Abstract
Bat-borne viral diseases are a major public health concern among newly emerging infectious diseases which includes severe acute respiratory syndrome, Nipah, Marburg and Ebola virus disease. During the survey for Nipah virus among bats at North-East region of India; Tioman virus (TioV), a new member of the Paramyxoviridae family was isolated from tissues of Pteropus giganteus bats for the first time in India. This isolate was identified and confirmed by RT-PCR, sequence analysis and electron microscopy. A range of vertebrate cell lines were shown to be susceptible to Tioman virus. Negative electron microscopy study revealed the “herringbone” morphology of the nucleocapsid filaments and enveloped particles with distinct envelope projections a characteristic of the Paramyxoviridae family. Sequence analysis of Nucleocapsid gene of TioV demonstrated sequence identity of 99.87% and 99.99% nucleotide and amino acid respectively with of TioV strain isolated in Malaysia, 2001. This report demonstrates the first isolation of Tioman virus from a region where Nipah virus activity has been noticed in the past and recent years. Bat-borne viruses have become serious concern world-wide. A Survey of bats for novel viruses in this region would help in recognizing emerging viruses and combating diseases caused by them.
Keywords: Tioman virus, Pteropus, Bat, Paramyxovirus, Nucleocapsid gene, PCR
Highlights
-
•
First isolation of Tioman virus from Pteropus bat in North East region of India.
-
•
Isolate was identified by RT-PCR, sequencing and electron microscopy.
-
•
TioV isolate showed highest sequence identity with Malaysia strain of TioV.
-
•
Bat-borne viruses are concern for human and animal health.
-
•
Survey of bats for novel viruses is needed to recognize emerging viruses.
1. Introduction
Bat-borne viruses are considered to be important emerging viruses, as they can pose a serious threat to human and animal health. Henipaviruses, coronaviruses, filoviruses and rabies-causing lyssaviruses are all transmissible from bats to humans. Bats are primary reservoir host and often the resulting human disease is fatal. They are known to harbor more zoonotic viruses per species than rodents and recognized as a significant source of zoonotic agents (Newman et al., 2011, Calisher et al., 2006, Mackenzie et al., 2003, Pavri et al., 1971, Mourya et al., 2014; Raut et al., 2012; Wynne and Wang, 2013).
Old World fruit bats of the family Pteropodidae, particularly species belonging to the genus Pteropus, have been considered as natural hosts for a large number of emerging viruses, especially of the family Paramyxoviridae (Calisher et al., 2006). Due to special characteristics, Pteropus bats are the perfect reservoir for most of the recently emerging zoonotic pathogens. They often live in large colonies or roosts and travel long distances; thus they are very effective in transmitting viruses among colony members and disseminating them over a considerable distance. Interactions among bats, humans and livestock are constantly increasing due to anthropogenic activities, thereby increasing the potential for transmission of viruses. Deforestation in tropical areas destroyed the natural habitats of these fruit bat species thus forcing them to live in the vicinity of human settlements. The resulting close contact is responsible for the emergence of highly pathogenic paramyxoviruses, like Hendra and Nipah virus (NiV) in human populations in Southeast Asia and Australia (Mackenzie et al., 2003).
Paramyxoviridae is a family of viruses that comprises important pathogens like Nipah virus, Measles virus, Human parainfluenza virus type 3 and Human respiratory syncytial virus (Aguilar and Lee, 2011). While investigating NiV in urine samples of giant fruit bats of the Pteropus genus on Tioman Island, Malaysia, in 2001, researchers isolated a novel virus which was placed in the Rubulavirus genus of the Paramyxoviridae family. The virus was named as Tioman virus (TioV) after the place of isolation from Malaysia (Chua et al., 2001).
In this communication, we report the isolation and confirmation of a Tioman virus isolated from Pteropus species of bats from North-East region of India.
