Highlights
-
•
Infectious bronchitis virus causes a respiratory disease in domestic chickens worldwide.
-
•
Recombination is thought to contribute to the emergence of IBV variants.
-
•
Strain ck/CH/LHLJ/140906 is originated from recombination events between 4/91- and H120-like strains.
-
•
Recombination of the S1 domain resulted in the emergence of a novel serotype of IBV.
Keywords: Infectious bronchitis coronavirus, Recombination, Genotype, Serotype, Vaccination-challenge test
Abstract
An infectious bronchitis coronavirus, designated as ck/CH/LHLJ/140906, was isolated from an infectious bronchitis virus (IBV) strain H120-vaccinated chicken flock, which presented with a suspected infectious bronchitis virus (IBV) infection. A phylogenetic analysis based on the S1 gene clustered ck/CH/LHLJ/140906 with the 793/B group; however, a pairwise comparison showed that the 5′ terminal of the S1 gene (containing hypervariable regions I and II) had high sequence identity with the H120 strain, while the 3′ terminal sequence was very similar to that of IBV 4/91 strain. A SimPlot analysis of the complete genomic sequence, which was confirmed by a phylogenetic analysis and nucleotide similarities using the corresponding gene fragments, suggested that isolate ck/CH/LHLJ/140906 emerged from multiple recombination events between parental IBV strains 4/91 and H120. Although the isolate ck/CH/LHLJ/140906 had slightly higher S1 amino acid sequence identity to strain 4/91 (88.2%) than to strain H120 (86%), the serotype of the virus was more closely related to that of the H120 strain (32% antigenic relatedness) than to the 4/91 strain (15% antigenic relatedness). Whereas, vaccination of specific pathogen-free chickens with the 4/91 vaccine provided better protection against challenge with ck/CH/LHLJ/140906 than did vaccination with the H120 strain according to the result of virus re-isolation. As the spike protein, especially in the hypervariable regions of the S1 domain, of IBVs contains viral neutralizing epitopes, the results of this study showed that recombination of the S1 domain resulted in the emergence of a new serotype.
1. Introduction
Infectious bronchitis virus (IBV), the prototype of the subfamily Coronavirinae, which is in the family Coronaviridae of the order Nidovirales, is an important pathogen in chickens, and it infects the respiratory tract, kidneys and oviduct, causing reduced performance, reduced egg quality and quantity, increased susceptibility to infection with other pathogens and increased mortality (Cavanagh, 2003). The genome of IBV is a single-stranded, positive-sense RNA of approximately 27.6 kb, one-third of which encodes four structural proteins, including the spike glycoprotein (S), the membrane glycoprotein (M), the phosphorylated nucleocapsid protein (N) and the small membrane protein (E). Interspersed among the structural protein genes are two small accessory protein genes (genes 3 and 5) that vary in number and sequence among IBVs. Two-thirds of the 5′ region in the IBV genome encodes the 1a and 1ab polyproteins, which are proteolytically cleaved by two virus-encoded replicase proteins (the papain-like and 3C-like proteinases) into 15 nonstructural proteins (nsp2–nsp16) (Ziebuhr et al., 2001). The 5′ and 3′ untranslated regions (UTRs) usually harbor important structural elements that are involved in replication and/or translation (Masters, 2006).
The S1 subunit of the S protein of IBV carries virus-neutralizing and serotype-specific determinants. IBVs exist as dozens of serotypes, which are defined by the virus neutralization test. In keeping with the large number of IBV serotypes, the S1 protein is very variable; the amino acid sequences of serotypes commonly differ by 20% to 25%, and can differ by up to 50%, (Cavanagh et al., 2005, Wickramasinghe et al., 2014). Massachusetts and 793/B are among the most important and prevalent serotypes of IBV worldwide (Jackwood, 2012). Until the mid-1950s, the Massachusetts serotype viruses were believed to be the only serotype found in the USA and other regions of the world, until a second IBV serotype, Connecticut, was reported. The use of commercially produced, Massachusetts-type modified, live virus vaccines began in the 1950s and has continued to the present. These types of vaccines, such as the Massachusetts (M41) and H120 strains, are the most commonly used around the world because such vaccines have been proven to confer protection against a wide range of IBV strains, including homologous and non-homologous IBV genotype/serotypes (Cavanagh and Gelb, 2008). The first known strain of the 793/B serotype, also known as 4/91 (Parsons et al., 1992) and CR88, was isolated in France in 1985 (Picault et al., 1995). This serotype may have entered the UK in the winter of 1990/91, when it was sometimes associated with deep pectoral muscle myopathy, in addition to the more usual manifestations of infectious bronchitis (Gough et al., 1992, Parsons et al., 1992). Subsequently, it was discovered that this serotype had been present in most European countries (de Wit et al., 2011). The first record of the 793/B serotype in Asia was in China in 2003; the virus isolate was named Taian-03, and the sequence of the S1 gene has been deposited in the GenBank database with the accession number AY837465. Currently, 793/B type viruses have been detected in several other Asian countries, including Japan (Mase et al., 2008), India (Sumi et al., 2012) and Iran (Bijanzed et al., 2013).
New serotypes and genotypes of IBV emerge frequently in different parts of the world (Al Tarcha et al., 1990, Cook, 1983, Gelb et al., 1991, Gough et al., 1992, Jia et al., 1995, Liu and Kong, 2004, Zanella et al., 2003). A number of factors, among which mutation and recombination are two major forces, account for the emergence and evolution of new genetic variants, and they play an important role in increasing the number of variants (Cavanagh, 1992, Estevez et al., 2003, Jia et al., 1995, Kusters et al., 1990, Wang et al., 1993). Although mutations are very common in IBV genomes, recombination between strains has also been widely reported (Davidson and Silva, 2008, Hewson et al., 2014, Jia et al., 1995, Kusters et al., 1990). It has been suggested that turkey coronavirus, another group of the gamma-corona viruses, emerged from a host infected with a known IBV strain and an uncharacterized coronavirus that resulted in recombination in the S1 gene and a subsequent host shift from chickens to turkeys (Jackwood et al., 2010).
Recently, a couple of “novel” IBVs have been isolated in China, and they were shown to have emerged from a recombination event between distant strains (Feng et al., 2014, Liu et al., 2013, Liu et al., 2014, Zhao et al., 2013, Zhou et al., 2014) by molecular characterization using sequencing and phylogenetic analysis. However, the biological characteristics, such as antigenicity and pathogenicity, of most of these IBVs have not been fully evaluated (Feng et al., 2014, Zhao et al., 2013, Zhou et al., 2014). In this study, a new variant of IBV, designated as ck/CH/LHLJ/140906, which was isolated in Heilongjiang province in China in 2014, was comprehensively analyzed by comparing its genome with those of two other IBV strains, 4/91 and H120, which were suspected of being the parental viruses of ck/CH/LHLJ/140906. In addition, the antigenicity of the isolate was further analyzed using cross virus-neutralization and vaccination-challenge tests.
