Abstract
Egg yolk constitutes a relevant alternative source of antibodies. It presents some advantages over mammalian serum immunoglobulins regarding productivity, animal welfare and specificity. The main immunoglobulin present in avian blood (IgY) is transmitted to their offspring and accumulates in egg yolks, which enables the non-invasive harvesting of high amounts of antibodies. Moreover, due to structural differences and phylogenetic distance, IgY is more suitable for diagnostic purposes than mammalian antibodies, since it does not react with certain components of the human immune system and displays greater avidity for mammalian conserved proteins. IgY has been extensively used in health researches, as both therapeutic and diagnostic tool. This article aims to review its applications in both human and veterinary health.
Keywords: Birds, Immunoglobulin Y, Diagnostics, Immunotherapy, Prophylaxis
Highlights
-
•
IgY-technology is a new frontier for biological products
-
•
IgY Abs is a non-invasive technology obtained from egg yolks of immunized hens.
-
•
A wide range of IgY applications has been successfully tested in both human and animal health.
-
•
IgY technology constitutes a relevant alternative for the mammalian antibodies production.
1. Introduction
Antibodies are protein molecules produced in response to an antigen. Due to their ability to bind to specific targets, they are widely used in research, diagnosis and therapy. Most of the currently available antibodies are produced in mammals, especially in small rodents [1]. However, the production of antibodies in mammals can be challenging due to the fact that some antigens elicit weak immune responses or are even completely non-immunogenic. Moreover, the production of antibodies in mammals involves procedures that cause pain and distress to animals; such as immunization, blood sample collection and sacrifice [2]. The search for more efficient and economical techniques, as well as for the reduction and refinement of the use of animals, has led to growing interest for egg yolk antibodies (IgY). Obtaining antibodies from egg yolk is a non-invasive method that eliminates the need for blood collection.
Hens produce a greater amount of antibodies compared to other animals, like rodents for example, considerably reducing the number of animals required for the production of antibodies [2,3]. This method presents several economical advantages over the use of other animals. For example, the cost of maintaining a hen is less expensive than maintaining animals like mice and rabbits; the amount of antibodies produced by chickens also corresponds to that of larger animals, such as goats and sheep. For instance, in one year, a hen lays about 300 eggs and produces an average of 18, 25 g of IgY [4]. Moreover, the specific IgY produced in chickens are about 1–10% of the total amount of antibodies [1]. Nonetheless, the amount of antibodies produced is correlated to the quantity of antigen applied to the hen, its immunogenicity and molecular weight [5,6]. Being a productive technique, as well as more refined from the point of view of animal welfare, IgY technology has been used for several purposes in human and veterinary health, such as in immunodiagnostics [7], immunotherapy [8], neutralization of toxins from venomous animals [9] and bacteria [10], and as functional food [11].
Considering the fast development of IgY technology, this work aims to review its applications in human and animal health, in addition to increasing the potential use of these antibodies among researchers and consequently promoting the reduced use of non-invasive procedures on animals.
2. Structural and biochemical properties of IgY
The specific protective effect of egg yolk extracts from immunized hens, attributed to the transfer of serum chicken antibodies to eggs, was first described in 1893 [12]. However, this knowledge remained without applications until it attracted the interest of the scientific community due to the search for animal welfare, which was driven by the works of Russel & Burch and the publication of the “Principles of Humane Experimental Technique” in 1959. The use of IgY increased in the 80s, possibly due to the development of commercially available secondary reagents, such as IgY purification kits and anti-IgY specific antibodies conjugated to alkaline phosphatase, fluorescein isothiocyanate and peroxidase markers [13]. In 1996, the European Center for the Validation of Alternative Methods (ECVAM) workshop recommended the use of IgY rather than mammalian IgG, with the purpose of minimizing the pain generated by invasive collection of serum antibodies [3].
IgY is present in birds, reptiles, amphibians and lungfish and is the evolutionary precursor of IgG and IgE, present only in mammals [14]. Over time, IgY was called IgG due to the supposed similarity between the two. However, Leslie & Clem emphasized the distinct differences between IgY and IgG, such as antigenic differences and the major size of IgY heavy chain, suggesting the use of this term instead of IgG [15].
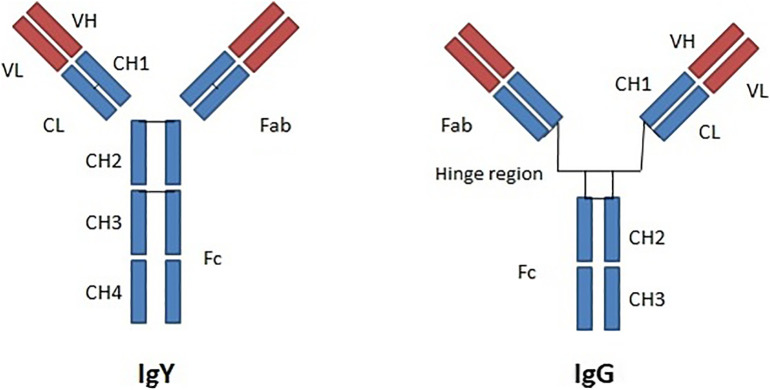
The IgY molecule has a structure similar to that of IgG, with two heavy chains (H), each one with a molecular weight of 67 to 70 kDa, and two light chains (L), with 25 kDa. The light chains have one constant region (CL) and one variable region (VL), similar to IgG. The major difference between IgY and IgG is found at the heavy chains. IgG has three constant regions at the heavy chains (CH1-CH3), while IgY has four constant regions (CH1-CH4) (Fig. 1 ). A further constant domain, with the corresponding carbohydrate chains, gives IgY a higher molecular weight (180 kDa) in comparison to IgG (150 kDa) [1].
Fig. 1.
Structure of IgY and IgG. V = variable domain of the light chain (VL) and the heavy chain (VH); C = constant domain of the light chain (CL) and the heavy chain (CH).
IgY is less flexible than IgG due to the absence of the hinge region between CH1 and CH2 [1], similar, in this aspect, to IgE [16,17]. The presence of glycine and proline residues at CH1-CH2 and CH2-CH3 regions also limits IgY flexibility [1]. One advantage of this higher chain stiffness is that it may be associated with increased IgY resistance to proteolytic degradation and fragmentation [17]. Also, IgY is more hydrophobic than IgG and has an isoelectric point between 5.7 and 7.6. The half-life of purified IgY is of months, retaining activity for up to six months at room temperature and one month at 37 °C. Moreover, affinity-purified and 21 biotinylated IgY retained high activity after five years of storage at 4 °C [1]. The structural and phylogenetic differences between IgY and IgG are manifested in different molecular and biochemical interactions. The Fc portion of IgY is unable to activate the human complement [18] and to bind to rheumatoid factor [19] and to protein G [20], it also does not react with human anti-mouse antibodies (HAMA) [21] nor with the erythrocyte agglutinogens A and B [22]. Due to phylogenetic distance, chickens produce a stronger immune response against conserved mammalian proteins [23]. Although IgY contains the variable (V), junction (J) and diversity (D) regions, the contribution of the V (D) J rearrangement to its immunogenetic diversity is small, due to the fact that only a single locus undergoes rearrangement [24]. Thus, the genetic diversity of IgY is guaranteed by gene hyperconversion, a process in which pseudogenes donate homologous sequences to the functional gene (V) after V (D) J rearrangement [25].
Even though it is a protein molecule, IgY is resistant to heat and pH, being stable between 30° and 70 °C and active between pH 3.5 and 11. However, the affinity of IgY to its antigen decreases with increasing temperature. Nonetheless, the addition of sucrose, maltose and glycine protects IgY from heat denaturation [8], while sorbitol stabilizes it at acid pH [26]. IgY is resistant to inactivation by the proteolytic enzymes trypsin and chymotrypsin, but is degraded by pepsin [27]. The digestion of IgY with papain leads to a Fc fragment plus two monovalent Fab fragments, like IgG. Nonetheless, IgY digestion with pepsin leads to a pFc′ fragment and two monovalent Fab fragments, unlike mammalian antibodies, which forms one bivalent Fab fragment when cleaved by pepsin [28]. The characteristics of IgY, in comparison to IgG, are summarized in Table 1 .
Table 1.
Comparison of IgY and IgG.
| IgY | IgG | |
|---|---|---|
| Species | Birds, reptiles, amphibians and lungfish | Mammals |
| Source | Serum and eggs | Serum |
| Concentration | 100–150 mg/egg (15 mL/yolk) |
200 mg/bleed (40 mL) |
| Molecular weight (kDa) | 180 | 150 |
| Constant domains (Heavy chain) | 4 | 3 |
| Hinge region | No | Yes |
| pH stability | 3.5–11.0 | 2.0–11.0 |
| Heat stability | Up to 70 °C | Up to 75°–80° |
| Proteolytic degradation | Pepsin and papain | Pepsin, papain, trypsin and chymotrypsin |
| Complement binding | No | Yes |
| Rheumatoid factor binding | No | Yes |
| Fc receptor binding | No | Yes |
| Binding to protein A and G | No | Yes |
Several methods have been developed to protect IgY from degradation by pH and pepsin present in the stomach, allowing it to arrive unscathed at the small intestine, which is especially important for the use of IgY against enteric pathogens. Among these treatments, chitosan and alginate microcapsules [29], β-cyclodextrin microcapsules [30,31], copolymers of methacrylic acid [32], liposomes [31,33], multiple emulsification [34], hydrogel [35] and carbon nanotubes with hydrogel [36] have been tested with different degrees of success. Nonetheless, unprotected IgY consumed as egg yolk in liquid [37] and powder forms [38] showed resistance to the gastrointestinal tract conditions in calves.
3. Production of IgY
The antibody titers are influenced by several factors, such as the antigen type and dose, the used adjuvant, the route of application, the inoculation frequency, age and stage of development in birds [39,40]. Several protocols for obtaining IgY, observing these different variables, have been applied [1,39]. Basically, multiple immunization protocols were tested for different antigens and animals, in order to obtain the highest antibody titer for each case [1].
Several antigen types have been used to produce specific IgY in birds, such as complex antigens (virus, bacteria and parasites) and single antigens (proteins, polysaccharides, peptides and nucleic acids) [40]. Different antigen concentrations can also be combined with adjuvant. However, it is common to use 10 to 100 μg of antigen per mL injected in two or three sites, with the age of hen ranging between seven and eight weeks [1].
