Abstract
Bovine coronaviruses (BCoVs) are widespread around the world and cause enteric or respiratory infections among cattle. The current study includes 13 samples from BCoVs collected in Normandy during an 11-year period (from 2003 to 2014), 16 French HCoV-OC43s, and 113 BCoVs or BCoVs-like sequence data derived from partial or complete genome sequences available on GenBank. According to a genotyping method developed previously for HCoV-OC43, BCoVs and BCoVs-like are distributed on three main sub-clusters named C1, C2, and C3. Sub-cluster C1 includes BCoVs and BCoVs-like from America and Asia. Sub-cluster C2 includes BCoVs from Europe. Sub-cluster C3 includes prototype, vaccine, or attenuated BCoV strains. The phylogenetic analyses revealed the monophyletic status of the BCoVs from France reported here for the first time. Moreover, BCoV exhibits a relative genetic stability when compared to HCoV-OC43 we previously described from the same region. The numerous recombination detected between HCoV-OC43 were much less frequent for BCoV. The analysis points thus to the influence of different evolutive constraints in these two close groups.
Keywords: Genotyping, Bovine coronavirus, Human coronavirus OC43, Evolution, Epidemiology, Recombination, Respiratory infection
Graphical abstract
Highlight
-
•
We develop a genotyping methodology for BCoVs.
-
•
We report first BCoV partial genomic data from France.
-
•
We observe a different evolutive patterns for the BCoV and HCoV-OC43.
-
•
We don't report the existence of recombinant variants among BCoVs.
-
•
We show a geographical distribution of BCoVs.
1. Introduction
Coronaviruses are the largest enveloped single-strand RNA viruses, ranging from 26 to 31 kilobases in size. They belong to the Coronaviridae family in the Nidovirales order, also including the families of Arteriviridae, Roniviridae, and the last described Mesoniviridae (Lauber et al., 2012, Gorbalenya et al., 2006).
Coronaviruses are divided into four genera named Alpha-, Beta-, Gamma- and Deltacoronavirus based on phylogenetic distance of highly conserved domains (Adams and Carstens, 2012, Woo et al., 2012). Betacoronavirus genus is divided into the four clades A to D. Coronaviruses infect a wide range of avian or mammalian species, and are responsible for enteric or respiratory infections (Woo et al., 2009). Interest in coronaviruses increased by the emergence of two human coronaviruses, Severe acute respiratory syndrome-related coronavirus (SARS-CoV) and Middle-East respiratory syndrome coronavirus (MERS-CoV) in 2002 and 2012, respectively. Both these coronaviruses belong to the Betacoronavirus genus, clades B and C, respectively, and cause severe respiratory diseases in humans. SARS-CoV emerged in human in 2002–2003, causing an outbreak with more than 8000 cases with a fatality rate of nearly 10% (Peiris et al., 2004). MERS-CoV emerged in 2012 in the Middle-East (Zaki et al., 2012). To date, 1413 laboratory cases of MERS-CoV infection have been confirmed, including 502 fatal cases, reported by the World Health Organization (WHO) (Institut National de Veille Sanitaire (INVS), 2015). Both SARS-CoV and MERS-CoV have a zoonotic origin, with an animal reservoir in close contact with the human population. The zoonotic potential of coronaviruses implies the need for surveillance of coronaviruses associated with domestic animals in close contact with human population.
Bovine coronavirus (BCoV) belongs to the Betacoronavirus genus clade A. This clade also contains other closely related coronaviruses known as Bovine-like coronavirus (BCoV-like), originating from captive wild ruminants such as waterbuck (Kobus ellypsiprimus), sambar deer (Cervus unicolor), white-tailed deer (Odocoileus virginianus), elk (Cervus elephus), giraffe (Giraffa camelopardalis), and sable antelope (Hipotragus niger) (Alekseev et al., 2008). These coronaviruses were antigenically, biologically, and genetically highly related to BCoV (Alekseev et al., 2008, Tsunemitsu et al., 1995, Majhdi et al., 1997, Hasoksuz et al., 2008). BCoVs-like were also detected from camelids such as alpaca (Vicugna pacos), llama (Lama glama), and dromedary camel (Camelius dromedarius). Particularly, a BCoV-like, named Dromedary camel coronavirus (DcCoV), was detected from dromedary camel feces in the United Arab Emirates in 2013, during an epidemiological investigation aiming to identify the animal reservoir of MERS-CoV (Cebra et al., 2003, Woo et al., 2014). BCoVs-like are globally responsible for enteric infection. However, in 2002 a BCoV-like responsible for respiratory infection was detected from a dog in United Kingdom (Erles et al., 2003). To date, only 15 complete genomes of BCoVs, including 13 from USA, one from Canada, and one from South Korea are available on GenBank. Moreover, 16 BCoVs-like complete genomes, including 12 from captive ruminants, one from a dog, and three from dromedary camels are available on GenBank (consulted on September 8th 2015). Much earlier, in 1988, a coronavirus closely related to BCoV was isolated from the feces of a diarrheic child in Germany. This coronavirus, tentatively named at first Human enteric coronavirus (HEC 4408), revealed itself to be closer to BCoVs than to BCoV-Like and to other HCoVs indeed. The phylogenetic position of this virus and the context of this case suggests the infection of the child by a cattle virus, consequently to a species barrier-crossing (Zhang et al., 1994).
