells were centrifuged onto microscope slides by a Cytospin centrifuge (Shandon Inc., Pittsburgh, PA), stained with Wright–Giemsa solution, and observed under light microscopy (Olympus, Tokyo, Japan). Photographs were taken with a digital camera and shown at a magnification of 1000 ×.
2.5. Morphological observation by transmission electronic microscopy
Macrophages were harvested by trypsinization, washed and fixed with cold 3% glutaraldehyde in 0.11 M cacodylate buffer for 30 min. After being rinsed in PBS, cells were postfixed in osmium tetroxide (1%) and embedded in Epon resin (Energy Beam Science, Agawam, Massachusetts). Semithin sections were cut, stained with 0.5% toluidine blue, and examined under a light microscope (BX51, Olympus, Tokyo, Japan). Ultrathin sections were stained with 2% uranyl acetate and Reynold's lead citrate and observed with a transmission electron microscope (JEM-1200EXII, JEOL Co., Tokyo, Japan).
2.6. Western blotting
Whole cell lysates were isolated from macrophages after treatment with various concentrations of sorafenib. The protein concentration was determined by a bicinchoninic acid (BCA) assay kit (Pierce, Rockford, Illinois). Equal amounts of proteins (50 μg in each lane) were electrophoresed in 10% SDS-polyacrylamide gels. Then, proteins were transferred onto a nylon blotting membrane. The membrane was blocked with 5% de-fatted milk and immunoblotted with primary antibodies against microtubule associated light chain protein 3B (LC-3B)-I and LC3B-II (Cell Signaling Technology, Danvers, MA) at room temperature for 3 h. This was followed by incubation with horseradish peroxidase-labeled second antibodies (Transduction Laboratories) and development using the enhanced chemiluminescence system (Amersham Pharmacia, Piscataway, New Jersey). The conjugated form of LC3 is called LC3-II to distinguish it from the unconjugated form (LC3-I) and has faster mobility on SDS gels.
2.7. Detection of cytokines produced by macrophages
The levels of IL6, IL10, TGF-β, and TNF-α in the cultured-macrophage supernatant were measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems) according to the manufacturer's instructions.
2.8. Phagocytosis
The phagocytic activity was measured according to the methods previously published. Briefly, yeast was heat-inactivated to diminish infectivity to avoid confusion with phagocytosis. Yeast suspension was prepared in PBS at a density of 1 × 108/mL as a stock. The macrophages collected from day 6 cultures were treated with lipopolysaccharide (LPS) (1 μM for 24 h), washed, re-suspended (1 × 106/mL) in an FCS-containing RPMI1640 medium and incubated with the yeast suspension (4 × 106/mL) at 37 °C for 30 min. Cells were then placed on a glass slide and observed under an inverted microscope (ECLIPSE TS100, Nikon, Tokyo, Japan). The total numbers of phagocytotic yeasts were counted out of 200 cells.
2.9. Statistical analysis
The results are expressed as means ± standard errors of the means (SEMs). Comparison in each experiment was performed using an unpaired Student's t-test. A p value of less than 0.05 was considered statistically significant.
3. Results
3.1. Sorafenib affected the viability of human macrophages
The viability of macrophages was mildly increased after treatment with low and intermediate doses of sorafenib (2.5 and 5 μM) in comparison with the control group. However, higher concentrations of sorafenib (10 μM) significantly inhibited the cell viability of macrophages (left panel of Fig. 1 ). In another set of experiment, 7.5 μM sorafenib was added and it showed that sorafenib significantly inhibited the viability of macrophages during the time course (right panel of Fig. 1).
Fig. 1.