The induction of high antibodies titers depends on the use of adjuvants. Though Freund's Complete Adjuvant (FCA) is the most potent to induce antibodies in laboratory animals, it can lead to severe inflammation at the injection site [40]. Although some studies have shown that the immunization with FCA is more tolerated by birds than by mammals and seems to not generate tissue damage in chickens [23,41], other studies using FCA for bird immunization contradict these results [42,43]. Since there is no consensus, one of the best substitutes for FCA is Freund's Incomplete Adjuvant (FIA), which, unlike FCA, does not contain mycobacteria. Shade et al. recommended the use of FIA, which can be used since the first immunization without any prejudice to the antibody titer [3]. In spite of this, the first inoculation is generally performed using FCA, while the subsequent inoculations are performed using FIA, without the occurrence of inflammation at the injection site [44,45].
The most common route of antigen administration for IgY production in chickens is the intramuscular route, where the inoculation is usually into the breast muscle. This technique is most suited for young hens, because subcutaneous injection into the neck is more difficult to perform and may cause more distress to the animal. The intramuscular injection into the leg must be avoided as it can lead to lameness [3]. Nonetheless, for a non-invasive method, the administration of antigen can also be performed orally [46]. The number of inoculations required depends on the type and dose of the antigen, as well as the adjuvant used. At least two inoculations must be performed before the laying period, with an interval of four to six weeks. The titer of IgY must be assessed 14 days after the last immunization. If the antibody titer decreases, further immunizations must be done during the entire laying period to increase antibody titers year round [3]. An increase in titer of egg yolk antibodies can be observed from the second week [47,48] or sometimes from the fifth week post antigen inoculation [49]. After a steady increase, stabilization of the antibody titer occurs, reaching a plateau, and from there on it decreases progressively [48]. Through booster inoculations, it is possible to keep high titers of egg yolk antibodies for more than 150 days [50].
The IgY extraction consists of the removal of lipids to form a water soluble fraction (WSF), followed by the precipitation of the antibodies contained therein. Several extraction methods are available for obtaining IgY from egg yolk and the choice of the suitable method depends on the purpose, which can require different purification degrees, as well as the extraction scale, cost and available technology [40].
A frequently used method for the extraction of IgY is the precipitation with polyethylene glycol (PEG) 6.000. The method consists on the delipidation by centrifuging egg yolk diluted in phosphate buffered saline (PBS) with 3.5% of PEG 6000. Next, the supernatant is twice centrifuged with 12% of PEG 6000 to precipitate the antibodies [6]. Akita & Nakai obtained highly purified IgY by removing the lipids from the yolks by means of six-fold dilution in water at pH 5.0, with an incubation time of 6 h at 4 °C, followed by IgY precipitation with 60% of ammonium sulfate (NH4)2SO4, which was supplemented by the addition of sodium chloride (NaCl) or by ultrafiltration prior to gel filtration or ion exchange chromatography. The authors also achieved highly purified IgY by means of ethanol precipitation at lower temperatures [51]. Nonetheless, a study conducted by Araújo et al. showed that the most suited concentration of (NH4)2SO4 for IgY's precipitation is 20%. In this study, higher concentrations of (NH4)2SO4 also caused the precipitation of non-specific proteins, while a lower concentration led to a greater loss of antibodies in the supernatant [52].
Since the Fc region of IgY does not bind to proteins A and G, which are commonly used in the affinity purification of mammalian IgG, affinity chromatographic methods for IgY purification requires other types of ligands. IgY can be purified through the adsorption of the immunizing antigen to the solid phase of the affinity column, which generates monospecific antibodies that can be eluted under acid [53] or alkaline conditions [54]. The purification of IgY can also be performed through thiophilic adsorption chromatography [55]. In contrast to other affinity purification methods, it can be performed at mild conditions [56].
Hens are able to produce between 100 and 150 mg of IgY antibodies by yolk, of which between 1 and 10% are specific [1]. In one year, a single laying hen produces between 17 and 35 g of total IgY [13]. Although the production of IgY for research is mainly done in chicken, other birds are also used for the same purpose, such as goose [57] and quail [58], using an immunization protocol similar to that used for chickens.
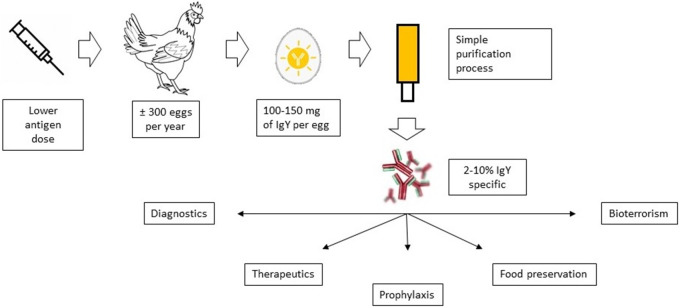
The production of IgY and its possible applications in both human and veterinary health are summarized in Fig. 2 .
Fig. 2.
Production and applications of IgY.
4. Therapeutic and prophylactic applications of IgY
4.1. Antibacterial activity
The use of polyclonal IgY against infectious diseases minimizes the risk of microbial resistance, since the antibody is directed to various antigens of the same microorganism, which requires multiple genes for its synthesis [8]. Therefore, specific IgY antibodies are a relevant alternative to use as antimicrobials in human and veterinary health in the face of the emergence of resistant bacteria. IgY antibacterial activity can be assessed by the inhibition of bacterial growth and biofilm formation in vitro, as well as adhesion ability, since they are prior conditions for successful colonization of a higher animal by bacteria and viruses [13]. IgY antibacterial activity against gastrointestinal pathogens has been widely investigated. Nasiri et al. extracted IgY from hens immunized with the recombinant protein FanC, from enterotoxigenic Escherichia coli (ETEC) and these antibodies bound specifically to FanC in ELISA, Western blot and Dot-blotting [59], demanding, thus, more investigations to evaluate its viability as a potential immunotherapeutic compound.
Li et al. evaluated the effect of specific and non-specific IgY against Salmonella typhimurium on the immune response of mice infected by the bacterium [60]. Specific IgY reduced the expression of the pro-inflammatory cytokines TNF-α and INF-γ and elevated that of the anti-inflammatory cytokine IL-10; while non-specific IgY also reduced the level of TNF-α, but did not alter the expression level of INF-γ and IL-10. Although both specific and non-specific antibodies reduced the number of T lymphocytes in the lamina propria and of CD4+, CD8+ and γδ T lymphocytes in the jejunum of mice, the animals treated with anti-S. typhimurium IgY had a longer survival rate than those treated with non-specific IgY.
IgY activity against bacterial spores was also investigated. Oral administration of IgY against Clostridium difficile spores both delayed the onset of diarrhea in rats treated prior to the infection and reduced its recurrence in infected rats. Thus, specific IgY could be used in the prophylaxis of C. difficile infection, as well as an immunotherapeutic to eliminate the remaining spores on the intestines of people who have already had the infection, which would reduce the recurrence of diarrhea [61].
The therapeutic action of IgY against Helicobacter pylori has been largely studied as well, through antibodies directed to both the whole cell and to specific proteins. Oral administration of Anti-HpUc (H. pylori urease) IgY inhibited the growth of H. pylori in vitro and reduced the inflammation of stomach in mice, with a significant reduction of neutrophils and lymphocytes infiltration on the tissue. A decrease in the serum level of antibodies against H. pylori was also observed in treated animals [62]. Similar results, as the reduction of serum levels of anti-H. pylori IgG and the absence of inflammation signs on the stomach were observed in mice that received oral IgY against the H. pylori vacuolating cytokine A (VacA) [63].
Specific IgY against HP-Nap protein, the main virulence factor of H. pylori, significantly inhibited the adhesion of the bacterium to an AGS cells culture [64]. Solhi et al. evaluated the occurrence of cross reaction between strain-specific IgY and four different H. pylori strains. The antibodies inhibited the cell growth and the urease enzyme not only of the strains for which they were specific, but also of other strains [65].
The therapeutic potential of IgY against bacterial respiratory tract infections has also been considered. IgY against Mycobacterium tuberculosis increased the proliferation of PBMC (peripheral blood mononuclear cells) in rats in a dose-dependent manner. An increased expression of cytokines that stimulate the cellular immune response was observed and an increase of mRNA level of IL-2 and IFN-γ was detected by RT-PCR, which indicates that anti-M. tuberculosis IgY is a potential immunotherapeutic that stimulates T-helper 1 immune response [66].
Shi et al. produced specific IgY against multi-drug resistant strains of Acinetobacter baumannii. The antibodies inhibited bacterial growth in vitro, significantly reduced the mortality of mice infected with the bacterium and attenuated the lung inflammation [67].
IgY has also been tested as a potential therapeutic resource in oral infections against Prevotella intermedia. Specific IgY inhibited bacterial growth in liquid medium and showed therapeutic activity in rats with P. intermedia induced gingivitis. The reduction of gingival inflammation, bacterial plaque and bleeding, as well as the normalization of WBC (White Blood Cells) levels in the blood, were observed in treated animals. Histopathological examination showed no signs of inflammation in the gums of rats treated with anti-P. intermedia IgY [68].
Additionally, studies have shown inhibitory growth effect of therapeutic IgY produced against Fusobacterium nucleatum in liquid medium and in the formation of biofilm in polyestyrene plates. Anti-F. nucleatum IgY also reduced bone loss in mice with periodontal disease when applied after the infection, thus presenting therapeutic function [69].
The therapeutic effect of IgY against acne was also evaluated. Revathy et al. produced and assessed the efficiency of specific IgY against the bacterium Propionibacterium acnes. The antibody inhibited bacterial growth and biofilm formation in vitro, proving to be a possible alternative to antibiotics in the treatment of acne [70].
More recently, IgY was tested for antibacterial effect in aquatic animals. Gao et al. produced IgY against Vibrio spp., the major cause of death in white shrimp (Litopenaeus vannamei), and demonstrated that powder egg yolk containing anti-Vibrio IgY significantly reduced the mortality of white shrimp infected with V. harveyi and V. parahaemolyticus [30], which indicates that therapeutic IgY can also be applied in aquafarming.
The prophylactic potential of IgY against dental caries has been widely studied. An interesting study carried by Bachtiar et al. evaluated the effect of soybean milk containing IgY against the cariogenic bacterium Streptococcus mutans in rats. The animals fed with this milk presented a decrease in the number of S. mutans in dental biofilm and fifteen days after milk intake IgY could still be detected in the saliva [71]. In other research, the effect of specific IgY against the cell-associated glucosyltransferase enzyme (CA-gtf), of S. mutans, was evaluated. As a result, Anti-CA-gtf IgY inhibited bacterial adhesion in vitro and suppressed the oral cavity colonization [72].
In addition, specific IgY action against the ComD protein, a quorum-sensing signals receptor of S. mutans, was also explored. It inhibited, in vitro, the development of biofilm of S. mutans from the oral cavity of people with and without caries, and changes in the protein expression pattern were found in the bacteria treated with anti-ComD IgY [73].
