Abstract
Objective:
Opioid prescriptions after surgery are effective for pain management but have been a significant contributor to the current opioid epidemic. Our objective is to review pragmatic approaches to develop and implement evidence-based guidelines based on a learning health system model.
Summary Background Data:
During the last 2 years there has been a preponderance of data demonstrating that opioids are overprescribed after surgery. This contributes to a number of adverse outcomes, including diversion of leftover pills in the community and rising rates of opioid use disorder.
Methods:
We conducted a MEDLINE/PubMed review of published examples and reviewed our institutional experience in developing and implementing evidence-based postoperative prescribing recommendations.
Results:
Thirty studies have described collecting data regarding opioid prescribing and patient-reported use in a cohort of 13,591 patients. Three studies describe successful implementation of opioid prescribing recommendations based on patient-reported opioid use. These settings utilized learning health system principles to establish a cycle of quality improvement based on data generated from routine practice. Key components of this pathway were collecting patient-reported outcomes, identifying key stakeholders, and continual assessment. These pathways were rapidly adopted and resulted in a 37% to 63% reduction in prescribing without increasing requests for refills or patient-reported pain scores.
Conclusion:
A pathway for creating evidence-based opioid-prescribing recommendations can be utilized in diverse practice environments and can lead to significantly decreased opioid prescribing without adversely affecting patient outcomes.
Keywords: learning health system, opioid prescribing, opioids, postoperative opioid prescribing, quality improvement
Opioid prescribing following surgical care has been a major factor driving the opioid epidemic in the United States.1 Excessive opioid prescribing has commonly occurred across surgical procedures and specialties, in part because of a lack of evidence about patients’ opioid requirements following surgery.2–6 Unfortunately, unused pills following procedural care are a major source of nonmedical opioid use, and the most common initial opioid exposure for individuals with opioid use disorder owing to heroin.7–11 Moreover, high levels of opioid prescribing in the immediate postoperative period are associated with prolonged opioid use among previously opioid-naïve patients.12–15 Although recent legislative measures now require providers to use prescription drug monitoring programs (PDMPs) and restrict prescribing for acute pain, these have been met with mixed success.16–19 Furthermore, these measures are neither patient-focused nor physician-driven, and fail to engage these primary stakeholders in the current opioid crisis.
A number of institutions have had success reducing excessive opioid prescribing utilizing a quality improvement framework.20–23 This framework is based on the principles of a learning health system, in which improvements in care are achieved by integrating data and experience generated by routine practice.24 In this model, a continuous cycle is developed in which data are analyzed to identify opportunities for quality improvement, measures are implemented based on this analysis, and then iterative change takes place based on reanalysis of new information (Fig. 1). A well-known example of this is the development of a National Trauma Care System with the goal of zero preventable deaths for patients who have sustained traumatic injury.25 It is estimated that this effort has the potential to save 100,000 lives in a 5-year period.26 Another successful example includes the Surgical Care and Outcomes Assessment Program, wherein electronic medical records are used to feedback outcomes and performance to surgeons in the state of Washington. This has resulted in decreased variability in care delivery, which in turn has decreased complications and improved cost savings.27
FIGURE 1.
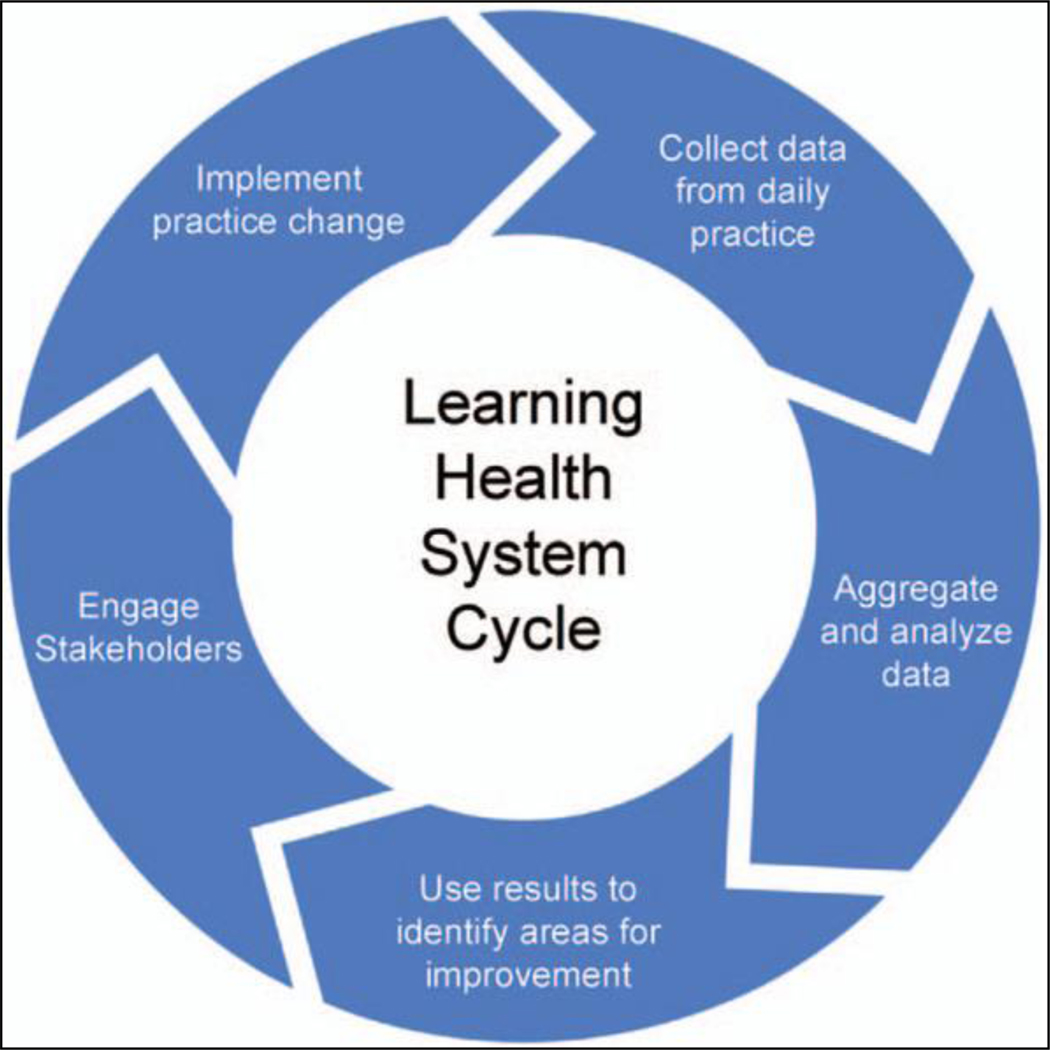
Cycle of continuous quality improvement in a learning health system.