2. Materials and methods
2.1. Viral isolation
An IBV strain, designated as ck/CH/LHLJ/140906, was isolated from swollen kidneys from diseased chickens in a chicken flock suspected of having an IBV infection during the course of our conventional surveillance activities for IBV in China in 2014. Three different ages of broilers, at 10-day intervals, were reared in the same house. All 1-day-old chickens were vaccinated against IBV with the commercial live attenuated H120 vaccine and then boosted when 14 days old. The broilers in two of the flocks showed early signs of respiratory disease when they were 15 and 25 days old, and the chickens in the third flock, which were 35 days old, showed no clinical signs. Gross examinations showed mild to severe tracheitis, nephritis and proventriculitis. The morbidities were 50% and 10%, respectively, and the mortalities were 20% and 5%, respectively.
The kidney samples of the diseased chickens were collected, pooled and used for virus isolation as previously described (Liu and Kong, 2004). Three blind passages were performed when the characteristic embryo changes appeared, such as dwarfing, stunting, or curling of the embryos, which were observed from 26 to 72 h. In addition, the IBV vaccine strains 4/91 and H120 were used in the cross virus-neutralization and vaccination-challenge tests in this study. Embryo-propagated viral stocks of ck/CH/LHLJ/140906, 4/91 and H120 were produced by inoculating the virus into embryonated specific pathogen-free (SPF) chicken eggs via the allantoic cavity, and the infectious allantoic fluid was collected 48 h post-inoculation as previously described (Liu and Kong, 2004). The titers of the three viruses were determined by inoculation of 10-fold dilutions into groups of five 10-day-old embryonated chicken eggs. The median embryo infectious dose (EID50) was calculated using the method of Reed and Muench (1938).
2.2. Eggs and chicks
White Leghorn SPF chickens and fertile SPF chicken eggs were obtained from the Harbin Veterinary Research Institute. The birds were maintained in isolators with negative pressure, and food and water were provided ad libitum. All experimental procedures were approved by the Ethical and Animal Welfare Committee of Heilongjiang province, China.
2.3. RNA extraction, amplification and sequencing
Genomic RNA was extracted from virus-infected allantoic fluid with the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s instructions. The extracted RNA was used as template in a reverse transcription PCR (RT-PCR). Overlapping primers were designed in a manner such that each pair of primers covered approximately 2 kb of the genome based on the conserved sequences among the LX4-, 793/B- and Massachusetts-type IBVs (Table 1 ). All gene fragments were amplified using the RT-PCR kit (Takara, Dalian, China) according to the manufacturer’s instructions, and the RT-PCR products were cloned into the pMD-18T vector (Takara). Rapid amplification of cDNA ends (RACE) was conducted to determine the 3′/5′-termini of ck/CH/LHLJ/140906 genomic RNA using the 3′/5′ RACE kit (Takara) according to the manufacturer’s instructions (Liu et al., 2014). For each amplicon, DNA from at least three independent clones was sequenced to exclude errors that can occur during the RT-PCR.
Table 1.
Sequences of primers used in the present study.
| Primer | Sequence | Position in genome# | Direction | Size (bp) |
|---|---|---|---|---|
| 0906-1F | GAAGACATCTTTGGTGTCTCTCC | 901–923 | Fw | 1979 |
| 0906-1R | GTCATCATCACTCTCATAATCAC | 2858–2880 | ReV | |
| 0906-2F | GTGATTATGAGAGTGATGATGAC | 2778–2800 | Fw | 2017 |
| 0906-2R | CAATGGCCACTGTTAGTAGAGCC | 4763–4795 | ReV | |
| 0906-3F | GATGCAAATAGTGTGGATGAGG | 4643–4664 | Fw | 2153 |
| 0906-3R | CAGGTTCCACTACTAATTGCTC | 6775–6796 | ReV | |
| 0906-4F | CACAGAGTGCGCATGCATTAGAG | 6677–6699 | Fw | 2109 |
| 0906-4R | GAGTAACGTGGTGGTGTATAC | 8766–8786 | ReV | |
| 0906-5F | CTTGCAGCGTATGCTAGAC | 8641–8659 | Fw | 2229 |
| 0906-5R | GAGTAACTGACTGTAAAACAG | 10850–10870 | ReV | |
| 0906-6F | CTTGCATCTGATGATGTTGGAG | 10729–10750 | Fw | 2036 |
| 0906-6R | CCTTATTATCTTCAAACCAC | 12746–12765 | ReV | |
| 0906-7F | CTACGCCTTGAGGCATTTTGAC | 12656–12677 | Fw | 2207 |
| 0906-7R | GCGATATAACGCTCCATAACAG | 14842–14863 | ReV | |
| 0906-8F | GCTAGTGGAGGTTGATGGTGAGC | 14735–14757 | Fw | 2089 |
| 0906-8R | CATCACGCTGACGCATGACAAC | 16803–16824 | ReV | |
| 0906-9F | CTGCGGATTCGCAGCATGCGCTG | 16729–16751 | Fw | 2150 |
| 0906-9R | CTACATATGAGTACCGCTTATAAC | 18856–18879 | ReV | |
| 0906-10F | CACAGATATCGAGCCAAATGGCC | 18752–18774 | Fw | 1564 |
| 0906-10R | CATCTCTTACCAGTAACTTAC | 20296–20316 | ReV | |
| S1Oligo3′ | TGAAAACTGAACAAAAGAC | 20248–20266 | Fw | 1719 |
| S1Oligo5′ | TTGCCCTTATGTTAGTTATG | 21948–21967 | ReV | |
| 0906-11F | CAGTTTGTAGTGTCTGGTGGC | 21802–21822 | Fw | 2147 |
| 0906-11R | GTCTAGACTGTAAGTTACTATT | 23928–23949 | ReV | |
| 0906-12F | GTAACTGAACAATACAGACC | 23767–23786 | Fw | 2266 |
| 0906-12R | CTTAGGCTGGGGTGAATTAAG | 26002–26033 | ReV |
Based on the H120 strain genome (FJ888351).
The complete genomic sequence of ck/CH/LHLJ/140906 reported in this study has been submitted to GenBank and has been assigned accession number KP036502.
2.4. Genomic sequence determination and phylogenetic analysis
Assembly of contiguous sequences was performed with the GeneDoc software (Ammayappan and Vakharia, 2009). Comparative sequence analyses of ck/CH/LHLJ/140906 with other IBVs were conducted using BLAST searches of the National Center for Biotechnology Information database and Vector NTI Advance 10 software. Phylogenetic analyses based on both the S1 gene and corresponding gene fragments were conducted using the MEGA4 program (Tamura et al., 2007). Phylogenetic trees were constructed from aligned nucleotide and amino acid sequences using the neighbor-joining method with 1000 bootstraps.
2.5. Similarity plot and sequence comparison
Similarity and breakpoint analyses of the complete genomic sequence of ck/CH/LHLJ/140906 were aligned with those of 4/91 and H120, and the multiple alignment results were introduced into SimPlot version 3.5.1 to identify likely recombination breakpoints (Lole et al., 1999). Furthermore, pairwise comparison of the complete genomic sequence of ck/CH/LHLJ/140906 was performed with those of the 4/91 and H120 strains to confirm the precise recombination breakpoints, and a phylogenetic tree was reconstructed for each recombinant fragment to avoid phylogenetic biases derived from ignoring recombination events.