The protective effect of IgY against opportunistic infections was also investigated by Thomsen et al., where, by means of chemiluminescence, the production of reactive oxygen species (ROS) in polymorphonuclear neutrophils (PMN) was evaluated as an indicator of the intensity of PMN mediated immune response against Pseudomonas aeruginosas in vitro, as well as the influence of anti-P. aeruginosas IgY in this process. The specific IgY opsonized the bacterium and intensified the cellular immune response, probably due to the recognition of IgY by receptors resembling avian IgY receptors or physical-chemical changes in the bacterium, since IgY does not activate mammalian Fc receptors. Anti-P. aeruginosas IgY could be used in the prophylaxis of P. aeruginosas infection in patients with Cystic fibrosis by increasing the cellular immune response [74].
Prophylaxis of bacterial infections in animals for consumption, using IgY, was also considered. Specific IgY incorporated into hydrogel added to carbon nanotubes and chitosan (H-CNT) showed protective activity against enterotoxigenic Escherichia coli (ETEC) in piglets [75]. Thus, Anti-ETEC IgY incorporated into H-CNT and orally applied could be used to prevent the intestinal infection in these animals.
In order to generate antibodies against the bacterium Campylobacter jejuni to be used as food additive aiming to control C. jejuni colonization in chickens, Thibodeau et al. produced anti-C. jejuni IgY in three different ways: oral administration of a pool of living bacteria from four C. jejuni strains, subcutaneous injection of extracts from the outer membrane of the bacterium or of formalin inactivated bacterium. The hens submitted to subcutaneous inoculation produced IgY more intensely, although the antibodies obtained through oral inoculation also showed good activity. Anti-C. jejuni IgY, obtained from the three ways, presented bactericidal activity in vitro [46].
IgY against the bacterium Aeromonas salmonicida, the causative agent of ulcers on fish skin, was added, in powder form, to the water in which Koi carp (Cyprinus carpio koi) ornamental fish were raised. Anti-A. salmonicida antibodies showed good prophylactic activity against the infection, and a small amount of specific IgY was enough to completely prevent the appearance of skin ulcers in healthy fish that cohabited with sick fish previously infected with A. salmonicida [76].
The potential of IgY technology to prevent bacterial infections in animals could be useful in the needful strategies to reduce the use of antibiotics in food animal husbandry, which has been linked to the selection and spread of resistant bacterial strains that threaten antibiotics as a therapeutic resource [77,78].
4.2. Antiviral activity
The therapeutic potential of IgY against viral infections of the gastrointestinal tract in animals has been extensively investigated. Oral administration of egg yolk powder rich in IgY against bovine group A Rotavirus (RVA) was performed on calves infected by the virus. As a result, disease attenuation occurred in treated animals, with a reduction of the period of diarrhea and hyperthermia and the absence of other symptoms observed in untreated calves, such as anorexia, dehydration and depression. The egg yolk powder enriched with anti-RVA IgY retained the activity after two years, when kept at 4 °C [38]. In another study, IgY against the S1 protein of porcine epidemical diarrhea virus (PEDV) was produced and orally administered in piglets previously infected with PEDV. Anti-S1 IgY reduced the severity of diarrhea and intestinal lesions caused by PEDV infection and nullified the mortality of piglets due to the disease [79].
Another possible therapeutic use of specific IgY is against dengue fever, as demonstrated by Fink et al. Anti-DENV2 IgY produced in goose was able to neutralize the virus in vitro and in vivo without binding to Fcγ receptors on myeloid cells and generating ADE (antibody dependent enhancement) in mice [57].
The protective effect of IgY against influenza has been widely studied. IgY against the avian influenza A virus (H5N1) were extracted from eggs available in supermarkets of Vietnam, where the vaccination of chickens against H5N1 virus is required. Anti-H5N1 IgY was administrated intranasally in mice before and after their infection with H5N1 and H5N2 and completely prevented the disease onset [80]. These results reveal that commercially available eggs that are produced in countries where anti-H5N1 vaccination is mandatory constitute a considerable source of IgY that could be used to prevent a potential pandemic of H5N1 virus.
Following this rationale, Wallach et al. produced IgY against H1N1, H3N2 and H5N1 strains of influenza virus, which were tested for their ability to neutralize homologous and heterologous strains in vitro and to prevent the infection in vivo. The antibodies inhibited only homologous strains in the seroneutralization and in the haemagglutination inhibition assay, except anti-H5N1 IgY, which also inhibited H1N1. Anti-H5N1 and anti-H1N1 IgY were applied intranasally in mice 1 h before the infection by influenza virus and showed prophylactic activity. Thus, IgY against influenza virus strains could be used in nasal, oral or spray applications to protect individuals and environments [81]. IgY against influenza B virus (IBV) was also tested and neutralized the activity of haemagglutinins and neuraminidase present in the virus and inhibited viral replication in vitro. When applied intranasally, anti-IBV IgY prevented influenza development in mice treated prior to virus exposure and attenuated the disease in those treated post-infection [48].
The prophylactic effect of IgY against other viral infections of the respiratory tract was also evaluated. IgY against Andes virus (ANDV), the causative agent of the Hantavirus Pulmonary Syndrome (HPS), was obtained from geese eggs by inoculating these animals with the DNA encoding the virus envelope glycoprotein. Anti-ANDV IgY was able to prevent HPS development in recently infected mice but failed when applied after the onset of viremia, thus presenting prophylactic and non-therapeutic activity [82].
The protective effects of IgY were also considered for viral infections in poultry. Aizenshtein et al. produced, in the same eggs, efficient IgY against the Newcastle disease virus (NDV), infectious bursal disease virus (IBDV), influenza and reovirus, which are pathogenic for birds [83], demonstrating that passive immunization with IgY against several viruses is possible; nonetheless, it is a technology that must be further explored.
4.3. Antifungal activity
A gel preparation for oral use containing IgY against Candida albicans was tested by Tekeuchi et al. and caused a reduction in the number of colony-forming units (CFU) on the oral cavity of elderly people, showing promise for prophylactic use against C. albicans oral infection [84]. In other research, specific IgY inhibited the adhesion of Candida albicans and Candida glabrata to denture base material. Anti-C. albicans IgY was more effective against C. albicans than anti-C. glabrata IgY, while both antibodies were equally effective in preventing the adhesion of C. glabrata [85].
4.4. Antiparasitic activity
Sampaio et al. produced a high avidity IgY against the protozoan Trypanosoma evansi. There was neither prophylactic action nor infection control in the treated rats, but anti-T. evansi IgY increased their survival rate when used concomitantly to anti-hematozoa drugs [86]. In another study, Grando et al. inoculated Trypanosoma cruzi trypomastigots in hens and extracted a high amount of anti-T. cruzi IgY that cross-reacted with T. evansi antigens in Western blot, showing that the anti-T. cruzi IgY is not reliable as a diagnostic tool, but deserves more investigations as a possible therapeutic resource for trypanosomes infection [5].
4.5. Antitumor activity
The phylogenetic distance between birds and mammals ensures a stronger immune response against mammalian antigens by birds [23]. Such a feature may be advantageous to produce IgY against human tumor antigens. Following this rationale, Amirijavidv et al. produced highly specific IgY against a sequence of 21 amino acids present on the ectodomain of the TRAIL (TNF-related apoptosis-inducing ligand) receptor TRAIL-R2 (DR5). The antibodies bound to the amino acid sequence and activated the DR5 receptors in human breast cancer cells MCF7, acting as a TRAIL agonist and inducing apoptosis [87]. IgY against other receptors, such as the HER2 receptor, was tested coupled to single walled carbon nanotubes (SWNTs) and specifically detected the HER2 receptors on the surface of SK-BR-3 cells. The binding of the complex to the receptors was measured by Raman signals emitted by the nanotubes. SWNT has a near infrared absorption (NIR), which can be used for tumor ablation, and, coupled to anti-HER2 IgY, was able to kill SK-BR-3 cells without needing internalization of the complex by the cell [88]. These findings show that IgY produced against tumor antigens is an attractive alternative for a more selective treatment of cancers and its use could, therefore, minimize the side effects of traditional chemotherapy.
4.6. Antiobesity activity
IgY raised against porcine pancreatic lipase was used against the enzyme in vitro and in vivo. Later, mice with obesity induced by high fat diet were orally treated with the antibody, which was given concomitantly with food, and a reduction of adipose tissue and liver fat level was observed, as well as an increase of fecal excretion of triglycerides and their decrease in blood plasma. Anti-lipase IgY inhibited the hydrolysis of diet fat and reduced its intestinal absorption, showing anti-obesity activity [89].
4.7. Antiallergic activity
Wei-xu et al. evaluated the antiallergic effect of specific IgY against the pro-inflammatory cytokines IL-β1 and TNF-α in guinea pigs with induced allergic rhinitis. A reduction of the eosinophils number in the blood and in the nasal and bronchial lavages was found, as well as a decrease of eosinophils, neutrophils and lymphocytes infiltration into the nasal mucosa and the lungs of animals treated with anti-IL-β1 and anti-TNF-α IgY, alone or jointly [90].
4.8. Anti-venom activity
One of the side effects that occur in individuals receiving anti-venom serum produced in goats, sheep and horses is due to the presence of serum proteins on the anti-venom serum derived from these animals, in which IgG is not sufficiently purified [52,91]. One advantage of using IgY in anti-venom serotherapy is that it is easily purified, which would minimize the occurrence of side effects due to non-specific proteins. Araujo et al. demonstrated this property when specific IgY was produced as anti-venom of the snake genus Bothrops sp. These antibodies neutralized a pool of venoms from five Bothrops species, with an ED50 of 150 μL/2LD50, showing little to no side effects in mice [52].
Mendonza et al. also produced IgY capable of neutralizing the venom of the peruvian snake Bothrops atox. The anti-venom IgY showed considerable cross reaction with the venom of Bothrops brazili and could be used not only as B. atox anti-venom, but also as a tool for the research of cross reaction with venoms from different species [9].
In another elegant work, Andrade et al. produced IgY against a pool of venoms from snakes of the genus Bothrops and against the venom from the species Crotalus durissus terrificus. Anti-venom IgY extracted from eggs was compared to the horse anti-venom IgG in Western blot. The results showed that specific egg's IgY recognized the same antigens as the equine anti-venom [92].
IgY against coral snake venom was first produced in response to a pool of venoms from different species of Micrurus. These antibodies recognized, by Western blot, venom proteins from several snakes: M. isozonus, M. surinamensis, M. f. fulvius, Naja kaouthia, N. pallida, Bothrops colombiensis, Crotalus durissus cumanensis and C. vegrandis and could, therefore, be used as a broad-spectrum snake anti-venom [93].