For opioid prescribing, similar projects have leveraged patient feedback on key outcomes, such as pain, satisfaction, and opioid consumption to create tailored guidelines for postoperative opioid prescribing.28 Encouragingly, this work has led to significant and sustained improvements in postoperative prescribing without negatively impacting patient satisfaction or pain.20,29,30 Randomized controlled trials (RCTs) could help augment these studies and establish a causal connection between prescribing recommendations based on patient outcomes, changes in prescribing practice, and stability in patient satisfaction. However, RCTs are costly, logistically challenging to execute, and often do not represent real-world practice given the constraints of creating study cohorts and comparison groups.31 In addition, it is imperative to ensure pain is effectively treated after surgery, and testing interventions that threaten adequate postoperative pain control can be ethically challenging.32
In the absence of high-level evidence, pragmatic quality improvement studies based on the principles of a learning health system may represent the best opportunity to garner robust, patient-centered evidence to efficiently change practice. We review a pathway that is meant to engage surgeons and patients in generating data to support changes in prescribing practice that are unique to the settings in which they are implemented. By utilizing analysis and feedback from providers and patients, practice change is more likely to be sustainable and reflect the values of patients and providers.33,34 The result is rapid improvement in quality of surgical care that integrates patient-reported outcomes to create practical opioid prescribing guidelines following surgery.
METHODS
We conducted a search of the PubMed/MEDLINE database to identify studies for inclusion. Primary inclusion criteria were original research studies that evaluated the amount of opioids prescribed and the amount of opioids consumed by patients after. The primary outcomes of interest were prescription size and opioid consumption by patients. The search query used was “(opioid[TIAB] OR opiate[TIAB]) AND (postoperative[TIAB] OR postsurgical[TIAB] OR surgery[TIAB] OR procedure[TIAB] OR procedures[TIAB]) AND (prescription OR prescribing) AND (use OR utilization OR consumption)” This was limited to English-language articles analyzing adult populations (18 years) published before March 2019. Of the 305 query results, 30 met inclusion criteria.2,3,7,23,30,4,35–40,41–58
RESULTS
Procedure and Patient Selection
The success of quality improvement projects depends in large part on their ability not to overextend stakeholders or be too methodologically complex.59 Therefore, the majority of literature has focused on developing guidelines for patients undergoing common surgical procedures in which a straightforward postoperative course is expected (eg, laparoscopic cholecystectomy, inguinal hernia repair).20,21 Studies addressing postoperative opioid prescribing have focused on a narrow range of procedures, such as common outpatient general surgical procedures, outpatient orthopedic surgery, minimally invasive gynecologic and urologic surgery, dental procedures, and dermatologic procedures (Table 1).2,7,20,30,35–40,60
TABLE 1.
Review of Studies Describing Data Collection Methods Regarding Opioid Prescription and Use.
| Study | Opioid Prescription Data Collection Method | Opioid Use Data Collection Method | Procedures | N |
|---|---|---|---|---|
| Bates et al, 201135 | Phone and mail-out survey | Phone and mail-out survey | Urologic surgery | 275 |
| Rodgers et al, 20127 | Medical record review | Phone survey | Upper extremity surgery | 287 |
| Harris et al, 201336 | Medical record review | Phone survey | Dermatologic surgery | 212 |
| Swenson et al, 201637 | Medical record review | Phone survey | Minimally invasive urogynecologic surgery | 50 |
| Maughan et al, 201638 | Medical record review | Text message survey | Impacted tooth extraction | 49 |
| Kim et al, 201639 | In-person survey | In-person survey | Upper extremity surgery | 1416 |
| Bartels et al, 201640 | Electronic or mail-out survey | Electronic or mail-out survey | Cesarean delivery, thoracic surgery | 63 |
| As-Sanie et al, 20174 | Medical record review | Phone survey | Hysterectomy | 102 |
| Osmundson et al, 201741 | Not collected | Email or phone survey | Cesarean delivery | 246 |
| Hill et al, 20172 | Medical record review | Phone survey | Elective surgical procedures | 642 |
| Howard et al, 201742 | Medical record review | Phone survey | Laparoscopic cholecystectomy | 370 |
| Bateman et al, 201715 | Phone survey | Phone survey | Cesarean delivery | 720 |
| Thiels et al, 201843 | Medical record review | Phone survey | Elective surgical procedures | 2486 |
| Swarup et al, 201844 | Medical record review | Mail-out survey | Outpatient anorectal surgery | 42 |
| Howard et al, 20183 | Medical record review | Phone survey | Common surgical and gynecologic procedures | 2392 |
| Wojahn et al, 201845 | Medical record review | Written questionnaire | Knee arthroscopy | 221 |
| Saini et al, 201823 | Medical record review | In-person survey | Orthopedic foot and ankle procedures | 988 |
| Hart et al, 201846 | Medical record review | Phone survey | Breast surgery | 95 |
| Sabatino et al, 201847 | Medical record review | Phone survey | Elective orthopedic procedures | 1159 |
| Cunningham et al, 201848 | Medical record review | In-person survey | Hip arthroscopy | 80 |
| Griffith et al, 201849 | Phone survey | Phone survey | Gynecologic procedures | 56 |
| Peters et al, 201850 | Phone survey | Phone survey | Carpal tunnel release | 49 |
| Hota et al, 201851 | Written questionnaire | Written questionnaire | Gynecologic procedures | 75 |
| Bartels et al, 201953 | Medical record review | Online, phone, or mail-out survey | Gastrointestinal procedures | 233 |
| Friedman et al, 201954 | In person | In person | Bariatric surgery | 115 |
| Rose et al, 201955 | Written questionnaire | Written questionnaire | Outpatient plastic surgery | 170 |
| Locketz et al, 201956 | Not collected | Written questionnaire | Sinus and nasal surgery | 219 |
| Hozack et al, 201957 | Not collected | In-person survey | Cubital tunnel decompression surgery | 100 |
| Bicket et al, 201958 | Medical record review | Phone survey | Joint and spine surgery | 140 |
| Fuijii et al, 201952 | Medical record review | Phone survey | Common surgical procedures | 539 |
In these examples, the focus on elective procedures and a relatively healthy, opioid-naïve patient population may have contributed to their success. Piloting practice change in a single or small group of related procedures may facilitate identifying and engaging the primary stakeholders (residents, faculty, nurses, midlevel providers) whose participation will be critical to success. After the success of initial pilot efforts, it may be possible to expand prescribing guidelines to more complex procedures or patient populations (such as those with preoperative opioid use or postoperative complications).