2. Materials and methods
2.1. Ethics statement
The Scientific Advisory Committee, Institutional Biosafety Committee, and Institutional Animal Ethical Committee of National Institute of Virology, Pune, India has approved this study. The Institute follows the guideline of Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, New Delhi. Prior permission for capturing bat was obtained from the Chief Conservators of Forests of West Bengal and Assam State.
2.2. Study area
To determine the presence of NiV in Pteropus bats, a survey was conducted in two states of North-East region, India i.e. West Bengal and Assam states that share boundaries with Bangladesh. The criteria for selection of the study areas were based on earlier reports of a NiV seropositive bat from the Myanaguri area in West Bengal and confirmed human cases from Siliguri and Nadia districts of West Bengal and roosting areas of Pteropus bats (Yadav et al., 2012, Chadha et al., 2006).
2.3. Bat capturing and sample processing in the field
Sixty-eight Pteropus bats were collected from Jalpaiguri (n = 8) and Cooch Behar (n = 39) districts of West Bengal and Dhubri district (n = 21) of Assam on two occasions from March to May 2015. Mist nets were used to capture the bats. After capturing the bats, species identification and morphometry was done. Further, the bats were euthanized and necropsies were performed in the field following proper biosafety measures. Blood, organs (kidney, liver, and spleen), throat swabs, rectal swabs and urine samples were collected from bats. Waste disposal was done following guidelines and proper precautionary measures. Organ specimens were frozen in liquid nitrogen immediately after necropsy, while blood samples were kept at room temperature for 30 min and Centrifuged for 10 min at approximately 1000 g. Separated serum was aliquoted into labeled cryovials. Vials of serum were transported at + 4 °C in Styrofoam box to National Institute of Virology (NIV), Pune, for further investigation.
2.4. Virus isolation from bat tissue specimens
Liver/spleen and kidney tissues of 68 bats were homogenized in sterile Minimum Essential Medium (MEM; Gibco) using a homogenizer (GenoGrinder 2000; BT&C Inc., Lebanon, NJ, USA). Further, tissue homogenates were centrifuged at 5000 rpm for 10 min, and 0.1 mL of the supernatants was inoculated on to monolayers of Vero CCL-81 cells grown in 24-well cell culture plates after removing the growth medium. The cells were incubated for 1 h at 37 °C to allow virus adsorption, with rocking every 10 min for uniform virus distribution. After the incubation, the inoculum was removed and the cells were washed with 1 × Phosphate buffer saline (PBS). Finally MEM supplemented with 2% Fetal bovine serum (FBS) was added to each well. The cultures were incubated further in 5% CO2 incubator at 37 °C and observed daily for cytopathic effects (CPE) under an inverted microscope. Cultures that showed CPE were harvested and the suspension was centrifuged at 5000 rpm for 30 min at 4 °C; the supernatants were processed immediately or stored at − 86 °C in 1 mL aliquots. Viral RNA was extracted using Tripure reagent and RNA extraction kit (Qiagen, Valencia, CA, USA) as per the manufacturer's instruction. Virus isolations were also attempted with other specimens of bats (throat and rectal swabs, urine), following the same protocol (Chua et al., 2002).
2.5. Electron microscopy
Cell culture supernatants and pellets of Vero CCL-81 infected cells showing distinct CPE were examined by negative-stain transmission electron microscopy (TEM) as described previously (Brenner and Horne, 1959, Gangodkar et al., 2010).
2.6. Confirmation of novel virus isolate by RT-PCR and sequencing
To identify the virus isolate, various diagnostic tests were undertaken targeting NiV and genus Paramyxovirus using specific primers by RT-PCR (Guillaume et al., 2004, Tong et al., 2008). Further, the isolates were screened by RT-PCR using primers targeting the nucleocapsid gene and phosphoprotein gene of paramyxoviruses, as described earlier (Chua et al., 2001). Amplified products were further sequenced targeting nucleocapsid and phosphoprotein gene. The sequences obtained by sequencing were curated using Sequencher 5.0 (Gene Codes Corporation, Ann Arbor, MI, USA) version software. The curated sequences were aligned using Clustal W (EMBL-EBI, Cambridgeshire, UK), and a phylogenetic tree was constructed using the neighbor-joining algorithm (Kimura 2-parameter model) with 500-bootstrap replicates as implemented by Mega v 6.0 software (Tamura et al., 2007).