Zolfagharian and Dounighi produced IgY by inoculating the Vipera lebetina snake venom, inactivated by γ radiation, in hens [94]. These antibodies were effective in neutralizing the crude venom of Vipera lebetina in mice.
Anti-venom IgY were also obtained from eggs of hens immunized with the venom of the snake Trimeresurus albolabris. These IgY recognized, by Western blot, most of the proteins present in the T. albolabris venom and neutralized it in mice in a dose-dependent manner [95].
More recently, Liu et al. extracted and purified IgY from eggs of hens inoculated with the venom of the Deinagkistrodon actus snake. These antibodies were able to neutralize the lethal effects of the venom, such as bleeding, edema formation and myotoxicity in a dose-dependent manner [96].
Da Rocha et al. produced IgY against ophidian toxins of Crotalus durissus terrificus, Bothrops jararaca and Bitis arietans. The antibodies were able to bind to specific components of the venoms in Western blot and protected 100% of the intoxicated mice when obtained after the ninth inoculation. The authors recommended the use of a small antigen dose (20 μL) applied in successive inoculations for IgY production, since this dose was enough to genetically alter the V(D)J segments on the naïve cells and to generate immunological memory [97].
However, IgY raised against the venom of the snake Oxyuranus scutellatus was less effective than equine IgG, being unable to neutralize the neurotoxic and coagulant effects of the venom [98]. Nonetheless, this result cannot be extended to IgY produced against other venoms.
IgY against the Tityus caripitensis scorpion venom, produced by Alvarez et al., neutralized not only the venom of T. caripitensis, but also that of other Tityus species (T. quirogae, T. discrepans and T. gonzalespongai), and inactivated the hyaluronidase, an enzyme that facilitates the toxin spread in the tissues, present in the T. serrulatus venom [99]. Thus, IgY raised against T. caripitensis venom could be used as a broad-spectrum anti-scorpionic serum.
4.9. Prophylaxis of Celiac disease
Another application of IgY technology, the prophylaxis of Celiac disease, was demonstrated by Gujral et al., who developed powdered egg yolk formula with protective sugars containing anti-gliadin IgY, among which, the formula with mannitol (EYP-M) retained its activity after being submitted, in vitro, to chemical conditions analogous to those of the stomach and small intestine. The formula IgY-EPY-M neutralized in vitro both the isolated gliadin and that present in food matrix and inhibited its intestinal absorption in mice, showing promising for the prevention of Celiac disease [100].
4.10. Prophylaxis of toxicity
Bobeck et al. used IgY against the human intestinal alkaline phosphatase (hIAP) to assess the influence of IAP on increased bioavailability of phytate phosphate in the presence of 1α-dihydroxycholecalciferol (vitamin D3) in chickens. Anti-hIAP IgY was ingested by chickens and reduced the absorption of phytate phosphate, which suggests that although it performed less adequately than sevelamer chorhydrate, already used for the same purpose, anti-hIAP IgY can be optimized for the prevention of phytate phosphate toxicity induced by the consumption of the active form of vitamin D [101].
5. Applications of IgY in diagnosis
5.1. Viral infections diagnosis
The ability of IgY to detect viral pathogens of the gastrointestinal tract in humans and animals has been widely studied. IgY raised against canine parvovirus viral like particles (CPV-VLPs) were used in ELISA and immunochromatography, showing sensitivity and specificity in the detection of canine parvovirus in dog fecal samples [102].
Specific IgY against the E2 protein of bovine viral diarrhea virus (BVDV) were used in ELISA to detect pathogens that cause diarrhea in cattle. Anti-E2 IgY showed a high specificity, recognizing only BVDV. ELISA and immunochromatography tests using these antibodies were efficient in detecting BVDV in serum samples of cows with diarrhea, showing a concordance of 95, 45% and 90% with RT-PCR, respectively [103].
Da Silva et al. used IgY against hepatitis A virus (HAV) in a competitive immunoenzymatic assay to detect anti-HAV IgG in serum samples, showing satisfactory sensitivity and specificity [104]. More recently, IgY against HAV was used to detect the virus in hepatic sections of infected rhesus monkeys by means of indirect immunofluorescence (IIF). Anti-HVA IgY was more efficient than the commercially available IgG for the detection of the same antigen [105].
IgY was also used to detect viral pathogens in aquatic animals. Specific IgY against the soft-shelled turtle systemic septicemia spherical virus (STSSSV) was used to compose a lateral flow assay to detect the virus in turtles. This assay was sensitive and specific, detecting STSSSV in all infected turtles in serum and feces samples [106].
A potential for diagnosis was presented by IgY raised against the nucleocapsid protein (NP) of coronavirus (CoV). Anti-NP IgY, used as capture antibody in ELISA for detecting NP, lowered its detection limit to a picogram level, which indicates that the antibody is promising for use in the diagnosis of acute respiratory syndrome associated to coronavirus (SARS-CoV) and is sensitive enough to detect small amounts of NP [107].
Moreover, the ability of IgY in diagnosing dengue fever was also evaluated. Figueiredo et al. produced IgY against the non-structural protein 1 (NS1) of dengue virus 2 (DENV2). These antibodies were used to compose an immunosensor that was effective in electrically detecting the NS1 protein of DENV2 in standard samples and could be used for dengue 2 diagnosis in biological samples [108].
5.2. Bacterial infections diagnosis
One of the most studied diagnostic potential of IgY is against Staphylococcus aureus. The fact that the Fc portion of IgG reacts with the staphylococcal protein A makes IgY a relevant resource for more specific detection of different S. aureus strains and their toxins, since, due to structural differences, the Fc portion of IgY does not react with protein A [109].
Following this rationale, Walczak et al. produced IgY against the fibrinogen binding protein (Efb) of Staphylococcus aureus and against a peptide epitope that encompasses the residues 127–140 of Efb protein. Anti-Efb and anti-Efb127–140 antibodies presented high titers in ELISA and strong avidity in Western blot, showing promising use in the diagnosis of S. aureus infection [110].
Another ELISA test using IgY against the staphylococcal enterotoxin B (SEB) of S. aureus as capture antibody and specific ssDNA aptamers coupled to biotin as revealing was developed by Mulidi et al. This assay was efficient in detecting SEB, but also reacted with others staphylococcal toxins, such as staphylococcal endotoxins A (SEA) and C (SEC), toxic shock syndrome toxin (TSST) and α-hemolysin [111].
IgY raised against α-hemolysin was applied as capture antibody in ELISA for detecting the toxin in the supernatant of S. aureus cultures. Anti-αhA IgY showed high specificity against the toxin, without reacting with protein A, which is present in all S. aureus strains [44].
The ability of IgY to diagnose resistant S. aureus was also investigated. Yamada et al. produced IgY against the penicillin binding protein (PBP) 2a, which is present in methicillin resistant S. aureus (MRSA) strains. Anti-PBP2a IgY was used in ELISA, lateral flow and latex to detect MRSA and other S. aureus strains sensitive to methicillin and beta-lactams. The antibody was MRSA specific and did not react with the sensitive strains that express significant amounts of protein A [112].
5.3. Parasitic infections diagnosis
Among the parasitic infections tested, Cakir-Koc evaluated the potential of IgY in detecting the protozoan Toxoplasma gondii by producing IgY against its surface protein SAG-1, which reacted with the target antigen in ELISA and Western blot [113]. Therefore, this study revealed a potential diagnostic test for toxoplasmosis. More recently, Anti-SAG1 IgY conjugated to fluorescein isothiocyanate detected T. gondii tachyzoites in a colony, by means of immunofluorescence, and may be used to detect the protozoan in other types of samples [114].
In other interesting research, an indirect ELISA using IgY to detect cathepsin F (CF), a cysteine protease present in the helminth Opisthorchirs viverrini, was developed. This assay showed good sensitivity in the detection of O. viverrini in fecal samples from humans living in endemics places; however, a cross reaction with Taenia spp., Echinostoma spp. and Minute Intestinal Fluke (MIF) was observed [115]. Therefore, improvements still have to be made before this technology is available.
Miura et al. produced IgY against the GP60 protein, from the Cryptosporidium hominis protozoan. These IgY bound to GP60 in Western blot and to C. parvum sporozoites in fecal samples by indirect immunofluorescence, suggesting that anti-GP60 IgY could be used in the diagnosis of cryptosporidiosis caused by both C. hominis and C. parvum [116].
5.4. Diagnosis of tumors
Several authors have been investigating the potential of IgY in the detection of tumor markers. IgY against the peptide antigen CA 15-3, a commonly used breast cancer marker, was used as secondary antibody in a sandwich ELISA aiming to detect CA 15-3, showing potential for clinical use [49]. Sun et al. produced IgY against two portions of the transmembrane glycoprotein HER2: HER2-A, proximal region, and HER2-B, distal region. Anti-HER2-A and anti-HER2-B IgY effectively detected the HER2 glycoprotein in cultured breast cancer SK-BR-3 cells by immunofluorescence and in sections of breast tumors over expressing HER2-B, using immunohistochemistry. In addition, the antibodies bound to the glycoprotein in ELISA and Western blot, which indicates that IgY against HER2 is promising for use in breast cancer diagnosis [117].
In another study, Łupicka-Słowik et al. developed a direct ELISA test using a lysate of human malignant tumor cells and IgY against bovine adenosine deaminase (c-ADA). The assay was efficient in detecting human adenosine deaminase (h-ADA) present in the tumor cells lysate, which was possible due to the high homology between c-ADA and h-ADA. The ELISA test using anti-c-ADA IgY could be used to diagnose several types of malignant tumors in humans, as well as be an alternative to currently employed enzymatic methods for the detection and quantification of ADA for pleural tuberculosis diagnosis [118].
Another IgY developed to detect prostatic specific antigen (PSA) and two peptide fragments of this protein demonstrated specificity and marked the antigen more strongly than the IgG counterpart using Western blot analysis. However, anti-PSA IgY had an unsatisfactory sensitivity when applied as secondary antibody in indirect ELISA [45], thus deserving more investigation.
In general, these findings suggest that the phylogenetic distance between birds and mammals, that ensures a stronger immune response against mammalian antigens by birds than by other mammals [23], makes the production of IgY against tumor antigens advantageous not only for therapeutic purposes, as described above, but also for usage in several types of immunoassays for tumor detection in humans.
5.5. Hematological tests
Hens immunized with umbilical cord sera produced specific IgY against IgG and the complement fractions C3b and C3d. These antibodies did not react with the C4b fraction - which configures a higher specificity, since anti-C4b antibodies often cross-reacts with the antigens of Chido/Rodgers RBC group - nor with erythrocyte antigens from ABO group. These antibodies are, therefore, promising as a reagent for Coombs test [119].
