Abstract
The antioxidant N-acetyl-l-cysteine (NAC) had been shown to inhibit replication of seasonal human influenza A viruses. Here, the effects of NAC on H9N2 swine influenza virus-induced acute lung injury (ALI) were investigated in mice. BALB/c mice were inoculated intranasally with 107 50% tissue culture infective doses (TCID50) of A/swine/HeBei/012/2008/(H9N2) viruses with or without NAC treatments to induce ALI model. The result showed that pulmonary inflammation, pulmonary edema, MPO activity, total cells, neutrophils, macrophages, TNF-α, IL-6, IL-1β and CXCL-10 in BALF were attenuated by NAC. Moreover, our data showed that NAC significantly inhibited the levels of TLR4 protein and TLR4 mRNA in the lungs. Pharmacological inhibitors of TLR4 (E5564) exerted similar effects like those determined for NAC in H9N2 swine influenza virus-infected mice. These results suggest that antioxidants like NAC represent a potential additional treatment option that could be considered in the case of an influenza A virus pandemic.
Keywords: Acute lung injury, H9N2 swine influenza virus, N-acetyl-l-cystine (NAC), Toll-like receptor 4
Highlights
-
•
NAC protects against H9N2 swine influenza virus-induced acute lung injury (ALI).
-
•
NAC protects against acute lung injury by inactivation of TLR4.
-
•
Eritoran (E5564), a TLR4 antagonist, also protects against acute lung injury.
1. Introduction
Influenza A viruses cause respiratory tract infections and are responsible for significant global morbidity and mortality [1]. Antivirals and strain-specific vaccination display clear efficacy for the treatment and prophylaxis of influenza infections [2]. The neuraminidase inhibitors oseltamivir and zanamivir as well as the adamantanes amantadin and rimantadin are licensed for the treatment of influenza [3], [4], [5], [6], [7]. However, emerging evidence suggests that the use of both drug classes is limited by the emergence of resistant virus strains and that, along with the long lag time for vaccine production and the ongoing threat of new pandemic strains, the global population is at risk.
Acute lung injury (ALI) and its most severe presentation, acute respiratory distress syndrome (ARDS), were the diseases induced by many extreme conditions including severe bacterial pneumonia, severe sepsis, trauma and burn [8], [9], [10]. Severe acute respiratory syndrome (SARS) virus and avian influenza A H5N1 viral infection of the lung has resulted in high mortality among humans because of the complication of ARDS [11], [12], [13]. Our previous work indicated that swine influenza A H9N2 viral infection has resulted in ALI [14]. Therefore, infectious factors, most of which are viruses, have become one of the most important causes of ALI in humans. The pathophysiological mechanism of ALI is believed to be associated with the uncontrolled inflammatory response in the lungs [8], [9], [10], [11], [12], [13], [14]. Meanwhile, studies found that the toll-like receptor 4 (TLR4) played a pivotal role on ALI [15]. TLR4 mediates many forms of ALI, such as ventilator-induced ALI and hemorrhagic shock-induced ALI [16], [17]. Once TLR4 binds with its ligands, it further activates NF-kB through a MyD88-dependent pathway that ultimately stimulates the expression of proinflammatory cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor (TNF)-α leading to pathological changes in ALI [18], [19]. Also, NAC is a known antioxidant and antioxidants were already shown to interfere with influenza A virus replication and virus-induced proinflammatory responses [20], [21], [22].
Notably, NAC was already shown to synergize with oseltamivir in the treatment of lethal seasonal influenza A virus infection in a mouse model [23]. Here, we investigated the effects of NAC as a prototype antioxidant on H9N2 swine influenza virus-induced ALI. Moreover, the influence of NAC on TLR4 signaling pathway was studied in mice. To demonstrate the influence of NAC on H9N2 swine influenza virus-induced ALI by inactivation of the TLR4 signaling pathway, as a control, this report also investigated the effects of Eritoran tetrasodium (E5564), a potent, well-tolerated, synthetic TLR4 antagonist, on H9N2 swine influenza virus-induced ALI.
2. Materials and methods
2.1. Ethics statement
The study was approved by the Animal Care and Use Committee of the Hebei North University (Zhangjiakou, Hebei). All animal procedures followed the ethics guidelines of the National Research Council Guide for the Care and Use of Laboratory Animals (1985).
2.2. Virus strains
The H9N2 influenza A/swine/HeBei/012/2008 virus was inoculated into 10-day-old SPF chicken embryos (Beijing Laboratory Animal Research Center, China) and was consecutively blind passaged for 3 generations. The allantoic fluid was collected and stored at − 80 °C. Virus titers were determined as 50% tissue culture infective doses (TCID50).
2.3. Drugs
N-acetyl-l-cysteine (NAC) was obtained from Alexis (distributed by Axxora, Germany), dissolved in unsupplemented MEM and adjusted to pH 7.4 with NaOH. Eritoran (E5564) was purchased from BOC Sciences (USA).
2.4. Murine model of H9N2 influenza virus-induced ALI
Two hundred six- to eight-week-old male BALB/c mice (Beijing Laboratory Animal Research Centre, Beijing, China) were randomly divided into 4 groups (n = 50), control group, H9N2 group (H9N2 virus-infected mice), NAC group (H9N2 virus + NAC), and E5564 group (H9N2 virus + E5564). According to the report, ALI was induced by H9N2 influenza A/swine/HeBei/012/2008 virus via intranasal inoculation. In brief, mice were lightly anesthetized with diethyl ether and inoculated intranasally (50 μL) with 107 50% tissue culture infective doses (TCID50) of H9N2 virus diluted in sterile saline. The mice in the control group were inoculated intranasally (50 μL) with an equivalent dilution of non-infectious allantoic fluid. NAC (200 mg/kg) was dissolved in sterile normal saline and injected intraperitoneally every 4.5 h, starting 1 h before challenge (five injections in total). 2 h postinfection, animals received E5564 (Eritoran, 200 μg/mouse in 100 μL, i.v.) once daily for 5 successive days. Mice were monitored for survival, weight loss, and clinical signs of illness (e.g., inactivity, ruffled fur, hunched posture, poor appetite, rapid shallow breathing, audible crackling) for 14 days.