BCoV is closely related to the Human coronavirus OC43 (HCoV-OC43), that was first isolated in 1967 (McIntosh et al., 1967). More precisely, BCoV and HCoV-OC43 share a global nucleotide identity of 96%. The genetic comparison of BCoV and HCoV-OC43 reveals that HCoV-OC43 results from a zoonotic transmission from bovine to human. Using a molecular clock analysis, Vijgen et al. estimated the tMRCA of these viruses around 1890 (Vijgen et al., 2005). The intraspecific variability of the HCoV-OC43 revealed the co-circulation of numerous recombinant variants (Kin et al., 2015). Given the similarity of HCoV-OC43 with BCoV, we were interested in testing the genetic variability of BCoV using the same methods. Thus, the objective of the current study was to investigate the intraspecific genetic variability of BCoV collected in Normandy from 2003 to 2014 and to test whether recombination remain a major mechanism of variability in BCoVs as seen for his sister clade HCoV-OC43.
2. Materials and methods
2.1. Samples and positive control
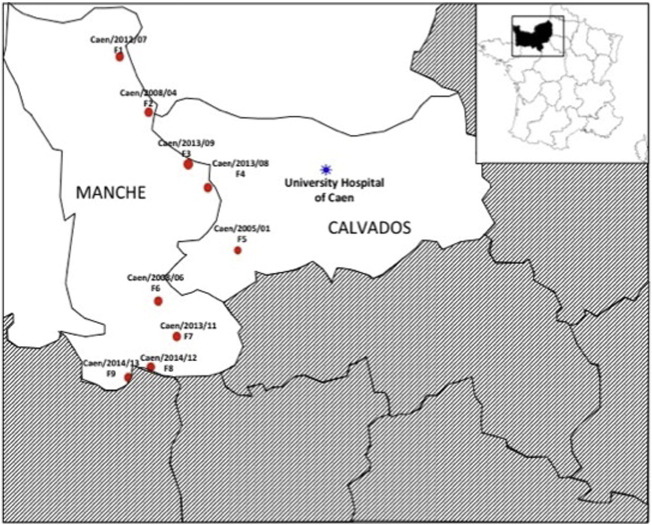
Thirteen field samples positive for BCoV and one cell-culture BCoV supernatant were included in this study. The 13 field samples were provided to us by laboratories of Normandy specializing in veterinary diagnostics. These samples include 12 diarrheic fecal samples and one nasal aspirate. The cattle herd of origin of nine samples are known precisely and depicted in Fig. 1 . The cell-culture supernatant is derived from a fecal sample propagated on HRT-18 cells in our laboratory since 1990s. We used this BCoV as positive control. Age, type of samples and counties of origin of specimens, when available, are indicated in Table 1 .
Fig. 1.
Location of the livestock where nine of our samples were collected. The name of sample was indicated above each farm of origin as follows: Caen/year of sampling/specimen number. The nine corresponding farms were named F1 to F9.
Table 1.
Features of the 14 BCoV samples used in this study. amo., month; d., days. bna, not available.
| Specimen | Age at time samplinga,b | Type of sample | Department of originb |
|---|---|---|---|
| Caen/2005/01 | na | Respiratory | Calvados |
| Caen/2005/02 | na | Enteric | Calvados |
| Caen/unknown/03⁎ | na | Culture supernatant (HRT18) | na |
| Caen/2008/04 | < 1 mo. | Enteric | Manche |
| Caen/2003/05 | na | Enteric | Calvados |
| Caen/2010/06 | 5 d. | Enteric | Manche |
| Caen/2012/07 | < 1 mo. | Enteric | Manche |
| Caen/2013/08 | 15 d. | Enteric | Manche |
| Caen/2013/09 | < 1 mo. | Enteric | Manche |
| Caen/2013/10 | < 1 mo. | Enteric | Manche |
| Caen/2013/11 | < 1 mo. | Enteric | Manche |
| Caen/2014/12 | < 1 mo. | Enteric | Manche |
| Caen/2014/13 | < 1 mo. | Enteric | Manche |
| Caen/2004/14 | 7 d. | Enteric | Calvados |
Propagated on cell culture since 1990s.
2.2. Sample preparation, nucleic-acid extraction, and real-time RT-PCR
Fecal samples were resuspended (10% suspension in sterile PBS Buffer) and centrifuged at 3000 rpm for 10 min. Total nucleic acids of 200 μl of fecal suspension, nasal aspirate, and culture supernatant were manually extracted using High Pure RNA Isolation Kit (Roche, Basel, Sweden) following the manufacturer's instructions. The determination of the cycle threshold (Ct) was performed using an in-house real-time RT-PCR, targeting a 128 nucleotide fragment of the BCoV M gene, for which the original primers and probe can be found in Table S1 (supplementary material). RT-PCR reactions were performed using One Step RT-PCR System (Qiagen, Holden, Germany). Reaction mixtures were performed in a final volume of 25 μl, composed of 5 μl of 5 × reaction buffer, 1 μl of a dNTP solution (0.4 mM each), 1 μl of Enzyme mix (Reverse transcriptase and Taq polymerase), and 12 μl of RNA free water, provided in the kit. We added 1.5 μl of MgSO4, 0.4 μM of each BCoV-M550F and BCoV-M650R primers, 0.2 μM of BCoV-M600 probe, and 2.5 μl of RNA template. Thermal cycling involved 50 °C for 30 min, followed by 95 °C for 15 min and then 45 of 95 °C for 30 s and 60 °C for 1 min.