5.6. Enzyme detection
IgY immunoassays were used to detect the hepatic expression of Cytochrome P450 2E1 (CYP2E1) in mice treated with medicinal herbs and products derived from plants rich in flavonoids. CYP2E1 metabolizes a wide variety of chemicals with different structures, in particular small and hydrophobic compounds, including potential cytotoxic and carcinogenic agents. Anti-CYP2E1 IgY was specific, without reacting with other cytochromes, and was able to detect the reduction of hepatic CYPE2E1 expression due to the ingestion of natural products with hepatoprotective effects [120].
5.7. Identification of substances
The ability of IgY in identifying harmful substances in consumer products has been evaluated. An ELISA test was developed to detect the staphylococcal enterotoxin G (SEG), using specific IgY, and showed satisfactory sensitivity and specificity, reducing the interference of protein A that occurs in IgG tests. This test was successfully used to detect SEG in milk and dairy products samples and could therefore be used to identify the toxin in food [121].
Bittner et al. used IgY in ELISA to detect potentially allergenic proteins in commercially available latex gloves. This assay presented similar results to that of the gold standard test, which uses mammalian IgG [122].
IgY can also be used in assays to identify antibiotic residues in food products of animal origin, as demonstrated in a study by He et al., in which produced anti-gentamycin IgY specifically detected the target antibiotic present in animal origin products [123]. Following this rationale, Li et al. used specific IgY to detect kanamycin and gentamycin residues in milk and meat samples by means of competition ELISA and FPIA (fluorescence polarization immunoassay) [124].
The potential of IgY in identifying substances has also been used to evaluate the toxicity of a natural product employed in the alternative medicine. IgY labeled with Quantum dot were successfully used in a lateral flow assay for the detection of rhein; a toxic substance found in the plant Rheum officinale, widely used in Chinese traditional medicine; in plant samples and serum from users [125].
6. Other important applications
6.1. Food preservation
IgY raised against the bacterium Listeria monocytogenes showed a significant inhibitory effect of bacterial growth in liquid medium and in fish samples stored between 0 and 6 °C in a dose-dependent manner, which indicates that anti-L. monocytogenes IgY is a potential antimicrobial for use in the food industry [47]. Taking into consideration the versatility and the range of IgY already tested against several bacteria, this result could be easily apply to other food poisoning bacteria and viruses.
6.2. Use of IgY in bioterrorism circumstance
Among the importance of this technology, LeClair et al. demonstrated that IgY produced against the staphylococcal enterotoxin B (SEB), a potential biological weapon, can save individuals exposed to this material. The results with Rhesus monkeys showed that animals that received anti-SEB IgY 30 min before or 4 h after exposure to a lethal SEB aerosol survived [10], which indicates that anti-SEB IgY could be used to protect populations in a hypothetical context of bioterrorism involving SEB [13].
7. Conclusion
The latest findings using IgY have clearly demonstrated the versatility of this technology. Obtaining IgY from birds presents several technical and economical advantages over mammalian IgG, and as described in this review, IgY technology has a broad spectrum of applications in human and veterinary health. It can be used in multiple types of therapies; it is useful in the prevention of various types of diseases and detects, by means of different techniques, several classes of antigens, such as microorganisms, tumor markers and substances.
Among the advantages of this technology, the replacement of invasive antibody collection by its extraction from eggs is one of the most interesting, considering the animal welfare benefits, with this technology it is possible to achieve great quantities of antibodies with a lower cost of production and less damage to animal welfare.
Due to its structural differences and phylogenetic distance, IgY is more specific for diagnostic use and displays greater avidity for mammalian conserved proteins than IgG, being, thus, an important alternative in the search for more effective diagnostics and therapies. In addition, in view of its proven ability to neutralize microorganisms, IgY represents an important therapeutic resource in times of increasing resistance to antibiotics and emergence of viral diseases for which there is no treatment.
Declaration of Competing Interest
There are no funding or competing interests to report.
References
- 1.Michael A., Meenatchisundaram S., Parameswari G., Subbraj T., Selvakumaran R., Ramalingam S. Chicken egg yolk antibodies (IgY) as an alternative to mammalian antibodies. Indian J. Sci. Technol. 2010;3(4):468–474. doi: 10.17485/ijst/2010/v3i4/29741. [DOI] [Google Scholar]
- 2.Narat M. Production of antibodies in chickens. Food Technol. Biotechnol. 2003;41(3):259–267. [Google Scholar]
- 3.Schade R., Staak C., Hendriksen C., Erhard M., Hugl H., Koch G. The production of avian (egg yolk) antibodies: IgY. The report and recommendations of ECVAM workshop 21. ATLA. 1996;24:925–934. [Google Scholar]
- 4.Pauly D., Chacana P.A., Calzado E.G., Brembs B., Schade R. IgY technology: extraction of chicken antibodies from egg yolk by polyethylene glycol (PEG) precipitation. J. Vis. Exp. 2011;1(51):1–6. doi: 10.3791/3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grando T.H., Baldissera M.D., de Sá M.F., do Carmo G., Porto B.C.Z., Aguirre G.S.V. Avian antibodies (IgY) against Trypanosoma cruzi: purification and characterization studies. J. Immunol. Methods. 2017;449:56–61. doi: 10.1016/j.jim.2017.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polson A., von Wechmar M.B., Fazakerley G. Antibodies to proteins from yolk of immunized hens. Immunol. Commun. 1980;9(5):495–514. doi: 10.3109/08820138009066011. [DOI] [PubMed] [Google Scholar]
- 7.Cai Y., Guo J., Chen S., Tian L., Steinmann P., Chen M. Chicken egg yolk antibodies (IgY) for detecting circulating antigens of Schistosoma japonicum. Parasitol. Int. 2012;61(3):385–390. doi: 10.1016/j.parint.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Rahman S., Nguyen S.V., Icatlo F.C., Jr., Umeda K., Kodama Y. Oral passive IgY-based immunotherapeutics: a novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 2013;9(5):1039–1048. doi: 10.4161/hv.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mendoza J.C., Vivas D., Rodríguez E., Inga R., Sandoval G., Lazo F. Eficacia Experimental de Anticuerpos IgY Producidos en Huevos, Contra el Veneno de la Serpiente Peruana Bothrops atrox. Rev. Peru. Med. Exp. Salud Publica. 2012;29(1):69–75. doi: 10.1590/s1726-46342012000100010. [DOI] [PubMed] [Google Scholar]
- 10.LeClaire R.D., Hunt R.E., Bavari S. Protection against bacterial superantigen staphylococcal enterotoxin B by passive vaccination. Infect. Immun. 2002;70(5):2278–2281. doi: 10.1128/IAI.70.5.2278-2281.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horie K., Horie N., Abdou A.M., Yang J., Yun S., Chun H. Suppressive effect of functional drinking yogurt containing specific egg yolk immunoglobulin on Helicobacter pylori in humans. J. Dairy Sci. 2004;87(12):4073–4079. doi: 10.3168/jds.S0022-0302(04)73549-3. [DOI] [PubMed] [Google Scholar]
- 12.Klemperer F. Ueber natürliche immunität und ihre verwerthung für die immunisirungstherapie. Archiv. Experiment. Phatol. Pharmakol. 1893;31:356–382. [Google Scholar]
- 13.Shade R., Terzolo H. EPC 2006-12th European Poultry Conference, Verona, Italy, 10–14 September. 2006. IgY-technology: Application and trends. [Google Scholar]
- 14.Warr G.W., Magor K.E., Higgins D.A. IgY: clues to the origins of modern antibodies. Immunol. Today. 1995;16(8):392–398. doi: 10.1016/0167-5699(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 15.Leslie G.A., Clem L.W. Phylogeny of immunoglobulin structure and function. J. Exp. Med. 1969;130(6):1337–1352. doi: 10.1084/jem.130.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parvari R., Avivi A., Lentner F., Ziv E., Tel-Or S., Burstein Y. Chicken immunoglobulin gamma-heavy chains: limited VH gene repertoire, combinatorial diversification by D gene segments and evolution of the heavy chain lócus. EMBO J. 1988;7(3):739–744. doi: 10.1002/j.1460-2075.1988.tb02870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spillner E., Braren I., Greunke K., Seismann H., Blank S., du Plessis D. Avian IgY antibodies and their recombinant equivalents in research, diagnostics and therapy. Biologicals. 2012;40(5):313–322. doi: 10.1016/j.biologicals.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larsson A., Wejåker P., Forsberg P., Lindahl T. Chicken antibodies: a tool to avoid interference by complement activation in ELISA. J. Immunol. Methods. 1992;156(1):79–83. doi: 10.1016/0022-1759(92)90013-j. [DOI] [PubMed] [Google Scholar]
- 19.Larsson A., Karlsson-Parra A., Sjöquist J. Use of chicken antibodies in enzyme immunoassays to avoid interference by rheumatoid factors. Clin. Chem. 1991;37(3):411–414. [PubMed] [Google Scholar]
- 20.Akerström B., Brodin T., Reis K., Björck L. Protein G: a powerful tool for binding and detection of monoclonal and polyclonal antibodies. J. Immunol. 1985;135(4):2589–2592. [PubMed] [Google Scholar]
- 21.Carlander D., Stålberg J., Larsson A. Chicken antibodies: a clinical chemistry perspective. Ups. J. Med. Sci. 1999;104(3):179–189. doi: 10.3109/03009739909178961. [DOI] [PubMed] [Google Scholar]