2.7. Susceptibility of different vertebrate cell lines to TioV
In order to study susceptibility of different vertebrate cells to TioV, the infectious virus titer was determined by estimating 50% tissue culture infective dose (TCID50) using Reed and Muench method (Reed and Muench, 1938). Four vertebrate cell lines Vero E-6 cells, Pipistrellus ceylonicus Bat Embryo cells, Baby Hamster Kidney-21 (BHK-21) and Madine Darbey Canine Kidney (MDCK) cells were used for virus infection. The 70–80% confluent monolayer of the cells was infected with 10 multiplicity of infection (m.o.i) virus and observed for seven post-infection days. The methodology of cell infection by the virus was similar as mentioned above in Section 2.4. Two passages of the virus were made in all cell lines in order to confirm the susceptibility of the cells. All the cells were studied for susceptibility based on CPE and further confirmed using Real-Time RT-PCR.
2.8. Studies on Tioman virus growth in embryonated chicken eggs
To explore the possibility of propagation in embryonated chicken eggs, 0.2 mL of Vero CCL-81 grown TioV was inoculated in the allantoic cavities of 10-day-old embryonated White-leghorn chicken eggs. The eggs were incubated at 37 °C for 5 days and were observed for sluggishness and mortality after every 24 h. Allantoic fluids from infected eggs were harvested after 5 days of incubation and stored at − 86 °C. First blind passage was performed and allantoic fluid was tested for Tioman virus by Real-time RT-PCR.