2.3. Sequencing of the three complete genes nsp12, S, and N and genotyping method
The sequences of BCoVs were named according to the following nomenclature: Virus name/FRA-EPI/location of sampling/year of sampling/specimen number. FRA corresponds to the acronym of France, EPI is an abbreviation for the EPICOREM consortium. In this study, the term “molecular isolate” was used for sequences of BCoV amplified directly from samples.
To determine the genomic sequence of nsp12, S, and, N genes, a set of 17 overlapping RT-PCR products, from 553 to 1260 nucleotides, were generated using a total of 42 primers (Lau et al., 2011, Hasoksuz et al., 2002, Martínez et al., 2012). RT-PCR reactions were performed using One Step RT-PCR System (Qiagen, Holden, Germany), from 2.5 μl of nucleic acid in a final volume of 25 μl, according the manufacturer's instructions. Briefly, reaction mixtures were composed of 5 μl of 5 × reaction buffer, 1 μl of a dNTP solution (0.4 mM each), 1 μl of Enzyme mix (Reverse transcriptase and Taq polymerase), and 13.5 μl of RNA free water, 0.4 μM of forward and reverse primers (Table S1, supplementary material), and 2.5 μl of RNA template. Thermal cycling involved 50 °C for 30 min, followed by 95 °C for 15 min and then 45 of 95 °C for 30 s, 56 °C to 60 °C (depending of primers used), and 72 °C for 1 min.
The RT-PCR products encompassed all three complete genes (Table S1, supplementary material). All the primers were tested in silico and in vitro with our control BCoV strain to test their efficacy and the primer abilities to provide us with quality sequencing products.
The bidirectional sequencing was performed at the Laboratory for Urgent Response to Biological Threats, Environment and Infectious Risks Unit, Institut Pasteur (Paris, France), following their sequencing protocol. The analysis of sequencing products was performed by the capillary electrophoresis 3730XL (Life Technologies, Carlsbad, CA, US).
2.4. Bioinformatic analysis
Sequences were assembled in contigs corresponding to the entire nsp12, S, or N genes with CodonCode Aligner Software, version 5.0.1 (CodonCode corporation, Centerville, MA, USA). Multiple sequence alignments were performed using Muscle algorithm, implemented in MEGA software, version 6.06 (http://www.megasoftware.net). Thirteen BCoV sequences from the USA, available in Genbank, were added to the alignment (Tables S2 and S3, supplementary material) (Chouljenko et al., 2001, Zhang et al., 2007, Mebus et al., 1973). Two additional prototype BCoV sequences from Japan and Canada, named Kakegawa and Quebec respectively, were also added (Gélinas et al., 2001, Yoo and Pei, 2001). Sixteen sequences from bovine-like coronaviruses were added as well, corresponding to coronaviruses collected from sable antelope, white-tailed deer, sambar deer, waterbuck, giraffe, alpaca, dog, and dromedary camel (Alekseev et al., 2008, Cebra et al., 2003, Woo et al., 2014, Erles et al., 2003, Hasoksuz et al., 2007). Finally, the sequence of HEC 4408 published by Zhang et al. and the 16 sequences of HCoV-OC43 previously published by our team were added (Zhang et al., 1994, Kin et al., 2015). The accession numbers corresponding to these sequences are given in Tables S2 and S3 (supplementary material). Note that there are no complete genomes of BCoV or BCoV-like isolates from Europe.
Maximum likelihood phylogenetic trees were constructed using the Tamura-Nei's (TN93) substitution model, according to Akaike Information Criterion (AIC) and Bayesian Information Criterion (BIC) values evaluated by a model testing (Aldrich, 1997, Tamura and Nei, 1993). A bootstrap test including 1000 replicates was performed for each tree (Felsenstein, 1985). All of these programs are implemented in MEGA6 software (Tamura et al., 2013). The topologies of trees were also double-checked using a neighbor-joining method (substitution model TN93), with a bootstrap test including 1000 replicates (Felsenstein, 1985, Saitou and Nei, 1987). The topologies of these trees constitute the basis of our genotyping methodology. The genotype of each BCoV or BCoV-like is defined based on the sub-cluster in which the three nsp12, S and N sequences of a same BCoV (or BCoV-like) is located on each tree. Finally, the genotype name is constructed by the juxtaposition of the sub-cluster names, C1, C2 or C3, on nsp12, S, and N tree respectively.
An additional maximum likelihood tree based on 143 complete sequences of S gene of BCoV, BCoV-like, and HCoV-OC43 from USA, Brazil, South Korea, China, Italy, Swedish, Denmark and France was constructed. To this end, 81S gene sequences from BCoVs and BCoVs-like were added to the previous S gene alignment (Martínez et al., 2012, Beaudeau et al., 2010, Decaro et al., 2009a, Jeong et al., 2005, Zhang et al., 1991, Park et al., 2007, Chung et al., 2011, Park et al., 2006). The accession numbers corresponding to these sequences are provided in Tables S2 and S3 (supplementary material). Phylogenetic trees were constructed using the maximum likelihood method with the substitution model TN93, implemented in MEGA6 (Aldrich, 1997, Tamura and Nei, 1993, Tamura et al., 2013). The topology of the tree was checked using a neighbor-joining (substitution model TN93) method, with a bootstrap test including 1000 replicates (Felsenstein, 1985).