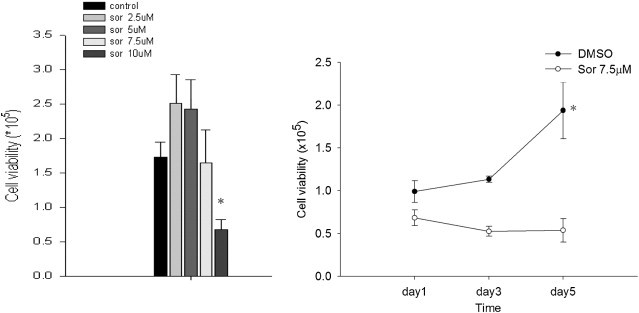
Effect of sorafenib on macrophage viability. After treatment with sorafenib (0, 2.5, 5.0, 7.5, and 10 μM), the monocyte-derived human macrophages were harvested on day 7 and the number of viable cells was counted using the trypan blue test. In the right panel, cells were treated with 7.5 mM sorafenib and harvested on days 1, 3, and 5 for the same test. Data from the three separate experiments were expressed as means ± SEMs. *p < 0.05 vs. DMSO control group.
3.2. Sorafenib induced autophagy in human macrophages
Morphologically, sorafenib-treated macrophages exhibited extensive development of cytoplasmic vacuoles (Fig. 2A). Such morphological changes raised the concern that autophagy might exist in sorafenib-treated macrophages. These cytoplasmic vacuoles were further examined under transmitted electron microscopy, and double-membrane structures containing organelle components, characteristics of autophagosomes, were identified in sorafenib-treated macrophages, whereas only empty single-layer vacuoles were noted in the control group (Fig. 2B). By using acridine orange staining, the formation of acidic vesicular organelles (AVO) in macrophages was demonstrated (Fig. 3 ). Microtubule-associated protein 1 light chain 3 (LC3) is the corresponding mammalian homologue of yeast autophagy protein Atg8, which aggregates to autophagosome membranes when autophagy occurs. Western blotting showed an increase in the conversion of free cytosolic LC3B (LC3B-I) to phosphatidylethanolamine-conjugated LC3B (LC3B-II) in a dose-dependent manner (Fig. 4 ). The increase of LC3 I lipidation persisted to at least day 7. Taken together, they suggest that sorafenib could induce autophagy in human macrophages.
Fig. 2.
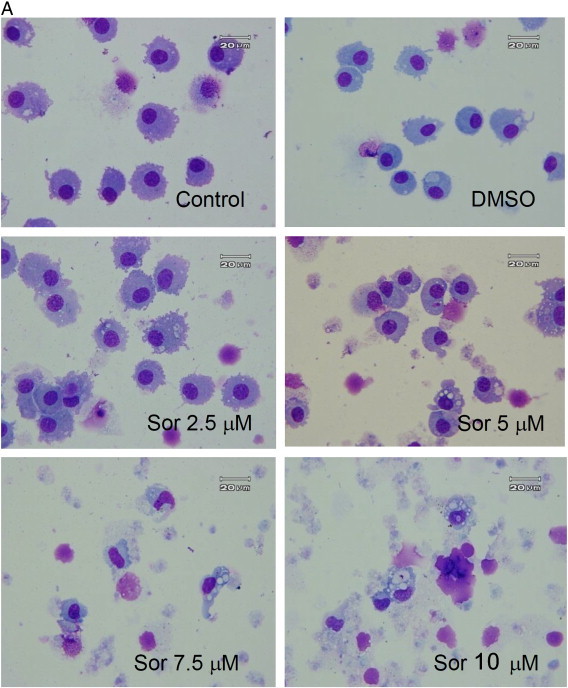
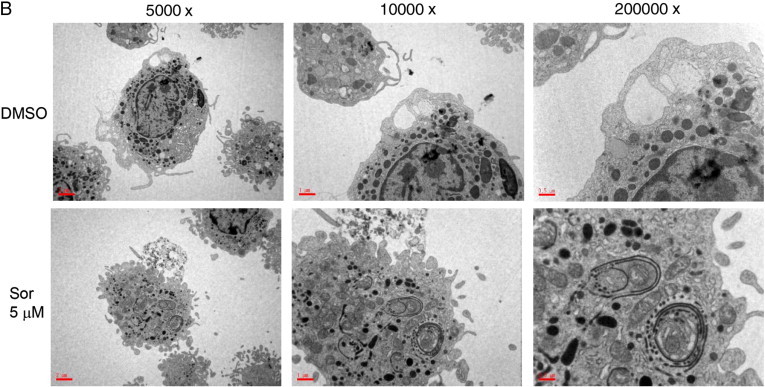
Morphological alteration of macrophages by sorafenib. (A) Morphology of macrophages, which were treated with 2.5, 5, 7.5, and 10 μM sorafenib (magnification 1000 ×). Macrophages were stained with Wright–Giemsa solution and observed under a light microscope. (B) Morphology of macrophages observed under transmitted electron microscopy. Macrophages were treated with 7.5 μM sorafenib for 4 days. Empty vacuoles with a monolayer membrane were observed in untreated cells. Numerous autophagic vacuoles with a typical double-layer membrane containing organelle remnants were noted.