3. Results
3.1. Isolation of virus from a Pteropus giganteus bat
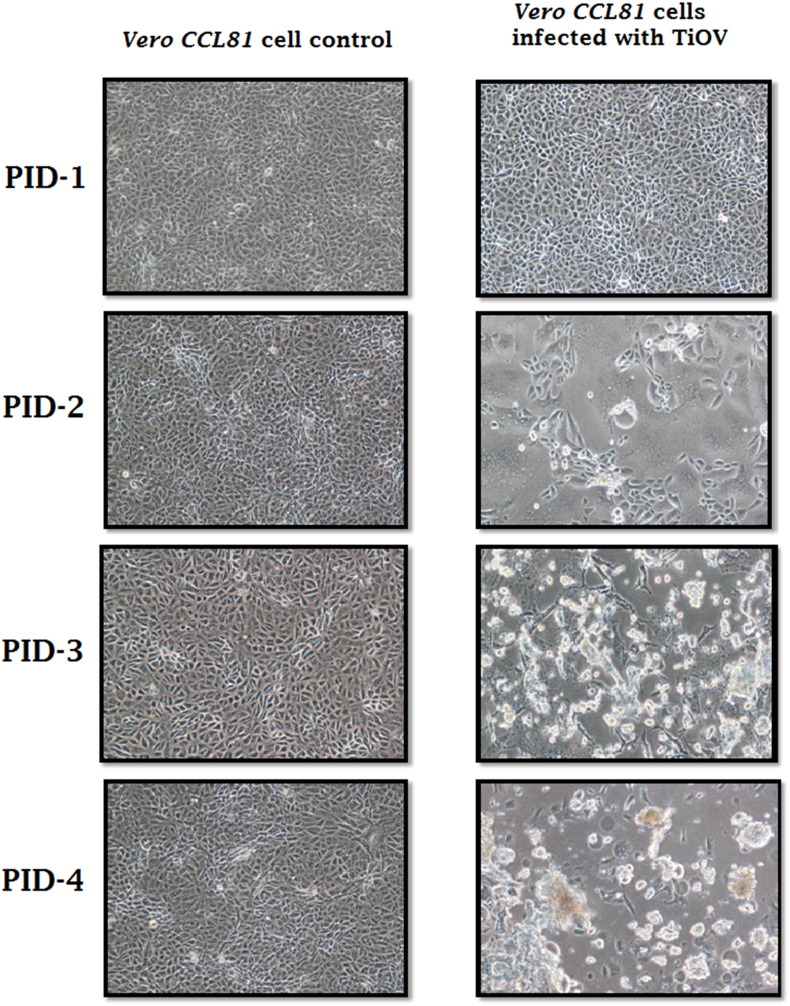
Cytopathic effect was (CPE) observed in Vero CCL-81cells inoculated with a kidney tissue homogenate of P. giganteus bat (NIVAN159672). The characteristics of CPE included cell fusion and formation of syncytium with aggregation of the nucleolus. CPE was prominent on post-infection-day 3 and cell detachment was observed on days post-infection-4 (DPI) (Isolation dated 21st April 2015) (Fig. 1 ). The supernatant was tested by PCR, sequencing and electron microscopy for identification of the suspected virus isolate.
Fig. 1.
Tioman virus propagation and cytopathic effects in Vero CCL-81 cells at different post infection days (PID).
3.2. Electron microscopy
Negative contrast electron microscopy of the cell supernatant of Vero CCL-81 infected with virus isolate showed the presence of virus particles with the typical paramyxovirus morphology. The “herringbone” morphology of the nucleocapsid filaments, a characteristic of the Paramyxoviridae family, was clearly visible (Fig. 2 ). Distinct enveloped paramyxovirus particles with envelope projections of approximately 9 nm in length were also visualized.
Fig. 2.
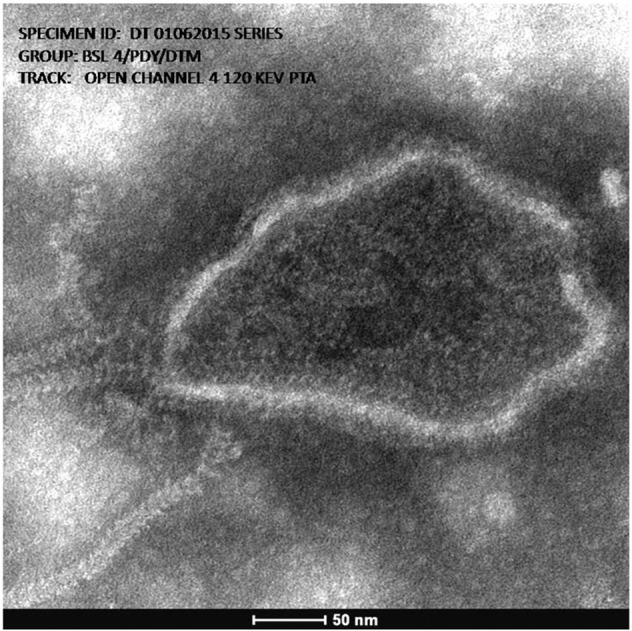
Representative transmission electron micrograph of Tioman virus showing typical Paramyxoviridae morphology. The classic “herringbone” (HB) structures and envelope projections can be seen.