3. Results
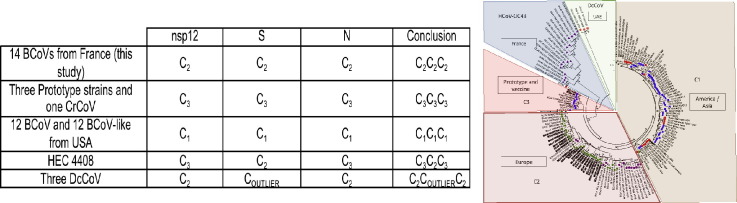
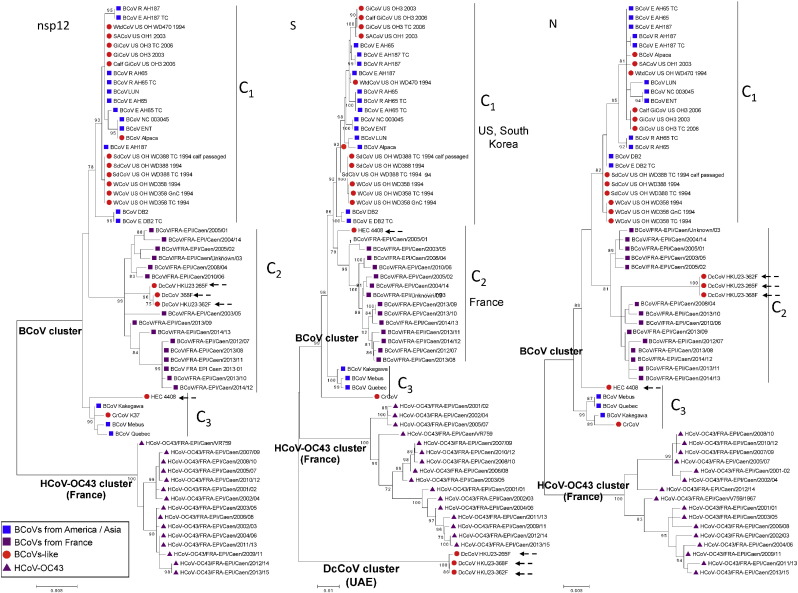
The phylogenetic analysis presented in Fig. 2 shows the nsp12, S, and N trees obtained by a maximum likelihood method. Two clusters, one grouping BCoVs and BCoVs-like and the other one containing HCoV-OC43s, are observed in the nsp12 and N trees. DcCoVs differentiates as a potential third cluster on the S tree. The BCoV cluster of the nsp12, S, and N maximum likelihood trees is divided into three sub-clusters. These sub-clusters are globally comprised of by the same molecular isolates for nsp12, S, and N trees. Nsp12, S, and N produced comparable tree topologies and therefore a globally congruent phylogenetic signal that does not suggest BCoV recombination events, contrary to HCoV-OC43 during the same period and in the same geographic area (Kin et al., 2015). The positions of BCoV and BCoV-like sequences on each tree are summarized in Table S2 (supplementary material). According to nsp12, S, and N trees, one sub-cluster named C1 contains BCoVs and BCoVs-like from the USA, constituting the genotype C1C1C1. A second sub-cluster, named C2, groups on each trees all the BCoV sequences generated in this study defining the genotype C2C2C2. Finally, the last sub-cluster, named C3, contains the prototype strains Kakegawa, Mebus, Quebec, and CrCoV on all trees, constituting the genotypes C3C3C3. Two exceptions are observed. First, the three DcCoVs are included on sub-cluster C2 on nsp12 and N tree, but are grouped in a potential outlier position from BCoV and HCoV-OC43 clusters, constituting a genotype named C2COUTLIERC2. The second exception is the HEC 4408 that is located on sub-cluster C3 on nsp12 and N trees, and in sub-clusters C2 on S tree, constituting the genotype C3C2C3. This analysis reveals a potential geographical distribution with a segregation of BCoV and BCoV-like sequences from US on one hand, and BCoV from France in another hand. An additional S tree, depicted in Fig. S4 (supplementary material), includes additional BCoV and BCoV-like sequences from the USA, Brazil, South Korea, China, Sweden, Denmark, and Italy. The topology of this additional S tree is concordant with the S tree presented in Fig. 2. The same tree clusters (BCoV and BCoV-like, HCoV-OC43, and DcCoV) were observed. In the same way, the three sub-clusters C1, C2, and C3 were observed inside to the BCoV cluster. The additional sequences include in this most detailed S tree allowed us to deeply characterize the geographic distribution of BCoV and BCoV-like. Indeed, in addition to USA sequences, the sub-cluser C1 contains BCoVs and BCoVs-like from both America and Asia continent, more precisely from China, South Korea, and Brazil. The sub-cluster C2 contains in addition to the 14 French BCoVs, 35 additional BCoVs from other European countries such as Sweden, Denmark, and Italy (cf. Tables S2 and S3 in supplementary material).
Fig. 2.
Phylogenetic analysis of the complete nsp12, S, and N genes of 29 BCoVs, 16 HCoV-OC43s, 17 bovine-like CoVs. The phylogenetic trees were constructed by the maximum likelihood method. Bootstrap values were calculated from 1000 replicates. Bootstrap values over 70% are shown. The evolutionary distances were computed using the Tamura Nei model and units are the number of base substitutions per site.