Fig. 3.
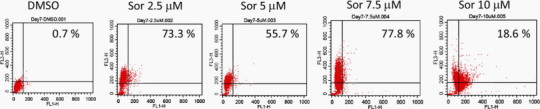
Acridine orange staining of acidic component of vacuoles. Representative flow cytometry data showed the acridine orange fluorescent intensities after treatment of sorafenib for 7 days. Similar results were obtained in three independent experiments.
Fig. 4.
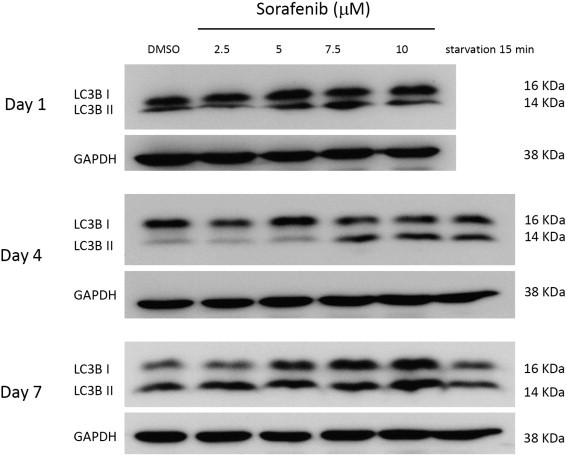
Lipidation of LC3-I in macrophages by immunoblotting. Macrophages were treated with sorafenib (0 to 10 μM for 1, 4, and 7 days) and serum starvation, and then subjected to immunoblot analysis using anti-LC-3B antibody and anti-GAPDH antibody.
3.3. Sorafenib suppressed the CD80 expression and function of activated macrophages
We next investigated whether sorafenib can affect classical activation of macrophages or not. Sorafenib downregulated the expression of surface co-stimulatory molecule CD80 in a dose-dependent manner (Fig. 5A). The function of activated macrophages was inhibited by sorafenib in terms of phagocytosis (Fig. 5B) and the production of reactive oxygen species (Fig. 5C).
Fig. 5.
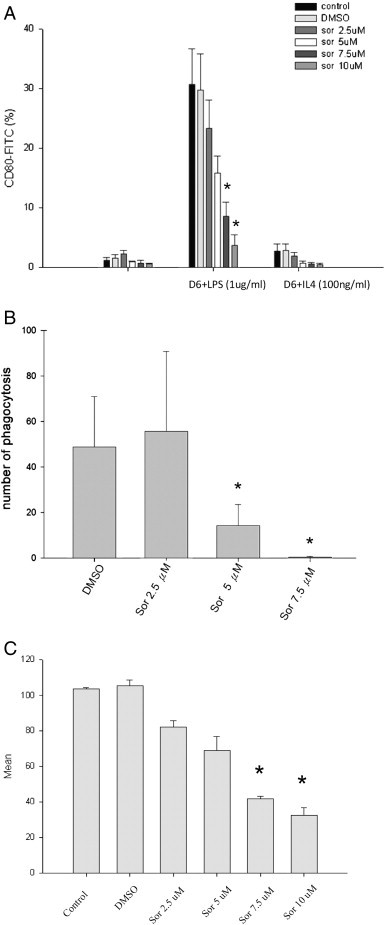
Expression of surface antigen CD80 and assessment of function in macrophages. (A) Expression of surface molecule CD80 on monocyte-derived macrophages. (B) Effect of sorafenib on phagocytosis. (C) Effect of sorafenib on LPS-induced production of reactive oxygen species. Data from three separate experiments were expressed as means ± SEMs. *p < 0.05 vs. DMSO control group.