Baseline Data Collection
Collecting data regarding existing postoperative prescribing practices provide a baseline description of institutional trends against which changes can be compared. This process uses medical record review to capture basic patient demographics, surgical procedures, and postoperative prescriptions, and is performed retrospectively in a defined period of time (6–12 months) to illustrate variability and preexisting trends. Most studies describe using chart review to collect data regarding the type and size of opioid prescription that patients receive.2–4,7,23,36–38,42–48,52,53,58 If this is not feasible, these data can also be collected directly from patients, for example, by asking them to read the label on the prescription bottle they received at discharge.30 PDMP registries could also be queried, which are now used in every state. This review can also be used to identify which providers are writing opioid prescriptions after surgery, which is important to know during implementation and engagement of stakeholders and may vary across environments.
Baseline data collection should also involve meeting with stakeholders involved in the patient’s care pathway. Qualitative data can be generated to understand the knowledge and motivations that drive current prescribing practices, such as environmental and social factors.61 For example, are surgeons prescribing a given amount because of strongly held beliefs, or would they be amenable to change? Interviews with nursing and other clinical staff, who may do the bulk of patient education about pain management, are also instrumental to garner perspectives and engagement.
Pain- and opioid-related postoperative outcomes
Patient-reported outcomes regarding opioid consumption, postoperative pain, refill requests, and patient satisfaction after surgery are the cornerstone of creating patient-centered guidelines for opioid prescribing. Depending on the resources and workflow of a given institution, studies that have implemented this pathway have described data collection via phone survey, electronic survey, postal mail survey, or even in person at a follow-up clinic visit (Table 1). At minimum, the following information is universally collected: type of medication taken after surgery, number of pills taken, number of days medication was taken after surgery, need for refills, pain after surgery, and satisfaction with surgery (Table 2). Patient-reported opioid use should be converted into milligrams of oral morphine equivalents (OMEs) to adjust for differing potency between medications.62 As with other retrospective studies, data collected are subject to recall bias. Previous work has described a typical survey interval of 1 month after surgery; however, earlier survey time may help reduce recall bias, and a majority of patients report cessation of opioid use within a few days after surgery.63 Some studies describe surveying patients as early as 2 days after discharge.58 Periodic reminders and even providing patients with a medication log may increase the accuracy of reporting. Additionally, prompting patients with objective data collected from their chart can also effectively reduce recall bias (eg, “I see you were prescribed 10 pills, how many did you take?”).64
TABLE 2.
Opioid- and pain-related outcomes survey.
| 1 Did you fill the opioid prescription that was given to you after surgery? |
| 2 How many pills did you use? |
| 3 For how many days did you take pills? |
| 4 Are you still taking the medication? |
| 5 How many leftover pills you have? |
| 6 If you still have leftover pills, where do you store it? |
| 7 If you disposed of the leftover pills, how did you dispose of them? |
| 8 Did you need to refill the prescription you were given after surgery? |
| 9 Did you take any pain medications other than what your doctor gave you after surgery? This includes over the counter medications such as Motrin (generic name: ibuprofen) and Tylenol (generic name: acetaminophen). |
| 10 Do you feel like you had enough pain medication? |
| 11 On a scale of 0 to 10 (0 being no pain, 10 being the worst pain imaginable), what was your average overall pain score in the week following your surgery? |
In addition to collecting data strictly related to postoperative opioid use and pain scores, patient survey can be a valuable tool to assess other important health behaviors related to opioids. Many patients report having and using leftover opioids from other procedures, so although they may not use opioid prescribed after a current surgery, they may take opioids from a previous surgery. For example, 1 in 4 adults aged 50 to 80 report filling an opioid prescription in the last 2 years, and 86% saved leftover pills for later use.65 Patient survey also allows for assessment of what patients were told by their provider regarding opioids and how patients store or plan to dispose of opioids, which has been found to be an area for significant improvement.40
There may be a necessary tradeoff between the amount of information collected from patients and available resources to conduct surveys. As data collection, especially involving patient surveys, can be time- and resource-intensive, utilization of administrative data abstractors, research residents, and even medical students has been described conduct this work effectively. In limited practice settings, data collection can be integrated into the postoperative visit, and limited to a few key pieces of information. Another strategy is to utilize a PDMP maintained by the state as a source of already collected data. Although some states only allow abstraction of deidentified data for research purposes, many states allow full access to PDMP registries as part of research and quality improvement initiatives. Lastly, in small or individual practices that lack resources, existing data sources for patient-reported outcomes such as the Consumer Assessment of Healthcare Providers and Systems (CAHPS) that have resulted from the Medicare Access and CHIP Reauthorization Act (MACRA) can be utilized to capture these patient data. This type of quality improvement initiative falls within the American Board of Surgery’s requirement that practicing surgeons continue to stay involved in practice improvement activities.
Generation of Prescribing Recommendations
After data collection regarding institutional prescribing practices and patient-reported opioid use after surgery, prescribing recommendations can be generated. For a single procedure, there will be a range of opioid use with significant outliers. A majority of studies collect p atient-reported outcomes from at least 100 patients. With a skew toward low opioid usage in most patients, a survey of 100 patients would achieve roughly a 10% margin of error with a 95% confidence level. Given the homogenous trends so far observed in postoperative opioid use across a number of study populations, even smaller sample sizes may still obtain a sufficient 80% power.20,21 The amount of medication that would cover the opioid needs of 80% of patients undergoing the chosen surgical procedures has been previously used as a benchmark.21 Other strategies have included defining evidence-based prescribing recommendations based on low, standard, and high opioid users, as well as using a process such as the Delphi method to develop expert consensus.43,66 Simply choosing the mean or median opioid use for a given procedure runs the risk of undertreating 50% of patients, particularly given the potential variation around point estimates.