4. Discussion
The world population of cattle is estimated at 1.43 billion (Robinson et al., 2014). In France, the cattle population extends to around 19 million among which 1.9 million constitute the livestock of Lower Normandy, where the present study emerges from. BCoV infections are not listed as “monitored transmittable veterinary infections”. Therefore, no public data measuring the impact of this infection on livestock cattle, national or international, are available despite BCoV being an ubiquitous virus. Partial genomic sequences available in GenBank come from 17 countries, unequally distributed on five continents. Fifteen complete BCoV genomes from North America and South Korea (14 and 1, respectively) are currently available (according to GenBank, consulted on September 8th, 2015) but no BCoV sequence from France was available before the present study. Among the few available epidemiological studies, Beaudeau et al. showed that in Sweden, the seroprevalence of BCoV is ranging from 43 to 65% in cattle less than12 months, and observed a winter circulation in the temperate zones of the Northern hemisphere (Beaudeau et al., 2010). This epidemiology shares common characteristics with that of the HCoV-OC43 infection in humans, namely a primary infection in young patients in winter. BCoV and HCoV-OC43 belong to the clade A of Betacoronavirus genus sharing a global similarity of 96% on a nucleotide level. The divergence between HCoV-OC43 and BCoV is estimated at the end of the 19th century, thus contemporary with a species barrier crossing from cattle to humans. Six different genotypes have been identified in France among the 15 HCoV-OC43 circulating strains (including numerous recombinant variants) in respiratory samples taken over a 13-year period (Kin et al., 2015). This evolutive pattern (deeply affected by recombination) has been commonly described for other coronaviruses (Infection bronchitis virus, Feline coronavirus, Canine coronavirus) and proves, in these contexts, to be a factor that favors the potential emergence of new variants (Cavanagh et al., 1992, Herrewegh et al., 1998, Decaro et al., 2009b). There is no published data for the potential localization of recombination hot spot(s) on the BCoV genome. Given the genetic relatedness between HCoV-OC43 and BCoV, we propose the hypothesis that these potential hot spots could be located in the same regions for these two viruses. Extensive sequencing of complete BCoV genomes would ascertain the existence of these hot spots, but was not performed in this study.
The addition of BCoV sequences in France into the phylogenetic analysis does not modify global phylogeny since HCoV-OC43 and BCoV remain monophyletic in our analysis. This holds true even when the prototype strains (propagated in cellular culture) are included in our analysis (data not shown). In this light, BCoVs from France are grouped in a non-ambiguous way with the other BCoVs but remain themselves monophyletic. Base on complete or partial genome phylogenetic trees, BCoVs-like form a sister group to BCoVs and include BCoVs from various animal origins (Alekseev et al., 2008, Woo et al., 2014).
The topological analysis of the three phylogenetic trees obtained, nsp12, S, and N, shows the existence of two clusters, one including HCoV-OC43 and the other including both BCoV and BCoV-like, respectively. The BCoV cluster is divided into three sub-clusters: C1, C2, and C3. Results obtained using the chosen methodology do not show the existence of circulating BCoV recombinant variants. Indeed, all of the BCoVs in our study are of non-recombinant genotype C2C2C2 (see Table 2 ). All of the sequences of the 24 BCoVs and BCoVs-like coming from the US and included in the phylogenetic analysis are of a non-recombinant C1C1C1 genotype. In the same way, the sequences of three BCoV prototypes and the CrCoV included in the study are of a non-recombinant C3C3C3 genotype. Within this analysis of putative recombinant detection, only the coronavirus strain HEC 4408 shows a recombinant genotype of type C3C2C3. It is worthwhile to highlight that the HEC 4408 finds his root among the French isolates of BCoV (Fig. 2) and thus represents an independent sub-group within the BCoV cluster. The HEC 4408, serologically distinct from HCoV-OC43, illustrates the capacity of BCoV for spill-over independently of its role in the emergence of HCoV-OC43.
Table 2.
Distribution of sequences of BCoV and BCoV-like among the sub-clusters on nsp12, S, and N tree.
| nsp12 | S | N | Conclusion | |
|---|---|---|---|---|
| 14 BCoVs from France (this study) | C2 | C2 | C2 | C2C2C2 |
| Three Prototype strains⁎ and one CrCoV | C3 | C3 | C3 | C3C3C3 |
| 12 BCoV and 12 BCoV-like from USA | C1 | C1 | C1 | C1C1C1 |
| HEC 4408 | C3 | C2 | C3 | C3C2C3 |
| Three DcCoV | C2 | COUTLIER | C2 | C2COUTLIERC2 |
BCoV Mebus, Kakegawa and Quebec.
In the end, these results suggest that HCoV-OC43 and BCoV do not seem to evolve along the same dynamics, as it points to a generation of recombinant variants that seems very active in HCoV-OC43 as compared to BCoV. Given that these two viruses are extremely close with respect to genetic and epidemiological characteristics, this difference could be linked to different environmental constraints (sensu-lato). In this light, one may conceive of a lesser genetic diversity of the animal host (animal livestock versus human) resulting from domestication and breeding practices by human, and a lesser geographic mobility of animals, thereby weakening the BCoV diversifying selection process and decreasing potential co-infection and thus recombination events. These hypotheses must however be tested on a greater viral genetic dataset.
The results involving DcCoV sequences available in GenBank show a potential genotype C2 « COUTLIER » C2 . The potential outlier topology describes the fact that, in the S gene tree, the DcCoV sequences form a potential third cluster that is distinct from that of BCoV and HCoV-OC43.