3.4. Sorafenib affected the secretion of cytokines of human macrophage
The level of secreted IL-10 was reduced by sorafenib treatments. However, the levels of IL-6, TNF-α and TGF-β demonstrated no significant changes (Fig. 6 ).
Fig. 6.
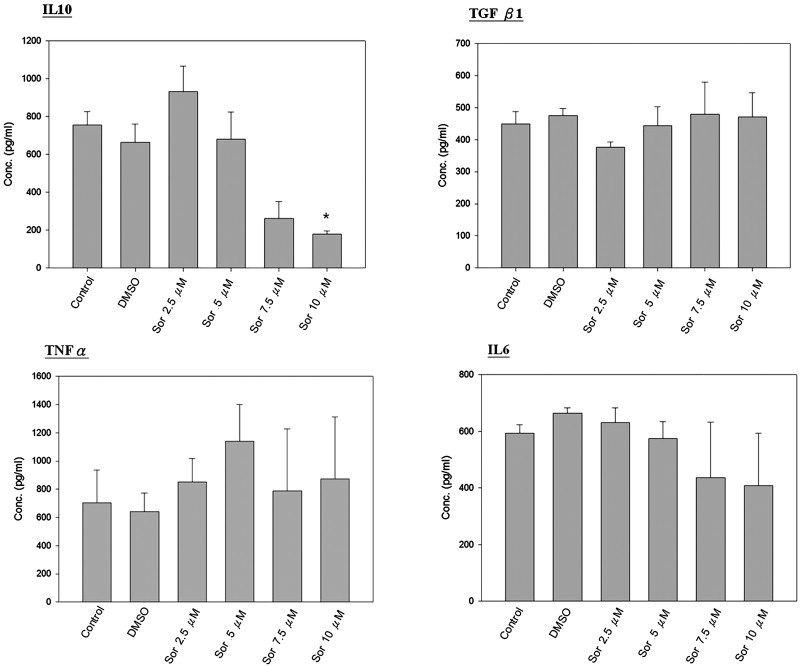
Production of cytokines by macrophages. The levels of IL-6, IL-10, TGF-β1 and TNF-α in the DC supernatant were measured using enzyme-linked immunosorbent assay (ELISA) (R&D Systems, HS120, D1000B) according to the manufacturer's instructions. *p < 0.05 vs. DMSO control group.
4. Discussion
Sorafenib, an oral small-molecule target therapeutics against cancer, induced autophagy in human macrophages accompanied by cell death and suppressed function.
The role of autophagy in macrophages has been characterized. The development of autophagy could recognize and restrict viral, bacterial, and parasitic infection inside macrophages [12], [13], [14]. By contrast, some viruses have the capability to subvert autophagy to survive, such as severe acute respiratory syndrome coronavirus [15]. Thus, the role and function of autophagy in macrophages remain controversial and need to be clarified. It implies that pharmacological manipulation of macrophages by inducing autophagy might have the potential to benefit the disease outcome of clinical immunological disorders. However, the phenomenon of autophagy induction and the candidate agents to induce autophagy in macrophages have not been reported. In our preliminary study, sorafenib could induce autophagy in both macrophages and dendritic cells (data not shown), indicating a potential role modulating innate immunity. Furthermore, the sorafenib-induced autophagy in human macrophages could be categorized as autophagy-associated cell death.
The polarization of macrophages by classical activation and alternative activation pathways differs by function and phenotype [16], [17]. For the classical activation of macrophages, endotoxin LPS has been applied as a standard activator. We demonstrated that sorafenib induced autophagy of macrophages in steady state. The function of classically activated macrophages was also declined. Whether this effect extends to that of the alternative activation of macrophages or not remains to be determined. Particularly, macrophages, which have been deviated to the phenotype of alternative activation within a tumor microenvironment, may have various responses to sorafenib treatments.
Given that sorafenib is a currently recommended targeted therapeutic agent against cancer, the safety and adverse effects have been elucidated. Our results suggest that the finding of autophagy-inducing activity in macrophages could be extended to two clinical aspects of use. Firstly, the safety consideration on immunotoxicity of sorafenib during cancer treatment could be further examined. Secondly, the immunosuppressive effect of sorafenib on macrophages may shed a light on treating diseases with unwanted immune responses, such as autoimmune disorders or rejection of organ transplantation.