2.5. Lung wet/dry weight ratio
The severity of pulmonary edema was assessed by the wet to dry ratio (W/D ratio) [24]. The W/D ratio was determined by weighing the right lung before and after oven desiccation at 80 °C.
2.6. H&E staining
Mice were weighed and sacrificed on days 3, 5, 6, 8, and 14 postinfection. The left lobes of the lungs were fixed in 10% formalin, embedded in paraffin, cut into 5 μm sections, and stained with hematoxylin and eosin (H&E). Lung injury score was measured by a blinded pathologist with a 0 to 4 point scale according to combined assessments of inflammatory cell infiltration, alveolar edema and interstitial edema. A score of 0 represented no damage; l represented mild damage; 2 represented moderate damage; 3 represented severe damage and 4 represented very severe histological changes [25].
2.7. Total and differential cell counts in bronchoalveolar lavage fluid (BALF)
The mice were euthanized by exsanguination, and BALF was collected by flushing the lungs three times with 0.5 mL sterile physiological saline (0.9% NaCl) via the tracheal cannula. The fluid recovery rate was more than 80%. After BALF collection, the samples were centrifuged at 420 ×g at 4 °C for 15 min, and the supernatant was stored at − 70 °C for subsequent analysis of cytokine levels. The cell pellets were resuspended in 1 mL PBS, and the total cell counts were performed using a Neubauer chamber. For neutrophils, macrophages and lymphocytes counts, cytospin slides were prepared and stained with Diff-Quick. 300 cells were counted per slide [26], [27].
2.8. Myeloperoxidase (MPO) activity assay
The lungs were collected and homogenized, and were centrifuged at 4500 g for 10 min and resuspended in 50 mM KPO4 buffer (PH 6.0) with 0.5% hexadecyl-trimethylammonium bromide (HTAB). Then, samples were sonicated and incubated at 60 °C for 2 h. Later, samples were assayed for activity in a H2O2/O-dianisidine buffer at 460 nm with a spectrophotometer. Results are expressed as units of MPO activity per gram of lung tissue.
2.9. TNF-α, IL-6, IL-1β, and CXCL-10 in BALF
The BALF supernatant was collected after centrifugation (for 4 min at 4500 g) and stored at − 70 °C before cytokine assay. Levels of TNF-α, IL-1β, IL-6 and CXCL-10 in the cell-free BALF were evaluated using ELISA in accordance with the manufacturer's instructions (R&D Systems, USA) [28].
2.10. Determination of total superoxide dismutase (T-SOD) activity and malondialdehyde (MDA) concentration in the lung
The lungs were collected and homogenized with 0.9% sterile physiological saline. The homogenate was centrifuged at 4500 g for 10 min, and then, the supernatant was collected for T-SOD activity, and MDA concentration assay.
T-SOD activity was measured according to the method of Sun et al. [29], by determining the inhibition of nitroblue tetrazolium (NBT) reduction with xanthine–xanthine oxidase as an O2 − generator. One unit of T-SOD is defined as the amount of protein that reduces the rate of NBT reduction by 50%. The results were expressed as units per milligram of protein (U/mg protein).
MDA levels were determined by the method of Hermann and Cheeseman [30] based on the reaction with thiobarbituric acid at 90–100 °C. After cooling, the absorbance was read at 532 nm. The concentrations were expressed as nanomoles per milligram of protein (nmol/mg protein).
2.11. RT-PCR (real time reverse-transcribed polymerase chain reaction)
Total RNA was isolated from the lung tissues by Trizol reagent (Invitrogen) according to the manufacturer's protocol. Real-time quantitative PCR mixture was prepared using SYBR® Premix Ex Taq™ II Kit (Takara), and amplification was performed according to the manufacturer's protocol via GeneAmp PCR system 7300 (Applied Biosystems). The primer sequences were as follows: TLR4 (forward) 5′-CTGTATTCCCTCAGCACTCTTG-3′ and (reverse) 5′-CCCACTGCAGGAATTTCTGATG-3′; GAPDH (forward) 5′-TTCACCACCATGGAGAAGGC-3′ and (reverse) 5′-GGCATGGACTGTGGTCATGA-3′. The GAPDH gene was used as an internal control.
2.12. Western blot analysis
The lungs were homogenized, and centrifuged at 11,000 g at 4 °C for 15 min, and the supernatant was collected. Protein was quantified with a BCA Protein Assay Kit (Thermo Scientific). For western blot, 20 μg of total protein was run on 10% SDS-PAGE. Proteins were then transferred to nitrocellulose filter membranes. The membranes were incubated in PBS containing 5% BSA for 2 h. The blots were then incubated for 2 h with primary antibodies for TLR4 (Cell Signaling Technology) and GAPDH (Cell Signaling Technology), respectively. The secondary antibody (horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin) was added and incubated at room temperature for 1 h. Peroxidase labeling was detected with the enhanced chemiluminescence Western blotting detection system (Amersham Pharmacia Biotech) and analyzed by a densitometry system. The relative protein level was normalized to GAPDH (Cell Signaling Technology).
2.13. Statistical analysis
All data were expressed as means ± SD and statistical analysis was performed with the SPSS statistical software package for Windows, version 13.0 (SPSS). Analysis of variance (ANOVA) followed by Tukey's multiple comparison tests was used. P < 0.05 was considered to be statistically significant. Kruskal–Wallis test was used in the lung injury score. For survival studies, a Log-Rank (Mantel–Cox) test was used.