The complementary phylogenetic analysis involving a great number of S gene sequences presented on Fig. S4 (supplementary material) highlights three clusters that group each of the HCoV-OC43s, BCoVs and BCoVs-like, and DcCoVs, respectively. In the cluster of BCoV and BCoV-like strains, a geographic distribution may be noted, having a so-called European sub-cluster and a so-called America-Asia sub-cluster. This distribution suggests that the divergence of the two sub-clusters of BCoVs is a relatively recent phenomenon with regards to the history of livestock cattle. Indeed, the Bos taurus taurus livestock cattle of the Old World (Europe, Asia, and Africa) are issued from the domestication facilities of the aurochs, a wild species living exclusively in the Old World. This domestication event occurred around 10,000 years ago. Livestock cattle arrived on the North and South American continent after the discovery of the New World in the XIth century (Bollongino et al., 2012, Loftus et al., 1994). The distinction of genetic clusters corresponding to the BCoV of different geographic origins could be attributed to the diffusion of strains favoring the exchange of animals incurred by international commerce. In Europe, France is the largest producer and exporter of cattle. Moreover, France exports live cattle for reproduction to Italy, Spain, Greece, and Germany, and also to Algeria, Tunisia, and Libya. The import–export of fresh and frozen beef between the US and the European Union has been minimal, especially after 1998 at which point the EU appealed to the World Trade Organization (WTO) to prohibit the import of beef for purposes of food safety. The final decision of the WTO was favorable to the European Union, but was not handed down until 2007. However, between 1998 and 2007, commerce between US and EU was greatly weakened.
The United States exports beef to Asia, especially to Japan and to South Korea. We recall that the BCoVs, as well as the BCoVs-like collected from ruminants in the USA and in South Korea are very similar. It is also worthwhile to note that the BCoV is not included in the WTO list of illness being monitored in international exchange.
In conclusion, the present epidemiological study has made it possible for the first time to obtain partial sequences of BCoV originating from France. The results obtained, despite their limits in methodology and in the number of sequences, suggest the existence of an evolutive pattern by recombination that is different between two very similar human and bovine coronaviruses. This study also draws light to the scarce availability of BCoV sequences and genetic analyses despite the fact that this pathogenic agent was identified over 50 years ago and that it seems to have a significant sanitary impact on livestock cattle.
The following are the supplementary data related to this article.
Primers and probes used for RT-PCR and full sequencing of nsp12, S, and N genes of 14 BCoVs. In bold are indicated the primers used by default. In non-bold are indicated the alternative primers. Primer locations are given based on the genome of BCoV Mebus strain (accession number U00735).
GenBank accession numbers associated with the complete genome sequences used in this study.
GenBank accession numbers associated to nsp12, S and N genes complete sequences used in this study.
Phylogenetic analysis of the complete S gene of 104 BCoV, 16 HCoV-OC43s, 23 BCoV-like. The phylogenetic trees were constructed using the maximum likelihood method. Bootstrap values were calculated from 1000 replicates. Bootstrap values of over 70% are shown. The evolutionary distances were computed using the Tamura Nei model and units are the number of base substitutions per site. Full lines delimit BCoV and BCoV-like, HCoV-OC43, and DcCoV clusters. Broken lines delimit sub-clusters C1, C2 and C3.
Funding
This work was supported by the French Agence Nationale de la Recherche (ANR) on the behalf of EPICOREM project (Eco-Epidemiology of Coronaviruses, from Wildlife to Human: Emergence Threat Assessment), grant ANR-13-BSV3-0013.
Acknowledgements
We are grateful to Stephane Pronost of departmental laboratory LABEO Franck Duncombe of Calvados and to Victor Carpinshi, Fabienne Benoît, and Delphine Esperet of departmental laboratory of Manche, for providing us the field samples. We thank all of our partners of the EPICOREM Consortium.
References
- Adams M.J., Carstens E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012) Arch. Virol. 2012;157:1411–1422. doi: 10.1007/s00705-012-1299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich J. Others RA Fisher and the making of maximum likelihood 1912–1922. Stat. Sci. 1997;12:162–176. [Google Scholar]
- Alekseev K.P., Vlasova A.N., Jung K., Hasoksuz M., Zhang X., Halpin R., Wang S., Ghedin E., Spiro D., Saif L.J. Bovine-like coronaviruses isolated from four species of captive wild ruminants are homologous to bovine coronaviruses, based on complete genomic sequences. J. Virol. 2008;82:12422–12431. doi: 10.1128/JVI.01586-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudeau F., Björkman C., Alenius S., Frössling J. Spatial patterns of bovine Corona Virus and bovine respiratory syncytial virus in the Swedish beef cattle population. Acta Vet. Scand. 2010;52:33. doi: 10.1186/1751-0147-52-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollongino R., Burger J., Powell A., Mashkour M., Vigne J.-D., Thomas M.G. Modern taurine cattle descended from small number of near-Eastern Founders. Mol. Biol. Evol. 2012;29:2101–2104. doi: 10.1093/molbev/mss092. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Cook J.K.A. Infectious bronchitis virus: evidence for recombination within the Massachusetts serotype. Avian Pathol. 1992;21:401–408. doi: 10.1080/03079459208418858. [DOI] [PubMed] [Google Scholar]