2.6. Cross virus-neutralization tests
Cross virus-neutralization tests were performed using constant (102 EID50) viral titers and diluted serum against each of the three viruses, ck/CH/LHLJ/140906, 4/91 and H120, in SPF chickens embryos for serotyping (Liu et al., 2013). The end-point of each serum sample was calculated using the methods of Reed and Muench (1938). The cross-reactivity R values were calculated according to the formula published by Archetti and Horsfall (1950). R value less than 50% constitutes a different serotype.
2.7. Experimental design
Forty, 1-day-old SPF White Leghorn chicks were housed in different isolators and divided into four groups, each containing 10 birds. Chickens in groups 1 and 2 were vaccinated with the 4/91 and H120 viruses, respectively, by oculonasal administration at 1-day-old of age with a dose of 104 EID50 per chick. Birds in groups 3 and 4 were mock-inoculated with sterile allantoic fluid. Blood samples were collected from all birds in each group at post-inoculation days 4, 8, 12, 16 and 20. At 20 days post-inoculation, birds in groups 1–3 were challenged by oculonasal application of 106 EID50/0.1 ml of ck/CH/LHLJ/140906 virus. Birds in group 4 were mock-inoculated with sterile allantoic fluid and served as the negative control. Nasopharyngeal swab and blood samples were collected from all birds at 4, 8, 12, 16 and 20 days after challenge. The nasopharyngeal swabs were placed into separate tubes containing 0.6 ml of phosphate-buffered saline (PBS) containing antibiotics (2000 U/ml penicillin G, 200 μg/ml gentamicin sulfate, 4 μg/ml amphotericin B; Sigma–Aldrich, St Louis, MO, USA) and stored at −80 °C until virus recovery. The serum collected in this study was stored at −70 °C until ELISA testing. Chicks were examined daily for signs of infection for 30 days after inoculation.
2.8. Virus recovery
Nasopharyngeal swab tubes were centrifuged at 6000g for 10 min, and the supernatant was collected for virus recovery. The supernatant samples were inoculated into two to five SPF embryonated eggs via the allantoic cavity (0.1 ml per egg). The eggs were candled daily to record embryo mortality. After 7 days, the remaining embryos were chilled at 4 °C and examined for characteristic IBV lesions, such as the dwarfing, stunting or curling of embryos. Embryo mortality recorded in the first 24 h post-inoculation was considered non-specific. Allantoic fluid from two of the inoculated embryos was collected for RT-PCR amplification as previously described (Liu and Kong, 2004) and the PCR productions were used for sequencing directly to verify the challenged virus. A positive sample was recorded if specific lesions were observed and the RT-PCR amplification was positive.
2.9. Serum antibody detection
Serum samples were tested for IBV antibodies using the IDEXX IBV AB test kit (IDEXX Corporation, Westbrook, ME, USA), which is an indirect enzyme-linked immunosorbent assay. The kit was used according to the manufacturer’s instructions. Each sample was tested in triplicate. Serum-to-positive ratios (S/P ratios) were calculated and evaluated as positive or negative according to the kit directions.
3. Results
3.1. Genome organization and phylogenetic analysis of IBV isolate ck/CH/LHLJ/140906
Complete genome sequence data for the IBV isolate ck/CH/LHLJ/140906 was obtained by sequence assembly. The size of the genome was 27,600 bases, excluding the poly-A tail at the 3′ end. The genome of the virus was similar overall in its coding capacities and genomic organizations to those of other IBVs. BLAST searches with the complete genomic sequence of ck/CH/LHLJ/140906 revealed that ck/CH/LHLJ/140906 was most closed related to the 4/91 vaccine and IBVUkr27-11-Ukraine strains, sharing 98% nucleotide identity with these IBV strains.
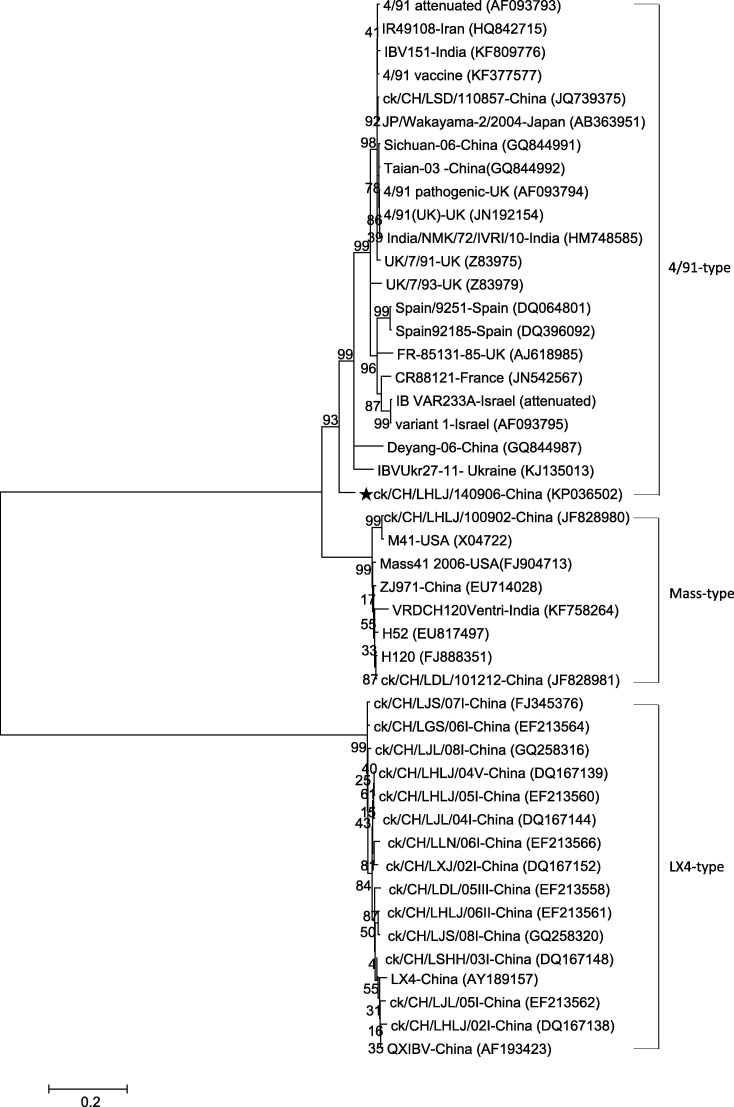
The phylogenetic trees constructed using the nucleotide sequences of the S1 subunit of the spike gene are shown in Fig. 1 . On the basis of the phylogenetic trees, isolate ck/CH/LHLJ/140906 was clustered with 793/B strains from different countries of the world, despite the fact that it showed diversity and had a close relationship with the 793/B-type strain IBVUkr27-11-Ukraine, which was isolated in the Ukraine in 2011. Similarly to the results of the phylogenetic trees, the BLAST searches that were conducted using the entire S1 gene revealed that ck/CH/LHLJ/140906 was most closely related to strain IBVUkr27-11-Ukraine (95%) (KJ135013); the other strains, including the 4/91 vaccine, did not share more than 91% nucleotide identity with ck/CH/LHLJ/140906.