Once the amount of medication is agreed upon, this should be translated into a number of pills depending on the type of medication, as potencies differ between medications. For example, 5/325 mg of hydrocodone/acetaminophen is equivalent to 5mg of oral morphine, whereas 5mg of oxycodone is equivalent to 7.5mg of oral morphine. Therefore, if the agreed-upon prescribing recommendation for the chosen procedure is 75mg OME, the equivalent prescription size would be 15 tablets of hydrocodone/acetaminophen 5/325mg or 10 tablets of oxycodone 5mg. This must also be balanced against ease of implementation. Recommending a single number of pills regardless of medication type—despite the relative difference in potency—may increase compliance by providers. Furthermore, opioid formulations that contain acetaminophen make it more difficult for patients to dose around-the-clock acetaminophen, so recommendations that favor non-acetaminophen-containing medications such as oxycodone may be preferable.
Developing prescribing guidelines should also be used as an opportunity to recommend a multimodal analgesic strategy after surgery, which is universally recommended.67 This evidence-based practice has been shown to decrease pain scores, improve patient satisfaction, and even improve outcomes after surgery compared to using opioids alone. Most medical record systems—electronic or otherwise—allow providers to add these over-the-counter medications to the patient’s postoperative medication list as a reminder to take these at scheduled times throughout the day.
Stakeholder Engagement
For many health systems, implementing evidence-based opioid prescribing practices will represent a significant change from the norm. Successful implementation of postoperative prescribing recommendations relies on familiarizing providers with this new practice. Critically, this effort must be aimed at all providers who will take part in the care of a patient undergoing the targeted procedure, from the first clinic visit to the final follow-up. Failure to focus on stakeholder engagement at all levels can lead to an experience whereby conflicting information is provided by surgeons, trainees, and other clinical staff members. For example, informing only prescribing providers of new changes could lead to significant confusion, inappropriate patient counseling, and even the belief that the prescription was entered in error if the postoperative nursing staff are not made aware that a new prescription size will be used which will likely be significantly smaller than those they have seen in the past.
Methods for disseminating this information can include departmental meetings, educational videos, email, website creation, and printed reference materials. In academic practice settings, regular meeting such as grand rounds or weekly educational conferences provide an appropriate forum to reach a large audience. Providing material online also offers a quick way for providers to reference new procedures. Educational brochures targeted at providers can also be developed and shared across institutions.68 There are also a number of process change strategies that can be used to maximize stakeholder involvement. Lean quality improvement methods, which are used at many health systems, suggest creating a value-stream map to identify stakeholders, or creating a stakeholder map to measure the reaction, needs, and effect on those involved in implementing a new care pathway.
Patient Education
Efforts by pharmaceutical companies and various professional societies in the 1990s have led to many patients holding the belief that surgery can be pain-free. Even today, older adults report that they are infrequently counseled on safe opioid use.69
There are several principles which should be communicated to patients, which we outline in Table 3. These principles can be communicated during pre- and postoperative clinic visits, throughout a patient’s hospital stay, and using engaging printed material.70 Patient education should focus on 3 critical elements: expectations for pain, risks and alternatives of opioid analgesics, and safe storage and disposal of opioids.
TABLE 3.
Patient Counseling Principles.
| Principle | Example |
|---|---|
| Set expectations | “Having some pain after surgery is normal. You will be sore for a few days, but this will gradually improve.” |
| Set norms | “We’ve found that most patients who undergo this procedure usually only need about 5 pills to control their pain.” |
| Non-opioids | “You’ll get the best pain control by taking over-the-counter pain medication such as acetaminophen and ibuprofen around the clock. You should take the stronger, prescription medication only for breakthrough pain.” |
| Appropriate use | “This prescription is only for pain related to your surgery. You should not use these pills to treat other pain like a headache or backache.” |
| Adverse effects | “It’s important to know that opioids can be addictive and harmful if used inappropriately, therefore we recommend taking only the minimum necessary amount.” |
| Safe disposal | “Most patients have leftover pills after surgery. These can be dangerous to others, like children, if left around the house. We recommend safely disposing of the pills when you no longer need them using the following resources: https://takebackday.dea.gov/” |
By informing patients that some pain is normal after surgery, and that the goal is adequate function as opposed to being pain free, patients are less likely to take as much medication or call for a refill.71 Setting expectations and norms can also be augmented by the data previously collected as part of the protocol. For example, if patientreportedoutcomesrevealthatmostpatientstakeXnumberofpillsafter a given procedure, providers can have a powerful impact on patient expectations by sharing, “We’ve found that most patients use X pills after this procedure.” Presenting information that helps set expectations has been shown to serve as an anchor for a patient’s own postoperative experience.72 To that end, patients can also be briefly introduced to the larger context of the intervention, that is, the ongoing changes and legislation surrounding postoperative opioids in the context of the opioid epidemic. Patients should also be counseled on the use of nonopioid, multimodal analgesia after surgery, including acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs). This should be emphasized as the primary method of pain control, with opioids being reserved for breakthrough pain.
Lastly, pre- and postoperative patient counseling provides a good opportunity to discuss appropriate use of opioids (ie, not taking them for unrelated conditions), risks of opioids, and how to safely dispose of opioids. This final piece of information may be particularly helpful, as most patients report not knowing how or where to dispose of leftover pills.1,35,36
Continual Assessment
A cycle of data analysis and practice change is central to a learning health system, and has been shown to lead to sustainable, patient-focused quality improvement.24 Prospective chart review of prescribing patterns should be conducted to assess the impact of opioid prescribing recommendations. This will demonstrate whether the new recommendations are being utilized and help identify areas for improvement. For example, chart review may identify that a given provider or hospital unit has not implemented new prescribing practices, giving project leaders an opportunity to identify barriers. This continual assessment is an important tenet of improving care delivery within a learning health system. The goal should be a cycle of continuous feedback that drives improvement on a regular basis.73
Continual data collection also helps ensure that new practices are beneficial to patients. It is necessary to continually assess the impact of prescribing changes on the patient experience. A survey instrument similar to the one used to collect preintervention data can be used to assess medication use, pain scores, and patient satisfaction after implementation of prescribing changes. This serves 2 major roles. First, it will allow providers to make sure that new prescribing practices have not resulted in decreased patient satisfaction, increased pain scores, or increased calls for medication refills. Second, it allows providers to adjust and improve postoperative prescribing practices based on evolving data. Smaller opioid prescriptions have been shown to be linked to decreased opioid consumption after surgery.3,30 Therefore, reducing opioid prescription size may lead to reduced opioid use by patients, allowing investigators to further tailor new prescribing recommendations based on this dynamic patient feedback. Again, in practice settings where continual assessment is not feasible, the aforementioned data sources such as CAHPS could be utilized to capture these patient data.