In conclusion, sorafenib can induce autophagy and modulate the function of human macrophages. The possible role of this unmet immunomodulatory effect needs further elucidation.
Conflict of interest
The authors declare no conflict of interest for this study.
Acknowledgments
This work was supported by grant MMH-E-101-10 from Mackay Memorial Hospital, Taipei, Taiwan.
Contributor Information
Chih-Hsiung Wu, Email: chwu@tmu.edu.tw.
Yu-Jen Chen, Email: chenmdphd@gmail.com.
References
- 1.Gordon S. Innate immune functions of macrophages in different tissue environments. J Innate Immun. 2012;4:409–410. doi: 10.1159/000339280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilhelm S.M., Carter C., Tang L., Wilkie D., McNabola A., Rong H. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B., Eisen T., Stadler W.M., Szczylik C., Oudard S., Siebels M. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh C.H., Jeng K.S., Lin C.C., Chen C.K., Liu C.Y., Lin C.P. Combination of sorafenib and intensity modulated radiotherapy for unresectable hepatocellular carcinoma. Clin Drug Investig. 2009;29:65–71. doi: 10.2165/0044011-200929010-00007. [DOI] [PubMed] [Google Scholar]
- 6.Brose M.S., Nutting C.M., Sherman S.I., Shong Y.K., Smit J.W., Reike G. Rationale and design of decision: a double-blind, randomized, placebo-controlled phase III trial evaluating the efficacy and safety of sorafenib in patients with locally advanced or metastatic radioactive iodine (RAI)-refractory, differentiated thyroid cancer. BMC Cancer. 2011;11:349. doi: 10.1186/1471-2407-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hipp M.M., Hilf N., Walter S., Werth D., Brauer K.M., Radsak M.P. Sorafenib, but not sunitinib, affects function of dendritic cells and induction of primary immune responses. Blood. 2008;111:5610–5620. doi: 10.1182/blood-2007-02-075945. [DOI] [PubMed] [Google Scholar]
- 8.Edwards J.P., Emens L.A. The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE(2) in murine macrophages. Int Immunopharmacol. 2010;10:1220–1228. doi: 10.1016/j.intimp.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klionsky D.J., Abdalla F.C., Abeliovich H., Abraham R.T., Acevedo-Arozena A., Adeli K. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophagy. 2012;8:445–544. doi: 10.4161/auto.19496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Y.J., Huang W.P., Yang Y.C., Lin C.P., Chen S.H., Hsu M.L. Platonin induces autophagy-associated cell death in human leukemia cells. Autophagy. 2009;5:173–183. doi: 10.4161/auto.5.2.7360. [DOI] [PubMed] [Google Scholar]
- 11.Mihalache C.C., Simon H.U. Autophagy regulation in macrophages and neutrophils. Exp Cell Res. 2012;318:1187–1192. doi: 10.1016/j.yexcr.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 12.English L., Chemali M., Duron J., Rondeau C., Laplante A., Gingras D. Autophagy enhances the presentation of endogenous viral antigens on MHC class I molecules during HSV-1 infection. Nat Immunol. 2009;10:480–487. doi: 10.1038/ni.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutierrez M.G., Master S.S., Singh S.B., Taylor G.A., Colombo M.I., Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Wilson D., Matthews S., Yap G.S. Rapid elimination of Toxoplasma gondii by gamma interferon-primed mouse macrophages is independent of CD40 signaling. Infect Immun. 2007;75:4799–4803. doi: 10.1128/IAI.00738-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prentice E., Jerome W.G., Yoshimori T., Mizushima N., Denison M.R. Coronavirus replication complex formation utilizes components of cellular autophagy. J Biol Chem. 2004;279:10136–10141. doi: 10.1074/jbc.M306124200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G., Yang H. Modulation of macrophage activation and programming in immunity. J Cell Physiol. 2013;228:502–512. doi: 10.1002/jcp.24157. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S., Martinez F.O. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]