Fig. 1.
Consensus phylogenetic tree resulting from the analysis of the nucleotide sequences of the S1 gene of IBV isolate ck/CH/LHLJ/140906 (black star), other 793/B reference strains, LX4- and Massachusetts-type viruses (accession numbers in parentheses). The trees were computed using the neighbor-joining method with 1000 bootstrap replicates using the MEGA4 program.
The predicted S1 amino acid sequence of the S1 gene of IBV isolate ck/CH/LHLJ/140906 was pairwise compared with those of six 793/B-type IBV strains: the prototype 793/B-type strain 4/91 (UK), 4/91 pathogenic and attenuated strains, the 4/91 vaccine strain, IBVUkr27-11-Ukraine, and Taian-03, which was the first isolated 793/B-type IBV in China, and two Massachusetts-type strains, H120 and M41 (Fig. 2 ). Isolate ck/CH/LHLJ/140906 had 88.2% amino acid sequence identity to the 4/91 vaccine strain and 86% identity when compared to the H120 vaccine strain. The amino acid sequence of the S1 domain at residues 1–192 of isolate ck/CH/LHLJ/140906, in which the hypervariable regions I (HVR I) and II (HVR II) were located, was closely related to those of H120 and M41 (it was more similar to that of H120 than M41) compared with that of the 793/B-type IBV. However, the virus was more similar to 793/B-type IBVs than to H120 at residues 199–573 of the S1 subunit of the spike protein, likely indicating that a recombinant event occurred during the origin and evolution of the ck/CH/LHLJ/140906 isolate.
Fig. 2.
Multiple sequence alignment of S1 amino acid sequences from six 4/91- and two Massachusetts-type IBVs. The numbers on the right of each alignment showed the nucleotide positions in S1 subunit of spike protein of each virus. The sequences of ck/CH/LHLJ/140906 are listed and the only amino acids differing from those of ck/CH/LHLJ/140906 are depicted. The three hypervariable regions (HVRs) are boxed. The deleted nucleotides are represented as –. The cleavage sites of S1 subunit of spike protein are underlined. The GenBank accession numbers are the same as those in Fig. 1.
3.2. Identification of recombination within the genome of IBV isolate ck/CH/LHLJ/140906
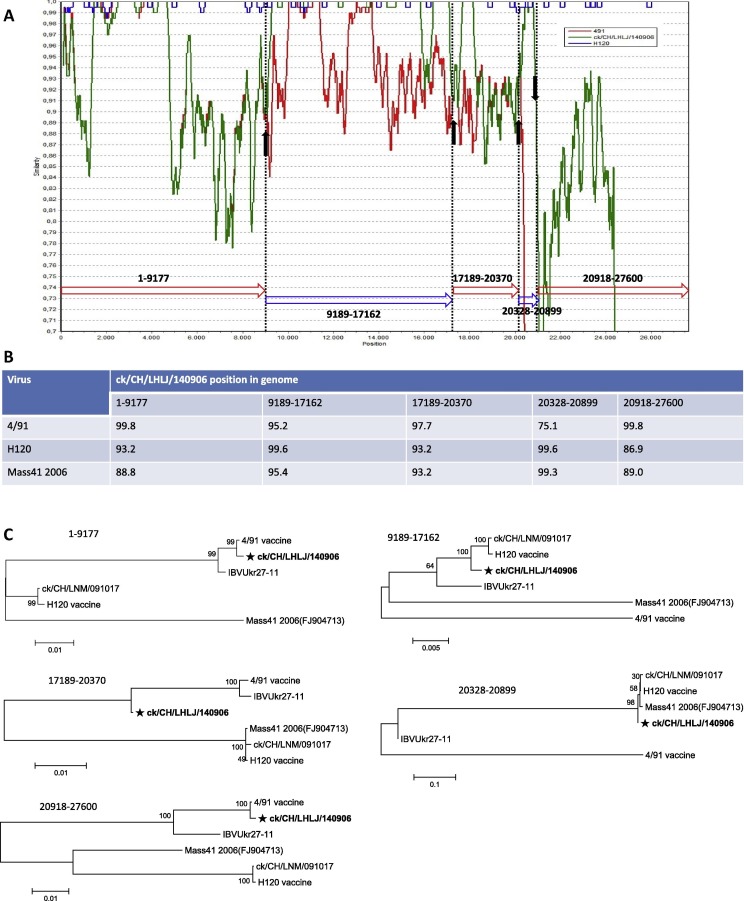
To identify the possible recombination events that occurred in the genome of IBV isolate ck/CH/LHLJ/140906 and the regions likely to have been involved in the recombination, similarity plots were performed using strains 4/91 and H120 as representatives of the two main groups of IBVs, while the ck/CH/LDL/101212 strain served as a query. SimPlot analysis was used to display the consecutive nucleotide identity and to illustrate the crossover events among the queried strain and the parental strains. As illustrated in Fig. 3 A, four crossover events were suggested by the similarity plot, and 4/91 and H120 served as the parental strains.
Fig. 3.
Recombination analysis of the IBV isolate ck/CH/LHLJ/140906. Similarity plot using ck/CH/LDL/101212 as the query sequence (A). The solid arrows showed the deduced recombination breakpoints. The hollow arrows showed the different fragments and their colors were the same as those of the parental viruses. The numbers showed the nucleotide positions of the corresponding fragments in the genome of isolate ck/CH/LHLJ/140906. The y-axis shows the percentage similarity within a sliding window of 200 bp centered on the position plotted, with a step size between plots of 20 bp. IBV isolate ck/CH/LHLJ/140906 was compared to 4/91 and H120 vaccine strains. Percentages of nucleotide sequence identity among ck/CH/LHLJ/140906, 4/91, H120 and Mass41 2006 (B). Percentages of nucleotide sequence identity of corresponding gene fragments are indicated. Phylogenetic analysis of genome positions 1-9177, 9189-17162, 17189-20370, 20328-20899 and 20918-27600 among ck/CH/LHLJ/140906, two 793/B-type (4/91 and IBVUkr27-11) and three Massachusetts-type (ck/CH/LNM/091017, H120 and Mass41 2006) strains (C). The trees were constructed using the neighbor-joining method. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To obtain a precise picture of these possible crossover points, the genomic sequence of the ck/CH/LHLJ/140906 isolate was carefully pairwise compared with those of the 4/91, H120 and M41viruses. In line with the results obtained from the SimPlot analysis, four recombination events with four crossover points in the genome of IBV isolate ck/CH/LHLJ/140906 were found, as shown in Fig. 4 . The first recombination breakpoint (nt 9178–9188) was located in the Nsp5 gene (Fig. 4A), the second was located in the Nsp14 gene (nt 17163–17188) (Fig. 4B), the third was located in the Nsp16 gene (nt 20371–20329), and the last was located 5′ of the S1 subunit coding sequences of the spike gene (Fig 4C) (nt 20900–20917) of isolate ck/CH/LHLJ/140906. In addition, data from nucleotide similarities using the corresponding gene fragments supported the above-mentioned results (Fig. 3B), and indicated that H120 showed higher similarity with isolate ck/CH/LHLJ/140906 than M41 in the corresponding gene fragments (Fig. 3B). Phylogenetic trees constructed using the corresponding gene fragments also confirmed the result (Fig. 3C). Taken together, our results clearly indicated that isolate ck/CH/LHLJ/140906 is a naturally recombinant virus that emerged from recombination between 4/91- and H120-like viruses.