The above 7 elements of this protocol are outlined in Table 4.
TABLE 4.
Framework for an Evidence-based Postoperative Opioid-prescribing Pathway.
| Pathway Element | Description/Examples |
|---|---|
| Choose procedure or patient cohort to pilot initiative | Elective, low variability, uncomplicated patients |
| Collect data on prescribing practices | Retrospective chart review, Prescription Drug Monitoring Programs |
| Collect data on patient-reported opioid use | Prospective patient surveys (medication use, pain scores, satisfaction) |
| Generate evidence-based prescribing recommendations | Using patient-reported medication use, identify potential prescribing cut-points (such as by percentile treated) |
| Stakeholder education | Engaging all providers involved in patient care (pre-op, peri-op, post-op residents, faculty, nurses, PAs, NPs) |
| Patient and caregiver education | Counseling patients and caregivers regarding safe postoperative opioid use, pain control, disposal |
| Continual assessment and update of recommendations | Prospective chart review to analyze impact of recommendations, patient surveys to analyze medication use and pain scores |
EXAMPLES OF PATHWAY IMPLEMENTATION
As outlined in Table 1, this common pathway to collect data regarding opioid-prescribing practices and patient use has been described in several studies. The majority of studies collected data regarding opioid prescribing through medical record review, then collected patient-reported outcomes through a survey instrument. Three studies have specifically described the creation of opioid prescribing guidelines utilizing this pathway.20–22
Hill et al chose 5 common surgical procedures to target for intervention: partial mastectomy, partial mastectomy with sentinel lymphnodebiopsy,laparoscopiccholecystectomy,laparoscopicinguinal hernia repair, and open inguinal hernia repair.21 Using electronic medical record review, data were collected regarding prescription size and medicals refills for these procedures. Phone surveys were used to collect patient-reported opioiduse. Investigators demonstrated universal overprescribing of opioids for all procedures. Prescribing recommendations were then developed based on the 80th percentile of patient-reported opioid use. The prescribing recommendations, in an equivalent dose of oxycodone 5mg, were: 5 pills for partial mastectomy, 10 pills for partial mastectomy with sentinel lymph node biopsy, and 15 pills for laparoscopic cholecystectomy, laparoscopic inguinal hernia repair, and open inguinal hernia repair.
These data regarding overprescribing and the guidelines were then shared at surgical grand rounds, departmental meetings, a resident forum, and by email. Prescribers were also instructed to encourage patients to use acetaminophen and NSAIDs as the primary form of pain control. Following implementation of these recommendations, prescription size decreased by 43% to 74% for these 5 procedures.21 Only 1 patient required a medication refill.
At our own institution, we implemented this pathway initially for elective laparoscopic cholecystectomy, a very common surgical procedure.20 Retrospective chart review identified postoperative prescribing patterns and phone surveys established postoperative use data. Opioid use at the 80th percentile was 75 OMEs. This is equivalent to 15 tablets of hydrocodone/acetaminophen 5/325mg or 10 tablets of oxycodone 5mg. For ease of implementation, we recommended 15 tablets of either medication. These data and recommendations were presented to faculty, residents, and staff at grand rounds, departmental meetings, email, and through an easily accessible website.74 Our standard preoperative patient education materials were also changed to encourage nonopioid analgesic use and address the risks of opioids. After publicizing these recommendations, we immediately began conducting prospective chart review and patient surveys to track prescription sizes and assess the impact of these guidelines. Postoperative prescribing fell by 63% without any change in requests for refills or patient-reported pain score.20
Lee et al22 described application of this pathway in five common surgical oncology procedures. Here, the investigators utilized the previously published recommendations from Hill et al, which eliminated the time-consuming process of surveying patients regarding postoperative opioid use. The recommendations, in tablets of oxycodone 5mg, were 20 tablets for melanoma wide local excision with or without sentinel lymph node biopsy (SLNB), 20 tablets for simple mastectomy with or without SLNB, 10 tablets for SLNB, 10 tablets for lumpectomy with SLNB, and 5 tablets for breast biopsy or lumpectomy without SLNB. Prescriber education was accomplished through a written protocol and mandatory educational conferences, and patients received standardized instructions regarding pain control. Again, continuous data collection after implementing the recommendations demonstrated reductions of 37% to 42% in prescription size without an increase in requests for refills.
IMPLICATIONS AND ADAPTABILITY
A pathway that utilizes patient-reported outcomes and physician-led practice change can result in significantly decreased postoperative opioid prescriptions without increasing pain or need for refills after surgery. In the studies references above, new prescribing practices were rapidly adopted by surgeons following brief educational interventions. This represents a powerful strategy by which to quickly and effectively mitigate excess opioid exposure among patients and communities without decreasing patient satisfaction. Although the studies identified in this review may not provide the quality of evidence available from a randomized controlled trial, they produce real results to address an urgent clinical problem.
An important element of this pathway is that it allows for development of improved prescribing in any practice setting. By collecting patient data and developing practice change based on those results, these changes can be implemented to serve the unique population in a given institution or community. Different patient populations may have different analgesic requirements following surgery and collecting patient-reported opioid use enables new prescribing practices to match this. Interestingly, however, the similarity in prescribing recommendations and outcomes at both of these institutions may suggest similar, procedure-specific pain needs. This would further lend to the generalizability of this type of prescribing recommendation.