- Cebra C.K., Mattson D.E., Baker R.J., Sonn R.J., Dearing P.L. Potential pathogens in feces from unweaned llamas and alpacas with diarrhea. J. Am. Vet. Med. Assoc. 2003;223:1806–1808. doi: 10.2460/javma.2003.223.1806. [DOI] [PubMed] [Google Scholar]
- Chouljenko V.N., Lin X.Q., Storz J., Kousoulas K.G., Gorbalenya A.E. Comparison of genomic and predicted amino acid sequences of respiratory and enteric bovine coronaviruses isolated from the same animal with fatal shipping pneumonia. J. Gen. Virol. 2001;82:2927–2933. doi: 10.1099/0022-1317-82-12-2927. [DOI] [PubMed] [Google Scholar]
- Chung J.-Y., Kim H.-R., Bae Y.-C., Lee O.-S., Oem J.-K. Detection and characterization of bovine-like coronaviruses from four species of zoo ruminants. Vet. Microbiol. 2011;148:396–401. doi: 10.1016/j.vetmic.2010.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro N., Campolo M., Mari V., Desario C., Colaianni M.L., Di Trani L., Cordioli P., Buonavoglia C. A candidate modified-live bovine coronavirus vaccine: safety and immunogenicity evaluation. New Microbiol. 2009;32:109–113. [PubMed] [Google Scholar]
- Decaro N., Mari V., Campolo M., Lorusso A., Camero M., Elia G., Martella V., Cordioli P., Enjuanes L., Buonavoglia C. Recombinant canine coronaviruses related to transmissible gastroenteritis virus of swine are circulating in dogs. J. Virol. 2009;83:1532–1537. doi: 10.1128/JVI.01937-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles K., Toomey C., Brooks H.W., Brownlie J. Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology. 2003;310:216–223. doi: 10.1016/S0042-6822(03)00160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- Gélinas A.-M., Boutin M., Sasseville A., Dea S. Bovine coronaviruses associated with enteric and respiratory diseases in Canadian dairy cattle display different reactivities to < i > anti </i > − HE monoclonal antibodies and distinct amino acid changes in their HE, S and ns4. 9 protein. Virus Res. 2001;76:43–57. doi: 10.1016/S0168-1702(01)00243-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Enjuanes L., Ziebuhr J., Snijder E.J. Nidovirales: evolving the largest RNA virus genome. Virus Res. 2006;117:17–37. doi: 10.1016/j.virusres.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Sreevatsan S., Cho K.-O., Hoet A.E., Saif L.J. Molecular analysis of the S1 subunit of the spike glycoprotein of respiratory and enteric bovine coronavirus isolates. Virus Res. 2002;84:101–109. doi: 10.1016/S0168-1702(02)00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Alekseev K., Vlasova A., Zhang X., Spiro D., Halpin R., Wang S., Ghedin E., Saif L.J. Biologic, antigenic, and full-length genomic characterization of a bovine-like coronavirus isolated from a giraffe. J. Virol. 2007;81:4981–4990. doi: 10.1128/JVI.02361-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz M., Vlasova A., Saif L.J. Detection of Group 2a Coronaviruses with Emphasis on Bovine and Wild Ruminant Strains. In: Cavanagh D., editor. SARS- and Other Coronaviruses. Humana Press; 2008. pp. 43–59. (Methods in Molecular Biology). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institut National de Veille Sanitaire (INVS) 2015. Bulletin hebdomadaire international N°517, du 12 au 18 août. [Google Scholar]
- Jeong J.-H., Kim G.-Y., Yoon S.-S., Park S.-J., Kim Y.-J., Sung C.-M., Shin S.-S., Lee B.-J., Kang M.-I., Park N.-Y., Koh H.-B., Cho K.-O. Molecular analysis of S gene of spike glycoprotein of winter dysentery bovine coronavirus circulated in Korea during 2002–2003. Virus Res. 2005;108:207–212. doi: 10.1016/j.virusres.2004.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin N., Miszczak F., Lin W., Gouilh M.A., Vabret A. Consortium, E. Genomic Analysis of 15 Human Coronaviruses OC43 (HCoV-OC43s) Circulating in France from 2001 to 2013 reveals a high intra-specific diversity with new recombinant genotypes. Viruses. 2015;7:2358–2377. doi: 10.3390/v7052358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S., Lee P., Tsang A.K.L., Yip C.C.Y., Tse H., Lee R.A., So L.-Y., Lau Y.-L., Chan K.-H., Woo P.C.Y., Yuen K.-Y. Molecular epidemiology of human coronavirus OC43 reveals evolution of different genotypes over time and recent emergence of a novel genotype due to natural recombination. J. Virol. 2011;85:11325–11337. doi: 10.1128/JVI.05512-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauber C., Ziebuhr J., Junglen S., Drosten C., Zirkel F., Nga P.T., Morita K., Snijder E.J., Gorbalenya A.E. Mesoniviridae: a proposed new family in the order Nidovirales formed by a single species of mosquito-borne viruses. Arch. Virol. 2012;157:1623–1628. doi: 10.1007/s00705-012-1295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus R.T., Machugh D.E., Bradley D.G., Sharp P.M., Cunningham P. Evidence for two independent domestications of cattle. Proc. Natl. Acad. Sci. 1994;91:2757–2761. doi: 10.1073/pnas.91.7.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majhdi F., Minocha H.C., Kapil S. Isolation and characterization of a coronavirus from elk calves with diarrhea. J. Clin. Microbiol. 1997;35:2937–2942. doi: 10.1128/jcm.35.11.2937-2942.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez N., Brandão P.E., de Souza S.P., Barrera M., Santana N., de Arce H.D., Pérez L.J. Molecular and phylogenetic analysis of bovine coronavirus based on the spike glycoprotein gene. Infect. Genet. Evol. 2012;12:1870–1878. doi: 10.1016/j.meegid.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntosh K., Dees J.H., Becker W.B., Kapikian A.Z., Chanock R.M. Recovery in tracheal organ cultures of novel viruses from patients with respiratory disease. Proc. Natl. Acad. Sci. U. S. A. 1967;57:933. doi: 10.1073/pnas.57.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mebus C.A., Stair E.L., Rhodes M.B., Twiehaus M.J. Pathology of neonatal calf diarrhea induced by a coronavirus-like agent. Vet. Pathol. Online. 1973;10:45–64. doi: 10.1177/030098587301000105. [DOI] [PubMed] [Google Scholar]