3. Results
3.1. NAC alleviates clinical symptoms and reduces mortality in mice
The infected mice exhibited similar clinical signs (inactivity and ruffled fur) on day 3 post-inoculation. By day 6, more severe clinical signs of the infected mice were present, including inactivity, ruffled fur, hunched posture, poor appetite, rapid shallow breathing, and audible crackling. E5564 or NAC treatments can relieve the clinical symptoms in mice. As shown in Fig. 1 , the infected mice began to die from day 4 post-inoculation, reaching a plateau on day 9 post-inoculation. The body weights of the infected mice were significantly decreased on days 6–8 post-inoculation compared with the control group (P < 0.05). However, survival rates and the body weight were higher in the NAC-treated mice and in the E5564-treated mice than those in the H9N2 group (P < 0.05), and there was no statistically significant difference between the NAC and E5564 groups (P > 0.05). Therefore, inhibition of the mortality rate and body weight loss were observed in NAC or E5564-treated mice. None of the mice in the control group showed any clinical signs or died, and their body mass increased over the course of the experiment.
Fig. 1.
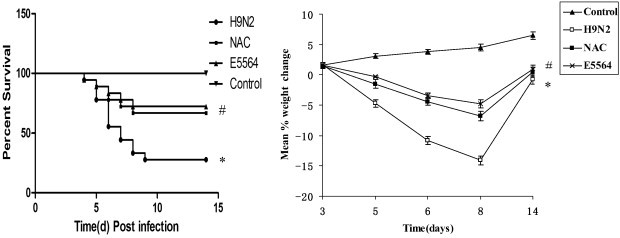
NAC treatment protects mice from lethal influenza challenge.
BALB/c mice were infected with H9N2 influenza A/swine/HeBei/012/2008 virus. Then, mice received NAC or E5564, and survival and body weight were monitored. ⁎P < 0.05 compared with the control group. #P < 0.05 compared with the H9N2 group.
3.2. NAC attenuates pulmonary inflammation and pulmonary edema in mice
To assess the pathological changes, H&E staining and lung injury score system were used in our study. Infected mice displayed a similar histopathologic pattern. On day 2 postinoculation, lung lesions of infected mice were characterized by interstitial edema around the small blood vessels (Fig. 2A and D, black triangle), and dropout of mucous epithelium adhering to the surface of bronchioles (Fig. 2A and D, black arrow). On day 3 postinoculation there was interstitial pneumonia, which showed edema and thickening of the alveolar walls, interstitial edema around the small blood vessels (Fig. 2E, black triangle) and peribronchiolitis with edema and inflammatory cellular infiltrate around the bronchioles (Fig. 2B, gray arrow). Dropout of mucous epithelium from the bronchioles is also seen (Fig. 2B, black arrow). On day 5 postinoculation, there was bronchiolitis (Fig. 2C, gray arrow) with inflammatory cellular infiltrate, peribronchiolitis with edema and inflammatory cellular infiltrate around the bronchioles (Fig. 2C and F, gray arrow), and interstitial edema around the small blood vessels (Fig. 2F, black triangle). However, after NAC treatments, the pathological changes in the lung tissues were relieved (Fig. 2D, E, and F). The mice in the control group showed no histological changes. As for pulmonary edema evaluation, lung wet:dry weight ratios (W/D) were employed. In the H9N2 group, lung wet:dry weight ratios were significantly increased compared with the control group on days 5–8 postinoculation (P < 0.01), reaching a peak on day 6 and declining thereafter (Fig. 3). Nevertheless, W/D significantly decreased after NAC administration (Fig. 3 ). E5564 has the same effect in W/D decrease.
Fig. 2.
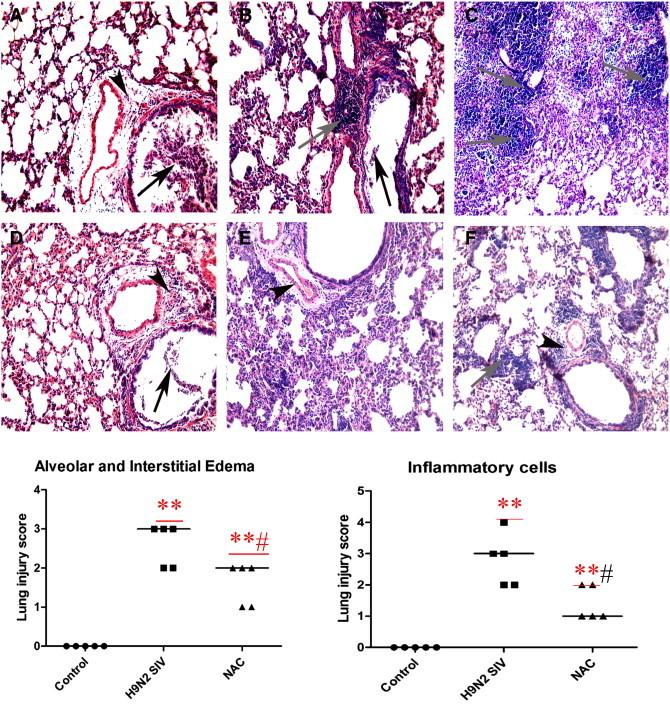
NAC attenuates pulmonary inflammation in mice.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with or without NAC treatment, mice were sacrificed and their lungs were fixed. Then, tissue sections were stained with hematoxylin and eosin (H&E). On day 2 postinoculation, there was interstitial edema around the small blood vessels (panels A and D, black triangle) and dropout of mucous epithelium adhering to the surface of bronchioles (panels A and D, black arrow). On day 3 postinoculation, the virus caused interstitial pneumonia and slight peribronchiolitis (panel B, gray arrow). On day 5 postinoculation, the virus caused severe bronchiolitis and peribronchiolitis (panels C and F, gray arrow). However, after NAC treatments, the pathological changes in the lung tissues were relieved. (panels D, E, and F). Objective magnification, × 200 (A–F). ⁎P < 0.05, ⁎⁎P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 compared with the H9N2 group.