This pathway can also be implemented at any scale of surgical practice and for a variety of surgical procedures. A small, individual practice that may not have the resources to conduct phone surveys could collect data regarding opioid consumption at in person followup visits, then adjust prescribing accordingly. Individual practices that may lack additional resources could further utilize prescribing data generated from a similar population, region, or practice, and again tailor the results to match their practice. In Michigan, for example, many community hospitals now use prescribing guidelines developed through data collection from large health systems around the state. This pathway can also be significantly upscaled to enable substantial practice change on a large scale. Again, in Michigan, patient-reported opioid consumption is now collected across the entire state through a large surgical collaborative.3 Prescribing recommendations are then developed based on more robust data and disseminated not just to one health system, but to a collaborative of >70 hospitals across the state. This has resulted in patient-focused prescribing recommendations being developed for roughly 25 common operations.75
This effort fulfills the definition of quality improvement in a learning health system, wherein data generated through routine practice are used to develop new knowledge that can then be integrated into practice.73 As a result, the measures put into place improve care quality and patient safety while being tailored to a specific patient population. Continual assessment ensures that these measures benefit patients. For example, it would be a great disservice to patients if implementing a protocol designed to improve surgical quality and safety negatively affected patients’ pain and satisfaction after surgery. What’s more, by driving practice change with institutional data and stakeholder feedback, these changes reflect the goals and motivations of all the providers involved, rather than being mandated by a small group. This framework has been shown to lead to quality improvement that is both sustainable and grounded in a sense of purpose among those involved.76 This is an inherently collaborative approach which relies on inclusion of providers at every level of care delivery, again increasing ownership of practice change among providers. Quality improvement initiatives that fail to effectively involve all levels of care—and not just leadership—have been shown to have only limited impact in clinical operations.
Although this pathway represents an important and practical step in combating the ongoing opioid epidemic, it is not without limitations. First, the prescribing recommendations developed through this pathway are based on patient-reported opioid use only. They do not account for other patient-specific factors such as presence of comorbidities, chronic pain conditions, preoperative opioid use, or complications. These factors should also be taken into consideration when providing a postoperative prescription to a patient to achieve maximal pain control. It is possible that future prescribing recommendations could be developed using a combination of these factors in a predictive model to allow for prescriptions that are tailored to individual patients. Additionally, to date this pathway has only been implemented at a limited number of institutions and may not have the same success at other health systems. However, by following a learning health system model, this pathway is designed to be adjustable depending on each institution’s unique resources and needs. Lastly, appropriate prescribing is only one part of a larger effort to address the opioid epidemic. In addition to this effort, other work continues to be critical, such as public awareness campaigns, opioid take-back drives, and access to addiction treatment.
CONCLUSION
A pathway that utilizes patient-reported outcomes enables physicians to dramatically reduce excessive opioid prescribing without negatively impacting patients’ experience after surgery. Importantly, this approach is generalizable to various settings, practical to implement, and leads to practice change that is patient-focused. Our own experience upscaling this pathway to a population level has already shown promising results. We encourage providers to utilize these principles and make them their own.
Acknowledgments
C.B., M.E., J.W. receive funding from the Michigan Department of Health and Human Services and the National Institute on Drug Abuse (RO1 DA042859). R.H. receives funding from the Blue Cross Blue Shield of Michigan Foundation. J.V. receives funding from the National Institutes of Health Ruth L. Kirschstein National Research Service Award (1F32DK115340–01A1). J.L. receives funding from the National Cancer Institute (5T32 CA009672–23). The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Michigan Department of Health and Human Services.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.Bicket MC, Long JJ, Pronovost PJ, et al. Prescription opioid analgesics commonly unused after surgery: a systematic review. JAMA Surg. 2017;152:1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill MV, McMahon ML, Stucke RS, et al. Wide variation and excessive dosage of opioid prescriptions for common general surgical procedures. Ann Surg. 2017;265:709–714. [DOI] [PubMed] [Google Scholar]
- 3.Howard R, Fry B, Gunaseelan V, et al. Association of opioid prescribing with opioid consumption after surgery in Michigan. JAMA Surg. 2018;e184234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.As-Sanie S, Till SR, Mowers EL, et al. Opioid prescribing patterns, patient use, and postoperative pain after hysterectomy for benign indications. Obstet Gynecol. 2017;130:1261–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waljee JF, Zhong L, Hou H, et al. The use of opioid analgesics following common upper extremity surgical procedures: a national, population-based study. Plast Reconstr Surg. 2016;137:355e–364e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thiels CA, Anderson SS, Ubl DS, et al. Wide variation and overprescription of opioids after elective surgery. Ann Surg. 2017;266:564–573. [DOI] [PubMed] [Google Scholar]
- 7.Rodgers J, Cunningham K, Fitzgerald K, et al. Opioid consumption following outpatient upper extremity surgery. J Hand Surg Am. 2012;37:645–650. [DOI] [PubMed] [Google Scholar]
- 8.Center for Behavioral Health Statistics and Quality. (2017). 2016 National Survey on Drug Use and Health: Detailed Tables. Substance Abuse and Mental Health Services Administration R, MD. [Google Scholar]