- Park S.-J., Jeong C., Yoon S.-S., Choy H.E., Saif L.J., Park S.-H., Kim Y.-J., Jeong J.-H., Park S.-I., Kim H.-H., Lee B.-J., Cho H.-S., Kim S.-K., Kang M.-I., Cho K.-O. Detection and characterization of bovine coronaviruses in fecal specimens of adult cattle with diarrhea during the warmer seasons. J. Clin. Microbiol. 2006;44:3178–3188. doi: 10.1128/JCM.02667-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.J., Lim G.K., Park S.I., Kim H.H., Koh H.B., Cho K.O. Detection and molecular characterization of calf diarrhoea bovine coronaviruses circulating in South Korea during 2004–2005. Zoonoses Public Health. 2007;54:223–230. doi: 10.1111/j.1863-2378.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- Peiris J.S.M., Guan Y., Yuen K.Y. Severe acute respiratory syndrome. Nat. Med. 2004;10:S88–S97. doi: 10.1038/nm1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T.P., Wint G.R.W., Conchedda G., Van Boeckel T.P., Ercoli V., Palamara E., Cinardi G., D'Aietti L., Hay S.I., Gilbert M. Mapping the global distribution of livestock. PLoS One. 2014;9 doi: 10.1371/journal.pone.0096084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 1993;10:512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsunemitsu H., El-Kanawati Z.R., Smith D.R., Reed H.H., Saif L.J. Isolation of coronaviruses antigenically indistinguishable from bovine coronavirus from wild ruminants with diarrhea. J. Clin. Microbiol. 1995;33:3264–3269. doi: 10.1128/jcm.33.12.3264-3269.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijgen L., Keyaerts E., Moes E., Thoelen I., Wollants E., Lemey P., Vandamme A.-M., Van Ranst M. Complete genomic sequence of human coronavirus OC43: molecular clock analysis suggests a relatively recent zoonotic coronavirus transmission event. J. Virol. 2005;79:1595–1604. doi: 10.1128/JVI.79.3.1595-1604.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Huang Y., Yuen K.-Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009;234:1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Lam C.S.F., Lau C.C.Y., Tsang A.K.L., Lau J.H.N., Bai R., Teng J.L.L., Tsang C.C.C., Wang M., Zheng B.-J., Chan K.-H., Yuen K.-Y. Discovery of seven novel mammalian and avian coronaviruses in the Genus deltacoronavirus supports Bat Coronaviruses as the Gene Source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012;86:3995–4008. doi: 10.1128/JVI.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo P.C.Y., Lau S.K.P., Wernery U., Wong E.Y.M., Tsang A.K.L., Johnson B., Yip C.C.Y., Lau C.C.Y., Sivakumar S., Cai J.-P., Fan R.Y.Y., Chan K.-H., Mareena R., Yuen K.-Y. Novel betacoronavirus in dromedaries of the Middle East, 2013. Emerg. Infect. Dis. 2014;20:560–572. doi: 10.3201/eid2004.131769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo D., Pei Y. Full-length genomic sequence of bovine coronavirus (31 kb). completion of the open reading frame 1a/1b sequences. Adv. Exp. Med. Biol. 2001;494:73–76. [PubMed] [Google Scholar]
- Zaki A.M., van Boheemen S., Bestebroer T.M., Osterhaus A.D.M.E., Fouchier R.A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang X.M., Kousoulas K.G., Storz J. Comparison of the nucleotide and deduced amino acid sequences of the S genes specified by virulent and avirulent strains of bovine coronaviruses. Virology. 1991;183:397–404. doi: 10.1016/0042-6822(91)90154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.M., Herbst W., Kousoulas K.G., Storz J. Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. J. Med. Virol. 1994;44:152–161. doi: 10.1002/jmv.1890440207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Hasoksuz M., Spiro D., Halpin R., Wang S., Vlasova A., Janies D., Jones L.R., Ghedin E., Saif L.J. Quasispecies of bovine enteric and respiratory coronaviruses based on complete genome sequences and genetic changes after tissue culture adaptation. Virology. 2007;363:1–10. doi: 10.1016/j.virol.2007.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers and probes used for RT-PCR and full sequencing of nsp12, S, and N genes of 14 BCoVs. In bold are indicated the primers used by default. In non-bold are indicated the alternative primers. Primer locations are given based on the genome of BCoV Mebus strain (accession number U00735).
GenBank accession numbers associated with the complete genome sequences used in this study.
GenBank accession numbers associated to nsp12, S and N genes complete sequences used in this study.
Phylogenetic analysis of the complete S gene of 104 BCoV, 16 HCoV-OC43s, 23 BCoV-like. The phylogenetic trees were constructed using the maximum likelihood method. Bootstrap values were calculated from 1000 replicates. Bootstrap values of over 70% are shown. The evolutionary distances were computed using the Tamura Nei model and units are the number of base substitutions per site. Full lines delimit BCoV and BCoV-like, HCoV-OC43, and DcCoV clusters. Broken lines delimit sub-clusters C1, C2 and C3.