Fig. 3.
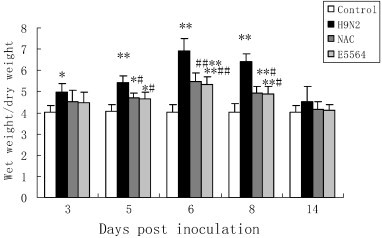
NAC improves pulmonary edema in mice.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with or without NAC treatment, mice were sacrificed and their lungs were collected at indicated times postinoculation, lung weights were recorded, and lung wet-to-dry weight ratios were calculated. White bars represent the control group; black bars, the H9N2 group; gray bars, the NAC group; and pale gray bars, the E5564 group. Bars represent means ± SD of 5 mice. ⁎P < 0.05, ⁎⁎P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 compared with the H9N2 group.
3.3. NAC reduces cell counts in BALF
The cell counts in BALF are shown in Fig. 4 . 3, 5, 6, 8 and 14 days after H9N2 swine influenza virus infection, total cells, neutrophils, macrophages, and lymphocytes were remarkably increased in BALF. However, NAC treatments reduced total cells, neutrophils, macrophages and lymphocytes in BALF. E5564 also reduced total cells, neutrophils, macrophages and lymphocytes in BALF.
Fig. 4.
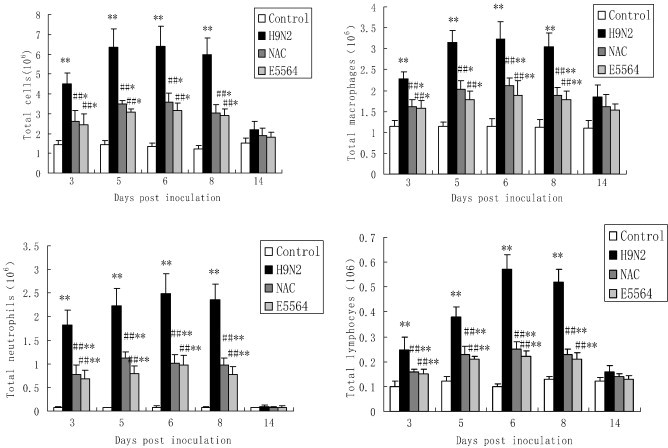
NAC reduces cellular counts in BALF.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with or without NAC treatment, mice were sacrificed and their lungs were lavaged. Cells in the BALF were collected and cytospin preparations were made. Total cells, neutrophils, macrophages and lymphocytes in BALF were analyzed. Each bar represents the mean ± SD of 5 mice. ⁎P < 0.05, ⁎⁎P < 0.01 compared with the control group. #P < 0.05, ##P < 0.01 compared with the H9N2 group.
3.4. NAC reduces myeloperoxidase (MPO) activity in the lung
The increased neutrophil infiltration is known to play an important role in the development of ALI, and the MPO activity was considered as a marker of influx of neutrophils. Our data also showed that elevation of lung MPO activity stimulated by H9N2 swine influenza virus infection was significantly inhibited by NAC treatment (Fig. 5 ). MPO activity was also significantly inhibited by E5564 treatment.
Fig. 5.
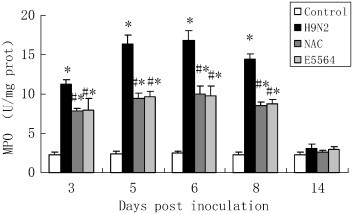
NAC reduces MPO activity in mice.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with or without NAC treatment, mice were sacrificed and their lungs were removed. MPO activity was detected as described in the Materials and methods section. Each bar represents the mean ± SD of 5 mice. ⁎P < 0.01 compared with the control group. #P < 0.01 compared with the H9N2 group.
3.5. Influence of NAC on concentration of MDA and T-SOD activity in the lung
The concentration of MDA in the mice in the H9N2 group increased markedly from day 3 to day 8 postinoculation compared with the mice in the control group. However, MDA concentrations were reduced by NAC treatment on days 3–8 postinoculation. Similar to the NAC, E5564 treatment result in a decrease in the MDA concentrations on days 3–8 postinoculation. However, no significant differences were observed in the concentration of MDA among the H9N2 group, E5564 group and NAC group at day 14 (Fig. 6 ).
Fig. 6.
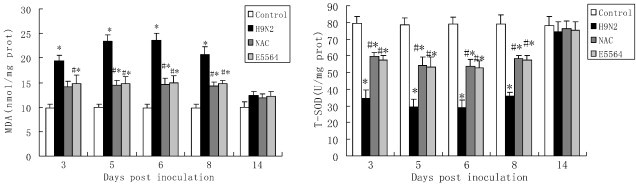
NAC improves T-SOD activity and reduces MDA concentrations in mice.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with either NAC or E5564 treatment, mice were sacrificed and their lungs were collected. T-SOD activity and MDA concentrations were detected as described in the Materials and methods section. Each bar represents the mean ± SD of 5 mice. ⁎P < 0.01 compared with the control group. #P < 0.01 compared with the H9N2 group.
T-SOD activity in the mice in the H9N2 group was significantly lower than that in the control group on days 3–8 postinoculation. NAC treatment results in an increase in the T-SOD activity on days 3–8 postinoculation. Similar to the NAC, E5564 treatment also results in an increase in the T-SOD activity on days 3–8 postinoculation. However, no significant differences were observed in T-SOD activity among the H9N2 group, E5564 group and NAC group at day 14 (Fig. 6).