Fig. 4.
Analysis of recombination among IBV ck/CH/LHLJ/140906, 4/91, H120 and Mass41 2006 strains. The numbers on the right of each alignment showed the nucleotide positions in genome of each virus. The sequences of ck/CH/LHLJ/140906 are listed and only the nucleotides differing from those of ck/CH/LHLJ/140906 are depicted. The region where the template switches (breakpoint) have taken place is underlined. The deleted nucleotides are indicated by -. The GenBank accession numbers are the same as those in Fig. 1.
3.3. Serotype of IBV isolate ck/CH/LHLJ/140906
Based on the molecular characterization results, cross virus-neutralization tests were employed to serotype the recombinant virus ck/CH/LHLJ/140906 and its deduced parental viruses, 4/91 and H120. The results showed that the R value between virus ck/CH/LHLJ/140906 and the 4/91 vaccine strain was 15%, indicating that ck/CH/LHLJ/140906 possessed a serotype distinct from that of the 4/91 strain (793/B serotype); however, ck/CH/LHLJ/140906 was antigenically closer to Massachusetts strain H120, which resulted in an R value of 32% (Table 2 ), than to strain 4/91. In addition, the results in this study demonstrated that isolate ck/CH/LHLJ/140906 was serotypically distinct from either of the parental viruses and represented a new serotype.
Table 2.
Titers were obtained in reciprocal β virus neutralization tests (diluted serum, constant virus).
| Virus | Serum |
||
|---|---|---|---|
| ck/CH/LHLJ/140906 | 4/91 | H120 | |
| ck/CH/LHLJ/140906 | 147.0 | 40.0 | 107.6 |
| 4/91 | 18.4 | 222.9 | <2 |
| H120 | 32 | <2 | 227.5 |
3.4. Protection provided by vaccination with the 4/91 and H120 strains
None of the chickens showed clinical signs when challenged with the IBV isolate ck/CH/LHLJ/140906 when they were 20 days old, indicating that this IBV strain is apathogenic to 20-day-old SPF chickens. Similarly, chickens vaccinated with either 4/91 or the H120 strains did not show clinical signs after challenge with ck/CH/LHLJ/140906. As listed in Table 3 , the challenged virus was re-isolated from the tracheas of 30% of the birds in the group vaccinated with the 4/91 vaccine at 4 days post-challenge, whereas 50% of the birds were positive for virus recovery in the group vaccinated with the H120 vaccine. In contrast, the challenged virus was recovered from nasopharyngeal swabs of nearly all birds in the non-vaccinated group at 4 and 8 days post-challenge. As expected, none of the chickens were positive for virus isolation in the negative control group. The serological responses induced by the IBV vaccines and challenge viruses are presented in Table 3. Most of the birds (70%) showed seroconversion at 8 days post-challenge. However, only 50% of the birds in each vaccinated group had seroconverted by 12 days post-vaccination with the 4/91 or H120 vaccine strains; by 20 days post-vaccination, all of the birds vaccinated with either strain 4/91 or the H120 vaccine strain had seroconverted.
Table 3.
Results of vaccination-challenge tests (vaccination with infectious bronchitis virus vaccine strains 4/91 and H120, followed by challenge with isolate ck/CH/LHLJ/140906).
| Group⁎ | Morbidity | Mortality | Antibody response |
Virus recovery† |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated |
Challenged |
4 d‡ | 8 d | 12 d | 16 d | |||||||||||
| 4 d‡ | 8 d | 12 d | 16 d | 20 d | 4 d‡ | 8 d | 12 d | 16 d | 20 d | |||||||
| 1 | 0/10 | 0/10 | 0/10 | 0/10 | 5/10 | 8/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 3/10 | 1/10 | 0/10 | 0/10 |
| 2 | 0/10 | 0/10 | 0/10 | 0/10 | 5/10 | 9/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 10/10 | 5/10 | 1/10 | 1/10 | 0/10 |
| 3 | 0/10 | 0/10 | – | – | – | – | – | 0/10 | 7/10 | 9/10 | 10/10 | 10/10 | 10/10 | 9/10 | 2/10 | 0/10 |
| 4 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 | 0/10 |
Birds in groups 1 and 2 were vaccinated with 4/91 and H120 vaccines, respectively, and challenged with isolate ck/CH/LHLJ/140906. Birds in group 3 were only challenged with IBV isolate ck/CH/LHLJ/140906. Birds in group 4 were not exposed to any viruses and served as negative controls.
Two procedures were used for virus recovery after challenge as described previously (Liu et al., 2009). First, embryos that had been inoculated with individual nasopharyngeal swab samples were observed for lesions. Second, reverse transcription PCR (RT-PCR) using a pair of oligonucleotide primers, N(−) and N(+), was conducted on RNA recovered from the allantoic fluid of the same eggs. The results from the two procedures were identical.
Days after vaccination/challenge.
4. Discussion
Recombination is a common phenomenon among coronaviruses. The high frequency of homologous recombination, together with the high mutation rates of the genome may lead to the adaptation of coronaviruses and allow the generation of new strains and genotypes (Jackwood et al., 2012, Pasternak et al., 2006). In this study, based on the SimPlot analysis, which was confirmed by the pairwise nucleotide identity comparison and phylogenetic analysis of different corresponding gene fragments, our results provide convincing evidence that recombination events occurred during the origin and evolution of the IBV isolate ck/CH/LHLJ/140906. Recombination between IBVs and its role in the emergence of new IBV variants have been reported previously (Cavanagh, 1992, Jia et al., 1995, Lee and Jackwood, 2000) and may occur at multiple sites (Jia et al., 1995, Lee and Jackwood, 2000). This is the case in the genome for IBV isolate ck/CH/LHLJ/140906, in which template switches may be occurred between H120- and 4/91-like viruses. It has been shown that crossover events in IBVs occur more frequently at the 3′ end of the S gene (Chen et al., 2009, Jia et al., 1995, Mondal and Cardona, 2007, Mardani et al., 2010). However, of the four recombination events investigated in this study, three presented in the ORF1 region (Nsp genes 5, 14 and 16), and the last was located at the 5′ end of the spike gene. It is particularly notable here that the recombination event in the S1 gene of the IBV isolate ck/CH/LHLJ/140906 made this virus a novel strain of IBV that is somewhat distinct from both parental viruses, H120 and 4/91 (Fig. 1), although it is 793/B genotype according to the phylogenetic analysis.