- 22.Calzado E.G., Mario E.C., Chávez T.S., Vázquez E.L., Ochoa Z.C., Schade R. Extraction of a monospecific coombs-reagent from chicken eggs. ALTEX. 2003;20(1):21–25. [PubMed] [Google Scholar]
- 23.Gassmann M., Thömmes P., Weiser T., Hübscher U. Efficient production of chicken egg yolk antibodies against a conserved mammalian protein. FASEB J. 1990;4(8):2528–2532. doi: 10.1096/fasebj.4.8.1970792. [DOI] [PubMed] [Google Scholar]
- 24.Ratcliffe M., Jacobsen K.A. Rearrangement of immunoglobulin genes in chicken B cell development. Semin. Immunol. 1994;6(3):175–184. doi: 10.1006/smim.1994.1023. [DOI] [PubMed] [Google Scholar]
- 25.McCormack W.T., Tjoelker L.W., Thompson C.B. Avian B-cell development: generation of an immunoglobulin repertoire by gene conversion. Annu. Rev. Immunol. 1991;9(1):219–241. doi: 10.1146/annurev.iy.09.040191.001251. [DOI] [PubMed] [Google Scholar]
- 26.Lee K.A., Chang S.K., Lee Y.J., Lee J.H., Koo N.S. Acid stability of anti-Helicobacter pyroli IgY in aqueous polyol solution. J. Biochem. Mol. Biol. 2002;35(5):488–493. doi: 10.5483/BMBRep.2002.35.5.488. [DOI] [PubMed] [Google Scholar]
- 27.Hatta H., Tsuda K., Akachi S., Kim M., Yamamoto T., Ebina T. Oral passive immunization effect of anti-human rotavirus IgY and its behavior against proteolytic enzymes. Biosci. Biotechnol. Biochem. 1993;57(7):1077–1081. doi: 10.1271/bbb.57.1077. [DOI] [PubMed] [Google Scholar]
- 28.Akita E.M., Nakai S. Production and purification of Fab′ fragments from chicken egg yolk immunoglobulin Y (IgY) J. Immunol. Methods. 1993;162(2):155–164. doi: 10.1016/0022-1759(93)90380-P. [DOI] [PubMed] [Google Scholar]
- 29.Li X., Jin L., McAllister T.A., Stanford K., Xu J., Lu Y. Chitosan-alginate microcapsules for oral delivery of egg yolk immunoglobulin (IgY) J. Agric. Food Chem. 2007;55(8):2911–2917. doi: 10.1021/jf062900q. [DOI] [PubMed] [Google Scholar]
- 30.Gao X., Zhang X., Lin L., Yao D., J Sun X. Du. Passive immune-protection of Litopenaeus vannamei against Vibrio harveyi e Vibrio parahaemolyticus infections with anti-Vibrio egg yolk (IgY)-encapsulated feed. Int. J. Mol. Sci. 2016;17(5):1–10. doi: 10.3390/ijms17050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chang H., Lee Y., Chen C.C., Tu Y. Microencapsulation protects immunoglobulin in yolk (IgY) specific against Helicobacter pylori urease. JFS. 2002;67(1):15–20. doi: 10.1111/j.1365-2621.2002.tb11351.x. [DOI] [Google Scholar]
- 32.Kovacs-nolan J., Mine Y. Microencapsulation for the gastric passage and controlled intestinal release of immunoglobulin Y. J. Immunol. Methods. 2005;296(1–2):199–209. doi: 10.1016/j.jim.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu M., Miwa Y., Hashimoto K., Goto A. Encapsulation of chicken egg yolk immunoglobulin G (IgY) by liposomes. Biosci. Biotechnol. Biochem. 1993;57(9):1445–1449. doi: 10.1271/bbb.57.1445. [DOI] [PubMed] [Google Scholar]
- 34.Cho Y., Lee J., Park I., Huh C., Baek Y., Park J. Protective effect of microencapsulation consisting of multiple emulsification and heat gelation processes on immunoglobulin in yolk. JFS. 2006;70(2):148–151. doi: 10.1111/j.1365-2621.2005.tb07088.x. [DOI] [Google Scholar]
- 35.Bellingeri R.V., Picco N.Y., Alustiza F.E., Canova J.V., Molina M.A., Acevedo D.F. Ph-responsive hydrogels to protect IgY from gastric conditions: in vitro evaluation. J. Food Sci. Technol. 2015;52(5):3117–3122. doi: 10.1007/s13197-014-1337-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bellingeri R., Alustiza F., Picco N., Acevedo D., Molina M.A., Rivero R. In vitro toxicity evaluation of hydrogel-carbon nanotubes composites on intestinal cells. J. Appl. Polym. Sci. 2015;132(5):41370–41377. doi: 10.1002/app.41370. [DOI] [Google Scholar]
- 37.Vega C., Bok M., Chacana P., Saif L., Fernandez F., Parreño V. Egg yolk IgY: protection against rotavirus induced diarrhea and modulatory effect on the systemic and mucosal antibody responses in newborn calves. Vet. Immunol. Immunophatol. 2011;142(3–4):156–169. doi: 10.1016/j.vetimm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vega C., Bok M., Saif L., Fernandez F., Parreño V. Egg yolk IgY antibodies: a therapeutic intervention against group a rotavirus in calves. Res. Vet. Sci. 2015;103:1–10. doi: 10.1016/j.rvsc.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schade R., Calzado E.G., Sarmiento R., Chacana P.A., Porankiewicz-Asplund J., Terzolo H.R. Chicken egg yolk antibodies (IgYtechnology): a review of progress in production and use in research and human and veterinary medicine. ATLA. 2005;33(2):129–154. doi: 10.1177/026119290503300208. [DOI] [PubMed] [Google Scholar]
- 40.Chalghoumi R., Beckers Y., Portetelle D., Théwis A. Hen egg yolk antibodies (IgY), production and use for passive immunization against bacterial enteric infections in chicken: a review. Biotechnol. Agro. Soc. Environ. 2009;13(2):295–308. [Google Scholar]
- 41.Bollen L.S., Crowley A., Stodulski G., Hau J. Antibody production in rabbits and chickens immunized with human IgG. A comparison of titre and avidity development in rabbit serum, chicken serum and egg yolk using three different adjuvants. J. Immunol. Methods. 1996;191(2):113–120. doi: 10.1016/0022-1759(96)00010-5. [DOI] [PubMed] [Google Scholar]
- 42.Wanke R., Schmidt P., Erhard M.H., Sprick-Sanjose A.M., Stangassinger M., Schmahl W. Freund's complete adjuvant in the chicken: efficient immunostimulation with severe local inflammatory reaction. Zentralbl Veterinarmed A. 1996;43(4):243–253. [PubMed] [Google Scholar]
- 43.Olbrich C., Müller R.H., Tabatt K., Kayser O., Schulze C., Schade R. Stable biocompatible adjuvants—a new type of adjuvant based on solid lipid nanoparticles: a study on cytotoxicity, compatibility and efficacy in chicken. Altern. Lab. Anim. 2002;30(4):443–458. doi: 10.1177/026119290203000407. [DOI] [PubMed] [Google Scholar]
- 44.Reddy P.K., Shekar A., Kingston J.J., Sripathy M.H., Batra H. Evaluation of IgY capture ELISA for sensitive detection of alphahemolysin of Staphylococcus aureus without staphylococcal protein A interference. J. Immunol. Methods. 2013;391(1–2):31–38. doi: 10.1016/j.jim.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Łupicka-Słowik A., Walczak M., Grzywa R., Bobrek K., Łęcka M., Boivin S. Generation and application of polyclonal IgY antibodies specific for full-length and nicked prostate-specific antigen. Bioanalysis. 2014;6(23):3197–3213. doi: 10.4155/bio.14.172. [DOI] [PubMed] [Google Scholar]
- 46.Thibodeau A., Fravalo P., Perron A., Laurent-Lewandowski S., Letellier A. Production and characterization of anti-Campylobacter jejuni IgY derived from egg yolks. Acta Vet. Scand. 2017;59(80):1–9. doi: 10.1186/s13028-017-0346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sui J., Cao L., Lin H. Antibacterial activity of egg yolk antibody (IgY) against Listeria monocytogenes and preliminary evaluation of its potential for food preservation. J. Sci. Food Agric. 2011;91(11):1946–1950. doi: 10.1002/jsfa.4381. [DOI] [PubMed] [Google Scholar]
- 48.Wen J., Zhao S., He D., Yang Y., Li Y., Zhu S. Preparation and characterization of egg yolk immunoglobulin Y specific to influenza B vírus. Antivir. Res. 2012;93(1):154–159. doi: 10.1016/j.antiviral.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 49.Grzywa R., Łupicka-Słowik A., Walczak M., Idzi M., Bobrek K., Boivin S. Highly sensitive detection of cancer antigen 15-3 using novel avian IgY antibodies. Altex. 2014;31(1):43–52. doi: 10.14573/altex.1309181. [DOI] [PubMed] [Google Scholar]
- 50.Meenatchisundaram S., Shanmugam V., Anjali V.M. Development of chicken egg yolk antibodies against Streptococcus mitis – purification and neutralizing efficacy. J. Basic Clin. Pharm. 2011;2(2):109–114. [PMC free article] [PubMed] [Google Scholar]
- 51.Akita E.M., Nakai S. Lmmunoglobulins from egg yolk: isolation and purification. J. Food Sci. 1992;57(3):629–634. doi: 10.1111/j.1365-2621.1992.tb08058.x. [DOI] [Google Scholar]
- 52.Araújo A.S., Lobato Z.I.P., Chávez-Olórtegui C., Velarde D.T. Brazilian IgY-Bothrops antivenom: studies on the development of a process in chicken egg yolk. Toxicon. 2010;55(4):739–744. doi: 10.1016/j.toxicon.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Tu Y., Chen C., Chang H. Isolation of immunoglobulin in yolk (IgY) and rabbit serum immunoglobulin G (IgG) specific against bovine lactoferrin by immunoaffinity chromatograph. Food Res. Int. 2001;34(9):783–789. doi: 10.1016/S0963-9969(00)00172-1. [DOI] [Google Scholar]
- 54.Kuronen I., Kokko H., Mononen I., Parviainen M. Hen egg yolk antibodies purified by antigen affinity under highly alkaline conditions provide new tools for diagnostics. Human intact parathyrin as a model antigen. Eur. J. Clin. Chem. Clin. Biochem. 1997;35(6):435–440. doi: 10.1515/cclm.1997.35.6.435. [DOI] [PubMed] [Google Scholar]
- 55.Santos F.N., Brum B.C., Cruz P.B., Molinaro C.M., Silva V.L., Chaves S.A.M. Production and characterization of IgY against canine IgG: prospect of a new tool for the immunodiagnostic of canine diseases. Braz. Arch. Biol. Technol. 2014;57(4):523–531. doi: 10.1590/S1516-89132014005000020. [DOI] [Google Scholar]
- 56.Hutchens T.W. Thiophilic adsorption chromatography. Methods Mol. Biol. 1992;11:1–15. doi: 10.1385/0-89603-213-2:1. [DOI] [PubMed] [Google Scholar]
- 57.Fink A.L., Williams K.L., Harris E., Alvine T.D., Henderson T., Schiltz J. Dengue virus specific IgY provides protection following lethal dengue virus challenge and is neutralizing in the absence of inducing antibody dependent enhancement. PLoS Negl. Trop. Dis. 2017;11(7):1–17. doi: 10.1371/journal.pntd.0005721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Najdi S., Brujeni G.N., Sheikhi N., Chakhkar S. Development of anti-Helicobacter pylori immunoglobulins Y (IgYs) in quail. Iran J. Vet. Res. 2016;17(2):106–110. [PMC free article] [PubMed] [Google Scholar]