3.6. Influence of NAC on cytokine and chemokine production in BALF
TNF-α, IL-6, IL-1β, and CXCL-10 in BALF were measured by ELISA. Mice in the control group produced low levels of all basal cytokines/chemokines tested. TNF-α, IL-6, IL-1β, and CXCL-10 in BALF in the mice with H9N2 swine influenza virus infection were markedly higher from day 3 to day 8 postinoculation than the mice in the control group. The H9N2 swine influenza virus-induced cytokine/chemokine secretion was reduced by NAC. Similar to the NAC, E5564 treatment also results in a decrease in the H9N2 swine influenza virus-induced cytokine/chemokine secretion. However, no significant differences were observed in TNF-α, IL-6, IL-1β, and CXCL-10 in BALF among the H9N2 group, E5564 group and NAC group at day 14 (Fig. 7 ).
Fig. 7.
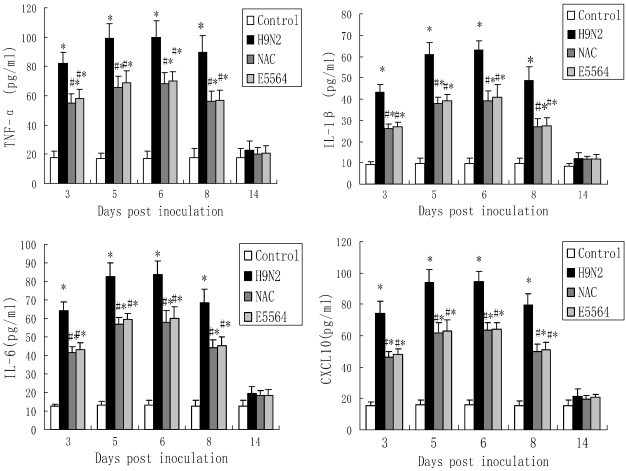
NAC down-regulates TNF-α, IL-6, IL-1β, and CXCL-10 in BALF.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with either NAC or E5564 treatment, mice were sacrificed, their lungs were lavaged and the BALF was collected. TNF-α, IL-6, IL-1β, and CXCL-10 were detected by ELISA. Each bar represents the mean ± SD of 5 mice. ⁎P < 0.01 compared with the control group. #P < 0.01 compared with the H9N2 group.
3.7. NAC inhibits toll-like receptor 4 (TLR4) expression
Five days after the H9N2 swine influenza virus infection, mRNA and protein expression of TLR4 were detected using real-time PCR and western blot. The result showed as follows: compared with the control group, enhanced mRNA and protein expression of TLR4 were noticed after the H9N2 swine influenza virus infection. Meanwhile, our data showed that H9N2 swine influenza virus-infected enhanced TLR4 expression was inhibited by NAC. Similar to the NAC, E5564 treatment also results in the inhibition of mRNA and protein expression of TLR4 (Fig. 8 ).
Fig. 8.
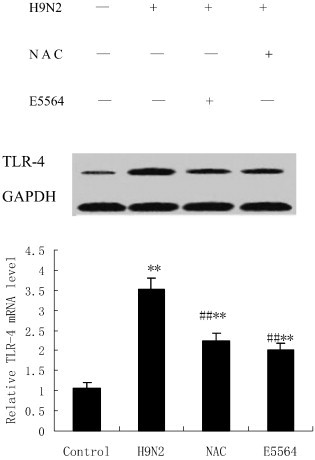
NAC inhibits TLR4 activation in mice.
After infection with A/swine/HeBei/012/2008/(H9N2) virus with either NAC or E5564 treatment, mice were sacrificed, and their lungs were removed. The lung tissues were subjected to western blotting analysis. GAPDH was used as a loading control. Each bar represents the mean ± SD of 5 mice. ⁎⁎P < 0.01 compared with the control group. ##P < 0.01 compared with the H9N2 group.
4. Discussion
Antioxidant molecules including reduced glutathione and its precursor N-acetyl-l-cysteine (NAC) are potentially useful against infection with influenza A viruses [20], [21], [23], [31]. In cell culture experiments NAC prevented influenza A (H3N2) virus-induced oxidative stress, cell death, and expression of inflammatory genes [32], [33]. Protective activity of NAC against seasonal influenza A infection was shown in animal studies by decreasing mortality of mice infected with the influenza A strain A/PR/8 (H1N1) [34]. In humans, NAC significantly reduced the incidence of clinically apparent A/H1N1 disease [20]. In the present study, we demonstrated that NAC attenuates H9N2 swine influenza virus-induced ALI.
ALI and its severe form, acute respiratory distress syndrome (ARDS), are an acute and progressive respiratory disease and are characterized by progressively diffuse bilateral pulmonary edema and inflammation, reduced pulmonary compliance, and hypoxemia. Pulmonary inflammation and pulmonary edema are two of the most important pathological findings in ALI [8], [9], [10], [35], [36], [37]. In the present paper, H9N2 swine influenza virus-infected mice experienced pulmonary edema and inflammation. Meanwhile, NAC reduced the pulmonary edema and inflammation.
Neutrophil and macrophages were the major inflammatory cell types in ALI [37], [38]. In the present paper, we observed a concurrent increase of total cells, neutrophils, macrophages, and lymphocytes in the BALF of H9N2 swine influenza virus-infected mice, and NAC reduced the total and differential cell counts. MPO activity in the lungs, reflecting the activation of neutrophils, was largely up-regulated in ALI condition. Accordingly, MPO activity was also notably suppressed by NAC. These findings might explain that the reduction in total cells, neutrophils, macrophages, and lymphocytes and down-regulation of MPO activation could prevent the animals from developing ALI.
Virus-induced cytokine/chemokine storm seems to contribute to severe pathogenesis of H5N1 infection in humans [39]. Various inflammatory mediators were involved in ALI. Among them, TNF-α, IL-6, IL-1β, and CXCL-10 were considered as the most important inflammatory mediators [7], [22], [40]. NAC decreased the production of proinflammatory molecules (CXCL8, CXCL10, CCL5 and interleukin-6 (IL-6)) in H5N1-infected A549 cells [22]. According to our data, TNF-α, IL-6, IL-1β, and CXCL-10 in BALF were noticeably raised by H9N2 swine influenza virus infection. Meanwhile, our data showed that NAC reduced the up-regulated TNF-α, IL-6, IL-1β, and CXCL-10 in BALF. These findings indicate that NAC effectively inhibited H9N2 swine influenza virus-induced infection, blocking the uncontrolled inflammatory process in ALI.