The emergence of the new ck/CH/LHLJ/140906 isolate was considered to be explained, as was the earlier emergence of novel viruses, by recombination between H120- and 4/91-like viruses. In China, only Massachusetts- and LDT3-A-based live vaccines are licensed for use. Based on field experience in large establishments, Massachusetts-based commercial IBV vaccines, especially the H120 strain, are extensively used and can persist in the field for long periods. In regard to the 4/91-like viruses, this type of vaccine was introduced to China in the late 1990s before the detection of this genotype in the field. Increased numbers of this type of virus have been isolated from chicken flocks of various bird farms in recent years in China. In addition, based on partial sequencing of the S1 gene, it appears that IBV strain 4/91 can change rapidly or that multiple introductions have occurred, e.g., the S1 genes of the 4/91 isolates were 97.8–98.6% identical to that of the IBV 4/91 vaccine in Canada in 2012; however, in 2013, the identity was 94.3–98.8% (Martin et al., 2014). The 4/91-like sequence from isolate ck/CH/LHLJ/140906 was more than 99.6% identical to the S1 gene of the 4/91 vaccine strain, suggesting that the vaccine was the likely source of the 4/91-like sequence. Therefore, it is noteworthy that the natural recombination event demonstrated here suggests that sometimes live vaccines can be involved in recombination.
In contrast to virus species belonging to the alpha- and beta-coronaviruses, which occur as only one or two different serotypes, there are many different serotypes of chicken IBV gamma-coronaviruses (Jackwood, 2012, de Wit et al., 2011). As the main antigenic viral protein containing epitopes for neutralization (Cavanagh, 2003), the high sequence diversity of the S1 domain accounts for this serotypical variation. Consequently, the analysis of the S1 subunit of the spike glycoprotein to distinguish between genotypes has allowed the IBVs actually present on farms to be readily identified, and this has allowed the vaccine genotype to match that of any current field threat. Furthermore, the technique has allowed the detection of new genotypes or variants, some of which might cause disease. A complicating factor in regard to genotyping IBVs is that when the IBV strain tested is the result of a recombination event in the S1 subunit regions of the spike protein between different IBV genotypes, an examination of different parts of S1 can result in the determination of a different genotype (Wang et al., 1993, Jia et al., 1995, Dolz et al., 2008). In this study, isolate ck/CH/LHLJ/140906 shared similar amino acid sequence identities of the S1 subunit of the spike protein to those of the 4/91 and H120 vaccine strains (88.2% to 4/91 and 86% to H120) due to a recombination event in the S1 gene, although it was clustered with 793/B-type viruses when the phylogenetic tree was constructed using S1 genes (Fig. 1). In contrast, the ck/CH/LHLJ/140906 was serologically more closely related to strain H120 than strain 4/91. Using monoclonal antibodies, five conformation-dependent, neutralizing antigenic sites were mapped on S1, as were other immunodominant regions in the N-terminal regions of S2 (Koch et al., 1990, Kusters et al., 1989, Lenstra et al., 1989). The five neutralizing antigenic sites on S1 co-locate within three hypervariable regions (HVRs) (Cavanagh et al., 1988, Cavanagh et al., 1992, Moore et al., 1997, Niesters et al., 1987), suggesting that the HVRs are involved in antigenicity and, hence, serotypical variation. In this study, the first two HVRs were found to be located in the H120-like sequences in ck/CH/LHLJ/140906, and the third HVR was in the 4/91-like sequence, suggesting that a recombination event in the S1 gene likely accounts for the serotype shift of the 4/91 genotype to a new serotype, although multiple recombination events were identified in the genome of the virus. Hence, genomic information is objective and provides essential information for epidemiological studies and evolutionary analyses in this case.
In general, IBV strains that have been shown to have the same serotype by virus neutralization may be able to induce complete immunity in chickens based on challenge studies, although in some cases, IBV strains of different serotypes can induce partial or complete immunity in chickens (Ladman et al., 2006). The broader degree of cross-protection in birds is likely to be related to cell-mediated immune responses to shared T-cell epitopes among some heterologous strains (Collisson et al., 2000, Seo et al., 2000). In this study, 4/91 vaccination conferred better protection against challenge with ck/CH/LHLJ/140906 than did the H120 vaccine in our vaccination-challenge test, which contrasts with the results that showed that the serotype of ck/CH/LHLJ/140906 was more closely related to that of the H120 strain. Generally, there is a higher chance of good cross-protection between strains with a high level of homology than between strains with low homology (de Wit et al., 2011), and a previous report showed that S1 sequence identity values were more strongly correlated with protective relatedness values than antigenic relatedness values (Ladman et al., 2006). In this study, IBV strain ck/CH/LHLJ/140906 had higher S1 amino acid identity with strain 4/91 than with strain H120. However, the regions in the S1 domain that were considered to contribute to the cross-protection and serotypical variation were HVRs, especially HVR I and HVR II (Ladman et al., 2006). These two HVRs in the S1 subunit of the spike protein of ck/CH/LHLJ/140906 were H120-like, which cannot explain why vaccination with strain 4/91 conferred better protection against ck/CH/LHLJ/140906 than did vaccination with strain H120, despite the fact that the serotype of ck/CH/LHLJ/140906 was more closely related to that of the H120 strain. However, isolate ck/CH/LHLJ/140906 shared higher sequence identity in the S2 domain and in the N protein with the 4/91 vaccine than with those of strain H120; these regions are also important for the immune response, especially cell-mediated immune responses (Seo et al., 2000, Wickramasinghe et al., 2014).
The IBV strain ck/CH/LHLJ/140906 in this study was isolated from H120-vaccinated broilers that showed signs of respiratory disease. However, the 20-day-old SPF chickens did not show clinical signs and mortality when challenged with this strain in this study. These results may not be in conflict with the field results because our observations were obtained from SPF chickens over short periods of time and only considered chickens infected with ck/CH/LHLJ/140906 alone. If other factors, such as secondary infections with pathogenic bacteria or co-infections with immunosuppressing viruses, such as infectious bursal disease virus, are thought to be important in commercial broilers in field conditions (Cavanagh and Gelb, 2008), the present results would have to be adjusted and re-evaluated. Otherwise, 20-day-old SPF white leghorn layers may be not susceptible to ck/CH/LHLJ/140906 strain. In addition, it was possible that ck/CH/LHLJ/140906 strain was indeed non-pathogenic giving that the virus emerged from recombination of vaccine strains and some other agent caused or contributed to the clinical signs observed in the field. Hence, challenge studies in younger chickens or in broilers will be needed to verify the possibility that the virus is indeed non-pathogenic.
The IBV strain ck/CH/LHLJ/140906 was isolated in September 2014 in China and, thus far, we do not know whether this virus has established itself to a significant extent in China. The implication of our results emphasizes, consequently, the importance of IBV surveillance in chicken flocks, although the ability of the virus to change continually by mutation or recombination challenges our ability to both diagnose and control it.