- 59.Nasiri K., Nassiri M.R., Tahmoorespur M., Haghparast A., Zibaee S. Production and characterization of egg yolk antibody (IgY) against recombinant VP8-S2 antigen. Pol. J. Vet. Sci. 2016;19(2):271–279. doi: 10.1515/pjvs-2016-0034. [DOI] [PubMed] [Google Scholar]
- 60.Li X., Yao Y., Wang X., Zhen Y., Thacker P.A., Wang L. Chicken egg yolk antibodies (IgY) modulate the intestinal mucosal immune response in a mouse model of Salmonella typhimurium infection. Int. Immunopharmacol. 2016;36:305–314. doi: 10.1016/j.intimp.2016.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pizarro-Guajardo M., Díaz-González F., Álvarez-Lobos M., Paredes-Sabja D. Characterization of chicken IgY specific to Clostridium difficile R20291 spores and the effect of oral administration in mouse models of initiation and recurrent disease. Front. Cell. Infect. Microbiol. 2017;7:1–16. doi: 10.3389/fcimb.2017.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Malekshahi Z.V., Gargari S.L.M., Rasooli I., Ebrahimizadeh W. Treatment of Helicobacter pylori infection in mice with oral administration of egg yolk-driven anti-UreC immunoglobulin. Micro. Pathog. 2011;51(5):366–372. doi: 10.1016/j.micpath.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 63.Hong K.S., Ki M., Ullah H.M.A., Lee E., Kim Y.D., Chung M. Preventive effect of anti-VacA egg yolk immunoglobulin (IgY) on Helicobacter pylori-infected mice. Vaccine. 2018;36(3):371–380. doi: 10.1016/j.vaccine.2017.11.082. [DOI] [PubMed] [Google Scholar]
- 64.Borhani K., Mobarez A.M., Khabiri A.R., Behmanesh M., Khoramabadi N. Inhibitory effects of rHP-NAP IgY against H. pylori attachment to AGS cell line. Microb. Pathog. 2016;97:231–235. doi: 10.1016/j.micpath.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 65.Solhi R., Alebouyeh M., Khafri A., Rezaeifard M., Aminian M. In vitro evaluation of cross-strain inhibitory effects of IgY polyclonal antibody against H. pylori. Microbial Pathog. 2017;110:682–687. doi: 10.1016/j.micpath.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 66.Sudjarwo S.A., Eraiko K., Sudjarwo G.W. Koerniasari, the activity of immunoglobulin Y anti-mycobacterium tuberculosis on proliferation and cytokine expression of rat peripheral blood mononuclear cells. Pharm. Res. 2017;9(1):5–8. doi: 10.4103/pr.pr_66_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shi H., Zhub J., Zouc B., Shic L., Duc L., Long Y. Effects of specific egg yolk immunoglobulin on pan-drug-resistant Acinetobacter baumannii. Biomed. Pharmacother. 2017;95:1734–1742. doi: 10.1016/j.biopha.2017.09.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hou Y., Zhen Y., Wang D., Zhu J., Sun D., Liu X. Protective effect of an egg yolk-derived immunoglobulin (IgY) against Prevotella intermedia-mediated gingivitis. J. Appl. Microbiol. 2014;116(4):1020–1027. doi: 10.1111/jam.12419. [DOI] [PubMed] [Google Scholar]
- 69.Xu F.X., Xu Y.P., Jin L.J., Liu H., Wang L.H., You J.S. Effectiveness of egg yolk immunoglobulin (IgY) against periodontal diseasecausing Fusobacterium nucleatum. J. Appl. Microbiol. 2012;113(4):983–991. doi: 10.1111/j.1365-2672.2012.05396.x. [DOI] [PubMed] [Google Scholar]
- 70.Revathy J., Karthika S., Sentila R., Michael A. In vitro evaluation of the efficacy of chicken egg yolk antibodies (IgY) generated against Propionibacterium acnes. Int. J. Cosmet. Sci. 2014;36(1):68–73. doi: 10.1111/ics.12097. [DOI] [PubMed] [Google Scholar]
- 71.Bachtiar E.W., Soejoedono R.D., Bachtiar B.M., Henrietta A., Farhana N., Yuniastuti M. Effects of soybean milk, chitosan, and anti-Streptococcus mutans IgY in malnourished rats' dental biofilm and the IgY persistencyin saliva. Interv. Med. Appl. Sci. 2015;7(3):118–123. doi: 10.1556/1646.7.2015.3.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nguyen S.V., Icatlo F.C., Nakano T., Isogai E., Hirose K., Mizugai H. Anti-cell-associated glucosyltransferase immunoglobulin Y suppression of salivary mutans streptococci in healthy young adults. JADA. 2011;142(8):943–949. doi: 10.14219/jada.archive.2011.0301. [DOI] [PubMed] [Google Scholar]
- 73.Bachtiar E.W., Bachtiar B.M., Soejoedono R.D., Wibawan I.W., Afdhal A. Biological and immunogenicity property of IgY Anti S. mutans ComD. Open Dent. J. 2016;10(1):308–314. doi: 10.2174/1874210601610010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomsen K., Christophersen L., Jensen P.Ø., Bjarnsholt T., Moser C., Høiby N. Anti-Pseudomonas aeruginosa IgY antibodies promote bacterial opsonization and augment the phagocytic activity of polymorphonuclear neutrophils. Hum. Vaccin. Immunother. 2016;12(7):1690–1699. doi: 10.1080/21645515.2016.1145848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alustiza F., Bellingeri R., Picco N., Motta C., Grosso M.C., Barbero C.A. IgY against enterotoxigenic Escherichia coli administered by hidrogel-carbon nanotubes composites to prevent neonatal diarrhoea in experimentally challenged piglets. Vaccine. 2016;34(28):3291–3297. doi: 10.1016/j.vaccine.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 76.Gan H., He H., Sato A., Hatta H., Nakao M., Somamoto T. Ulcer disease prophylaxis in koi carp by bath immersion with chicken egg yolk containing anti-Aeromonas salmonicida IgY. Res. Vet. Sci. 2015;99:82–86. doi: 10.1016/j.rvsc.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 77.Witte W. Medical consequences of antibiotic use in agriculture. Sci. 1998;279(5353):996–997. doi: 10.1126/science.279.5353.996. [DOI] [PubMed] [Google Scholar]
- 78.Silbergeld E.K., Graham J., Price L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health. 2008;29:151–169. doi: 10.1146/annurev.publhealth.29.020907.090904. [DOI] [PubMed] [Google Scholar]
- 79.Lee D.H., Jeon Y., Park C., Kim S., Lee D.S., Lee C. Immunoprophylactic effect of chicken egg yolk antibody (IgY) against a recombinant S1 domain of the porcine epidemic diarrhea vírus spike protein in piglets. Arch. Virol. 2015;160(9):2197–2207. doi: 10.1007/s00705-015-2494-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nguyen H.H., Tumpey T.M., Park H., Byun Y., Tran L.D., Nguyen V.D. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One. 2010;5(4):1–11. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wallach M.G., Webby R.J., Islam F., Walkden-Brown S., Emmoth E., Feinstein R. Cross-protection of chicken immunoglobulin Y antibodies against H5N1 and H1N1 viruses passively administered in mice. Clin. Vaccine Immunol. 2011;18(7):1083–1090. doi: 10.1128/CVI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Haese N., Brocato R.L., Henderson T., Nilles M.L., Kwilas S.A., Josleyn M.D. Antiviral biologic produced in DNA vaccine/goose platform protects hamsters against hantavirus pulmonary syndrome when administered post-exposure. PLoS Negl. Trop. Dis. 2015;9(6):1–19. doi: 10.1371/journal.pntd.0003803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aizenshtein E., Yosipovicha R., Kvint M., Shadmona R., Krispel S., Shuster E. Practical aspects in the use of passive immunization as an alternative to attenuated viral vacines. Vaccine. 2016;34(22):2513–2518. doi: 10.1016/j.vaccine.2016.03.051. [DOI] [PubMed] [Google Scholar]
- 84.Takeuchi S., Motohashi J., Kimori H., Nakagawa Y., Tsurumoto A. Effects of oral moisturising gel containing egg yolk antibodies against Candida albicans in older people. Gerodontology. 2014;33(1):128–134. doi: 10.1111/ger.12139. [DOI] [PubMed] [Google Scholar]
- 85.Kamikawa Y., Fujisaki J., Nagayama T., Kawasaki K., Hirabayashi D., Hamada T. Use of Candida-specific chicken egg yolk antibodies to inhibit the adhering of Candida to denture base materials: prevention of denture stomatitis. Gerontology. 2016;33(3):342–347. doi: 10.1111/ger.12163. [DOI] [PubMed] [Google Scholar]
- 86.Sampaio L.C.L., Baldissera M.D., Grando T.H., Gressler L.T., Capeleto D.M., de Sá M.F. Production, purification and therapeutic potential of egg yolk antibodies for treating Trypanosoma evansi infection. Vet. Parasitol. 2014;204(3–4):96–103. doi: 10.1016/j.vetpar.2014.05.032. [DOI] [PubMed] [Google Scholar]
- 87.Amirijavid S., Entezari M., Movafagh A., Hashemi M., Mosavi-Jarahi A., Dehghani H. Apoptotic killing of breast cancer cells by IgYs produced against a small 21 aminoacid epitope of the human TRAIL-2 receptor, Asian Pac. J. Cancer Prev. 2016;17(3):293–297. doi: 10.7314/apjcp.2016.17.s3.293. [DOI] [PubMed] [Google Scholar]