However, cytokine and chemokine knockout mice or steroid-treated wild-type mice did not have survival advantage over wild-type mice after viral challenge [41]. These data indicate that suppression of cytokine/chemokine expression alone is not sufficient to improve disease outcome. Oxygen free radicals (OFRs) play an important role in the pathogenesis of ALI [42]. In unchallenged physiological states, production and scavenging of OFRs maintain their homeostasis. In disease states, however, OFRs may be generated in quantities sufficient to overwhelm antioxidant enzyme systems such as SOD. Over production can lead to various forms of cell damage and death [43] and may aggravate the development of disease [44]. In this study, we found that the concentrations of MDA were significantly higher in the lungs of mice with H9N2 swine influenza virus infection than those in the mice in the control group, but SOD activity in the mice with swine influenza virus infection was significantly lower than that in the mice in the control group. These results suggest that increased MDA levels should be correlated with the reduction in SOD activity in H9N2 swine influenza virus-infected mice, and we deduced that OFRs may be involved in the pathogenesis of H9N2 swine influenza virus-induced pneumonia.
Toll-like receptor (TLR), a receptor protein found on the surface of animal cells, plays a critical role in the innate immune system. When microbes invade the host, TLR recognizes the pathogen-associated molecular patterns (PAMPs), such as lipopolysaccharide (LPS), lipoprotein, flagellin of the flagellum, and double-stranded viral RNA (e.g., H9N2 influenza). While ssRNA viruses are known to recognize by Toll-like receptor (TLR) 7/8 [45], dsRNA viruses recognize TLR3 [46]. Detection of PAMPs by TLR proteins activates immune cell responses. Imai et al. identified that induction of ALI, induced by acid aspiration and infection by respiratory viruses and bacteria, was mediated by a common signaling pathway: NADPH oxidase-dependent production of reactive oxygen species generated oxidized host phospholipids, e.g., oxidized 1-palmitoyl-2-arachidonoyl-phosphaticylcholine (OxPAPC), that, in turn, potently stimulated TLR4. They proposed that the ensuing cytokine storm, and in particular, IL-6, mediated ALI [47]. Other studies help confirm that TLR4 has been well documented as a pattern recognition receptor in acute infection-induced lung injury [48], [49], and TLR4 signaling is actively involved in the pathogenesis of noninfectious lung injury [50], [51]. TLR4−/− mice were protected from lethality caused by mouse-adapted influenza virus [52]. Imai Y et al. identified TLR4 as a key pathway that controls the severity of ALI induced H5N1 avian influenza virus [47]. The finding of Shirley et al. that the TLR4 antagonist, Eritoran, is highly protective when administered therapeutically to mice infected with a lethal dose of influenza [53], let us to know that blocking TLR4 therapeutically would protect against influenza infection. Herein, we hypothesize that NAC antiviral activity against H9N2 virus results from its ability to inhibit activation of TLR4 pathways. However, previous studies have reported that NAC is unable to alter the course of a fatal influenza pneumonia caused by inoculation of a murinized swine H1N1 influenza virus [54]. Surprisingly, our present study shows that NAC's protective effect against H9N2 virus-induced lung injury results from its ability to inhibit the activation of TLR4 pathways. In animal studies, proinflammatory cytokines as well as TLR4 protein and mRNA increased significantly after H9N2 swine influenza virus infection, and the lung tissue had clear pathological changes. In addition, similar to the TLR4 inhibitor E5564, after treatment NAC, rats exhibited reduced lung injury as well as levels of proinflammatory cytokines in the lung tissue. Moreover, similar to the TLR4 inhibitor E5564, NAC treatment also result in the inhibition of mRNA and protein expression of TLR4.
Our data showed that NAC treatment reduced the severity of H9N2 swine influenza virus-induced ALI, more likely by virtue of NAC-mediated TLR4 inhibition. The results suggest that NAC may show its beneficial effects for the potential treatment of H9N2 swine influenza virus-induced ALI.
Acknowledgments
This work was supported by the Natural Science Foundation of Hebei Province, China (Grant number: C2011405002), the Natural Science Research Key Programs of Educational Department of Hebei Province (Grant number: ZD20131045) and Key Programs of Hebei North University (Grant number: ZD201306).