3.3. Characterization of the TioV isolate by RT-PCR and sequencing
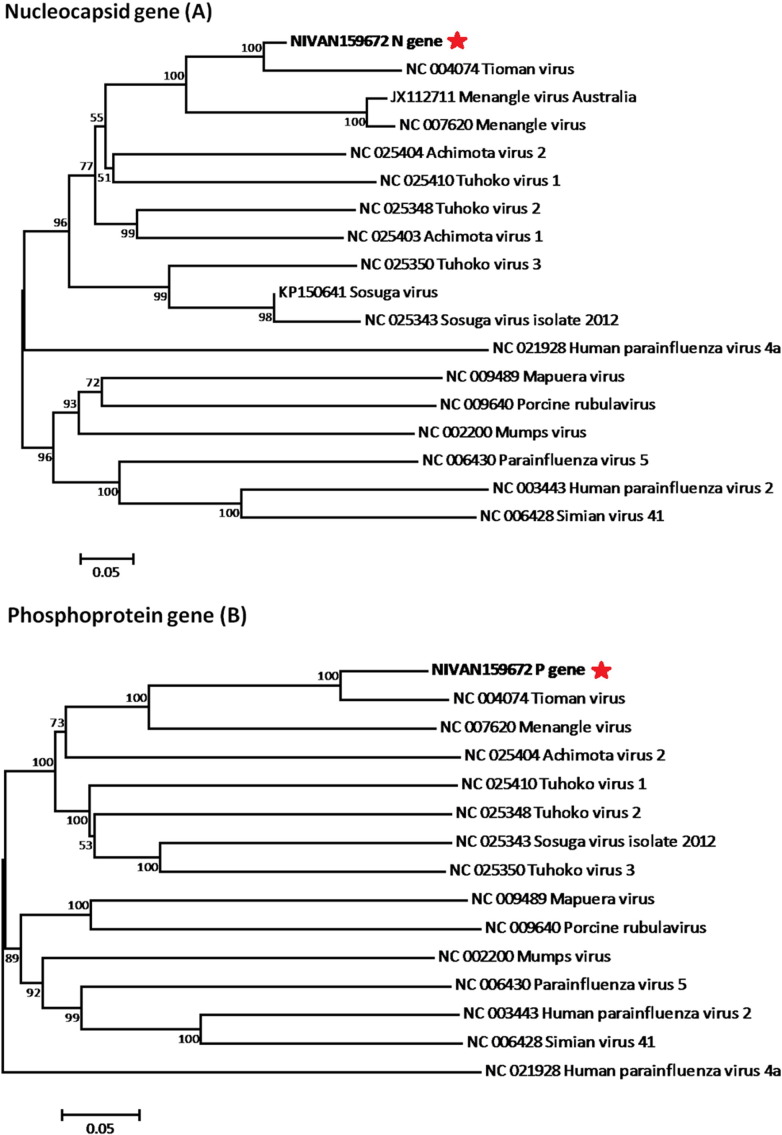
Out of 68 bat sample processed, kidney samples from two bats (NIVAN159672, NIVAN151165) were found to be positive by RT-PCR. PCR products of 473 bp were observed for nucleocapsid gene of TioV. Amplified products were further confirmed by sequencing partial Nucleocapsid and Phosphoprotein genes of TioV (gene bank accession no. KT601212, KT601213, KT601214. KT601215). Sequence analysis showed 99.87% nucleotide sequence identity with both Nucleoprotein gene and Phosphoprotein gene sequences of the Malaysian TioV isolate respectively (Bat/2002/Genbank AF298895) (Fig. 3 ). Partial sequences of TioV phosphoprotein and nucleocapsid gene revealed that TioV strains from India and Malaysia are from one lineage, it also makes up a clade with Menagle virus while Tuhoko, Achimota and Sosuga viruses make up a separate clade.
Fig. 3.
Phylogenetic tree of Tioman virus from Pteropus giganteus bat from India and other reported TiV and paramyxoviruses from GenBank using Neighbor joining method with 1000 bootstrap replicates for nucleocapsid gene (A); and phosphoprotein gene (B).