- 9.Gomes T, Mamdani MM, Dhalla IA, et al. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med. 2011;171:686–691. [DOI] [PubMed] [Google Scholar]
- 10.Cicero TJ, Ellis MS, Harney J. Shifting patterns of prescription opioid and hero in abuse in the United States. N Engl J Med. 2015;373:1789–1790. [DOI] [PubMed] [Google Scholar]
- 11.Cicero TJ, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821–826. [DOI] [PubMed] [Google Scholar]
- 12.Brummett CM, Waljee JF, Goesling J, et al. New persistent opioid use after minor and major surgical procedures in US adults. JAMA Surg. 2017;152:e170504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine (Phila Pa 1976). 2007;32:2127–2132. [DOI] [PubMed] [Google Scholar]
- 14.Waljee JF, Li L, Brummett CM, et al. Iatrogenic opioid dependence in the United States: are surgeons the gatekeepers? Ann Surg. 2017;265:728–730. [DOI] [PubMed] [Google Scholar]
- 15.Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naive women. Am J Obstet Gynecol. 2016;215 353 e351–353 e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meara E, Horwitz JR, Powell W, et al. State legal restrictions and prescription-opioid use among disabled adults. N Engl J Med. 2016;375:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kertesz SG, Gordon AJ. A crisis of opioids and the limits of prescription control: United States. Addiction. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Lin HC, Wang Z, Boyd C, et al. Associations between statewide prescription drug monitoring program (PDMP) requirement and physician patterns of prescribing opioid analgesics for patients with non-cancer chronic pain. Addict Behav. 2018;76:348–354. [DOI] [PubMed] [Google Scholar]
- 19.Habbouche J, Lee J, Steiger R, et al. Association of hydrocodone schedule change with opioid prescriptions following surgery. JAMA Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard R, Waljee J, Brummett C, et al. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2018;153:285–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MV, Stucke RS, McMahon ML, et al. An educational intervention decreases opioid prescribing after general surgical operations. Ann Surg. 2018;267:468–472. [DOI] [PubMed] [Google Scholar]
- 22.Lee JS, Howard RA, Klueh MP, et al. The impact of education and prescribing guidelines on opioid prescribing for breast and melanoma procedures. Ann Surg Oncol. 2019;26:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saini S, McDonald EL, Shakked R, et al. Prospective evaluation of utilization patterns and prescribing guidelines of opioid consumption following orthopedic foot and ankle surgery. Foot Ankle Int. 2018;39:1257–1265. [DOI] [PubMed] [Google Scholar]
- 24.Learning Health Systems. Content last reviewed November 2017. Agency for Healthcare Research and Quality, Rockville, MD: Available at: http://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. [Google Scholar]
- 25.Committee on Military Trauma Care’s Learning Health System and Its Translation to the Civilian Sector; Board on Health Sciences Policy; Board on the Health of Select Populations; Health and Medicine Division; National Academies of Sciences, Engineering, and Medicine, Berwick D, Downey A, Cornett E. A National Trauma Care System: integrating military and civilian trauma systems to achieve zero preventable deaths after injury. Mil Med. 2017;182:1563–1565.29087893 [Google Scholar]
- 26.Hashmi ZG, Haut ER, Efron DT, et al. A target to achieve zero preventable trauma deaths through quality improvement. JAMA Surg. 2018;153:686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scoap Collaborative WGftSC, Kwon S, Florence M, Grigas P, et al. Creating a learning healthcare system in surgery: Washington State’s Surgical Care and OutcomesAssessmentProgram(SCOAP)at5years.Surgery.2012;151:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grumbach K, Lucey CR, Johnston SC. Transforming from centers of learning to learning health systems: the challenge for academic health centers. JAMA. 2014;311:1109–1110. [DOI] [PubMed] [Google Scholar]
- 29.Duncan RW, Smith KL, Maguire M, et al. Alternatives to opioids for pain management in the emergency department decreases opioid usage and maintains patient satisfaction. Am J Emerg Med. 2019;37:38–44. [DOI] [PubMed] [Google Scholar]
- 30.Bateman BT, Cole NM, Maeda A, et al. Patterns of opioid prescription and use after cesarean delivery. Obstet Gynecol. 2017;130:29–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanson-Fisher RW, Bonevski B, Green LW, et al. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am J Prev Med. 2007;33:155–161. [DOI] [PubMed] [Google Scholar]
- 32.Savulescu J, Wartolowska K, Carr A. Randomised placebo-controlled trials of surgery: ethical analysis and guidelines. J Med Ethics. 2016;42:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Etheredge LM. A rapid-learning health system. Health Aff (Millwood). 2007;26:w107–w118. [DOI] [PubMed] [Google Scholar]
- 34.Taylor MJ, McNicholas C, Nicolay C, et al. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bates C, Laciak R, Southwick A, et al. Overprescription of post operative narcotics: a look at postoperative pain medication delivery, consumption and disposal in urological practice. J Urol. 2011;185:551–555. [DOI] [PubMed] [Google Scholar]
- 36.Harris K, Curtis J, Larsen B, et al. Opioid pain medication use after dermatologic surgery: a prospective observational study of 212 dermatologic surgery patients. JAMA Dermatol. 2013;149:317–321. [DOI] [PubMed] [Google Scholar]
- 37.Swenson CW, Kelley AS, Fenner DE, et al. Outpatient narcotic use after minimally invasive urogynecologic surgery. Female Pelvic Med Reconstr Surg. 2016;22:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maughan BC, Hersh EV, Shofer FS, et al. Unused opioid analgesics and drug disposal following outpatient dental surgery: a randomized controlled trial. Drug Alcohol Depend. 2016;168:328–334. [DOI] [PubMed] [Google Scholar]
- 39.Kim N, Matzon JL, Abboudi J, et al. A prospective evaluation of opioid utilization after upper-extremity surgical procedures: identifying consumption patterns and determining prescribing guidelines. J Bone Joint Surg Am. 2016;98:e89. [DOI] [PubMed] [Google Scholar]
- 40.Bartels K, Mayes LM, Dingmann C, et al. Opioid use and storage patterns by patients after hospital discharge following surgery. PLoS One. 2016;11:e0147972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Osmundson SS, Schornack LA, Grasch JL, et al. Postdischarge opioid use after cesarean delivery. Obstet Gynecol. 2017;130:36–41. [DOI] [PubMed] [Google Scholar]