References
- 1.WHO Influenza fact sheet no. 211. http://wwwwhoint/mediacentre/factsheets/fs211/en/indexhtml2009 (accessed 19 September 2011). Available from:
- 2.Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- 3.Cinatl J., Jr., Michaelis M., Doerr H.W. The threat of avian influenza A(H5N1). Part I: epidemiologic concerns and virulence determinants. Med Microbiol Immunol. 2007;196:181–190. doi: 10.1007/s00430-007-0042-5. [DOI] [PubMed] [Google Scholar]
- 4.Cinatl J., Jr., Michaelis M., Doerr H.W. The threat of avian influenza A(H5N1). Part II: clues to pathogenicity and pathology. Med Microbiol Immunol. 2007;196:191–201. doi: 10.1007/s00430-007-0045-2. [DOI] [PubMed] [Google Scholar]
- 5.Cinatl J., Jr., Michaelis M., Doerr H.W. The threat of avian influenza A(H5N1). Part III: antiviral therapy. Med Microbiol Immunol. 2007;196:203–212. doi: 10.1007/s00430-007-0048-z. [DOI] [PubMed] [Google Scholar]
- 6.Cinatl J., Jr., Michaelis M., Doerr H.W. The threat of avian influenza A(H5N1). Part IV: development of vaccines. Med Microbiol Immunol. 2007;196:213–225. doi: 10.1007/s00430-007-0052-3. [DOI] [PubMed] [Google Scholar]
- 7.Michaelis M., Doerr H.W., Cinatl J., Jr. Of chickens and men: avian influenza in humans. Curr Mol Med. 2009;9:131–151. doi: 10.2174/156652409787581565. [DOI] [PubMed] [Google Scholar]
- 8.Zhu T., Zhang W., Wang D.X. Insulin up-regulates epithelial sodium channel in LPS-induced acute lung injury model in rats by SGK1 activation. Injury. 2012;43:1277–1283. doi: 10.1016/j.injury.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Deng J., Wang D.X., Deng W., Li C.Y., Tong J., Ma H. Regulation of alveolar fluid clearance and ENaC expression in lung by exogenous angiotensin II. Respir Physiol Neurobiol. 2012;181:53–61. doi: 10.1016/j.resp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Ware L.B., Matthay M.A. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell M.L., Melisse S.B., Gordon R.B., Rob F., Teri J.F., Frederick G.H. Clinical issues and research in respiratory failure from severe acute respiratory syndrome. Am J Respir Crit Care Med. 2005;171:518–526. doi: 10.1164/rccm.200405-621WS. [DOI] [PubMed] [Google Scholar]
- 12.Chen C.Y., Lee C.H., Liu C.Y., Wang J.H., Wang L.M., Perng R.P. Clinical features and outcomes of severe acute respiratory syndrome and predictive factors for acute respiratory distress syndrome. J Chin Med Assoc. 2005;68:1–3. doi: 10.1016/S1726-4901(09)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subbarao K., Klimov A., Katz J., Regnery H., Lim W., Hall H. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 14.Dong W., Li-Feng X., Cun-Lian W., Ming-Ju X., Rui-Hua Z., Ying L. A mouse model of swine influenza virus H9N2 infection with acute lung injury. Acta Virol. 2012;56:227–233. doi: 10.4149/av_2012_03_227. [DOI] [PubMed] [Google Scholar]
- 15.Deng Y., Yang Z., Gao Y., Xu H., Zheng B., Jiang M. Toll-like receptor 4 mediates acute lung injury induced by high mobility group box-1. PLoS One. 2013;8:e64375. doi: 10.1371/journal.pone.0064375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv T., Shen X., Shi Y., Song Y. TLR4 is essential in acute lung injury induced by unresuscitated hemorrhagic shock. J Trauma. 2009;66:124–131. doi: 10.1097/TA.0b013e318181e555. [DOI] [PubMed] [Google Scholar]
- 17.Hu G., Malik A.B.., Minshall R.D. Toll-like receptor 4 mediates neutrophil sequestration and lung injury induced by endotoxin and hyperinflation. Crit Care Med. 2010;38:194–201. doi: 10.1097/CCM.0b013e3181bc7c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gribar S.C., Richardson W.M., Sodhi C.P., Hackam D.J. No longer an innocent bystander: epithelial toll-like receptor signaling in the development of mucosal inflammation. Mol Med. 2008;14:645–659. doi: 10.2119/2008-00035.Gribar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perros F., Lambrecht B.N., Hammad H. TLR4 signalling in pulmonary stromal cells is critical for inflammation and immunity in the airways. Respir Res. 2011;12:125. doi: 10.1186/1465-9921-12-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Flora S., Grassi C., Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10:1535–1541. doi: 10.1183/09031936.97.10071535. [DOI] [PubMed] [Google Scholar]
- 21.Cai J., Chen Y., Seth S., Furukawa S., Compans R.W., Jones D.P. Inhibition of influenza infection by glutathione. Free Radic Biol Med. 2003;34:928–936. doi: 10.1016/s0891-5849(03)00023-6. [DOI] [PubMed] [Google Scholar]
- 22.Geiler J., Michaelis M., Naczk P., Leutz A., Langer K., Doerr H.W. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza A virus. Biochem Pharmacol. 2010;79:413–420. doi: 10.1016/j.bcp.2009.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Garozzo A., Tempera G., Ungheri D., Timpanaro R., Castro A. N-acetylcysteine synergizes with oseltamivir in protecting mice from lethal influenza infection. Int J Immunopathol Pharmacol. 2007;20:349–354. doi: 10.1177/039463200702000215. [DOI] [PubMed] [Google Scholar]
- 24.Majeski E.I., Paintlia M.K., Lopez A.D., Harley R.A., London S.D., London L. Respiratory reovirus 1/L induction of intraluminal fibrosis, a model of bronchiolitis obliterans organizing pneumonia, is dependent on T lymphocytes. Am J Pathol. 2003;163:1467–1479. doi: 10.1016/S0002-9440(10)63504-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsey M.V., Tuder R.M., Abraham E. Neutrophils are major contributors to intraparenchymal lung IL-1b expression after hemorrhage and endotoxemia. J Immunol. 1998;160:1007–1013. [PubMed] [Google Scholar]
- 26.Vieira R.P., Claudino R.C., Duarte A.C., Santos A.B.., Perini A., Faria Neto H.C. Aerobic exercise decreases chronic allergic lung inflammation and airway remodeling in mice. Am J Respir Crit Care Med. 2007;176:1–7. doi: 10.1164/rccm.200610-1567OC. [DOI] [PubMed] [Google Scholar]