3.4. Susceptibility of vertebrate cells to TioV
TioV isolated from kidney tissue homogenate of bat showed a titer of 104.61/100 μL by TCID50 in Vero CCL-81 cell line. CPE-based susceptibility studies showed that all the studied vertebrate cell lines were susceptible to TioV with varying productivity. CPE in Vero CCL-81 cell line became evident by 2nd DPI and there was total degeneration of cells by 4th DPI. Vero E-6 cells, Pipistrellus ceylonicus bat Embryo cells and BHK-21 cell line showed CPE by 5th DPI. PS (Porcine stable cell line) cells did not show CPE in first passage; however it showed distinct CPE at 7th DPI in the second passage. MDCK cells showed growth of TioV with rounding and detachment of cells within 72h post-infection. However, susceptibility study by CPE showed that TioV grows faster in Vero CCL-81 cells in comparison with other vertebrate cells. The study of susceptibility of different vertebrate cells to TioV indicated that Vero CCL-81 cell lines are best suited for propagation of TioV. This may be useful for viral replication studies in future.
3.5. Susceptibility of embryonated eggs to TioV
Embryonated eggs did not show any sluggishness or mortality in the initial passage and first blind passage. Real-time RT-PCR using TioV specific primers and probe on the allantoic fluid of both the passages did not show any virus amplification. This showed that TioV did not grow in embryonated eggs.
4. Discussion
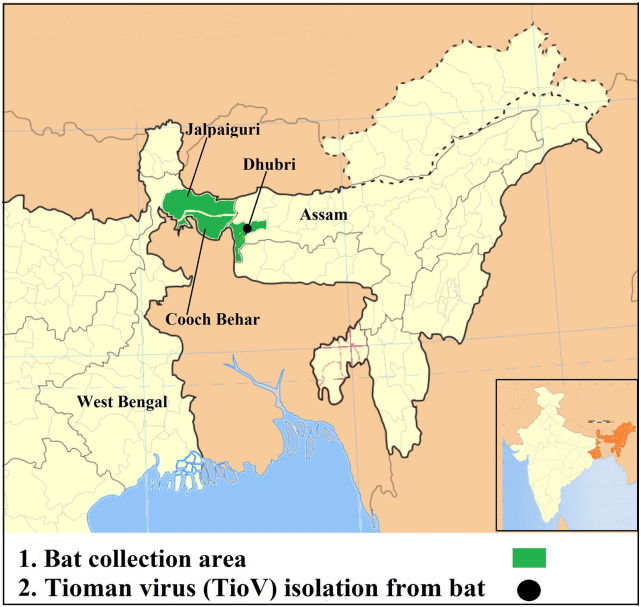
The present study reports the isolation of TioV from Pteropus giganteus bat from Dhubri, Assam, India (Fig. 4 ); this is the second report of TioV isolation besides Malaysia (Chua et al., 2001). TioV is antigenically related to Menangle virus (Bowden and Boyle, 2005) which is also harbored by pteropid fruit bats; the Menangle virus caused an outbreak of fetal deformities in pigs in Australia in 1997 (Philbey et al., 1998). All the above-named Pteropus-borne viruses group in a single clade, which separates them from other paramyxoviruses.
Fig. 4.
Geographic locations of bat collection and Tioman virus positivity in Pteropus giganteus bat, North-East, India.
The bats and flying foxes belonging to the order Chiroptera are ecologically remarkable. They are among the most abundant, diverse and geographically dispersed vertebrates and are natural reservoirs for a number of highly pathogenic zoonotic viruses. Bats are known to have persistent viral infections at a rate higher than other mammals, possibly due to shorter antibody half-life in these animals (Calisher et al., 2006). Detailed studies are needed on their importance as reservoirs of viruses and their potential to harbor important pathogen causing human and animal diseases.