Acknowledgment
This work was supported by grants from the China Agriculture Research System (No. CARS-41-K12), National “Twelfth Five-Year” Plan for Science & Technology Support (2015BAD12B03), and Special Fund for Agro-scientific Research in the Public Interest (No. 201303033).
References
- Ammayappan A., Vakharia V.N. Complete nucleotide analysis of the structural genome of the infectious bronchitis virus strain Md27 reveals its mosaic nature. Viruses. 2009;1:1166–1177. doi: 10.3390/v1031166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archetti I., Horsfall F.L. Persistent antigenic variation of influenza A viruses after complete neutralization with heterologous immune serum. J. Exp. Med. 1950;92:441–458. doi: 10.1084/jem.92.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Tarcha B., Kojnok J., Varro C. Isolation and characterization of new infectious bronchitis virus variants in Hungary. Acta Vet. Hung. 1990;38:287–298. [PubMed] [Google Scholar]
- Bijanzed P., Momayez R., Bozorgmehrifard M.H., Habloivarid M.H., Pourbakhsh S.A. Experimental study on histopathological changes and tissue tropism of Iranian infectious bronchitis serotype 793/B-like virus in SPF chickens. J. S. Afr. Vet. Assoc. 2013;84:1–7. doi: 10.4102/jsava.v84i1.970. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Gelb J. Infectious bronchitis. In: Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K., Swayne D.E., editors. Diseases of Poultry. twelfth ed. Wiley-Blackwell Publishing; Iowa: 2008. pp. 117–135. [Google Scholar]
- Cavanagh D. Recent advances in avian virology. Br. Vet. J. 1992;148:199–222. doi: 10.1016/0007-1935(92)90045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Severe acute respiratory syndrome vaccine development: experiences of vaccination against avian infectious bronchitis coronavirus. Avian Pathol. 2003;32:567–582. doi: 10.1080/03079450310001621198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K., Li D., Kant A., Koch G. Location of the amino acid differences in the S1 spike glycoprotein subunit of closely related serotypes of infectious bronchitis virus. Avian Pathol. 1992;21:33–43. doi: 10.1080/03079459208418816. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Mockett A.P. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res. 1988;11:141–150. doi: 10.1016/0168-1702(88)90039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Picault J.P., Gough R., Hess M., Mawditt K., Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005;34:20–25. doi: 10.1080/03079450400025414. [DOI] [PubMed] [Google Scholar]
- Collisson E.W., Pei J., Dzielawa J., Seo S.H. Cytotoxic T lymphocytes are vital in the control of infectious bronchitis virus in poultry. Dev. Comp. Immunol. 2000;24:187–200. doi: 10.1016/s0145-305x(99)00072-5. [DOI] [PubMed] [Google Scholar]
- Chen H.W., Huang Y.P., Wang C.H. Identification of Taiwan and China-like recombinant avian infectious bronchitis viruses in Taiwan. Virus Res. 2009;140:121–129. doi: 10.1016/j.virusres.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J.K. Isolation of a new serotype of infectious bronchitis-like virus from chickens in England. Vet. Rec. 1983;112:104–105. doi: 10.1136/vr.112.5.104. [DOI] [PubMed] [Google Scholar]
- Davidson I., Silva R. Creation of diversity in the animal virus world by inter-species and intra-species recombinations: lessons learned from poultry viruses. Virus Genes. 2008;36:1–9. doi: 10.1007/s11262-007-0165-1. [DOI] [PubMed] [Google Scholar]
- de Wit J.J.S., Cook J.K., van der Heijden H.M. Infectious bronchitis virus variants: a review of the history, current situation and control measures. Avian Pathol. 2011;40:223–235. doi: 10.1080/03079457.2011.566260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolz R., Pujols J., Ordonez G., Porta R., Majo N. Molecular epidemiology and evolution of avian infectious bronchitis virus in Spain over a fourteen-year period. Virology. 2008;374:50–59. doi: 10.1016/j.virol.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez C., Villegas P., El-Attrache J. A recombination event, induced in ovo, between a low passage infectious bronchitis virus field isolate and a highly embryo adapted vaccine strain. Avian Dis. 2003;47:1282–1290. doi: 10.1637/5919. [DOI] [PubMed] [Google Scholar]
- Feng K., Xue Y., Wang F., Chen F., Shu D., Xie Q. Analysis of S1 gene of avian infectious bronchitis virus isolated in southern China during 2011–2012. Virus Genes. 2014;49:292–303. doi: 10.1007/s11262-014-1097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelb J., Jr., Wolff J.B., Moran C.A. Variant serotypes of infectious bronchitis virus isolated from commercial layer and broiler chickens. Avian Dis. 1991;35:82–87. [PubMed] [Google Scholar]
- Gough R.E., Randall C.J., Dagless M., Alexander D.J., Cox W.J., Pearson D. A ‘new’ strain of infectious bronchitis virus infecting domestic fowl in Great Britain. Vet. Rec. 1992;130:493–494. doi: 10.1136/vr.130.22.493. [DOI] [PubMed] [Google Scholar]
- Hewson K.A., Noormohammadi A.H., Devlin J.M., Browning G.F., Schultz B.K., Ignjatovic J. Evaluation of a novel strain of infectious bronchitis virus emerged as a result of spike gene recombination between two highly diverged parent strains. Avian Pathol. 2014;43:249–257. doi: 10.1080/03079457.2014.914624. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W. Review of infectious bronchitis virus around the world. Avian Dis. 2012;56:634–641. doi: 10.1637/10227-043012-Review.1. [DOI] [PubMed] [Google Scholar]
- Jackwood M.W., Boynton T.O., Hilt D.A., McKinley E.T., Kissinger J.C., Paterson A.H., Robertson J., Lemke C., McCall A.W., Williams S.M., Jackwood J.W., Byrd L.A. Emergence of a group 3 coronavirus through recombination. Virology. 2010;398:98–108. doi: 10.1016/j.virol.2009.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackwood M.W., Hall D., Handel A. Molecular evolution and emergence of avian gamma coronaviruses. Infect. Genet. Evol. 2012;12:1305–1311. doi: 10.1016/j.meegid.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Karaca K., Parrish C.R., Naqi S.A. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch. Virol. 1995;140:259–271. doi: 10.1007/BF01309861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G., Hartog L., Kant A., van Roozelaar D.J. Antigenic domains on the peplomer protein of avian infectious bronchitis virus: correlation with biological functions. J. Gen. Virol. 1990;71:1929–1935. doi: 10.1099/0022-1317-71-9-1929. [DOI] [PubMed] [Google Scholar]