- 27.Kasahara D.I., Poynter M.E., Othman Z., Hemenway D., van der Vliet A. Acrolein inhalation suppresses lipopolysaccharide-induced inflammatory cytokine production but does not affect acute airways neutrophilia. J Immunol. 2008;18:736–745. doi: 10.4049/jimmunol.181.1.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Menezes S.L., Bozza P.T., Neto H.C., Laranjeira A.P., Negri E.M., Capelozzi V.L. Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol. 2005;98:1777–1783. doi: 10.1152/japplphysiol.01182.2004. [DOI] [PubMed] [Google Scholar]
- 29.Sun Y., Oberley L.W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:479–500. [PubMed] [Google Scholar]
- 30.Hermann E., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 31.GhezziP UngheriD. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol. 2004;17:99–102. doi: 10.1177/039463200401700114. [DOI] [PubMed] [Google Scholar]
- 32.Knobil K., Choi A.M., Weigand G.W., Jacoby D.B. Role of oxidants in influenza virus-induced gene expression. Am J Physiol. 1998;274:134–142. doi: 10.1152/ajplung.1998.274.1.L134. [DOI] [PubMed] [Google Scholar]
- 33.Kujime K., Hashimoto S., Gon Y., Shimizu K., Horie T. p38 Mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol. 2000;164:3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- 34.Ungheri D., Pisani C., Sanson G., Bertani A., Schioppacassi G., Delgado R. Protective effect of n-acetylcysteine in a model of influenza infection in mice. Int J Immunopathol Pharmacol. 2000;13:123–128. [PubMed] [Google Scholar]
- 35.Deng W., Wang D.X., Zhang W., Li C.Y. Regulation of epithelial sodium channel a-subunit expression by adenosine receptor A2a in alveolar epithelial cells. Chin Med J (Engl) 2011;124:1551–1555. [PubMed] [Google Scholar]
- 36.Deng W., Li C.Y., Tong J., Zhang W., Wang D.X. Regulation of ENaC-mediated alveolar fluid clearance by insulin via PI3K/Akt pathway in LPS-induced acute lung injury. Respir Res. 2012;13:29. doi: 10.1186/1465-9921-13-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hidalgo M.A., Romero A., Figueroa J., Cortés P., Concha I.I., Hancke J.L. Andrographolide interferes with binding of nuclear factor-kB to DNA in HL-60-derived neutrophilic cells. Br J Pharmacol. 2005;144:680–686. doi: 10.1038/sj.bjp.0706105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee L., Kelher M.R., Moore E.E., Banerjee A., Silliman C.C. Hypertonic saline inhibits arachidonic acid priming of the human neutrophil oxidase. J Surg Res. 2012;174:24–28. doi: 10.1016/j.jss.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michaelis M., Doerr H.W., Cinatl J., Jr. Of chickens and men: avian influenza in humans. Curr Mol Med. 2009;9:131–151. doi: 10.2174/156652409787581565. [DOI] [PubMed] [Google Scholar]
- 40.Christofidou-Solomidou M., Kennel S., Scherpereel A., Wiewrodt R., Solomides C.C., Pietra G.G. Vascular immunotargeting of glucose oxidase to the endothelial antigens induces distinct forms of oxidant acute lung injury. Am J Pathol. 2002;160:1155–1169. doi: 10.1016/S0002-9440(10)64935-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salomon R., Hoffmann E., Webster R.G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc Natl Acad Sci U S A. 2007;104:12479–12481. doi: 10.1073/pnas.0705289104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.He G., Dong C., Luan Z., McAllan B.M., Xu T., Zhao L. Oxygen free radical involvement in acute lung injury induced by H5N1 virus in mice. Influenza Other Respi Viruses. 2013;7:945–953. doi: 10.1111/irv.12067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Slemmer J.E., Shacka J.J., Sweeney M.I., Weber J.T. Antioxidants and free radical scavengers for the treatment of stroke, traumatic brain injury and aging. Curr Med Chem. 2008;15:404–414. doi: 10.2174/092986708783497337. [DOI] [PubMed] [Google Scholar]
- 44.Rao A.V., Balachandran B. Role of oxidative stress and antioxidants in neurodegenerative diseases. Nutr Neurosci. 2002;5:291–309. doi: 10.1080/1028415021000033767. [DOI] [PubMed] [Google Scholar]
- 45.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 46.Alexopoulou L1., Holt A.C., Medzhitov R., Flavell R.A. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 47.Imai Y., Kuba K., Neely G.G., Yaghubian-Malhami R., Perkmann T., van Loo G. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133:235–249. doi: 10.1016/j.cell.2008.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akira S. Mammalian Toll-like receptors. Curr Opin Immunol. 2003;15:5–11. doi: 10.1016/s0952-7915(02)00013-4. [DOI] [PubMed] [Google Scholar]
- 49.Medzhitov R., Janeway C., Jr. Innate immunity. N Engl J Med. 2000;343:338–344. doi: 10.1056/NEJM200008033430506. [DOI] [PubMed] [Google Scholar]
- 50.Baudouin S. Innate immune defense on the attack in acute lung injury. Crit Care Med. 2010;38:328–329. doi: 10.1097/CCM.0b013e3181b3a81e. [DOI] [PubMed] [Google Scholar]
- 51.Kaczorowski D.J., Mollen K.P., Edmonds R., Billiar T.R. Early events in the recognition of danger signals after tissue injury. J Leukoc Biol. 2008;83:546–552. doi: 10.1189/jlb.0607374. [DOI] [PubMed] [Google Scholar]
- 52.Nhu Q.M., Shirey K., Teijaro J.R., Farber D.L., Netzel-Arnett S., Antalis T.M. Novel signaling interactions between proteinase-activated receptor 2 and Toll-like receptors in vitro and in vivo. Mucosal Immunol. 2010;3:29–39. doi: 10.1038/mi.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shirey K.A., Lai W., Scott A.J., Lipsky M., Mistry P., Pletneva L.M. The TLR4 antagonist Eritoran protects mice from lethal influenza infection. Nature. 2013;497:498–502. doi: 10.1038/nature12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garigliany M.M., Desmecht D.J. N-acetylcysteine lacks universal inhibitory activity against influenza A viruses. J Negat Results Biomed. 2011;10:5. doi: 10.1186/1477-5751-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