- 42.Howard R, Waljee J, Brummett C, et al. Reduction in opioid prescribing through evidence-based prescribing guidelines. JAMA Surg. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thiels CA, Ubl DS, Yost KJ, et al. Results of a prospective, multicenter initiative aimed at developing opioid-prescribing guidelines after surgery. Ann Surg. 2018;268:457–468. [DOI] [PubMed] [Google Scholar]
- 44.Swarup A, Mathis KA, Hill MV, et al. Patterns of opioid use and prescribing for outpatient anorectal operations. J Surg Res. 2018;229:283–287. [DOI] [PubMed] [Google Scholar]
- 45.Wojahn RD, Bogunovic L, Brophy RH, et al. Opioid consumption after knees arthroscopy. J Bone Joint Surg Am. 2018;100:1629–1636. [DOI] [PubMed] [Google Scholar]
- 46.Hart AM, Broecker JS, Kao L, et al. Opioid use following outpatient breast surgery: are physicians part of the problem? Plast Reconstr Surg. 2018;142:611–620. [DOI] [PubMed] [Google Scholar]
- 47.Sabatino MJ, Kunkel ST, Ramkumar DB, et al. Excess opioid medication and variation in prescribing patterns following common orthopaedic procedures. J Bone Joint Surg Am. 2018;100:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunningham D, Lewis B, Hutyra C, et al. Prospective, observational study of opioid use after hip arthroscopy for femoroacetabular impingement syndrome. Arthroscopy. 2018;34 1488–1497 e1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Griffith KC, Clark NV, Zuckerman AL, et al. Opioid prescription and patient use after gynecologic procedures: a survey of patients and providers. J Minim Invasive Gynecol. 2018;25:684–688. [DOI] [PubMed] [Google Scholar]
- 50.Peters B, Izadpanah A, Islur A. Analgesic consumption following outpatient carpal tunnel release. J Hand Surg Am. 2018;43 189 e181–189 e185. [DOI] [PubMed] [Google Scholar]
- 51.Hota LS, Warda HA, Haviland MJ, et al. Opioid use following gynecologic and pelvic reconstructive surgery. Int Urogynecol J. 2018;29:1441–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujii MH, Hodges AC, Russell RL, et al. Post-discharge opioid prescribing and use after common surgical procedure. J Am Coll Surg. 2018;226:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bartels K, Mahoney K, Raymond KM, et al. Opioid and non-opioid utilization at home following gastrointestinal procedures: a prospective cohort study. Surg Endosc. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman DT, Ghiassi S, Hubbard MO, et al. Postoperative opioid prescribing practices and evidence-based guidelines in bariatric surgery. Obes Surg. 2019;29:2030–2036. [DOI] [PubMed] [Google Scholar]
- 55.Rose KR, Christie BM, Block LM, et al. Opioid prescribing and consumption patterns following outpatient plastic surgery procedures. Plast Reconstr Surg. 2019;143:929–938. [DOI] [PubMed] [Google Scholar]
- 56.Locketz GD, Brant JD, Adappa ND, et al. Postoperative opioid use in sinonasal surgery. Otolaryngol Head Neck Surg. 2019;160:402–408. [DOI] [PubMed] [Google Scholar]
- 57.Hozack BA, Abboudi J, Gallant G, et al. Prospective evaluation of opioid consumption following cubital tunnel decompression surgery. Hand (N Y). 2019;14:42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bicket MC, White E, Pronovost PJ, et al. Opioid oversupply after joint and spine surgery: a prospective cohort study. Anesth Analg. 2019;128:358–364. [DOI] [PubMed] [Google Scholar]
- 59.Curtis JR, Cook DJ, Wall RJ, et al. Intensive care unit quality improvement: a “how-to” guide for the interdisciplinary team. Crit Care Med. 2006;34:211–218. [DOI] [PubMed] [Google Scholar]
- 60.Wetzel M, Hockenberry J, Raval MV. Interventions for postsurgical opioid prescribing: a systematic review. JAMA Surg. 2018;153:948–954. [DOI] [PubMed] [Google Scholar]
- 61.Lee JS, Parashar V, Miller JB, et al. Opioid prescribing after curative-intents urgery: a qualitative study using the theoretical domains framework. Ann Surg Oncol. 2018;25:1843–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gammaitoni AR, Fine P, Alvarez N, et al. Clinical application of opioide quianalgesic data. Clin J Pain. 2003;19:286–297. [DOI] [PubMed] [Google Scholar]
- 63.Frost MH, Reeve BB, Liepa AM, et al. , Mayo FDAP-ROCMG. What is sufficient evidence for the reliability and validity of patient-reported outcome measures? Value Health. 2007;10(suppl 2):S94–S105. [DOI] [PubMed] [Google Scholar]
- 64.Coughlin SS. Recall bias in epidemiologic studies. J Clin Epidemiol. 1990;43:87–91. [DOI] [PubMed] [Google Scholar]
- 65.Harbaugh C, Malani P, Singer D, Kirch M, Solway E, Clark S, Waljee J. Older Adults’ Experiences with Opioid Prescriptions, Institute for Healthcare Policy an Innovation, University of Michigan and Innovation; July 2018. Available at: https://www.healthyagingpoll.org/sites/default/files/2018-10/NPHA-OpioidsReport_072518.pdf. [Google Scholar]
- 66.Overton HN, Hanna MN, Bruhn WE, et al. Opioid-prescribing guidelines for common surgical procedures: an expert panel consensus. J Am Coll Surg. 2018;227:411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152:691–697. [DOI] [PubMed] [Google Scholar]
- 68.4 evidence-based reasons for changing the way you prescribe opioids. MOPEN. Available at http://michigan-open.org/wp-content/uploads/2018/03/M-OPEN-SurgeonBrochure-FINAL-nobleed-032118.pdf.
- 69.Harbaugh C, Malani P, Solway E, et al. University of Michigan National Poll on Healthy Aging. Jul-Aug 2018. Available at: http://hdl.handle.net/2027.42/145684. [Google Scholar]
- 70.Michigan Opioid Prescribing and Engagement Network. Patient Resources. Available at: http://michigan-open.org/patient-resources/. Accessed December 1.
- 71.Gawande AA. It’s time to adopt electronic prescriptions for opioids. Ann Surg. 2017;265:693–694. [DOI] [PubMed] [Google Scholar]
- 72.Oussedik E, Cardwell LA, Patel NU, et al. An anchoring-based intervention to increase patient willingness to use injectable medication in psoriasis. JAMA Dermatol. 2017;153:932–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Learning Health Systems. Content last reviewed November 2017. Agency for Healthcare Research and Quality R, MD; Available at: http://www.ahrq.gov/professionals/systems/learning-health-systems/index.html. [Google Scholar]
- 74.Michigan Opioid Prescribing and Engagement Network. Opioid Prescribing Recommendations for Surgery. Available at: https://opioidprescribing.info. Published October 2017. Accessed April 7, 2019.
- 75.Opioid Prescribing Recommendations For Surgery. Opioid Prescribing Recommendations for Surgery. 2018. Web. December 7, 2018. [Google Scholar]
- 76.Schouten LM, Hulscher ME, van Everdingen JJ, et al. Evidence for the impact of quality improvement collaboratives: systematicreview.BMJ.2008;336:1491–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]