There is scanty information available regarding the hosts, reservoirs and transmission of TioV, though direct transmission via ingestion of fruit by humans has been suggested (Lehle et al., 2007). However, bat-to-human transmission of TioV has not yet been reported. Neutralizing antibodies against TioV have been detected in human serum samples from Tioman Island in Malaysia, from where the virus was first isolated (Yaiw et al., 2007). TioV's estimated prevalence of 1.8% is suggestive of its potential to cause subclinical infection in humans. Experimental studies have shown that TioV is capable of infecting and replicating in pigs and its main cellular targets are lymphocytes, thymic epithelioreticular cells and the tonsillar epithelium in these animals (Yaiw et al., 2008). Hence, pigs could act as an intermediate or amplifying host for human transmission, as has happened during Menangle Virus and NiV outbreaks (Parashar et al., 2000). During NiV outbreaks in Malaysia, pigs played a critical role in transmitting the disease to pig handlers by direct contact. Pig farms are a source of daily livelihood for a large number of populations in Assam and other states like Nagaland. The Nagaland pig production and marketing project is funded by the National Agricultural Innovation Project with a contribution from the International Fund for Agricultural Development and aims to develop sustainable solutions to livelihood improvement in one of the poorest districts in India. Pig farming was rampant during the year 2008 and being a good reservoir of many diseases in recent past the number of Japanese encephalitis cases and outbreaks were increased in these areas. Undetected mild TioV infection could occur in naturally infected pigs and this could facilitate viral transmission to humans via contact with oral secretions; this transmission could cause serious illness by crossing the species barrier. Therefore, the role of bats and pigs in transmitting viruses to humans in Asia needs to be determined. Although no evidence of TioV illness in humans or animals exists, TioV's close relationship to other disease-causing bat paramyxoviruses, including NiV, suggests the possibility that it too may cross the species barrier (Bowden and Boyle, 2005).
5. Conclusions
Our study has shown the presence of TioV by highlighting its isolation from Pteropus bat from Dhubri District, Assam India. The presence of large colonies of Pteropus bats in close proximity of human settlements warrants implementation of necessary steps for detection and identification of emerging bat-borne viruses circulating in North-East region of India.
Conflicts of interest
The authors declare that they have no competing interests.
Acknowledgments
Authors express their sincere gratitude to the Secretary and Director General, Indian Council of Medical Research, New Delhi for her continuous support. We would like to acknowledge ICMR for funding extramural project ‘Multi-site epidemiological and virological survey of Nipah virus: special emphasis on North-East region of India’ (Grant number 2013:1445). Authors are grateful to Dr. MS Chadha (Scientist ‘F’& head of department), Influenza department for continuous guidance and support and Dr. R Laxminarayanan, Senior Administrative Officer, NIV, Pune, for rendering logistic support. Technical assistance rendered by Divya Bhattad, Kumar Bagmare, Amita bargat, Shital Melag and UK Shende (Laboratory) is gratefully acknowledged.
References
- Aguilar H.C., Lee B. Emerging paramyxoviruses: molecular mechanisms and antiviral strategies. Expert Rev Mol Med. 2011;13 doi: 10.1017/S1462399410001754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowden T.R., Boyle D.B. Completion of the full-length genome sequence of Menangle virus: characterization of the polymerase gene and genomic 5-trailer region. Arch. Virol. 2005;150:2125–2137. doi: 10.1007/s00705-005-0552-7. [DOI] [PubMed] [Google Scholar]
- Brenner S., Horne W.R. A negative staining method for high resolution electron microscopy of viruses. Biochim. Biophys. Acta. 1959;34:103–110. doi: 10.1016/0006-3002(59)90237-9. [DOI] [PubMed] [Google Scholar]