- 88.Xiao Y., Gao X., Taratula O., Treado S., Urbas A., Holbrook R.D. Anti-HER2 IgY antibody-functionalized single-walled carbon nanotubes for detection and selective destruction of breast cancer cells. BMC Cancer. 2009;9(1):351–361. doi: 10.1186/1471-2407-9-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hirose M., Ando T., Shofiqur R., Umeda K., Kodama Y., Nguyen S.V. Anti-obesity activity of hen egg anti-lipase immunoglobulin yolk, a novel pancreatic lipase inhibitor. Nutr. Metab. 2013;10(1):1–6. doi: 10.1186/1743-7075-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wei-xu H., Wen-yun Z., Xi-ling Z., Zhu W., Li-hua W., Xiao-mu W. Anti-Interleukin-1 beta/tumor necrosis factor-alpha IgY antibodies reduce pathological allergic responses in guinea pigs with allergic rhinitis. Mediat. Inflamm. 2016;2016:1–11. doi: 10.1155/2016/3128182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sjostrom L., Al-Abdulla I.H., Rawat S., Smith D.C., Landon J. A comparison of ovine and equine antivenoms. Toxicom. 1994;32(4):427–433. doi: 10.1016/0041-0101(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 92.de Andrade F.G., Eto S.F., Ferraro A.C.N.S., Marioto D.T.G., Vieira N.J., Cheirubim A.P. The production and characterization of antibothropic and anti-crotalic IgY antibodies in laying hens: a long term experiment. Toxicon. 2013;66:18–24. doi: 10.1016/j.toxicon.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 93.Aguilar I., Sánchez E.E., Girón M.E., Estrella A., Guerrero B., Rodriguez-Acosta F.A. Coral snake antivenom produced in chickens (Gallus domesticus) Rev. Inst. Med. Trop. Sao Paulo. 2014;56(1):61–66. doi: 10.1590/S0036-46652014000100009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zolfagharian H., Dounighi N.M. Study on development of Vipera lebetina snake anti-venom in chicken egg yolk for passive immunization. Hum. Vaccin. Immunother. 2015;11(11):2734–2739. doi: 10.4161/21645515.2014.985492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Duan H., He Q., Zhou B., Wang W., Li B., Zhang Y. Anti-Trimeresurus albolabris venom IgY antibodies: preparation, purification and neutralization efficacy. J. Venom Anim. Toxins Incl. Trop. Dis. 2016;22(1):4–9. doi: 10.1186/s40409-016-0078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu J., He Q., Wang W., Zhou B., Li B., Zhang Y. Preparation and neutralization efficacy of IgY antibodies raised against Deinagkistrodon acutus venom. J. Venom Anim. Toxins Incl. Trop. Dis. 2017;23(1):1–9. doi: 10.1186/s40409-017-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.da Rocha D.G., Fernandez J.H., de Almeida C.M.C., da Silva C.L., Magnoli F.C., da Silva O.E. Development of IgY antibodies against anti-snake toxins endowed with highly lethal neutralizing activity. Eur. J. Pharm. Sci. 2017;106:404–412. doi: 10.1016/j.ejps.2017.05.069. [DOI] [PubMed] [Google Scholar]
- 98.Navarro D., Vargas M., Herrera M., Segura A., Gómez A., Villalta M. Development of a chicken-derived antivenom against the taipan snake (Oxyuranus scutellatus) venom and comparison with an equine antivenom. Toxicon. 2016;120:1–8. doi: 10.1016/j.toxicon.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 99.Alvarez A., Montero Y., Jimenez E., Zerpa N., Parrilla P., Malavé C. Antibodies anti-Tityus caripitensis venom: purification and neutralization efficacy. Toxicon. 2013;74:208–214. doi: 10.1016/j.toxicon.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 100.Gujral N., Löbenberg R., Suresh M., Sunwoo H. In-vitro and in-vivo binding activity of chicken egg yolk immunoglobulin Y (IgY) against gliadin in food matrix. J. Agric. Food Chem. 2012;60(12):3166–3172. doi: 10.1021/jf205319s. [DOI] [PubMed] [Google Scholar]
- 101.Bobeck E.A., Hellestad E.M., Helvig C.F., Petkovich P.M., Cook M.E. Oral antibodies to human intestinal alkaline phosphatase reduce dietary phytate phosphate bioavailability in the presence of dietary 1α-hydroxycholecalciferol. Poult. Sci. 2016;95(3):570–580. doi: 10.3382/ps/pev341. [DOI] [PubMed] [Google Scholar]
- 102.He J., Wang Y., Sun S., Zhang X. Evaluation of chicken IgY generated against canine parvovirus viral-like particles and development of enzyme-linked immunosorbent assay and immunochromatographic assay for canine parvovirus detection. Viral Immunol. 2015;28(9):489–494. doi: 10.1089/vim.2015.0030. [DOI] [PubMed] [Google Scholar]
- 103.Zhang X., Diraviyam T., Li X., Yao G., Michael A. Preparation of chicken IgY against recombinant E2 protein of bovine viral diarrhea virus (BVDV) and development of ELISA and ICA for BVDV detection. Biosci. Biotechnol. Biochem. 2016;80(12):2467–2472. doi: 10.1080/09168451.2016.1217144. [DOI] [PubMed] [Google Scholar]
- 104.da Silva A.S., de Vasconcelos G.A.L.B.M., Kappel L.A., Pinto M.A., de Paula V.S. An immunoenzymatic assay for the diagnosis of hepatitis A utilising immunoglobulin Y. Mem. Inst. Oswaldo Cruz. 2012;107(7):960–963. doi: 10.1590/s0074-02762012000700022. [DOI] [PubMed] [Google Scholar]
- 105.Bentes G.A., Lanzarini N.M., Lima L.R.P., Manso P.P.A., Silva A.S. da, Mouta Junior S.S. Using immunoglobulin Y as an alternative antibody for the detection of hepatitis A virus in frozen liver sections. Mem. Inst. Oswaldo Cruz. 2015;110(4):577–579. doi: 10.1590/0074-02760140457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L., Li D., Liu L., Fang J., Xua R., Zhang G. Development of a colloidal gold immunochromatographic strip for the rapid detection of soft-shelled turtle systemic septicemia spherical vírus. J. Virol. Methods. 2015;221:39–45. doi: 10.1016/j.jviromet.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 107.Palaniyappan A., Das D., Kammila S., Suresh M.R., Sunwoo H.H. Diagnostics of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) nucleocapsid antigen using chicken immunoglobulin Y. Poult. Sci. 2012;91(3):636–642. doi: 10.3382/ps.2011-01916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Figueiredo A., Vieira N.C.S., dos Santos J.F., Janegitz B.C., Aoki S.M., Junior P.P. Electrical detection of dengue biomarker using egg yolk immunoglobulin as the biological recognition element. Sci. Rep. 2015;5(1):1–5. doi: 10.1038/srep07865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Richman D.D., Cleveland P.H., Oxman M.N., Johnson K.M. The binding of staphylococcal protein A by the sera of different animal species. J. Immunol. 1982;128(5):2300–2305. [PubMed] [Google Scholar]
- 110.Walczak M., Grzywa R., Łupicka-Słowik A., Skorenski M., Bobrek K., Nowak D. Method for generation of peptide-specific IgY antibodies directed to Staphylococcus aureus extracellular fibrinogen binding protein epitope. Biopolymers. 2015;104(5):552–559. doi: 10.1002/bip.22695. [DOI] [PubMed] [Google Scholar]
- 111.Mudili V., Makam S.S., Sundararaj N., Siddaiah C., Gupta V.K., Rao P.V.L. A novel IgY-aptamer hybrid system for cost-effective detection of SEB and its evaluation on food and clinical samples. Sci. Rep. 2015;5(1):1–12. doi: 10.1038/srep15151. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Yamada K., Wanchun J., Ohkura T., Murai A., Hayakawa R., Kinoshita K. Detection of methicillin-resistant staphylococcus aureus using a specific anti-PBP2a chicken IgY antibody. Jpn. J. Infec. Dis. 2013;66(2):103–108. doi: 10.7883/yoken.66.103. [DOI] [PubMed] [Google Scholar]
- 113.Cakir-Koc R. Production of anti-SAG-1 IgY antibody against Toxoplasma gondii parasites and evaluation of antibody activity by ELISA method. Parasitol. Res. 2016;115(8):2947–2952. doi: 10.1007/s00436-016-5047-9. [DOI] [PubMed] [Google Scholar]
- 114.Sert M., Cakir-Koc R., Kilinc Y.B. Novel Fitc-labeled Igy antibody: fluorescence imaging Toxoplasma gondii in vitro. Sci. Rep. 2017;7(1):1–7. doi: 10.1038/s41598-017-00930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Teimoori S., Arimatsu Y., Laha T., Kaewkes S., Sereerak P., Sripa M. Chicken IgY-based coproantigen capture ELISA for diagnosis of human opisthorchiasis. Parasitol. Int. 2017;66(4):443–447. doi: 10.1016/j.parint.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Miura V.C., Aoki S.M., Junior P.P., Pires L.C., Dalmagro P., Nakamura A.A. Evaluation of recombinant Cryptosporidium hominis GP60 protein and anti-GP60 chicken polyclonal IgY for research and diagnostic purposes. Rev. Bras. Parasitol. Vet. 2017;26(2):205–210. doi: 10.1590/S1984-29612017032. [DOI] [PubMed] [Google Scholar]
- 117.Sun Y., Yang Y., Wang L., Lv L., Zhu J., Han W. Highly sensitive detection of cancer antigen human epidermal growth factor receptor 2 using novel chicken egg yolk immunoglobulin. Biologicals. 2015;43(3):165–170. doi: 10.1016/j.biologicals.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 118.Łupicka-Słowik A., Psurski M., Grzywa R., Bobrek K., Smok P., Walczak M. Development of adenosine deaminase-specific IgY antibodies: diagnostic and inhibitory application. Appl. Biochem. Biotechnol. 2017;184(4):1–17. doi: 10.1007/s12010-017-2626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Calzado E.J.G., Heredia M.T., Duharte J.F., Zarnani A. Evaluation of IgY antibody as a polyspecific coombs-reagent. Aviccena J. Med. Biotechnol. 2017;9(2):87–93. [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang Z., Jiang X., Li C., Xue H., Zhang X. Development of an IgY antibody-based immunoassay for the screening of the CYP2E1 inhibitor/enhancer from herbal medicines. Front. Pharmacol. 2016;7:1–11. doi: 10.3389/fphar.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Nagaraj S., Ramlal S., Kingston J., Batr H.V. Development of IgY based sandwich ELISA for the detection of staphylococcal enterotoxin G (SEG), an egc toxin. Int. J. Food Microbiol. 2016;237:136–141. doi: 10.1016/j.ijfoodmicro.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 122.Bittner C., Garrido M.V., Krach L.H., Harth V. Content of asthmagen natural rubber latex allergens in commercial disposable gloves. Adv. Exp. Med. Biol. 2016;921:37–44. doi: 10.1007/5584_2016_227. [DOI] [PubMed] [Google Scholar]
- 123.He J., Hu J., Thirumalai D., Schade R., Du E., Zhang X. Development of indirect competitive ELISA using egg yolk-derived immunoglobulin (IgY) for the detection of gentamicin residues. J. Environ. Sci. Health, Part B. 2016;51(1):8–13. doi: 10.1080/03601234.2015.1080479. [DOI] [PubMed] [Google Scholar]
- 124.Li C., Zhang Y., Eremin S.A., Yakup O., Yao G., Zhang X. Detection of kanamycin and gentamicin residues in animal-derived food using IgY antibody based ic-ELISA and FPIA. Food Chem. 2017;227:48–54. doi: 10.1016/j.foodchem.2017.01.058. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Kong H., Liu X., Cheng J., Zhang M., Wang Y. Quantum dot-based lateral-flow immunoassay for rapid detection of rhein using specific egg yolk antibodies. Artif. Cells Nanomed. Biotechnol. 2017;46(8):1685–1693. doi: 10.1080/21691401.2017.1389749. [DOI] [PubMed] [Google Scholar]