- Kusters J.G., Jager E.J., Niesters H.G., van der Zeijst B.A. Sequence evidence for RNA recombination in field isolates of avian coronavirus infectious bronchitis virus. Vaccine. 1990;8:605–608. doi: 10.1016/0264-410X(90)90018-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusters J.G., Niesters H.G., Lenstra J.A., Horzinek M.C., van der Zeijst B.A. Phylogeny of antigenic variants of avian coronavirus IBV. Virology. 1989;169:217–221. doi: 10.1016/0042-6822(89)90058-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladman B.S., Loupos A.B., Gelb J., Jr. Infectious bronchitis virus S1 gene sequence comparison is a better predictor of challenge of immunity in chickens than serotyping by virus neutralization. Avian Pathol. 2006;35:127–133. doi: 10.1080/03079450600597865. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Jackwood M.W. Evidence of genetic diversity generated by recombination among avian coronavirus IBV. Archives Virol. 2000;145:2135–2148. doi: 10.1007/s007050070044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenstra J.A., Kusters J.G., Koch G., van der Zeijst B.A. Antigenicity of the peplomer protein of infectious bronchitis virus. Mol. Immunol. 1989;26:7–15. doi: 10.1016/0161-5890(89)90014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and nonvaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Xu Q., Han Z., Liu X., Li H., Guo H., Sun N., Shao Y., Kong X. Origin and characteristics of the recombinant novel avian infectious bronchitis coronavirus isolate ck/CH/LJL/111054. Infect. Genet. Evol. 2014;23:189–195. doi: 10.1016/j.meegid.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Zhang X., Gong L., Yan B., Li C., Han Z., Shao Y., Li H., Kong X. Altered pathogenicity, immunogenicity, tissue tropism and 3′–7 kb region sequence of an avian infectious bronchitis coronavirus strain after serial passage in embryos. Vaccine. 2009;27:4630–4640. doi: 10.1016/j.vaccine.2009.05.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Ma H., Xu Q., Sun N., Han Z., Sun C., Guo H., Shao Y., Kong X., Liu S. Characterization of a recombinant coronavirus infectious bronchitis virus with distinct S1 subunits of spike and nucleocapsid genes and a 3′ untranslated region. Vet. Microbiol. 2013;162:429–436. doi: 10.1016/j.vetmic.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lole K.S., Bollinger R.C., Paranjape R.S., Gadkari D., Kulkarni S.S., Novak N.G., Ingersoll R., Sheppard H.W., Ray S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999;73:152–160. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.A.K., Brash M.L., Hoyland S.K., Coventry J.M., Sandrock C., Guerin M.T., Ojkic D. Genotyping of infectious bronchitis viruses identified in Canada between 2000 and 2013. Avian Pathol. 2014;43:264–268. doi: 10.1080/03079457.2014.916395. [DOI] [PubMed] [Google Scholar]
- Mardani K., Noormohammadi A.H., Ignjatovic J., Browning G.F. Naturally occurring recombination between distant strains of infectious bronchitis virus Archives. Virology. 2010;155:1581–1586. doi: 10.1007/s00705-010-0731-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mase M., Inoue T., Yamaguchi S., Imada T. Existence of avian infectious bronchitis virus with a European-prevalent 4/91 genotype in Japan. J. Vet. Med. Sci. 2008;70:1341–1344. doi: 10.1292/jvms.70.1341. [DOI] [PubMed] [Google Scholar]
- Masters P.S. The molecular biology of coronaviruses. Adv. Virus Res. 2006;66:193–292. doi: 10.1016/S0065-3527(06)66005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal S.P., Cardona C.J. Genotypic and phenotypic characterization of the California 99 (Cal99) variant of infectious bronchitis virus. Virus Gene. 2007;34:327–341. doi: 10.1007/s11262-006-0014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.M., Jackwood M.W., Hilt D.A. Identification of amino acids involved in a serotype and neutralization specific epitope within the s1 subunit of avian infectious bronchitis virus. Arch. Virol. 1997;142:2249–2256. doi: 10.1007/s007050050239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesters H.G., Bleumink-Pluym N.M., Osterhaus A.D., Horzinek M.C., van der Zeijst B.A. Epitopes on the peplomer protein of infectious bronchitis virus strainM41 as defined by monoclonal antibodies. Virology. 1987;161:511–519. doi: 10.1016/0042-6822(87)90145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons D., Ellis M.M., Cavanagh D., Cook J.K.A. Characterisation of an avian infectious bronchitis virus isolated from IB-vaccinated broiler breeder flocks. Vet. Rec. 1992;131:408–411. doi: 10.1136/vr.131.18.408. [DOI] [PubMed] [Google Scholar]
- Pasternak A.O., Spaan W.J., Snijder E.J. Nidovirus transcription: how to make sense…? J. Gen. Virol. 2006;87:1403–1421. doi: 10.1099/vir.0.81611-0. [DOI] [PubMed] [Google Scholar]
- Picault, J.P., Drouin, P., Lamande, J., Allee, C., Toux, J.Y., Le Coq, H., Guittet, M., Bennejean, G., 1995. L’epizootie recente de bronchite infectieuse aviaire en France: importance, evolution et etiologie. Proceedings of the 1eres Journée de la Recherche Avicole, Angers, 28–29 March, 177–179.
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Seo S.H., Pei J., Briles W.E., Dzielawa J., Collisson E.W. Adoptive transfer of infectious bronchitis virus primed alpha beta T cells bearing CD8 antigen protects chicks from acute infection. Virology. 2000;30:183–189. doi: 10.1006/viro.2000.0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi V., Singh S.D., Dhama K., Growthaman V., Barathidasan R., Sukumar K. Isolation and molecular characterization of infectious bronchitis virus from recent outbreaks in broiler flocks reveals emergence of novel strain in India. Trop. Anim. Health Prod. 2012;44:1791–1795. doi: 10.1007/s11250-012-0140-2. [DOI] [PubMed] [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Wang L., Junker D., Collisson E.W. Evidence of natural recombination within the S1 gene of infectious bronchitis virus. Virology. 1993;192:710–716. doi: 10.1006/viro.1993.1093. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe I.N., van Beurden S.J., Weerts E.A., Verheije M.H. The avian coronavirus spike protein. Virus Res. 2014;194:37–48. doi: 10.1016/j.virusres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella A., Lavazza A., Marchi R., Moreno Martin A., Paganelli F. Avian infectious bronchitis: characterization of new isolates from Italy. Avian Dis. 2003;47:180–185. doi: 10.1637/0005-2086(2003)047[0180:AIBCON]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Zhao F., Zou N., Wang F., Guo M., Liu P., Wen X., Cao S., Huang Y. Analysis of a QX-like avian infectious bronchitis virus genome identified recombination in the region containing the ORF 5a, ORF 5b, and nucleocapsid protein gene sequences. Virus Genes. 2013;46:454–464. doi: 10.1007/s11262-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziebuhr J., Theil V., Gorbalenya A.E. The autolytic release of a putative RNA virus transcription factor from its polyprotein precursor involves two paralogus papin-like proteinase that cleave the same peptide bond. J. Biol. Chem. 2001;276:33220–33232. doi: 10.1074/jbc.M104097200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Tang M., Jiang Y., Chen X., Shen X., Li J., Dai Y., Zou J. Complete genome sequence of a novel infectious bronchitis virus strain circulating in China with a distinct S gene. Virus Genes. 2014;49:152–156. doi: 10.1007/s11262-014-1063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]