- Calisher C.H. Bats: important reservoir hosts of emerging viruses. Clin. Microbiol. Rev. 2006;19:531–545. doi: 10.1128/CMR.00017-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadha M.S. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg. Infect. Dis. 2006;12:235–240. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua K.B. Tioman virus, a novel paramyxovirus isolated from fruit bats in Malaysia. Virology. 2001;283:215–229. doi: 10.1006/viro.2000.0882. [DOI] [PubMed] [Google Scholar]
- Chua K.B. Isolation of Nipah virus from Malaysian island flying-foxes. Microbes Infect. 2002;4(145–51):10. doi: 10.1016/s1286-4579(01)01522-2. [DOI] [PubMed] [Google Scholar]
- Gangodkar S. Dengue virus induced autophagosomes and changes in endo-membrane ultrastructure imaged by electron tomography and whole-mount-grid cell culture techniques. J. Electron Microsc. 2010;59(6):503–511. doi: 10.1093/jmicro/dfq063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaume V. Specific detection of Nipah virus using real-time RT-PCR (Taq Man) J. Virol. Methods. 2004;120:229–237. doi: 10.1016/j.jviromet.2004.05.018. [DOI] [PubMed] [Google Scholar]
- Lehle C. Henipavirus and Tioman virus antibodies in Pteropodid bats, Madagascar. Emerg. Infect. Dis. 2007;13:159–161. doi: 10.3201/eid1301.060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie J.S., Field H.E., Guyatt K.J. Managing emerging diseases borne by fruit bats (flying foxes), with particular reference to henipaviruses and Australian bat lyssavirus. J. Appl. Microbiol. 2003;94:59S–69S. doi: 10.1046/j.1365-2672.94.s1.7.x. [DOI] [PubMed] [Google Scholar]
- Mourya D.T. Malsoor virus, a novel bat phlebovirus, is closely related to severe fever with thrombocytopenia syndrome virus and heartland virus. J. Virol. 2014;88(6):3605–3609. doi: 10.1128/JVI.02617-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman S.H., Field H., Epstein J. FAO Animal Production and Health Manual No. 12. Food and Agriculture Organization of the United Nations; Rome, Italy: 2011. Investigating the role of bats in emerging zoonoses: balancing ecology, conservation and public health interest.http://www.fao.org/docrep/014/i2407e/i2407e00.pdf [Google Scholar]
- Parashar U.D. Case-control study of risk factors for human infection with a new zoonotic paramyxovirus, Nipah virus, during a 1998–1999 outbreak of severe encephalitis in Malaysia. J. Infect. Dis. 2000;181:1755–1759. doi: 10.1086/315457. [DOI] [PubMed] [Google Scholar]
- Pavri K.M., Singh K.R., Hollinger F.B. Isolation of a new parainfluenza virus from a frugivorous bat, Rousettus leschenaulti, collected at Poona, India. Am.J.Trop. Med. Hyg. 1971;20:125–130. doi: 10.4269/ajtmh.1971.20.125. [DOI] [PubMed] [Google Scholar]
- Philbey A.W. An apparently new virus (family Paramyxoviridae) infectious for pigs, humans, and fruit bats. Emerg. Infect. Dis. 1998;4:269–271. doi: 10.3201/eid0402.980214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raut C.G. Isolation of a novel adenovirus from Rousettus leschenaultii bats from India. Intervirology. 2012;55:488–490. doi: 10.1159/000337026. [DOI] [PubMed] [Google Scholar]
- Reed L.J., Muench H. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- Tamura K., Dudley J., Nei M., Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tong S. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 2008;46(8):2652–2658. doi: 10.1128/JCM.00192-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne J.W., Wang L.F. Bats and viruses: friend or foe? PLoS Pathog. 2013;9(10) doi: 10.1371/journal.ppat.1003651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav P.D. Detection of Nipah virus RNA in fruit bat (Pteropus giganteus) from India. Am.J.Trop. Med. Hyg. 2012;87:576–578. doi: 10.4269/ajtmh.2012.11-0416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaiw K.C. Serological evidence of possible human infection with Tioman virus, a newly described Paramyxovirus of bat origin. J. Infect. Dis. 2007;196:884–886. doi: 10.1086/520817. [DOI] [PubMed] [Google Scholar]
- Yaiw K.C. Tioman virus, a paramyxovirus of bat origin, causes mild disease in pigs and has a predilection for lymphoid tissues. J. Virol. 2008;82(1):565–568. doi: 10.1128/JVI.01660-07. [DOI] [PMC free article] [PubMed] [Google Scholar]